Aliens and Returnees: Review of Neobiotic Species of Freshwater Mollusks in Siberia from the Kazakhstan Steppe to the Arctic Tundra
Abstract
1. Introduction
2. Material and Methods
3. Results
Species Composition and Current Distribution of Neobiotic Mollusks of Siberia
| Species | Native Range | Invasive Range | Locality No. | First Finds in Siberia and Neighboring Regions | Coordinates | References | ||
|---|---|---|---|---|---|---|---|---|
| Date 1 | Site | Latitude | Longitude | |||||
| Dreissena polymorpha (Pallas, 1771) | Ponto-Caspian and Aral Sea regions; fresh waters of Balkan Peninsula [32,33,34] | North America, Europe, Middle and Southern Urals, Western Siberia [17,29] | 1 | 2018 | Beloyarsk Reservoir (Pyshma River basin, Middle Urals) | 56.8421 | 61.2780 | [35] |
| 2 | 2019 | Iriklinsky Reservoir (Ural River basin, Southern Urals) | 51.7768 | 58.7740 | [36] | |||
| 3 | 2021 | Pyshma River near Tyumen (Tura River basin, Western Siberia) | 56.9739 | 65.3721 | [17] | |||
| Sinanodonta lauta (E. von Martens, 1877) | Korea, Japan, and the coastal rivers of the South Primorye Region in Russia [37,38] | Ob’, Volga and Yenisei basins in Russia, Lake Balkhash basin in Kazakhstan, Borneo [13,14,38,39] | 4 | 2016 | Yenisei River at the outlet of the warm water of Krasnoyarsk TPP (Arctic Ocean basin, Eastern Siberia) | 55.9892 | 92.8699 | [13] |
| 5 | 2019 | Cooling reservoir and the channel of the Belovo Power Plant (Inya River basin, Western Siberia) | 54.4125 54.4344 | 86.4643 86.4500 | [39] | |||
| 6 | 2019 | Inya River just below the Belovo Reservoir (Ob’ River basin, Western Siberia) | 54.4571 | 86.4311 | [39] | |||
| Sinanodonta woodiana (I. Lea, 1834) | Indochina, China, Korea, and the Amur Basin, Russia [37] | Europe, North America, Central America and Caribbean, Japan, Indonesia, Philippines, Southeast Asia, Central Asia including Kazakhstan and Uzbekistan, Siberia [14,39] | 7 | 2016 | Yenisei River at the outlet of the warm water of Krasnoyarsk TPP (Arctic Ocean basin, Eastern Siberia) | 55.9892 | 92.8699 | [13] |
| 8 | 2019 | Cooling reservoir and the channel of the Belovo Power Plant (Inya River basin, Western Siberia) | 54.4125 54.4344 | 86.4643 86.4500 | [39] | |||
| Unio pictorum (Linnaeus, 1758) | Northern, Eastern and Western Europe, European Russia, Western Urals [37] | Middle and Southern Urals, southwest of Western Siberia, upper reaches of the Irtysh River basin (Eastern Kazakhstan) 2, Lake Kenon (Amur Basin, Transbaikalia) [11,16,37] | 9 | 1997 | Bukhtarma Reservoir (Irtysh River basin, Eastern Kazakhstan) 2 | 49.0259 | 83.9095 | [6,11] |
| 10 | 1998 | Lake Isetskoye and Verkh-Isetsky Pond (Iset’ River basin, Middle Urals) | 57.0167 56.8652 | 60.4667 60.5000 | [16,27] | |||
| 11 | 2001 | Lake Kenon (Amur River basin, Transbaikalia) | 52.0486 | 113.3726 | [9,40] | |||
| 12 | 2002 | Uy River in Chelyabinsk region (Tobol River basin, Southern Urals) | 54.1167 | 61.1000 | [16] | |||
| 13 | 2007 | Tobol River in Tyumen region (Irtysh River basin, Western Siberia) | 56.5175 | 66.3948 | [41] | |||
| U. tumidus Philipsson in Retzius, 1788 | Northern, Eastern and Western Europe, European Russia, Western Urals, Ural River in Russia and Kazakhstan, upper reaches of the Irtysh River (Eastern Kazakhstan) [16,37] | Southern Urals, extreme southwest of Western Siberia, upper reaches of the Irtysh River (Eastern Kazakhstan), Lake Kenon (Amur Basin, Transbaikalia) [16,37] | 14 | 1829 | ? Barnaul (probably Black Irtysh River basin, see [16]) | 53.3728? | 83.7318? | [16,42] |
| 15 | 2001 | Lake Kenon (Amur River basin, Transbaikalia) | 52.0486 | 113.3726 | [9,40] | |||
| 16 | 2005 | Uvel’ka River and Uy River in Chelyabinsk region (Tobol River basin, Southern Urals) | 54.0884 54.1000 | 61.5069 61.1333 | [7,16] | |||
| 17 | 2018 | Black Irtysh River in East-Kazakhstan region (Irtysh River basin, Eastern Kazakhstan) | 47.9000 | 84.9000 | [16,37] | |||
| U. crassus Philipsson in Retzius, 1788 | Northern, Eastern and Western Europe, European Russia (water bodies of Baltic, Black, Azov, and Caspian Sea drainage basins), Western Urals, Ural River in Russia and Kazakhstan [37] | Unknown 3 | 18 | 2006 | Pond of Tagil River (Tura River basin, Middle Urals) | 57.8000 | 60.0167 | [16] |
