Abstract
Wound care will always be among the main tasks in all surgical specialties. Several medicinal plants have proven efficacy to cure wounds. Ethnobotanical research and ethnopharmacological research have virtually endless potential to find new lead compounds. The aim of this research review is to assess the potential of some Gentiana species as sources of promising active compounds to support wound healing. Gentians are among the most popular medicinal plants used in many countries for a wide spectrum of health conditions. Traditionally, those used to cure wounds are Gentiana lutea, G. punctata, G. asclepiadea, G. cruciata, G. oliverii, G. septemphida, and G. gelida. Candidate compounds with skin regeneration and wound-healing potential isolated from gentians are isogentisin, isoorientin, mangiferin, lupeol, pinoresinol, syringaresinol, eustomoside, and sweroside. Based on the rich source of traditional knowledge on the properties of gentians to cure various skin and soft tissue complications; only very few modern pharmacological studies have been performed to test this potential. Our review demonstrates that this field deserves further investigation. Many gentians are declining in number and have high IUCN conservation status, and cultivation and micropropagation methods are the only solution for the development of new drugs based on gentian extracts.
1. Introduction
Regardless of how far medical advancement proceeds, wound care will always be among the main tasks in all surgical specialties. Even as we find noninvasive or minimally invasive approaches to replace major surgery, trauma continues to exist alongside the chronic wounds, such as arterial and venous leg ulcers, diabetic foot, and pressure ulcers. Acute wounds are categorized as open traumatic injuries, and the standard approach in coping with them is usually mechanical: hemostasis, necrectomy, disinfection, drainage, and closure by means of certain devices (such as stitches, staplers, etc.). They might be complicated with infection, usually treated with antibiotics and antiseptics.
Chronic wounds, also known as ulcers, are of considerable medical, social, and economic importance. Brownrigg et al. [1] and Richmond et al. [1,2] report that approximately 2.5–4.5 million people suffer from chronic wounds in the USA alone. On average, these wounds persist for 12–13 months and reoccur in up to 70% of the patients, which leads to disability and a significantly poorer quality of life [2,3]. The main pathophysiological factors making these wounds so challenging include uncontrollable inflammation, infection, formation of nonpermeable biofilms, and inability of the tissue to adequately respond to reparative stimuli [4,5,6,7,8]. Typical approaches to their treatment involve prolonged use of antiseptics and some specific topical agents, which aim to destabilize the biofilm and promote tissue growth (such as providing a favorable moist environment, application of proteolytic enzymes, etc.). Nonchemical options include vacuum suction, physiotherapy (UV light and cold plasma), and even use of maggot larva to clean the wound bed [9]. However, most of the approaches currently used to cure both acute and chronic wounds lack evidence for their efficacy [10,11]. Medicinal plants, such as Curcuma longa, Terminalia arjuna, Centella asiatica, Bidens pilosa, Aloe barbadensis, Rauwolfia serpentina, Symphitum officinale, Hypericum perforatum, and Plantago major, are well known for healing wounds. Around the world, some of them are used in registered medications [12,13,14,15,16,17,18]. The aim of this review research is to assess the potential of some Gentiana species as a source of promising active compounds to support wound healing.
2. Materials and Methods
We accessed Google Scholar, Web of Science, and PubMed to identify publications for the period 1960–2022 with the search string: “Gentiana + ethnobotany”, “Gentiana + wounds”, “Gentiana + secondary metabolites”, “iridoid(s)” “secoiridoid(s)”, “flavonoid(s)”, “alkaloid(s)”, “xanthone(s)”, “lignans”, etc. Following the PRISMA 2000 guidelines, the records were assessed for eligibility, and the inappropriate ones were excluded. In addition, the Latin version of the text of Dioscorides was checked for the application of “gentian” as well as some local ethnobotanical publications [19,20,21,22,23,24,25,26,27,28,29,30]. Additionally, distribution maps were built for five species found to have applications to cure wounds, together with their IUCN conservation status assessments.
3. Results and Discussion
3.1. Ethnobotany—Gentiana Species Used in Folk Medicine in General
The “Traditional medicinal uses” of Gentiana species are summarized in Table 1. In general, gentians appear to be popular medicinal plants throughout Europe, with some species being used in different regions for diverse conditions. For instance, G. cruciata is the plant with the highest number of phytotherapeutic uses in the region of Suva Planina in Southeastern Serbia [19]. It is also among the most popular medicinal plants in Bulgaria [20].
However, the relative importance of a given Gentiana species as a medicinal plant may vary across the continent. This is the case for G. lutea, a plant from which preparations are very well established in the formulation of suitable remedies (e.g., to prepare brandy, Table 1). Indeed, whereas G. lutea has a wide spectrum of traditional uses in the Pyrenees, in the Suva Planina mountains, the species use is restricted to far fewer health conditions [21]. In summary, the popular traditional applications of gentians in Europe are for loss of appetite, as a stomachic, for gastrointestinal disorders, and for gallbladder and liver diseases. The traditional utilization of G. lutea is reflected by its being listed in the European Pharmacopoeia 2016, 2020 (as plant substance Gentianae Radix: 1365, Gentian tincture: 1366, 1870) as well as being listed in the European Medicines Agency handbook (EMA) 2018. In the Himalayan region, gentians are used to cure jaundice (Table 1).
In practice, both G. lutea and G. punctata can be replaced by G. asclepiadea and G. cruciata, respectively [22,23,24]. Other species, such as G. pneumonanthe, are also used as traditional remedies [25]. G. lutea is also among the most often traditionally used plants in Serbia and the Pyrenees [26]. The most popular application of this plant is as an appetizer. Other uses include as an antipyretic, for sores and minor wound healing; for stomach ulcers, as hepatoprotective; as hypoglycemic; as antianemic; as an immune stimulant; as cardiotonic; and as an astringent [27]. G. cruciata is one of the most popular medicinal plants in traditional Bulgarian medicine, and it has a wide spectrum of uses (Table 1) [20].
3.2. Ethnobotany—Gentiana Species Used in Folk Medicine to Cure Wounds
According to Dioscorides, the gentian (not perfectly clear exactly which species) is “a wound remedy, cures deep ulcers and helps with eye inflammation” [28]. In addition to this, he mentions many other properties, such as an antidote against venomous bites probably as a pain killer (he lists hernia, falls from heights, and dolor laterum—literally “pain in the sides”—this might be kidney stone pain or intercostal neuralgia), liver ailments, gastritis, and convulsions and as an abortifacient and against certain skin conditions (probably a form of leprosy). He also describes an interesting method of extraction: the roots are bruised and steeped in water for five days, then boiled, strained, and finally reduced to a thick liquid with the consistency of honey. The final product is stored in ceramic jars for further use.
Gentians are used to cure wounds in traditional medicine practices mainly in the Middle East, Caucasian and Mediterranean regions especially Iran, Turkey, Georgia, Bulgaria, and Greece, and also in the Cantabrian Mountains (Table 1). More detailed information about the wound-healing application of gentians in Bulgaria (Table 1), where we find the uses in the records of the Materials for Bulgarian Botanical Dictionary [20]. This valuable collection of common names and ethnobotanical data, such as the plant’s application for remedial purposes and also their use in traditional spells and magical rituals, were recorded by teachers, university professors, naturalists, folklorists, and physicians during 19th and 20th centuries [20].
The gentian used as poultices to cure eczema, ulcers, boils, purulent wounds, burns, and hard-to-cure wounds in Bulgaria is G. cruciata, while G. lutea, G. punctata, and G. asclepiadea are listed as substitutes (Table 1). According to some traditional Iranian texts, such as Makhzan Al Advieh, drinking a water extract of gentian root alone, or combined with wine, pepper, and rue, was used in traditional Persian medicine for wound healing, soft tissue inflammation, and skin and soft tissue wounds and animal bite treatments [29]. The plaster of gentian root with vinegar is used in traditional Iranian medicine to cure infected wounds in addition to other applications, such as the treatment of urinary retention, menstrual, liver, and spleen dysfunctions and the detoxification of animal (scorpion and viper) poisons [30]. The Gentians, which these authors list, in relation to their activity are G. olivieri., G. gelida, and G. septemfida as well as Gentianella caucasica.
More than ten Gentiana species occur in the Anatolian and Iranian Plateaus among which are G. aquatica L., G. cruciata L., G. gelida M.Bieb., G. olivieri Griseb., G. verna subsp. oschtenica (Kusn.) Halda., G. prostrata Haenke., G. riparia Kar. & Kir. and G. septemfida Pall [31,32]. In addition, G. septemfida in Georgia is often used against hemorrhoids, while in Turkey, several other gentians in addition to this one are applied for this purpose [33] (Table 1). Gentians are not mentioned for any medicinal application by Watkins in her research on native British plants used in the 10th century by Anglo Saxons [34]. However, to cure chronic wounds, they used a topical preparation of Centaurium erythraea, in addition to its other applications for snakebite, eye pain, poor vision, ‘anyone dangerously ill’, and possibly a specific medical condition, such as fever or nerve spasms.

Table 1.
Application of Gentiana species in various traditional medicines and regions.
Table 1.
