Chitons from Deep-Water Mollusk-Rich Deposits in the Southwestern Adriatic Sea (Mollusca, Polyplacophora) †
Abstract
1. Introduction
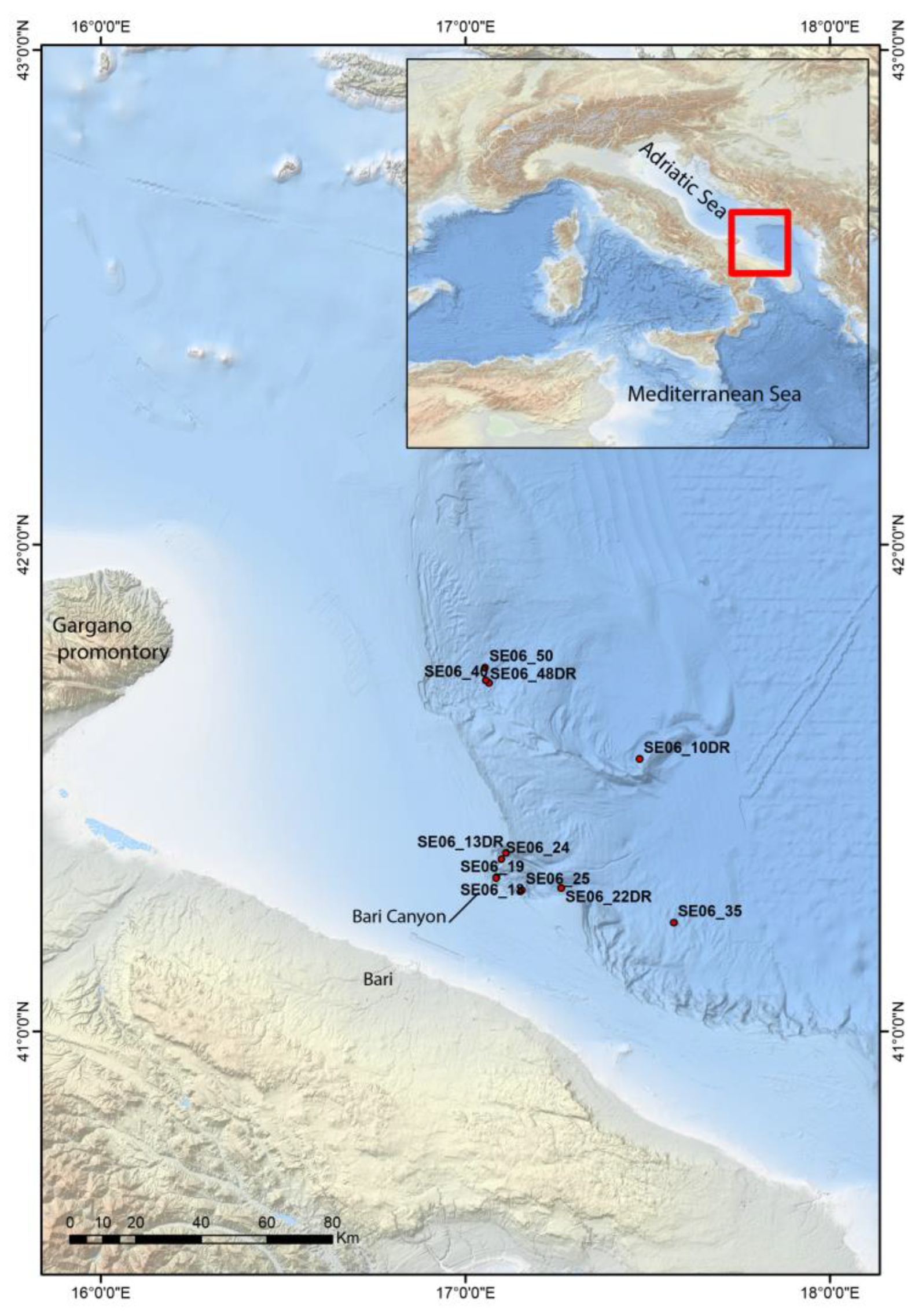
2. Material and Methods
3. Systematics
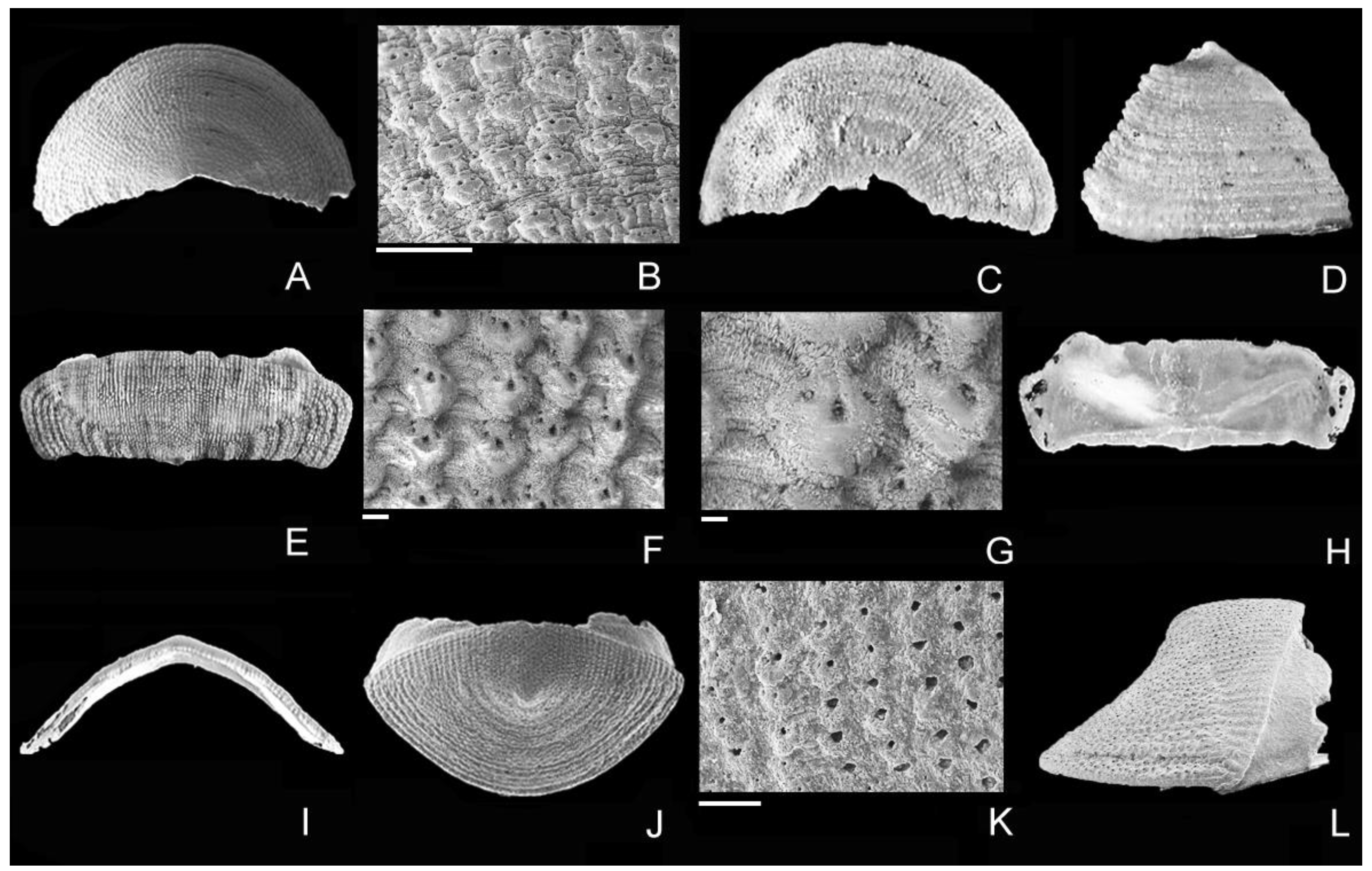
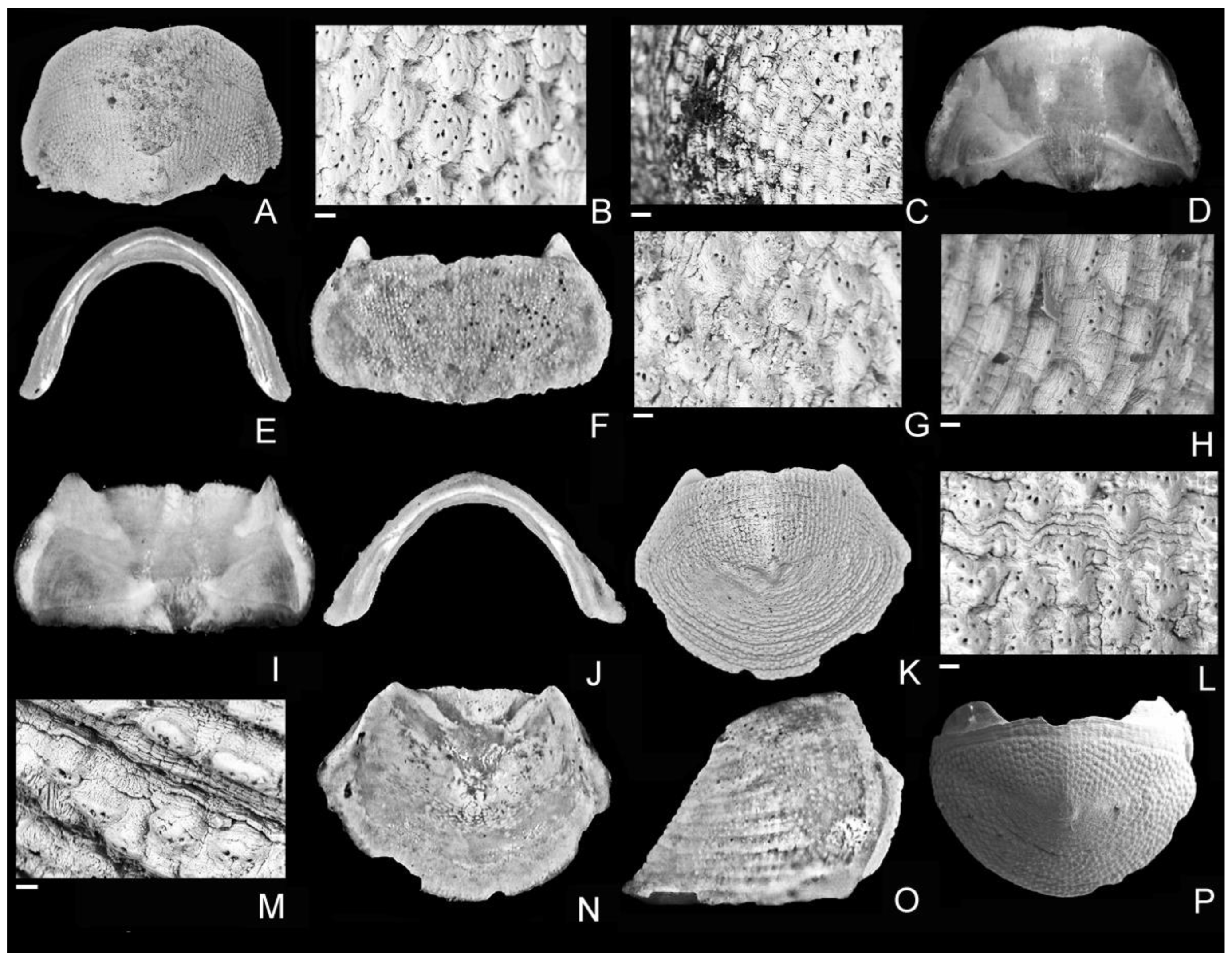
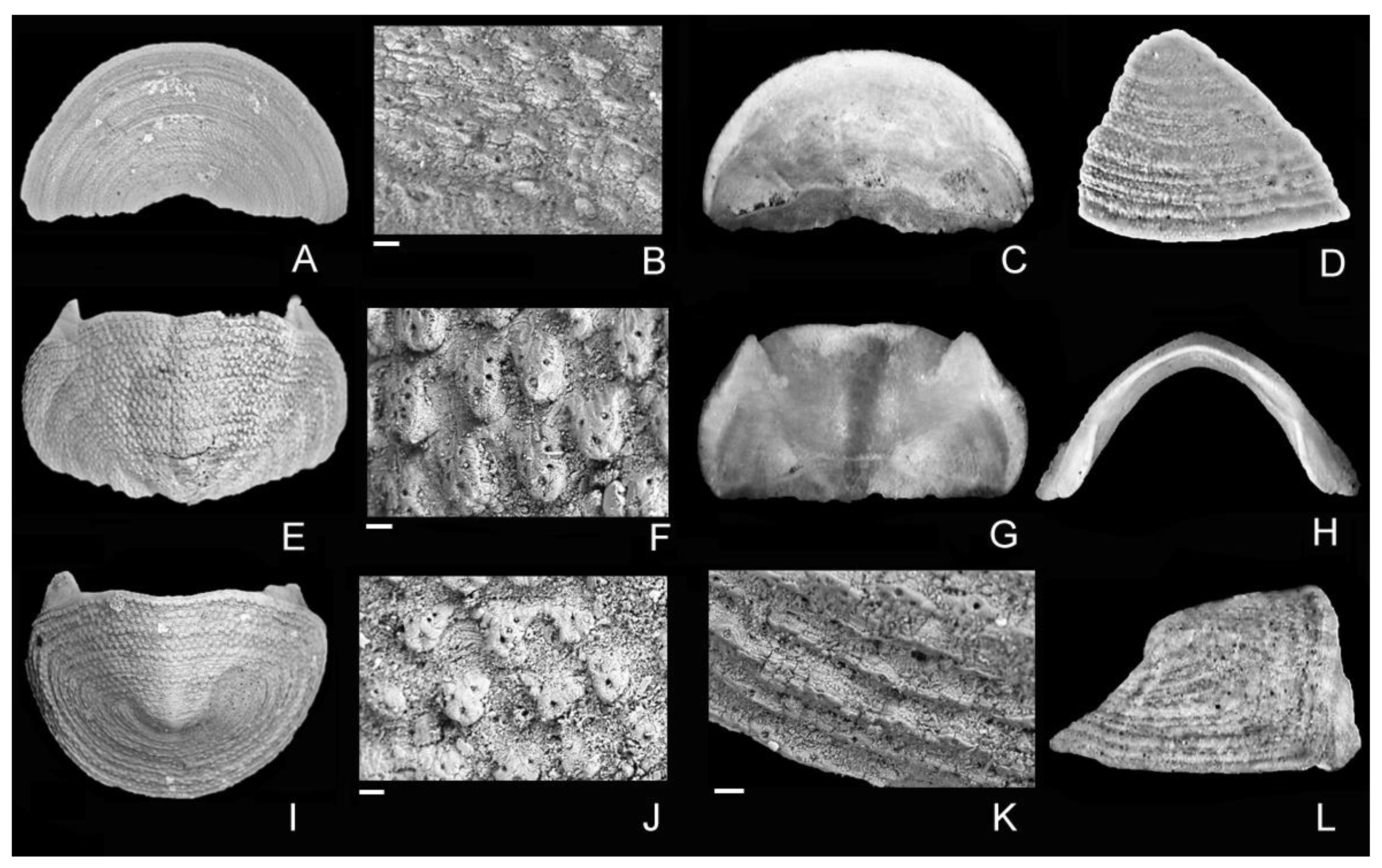
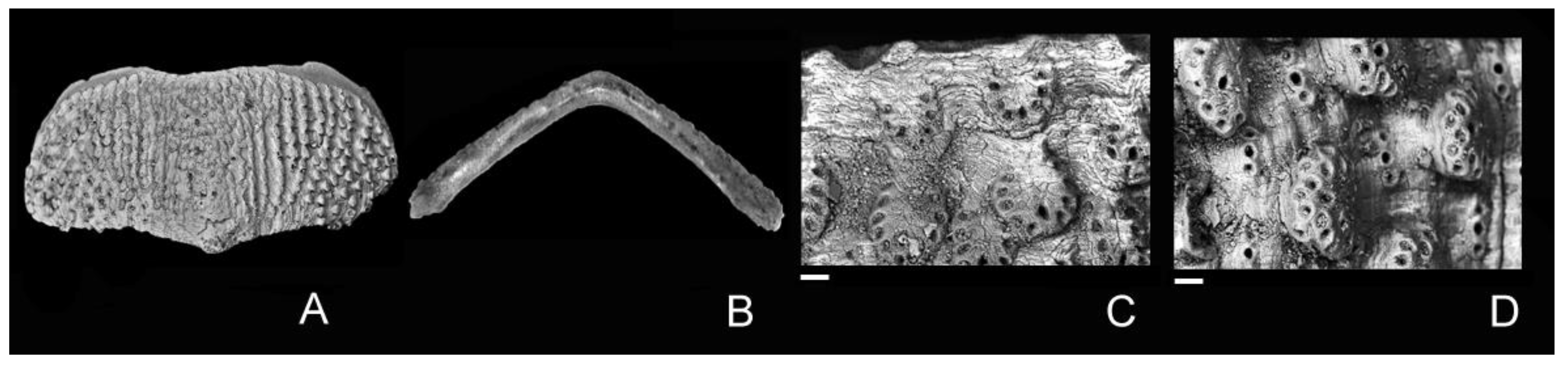
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fabbri, A.; Gallignani, P. Ricerche geomorfologiche e sedimentologiche nell’Adriatico meridionale. Giorn. Geol. 1972, 38, 53–498. [Google Scholar]
- Colantoni, P.; Gallignani, P. Quaternary evolution of the continental shelf off the coast of Bari (South Adriatic Sea): Shallow seismic, sedimentological and faunal evidences. Géol. Méditer. 