| Borysthenia naticina (Menke, 1845) | Eastern Central Europe, Eastern Europe and Turkey [29] | Southwest of Western Siberia, upper reaches of the Irtysh River (Eastern Kazakhstan) [1,11] | 19 | 2004? 2009 | Bukhtarma Reservoir (Irtysh River basin, Eastern Kazakhstan) | 49.0259 | 83.9095 | [6,11,18,43] |
| 20 | 2005 | Cooling reservoir of the Tyumen Heat and Power Plant No. 1 (Tura River basin, Western Siberia) | 57.1500 | 65.6241 | [1,18] | |||
| Ferrissia californica (Rowell, 1863) | North America: United States, southern Ontario, southern Quebec, southwest of British Columbia [29] | Many European countries, Israel, Syria and south-eastern Asia, southern part of the Western Siberia, Transcaucasia and Central Asia [29] | 21 | 2005 | Cooling reservoir of the Tyumen Heat and Power Plant No. 1 (Tura River basin, Western Siberia) | 57.1500 | 65.6241 | [1,18] |
| 22 | 2010 | Cooling reservoir of the Belovo Power Plant (Inya River basin, Western Siberia) | 54.4328 | 86.4647 | [3,18] | |||
| Helisoma anceps (Menke, 1830) | North America from Mexico to central Canada [29] | Europe, Middle Urals and Western Siberia [29] | 23 | 1999 | Riverbank drifts at Omsk (Irtysh River basin, Western Siberia) | 54.9912 | 73.3642 | [8,28] |
| 24 | 2010 | Cooling reservoir of the Belovo Power Plant (Inya River basin, Western Siberia) | 54.4328 | 86.4647 | [3,18] | |||
| 25 | 2013 | Cooling reservoirs around Nizhny Tagil (Tura River basin, Middle Urals) | 57.9105 | 59.9726 | [28] | |||
| Lithoglyphus naticoides (C. Pfeiffer, 1828) | Ponto-Azov basin: rivers northwest of the Black Sea [44] | Eastern and Central Europe, European Russia, upper reaches of the Irtysh River (Eastern Kazakhstan) [11,29,44] | 26 | 1998 | Bukhtarma Reservoir (Irtysh River basin, Eastern Kazakhstan) | 49.0259 | 83.9095 | [6,11,18] |
| Melanoides tuberculata (O. F. Müller, 1774) | Africa to Southeast Asia [29] | Australia and New Zealand, North America, South America, Europe and Azores, Western Siberia [29,45,46,47,48] | 27 | 2010 | Cooling reservoir of the Belovo Power Plant (Inya River basin, Western Siberia) | 54.4328 | 86.4647 | [3,18] |
| Physella acuta (Draparnaud, 1805) | North America (Canada, the Great Lakes region and Dakota) [29] | Europe, Asia, Africa, Australia, and South America (the Titicaca Lake), European Russia, Western Siberia and the Russian Far East [29] | 28 | 1969 | Floodplain waterbodies and rivers near Petropavl and Astana (Ishim River basin, Northern Kazakhstan) | 54.8765 51.1422 | 69.1195 71.4047 | [49,50,51] |
| 29 | 1979 | Upper reaches of the Irtysh River basin (East Kazakhstan) | 49.9533 | 82.5941 | [52] | |||
| 30 | 2005 | Cooling reservoir of the Tyumen Heat and Power Plant No. 1 (Tura River basin, Western Siberia) | 57.1500 | 65.6241 | [1,18] | |||
| 31 | 2010 | Cooling reservoir of the Belovo Power Plant (Inya River basin, Western Siberia) | 54.4328 | 86.4647 | [3,18] | |||
| 32 | 2013 | Cooling reservoirs and ponds near Nizhny Tagil, Yekaterinburg and Magnitogorsk (Irtysh River and Ural River basins, Southern and Middle Urals) | 57.9105 56.8421 53.4075 | 59.9726 61.2780 58.9784 | [28] | |||
| 33 | 2013 | Water bodies of the Korgalzhyn Reserve (Ishim River basin and basin of the endorheic lake Tengiz, Northern and Central Kazakhstan) | 50.4333 | 69.1889 | [8] | |||
| 34 | 2021 | Lake Dolgoye and warm stream inflowing the lake in Noril’sk (Pyasina River basin, Eastern Siberia) | 69.3377 | 88.2052 | This study | |||
| 35 | 2022 | Swampy oxbow of the Ishim River (Irtysh River basin, Western Siberia) | 56.0620 | 69.4772 | This study | |||
| Planorbella duryi (Wetherby, 1879) | Florida in North America [29] | South America, Hawaii, Northern, Eastern and South Africa, Europe, Northern coast of the Black See, Angara River basin [29] | 36 | 2010 | Heated stream running from the Ust-Ilimsk Pulp and Paper Plant (Angara River basin, Eastern Siberia) | 58.0323 | 102.7512 | [10] |
| Planorbella sp. | North and South America, northeastern Asia [29] | Widely distributed throughout the world (as aquarium animals, often end up in natural habitats) [29] | 37 | 2021 | Lake Dolgoye in Noril’sk (Pyasina River basin, Eastern Siberia) | 69.3377 | 88.2052 | This study |
| Pomacea canaliculata (Lamarck, 1822) | Southern South America (lower del Plata basin) [53] | South America (other parts), North America, Europe, Asia and many other regions, including Western Siberia [2,53] | 38 | 2002 | Cooling reservoir of the Belovo Power Plant (Inya River basin, Western Siberia) | 54.4328 | 86.4647 | [2,54] |