Application of Gentiana species in various traditional medicines and regions.
| Region | Part(s) Used | Application Mode and Indications | References |
|---|---|---|---|
| Gentiana spp. | |||
| Epirus, Greece | -- | On wounds, digestive system ailments, anthelminthic | [35] |
| Traditional Persian medicine | Root | Water extract to treat different skin and soft tissue wounds and animal bites Widely recorded for hemorrhoids | [29] |
| G. lutea L. | |||
| Bukovina Central Eastern Carpathians Ukrainian part | Roots | Tea, diabetes, stomach ache, potency problems, and gastric ulcers | [36] |
| Infused in alcohol and good for the liver | [37] | ||
| Bukovina Central Eastern Carpathians Romanian part | Roots | Infused in strong alcohol—beverage | [37] |
| Peshkopia, Rraice, and Mokra (Eastern Albania). | Aerial parts | Tea Cardiotonic | [26] |
| Pirot District, Serbia | Roots | Cold water and strong alcohol (raki) macerates improving the immune system, against circulatory disorders, aphrodisiac, digestive, stomach ache and ulcers, gastrointestinal disorders, beneficial effects in gall and liver diseases, blood purification, heart diseases, anemia, and as a mild sedative “lincura” | [38] |
| Suva Planina Mts. Serbia | Roots | Abdominal pains, digestive, strengthening the immune system (lincura—‘gentian brandy’) | [19] |
| Kosovo (only in Serbian villages) | Aerial parts | Tea for blood | [39] |
| Prokletije Mts. Montenegro | Root | Loss of appetite, as a stomachic, as well as a component in preparations showing beneficial effects in gall and liver diseases loss of appetite, flatulence, and digestive ailments, for worms | [40] |
| North and northeast Bosnia and Herzegovina | Root | Ailments: loss of appetite, flatulence, and digestive ailments, for worms, influenza infection | [41] |
| Friulians in northeast Italy and Slovenia, | As ointments obtained from plant maceration in grappa | [42] | |
| Swiss Alps | Recreational grappa | [42] | |
| Cantabrian Mountains Spain | Root | Decoction for purulent wounds | [43] |
| G. lutea subsp. symphiandra (Murb.) Hayek | |||
| Bulgaria | Root | “Same application like G. cruciata, but it has stronger activity” (see below for Bulgaria) incl. wounds | [20] |
| Pešter Plateau (southwestern Serbia) both Serbian and Albanian communities | Roots | Tea or cold macerate in water—digestive troubles, stomach ache, diarrhea | [27] |
| Northern Albanian Alps | Root | Macerated in raki (strong alcohol) and taken to treat heart diseases | [44] |
| Bosnia and Herzegovina Dinaric Alps | Root | Stomach and heart disorders, recovery, macerate, decoction, tincture | [45,46] |
| Aosta Valley, Western Alps, Italy | Root | Liqueur and grappa as digestive root decoction against digestive and liver problems, as tonic and appetizer, root decoction in wine as invigorating and against anemia | [47] |
| Spain | Roots | Boiled or soaked in wine or in a liqueur—digestive beverage | [48] |
| North and northeast Bosnia and Herzegovina | Root | Ailments: loss of appetite, flatulence, and digestive ailments, for worms, cardiovascular system disorders | [41] |
| G. punctata L. | |||
| Bulgaria | Root | “Identical application like G. lutea” (see above for Bulgaria) incl. wounds | [20] |
| Pirot District, Serbia | Roots | Infused in strong alcohol—beverage, loss of appetite, gastrointestinal disorders, beneficial effects in gall and liver diseases, anemia, improves digestion, against circulatory disorders, and as a mild sedative. | [38] |
| Prokletije Mts. Montenegro | Roots | Loss of appetite, as a stomachic, as well as a component in preparations showing beneficial effects in gall and liver diseases | [40] |
| Aosta Valley, Western Alps, Italy | Root | Liqueur and grappa as digestive root decoction against digestive and liver problems, as tonic and appetizer, root decoction in wine as invigorating and against anemia | [47] |
| G. asclepiadea L. | |||
| Bulgaria | Root | “Same application like G. cruciata” (see below for Bulgaria) incl. wounds | [20] |
| Bukovina Central Eastern Carpathians Romanian part | Aerial parts | Infused in strong alcohol—beverage | [37] |
| Serbia | Root “the underground parts yellow as wax” | Remedy against jaundice, after jaundice, digestive disorders, to improve appetite, against gall and liver diseases, against anemia, and for general strengthening of the immune system | [38] |
| Prokletije Mts. Montenegro | Roots | Loss of appetite, as a stomachic, component in preparations showing beneficial effects in gall and liver diseases | [40] |
| Bosnia and Herzegovina Dinaric Alps | Roots | Infusion, decoction, tincture for diseases of internal organs, especially liver and pancreas | [45] |
| G. verna L. | |||
| Aosta Valley, Western Alps, Italy | Flowers (fresh or dried) | Liqueur and grappa as digestive and appetizer, invigorating against headaches caused by cold and respiratory problems | [47] |
| G. cruciata L. | |||
| Bulgaria | Aerial parts | Decoction, improves appetite, abdominal pain, gastrointestinal disorders, diarrhea, constipation, beneficial effects in gall and liver diseases, jaundice, anemia, blood purification, malaria, tuberculosis, gout, dysmenorrhea, amenorrhea, kidney sand stone and inflammation, bronchitis, improves lactation, calming on the CNS, against hysteria and hypochondria, vertigo, against worms, hemorrhoids, decoction or tincture for poultices to cure eczema, ulcers, boils, purulent wounds, burns, hard-to-cure wounds. | [20] |
| Pirot District, Serbia | Aerial parts | Cold water or strong homemade alcohol macerate—cancer, cold, and fever; improves the immune system, diabetes, inflammation, blood purification, high blood pressure, lung disease. | [38] |
| Suva Planina Mts. Serbia | Whole plant in flower | Women’s illnesses, cholesterol, diabetes, to improve digestion, liver and gallbladder complaints, stomach ailments (stomach ulcers), diseases related to the throat and esophagus, diseases of the chest, blood detoxification, improves appetite and immune system, antianemic (tea) | [19] |
| Prokletije Mts. Montenegro | Roots, aerial parts | Loss of appetite, as a stomachic, as well as a component in preparations showing beneficial effects in gall and liver diseases | [40] |
| Mountain Regions of Far Eastern Europe (Caucasus to Middle Urals) | Whole plant, eaves | Infusion is used for epigastric pains, rabies, and plague, as antifebrile and anthelmintic, for wound healing, rheumatoid arthritis, gout, and early chlorosis and topically for purulent wounds; used for liver, gallbladder, and stomach ailments | [49,50,51] |
| Transcaucasus | Decoction is used for diseases of the stomach, malaria, hemorrhoids, infertility, and as hemostatic | [49,50,51] | |
| Middle Urals | Infusion is used for headaches and as anthelmintic | [49,50,51] | |
| G. kochiana Perr. et Song. | |||
| Prokletije Mts. Montenegro | Roots, aerial parts | Loss of appetite, as a stomachic, as well as a component in preparations showing beneficial effects in gall and liver diseases | [40] |
| G. acaulis L. | |||
| Aosta Valley, Western Alps, Italy | Flowers (fresh or dried) | Liqueur and grappa as digestive and as appetizer, invigorating against headaches caused by cold and respiratory problems | [47] |
| G. pneumonanthe L. | |||
| Deliblato Sands, Serbia | Rhizome | Tea is used as a tonic, for strengthening the digestive organs and improving appetite | [25] |
| G. septemfida Pall. | |||
| Mountain Regions of Far Eastern Europe | Leaves Roots | Cholagogue, for stomach pain and liver and gallbladder ailments Water extract and the decoction are used to treat malaria and for stomach problems | [49,50,51] |
| Azerbaijan and Georgia | Roots Leaves | Decoction is used to treat malaria and for stomach problems Prepared as tea and used as cholagogue, for stomach pain and liver and gallbladder ailments | [23,52,53,54,55] |
| Georgia | -- | Cardiovascular, etc. | [33] |
| Turkey | -- | Cardiovascular, hemorrhoids, etc. | [33] |
| Iran | Root | Plaster with vinegar against bites of poisonous animals and to cure infected wounds, to treat urinary retention, menstrual, liver and spleen dysfunctions. | [30] |
| G. olivieri Griseb. | |||
| Turkey | Flowering aerial parts, macerate | Lowering the blood glucose in type II diabetes (methanol extract showed potent hypoglycaemic activity) | [56] |
| Turkey | Aerial parts, raw leaf | Wound healing | [57] |
| Iran | Root | Plaster with vinegar against bites of poisonous animals and to cure infected wounds, to treat urinary retention, menstrual, liver and spleen dysfunctions. | [30] |
| G. gelida Bieb | |||
| Turkey | Flowering aerial parts, decoction | Bronchitis, uretic | [58] |
| Turkey | Aerial parts, powder (eaten with honey) or decoction | Against jaundice | [59] |
| Iran | Root | Plaster with vinegar against bites of poisonous animals and to cure infected wounds, to treat urinary retention, menstrual, liver, and spleen dysfunctions. | [30] |
| G. kurroo Royle | |||
| Endemic to Western and North-western Himalayas | Root and rhizome Leaves | Stomach ache and urinary infections, fever Oil extract applied on ulcers and fungal infections | [60] |
| Pabbar Valley Western Himalayas | Root | Diabetes, digestive disorders, toothache (root powder orally) | [61] |
| G. moorcroftiana Wall. ex G. Don. | |||
| Indian Western Himalayas | Aerial parts, juice | Jaundice and blood purification | [62] |
| G. tubiflora (G. Don.) Griseb. | |||
| Indian Western Himalayas | Aerial parts, juice | Jaundice | [62] |
| 10 species of the Section Cruciata G. dahurica Fisch., G. crassicaulis Duthie ex Burk., G. siphonantha Maxim. ex Kusnez., G. waltonii Burk. G. lhassica Burk., G. straminea Maxim., G. robusta King ex Hook. f., G. dendrologi Marq., G. tibetica King ex Hook. f., G. officinalis H. Smith | |||
| Qinghai–Tibet Plateau | Treatment of Chi-Ba disease with symptoms of jaundice | [63] | |
3.3. Phytochemistry of Gentiana Species Used Traditionally to Cure Wounds
Many of the useful therapeutic effects produced by the Gentiana genus compositions can be attributed to the structure–activity relationship (SAR) of these chemical compounds. This genus is characterized by the presence of various secoiridoids, iridoids, xanthones, flavonoids, terpenoids, and their glycosidic forms, which contribute to wound healing and other anti-inflammatory properties [64,65,66]. Many studies revealed the effectiveness of these metabolites in the wound-healing process via different mechanisms, for example, an increase in the stimulation of collagen production, fibroblast cell growth stimulation, and re-epithelization [67,68,69]. Several investigations on certain compounds (which also reported on various Gentiana spp.), such as isogentisin (1), isoorientin (2), mangiferin (3), lupeol (4), pinoresinol (5), syringaresinol (6), eustomoside (7), and sweroside (8) revealed their potential in wound healing and skin regeneration procedures (Figure 1, Table 2) [67,70,71,72,73,74,75]. These molecules advance skin and wound healing through a variety of mechanisms, including sweroside’s inhibition of reactive oxygen species (ROS) and increased formation of the procollagen complex [76]. According to Harish et al.’s study, lupeol increased the epithelialization rate of the incision wound via the Wnt signaling pathway via glycogen synthase kinase 3-protein [77]. Another study identified that isoorientin has an impact on skin regeneration by upregulating epidermal involucrin via activating the expression of protein kinase C, p38, and ERK 1/2 [78]. Similar findings were also reported about mangiferin’s impacts on IL-1β, TNFα, and COX-2 and an immunohistochemical marker of angiogenesis in wound healing [79].