1978, 5, 327–338. [Google Scholar] [CrossRef]
- Trincardi, F.; Foglini, F.; Verdicchio, G.; Asioli, A.; Correggiari, A.; Minisini, D.; Piva, A.; Remia, A.; Ridente, D.; Taviani, M. The impact of cascading currents on the Bari Canyon System, SW-Adriatic Margin (Central Mediterranean). Mar. Geol. 2007, 246, 208–230. [Google Scholar] [CrossRef]
- Freiwald, A.; Beuck, L.; Rüggeberg, A.; Taviani, M.; Hebbeln, D.; R/V Meteor Cruise M70-1 participants. The white coral community in the central Mediterranean Sea revealed by ROV surveys. Oceanography 2009, 22, 58–74. [Google Scholar] [CrossRef]
- Angeletti, L.; Taviani, M.; Canese, S.; Foglini, F.; Mastrototaro, F.; Argnani, A.; Trincardi, F.; Bakran-Petricioli, T.; Ceregato, A.; Chimienti, G.; et al. New deep-water cnidarian sites in the southern Adriatic Sea. Mediter. Mar. Sci. 2014, 15, 263–273. [Google Scholar] [CrossRef]
- Angeletti, L.; Prampolini, M.; Foglini, F.; Grande, V.; Taviani, M. Cold-water coral habitat in the Bari Canyon System, Southern Adriatic Sea (Mediterranean Sea). In Seafloor Geomorphology as Benthic Habitat, Chapter 49, 2nd ed.; Harris, P., Baker, E., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 811–824, Geohab Atlas and Seafloor Geomorphic Features and Benthic Habitats. [Google Scholar]