| Viviparus viviparus (Linnaeus, 1758) | Europe except for extreme North and South, Western Transcaucasia [29] | Crimean Peninsula, Western Siberia, Eastern Kazakhstan [19,29] | 39 | 1990s | Novosibirsk Reservoir (Ob’ River basin, Western Siberia) | 54.7344 | 82.8416 | [4,5,8] |
| 40 | 1994 | Bukhtarma Reservoir (Irtysh River basin, Eastern Kazakhstan) | 49.0259 | 83.9095 | [5,6,11,18] | |||
| 41 | 2009 | Oxbow lake near Tobol’sk (Irtysh River basin, Western Siberia) | 58.2038 | 68.2463 | [8] | |||
| 42 | 2015 | Tura River at Tyumen (Tobol River basin, Western Siberia) | 57.1563 | 65.5395 | [12] | |||
| 43 | 2022 | Ob’ River at Barnaul (Arctic Ocean basin, Western Siberia) | 53.3728 | 83.7318 | This study | |||
4. Discussion
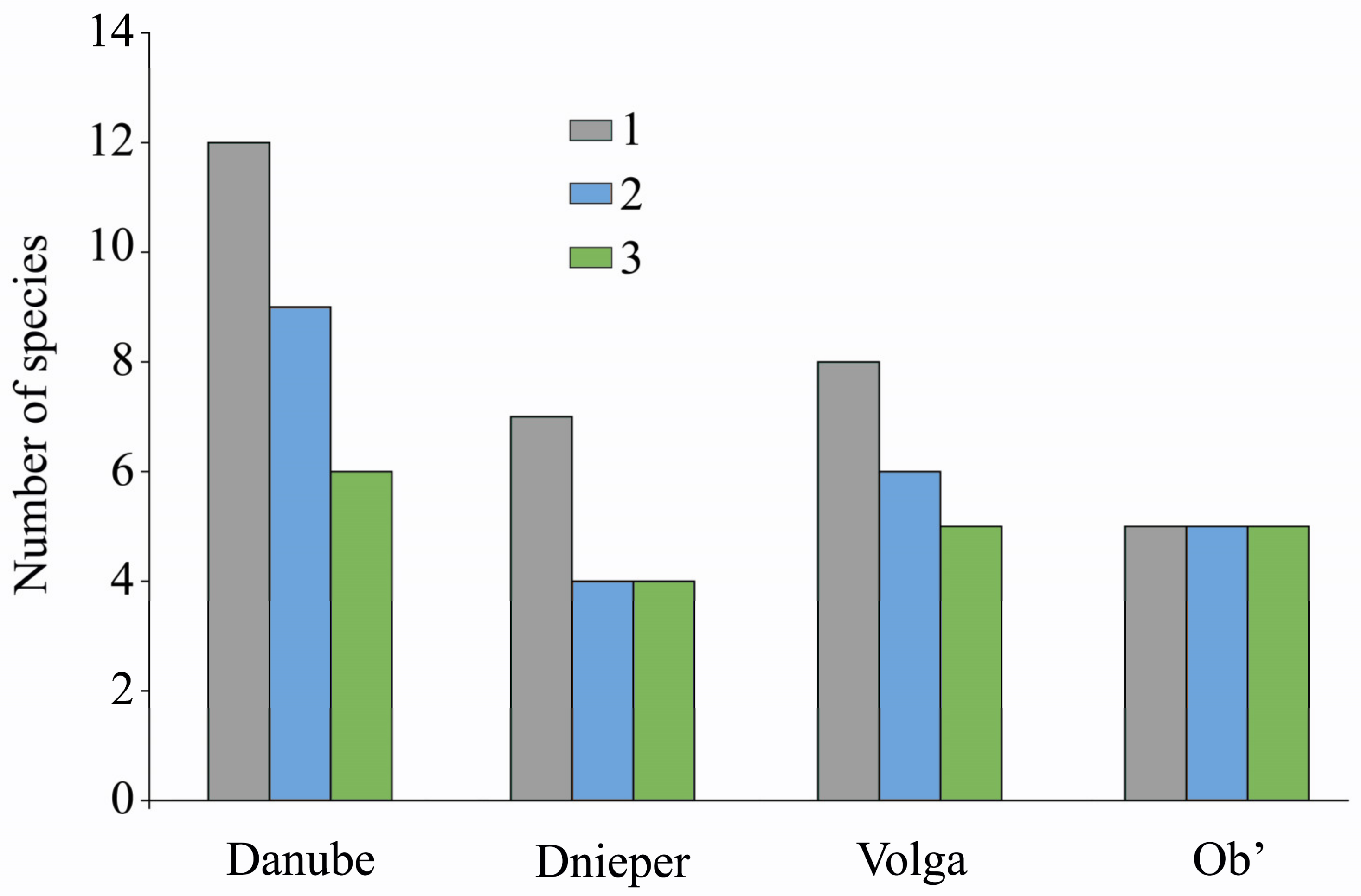
4.1. An Analysis of Neobiotic Malacofauna of Siberia
4.2. Forecast of Changes in Freshwater Malacofauna of Siberia in the Coming Decades
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharapova, T.A. The peculiarities of distribution and ecology of invasive mollusks in the cooling reservoir of the Tymen Power Plant-1 in Western Siberia. Vestn. Zool. 2008, 42, 185–187. (In Russian) [Google Scholar]
- Yanygina, L.V.; Kirillov, V.V.; Zarubina, E.Y. Invasive species in the biocenosis of the cooling reservoir of Belovskaya Power Plant (Southwest Siberia). Russ. J. Biol. Invasions 2010, 1, 50–54. [Google Scholar] [CrossRef]
- Yanygina, L.V.; Vinarski, M.V. Macroinvertebrates invasion in aquatic ecosystems of the upper Ob’ basin. In The III International Symposium “Invasion of Alien Species in Holarctic. Borok-3”. October 5th—9th. Programme and Book of Abstracts; Myshkin: Borok, Russia, 2010; pp. 98–99. [Google Scholar]
- Andreev, N.I.; Andreeva, S.I.; Vinarski, M.V.; Lazutkina, E.A.; Selezneva, M.V. Viviparus viviparus (L. 1758) (Mollusca: Gastropoda) is a new species for fauna of Novosibirsk reservoir. In Materialy Mezhdunarodnoy Konferentsii “Sovremennoe Sostoyanie Vodnykh Bioresursov” (Proc. Int. Conf. “Modern Status of Water Biological Resources”); Agros: Novosibirsk, Russia, 2008; pp. 118–120. (In Russian) [Google Scholar]
- Yanygina, L.V. The role of Viviparus viviparus (L.) (Gastropoda, Viviparidae) in formation of macrozoobenthos communities in the Novosibirsk Reservoir. Russ. J. Biol. Invasions 2012, 3, 64–70. [Google Scholar] [CrossRef]
- Devyatkov, V.I. Macrozoobenthos. In Suktsessii Biotsenozov Bukhtarminskogo Vodokhranilishcha (Successions of the Bukhtarma Reservoir Biocenoses); Bazhenova, O.P., Ed.; Omsk State Agricultural University: Omsk, Russia, 2009; pp. 95–119. (In Russian) [Google Scholar]
- Andreeva, S.I.; Vinarski, M.V.; Karimov, A.V. The first record of Unio species (Bivalvia: Unionidae) in the Irtysh River basin (Western Siberia, Russia). Mollusca 2009, 27, 87–91. [Google Scholar]
- Vinarski, M.V.; Andreev, N.I.; Andreeva, S.I.; Kazantsev, I.E.; Karimov, A.V.; Lazutkina, E.A. Alien mollusk species in the aquatic ecosystems of Western Siberia: A review. Rus. J. Biol. Invasions 2015, 6, 137–147. [Google Scholar] [CrossRef]
- Klishko, O.K. Zoobentos Ozior Zabaikalya. Chast’ 1. Vidovoye Raznoobraziye, Rasprostraneniye i Strukturnaya Organizatsiya (Zoobenthos of the Transbaikalia Lakes. Part 1. Species Diversity, Distribution and Structural Organization); Baikal Scientific Centre: Ulan-Ude, Russia, 2001; pp. 1–208. (In Russian) [Google Scholar]
- Sitnikova, T.; Soldatenko, E.; Kamaltynov, R.; Riedel, F. The finding of North American freshwater gastropods of the genus Planorbella Haldeman, 1842 (Pulmonata: Planorbidae) in East Siberia. Aquat. Invasions 2010, 5, 201–205. [Google Scholar] [CrossRef]
- Devyatkov, V.I. Macrozoobenthos of Bukhtarma reservoir in 2005–2009. Selevinia 2013, 21, 43–48. (In Russian) [Google Scholar]
- Babushkin, E.S.; Vinarski, M.V. The first find of the river snail Viviparus viviparus in the Tura River (Tyumen Region). Fauna Ural. Sib. 2017, 1, 19–24. (In Russian) [Google Scholar]
- Bespalaya, Y.V.; Bolotov, I.N.; Aksenova, O.V.; Gofarov, M.Y.; Kondakov, A.V.; Vikhrev, I.V.; Vinarski, M.V. DNA barcoding reveals invasion of two cryptic Sinanodonta mussel species (Bivalvia: Unionidae) into the largest Siberian River. Limnologica 2018, 69, 94–102. [Google Scholar] [CrossRef]
