Seasonality and Long-Term Effect of Environmental Variables on the Orb Weaver Spider Community of a Tropical Dry Forest in the Balsas Basin, Mexico
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sampling Protocol
2.3. Taxonomic Determination
2.4. Vegetation Characterization
2.5. Environmental Data and Seasonality Delimitation
2.6. Diversity Analysis
Description and Evaluation of the Spider Community Structure
2.7. Spider and Plant Beta Diversity
2.8. Seasonality and Phenological Analysis
2.9. Effect of the Environmental Variables on the Spider Community
2.10. Non-Parametric Richness Estimators
2.11. Species Accumulation Curves Based on the Clench Model
3. Results
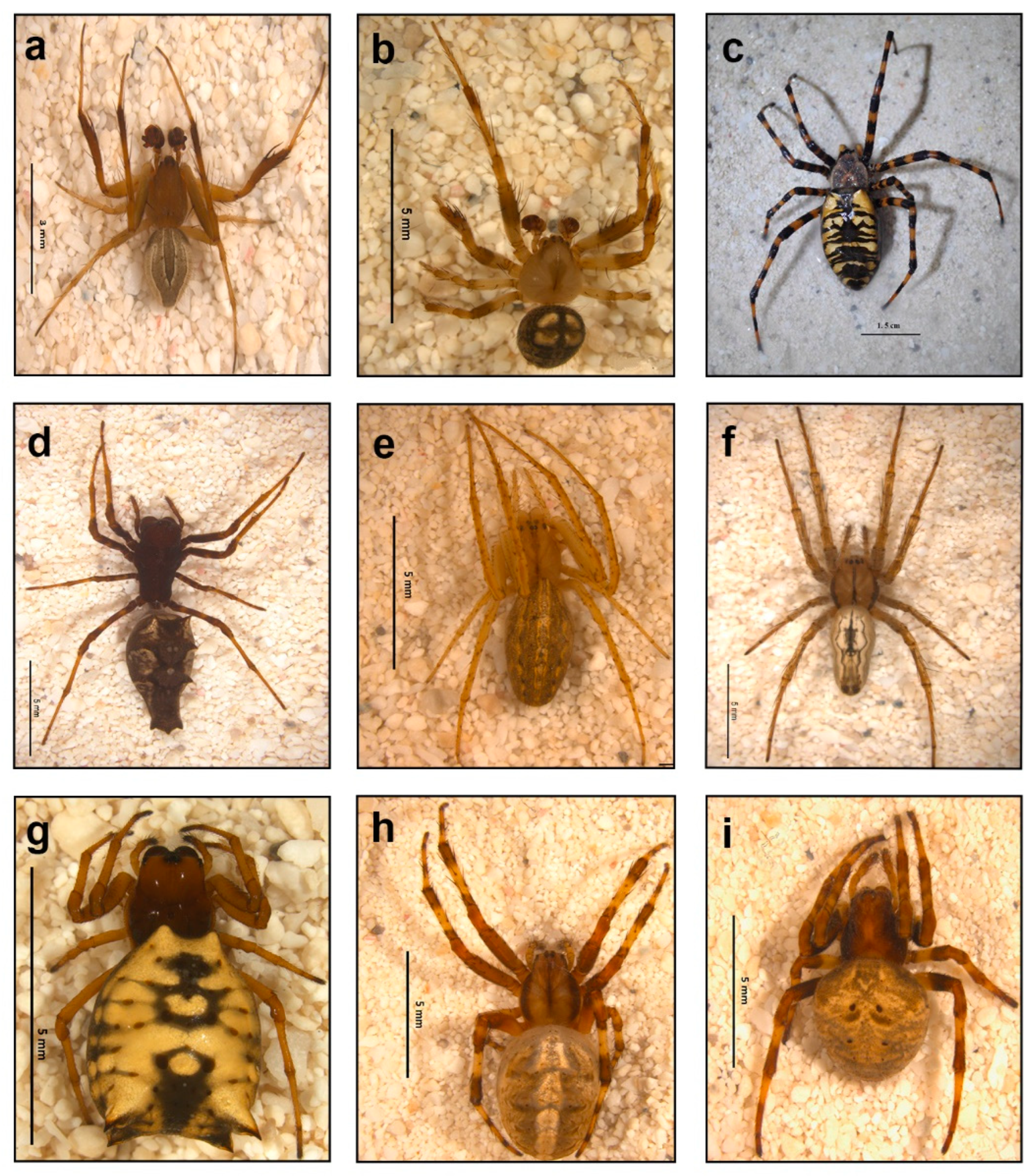
3.1. Spider Community
- SME
- 2.
- HA
- 3.