- Taviani, M.; Angeletti, L.; Beuck, L.; Campiani, E.; Canese, S.; Foglini, F.; Freiwald, A.; Montagna, P.; Trincardi, F. Reprint of ‘On and off the beaten track: Megafaunal sessile life and Adriatic cascading processes’. Mar. Geol. 2016, 375, 146–160. [Google Scholar] [CrossRef]
- Foglini, F.; Grande, V.; Marchese, F.; Bracchi, V.A.; Prampolini, M.; Angeletti, L.; Castellan, G.; Chimienti, G.; Hansen, I.M.; Gudmundsen, M.; et al. Application of Hyperspectral Imaging to Underwater Habitat Mapping, Southern Adriatic Sea. Sensors 2019, 19, 2261. [Google Scholar] [CrossRef]
- Taviani, M.; Angeletti, L.; Foglini, F.; Corselli, C.; Nasto, I.; Pons-Branchu, E.; Montagna, P. U/Th dating records of cold-water coral colonization in submarine canyons and adjacent sectors of the southern Adriatic Sea since the Last Glacial Maximum. Progr. Oceanogr. 2019, 175, 300–308. [Google Scholar] [CrossRef]
- Taviani, M. Studio di Una Tanatocenosi Pleistocenica Dragata nel Basso Adriatico. Master’s Thesis (Laurea), Facoltà di Scienze Matematiche, Fisiche e Naturali, University of Bologna, Bologna, Italy; 1976. p. 145, unpublished.
- Colantoni, P.; Noto, P.; Taviani, M. Prime datazioni assolute di una fauna fossile a Pseudamussium septemradiatum dragata nel basso Adriatico. Giorn. Geol. 1975, 40, 133–140. [Google Scholar]
- Taviani, M. Nota sul ritrovamento di cinque specie di Molluschi Gastropoda, Prosobranchia poco conosciuti o nuovi per le acque del Mediterraneo. Quad. Civ. Staz. Idrobiol. Milano 1974, 5, 39–50. [Google Scholar]
- Taviani, M. Associazioni a Molluschi pleistoceniche-attuali dragate nell’Adriatico meridionale. Boll. Zool. 1978, 45, 297–306. [Google Scholar] [CrossRef]
- Panetta, P.; Mastrototaro, F.; Chimienti, G.; Angeletti, L.; D’Onghia, G. Tanatocenosi wurmiana nel Canyon di Bari (Mar Adriatico). Biol. Mar. Mediter. 2013, 20, 148–149. [Google Scholar]
- Trincardi, F.; Ridente, D. SETE-06 Cruise Report R/V Urania, Monopoli, May 06—Ancona, May, 23, 2007. HERMES, Unpublished Report. Available online: https://gismarcloud.myqnapcloud.com/share.cgi?ssid=1b510bc2f0ab4ee6b3612e4aac2d441a (accessed on 15 January 2023).