- Kondakov, A.V.; Bespalaya, Y.V.; Vikhrev, I.V.; Konopleva, E.S.; Gofarov, M.Y.; Tomilova, A.A.; Vinarski, M.V.; Bolotov, I.N. The Asian Pond mussels rapidly colonize Russia: Successful invasion of two cryptic species to the Volga and Ob’ rivers. Bionvasions Rec. 2020, 9, 504–518. [Google Scholar] [CrossRef]
- Kondakov, A.V.; Bolotov, I.N.; Vikhrev, I.V.; Konopleva, E.S.; Tomilova, A.A.; Khrebtova, I.S.; Aksenova, O.V. A tropical biodiversity hotspot in Siberia: Alien freshwater molluscs in an artificially heated channel of a thermal power plant. In Invasion of Alien Species in Holarctic. Borok-VI: Abstracts of the Sixth International Symposium; Dgebuadze, Y.Y., Krylov, A.V., Petrosyan, V.G., Karabanov, D.P., Eds.; Buk: Kazan, Russia, 2021; p. 114. [Google Scholar]
- Babushkin, E.S.; Vinarski, M.V.; Kondakov, A.V.; Tomilova, A.A.; Grebennikov, M.E.; Stolbov, V.A.; Bolotov, I.N. European freshwater mussels (Unio spp., Unionidae) in Siberia and Kazakhstan: Pleistocene relicts or recent invaders? Limnologica 2021, 90, 125903. [Google Scholar] [CrossRef]
- Babushkin, E.S.; Vinarski, M.V.; Gerasimova, A.A.; Ivanov, S.N.; Sharapova, T.A. First finding of Dreissena polymorpha (Pallas, 1771) (Mollusca, Bivalvia) in Siberia. Rus. J. Biol. Invasions 2022, 13, 167–173. [Google Scholar] [CrossRef]
- Yanygina, L.V. Regional features of alien macroinvertebrate invasion into the water ecosystems of the Ob’ River basin. Contemp. Probl. Ecol. 2016, 9, 384–390. [Google Scholar] [CrossRef]
- Yanygina, L.V. Community-level effects of a Viviparus viviparus L. (Gastropoda, Viviparidae) invasion in the Novosibirsk reservoir. Limnology 2020, 21, 165–171. [Google Scholar] [CrossRef]
- Yanygina, L.V. Mass mortality of invasive snails: Impact of nutrient release on littoral water quality. Diversity 2021, 13, 362. [Google Scholar] [CrossRef]
- Yanygina, L.V.; Kotovshchikov, A.V.; Kipriyanova, L.M.; Volgina, D.D. Factors of spatial distribution and risk assessment of Viviparus viviparus L. invasion in aquatic ecosystems of the Ob’ River basin. Contemp. Probl. Ecol. 2020, 13, 162–171. [Google Scholar] [CrossRef]
- Babushkin, E.S.; Vinarski, M.V.; Kondakov, A.V.; Tomilova, A.A.; Grebennikov, M.E.; Stolbov, V.A.; Bolotov, I.N. Freshwater mussels of the genus Unio Retzius, 1788 (Bivalvia: Unionidae) in Siberia and Kazakhstan: Relicts or conquerors? In Invasion of Alien Species in Holarctic. Borok-VI: Abstracts of the Sixth International Symposium; Dgebuadze, Y.Y., Krylov, A.V., Petrosyan, V.G., Karabanov, D.P., Eds.; Buk: Kazan, Russia, 2021; p. 33. [Google Scholar]
- Zhadin, V.I. Methods of Hydrobiological Research; Vysshyaya Shkola Publishers: Moscow, Russia, 1960; pp. 1–191. (In Russian) [Google Scholar]
- Starobogatov, Y.I.; Prozorova, L.A.; Bogatov, V.V.; Saenko, E.M. Molluscs. In Identification Key to Freshwater Invertebrates of Russia and Adjacent Areas; Tsalolikhin, S.Y., Ed.; Nauka Publishers: St. Petersburg, Russia, 2004; Volume 6, pp. 9–492. (In Russian) [Google Scholar]
- Andreyeva, S.I.; Andreyev, N.I.; Vinarski, M.V. Key to freshwater gastropods of Western Siberia (Mollusca: Gastropoda). V. 1. Gastropoda: Pulmonata. Fasc. 1. Families Acroloxidae and Lymnaeidae; Omsk Regional Printing House: Omsk, Russia, 2010; pp. 1–200. (In Russian) [Google Scholar]
- Glöer, P. Süsswassermollusken; 14. Auflage; Deutscher Jugendbund für Naturbeobachtung: Hamburg, Germany, 2015; pp. 1–132. [Google Scholar]
- Khokhutkin, I.M.; Vinarski, M.V.; Grebennikov, M.E. Molluscs of the Urals and the Adjacent Areas. Fasc. 1. The Family Lymnaeidae (Gastropoda, Pulmonata, Lymnaeiformes); Goshchitsky Publishers: Yekaterinburg, Russia, 2009; pp. 1–162. (In Russian) [Google Scholar]
- Khokhutkin, I.M.; Vinarski, M.V. Molluscs of the Urals and the Adjacent Areas. Fasc. 2. The Families Acroloxidae, Physidae, Planorbidae (Gastropoda, Pulmonata, Lymnaeiformes); Goshchitsky Publishers: Yekaterinburg, Russia, 2013; pp. 1–184. (In Russian) [Google Scholar]
- Vinarski, M.V.; Kantor, Y.I. Analytical Catalogue of Fresh and Brackish Water Molluscs of Russia and Adjacent Countries; A.N. Severtsov Institute of Ecology and Evolution of RAS: Moscow, Russia, 2016; pp. 1–544. [Google Scholar]
- MolluscaBase. 2023. Available online: https://www.molluscabase.org (accessed on 21 January 2023).