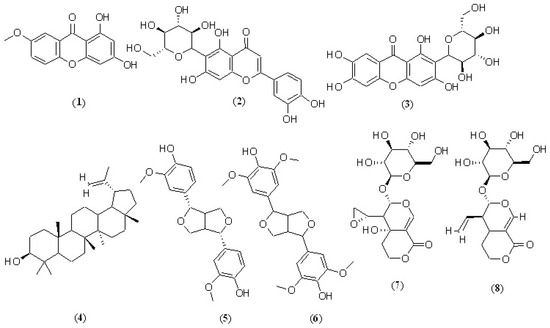
Figure 1.
Candidate Gentian compounds with skin regeneration and wound-healing potential. isogentisin (1), isoorientin (2), mangiferin (3), lupeol (4), pinoresinol (5), syringaresinol (6), eustomoside (7), and sweroside (8).

Table 2.
Some of the most important compounds in seven European and Asia Minor Gentiana species (Gentiana lutea, G. punctata, G. asclepiadea, G. septemfida cruciata, G. oliverii, and G. gelida), which were found to be used in relation to wound-healing practices in traditional medicine (see Table 1). Legend: This compound is of uncertain origin and is rather an artifact of the extraction process.
Table 2.
Some of the most important compounds in seven European and Asia Minor Gentiana species (Gentiana lutea, G. punctata, G. asclepiadea, G. septemfida cruciata, G. oliverii, and G. gelida), which were found to be used in relation to wound-healing practices in traditional medicine (see Table 1). Legend: This compound is of uncertain origin and is rather an artifact of the extraction process.
| Iridoid and Secoiridoid Aglycones and Glycosides | |||||||
|---|---|---|---|---|---|---|---|
| lutea | punctata | asclepiadea | septemfida | cruciata | oliverii | gelida | |
| Amarogentin | Roots [80,81,82,83,84] Leaves [83] | Roots [85] | |||||
| Amaroswerin | Roots [84,86] | ||||||
| (-)-Gentiolactone | Roots [87] | ||||||
| Gentioflavoside | Roots [88,89] | ||||||
| Gentiolutelin | Fresh roots [80] | ||||||
| Gentiolutelin dimethylacetal | Fresh roots [80] | ||||||
| Gentiopicrin | Aerial parts, Roots [90,91] | Aerial parts [92] | |||||
| 6’-O- Gentiopicrin | Roots [90] | ||||||
| Olivierigenin | Aerial parts [93] | ||||||
| Gentiopicroside | Seeds [94] Roots [67,83,84,86,95,96,97,98] Leaves [83,99,100] Flowers [101] | Roots [88,89,97] | Roots [92] | Roots [92,102] | Aerial parts [93] | Aerial parts [103] | |
| Olivieroside A | Aerial parts [93] | ||||||
| Olivieroside B | Aerial parts [93] | ||||||
| Olivieroside C | Whole plant [104] | ||||||
| 6’-O-β-D-glucosyl- gentiopicroside | Whole plant [104] | ||||||
| Swertiapunimarin | Whole plant [104] | ||||||
| Gelidoside | Aerial parts [103] | ||||||
| Gentomoside | Aerial parts [103] | ||||||
| Gentiopicroside (6-O-β-D-glucopyranoside of) | Roots [105] | ||||||
| Gentioside | Roots [106] | Roots [88,89,97,107] | Roots [92] (isomers) | ||||
| Eustomorusside | Leaves [99] | Whole plant [104] | Aerial parts [103] | ||||
| Eustomoside | Leaves [99] | Whole plant [104] | Aerial parts [103] | ||||
| Iridoid A | Roots [107] | ||||||
| Loganic acid | Roots [81,95,96,100,105] | Roots [107] | Roots [102] | ||||
| Loganin | Roots [107] | ||||||
| Trifloroside | Seeds [94] | Aerial parts [108] | Aerial parts [108] | ||||
| Scabran G3 | Fresh roots [81] | Aerial parts [103] | |||||
| ScabranG4 | Fresh roots [81] | ||||||
| Septemfidoside | Leaves [99] Flowers [101] | Whole plant [104] | |||||
| Sweroside | Seeds [94,95,96] Roots [67,97] | Roots [88,89,97,109] | Roots [92] | Aerial parts [91] | Roots [92,102] Aerial parts [92] | Whole plant [104] | |
| Sweroside (6-O-β-D-glucopyranoside) | Roots [105] | ||||||
| Swertiamarin | Roots [67,81,83,84,95,97,100] | Roots [88,89,97,109] | Roots [92] | Aerial parts [91] | Roots [92,102] Aerial parts [92] | Aerial parts [103] | |
| Flavonoids | |||||||
| lutea | punctata | asclepiadea | septemfida | cruciata | oliverii | gelida | |
| Homorientin | Leaves [83] | ||||||
| Isoorientin | Plant parts [89] | Leaves [89,110] | Aerial parts, Roots [91] | Aerial parts [93,111] | |||
| Luteolin | Roots [85] | ||||||
| Luteolin-7-O-glucoside | Roots [85] | ||||||
| Chrysin | Roots [85] | ||||||
| Apigenin | Roots [85] | ||||||
| Quercetin | Roots [85] | ||||||
| Rutoside | Roots [85] | ||||||
| Naringenin | Roots [85] | ||||||
| Isoorientin-4′-O-β-D-glucoside | Leaves [110] | ||||||
| Isoorientin-2′’-O-β-D-glucoside | Leaves [112] | ||||||
| trans-Cafeoyl- 2″-isoorientin-4′-0-β-D-glucoside | Leaves [112] | ||||||
| trans-Feruloyl-2″-isoorientin-4′-O-β-D-glucoside | Leaves [112] | ||||||
| Isosaponarin | Roots [113] | ||||||
| Isosaponarin-6′’-O-β-D-xylopyranoside | Roots [113] | ||||||
| Isovitexin | Leaves [83,89,99] | Leaves [89,114] | Aerial parts, Roots [90,91] | ||||
| Isovitexin-4′-O-glucoside | Leaves [14] | ||||||
| Isoscoparin | Leaves [112] | ||||||
| trans-Feruloyl-2″-isovitexin | Leaves [112] | ||||||
| trans-Feruloyl-2′-isovitexine-4′-O-β-D-glucoside | Leaves [112] | ||||||
| Alkaloids | |||||||
| lutea | punctata | asclepiadea | septemfida | cruciata | oliverii | gelida | |
| Gentianadine | Roots [115] | Roots [116] | Aerial parts [117] | ||||
| Gentianine | Roots [115,118] | Roots [116] | Roots [90] | Aerial parts [117] | |||
| Gentiananine | Aerial parts [119] | ||||||
| Oliverine | Aerial parts [119] | ||||||
| Gentioflavine | Roots [118] | Roots [116] | |||||
| Alkaloid I | Aerial parts [120] | ||||||
| Alkaloid II | Aerial parts [120] | Aerial parts [120] | |||||
| Alkaloid V | Aerial parts [118] | Aerial parts [118] | |||||
| Dehydrogentioflavine | Roots [116] | ||||||
| Gentiotibetine | Roots [116] | Aerial parts [119] | |||||
| 3,4-dihydro-1H,6H,8H-naphtho [1,2-c:4,5-c’,d’]dipyrano-1,8-dione | Roots [121] | ||||||
| Xanthones | |||||||
| lutea | punctata | asclepiadea | septemfida | cruciata | oliverii | gelida | |
| Gentianin | Roots [82,97,115] | ||||||
| Gentisein | Roots [89,122] Fresh roots [81] | Roots [89] | |||||
| 2-Hydroxygentisein | Roots [89] | ||||||
| 8-Hydroxygentisein | Roots [89] | ||||||
| Gentisin | Roots [95,122] Fresh roots [81] | ||||||
| 7-Hydroxy-3-methoxy-1-O-primeverosylxanthone | Roots [106] | ||||||
| 1-Hydroxy-3-methoxy-7-O-primeverosylxanthone | Roots [106] | ||||||
| Isogentisin | Roots [80,82,95,122] Fresh roots [81] Flowers [101] Leaves [82] | Roots [90] | |||||
| Mangiferin | Leaves [82,83] | Aerial parts, Roots [90,91] | |||||
| 2,3′,4,6-Tetrahydroxybenzophenone | Fresh roots [81] | ||||||
| Lignans and related compounds | |||||||
| lutea | punctata | asclepiadea | septemfida | cruciata | oliverii | gelida | |
| (–)-Berchemol | Fresh roots [81] | ||||||
| (–)-Berchemol | Fresh roots [81] | ||||||
| Gentioluteol | Fresh roots [81] | ||||||
| (+)–1-Hydroxysyringaresinol | Fresh roots [81] | ||||||
| (+)-Fraxiresinol | Fresh roots [81] | ||||||
| (+)-1-Hydroxypinoresinol | Fresh roots [81] | ||||||
| Syringaresinol 4′-O-β-D-glucopyranoside | Fresh roots [81] | ||||||
| Anofinic acid | Fresh roots [81] | ||||||
| 2-Hydroxyanofinic acid | Fresh roots [81] | ||||||
| 2-Methoxyanofinic acid | Fresh roots [81] | ||||||
| Syringaresinol | Fresh roots [81] | ||||||
| (+)-Medioresinol | Fresh roots [81] | ||||||
| (–)-Pinoresinol | Fresh roots [81] | ||||||
| Gentiolutelin dimethylacetal | Fresh roots [81] | ||||||
| Gentiolutelin | Fresh roots [81] | ||||||
| Olivieridepside | Aerial parts [93] | ||||||
3.4. Pharmacological Tests for Wound Healing by Gentiana Species
Although many of the world’s traditional systems, such as traditional European medicine and traditional Iranian medicine, emphasize gentian’s use in various skin and soft tissue complications, some studies have been conducted on the potential of gentian for wound healing.