- EL
3.2. Plant Community Description
3.3. Spider and Plant Beta Diversity (β)
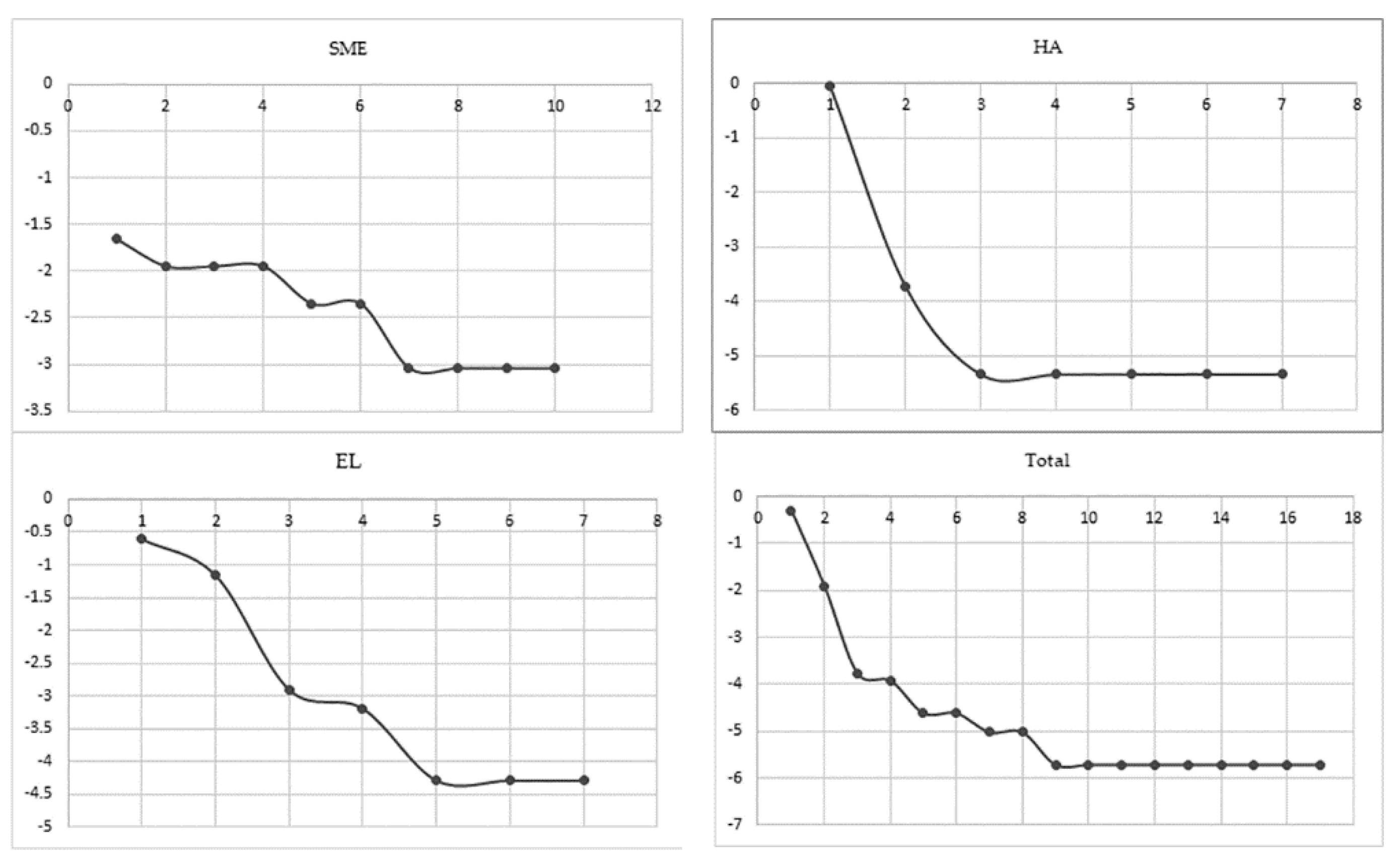
3.4. Seasonality and Phenology
3.4.1. SME
3.4.2. HA
3.4.3. EL
3.5. Effect of Environmental Variables
3.6. Richness Estimators
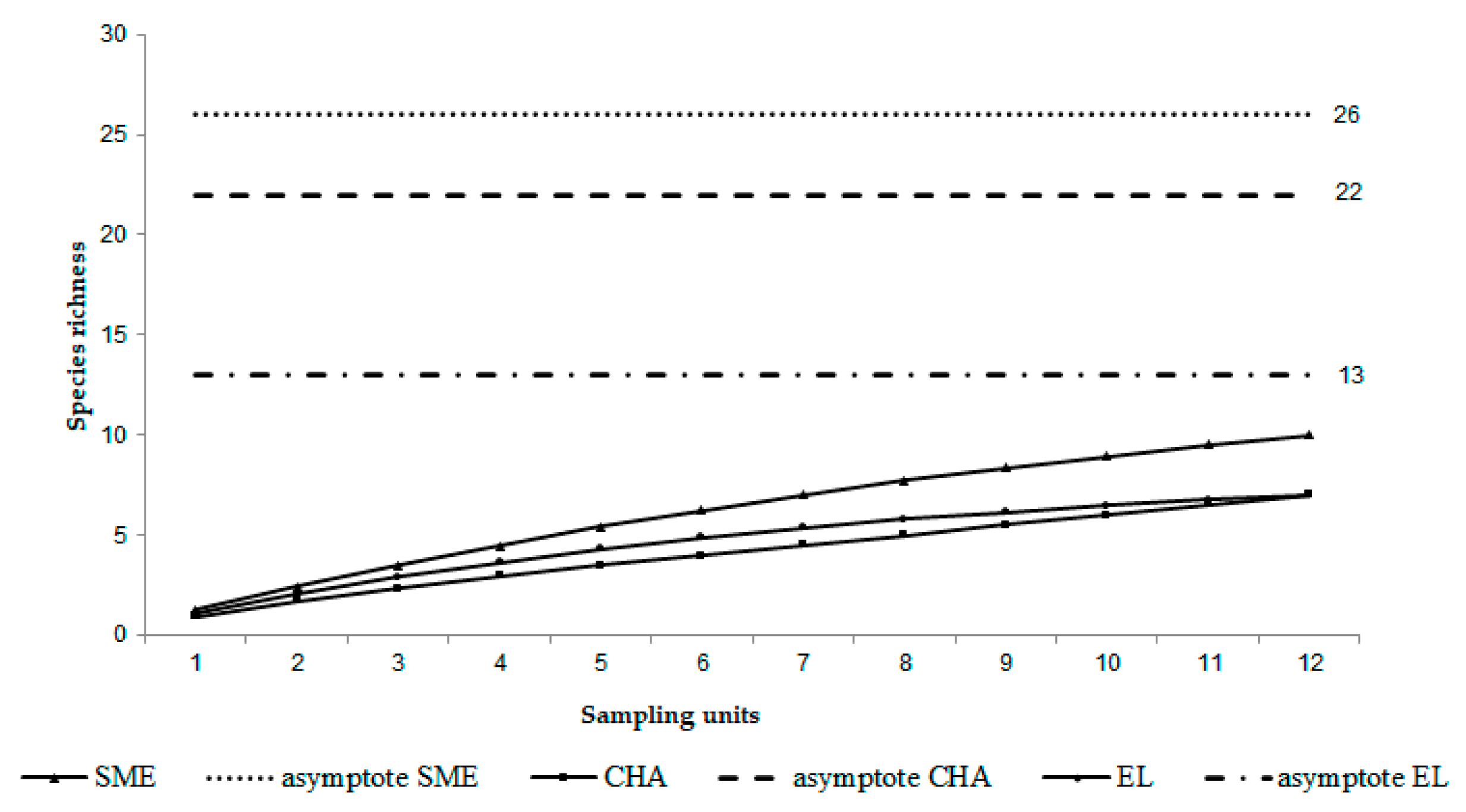
3.7. Species Accumulation Curve
4. Discussion
Non-Parametric Estimators and Species Accumulation Curves
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Riechert, S.; Lockley, T. Spiders as biological controls agents. Annu. Rev. Entomol. 1984, 29, 299–320. [Google Scholar] [CrossRef]
- Nyffeler, M.; Benz, G. Spiders in natural pest control: A review. J. Appl. Entomol. 1987, 103, 321–339. [Google Scholar] [CrossRef]
- Coddington, J.A.; Levi, H.W. Systematics and evolution of spiders (Araneae). Annu. Rev. Ecol. Evol. Syst. 1991, 22, 565–592. [Google Scholar] [CrossRef]
- Wimp, G.M.; Martinsen, G.D.; Floate, K.D.; Bangert, R.K.; Whitham, T.G. Plant genetic determinants of arthropod community structure and diversity. Evolution 2005, 59, 61–69. [Google Scholar] [PubMed]
- Wojtowicz, T.; Compson, Z.G.; Lamit, L.J.; Whitham, T.G.; Gehring, C.A. Plant genetic identity of foundation tree species and their hybrids affects a litter-dwelling generalist predator. Oecologia 2014, 176, 799–810. [Google Scholar] [CrossRef]
- Carvalho, L.S.; Sebastian, N.; Araújo, H.F.; Dias, S.C.; Venticinque, E.; Brescovit, A.D.; Vasconcellos, A. Climatic variables do not directly predict spider richness and abundance in semiarid Caatinga vegetation, Brazil. Environ. Entomol. 2015, 44, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Quijano-Cuervo, L.; Rangel-Acosta, J.; Martínez-Hernández, N.; Sabogal-González, A. Estratificación vertical de arañas tejedoras (Araneae) en fragmentos de bosque seco tropical del Caribe colombiano. Rev. Biol. Trop. 2019, 67, 224–242. [Google Scholar] [CrossRef]
- Michalko, R.; Košulič, O.; Martinek, P.; Birkhofer, K. Disturbance by invasive pathogenic fungus alters arthropod predator–prey food webs in ash plantations. J. Anim. Ecol. 2021, 90, 2213–2226. [Google Scholar] [CrossRef]
- Michalko, R.; Košulič, O.; Wongprom, P.; Songsangchote, C.; Saksongmuang, V.; Trisurat, Y. Reforestations of tropical forests alter interactions between web-building spiders and their prey. Ecosystems 2021, 24, 1962–1975. [Google Scholar] [CrossRef]
- Leroy, B.; Paschetta, M.; Canard, A.; Bakkenes, M.; Isaia, M.; Ysnel, F. First assessment of effects of global change on threatened spiders: Potential impacts on Dolomedes plantarius (Clerck) and its conservation plans. Biol. Conserv. 2013, 161, 155–163. [Google Scholar] [CrossRef]
- Spiller, D.A.; Schoener, T.W. Control climático de la fuerza de interacción trófica: El efecto de las lagartijas sobre las arañas. Ecologia 2008, 154, 763–771. [Google Scholar]
- Janzen, D.H. Tropical dry forests: The most endangered major tropical ecosystem. In Biodiversity; Wilson, E.O., Peters, F.M., Eds.; National Academy Press: New York, NY, USA, 1988; pp. 130–137. [Google Scholar]
- Ceballos, G.; Valenzuela, D. Diversidad, Ecología y Conservación de los Mamíferos de Latinoamérica. In Diversidad, Amenazas y Áreas Prioritarias para la Conservación de las Selvas Secas del Oeste de México; Ceballos, G., García, A., Martínez, L., Espinoza, E., Bezaury Creel, J., Dirzo, R., Eds.; National Commission for the Knowledge and Use of Biodiversity/National Autonomous University of Mexico: Mexico City, Mexico, 2010; pp. 93–118. [Google Scholar]