- Nekhaev, I.O.; Palatov, D.M. From the Black Sea to the White Sea: The first record of the invasive mollusc Physella acuta in the extreme North of the Europe. Russ. J. Biol. Invasions 2016, 7, 351–354. [Google Scholar] [CrossRef]
- Rajagopal, S.; Pollux, B.J.A.; Peters, J.L.; Cremers, G.; Moonvan der Staay, S.Y.; van Alen, T.; Eygensteyn, J.; Van Hoek, A.; Palau, A.; Bij de Vaate, A.; et al. Origin of Spanish invasion by the zebra mussel, Dreissena polymorpha (Pallas, 1771) revealed by amplified fragment length polymorphism (AFLP) fingerprinting. Biol. Invasions 2009, 11, 2147–2159. [Google Scholar] [CrossRef]
- Son, M.O. Molluski-Vselentsy v Presnykh i Solonovatykh Vodakh Severnogo Prichernomor’ya (Alien Mollusks of Fresh and Saline Waters of Northern Black Sea Region); Druk: Odessa, Ukraine, 2007; pp. 1–132. (In Russian) [Google Scholar]
- Starobogatov, Y.I.; Andreeva, S.I. The species’ range and its history. In Dreissena: Sistematika, Ekologiya, Prakticheskoe Znachenie (Dreissena: Systematics, Ecology, Practical Significance); Starobogatov, Y.I., Ed.; Nauka: Moscow, Russia, 1994; pp. 47–55. (In Russian) [Google Scholar]
- Eremkina, T.V.; Tsurikhin, E.A.; Chechulina, N.V.; Klimova, N.B.; Izimetova, M.P. Changes in the ecosystem of the Beloyarskoe Reservoir (Middle Ural) in the conditions of formation of the population of the invasive species Dreissena polymorpha (Pallas, 1771). In Invasion of Alien Species in Holarctic. Borok-VI: Abstracts of the Sixth International Symposium; Dgebuadze, Y.Y., Krylov, A.V., Petrosyan, V.G., Karabanov, D.P., Eds.; Buk: Kazan, Russia, 2021; p. 67. [Google Scholar]
- Kolozin, V.A.; Filinova, E.I.; Meleshin, D.I. First finding of Dreissena polymorpha (Pallas, 1771) in the Iriklinsky Reservoir. Russ. J. Biol. Invasions 2021, 12, 283–288. [Google Scholar] [CrossRef]
- Bolotov, I.N.; Kondakov, A.V.; Konopleva, E.S.; Vikhrev, I.V.; Aksenova, O.V.; Aksenov, A.S.; Bespalaya, Y.V.; Borovskoy, A.V.; Danilov, P.P.; Dvoryankin, G.A.; et al. Integrative taxonomy, biogeography and conservation of freshwater mussels (Unionidae) in Russia. Sci. Rep. 2020, 10, 3072. [Google Scholar] [CrossRef]
- Kondakov, A.V.; Palatov, D.M.; Rajabov, Z.P.; Gofarov, M.Y.; Konopleva, E.S.; Tomilova, A.A.; Vikhrev, I.V.; Bolotov, I.N. DNA analysis of a non-native lineage of Sinanodonta woodiana species complex (Bivalvia: Unionidae) from Middle Asia supports the Chinese origin of the European invaders. Zootaxa 2018, 4462, 511–522. [Google Scholar] [CrossRef] [PubMed]
- Kondakov, A.V.; Konopleva, E.S.; Vikhrev, I.V.; Bespalaya, Y.V.; Gofarov, M.Y.; Kabakov, M.B.; Tomilova, A.A.; Vinarski, M.V.; Bolotov, I.N. Phylogeographic affinities, distribution and population status of the non-native Asian pond mussels Sinanodonta lauta and S. woodiana in Kazakhstan. Ecol. Montenegrina 2020, 27, 22–34. [Google Scholar] [CrossRef]
- Klishko, O.; Lopes-Lima, M.; Froufe, E.; Bogan, A.; Vasiliev, L.; Yanovich, L. Taxonomic reassessment of the freshwater mussel genus Unio (Bivalvia: Unionidae) in Russia and Ukraine based on morphological and molecular data. Zootaxa 2017, 4286, 93–112. [Google Scholar] [CrossRef]
- Stolbov, V.A.; Voronova, K.P. The infestation of mussels (Bivalvia: Unionidae) by water mites of the genus Unionicola (Acari: Hydrachnidia: Unionicolidae) in water bodies of the south of Western Siberia. Parazitologiya 2019, 53, 220–229. (In Russian) [Google Scholar] [CrossRef]
- Von Martens, E. Russische und Sibirische Conchylien von Ehrenberg Gesammelt. Sitz. Ges. Nat. Freunde Berl. 1875, 88–96. [Google Scholar]
- Devyatkov, V.I.; Evseeva, A.A. Status of zooplankton and zoobenthos of the Bukhtarma reservoir. In Fishery Research in the Republic of Kazakhstan: History and the Current State; Bastau: Almaty, Kazakhstan, 2005; pp. 417–427. (In Russian) [Google Scholar]
- Guseva, D.O.; Gusev, A.A.; Feneva, I.Y. Lithoglyphus naticoides (C. Pfeiffer, 1828), Gravel snail. In The Most Dangerous Invasive Species of Russia (TOP-100); Dgebuadze, Y.Y., Petrosyan, V.G., Khlyap, L.A., Eds.; KMK Scientific Press: Moscow, Russia, 2018; pp. 312–321. (In Russian) [Google Scholar]
- Duggan, I.C. First record of a wild population of the tropical snail Melanoides tuberculata in New Zealand natural waters. N. Z. J. Mar. Freshw. Res. 2002, 36, 825–829. [Google Scholar] [CrossRef]
- Peso, J.G.; Pérez, D.C.; Vogler, R.E. The invasive snail Melanoides tuberculata in Argentina and Paraguay. Limnologica 2011, 41, 281–284. [Google Scholar] [CrossRef]