Although Gentiana lutea’s most common and traditional use is to treat digestive disorders, it is worth paying attention to its many other biological effects. Mathew et al. examined the ethanol and petroleum ether rhizome extracts of G. lutea for wound-healing activity at oral doses of 300 and 500 mg/kg/day [123]. According to their findings, both extracts significantly increased the incision and excision wound models. Other research found that three secoiridoid glycosides isolated from G. lutea ssp. symphyandra (gentiopicroside, sweroside, and swertiamarine) stimulated collagen production and mitotic activity in cultured chicken embryonic fibroblasts [67]. An ethanolic extract of G. lutea rhizomes increased the activity of human keratinocyte lipid and ceramide synthesis, which is required for the formation of the epidermal barrier [124,125]. According to one study, the extracts of G. lutea root have anti-inflammatory, antiproliferative, and antifungal properties [126]. Other Gentiana species are also promising for wound cure. A methanolic extract of G. macrophylla root demonstrated notable activity against bacterial strains isolated from burn wounds with a phenolic and flavonoid content of the extract of 26.70 ± 1.5 mg gallic acid equivalent (GAE)/g·DW and 10.11 ± 0.8 mg quercetin equivalent/g·DW, respectively [127]. Research on G. scabra saw ethanolic root extract demonstrating beneficial effects on skin lesions and thickness in a mouse contact dermatitis model [128].
3.5. Conservation Status of Gentiana Species Used Traditionally to Cure Wounds
Medicinal plants associated with traditional uses are often wild-harvested, and thus ethnobotanical research should consider conservation issues. For example, it is well-known that G. lutea is still collected in the wild in the Pyrenees for medicinal uses despite legal protection [21]. Furthermore, since locals may use G. lutea and G. burseri Lap. indiscriminately, with the latter being much rarer than the former, there is also a risk of these species becoming endangered [21] (Figure 2 and Figure 3).
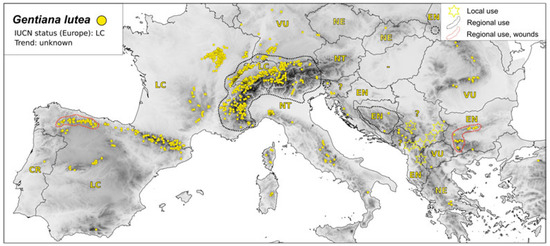
Figure 2.
Map of distribution of G. lutea—conservation status and medicinal use, Legend: IUCN status—Least Concern (LC); Near Threatened (NT); Vulnerable (VU); Endangered (EN); Critically Endangered (CR); and Not Evaluated (NE).
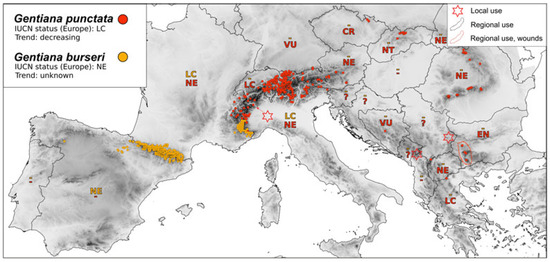
Figure 3.
Map of distribution G. punctata and G. burseri—conservation status and medicinal use, Legend: IUCN status—Least Concern (LC); Near Threatened (NT); Vulnerable (VU); Endangered (EN); Critically Endangered (CR); and Not Evaluated (NE).
In the Suva Planina range, ethnobotanical research established that G. lutea has the highest Use Value [UV = 1], calculated as a quotient of number of citations per species and number of informants, which indicates a strong anthropogenic pressure on this species in Serbia as well [19] (Figure 2). It is dried and sold in the mountain villages of Peshkopia, Eastern Albania [26]. The situation is similar in other parts of its range [46]. Wild yellow gentians are also collected to sell in Italy [129]. In addition, high UV is reported for G. lutea subsp. symhyandra in Bosnia and Herzegovina [45] where this taxon is evaluated as endangered (Figure 2).
Many gentians are declining in population and have a high International Union for Conservation of Nature (IUCN) conservation status in several European countries, but in others, they are considered Least Concerned and are not even listed in the Red Data books (Figure 2, Figure 3, Figure 4 and Figure 5). Populations of Gentiana lutea as well as G. punctata are declining in most territories of Europe (Figure 2 and Figure 3). G. lutea subsp. lutea L. is rare in Romania and Slovenia, where it is evaluated as Endangered, and in Portugal, where it is Critically Endangered [130] (Figure 2).
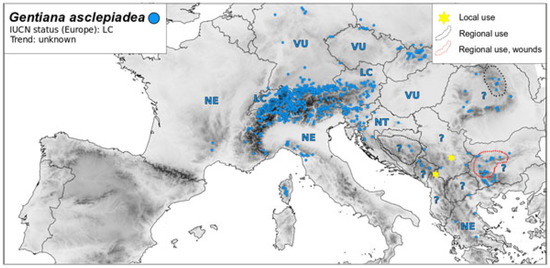
Figure 4.
Map of distribution G. asclepiadea—conservation status and medicinal use, Legend: IUCN status—Least Concern (LC); Near Threatened (NT); Vulnerable (VU); Endangered (EN); Critically Endangered (CR); and Not Evaluated (NE).
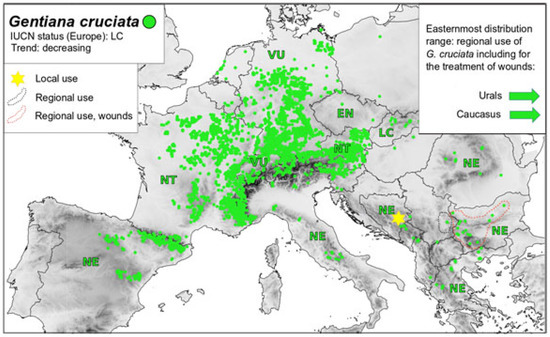
Figure 5.
Map of distribution G. cruciata—conservation status and medicinal use, Legend: IUCN status—Least Concern (LC); Near Threatened (NT); Vulnerable (VU); Endangered (EN); Critically Endangered (CR); and Not Evaluated (NE).
The typical subspecies has the Least Concern IUCN category, for instance, in the Czech Republic [131] and according to the European Environmental Agency, basically for most of the territory of Europe. However, this information needs revision because in Italy, G. lutea. subsp. lutea is considered Near Threatened, and in Sardinia, it is Endangered [132]. This subspecies does not occur in Bulgaria (although it is listed as Least Concerned) [130], and the only taxon growing in the mountains there is G. lutea subsp. symhyandra (Murb.) Hayek, which is evaluated as Endangered [133]. It is protected by the Biodiversity Act of the country but still collected in the wild by poachers according to Peev and coauthors [134] and Kozuharova (pers. comm. with a ranger in Pirin National Park in 2022).
G. asclepiadea is considered common in Bulgaria (and has not been evaluated in accordance with IUCN criteria). However, the popularity of this plant has led to instances of overharvesting, and it is now strictly protected in Poland [135], (Figure 4). Other gentians, such as G. cruciata (see Figure 5) and G. pneumonanthe (not shown), are considered common and thus are not evaluated in Bulgaria, Greece, Bosnia and Herzegovina, Italy, etc., but they are rare and threatened in the territory of other European countries (Figure 5) with all the consequences for biodiversity as gentians are host plants for highly specialized Blue Butterflies [136,137,138,139,140,141,142,143,144].
3.6. Cultivation and Micropropagation of Gentiana Species Used Traditionally to Cure Wounds
G. lutea has been cultivated in France for years [145]. Based on the ethnobotanical research in the mountain regions of the Pirot District, Serbia (Table 1), the cultivation of G. lutea and G. cruciata ought to be possible, due to the similarity of natural habitats in both France and Serbia. Thus, it could support the conservation of the wild populations and improve the living standard of the rural communities in the area [38]. As a result, the cultivation of G. lutea has been in progress for six years in Mount Tara (Serbia) [146].
Based on bioecological studies of G. lutea subsp. symhyandra in Bulgaria, Peev et al. (2018) concluded that this plant needs to be cultivated under conditions closely matching those of the natural habitats [134]. Micropropagation methods have already been developed for G. pneumonanthe [147], G. kurroo, G. cruciata, G. pannonica, and G. tibetica using somatic embryos [80], or by organogenesis for 11 other species (G. acaulis, G. lutea, G. purpura, G. cruciata, G. cerina, G. corymbifera, G. kurroo, G. punctata, G. tibetica, G. pneunomonante, and G. triforia [148]. There are no publications, other than two, about G. asclepiadea, published by Zając and Pindel [134,135,149]. Shoot multiplication of G. kurroo was achieved in vitro using shoot tips and nodal segments as explants [150]. An efficient protocol for callus regeneration and micropropagation has been developed [151].
4. Conclusions
There is rich traditional knowledge on the properties of gentians to cure various skin and soft tissue complications. However, only a few modern pharmacological studies have been performed to test this wound-healing potential. Nevertheless, they confirm the traditional practice to use gentians for wound healing. Limitations appeared also to be the insufficient phytochemical studies of some Gentiana species. Our review indicates that this field deserves further investigation. Many gentians are a declining species and have high IUCN conservation status. This is another limitation. However, our study points to prospects in cultivation and micropropagation methods, which are promising solutions for the development of new drugs based on gentian extracts.
Author Contributions
Conceptualization, E.K., A.P. and Z.N.; methodology, E.K., A.P., P.K., D.B., A.F. and Z.N.; software, A.F.; validation, A.P., A.H. and I.A.; investigation, D.B., P.K., E.K., A.H., A.P., Z.N. and A.F.; writing—original draft preparation, E.K. and A.P.; writing—review and editing, A.P., E.K. and P.K.; visualization, A.F.; supervision, E.K.; project administration, V.D. and I.A.; funding acquisition, V.D. All authors have read and agreed to the published version of the manuscript.