- Challenger, A.; Soberón, J. Los ecosistemas terrestres. In Capital Natural de México; Conocimiento Actual de la Biodiversidad; Comisión Nacional para el Conocimiento y Uso de la Biodiversidad: Mexico City, Mexico, 2008; Volume 1, pp. 87–108. [Google Scholar]
- Pérez-García, E.A.; Meave, J.A.; Cevallos-Ferriz, S.R. Flora and vegetation of the seasonally dry tropics in Mexico: Origin and biogeographical implications. Acta Bot. Mex. 2012, 100, 149–193. [Google Scholar]
- Maldonado, B.; Caballero, J.; Delgado-Salinas, A.; Lira, R. Relationship between Use Value and Ecological Importance of Floristic Resources of Seasonally Dry Tropical Forest in the Balsas River Basin, México. Econ. Bot. 2013, 67, 17–29. [Google Scholar] [CrossRef]
- Estrada-Márquez, A.S.; Villaseñor, J.L.; Escalante, T. Áreas de endemismo de Magnoliophyta en Morelos, México. Bot. Sci. 2020, 98, 377–392. [Google Scholar] [CrossRef]
- Trejo, I. Las selvas secas del Pacífico mexicano. In Diversidad, Amenazas y Áreas Prioritarias para la Conservación de las Selvas Secas del Oeste de México; Ceballos, G., García, A., Martínez, L., Espinoza, E., Bezaury Creel, J., Dirzo, R., Eds.; National Commission for the Knowledge and Use of Biodiversity/National Autonomous University of Mexico: Mexico City, Mexico, 2010; pp. 41–51. [Google Scholar]
- Morrone, J.J. Hacia una síntesis biogeográfica de México. Rev. Mex. Biodivers. 2005, 76, 207–252. [Google Scholar] [CrossRef]
- Luna-Reyes, M.; Llorente-Bousquets, J.; Luis-Martinez, A. Papilionoidea de la Sierra de Huautla, Morelos y Puebla, México (Insecta: Lepidoptera). Rev. Biol. Trop. 2008, 56, 1677–1716. [Google Scholar] [CrossRef] [PubMed]
- Nieto-Castañeda, I.G.; Pérez-Miguel, L.; García-Cano, A.A. New records of spiders (Arachnida: Araneae) from the Balsas Basin in central Mexico. Southwest. Nat. 2014, 59, 426–430. [Google Scholar] [CrossRef]
- Rosas-Echeverria, M.V.; Coyote-Ávila, C.A.; Aguilar-Dorantes, K.M.; Martínez-Peralta, C. Diversity of butterflies (Lepidoptera) in Sierra de Huautla, Morelos, México: A conservation approach. Ann. Entomol. Soc. Am. 2019, 112, 409–417. [Google Scholar] [CrossRef]
- Brown, K.M. Foraging ecology and niche partitioning in orb-weaving spiders. Oecologia 1981, 50, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Wise, D.H. Spiders in Ecological Webs; Cambridge University Press: New York, NY, USA, 1993; 328p. [Google Scholar]
- Zaragoza Caballero, S.; Noguera, F.A.; Gonzalez-Soriano, E.; Ramirez-Garcia, E.; Rodriguez-Palafox, A. Insectos. In Diversidad, Amenazas y Áreas Prioritarias para la Conservación de las Selvas Secas del Pacífico de México; Ceballos, G., Martinez, L., Garcia, A., Espinoza, E., Bezaury Creel, J., Dirzo, R., Eds.; National Commission for the Knowledge and Use of Biodiversity/National Autonomous University of Mexico: Mexico City, Mexico, 2010; pp. 195–214. [Google Scholar]
- Peñaloza-Villadiego, A.; García-Garrido, J.; Flórez-Daza, E.; Sampedro-Marin, A. Araneofauna de la Reserva Forestal Protectora Serranía de Coraza, Sucre-Colombia. Rev. Colomb. Cienc. Anim. 2013, 5, 36–47. [Google Scholar] [CrossRef]
- World Spider Catalog. Version 23.5. Natural History Museum Bern. 2023. Available online: http://wsc.nmbe.ch (accessed on 8 January 2023).