- Coelho, P.N.; Fernandez, M.A.; Cesar, D.A.S.; Ruocco, A.M.C.; Henry, R. Updated distribution and range expansion of the gastropod invader Melanoides tuberculata (Müller, 1774) in Brazilian waters. BioInvasions Rec. 2018, 7, 405–409. [Google Scholar] [CrossRef]
- Quirós-Rodríguez, J.A.; Yepes-Escobar, J.; Santafé-Patiño, G. The invasive snail Melanoides tuberculata (Müller, 1774) (Gastropoda, Thiaridae) in the lower basin of the Sinú River, Córdoba, Colombian Caribbean. Check List 2018, 14, 1089–1094. [Google Scholar] [CrossRef]
- Frolova, E.S. A study of freshwater mollusks of the Ishim River basin. In Voprosy Malakologii Sibiri (The Questions of Malacology in Siberia); Johanzen, B.G., Ed.; Tomsk State University: Tomsk, Russia, 1969; pp. 51–52. (In Russian) [Google Scholar]
- Frolova, E.S. Freshwater Mollusks of Northern Kazakhstan. Ph.D. Thesis, Tomsk State University, Tomsk, Russia, 1973; pp. 1–254. (In Russian). [Google Scholar]
- Frolova, E.S. Freshwater mollusks of Northern Kazakhstan and their role in benthos biomass in natural complexes. In Zametki po Faune i Flore Sibiri (Notes on the Flora and Fauna of Siberia); Petlina, A.P., Ed.; Tomsk State University: Tomsk, Russia, 1984; pp. 42–50. (In Russian) [Google Scholar]
- Krivosheina, L.V. Zoogeographic characteristics of freshwater malacofauna of Upper Irtush River. In Priroda i Khozyaistvo Vostochnogo Kazakhstana (Nature and Economics of Eastern Kazakhstan); Nauka: Alma-Ata, Russia, 1979; pp. 100–107. (In Russian) [Google Scholar]
- Seuffert, M.E.; Martín, P.R. Exceeding its own limits: Range expansion in Argentina of the globally invasive apple snail Pomacea canaliculata. Hydrobiologia 2021, 848, 385–401. [Google Scholar] [CrossRef]
- Yanygina, L.V.; Kirillov, V.V.; Zarubina, E.Y. The Role of Alien Species in the Formation of the Biocoenosis of the Cooling Reservoir of Belovskaya Power Plant. The II International Symposium “Invasion of Alien Species in Holarctic. Borok-2”. September 27th—October 1th. Programme and Book of Abstracts; Borok: Rybinsk, Russia, 2005; pp. 110–111. (In Russian) [Google Scholar]
- Vinarski, M.V. The history of an invasion: Phases of the explosive spread of the physid snail Physella acuta through Europe, Transcaucasia and Central Asia. Biol. Invasions 2017, 19, 1299–1314. [Google Scholar] [CrossRef]
- Vinarski, M.V. A great journey of a tiny snail. Priroda 2018, 2, 10–19. (In Russian) [Google Scholar]
- Zhadin, V.I. Fresh- and brackishwater Mollusca of the USSR. Opredeliteli Faune SSSR Izd. Zool. Inst. AN SSSR 1952, 46, 1–376. (In Russian) [Google Scholar]
- Starobogatov, Y.I. Fauna Mollyuskov i Zoogeograficheskoye Rayonirovaniye Vodoyemov Zemnogo Shara [Fauna of Mollusks and the Zoogeographic Regionalization of Fresh Water Bodies of the World]; Nauka: Saint Petersburg, Russia, 1970; pp. 1–372. (In Russian) [Google Scholar]
- Mozley, A. The freshwater and terrestrial Mollusca of Northern Asia. Trans. R. Soc. Edinb. 1936, 58, 605–695. [Google Scholar] [CrossRef]
- Vinarski, M.V. A historical outline of study of Siberian freshwater malacofauna (end of XVIII—Middle of XX centuries). Ruthenica Russ. Malacol. J. 2010, 20, 45–67. (In Russian) [Google Scholar]
- Zykin, V.S. Stratigrafiya i Evolyutsiya Prirodnoy Sredy i Klimata v Pozdnem Kaynozoye Yuga Zapadnoy Sibiri [Stratigraphy and Evolution of Environments and Climate during Late Cenozoic in the Southern West Siberia]; GEO: Novosibirsk, Russia, 2012; pp. 1–488. (In Russian) [Google Scholar]
- Makhrov, A.A.; Vinarski, M.V.; Gofarov, M.Y.; Dvoriankin, G.A.; Novoselov, A.P.; Bolotov, I.N. Faunal exchanges between the basins of the Arctic Ocean and the Caspian Sea: Their history and current processes. Biol. Bull. 2021, 48, 892–906. [Google Scholar] [CrossRef]
- Makhrov, A.A.; Bolotov, I.N.; Vinarski, M.V.; Artamonova, V.S. Origin of Glacial Relicts in Northern and Central Europe: Four Waves of Introduction of Cold-Water Species from Asia (Review). Inland Water Biol. 2022, 15, 707–728. [Google Scholar] [CrossRef]
- Artamonova, V.S.; Bolotov, I.N.; Vinarski, M.V.; Makhrov, A.A. Fresh- and Brackish-Water Cold-Tolerant Species of Southern Europe: Migrants from the Paratethys That Colonized the Arctic. Water 2021, 13, 1161. [Google Scholar] [CrossRef]
- Bogachev, V.V. Materialy k Istorii Presnovodnoi Fauny Evrazii [Materials on the History of the Freshwater Fauna of Eurasia]; Izdatel’stvo Akademii Nauk Ukrainskoi SSR: Kiev, Ukraine, 1961; pp. 1–404. (In Russian) [Google Scholar]