Funding
This study was conducted with the help of the project “Conservation of European Biodiversity through Exploitation of Traditional Herbal Knowledge for the Development of Innovative Products”, Horizon 2020, H2020-MSCA-RISE-2018, Proposal number: 823973, and Proposal acronym: EthnoHERBS.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Brownrigg, J.; Apelqvist, J.; Bakker, K.; Schaper, N.; Hinchliffe, R. Evidence-based management of PAD & the diabetic foot. Eur. J. Vasc. Endovasc. Surg. 2013, 45, 673–681. [Google Scholar]
- Richmond, N.A.; Maderal, A.D.; Vivas, A.C. Evidence-based management of common chronic lower extremity ulcers. Dermatol. Ther. 2013, 26, 187–196. [Google Scholar] [CrossRef]
- Canadian Agency for Drugs and Technologies in Health. Optimal Care of Chronic, Non-Healing, Lower Extremity Wounds: A Review of Clinical Evidence and Guidelines; Canadian Agency for Drugs and Technologies in Health: Ottawa, ON Canada, 2013. [Google Scholar]
- Woo, K.; Ayello, E.A.; Sibbald, R.G. The edge effect: Current therapeutic options to advance the wound edge. Adv. Ski. Wound Care 2007, 20, 99–117. [Google Scholar] [CrossRef]
- Stojadinovic, A.; Carlson, J.W.; Schultz, G.S.; Davis, T.A.; Elster, E.A. Topical advances in wound care. Gynecol. Oncol. 2008, 111, S70–S80. [Google Scholar] [CrossRef]
- Attinger, C.E.; Janis, J.E.; Steinberg, J.; Schwartz, J.; Al-Attar, A.; Couch, K. Clinical approach to wounds: Debridement and wound bed preparation including the use of dressings and wound-healing adjuvants. Plast. Reconstr. Surg. 2006, 117, 72S–109S. [Google Scholar] [CrossRef]
- Demidova-Rice, T.N.; Salomatina, E.V.; Yaroslavsky, A.N.; Herman, I.M.; Hamblin, M.R. Low-level light stimulates excisional wound healing in mice. Lasers Surg. Med. 2007, 39, 706–715. [Google Scholar] [CrossRef]
- Edmonds, M. Body of knowledge around the diabetic foot and limb salvage. J. Cardiovasc. Surg. 2012, 53, 605–616. [Google Scholar]
- Gupta, S.; Andersen, C.; Black, J.; de Leon, J.; Fife, C.; Lantis, J.I.; Niezgoda, J.; Snyder, R.; Sumpio, B.; Tettelbach, W. Management of Chronic Wounds: Diagnosis, Preparation, Treatment, and Follow-up. Wounds 2017, 29, S19–S36. [Google Scholar]
- Kramer, A.; Dissemond, J.; Kim, S.; Willy, C.; Mayer, D.; Papke, R.; Tuchmann, F.; Assadian, O. Consensus on wound antisepsis: Update 2018. Ski. Pharmacol. Physiol. 2018, 31, 28–58. [Google Scholar] [CrossRef]
- FrykbergRobert, G. Challenges in the treatment of chronic wounds. Adv. Wound Care 2015, 4, 560–582. [Google Scholar] [CrossRef]
- Phillipson, J.D. Phytochemistry and medicinal plants. Phytochemistry 2001, 56, 237–243. [Google Scholar] [CrossRef]
- Barna, M.; Kucera, A.; Hladicova, M.; Kucera, M. Wound healing effects of a Symphytum herb extract cream (Symphytum × uplandicum NYMAN:): Results of a randomized, controlled double-blind study. Wien. Med. Wochenschr. 2007, 157, 569–574. [Google Scholar] [CrossRef]
- González, J.A.; García-Barriuso, M.; Amich, F. Ethnobotanical study of medicinal plants traditionally used in the Arribes del Duero, western Spain. J. Ethnopharmacol. 2010, 131, 343–355. [Google Scholar] [CrossRef]
- Müller-Löbnitz, C.; Göthel, D. Review of the clinical efficacy of the multicomponent combination medication Traumeel and its components. Altern. Ther. Health Med. 2011, 17, S18–S31. [Google Scholar]
- Maver, T.; Maver, U.; Kleinschek, K.S.; Smrke, D.M.; Kreft, S. A review of herbal medicines in wound healing. Int. J. Dermatol. 2015, 54, 740–751. [Google Scholar] [CrossRef]
- Shedoeva, A.; Leavesley, D.; Upton, Z.; Fan, C. Wound healing and the use of medicinal plants. Evid.-Based Complement. Altern. Med. 2019, 2019, 2684108. [Google Scholar] [CrossRef]
- Sharma, A.; Khanna, S.; Kaur, G.; Singh, I. Medicinal plants and their components for wound healing applications. Future J. Pharm. Sci. 2021, 7, 53. [Google Scholar] [CrossRef]
- Jarić, S.; Mačukanović-Jocić, M.; Djurdjević, L.; Mitrović, M.; Kostić, O.; Karadžić, B.; Pavlović, P. An ethnobotanical survey of traditionally used plants on Suva planina mountain (south-eastern Serbia). J. Ethnopharmacol. 2015, 175, 93–108. [Google Scholar] [CrossRef] [PubMed]
- Davidov, B.; Yavashev, A.; Akhtarov, B. Materials for Bulgarian Botanical Dictionary; Royal Press: Nitriansky, Slovakia, 1939. [Google Scholar]
- Agelet, A.; Vallès, J. Studies on pharmaceutical ethnobotany in the region of Pallars (Pyrenees, Catalonia, Iberian Peninsula). Part I. General results and new or very rare medicinal plants. J. Ethnopharmacol. 2001, 77, 57–70. [Google Scholar] [CrossRef]
- Tuka, M.; Redzic, S.; Babic, A. Basic pharmacognostic research of Gentiana cruciata L. species from Bosnia and Herzegovina (W. Balkan). Planta Med. 2011, 77, PL60. [Google Scholar] [CrossRef]
- Agelet, A.; Valles, J. Studies on pharmaceutical ethnobotany in the region of Pallars (Pyrenees, Catalonia, Iberian Peninsula). Part II. New or very rare uses of previously known medicinal plants. J. Ethnopharmacol. 2003, 84, 211–227. [Google Scholar] [CrossRef] [PubMed]
- Bonet, M.À.; Valles, J. Ethnobotany of Montseny biosphere reserve (Catalonia, Iberian Peninsula): Plants used in veterinary medicine. J. Ethnopharmacol. 2007, 110, 130–147. [Google Scholar] [CrossRef] [PubMed]
- Popović, Z.; Smiljanić, M.; Kostić, M.; Nikić, P.; Janković, S. Wild flora and its usage in traditional phytotherapy (Deliblato Sands, Serbia, South East Europe). Indian J. Tradit. Knowl. 2014, 13, 9–35. [Google Scholar]
- Pieroni, A.; Ibraliu, A.; Abbasi, A.M.; Papajani-Toska, V. An ethnobotanical study among Albanians and Aromanians living in the Rraicë and Mokra areas of Eastern Albania. Genet. Resour. Crop Evol. 2015, 62, 477–500. [Google Scholar] [CrossRef]
- Pieroni, A.; Giusti, M.E.; Quave, C.L. Cross-cultural ethnobiology in the Western Balkans: Medical ethnobotany and ethnozoology among Albanians and Serbs in the Pešter Plateau, Sandžak, South-Western Serbia. Hum. Ecol. 2011, 39, 333. [Google Scholar] [CrossRef]
- Dioscorides, P. Dioscoridis Libri Octo Graecae et Latinae; Castigationes in Eosdem Libros. Parisiis, Impensis A; Birkmanni: Paris, France, 1549. [Google Scholar]
- Khorasani, M.H.A.A. Makhzan al-Advieh; Tehran University of Medical Sciences: Tehran, Iran, 2009; Volume 328. [Google Scholar]
- Mirzaee, F.; Hosseini, A.; Jouybari, H.B.; Davoodi, A.; Azadbakht, M. Medicinal, biological and phytochemical properties of Gentiana species. J. Tradit. Complement. Med. 2017, 7, 400–408. [Google Scholar] [CrossRef]
- Assadi, M.; Maassoumi, A.R.; Khatamsaz, M. Flora of Iran; Ministry of Agriculture, Research Institute of Forests and Rangelands: Tehran, Iran, 1989. [Google Scholar]
- Mozaffarian, V. A Dictionary of Iranian Plant Names; Farhang Moaser: Tehran, Iran, 1996; Volume 396. [Google Scholar]
- Kazancı, C.; Oruç, S.; Mosulishvili, M. Medicinal ethnobotany of wild plants: A cross-cultural comparison around Georgia-Turkey border, the Western Lesser Caucasus. J. Ethnobiol. Ethnomed. 2020, 16, 71. [Google Scholar] [CrossRef]
- Watkins, F. Investigation of Antimicrobials from Native British Plants Used in 10th Century Anglo-Saxon Wound Healing Formulations. Ph.D. Thesis, University of East London, London, UK, 2013. [Google Scholar]
- Vokou, D.; Katradi, K.; Kokkini, S. Ethnobotanical survey of Zagori (Epirus, Greece), a renowned centre of folk medicine in the past. J. Ethnopharmacol. 1993, 39, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Sõukand, R.; Pieroni, A. The importance of a border: Medical, veterinary, and wild food ethnobotany of the Hutsuls living on the Romanian and Ukrainian sides of Bukovina. J. Ethnopharmacol. 2016, 185, 17–40. [Google Scholar] [CrossRef] [PubMed]
- Mattalia, G.; Stryamets, N.; Pieroni, A.; Sõukand, R. Knowledge transmission patterns at the border: Ethnobotany of Hutsuls living in the Carpathian Mountains of Bukovina (SW Ukraine and NE Romania). J. Ethnobiol. Ethnomed. 2020, 16, 41. [Google Scholar] [CrossRef]
- Marković, M.; Pljevljakušić, D.; Menković, N.; Matejić, J.; Papović, O.; Jovanović, V.S. Traditional knowledge on the medicinal use of plants from genus Gentiana in the Pirot County (Serbia). Lek. Sirovine 2021, 41, 46–53. [Google Scholar] [CrossRef]
- Mustafa, B.; Hajdari, A.; Pulaj, B.; Quave, C.L.; Pieroni, A. Medical and food ethnobotany among Albanians and Serbs living in the Shtërpcë/Štrpce area, South Kosovo. J. Herb. Med. 2020, 22, 100344. [Google Scholar] [CrossRef]