- Agnarsson, I.; Coddington, J.A.; Kuntner, M. Systematics: Progress in the study of spider diversity and evolution. In Spider Research in the 21st Century: Trends and Perspectives; Penney, D., Ed.; Siri Scientific Press: Manchester, UK, 2013; pp. 58–111. [Google Scholar]
- Gilede-Moncayo, O.; Bello-Silva, J.C. La familia Araneidae (Araneoidea: Orbicularie) en el departamento del Meta, Colombia. Biota Neotrop. 2000, 1, 125–130. [Google Scholar]
- Levi, H.W. Keys to the genera of Araneid Orbweavers (Araneae, Araneidae) of the Americas. J. Arachnol. 2002, 30, 527–562. [Google Scholar] [CrossRef]
- Jimenez-Valverde, A.; Lobo, J.M.; Lopez-Martos, M.L. Listado actualizado de especies de araneidos y tomísidos (Araneae, Araneidae y Thomisidae) de la comunidad de Madrid: Mapas de distribución conocida, potencial y patrones de riqueza. Graellsia 2006, 62, 461–481. [Google Scholar] [CrossRef]
- Levi, H.W.; Kallal, R.J. Araneidae. In Spiders of North America: An Identification Manual; Ubick, D., Paquin, P., Cushing, P.E., Roth, V., Eds.; American Arachnological Society: Keene, NH, USA, 2017; pp. 77–84. [Google Scholar]
- Esquivel-Gómez, L.; Abdala-Roberts, L.; Pinkus-Rendón, M.; Parra-Tabla, V. Effects of tree species diversity on a community of weaver spiders in a tropical forest plantation. Biotropica 2017, 49, 63–70. [Google Scholar] [CrossRef]
- Dorado, O.; Maldonado, B.; Arias, D.M.; Sorani, V.; Ramirez, R.; Leyva, E.; Valenzuela, D. Programa de Conservación y Manejo Reserva de la Biosfera Sierra de Huautla. México; Comisión Nacional de Áreas Naturales Protegidas-Secretaría de Medio Ambiente y Recursos Naturales (CONANP-SEMARNAT), National Commission of Natural Protected Areas-Ministry of Environment and Natural Resources: Mexico City, Mexico, 2005; 202p. [Google Scholar]
- Miranda, F.; Hernandez, X.E. Los tipos de vegetación de México y su clasificación. Bol. Soc. Bot. 1963, 28, 29–179. [Google Scholar] [CrossRef]
- Challenger, A.; Caballero, J.; Zarate, S.; Elizondo, R. Utilización y Conservación de los Ecosistemas Terrestres de México: Pasado, Presente y Futuro; Agrupación Sierra Madre, National Commission for the Knowledge and Use of Biodiversity/Biology Institute/National Autonomous University of Mexico: Mexico City, MX, 1998; 847p. [Google Scholar]
- García, E. Modificaciones al Sistema de Clasificación Climática de Köppen, 5th ed.; Instituto de Geografía, UNAM: Mexico City, Mexico, 2004; 155p. [Google Scholar]
- Cushing, P.E. Introduction. In Spiders of North America: An Identification Manual; Ubick, D., Paquin, P., Cushing, P.E., Roth, V., Eds.; American Arachnological Society: Keene, NH, USA, 2017; pp. 1–16. [Google Scholar]
- Coddington, J.A.; Griswold, C.E.; Davila, D.S.; Peñaranda, E.; Larcher, S.F. Designing and testing sampling protocols to estimate biodiversity in tropical Ecosystems. In The Unity of Evolutionary Biology, Proceedings of the Fourth International Congress Of systematic and Evolutionary Biology, College Park, MD, USA, 1–7 July 1990; Dudley, E.C., Ed.; Dioscorides Press: Portland, OR, USA, 1991; pp. 44–60. [Google Scholar]
- Uetz, G.W.; Unzicker, J.D. Pitfall trapping in ecological studies of wandering spiders. J. Aracnol. 1975, 3, 101–111. [Google Scholar]