- Starobogatov, Y.I. Fauna of the lakes as the data source on their history. In Obshchie Zakonomernosti Vozniknoveniya i Razvitiya Ozer. Metody Izucheniya Istorii Ozer (General Patterns of Emergence and Development of the Lakes: Study Methods of the Lake History); Kvasov, D.D., Ed.; Gidrometeoizdat: Saint Petersburg, Russia, 1986; pp. 33–50. (In Russian) [Google Scholar]
- Frey, K.E.; Smith, L.C. Recent temperature and precipitation increases in West Siberia and their association with the Arctic Oscillation. Polar Res. 2003, 22, 287–300. [Google Scholar] [CrossRef]
- Degefie, D.T.; Fleischer, E.; Klemm, O.; Soromotin, A.V.; Soromotina, O.V.; Tolstikov, A.V.; Abramov, N.V. Climate extremes in south Western Siberia: Past and future. Stoch. Environ. Res. Risk A 2014, 28, 2161–2173. [Google Scholar] [CrossRef]
- Sada, R.; Schmalz, B.; Kiesel, J.; Fohrer, N. Projected changes in climate and hydrological regimes of the Western Siberian lowlands. Environ. Earth Sci. 2019, 78, 1–15. [Google Scholar] [CrossRef]
- Brandes, U.; Furevik, B.B.; Nielsen, L.R.; Kjær, E.D.; Rosef, L.; Fjellheim, S. Introduction history and population genetics of intracontinental scotch broom (Cytisus scoparius) invasion. Divers. Distrib. 2019, 25, 1773–1786. [Google Scholar] [CrossRef]
- Muller, J.M.; Hermann, J.J. An assessment of invasion risk from assisted migration. Conserv. Biol. 2008, 22, 562–567. [Google Scholar] [CrossRef]
- Der Sarkissian, C.; Möller, P.; Hofman, C.; Ilsøe, P.; Rick, T.; Schiøtte, T.; Sørensen, M.V.; Dalén, L.; Orlando, L. Unveiling the ecological applications of ancient DNA from mollusk shells. Front. Ecol. Evol. 2020, 8, 37. [Google Scholar] [CrossRef]
- Popova, S.M. Kaynozoyskaya Kontinental’naya Malakofauna Yuga Sibiri i Sopredel’nykh Territoriy [The Cenozoic Continental Malacofauna of Southern Siberia and Adjacent Territories]; Nauka: Moscow, Russia, 1981; pp. 1–181. (In Russian) [Google Scholar]
- Maderni, U.N. Mollyuski Kontinental’nogo Kainozoya Turgaiskogo Progiba i Smezhnykh Regionov (Mollusks of Continental Cainozoic of Turgay Deflection and Adjacent Regions); Nedra: Saint Petersburg, Russia, 1990; pp. 1–191. (In Russian) [Google Scholar]
- Yanygina, L.V.; Vizer, A.M. Long-term dynamics and current distribution of the river snail (Viviparus viviparus) in the Novosibirsk reservoir. Tomsk. State Univ. J. Biol. 2020, 49, 149–165. (In Russian) [Google Scholar] [CrossRef]
- Kozhov, M. Lake Baikal and Its Life; W. Junk: The Hague, The Netherlands, 1963; pp. 1–344. [Google Scholar]
- Sitnikova, T.Y.; Starobogatov, Y.I.; Shirokaya, A.A.; Shibanova, I.V.; Korobkova, N.V.; Adov, F.V. Snails (Gastropoda). In Index of Animal Species Inhabiting Lake Baikal and Its Catchment Area; Timoshkin, O.A., Ed.; Nauka: Novosibirsk, Russia, 2004; Volume 1, pp. 937–1002. (In Russian) [Google Scholar]
- Stift, M.; Michel, E.; Sitnikova, T.Y.; Mamonova, E.Y.; Sherbakov, D.Y. Palaearctic gastropod gains a foothold in the dominion of endemics: Range expansion and morphological change of Lymnaea (Radix) auricularia in Lake Baikal. Hydrobiologia 2004, 513, 101–108. [Google Scholar] [CrossRef]
- Schniebs, K.; Sitnikova, T.Y.; Vinarski, M.V.; Müller, A.; Khanaev, I.V.; Hundsdoerfer, A. Morphological and genetic variability in Radix auricularia (Mollusca: Gastropoda: Lymnaeidae) of Lake Baikal, Siberia: The story of an unfinished invasion into the ancient deepest lake. Diversity 2022, 14, 527. [Google Scholar] [CrossRef]
- Rajagopal, S.; van der Velde, G.; de Vaate, A.B. Reproductive biology of the Asiatic clams Corbicula fluminalis and Corbicula fluminea in the river Rhine. Arch. Hydrobiol. 2000, 149, 403–420. [Google Scholar] [CrossRef]
- Marescaux, J.; Pigneur, L.-M.; Van Doninck, K. New records of Corbicula clams in French rivers. Aquat. Invasions 2010, 5 (Suppl. 1), S35–S39. [Google Scholar] [CrossRef]
- Bespalaya, Y.V.; Bolotov, I.N.; Aksenova, O.V.; Kondakov, A.V.; Gofarov, M.Y.; Laenko, T.M.; Sokolova, S.E.; Shevchenko, A.R.; Travina, O.V. Aliens are moving to the Arctic frontiers: An integrative approach reveals selective expansion of androgenic hybrid Corbicula lineages towards the North of Russia. Biol. Invasions 2018, 20, 2227–2243. [Google Scholar] [CrossRef]
- Müller, O.; Baur, B. Survival of the invasive clam Corbicula fluminea (Müller) in response to winter water temperature. Malacologia 2011, 53, 367–371. [Google Scholar] [CrossRef]