- Menković, N.; Šavikin, K.; Tasić, S.; Zdunić, G.; Stešević, D.; Milosavljević, S.; Vincek, D. Ethnobotanical study on traditional uses of wild medicinal plants in Prokletije Mountains (Montenegro). J. Ethnopharmacol. 2011, 133, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Šarić-Kundalić, B.; Dobeš, C.; Klatte-Asselmeyer, V.; Saukel, J. Ethnobotanical survey of traditionally used plants in human therapy of east, north and north-east Bosnia and Herzegovina. J. Ethnopharmacol. 2011, 133, 1051–1076. [Google Scholar] [CrossRef]
- Mattalia, G.; Sõukand, R.; Corvo, P.; Pieroni, A. Dissymmetry at the border: Wild food and medicinal ethnobotany of slovenes and friulians in NE Italy. Econ. Bot. 2020, 74, 1–14. [Google Scholar] [CrossRef]
- Alonso, A.; Acedo, C.; Llamas, F. Ethnobotany from the Cantabrian mountains: Cofiñal (León, Spain), 11th Congress of the International Society of Ethnopharmacology celebrated in Albacete (Spain). Rev. Fitoter. 2010, 10, 57. [Google Scholar]
- Pieroni, A. Local plant resources in the ethnobotany of Theth, a village in the Northern Albanian Alps. Genet. Resour. Crop Evol. 2008, 55, 1197–1214. [Google Scholar] [CrossRef]
- Redžić, S.S. The ecological aspect of ethnobotany and ethnopharmacology of population in Bosnia and Herzegovina. Coll. Antropol. 2007, 31, 869–890. [Google Scholar]
- Ferrier, J.; Saciragic, L.; Trakić, S.; Chen, E.C.; Gendron, R.L.; Cuerrier, A.; Balick, M.J.; Redžić, S.; Alikadić, E.; Arnason, J.T. An ethnobotany of the Lukomir highlanders of Bosnia & Herzegovina. J. Ethnobiol. Ethnomed. 2015, 11, 81. [Google Scholar]
- Danna, C.; Poggio, L.; Smeriglio, A.; Mariotti, M.; Cornara, L. Ethnomedicinal and Ethnobotanical Survey in the Aosta Valley Side of the Gran Paradiso National Park (Western Alps, Italy). Plants 2022, 11, 170. [Google Scholar] [CrossRef]
- Valverde, R.M.; Tardío, J.; de Santayana, M.P. Ethnobotanical review of wild edible plants in Spain. Bot. J. Linn. Soc. 2006, 152, 27–71. [Google Scholar]
- Batsatsashvili, K.; Mehdiyeva, N.; Kikvidze, Z.; Khutsishvili, M.; Maisaia, I.; Sikharulidze, S.; Tchelidze, D.; Alizade, V.; Zambrana, N.Y.P.; Bussmann, R.W. Gentiana septemfida Pall.; Springer International Publishing: Cham, Switzerland, 2017. [Google Scholar]
- Bussmann, R.W.; Batsatsashvili, K.; Kikvidze, Z.; Paniagua-Zambrana, N.Y.; Khutsishvili, M.; Maisaia, I.; Sikharulidze, S.; Tchelidze, D. Gentiana cruciata L. Gentiana septemfida Pall. Gentianaceae. In Ethnobotany of the Mountain Regions of Far Eastern Europe: Ural, Northern Caucasus, Turkey, Iran; Springer: Berlin/Heidelberg, Germany, 2020; pp. 441–446. [Google Scholar]
- Bussmann, R.W.; Batsatsashvili, K.; Kikvidze, Z. Gentiana cruciata L. Gentiana septemfida Pall. Gentianaceae. In Ethnobotany of the Mountain Regions of Far Eastern Europe: Ural, Northern Caucasus, Turkey, and Iran; Springer: Berlin/Heidelberg, Germany, 2020; pp. 355–360. [Google Scholar]
- Bussmann, R.W.; Zambrana, N.Y.P.; Sikharulidze, S.; Kikvidze, Z.; Kikodze, D.; Tchelidze, D.; Khutsishvili, M.; Batsatsashvili, K.; Hart, R.E. A comparative ethnobotany of Khevsureti, Samtskhe-Javakheti, Tusheti, Svaneti, and Racha-Lechkhumi, Republic of Georgia (Sakartvelo), Caucasus. J. Ethnobiol. Ethnomed. 2016, 12, 43. [Google Scholar] [CrossRef] [PubMed]
- Bussmann, R.W.; Zambrana, N.P.; Sikharulidze, S.; Kikvidze, Z.; Kikodze, D.; Tchelidze, D.; Batsatsashvili, K.; Hart, R. Medicinal and food plants of Svaneti and Lechkhumi, Sakartvelo (Republic of Georgia), Caucasus. Med. Aromat Plants 2016, 5, 2167-0412. [Google Scholar]
- Bussmann, R.W.; Paniagua, Z.; Narel, Y.; Sikharulidze, S.; Kikvidze, Z.; Kikodze, D.; Tchelidze, D.; Batsatsashvili, K.; Robbie, E. Plants in the spa–the medicinal plant market of Borjomi, Sakartvelo (Republic of Georgia), Caucasus. Indian J. Tradit. Knowl. 2017, 16, 25–34. [Google Scholar]
- Bussmann, R.W.; Paniagua, Z.; Narel, Y.; Sikharulidze, S.; Kikvidze, Z.; Kikodze, D.; Tchelidze, D.; Batsatsashvili, K.; Robbie, E. Plant and fungal use inTusheti, Khevsureti and Pshavi, Sakartvelo (Republic of Georgia), Caucasus. Acta Soc. Bot. Pol. 2017, 86, 1–45. [Google Scholar]
- Yesilada, E. Natural remedies from Turkey: Perspectives of safety and efficacy. In Evaluation of Herbal Medicinal Products Perspective of Quality, Safety, and Efficacy; Houghton, P., Mukherjee, P.K., Eds.; Royal Society of Great Britain: London, UK; The Pharmaceutical Press: London, UK, 2009; pp. 28–41. [Google Scholar]
- Görhan, K.Ö.; Öztürk, F. Ethnopharmacological survey of medicinal and foods plants in Derecik (Hakkari-Turkey). Indian J. Tradit. Knowl. 2021, 20, 416–425. [Google Scholar]
- Güneş, F.; Özhatay, N. An ethnobotanical study from Kars Eastern Turkey. Biyol. Çeşitlilik Ve Koruma 2011, 4, 30–41. [Google Scholar]
- Özgen, U.; Kaya, Y.; Houghton, P. Folk medicines in the villages of Ilıca District (Erzurum, Turkey). Turk. J. Biol. 2012, 36, 93–106. [Google Scholar] [CrossRef]
- Skinder, B.M.; Ganai, B.A.; Wani, A.H. Scientific study of Gentiana kurroo Royle. Medicines 2017, 4, 74. [Google Scholar] [CrossRef]
- Chauhan, P.; Nigam, A.; Santvan, V.K. Ethnobotanical Uses of Medicinal Plants among the Rural People of Pabbar Valley in District Shimla, Himachal Pradesh, India. Plant Arch. 2020, 20, 3707–37019. [Google Scholar]
- Singh, K.N. Traditional knowledge on ethnobotanical uses of plant biodiversity: A detailed study from the Indian western Himalaya. Biodivers. Res. Conserv. 2012, 28, 63. [Google Scholar] [CrossRef]
- Zhao, Z.; Dorje, G.; Wang, Z. Identification of medicinal plants used as Tibetan traditional medicine Jie-Ji. J. Ethnopharmacol. 2010, 132, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Gobbi, S.; Hu, Q.; Foschi, G.; Catanzaro, E.; Belluti, F.; Rampa, A.; Fimognari, C.; Hartmann, R.W.; Bisi, A. Benzophenones as xanthone-open model CYP11B1 inhibitors potentially useful for promoting wound healing. Bioorganic Chem. 2019, 86, 401–409. [Google Scholar] [CrossRef]
- Viljoen, A.; Mncwangi, N.; Vermaak, I. Anti-inflammatory iridoids of botanical origin. Curr. Med. Chem. 2012, 19, 2104–2127. [Google Scholar] [CrossRef]
- Villegas, L.; Marcalo, A.; Martin, J.; Fernandez, I.; Maldonado, H.; Vaisberg, A.; Hammond, G. epi-Alpha-bisabolol [correction of bisbolol] is the wound-healing principle of Peperomia galioides: Investigation of the in vivo wound-healing activity of related terpenoids. J. Nat. Prod. 2001, 64, 1357–1359. [Google Scholar] [CrossRef]
- Öztürk, N.; Korkmaz, S.; Öztürk, Y.; Başer, K.H. Effects of gentiopicroside, sweroside and swertiamarine, secoiridoids from gentian (Gentiana lutea ssp. symphyandra), on cultured chicken embryonic fibroblasts. Planta Med. 2006, 72, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Shim, K.M.; Choi, S.H.; Jeong, M.-J.; Kang, S.S. Effects of aucubin on the healing of oral wounds. Vivo 2007, 21, 1037–1041. [Google Scholar]
- Stevenson, P.C.; Simmonds, M.S.; Sampson, J.; Houghton, P.J.; Grice, P. Wound healing activity of acylated iridoid glycosides from Scrophularia nodosa. Phytother. Res. 2002, 16, 33–35. [Google Scholar] [CrossRef]
- Jiang, X.; Chen, W.; Shen, F.; Xiao, W.; Guo, H.; Su, H.; Xiu, J.; Sun, W. Pinoresinol promotes MC3T3-E1 cell proliferation and differentiation via the cyclic AMP/protein kinase A signaling pathway. Mol. Med. Rep. 2019, 20, 2143–2150. [Google Scholar] [CrossRef]
- Konya, R.; Reis, R.; Sipahi, H.; Barta, A.; Hohmann, J.; Kırmızıbekmez, H. Secondary metabolites from Gentiana cruciata L. and their anti-inflammatory and analgesic activities. Nat. Prod. Res. 2022, 1–8. [Google Scholar] [CrossRef]
- Patel, S.; Srivastava, S.; Singh, M.R.; Singh, D. Preparation and optimization of chitosan-gelatin films for sustained delivery of lupeol for wound healing. Int. J. Biol. Macromol. 2018, 107, 1888–1897. [Google Scholar] [CrossRef] [PubMed]
- Schmieder, A.; Schwaiger, S.; Csordas, A.; Backovic, A.; Messner, B.; Wick, G.; Stuppner, H.; Bernhard, D. Isogentisin—A novel compound for the prevention of smoking-caused endothelial injury. Atherosclerosis 2007, 194, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Sutar, I.; Acikara, O.B.; Citoglu, G.S.; Keles, H.; Ergene, B.; Akkol, E.K. In vivo and in vitro evaluation of the therapeutic potential of some Turkish Scorzonera species as wound healing agent. Curr. Pharm. Des. 2012, 18, 1421–1433. [Google Scholar] [CrossRef]