- Ubick, D. Key to spider families of North America North of Mexico. In Spiders of North America: An Identification Manual; Ubick, D., Paquin, P., Cushing, P.E., Roth, V., Eds.; American Arachnological Society: Keene, NH, USA, 2017; pp. 30–48. [Google Scholar]
- Bradley, R.A.; Buchanan, S. Common Spiders of North America; University of California Press: Berkeley, CA, USA, 2013; 288p. [Google Scholar]
- Levi, H.W. The Ravilla group of the orbweaver genus Eriophora in North America (Araneae: Araneidae). Psyche 1970, 77, 280–302. [Google Scholar] [CrossRef]
- Levi, H.W. Small orb-weavers of the genus Araneus North of Mexico (Araneae: Araneidae). Bull. Mus. Comp. Zool. 1973, 145, 473–552. [Google Scholar]
- Levi, H.W. The American orb-weaver genera Cyclosa, Metazygia, and Eustala North of Mexico (Araneae: Araneidae). Bull. Mus. Comp. Zool. 1977, 148, 61–127. [Google Scholar]
- Levi, H.W. The spiny orb-weaver genera Micrathena and Chaetacis (Araneae: Araneidae). Bull. Mus. Comp. Zool. 1985, 150, 429–618. [Google Scholar]
- Levi, H.W. The Neotropical orb-weaver genera Edricus and Wagneriana (Araneae: Araneidae). Bull. Mus. Comp. Zool. 1991, 152, 363–415. [Google Scholar]
- Levi, H.W. American Neoscona and corrections to previous revisions of Neotropical orb-weavers (Araneae: Araneidae). Psyche 1992, 99, 221–239. [Google Scholar] [CrossRef]
- Levi, H.W. The Neotropical orb-weaving spiders of the genera Wixia, Pozonia, and Ocrepeira (Araneae: Araneidae). Bull. Mus. Comp. Zool. 1993, 153, 47–141. [Google Scholar]
- Levi, H.W. The American orb weavers of the genera Mecynogea, Manogea, Kapogea, and Cyrtophora (Araneae: Araneidae). Bull. Mus. Comp. Zool. 1997, 155, 215–255. [Google Scholar]
- Levi, H.W. Comments and new records for the American genera Gea and Argiope with the description of new species (Araneae: Araneidae). Bull. Mus. Comp. Zool. 2004, 158, 47–66. [Google Scholar] [CrossRef]
- Levi, H.W. The orb-weaver genus Mangora of Mexico, Central America, and The West Indies (Araneae: Araneidae). Bull. Mus. Comp. Zool. 2005, 158, 139–182. [Google Scholar] [CrossRef]
- Harrod, J.C.; Levi, H.W.; Leibensperger, L.B. The Neotropical orbweavers of the genus Larinia (Araneae: Araneidae). Psyche 1990, 97, 241–265. [Google Scholar] [CrossRef]
- Glueck, S. A taxonomic revision of the orb-weaver genus Acacesia (Araneae: Araneidae). Psyche 1994, 101, 59–84. [Google Scholar] [CrossRef]
- Dorado, O.; Arias, D.M.; Ramírez, R.; Sousa, M. Leguminosas de la Sierra de Huautla; Centro de Educación Ambiental y Conservación Sierra de Huautla, Universidad Autónoma del Estado de Morelos: Mexico City, Mexico, 2005; 176p. [Google Scholar]
- Domínguez-Domínguez, Q.A. La Familia Burseraceae (Kunth) en el Estado de Morelos. Bachelor’s Thesis, Universidad Autónoma del Estado de Morelos, Mexico City, Mexico, 2013. [Google Scholar]
- An Online Flora of All Known Plants Supporting the Global Strategy for Plant Conservation. Available online: http://www.worldfloraonline.org/ (accessed on 12 April 2022).
- Comisión Nacional del Agua, Servicio Meteorológico Nacional (CONAGUA-SMN). Estación Operando # 17038, 18.52; -99.145, 2015.
- Comisión Nacional del Agua, Servicio Meteorológico Nacional (CONAGUA-SMN). Estación operando # 17008, 18.44; -99.025, 2015.
- Comisión Nacional del Agua, Servicio Meteorológico Nacional (CONAGUA-SMN). Estación operando # 17057, 18.5311; -98.9375, 2015.