- Denton, M.E.; Chandra, S.; Wittmann, M.E.; Reuter, J.; Baguley, J.G. Reproduction and population structure of Corbicula fluminea in an oligotrophic subalpine lake. J. Shellfish Res. 2012, 31, 145–152. [Google Scholar] [CrossRef]
- Gama, M.; Crespo, D.; Dolbeth, M.; Anastácio, P.M. Ensemble forecasting of Corbicula fluminea worldwide distribution: Projections of the impact of climate change. Aquat. Conserv. 2017, 27, 675–684. [Google Scholar] [CrossRef]
- Bespalaya, Y.V.; Aksenova, O.V.; Kropotin, A.V.; Shevchenko, A.R.; Travina, O.V. Reproduction of the androgenetic population of the Asian Corbicula clam (Bivalvia: Cyrenidae) in the Northern Dvina River basin, Russia. Diversity 2021, 13, 316. [Google Scholar] [CrossRef]
- Kursalova, V.A.; Starobogatov, Y.I. Mollusks of the genus Corbicula of Anthropogen of Northern and Western Asia and Europe. In Mollusks: Techniques, Methods, and Results of Their Study. Abstracts of Papers of the All-Union Meeting on Study of Mollusks; Likharev, I.M., Ed.; Nauka: Saint Petersburg, Russia, 1971; pp. 93–96. (In Russian) [Google Scholar]
- Cianfanelli, S.; Stasolla, G.; Inghilesi, A.F.; Tricarico, E.; Goti, E.; Strangi, A.; Bodon, M. First European record of Sinotaia cf. quadrata (Benson, 1842), an alien invasive freshwater species: Accidental or voluntary introduction? (Caenogastropoda: Viviparidae). Boll. Malacol. 2017, 53, 150–160. [Google Scholar]
- Collas, F.P.L.; Breedveld, S.K.D.; Matthews, J.; van der Velde, G.; Leuwen, R.S.E.W. Invasion biology and risk assessment of the recently introduced Chinese mystery snail, Bellamya (Cipangopaludina) chinensis (Gray, 1834), in the Rhine and Meuse River basins in Western Europe. Aquat. Invasions 2017, 12, 275–286. [Google Scholar] [CrossRef]
- Arias, A.; Fernández-Rodríguez, I.; Sánchez, O.; Borrell, Y.J. Integrative taxonomy reveals the occurrence of the Asian freshwater snail Sinotaia cf. quadrata in inland waters of SW Europe. Aquat. Invasions 2020, 15, 616–632. [Google Scholar] [CrossRef]
- Hernández, J.; Úbeda, C.; Ferrero, L.; Deltoro, V.; Quiñonero-Salgado, S.; López-Soriano, J. Primera población de Cipangopaludina chinensis (Gray, 1834) (Gastropoda: Viviparidae) en la península Ibérica. Spira 2020, 7, 187–190. [Google Scholar]
- O’Leary, E.; Jojo, D.; David, A.A. Another mystery snail in the Adirondacks: DNA barcoding reveals the first records of Sinotaia cf. quadrata (Caenogastropoda: Viviparidae) from North America. Am. Malac. Bull. 2021, 38, 1–5. [Google Scholar] [CrossRef]
- Quiñonero-Salgado, S.; Núñez de Arenas, J.H.; López-Soriano, J. Primer registre de Sinotaia quadrata (Benson, 1842) (Gastropoda: Viviparidae) al País Valencià. Nemus 2022, 12, 281–283. [Google Scholar]


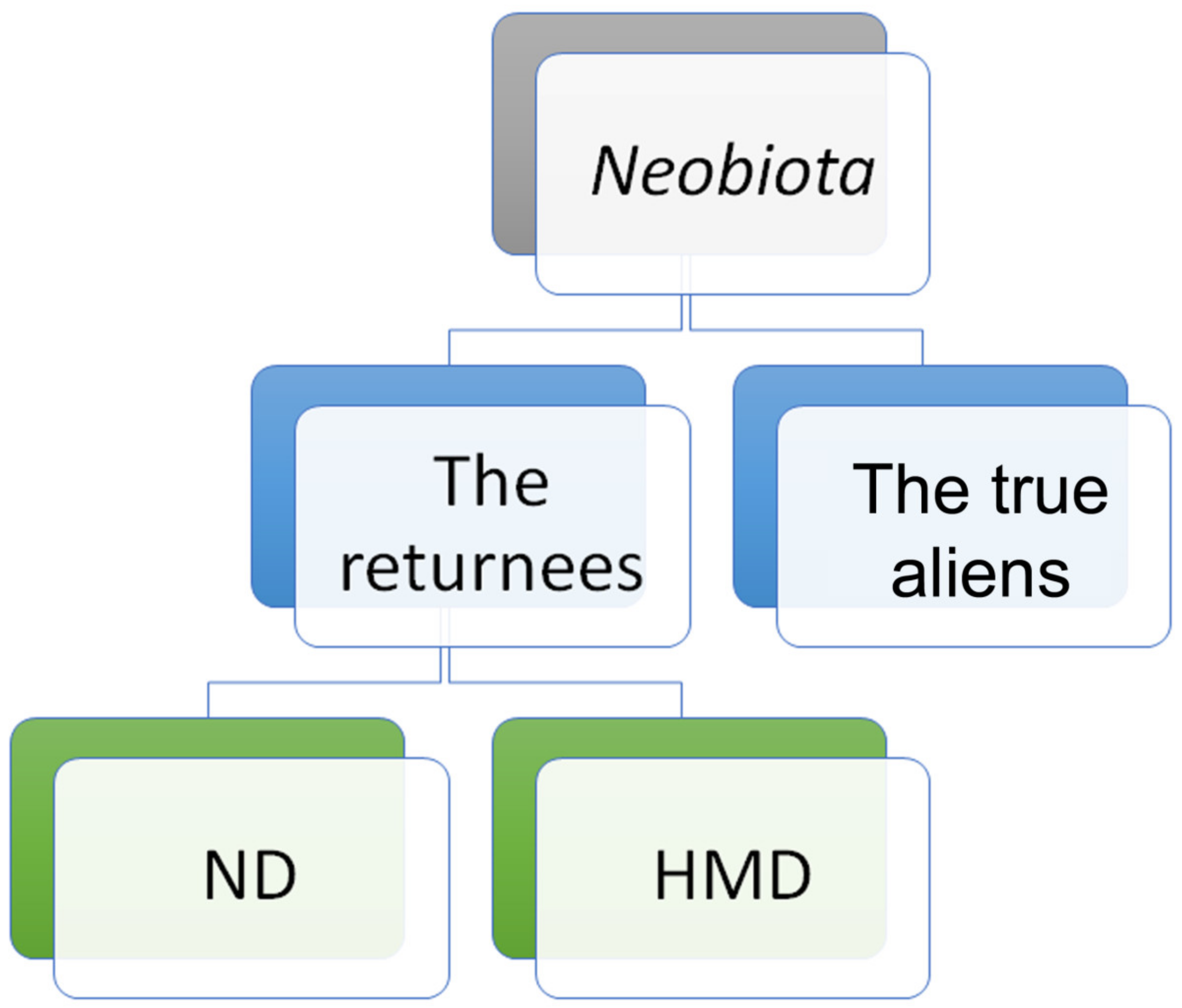
| True Aliens or Non-Indigenous | The Returnees | ||
|---|---|---|---|
| AT | FF | ND | HMD |
| Ferrissia californica, Melanoides tuberculata, Physella acuta, Helisoma anceps, Planorbella duryi, Planorbella sp., Pomacea canaliculata | Dreissena polymorpha (?), Sinanodonta lauta, S. woodiana | Unio crassus, U. pictorum (partly), U. tumidus (partly) | Borysthenia naticina, Lithoglyphus naticoides, Unio pictorum (partly), U. tumidus (partly), Viviparus viviparus |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Babushkin, E.S.; Nekhaev, I.O.; Vinarski, M.V.; Yanygina, L.V. Aliens and Returnees: Review of Neobiotic Species of Freshwater Mollusks in Siberia from the Kazakhstan Steppe to the Arctic Tundra. Diversity 2023, 15, 465. https://doi.org/10.3390/d15030465
Babushkin ES, Nekhaev IO, Vinarski MV, Yanygina LV. Aliens and Returnees: Review of Neobiotic Species of Freshwater Mollusks in Siberia from the Kazakhstan Steppe to the Arctic Tundra. Diversity. 2023; 15(3):465. https://doi.org/10.3390/d15030465
Chicago/Turabian StyleBabushkin, Evgeny S., Ivan O. Nekhaev, Maxim V. Vinarski, and Liubov V. Yanygina. 2023. "Aliens and Returnees: Review of Neobiotic Species of Freshwater Mollusks in Siberia from the Kazakhstan Steppe to the Arctic Tundra" Diversity 15, no. 3: 465. https://doi.org/10.3390/d15030465
APA StyleBabushkin, E. S., Nekhaev, I. O., Vinarski, M. V., & Yanygina, L. V. (2023). Aliens and Returnees: Review of Neobiotic Species of Freshwater Mollusks in Siberia from the Kazakhstan Steppe to the Arctic Tundra. Diversity, 15(3), 465. https://doi.org/10.3390/d15030465