- Zheng, H.; Zhang, M.; Luo, H.; Li, H. Isoorientin alleviates UVB-induced skin injury by regulating mitochondrial ROS and cellular autophagy. Biochem. Biophys. Res. Commun. 2019, 514, 1133–1139. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.A.; Kim, D.H.; Park, C.B.; Park, T.S.; Park, B.J. Anti-aging effects of sweroside isolated from Nymphoides indica. J. Soc. Cosmet. Sci. Korea 2018, 44, 103–110. [Google Scholar]
- Harish, B.; Krishna, V.; Kumar, H.S.; Ahamed, B.K.; Sharath, R.; Swamy, H.K. Wound healing activity and docking of glycogen-synthase-kinase-3-β-protein with isolated triterpenoid lupeol in rats. Phytomedicine 2008, 15, 763–767. [Google Scholar] [CrossRef] [PubMed]
- Razia, S.; Park, H.; Shin, E.; Shim, K.-S.; Cho, E.; Kim, S.-Y. Effects of Aloe vera flower extract and its active constituent isoorientin on skin moisturization via regulating involucrin expression: In vitro and molecular docking studies. Molecules 2021, 26, 2626. [Google Scholar] [CrossRef]
- Yadav, V.P.; Shukla, A.; Choudhury, S.; Singh, R.; Anand, M.; Prabhu, S.N. IL1β/TNFα/COX-2/VEGF axis responsible for effective healing potential of C-glucoside xanthone (mangiferin) based ointment in immunocompromised rats. Cytokine 2022, 158, 156012. [Google Scholar] [CrossRef]
- Aberham, A.; Pieri, V.; Croom, E.M., Jr.; Ellmerer, E.; Stuppner, H. Analysis of iridoids, secoiridoids and xanthones in Centaurium erythraea, Frasera caroliniensis and Gentiana lutea using LC–MS and RP-HPLC. J. Pharm. Biomed. Anal. 2011, 54, 517–525. [Google Scholar] [CrossRef]
- Ando, H.; Hirai, Y.; Fujii, M.; Hori, Y.; Fukumura, M.; Niiho, Y.; Nakajima, Y.; Shibata, T.; Toriizuka, K.; Ida, Y. The chemical constituents of fresh Gentian root. J. Nat. Med. 2007, 61, 269–279. [Google Scholar] [CrossRef]
- Citová, I.; Ganzera, M.; Stuppner, H.; Solich, P. Determination of gentisin, isogentisin, and amarogentin in Gentiana lutea L. by capillary electrophoresis. J. Sep. Sci. 2008, 31, 195–200. [Google Scholar] [CrossRef]
- Kušar, A.; Šircelj, H.; Bariĉeviĉ, D. Determination of seco-iridoid and 4-pyrone compounds in hydro-alcoholic extracts of Gentiana lutea L. subsp. symphyandra Murb. leaves and roots by using high performance liquid chromatography. Isr. J. Plant Sci. 2010, 58, 291–296. [Google Scholar] [CrossRef]
- Niiho, Y.; Yamazaki, T.; Nakajima, Y.; Yamamoto, T.; Ando, H.; Hirai, Y.; Toriizuka, K.; Ida, Y. Gastroprotective effects of bitter principles isolated from Gentian root and Swertia herb on experimentally-induced gastric lesions in rats. J. Nat. Med. 2006, 60, 82–88. [Google Scholar] [CrossRef]
- Buza, V.; Niculae, M.; Hanganu, D.; Pall, E.; Burtescu, R.F.; Olah, N.-K.; Matei-Lațiu, M.-C.; Vlasiuc, I.; Iozon, I.; Szakacs, A.R. Biological Activities and Chemical Profile of Gentiana asclepiadea and Inula helenium Ethanolic Extracts. Molecules 2022, 27, 3560. [Google Scholar] [CrossRef]
- Skrzypczak, L.; Wesolowska, M.; Skrzypczak, E. Gentiana species: In vitro culture, regeneration, and production of secoiridoid glucosides. In Medicinal and Aromatic Plants IV; Springer: Berlin/Heidelberg, Germany, 1993; pp. 172–186. [Google Scholar]
- Kakuda, R.; Machida, K.; Yaoita, Y.; Kikuchi, M.; Kikuchi, M. Studies on the constituents of Gentiana species. II. A new triterpenoid, and (S)-(+)-and (R)-(−)-gentiolactones from Gentiana lutea. Chem. Pharm. Bull. 2003, 51, 885–887. [Google Scholar] [CrossRef]
- Do, T.; Popov, S.; Marekov, N.; Trifonov, A. Iridoids from Gentianaceae plants growing in Bulgaria. Planta Med. 1987, 53, 580. [Google Scholar] [CrossRef]
- Jensen, S.R.; Schripsema, J. Chemotaxonomy and pharmacology of Gentianaceae. In Gentianaceae: Systematics and Natural History; Cambridge University Press: Cambridge, UK, 2002; Volume 5, pp. 574–631. [Google Scholar]
- Kitanov, G.; Van, D.T.; Asenov, I. Chemical composition of the roots of Gentiana asclepiadea. Chem. Nat. Compd. 1991, 27, 369–370. [Google Scholar] [CrossRef]
- Popović, Z.; Krstić-Milošević, D.; Marković, M.; Vidaković, V.; Bojović, S. Gentiana asclepiadea L. from Two High Mountainous Habitats: Inter-and Intrapopulation Variability Based on Species’ Phytochemistry. Plants 2021, 10, 140. [Google Scholar] [CrossRef]
- Szucs, Z.; Danos, B.; Nyiredy, S. Comparative analysis of the underground parts of Gentiana species by HPLC with diode-array and mass spectrometric detection. Chromatographia 2002, 56, S19–S23. [Google Scholar] [CrossRef]
- Kırmızıbekmez, H.; Tatar, D.; Erdoğan, M.; Kúsz, N.; Hohmann, J. A new depside and a new secoiridoid from the aerial parts of Gentiana olivieri from flora of Turkey. Nat. Prod. Res. 2022, 36, 2208–2214. [Google Scholar] [CrossRef] [PubMed]
- Bianco, A.; Ramunno, A.; Melchioni, C. Iridoids from seeds of Gentiana lutea. Nat. Prod. Res. 2003, 17, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Aberham, A.; Schwaiger, S.; Stuppner, H.; Ganzera, M. Quantitative analysis of iridoids, secoiridoids, xanthones and xanthone glycosides in Gentiana lutea L. roots by RP-HPLC and LC–MS. J. Pharm. Biomed. Anal. 2007, 45, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, A.M.; Caprioli, G.; Ricciutelli, M.; Maggi, F.; Marín, R.; Vittori, S.; Sagratini, G. Comparative HPLC/ESI-MS and HPLC/DAD study of different populations of cultivated, wild and commercial Gentiana lutea L. Food Chem. 2015, 174, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Sood, H.; Chauhan, R.S. Detection of intermediates through high-resolution mass spectrometry for constructing biosynthetic pathways for major chemical constituents in a medicinally important herb, Swertia chirayita. Nat. Prod. Res. 2015, 29, 1449–1455. [Google Scholar] [CrossRef]
- Keller, F. Gentiopicroside is located in the vacuoles of root protoplasts of Gentiana lutea. J. Plant Physiol. 1986, 122, 473–476. [Google Scholar] [CrossRef]
- Balijagić, J.; Janković, T.; Zdunić, G.; Bošković, J.; Šavikin, K.; Goćevac, D.; Stanojković, T.; Jovančević, M.; Menković, N. Chemical profile, radical scavenging and cytotoxic activity of yellow Gentian leaves (Genitaneae luteae folium) grown in northern regions of Montenegro. Nat. Prod. Commun. 2012, 7, 1487–1490. [Google Scholar] [CrossRef]
- Carnat, A.; Fraisse, D.; Carnat, A.P.; Felgines, C.; Chaud, D.; Lamaison, J.L. Influence of drying mode on iridoid bitter constituent levels in gentian root. J. Sci. Food Agric. 2005, 85, 598–602. [Google Scholar] [CrossRef]
- Venditti, A.; Guarcini, L.; Altieri, A.; Bianco, A. Phytochemical pattern of Gentiana species of Appennino in Central Italy. Nat. Prod. Res. 2013, 27, 2063–2065. [Google Scholar] [CrossRef]
- Hayta, S.; Akgun, I.H.; Ganzera, M.; Bedir, E.; Gurel, A. Shoot proliferation and HPLC-determination of iridoid glycosides in clones of Gentiana cruciata L. Plant Cell Tissue Organ Cult. 2011, 107, 175–180. [Google Scholar] [CrossRef]
- Çaliş, İ.; Rüegger, H.; Chun, Z.; Sticher, O. Secoiridoid glucosides isolated from Gentiana gelida. Planta Med. 1990, 56, 406–409. [Google Scholar] [CrossRef]
- Takeda, Y.; Masuda, T.; Honda, G.; Takaishi, Y.; Ito, M.; Ashurmetov, O.A.; Khodzhimatov, O.K.; Otsuka, H. Secoiridoid glycosides from Gentiana olivieri. Chem. Pharm. Bull. 1999, 47, 1338–1340. [Google Scholar] [CrossRef]
- Ueno, C.; Kakuda, R.; Kikuchi, M.; Yaoita, Y.; Machida, K.; Kikuchi, M. On the chemical constituents of Gentiana radix III. J. Tohoku Pharm. Univ. 2003, 50, 81–84. [Google Scholar]
- Hayashi, T.; Yamagishi, T. Two xanthone glycosides from Gentiana lutea. Phytochemistry 1988, 27, 3696–3699. [Google Scholar] [CrossRef]
- Rodriguez, S.; Marston, A.; Wolfender, J.; Hostettmann, K. Iridoids and secoiridoids in the Gentianaceae. Curr. Org. Chem. 1998, 2, 627–648. [Google Scholar] [CrossRef]
- Calis, I.; Rüegger, H.; Sticher, O. A new bitter secoiridoid glucoside from Gentiana gelida. Planta Med. 1989, 55, 106. [Google Scholar] [CrossRef]
- Handjieva, N.; Saadi, H.; Popov, S.; Baranovska, I. Separation of iridoids by vacuum liquid chromatography. Phytochem. Anal. 1991, 2, 130–133. [Google Scholar] [CrossRef]
- Hostettmann, K.; Bellmann, G.; Tabacchi, R.; Jacot-Guillarmod, A. Contribution à la phytochimie du genre Gentiana III. Etude des composés flavoniques et xanthoniques dans les feuilles de Gentiana lutea L.(2me communication). Helv. Chim. Acta 1973, 56, 3050–3054. [Google Scholar] [CrossRef]