- Stephen, H.S.; Barnes, B.V. Forest Ecology, 3rd ed.; John Wiley & Sons: New York, NY, USA, 1980; 687p. [Google Scholar]
- Jost, L. Entropy and diversity. Oikos 2006, 113, 363–375. [Google Scholar] [CrossRef]
- Moreno, C.; Barragán, E.F.; Pineda, E.; Pavón, N.P. Reanálisis de la diversidad alfa: Alternativas para interpretar y comparar información sobre comunidades ecológicas. Rev. Mex. Biodivers. 2011, 82, 1249–1261. [Google Scholar] [CrossRef]
- Jost, L.; González-Oreja, J.A. Midiendo la diversidad biológica: Más allá del índice de Shannon. Acta Zool. Lilloana 2012, 56, 3–14. [Google Scholar]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. Paleontological Statistics (Past) Software Versión 3.14; Natural History Museum; University of Oslo: Oslo, Norway, 2001. [Google Scholar]
- Koleff, P.; Gaston, K.J.; Lennon, J.J. Measuring beta diversity for presence–absence data. J. Anim. Ecol. 2003, 72, 367–382. [Google Scholar] [CrossRef]
- Moreno, C. Métodos para Medir la Biodiversidad; Serie Manuales y Tesis SEA, Programa Iberoamericano de Ciencia y Tecnología para el Desarrollo; Oficina Regional de Ciencia y Tecnología para América Latina y el Caribe de UNESCO y Sociedad Entomológica Aragonesa: Zaragoza, Spain, 2001; 84p. [Google Scholar]
- Lennon, J.J.; Koleff, P.; Greenwood, J.J.D.; Gaston, K.J. The geographical structure of British bird distributions: Diversity, spatial turnover and scale. J. Anim. Ecol. 2001, 70, 966–979. [Google Scholar] [CrossRef]
- Chao, A.; Chiu, C.H. Nonparametric Estimation and Comparison of Species Richness. In eLS; John Wiley & Sons, Ltd: Chichester, UK, 2016. [Google Scholar] [CrossRef]
- Colwell, R.K.; EstimateS. Version 8.2: Statistical Estimation of Species Richness and Shared Species from Samples. Software and User´s Guide. 2013. Available online: http://viceroy.eeb.uconn.edu/EstimateS (accessed on 8 May 2018).
- Moreno, C.E.; Halffter, G. On the measure of sampling effort used in species accumulation curves. J. Appl. Ecol. 2001, 38, 487–490. [Google Scholar] [CrossRef]
- StatSoft. STATISTICA Version 7.0. Data Analysis Software System and Computer Program Manual; StatSoft, Inc.: Tulsa, OK, USA, 2004. [Google Scholar]
- Hoffmann, A. Relación Bibliográfica Preliminar de las Arañas de México (Arachnida: Araneae); Instituto de Biología, Universidad Nacional Autónoma de México: Mexico City, Mexico, 1976; 117p. [Google Scholar]
- González-Tokman, D.; Martínez-Garza, C. Effect of ecological restoration on body condition of a predator. PLoS ONE 2015, 10, e0133551. [Google Scholar] [CrossRef]
- Candia-Ramírez, D.T.; Valdez-Mondragón, A. Spiders of the Orbiculariae clade (Araneae: Araneomorphae) from Calakmul municipality, Campeche, Mexico. Rev. Mex. Biodivers. 2017, 88, 154–162. [Google Scholar] [CrossRef]
- De La Cruz-Pérez, A.S.; Sánchez-Soto, C.F.; Ortiz-García, M.; Pérez-De La, C. Diversidad y distribución de arañas tejedoras diurnas (Arachnida: Araneae) en los microhábitats del agroecosistema cacao en Tabasco, México. Bol. Mus. Entomol. Univ. 2009, 10, 1–9. [Google Scholar]
- Cabra-García, J.; Chacón, P.; Valderrama-Ardila, C. Additive partitioning of spider diversity in a fragmented tropical dry forest (Valle del Cauca, Colombia). J. Arachnol. 2010, 38, 192–205. [Google Scholar] [CrossRef]
- Corcuera, P.; Jiménez, M.L.; Valverde, P.L. Does the microarchitecture of Mexican dry forest foliage influence spider distribution? J. Arachnol. 2008, 36, 552–556. [Google Scholar] [CrossRef]
- Theron, K.J.R.; Gaigher, J.S.; Pryke, M.J. Samways. High quality remnant patches in a complex agricultural landscape sustain high spider diversity. Biol. Conserv. 2020, 243, 108480. [Google Scholar] [CrossRef]
- Hernández-Silva, Y.G. Araneofauna de Vegetación en Tratamientos de Restauración Ecológica en Selva Estacional de Sierra de Huautla, Morelos. Bachelor’s Thesis, Facultad de Ciencias Biológicas, Universidad Autónoma del Estado de Morelos, Mexico City, Mexico, 2016. [Google Scholar]
- Menéndez-Acuña, M. Efecto de la Diversidad Genética de Una Especie Fundadora sobre la Comunidad de Plantas y Arañas Asociadas: El Caso de Bursera copallifera en Morelos, México. Master’s Thesis, Centro de Investigación en Biodiversidad y Conservación, Universidad Autónoma del Estado de Morelos, Mexico City, Mexico, 2017. [Google Scholar]
- Villa-Cosme, E.; Menéndez-Acuña, M. Diversidad y composición de la araneofauna (Arachnida: Araneae) en la zona arqueológica de Xochicalco, Morelos, México; Facultad de Biología, Universidad Autónoma del Estado de Morelos: Cuernavaca, Mexico, 2021; status (manuscript in preparation; to be submitted). [Google Scholar]
- Balfour, R.A.; Rypstra, A.L. The influence of habitat structure on spider density in a no-till soybean agroecosystem. J. Arachnol. 1998, 26, 221–226. [Google Scholar]
- Halaj, J.; Ross, D.W.; Moldenke, A.R. Habitat structure and prey availability as predictors of the abundance and community organization of spiders in Western Oregon forest canopies. J. Arachnol. 1998, 26, 203–220. [Google Scholar]
- Guerrero-Fuentes, D.R.; Desales-Lara, M.A. Arañas edáficas del clado RTA (Arachnida:Araneae) del sur de Tonatico, Estado de México, México. Acta Zool. Mex. Nueva Ser. 2021, 37, 1–25. [Google Scholar]
- Jiménez-Valverde, A.; Hortal, J. Las curvas de acumulación de especies y la necesidad de evaluar la calidad de los inventarios biológicos. Rev. Iber. Aracnol. 2003, 8, 151–161. [Google Scholar]
- Martínez-Hernández, J.G.; Brailovsky, H.; Corona-López, A.M.; Flores-Palacios, A.; Toledo-Hernández, V.H. Diversidad y estacionalidad de Coreidae (Hemiptera: Heteroptera) en el bosque tropical caducifolio en Morelos, México. Rev. Mex. Biodivers. 2020, 91, e913244. [Google Scholar] [CrossRef]
- Desales-Lara, M.A.; Durán-Barrón, C.G.; Mulia-Solano, C.E. Nuevos registros de aranéidos y terídidos (Araneae: Araneidae, Theridiidae) del Estado de México. Dugesiana 2008, 15, 1–6. [Google Scholar]
- Peña-Mendoza, M.T.; Cano-Santana, Z.; Saucedo-Morquecho, E.A.; Villeda-Hernandez, M.; Valdez, E. Densidad poblacional de Sphenarium purpurascens (Orthoptera: Pyrgomorphophidae) y Neoscona oaxacensis (Araneae: Araneidae) en dos zonas sujetas a restauración ecológica en la Reserva del Pedregal de San Ángel, D.F. México. Entomol. Mex. 2009, 8, 303–308. [Google Scholar]
- Berman, J.D.; Levi, H. The orb-weaver genus Neoscona in North America (Araneae: Araneidae). Bull. Mus. Comp. Zool. 1971, 141, 465–500. [Google Scholar]
- Nieto-Castañeda, I.G. Inventario de arañas de algunas localidades de los estados de Puebla y Morelos en la parte Alta del Balsas. Bachelor’s Thesis, FES-Zaragoza, Universidad Nacional Autónoma de México, Mexico City, Mexico, 2000. [Google Scholar]
- Maya-Morales, J.; Ibarra-Nuñez, G. Gremios de arañas de la vegetación del bosque mesófilo de montaña de la Reserva de la Biosfera Volcán Tacana, Chiapas, México. Entomol. Mex. 2012, 11, 53–58. [Google Scholar]
- Perez-Hernandez, C.X.; Zaragoza-Caballero, S. Diversidad alfa y beta de Cantharidae (Coleoptera) en el bosque tropical caducifolio de la vertiente del Pacífico mexicano. Rev. Mex. Biodivers. 2015, 86, 771–781. [Google Scholar] [CrossRef]
- Jiménez-Valverde, A.; Baselga, A.; Melic, A.; Txasko, N. Climate and regional beta-diversity gradients in spiders: Dispersal capacity has nothing to say? Insect. Conserv. Divers. 2010, 3, 51–60. [Google Scholar] [CrossRef]
- Dirzo, R.; Young, H.S.; Mooney, H.A.; Ceballos, G. Bosques Tropicales Estacionalmente Secos: Ecología y Conservación; Island Press: Washington, DC, USA, 2011; 392p. [Google Scholar]
- Trejo, I.; Dirzo, R. Diversidad florística de los bosques tropicales estacionalmente secos de México. Biodivers. Conserv. 2002, 11, 2063–2084. [Google Scholar] [CrossRef]
- Linares-Palomino, R.; Oliveira-Filho, A.T.; Pennington, R.T. Neotropical seasonally dry forests: Diversity, endemism, and biogeography of woody plants. In Seasonally Dry Tropical Forests, Dirzo, R., Young, H.S., Mooney, H.A., Ceballos, G., Eds.; Island Press: Washington, DC, USA, 2011; pp. 3–21. [Google Scholar]
- Sosa-Romero, M.; Menéndez-Acuña, M.; Burgos-Solorio, A. Fenología y estacionalidad del género Mexigonus Edwards, 2002 (Araneae: Salticidae) en un bosque templado al norte de Cuernavaca, Morelos, México. Entomol. Mex. 2016, 3, 919–923. [Google Scholar]
- Campuzano, E.F.; Padilla-Ramírez, J.R. Spatial and seasonal patterns of ground-dwelling spider assemblages belonging to the retrolateral tibial apophysis clade (Araneae: Araneomorphae) in two remnants of pine-oak forest from Mexico. Semental. Neotrop. Entorno Faunístico 2021, 56, 220–233. [Google Scholar] [CrossRef]
- Levi, H.; Levi, L. Arácnidos y Otros Artrópodos; Trillas: Mexico City, Mexico, 1993; 166p. [Google Scholar]
- Luna-Reyes, M.; Llorente-Bousquets, J.; Luis-Martínez, A.; Vargas, I. Composición faunística y fenología de las mariposas (Rhopalocera: Papilionoidea) de Cañón de Lobos, Yautepec, Morelos, México. Rev. Mex. Biodivers. 2010, 81, 315–342. [Google Scholar] [CrossRef]