- Sezik, E.; Aslan, M.; Yesilada, E.; Ito, S. Hypoglycaemic activity of Gentiana olivieri and isolation of the active constituent through bioassay-directed fractionation techniques. Life Sci. 2005, 76, 1223–1238. [Google Scholar] [CrossRef]
- Luong, M.D.; Jacot-Guillarmod, A. Contribution à la phytochimie du genre Gentiana XXI.; Les cinnamoyl-C-glucosylflavones et leurs O-glucosides dans Gentiana punctata L. Helv. Chim. Acta 1977, 60, 2099–2103. [Google Scholar] [CrossRef]
- Yamada, S.; Kakuda, R.; Yaoita, Y.; Kikuchi, M. A new flavone C-glycoside from Gentiana lutea. Nat. Med. 2005, 59, 189–192. [Google Scholar]
- Davoust, D.; Massias, M.; Molho, D. 13C NMR investigation of flavonoid C-β-D-glucosides. Detection of a conformational equilibrium. Org. Magn. Reson. 1980, 13, 218–219. [Google Scholar] [CrossRef]
- Singh, A. Phytochemicals of Gentianaceae: A review of pharmacological properties. Int. J. Pharm. Sci. Nanotechnol. 2008, 1, 33–36. [Google Scholar] [CrossRef]
- Popov, V.A.; Mollova, N.N.; Veleva, S.M.; Popov, S.S. Chemical ionization mass spectrometry of some monoterpene gentianine alkaloids and their derivatives. Biol. Mass Spectrom. 1992, 21, 358–362. [Google Scholar] [CrossRef]
- Mansoor, A.; Zaidi, M.I.; Malghani, M. Isolation of bioactive alkaloids from Gentiana olivieri and its non-toxic effect. Pak. J. Bot. 2000, 32, 105–110. [Google Scholar]
- Marekov, N.; Popov, S. The structure of gentioflavine, a new alkaloid of some Gentiana species. Tetrahedron 1968, 24, 1323–1326. [Google Scholar] [CrossRef]
- Rakhmatullaev, T.; Yunusov, S.Y. Alkaloids of Gentiana olivieri. Chem. Nat. Compd. 1973, 9, 56–58. [Google Scholar] [CrossRef]
- Mollov, N.; Marekov, N.; Popov, S.; Kouzmanov, B. Alkaloids of Some Gentiana Species. Comptes Rendus L’Academie Bulg. Sci. 1965, 18, 947. [Google Scholar]
- Kitanov, G.M.; Spassov, S.L. A naphthodipyranodione from Gentiana asclepiadea. Phytochemistry 1992, 31, 1067–1068. [Google Scholar] [CrossRef]
- Atkinson, J.; Gupta, P.; Lewis, J. Some phenolic constituents of Gentiana lutea. Tetrahedron 1969, 25, 1507–1511. [Google Scholar] [CrossRef] [PubMed]
- Mathew, A.; Taranalli, A.; Torgal, S. Evaluation of anti-inflammatory and wound healing activity of Gentiana lutea rhizome extracts in animals. Pharm. Biol. 2004, 42, 8–12. [Google Scholar] [CrossRef]
- Gendrisch, F.; Nováčková, A.; Sochorová, M.; Haarhaus, B.; Vávrová, K.; Schempp, C.M.; Wölfle, U. Gentiana lutea extract modulates ceramide synthesis in primary and psoriasis-like keratinocytes. Molecules 2020, 25, 1832. [Google Scholar] [CrossRef]
- Wölfle, U.; Haarhaus, B.; Seiwerth, J.; Cawelius, A.; Schwabe, K.; Quirin, K.-W.; Schempp, C.M. The herbal bitter drug Gentiana lutea modulates lipid synthesis in human keratinocytes in vitro and in vivo. Int. J. Mol. Sci. 2017, 18, 1814. [Google Scholar] [CrossRef]
- Nastasijević, B. Biological Effects of Gentian Root Extracts (Gentiana lutea)-Enzyme Inhibition, Antioxidative and Antimicrobial Activity; University of Belgrade Faculty of Technology and Metallurgy: Beograd, Serbia, 2016. [Google Scholar]
- Yin, C.; Xie, L.; Guo, Y. Phytochemical analysis and antibacterial activity of Gentiana macrophylla extract against bacteria isolated from burn wound infections. Microb. Pathog. 2018, 114, 25–28. [Google Scholar] [CrossRef]
- Yang, B.; Kim, S.; Kim, J.-H.; Lim, C.; Kim, H.; Cho, S. Gentiana scabra Bunge roots alleviates skin lesions of contact dermatitis in mice. J. Ethnopharmacol. 2019, 233, 141–147. [Google Scholar] [CrossRef]
- Mattalia, G.; Sõukand, R.; Corvo, P.; Pieroni, A. “We became rich and we lost everything”: Ethnobotany of remote mountain villages of Abruzzo and Molise, Central Italy. Hum. Ecol. 2021, 49, 217–224. [Google Scholar] [CrossRef]
- European Environment Agency. Gentiana lutea L. Available online: https://eunis.eea.europa.eu/species/172882 (accessed on 15 June 2022).
- Pladias. Database of the Czech Flora and Vegetation. Available online: www.pladias.cz (accessed on 15 June 2022).
- Fois, M.; Cuena-Lombrana, A.; Fenu, G.; Cogoni, D.; Bacchetta, G. The reliability of conservation status assessments at regional level: Past, present and future perspectives on Gentiana lutea L. ssp. lutea in Sardinia. J. Nat. Conserv. 2016, 33, 1–9. [Google Scholar] [CrossRef]
- Evstatieva, L. Gentiana lutea L. In Red Data Book of the Republic of Bulgaria, Plants and Fungi; Peev, D., Ed.; Institute of Biodiversity and Ecosystem Research: Sofia, Bulgaria, 2015; Volume 1, p. 881. [Google Scholar]
- Peev, D.R.; Vitkova, A.A.; Valyovska, N.V. New data of Gentiana lutea ssp. symphyandra (Gentianacea) in Bulgaria. Annu. Sofia Univ. “St. Kliment Ohridski” 2018, 2, 74–89. [Google Scholar]
- Zając, A.; Pindel, A. Review of the Willow Gentian, Gentiana asclepiadea L. Biodiversity 2011, 12, 181–185. [Google Scholar] [CrossRef]
- Petanidou, T.; Den Nijs, J.; Oostermeijer, J. Pollination ecology and constraints on seed set of the rare perennial Gentiana cruciata L. in the Netherlands. Acta Bot. Neerl. 1995, 44, 55–74. [Google Scholar] [CrossRef]
- Raijmann, L.E.; Van Leeuwen, N.C.; Kersten, R.; Oostermeijer, J.G.B.; Den Nijs, H.C.; Menken, S.B. Genetic variation and outcrossing rate in relation to population size in Gentiana pneumonanthe L. Conserv. Biol. 1994, 8, 1014–1026. [Google Scholar] [CrossRef]
- Křenová, Z.; Lepš, J. Regeneration of a Gentiana pneumonanthe population in an oligotrophic wet meadow. J. Veg. Sci. 1996, 7, 107–112. [Google Scholar] [CrossRef]
- Oostermeijer, J.G.B.; Brugman, M.L.; De Boer, E.R.; Den Nijs, H.C. Temporal and spatial variation in the demography of Gentiana pneumonanthe, a rare perennial herb. J. Ecol. 1996, 84, 153–166. [Google Scholar] [CrossRef]
- Oostermeijer, J.G.B.; Luijten, S.H.; Křenová, Z.V.; Den Nijs, H.C. Relationships between population and habitat characteristics and reproduction of the rare Gentiana pneumonanthe L. Conserv. Biol. 1998, 12, 1042–1053. [Google Scholar] [CrossRef]
- Kéry, M.; Matthies, D.; Fischer, M. The effect of plant population size on the interactions between the rare plant Gentiana cruciata and its specialized herbivore Maculinea rebeli. J. Ecol. 2001, 89, 418–427. [Google Scholar] [CrossRef]
- Kostrakiewicz-Gierałt, K. The effect of vegetation character on abundance and structure of subpopulations of rare herb species Gentiana pneumonanthe L. Pol. J. Ecol. 2013, 61, 35–46. [Google Scholar]
- Wójcik, T.; Piatek, K. New locality of Gentiana cruciata L. in the Strzyzowskie Foothills (Western Carpathians). Steciana 2015, 19, 67–73. [Google Scholar] [CrossRef]
- Wójcik, T.; Towpasz, K. Occurrence of Gentiana cruciata in dry grassland (Festuco-Brometea) in Kołaczyce (Strzyżowskie Foothills). Ecol. Quest. 2019, 30, 9–19. [Google Scholar] [CrossRef]
- Desmarest, P.; Derchue, D. La culture de la gentiane jaune en France. Techniques de production et amelioration. In Proceedings of the Sixieme Colloque International Consacré aux Plantes Médicinales et Substances D’origine Naturelle, Angers, France, 28 October 2015; pp. 33–40. [Google Scholar]
- Marković, T.; Radanović, D.; Nastasijević, B.; Antić-Mladenović, S.; Vasić, V.; Matković, A. Yield, quality and safety of yellow gentian roots produced under dry-farming conditions in various single basal fertilization and planting density models. Ind. Crop. Prod. 2019, 132, 236–244. [Google Scholar] [CrossRef]
- Pawłowska, B.; Bach, A. In vitro propagation of protected species Gentiana pneumonanthe L. for ornamental horticultural uses. Folia Hortic. 2003, 15, 113–122. [Google Scholar]
- Fiuk, A.; Rybczynski, J. Application of modern methods for plant material studies of Gentiana kurroo (Royle) somatic embryogenesis. Biotechnologia 2006, 4, 95–106. [Google Scholar]
- Devic, M.; Momcilovic, I.; Krstic, D.; Maksimovic, V.; Konjevic, R. In vitro multiplication of willow gentian (Gentiana asclepiadea L.) and the production of gentiopicrine and mangiferin. Phyton (Horn) 2006, 46, 45. [Google Scholar]
- Sharma, N.; Chandel, K.; Paul, A. In vitro propagation of Gentiana kurroo—An indigenous threatened plant of medicinal importance. Plant Cell Tissue Organ Cult. 1993, 34, 307–309. [Google Scholar] [CrossRef]
- Sharma, A.; Kaur, R.; Sharma, N. In vitro morphogenic response of different explants of Gentiana kurroo Royle from Western Himalayas—An endangered medicinal plant. Physiol. Mol. Biol. Plants 2014, 20, 249–256. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).