- Jiménez-Valverde, A.; Lobo, J.M. Determinants of local spider (Araneidae and Thomisidae) species richness on a regional scale: Climate and altitude vs. habitat structure. Ecol. Entomol. 2007, 32, 113–122. [Google Scholar] [CrossRef]
- Obuid-Allah, A.H.; El-Shimy, N.A.; Hussien, E.H.; Aboulnasr, G.N. Factors affecting the spatial and seasonal distribution of spiders in assiut, Egypt. Indian J. Arachnol. 2017, 6, 55–68. [Google Scholar]
- Avila, C.A.; Stenert, C.; Rodrigues, E.N.L.; Maltchik, L. Habitat structure determines spider diversity in highland ponds. Ecol. Res. 2017, 32, 359–367. [Google Scholar] [CrossRef]
- Ferretti, N.E.; Arnedo, M.; González, A. Impact of climate change on spider species distribution along the La Plata River basin, southern South America: Projecting future range shifts for the genus Stenoterommata (Araneae, Mygalomorphae, Nemesiidae). Ann. Zool. Fenn. 2018, 55, 123–133. [Google Scholar] [CrossRef]
- Benavides, L.; Florez, E. Comunidades de arañas (Arachnida: Araneae) en microhábitats de dosel en bosques de tierra firme e Igapó de la Amazonía Colombiana. Rev. Iber. Aracnol. 2006, 14, 49–62. [Google Scholar]
- Silva, D.; Coddington, J.A. Spiders of Pakitza (Madre de Dios, Perú): Species richness and notes on community structure. In The Biodiversity of Southeastern Perú; Wilson, D.E., Sandoval, A., Eds.; Smithsonian Institution: Washington, DC, USA, 1996; pp. 253–311. [Google Scholar]
- New, T.R. Untangling the web: Spiders and the challenge of invertebrate conservation. J. Insect Conserv. 1999, 3, 251–256. [Google Scholar] [CrossRef]



| Locality | |||
|---|---|---|---|
| Characteristics | SME | HA | EL |
| Coordinates | 18°30′57.34″ N −99°06′46.26″ W | 18°27′58.82″ N −98°59′32.1″ W | 18°32′52.93″ N −98°55′57.97″ W |
| Altitude (m) | 859 | 1036 | 1323 |
| Municipality | Tlaquiltenango | Tlaquiltenango | Tepalcingo |
| Annual average temperature | 23.46 °C | 23.56 °C | 23.16 °C |
| Annual average precipitation (mm) | 2.27 mm | 2.82 mm | 2.65 mm |
| Within REBIOSH | No | Yes | Yes |
| Locality | Number of Genera | Number of Species | Total Adult Individuals | Total Juvenile Individuals |
|---|---|---|---|---|
| SME | 10 | 10 | 21 | 58 |
| HA | 7 | 7 | 208 | 201 |
| EL | 6 | 7 | 73 | 113 |
| Total | - | - | 302 | 372 |
| Locality Estimator | SME | HA | EL |
|---|---|---|---|
| 0D | 10 | 7 | 7 |
| 1D | 8.828 | 1.303 | 3.189 |
| 2D | 8.019 | 1.102 | 2.470 |
| Chao 1 | 14 | 17 | 10 |
| Chao 2 | 16 | 20.75 | 9.25 |
| ßsim | ||||
|---|---|---|---|---|
| ß Diversity | SME | HA | EL | |
| SME | 0.78 | 0.7 | ||
| ßj | HA | 0.87 | 0.7 | |
| EL | 0.79 | 0.73 | ||
| ßsim | ||||
|---|---|---|---|---|
| ß Diversity | SME | HA | EL | |
| SME | 0.88 | 0.85 | ||
| ßj | HA | 0.93 | 0.74 | |
| EL | 0.92 | 0.81 | ||
| Locality | |||
|---|---|---|---|
| Parameter | SME | HA | EL |
| Sobs | 10 | 7 | 7 |
| Samples | 12 | 12 | 12 |
| a | 1.352 | 0.826 | 1.259 |
| b | 0.051 | 0.037 | 0.094 |
| Asymptote (spp) | 26 | 22 | 13 |
| Slope | 0.518 | 0.395 | 0.275 |
| % fauna recorded | 38 | 31 | 53 |
| Estimated sampling effort units required to record 95% of fauna (SEU) | 371 | 513 | 201 |
| Additional sampling effort units required | 359 | 501 | 189 |
| Time (years) | 30 | 42 | 16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Menéndez-Acuña, M.; Salas-Rodríguez, M.; Montiel-Parra, G.; Sotuyo, S.; Rosas-Echeverría, M.V. Seasonality and Long-Term Effect of Environmental Variables on the Orb Weaver Spider Community of a Tropical Dry Forest in the Balsas Basin, Mexico. Diversity 2023, 15, 466. https://doi.org/10.3390/d15030466
Menéndez-Acuña M, Salas-Rodríguez M, Montiel-Parra G, Sotuyo S, Rosas-Echeverría MV. Seasonality and Long-Term Effect of Environmental Variables on the Orb Weaver Spider Community of a Tropical Dry Forest in the Balsas Basin, Mexico. Diversity. 2023; 15(3):466. https://doi.org/10.3390/d15030466
Chicago/Turabian StyleMenéndez-Acuña, Miguel, Mónica Salas-Rodríguez, Griselda Montiel-Parra, Solange Sotuyo, and Ma Ventura Rosas-Echeverría. 2023. "Seasonality and Long-Term Effect of Environmental Variables on the Orb Weaver Spider Community of a Tropical Dry Forest in the Balsas Basin, Mexico" Diversity 15, no. 3: 466. https://doi.org/10.3390/d15030466
APA StyleMenéndez-Acuña, M., Salas-Rodríguez, M., Montiel-Parra, G., Sotuyo, S., & Rosas-Echeverría, M. V. (2023). Seasonality and Long-Term Effect of Environmental Variables on the Orb Weaver Spider Community of a Tropical Dry Forest in the Balsas Basin, Mexico. Diversity, 15(3), 466. https://doi.org/10.3390/d15030466