- Gray, J.E. A list of the genera of Recent Mollusca, their synonyms and types. Proc. Zool. Soc. 1847, 15, 129–206. [Google Scholar]
- Laghi, G.F. Upper Triassic chitons from the Italian Dolomites. Lav. Soc. Venez. Scie. Natur. 2005, 30, 79–84. [Google Scholar]
- Sirenko, B.I. Four new species and one new genus of Jurassic chitons (Mollusca: Polyplacophora: Lepidopleurida) from the Middle Russian Sea. Proc. Zool. Inst. Russ. Acad. Sci. 2013, 317, 30–44. [Google Scholar] [CrossRef]
- Gmelin, J.F. Caroli a Linnei Systema Natura Per Regna Tria Naturae, Secundum Classes, Ordines, Genera, Species, Cum Characteribus, Differentis, Synonymis, Locis etc., 13th ed.; Beer, G.E., Ed.; Lipsia, Germany, 1791; pp. 3021–4120, Editio decima tertia, aucta, reformata, cura J.F. Gmelin, 1(6). Vermes testacea. [Google Scholar]
- Kaas, P.; Knudsen, J. Lorentz Spengler’s descriptions of chitons (Mollusca: Polyplacophora). Zool. Med. 1992, 66, 49–90. [Google Scholar]
- Malatesta, A. Mediterranean Polyplacophora Cenozoic and Recent. Geol. Rom. 1962, 1, 145–171. [Google Scholar]
- Dell’Angelo, B.; Palazzi, S. Considerazioni sulla famiglia Leptochitonidae Dall, 1889 (Mollusca: Polyplacophora). III. Le specie terziarie e quaternarie europee, con note sistematiche e filogenetiche. In Atti Prima Giorn. di Studi Malacol. CISMA; Centro Italiano di Studi Malacologici: Rom, Italy, 1989; pp. 19–140. [Google Scholar]
- Dell’Angelo, B.; Smriglio, C. Chitoni Viventi del Mediterraneo; Edizioni Evolver: Rome, Italy, 1999; p. 256, (English Ed. 2001: Living chitons of the Mediterranean). [Google Scholar]
- Dell’Angelo, B.; Giusti, F. I Polyplacophora di una tafocenosi profonda del Mar Ligure meridionale. Parte II. La Conchiglia 2000, 32, 53–57. [Google Scholar]
- Kaas, P. Scandinavian species of Leptochiton Gray, 1847 (Mollusca: Polyplacophora). Sarsia 1981, 66, 217–229. [Google Scholar] [CrossRef]
- Strack, H.L. Polyplacophora (Keverslakken). In De Fossiele Schelpen van de Nederlandse Kust; Wesselingh, F.P., Moerdijk, P.W., Eds.; Nederlands Centrum voor Biodiversiteit Naturalis: Leiden, The Netherlands, 2010; Chapter 10; pp. 61–66. [Google Scholar]
- Kaas, P.; Van Belle, R.A. Monograph of Living Chitons (Mollusca: Polyplacophora). Vol. 1. Order Neoloricata: Lepidopleurina; Brill, E.J., Backuys, W., Eds.; Leiden, The Netherlands, 1985; p. 240. [Google Scholar]
- Sturrock, M.G.; Baxter, J.M. The ultrastructure of the aesthetes of Leptochiton asellus (Polyplacophora: Lepidopleurina). J. Zool. 1993, 230, 49–61. [Google Scholar] [CrossRef]
- Chemnitz, J.H. Neues Systematisches Conchylien Cabinet; Nürnberg, Germany, 1795; pp. 4–11, 4: (24) + 344 pp. pls CXXII-CLIX (1780); 5: (20) + 324 pp. pls CLXCLXXX–XIII (1781); 6: (12) + 375 pp. pls 1-36 (1782); 7: (12) + 356 pp. pls 37-69 (1784); 8: (16) + 372 pp. pls 70-102 (1785); 9(1): (12) + 151 pp. pls 103-116 (1786); 9(2): Xxvi + 194 pp. pls 117-136 (1786); 10: (20) + 376 pp. pls 137-173 (1788); 11: (20) + 310 pp. pls 174-213. [Google Scholar]
- Spengler, L. Udförlig beskrivelse over det mangeskallede konkylie-slaegt, af Linnaeus kaldet Chiton; met endeel nye Arter og Varieteter. Skrivt. Natur. Selsk. 1797, 4, 62–103. [Google Scholar]
- Leloup, E. Contributions à l’étude de la faune belge. IV. Les Polyplacophores de la côte belge. Bull. Mus. R. Hist. natur. Belg. 1934, 10, 1–23. [Google Scholar]
- Fredj, G. Essai de Stockage et d’Exploitation des Données en Ecologie Marine. Application à l’Etude Biogéographique du Benthos Mediterranéen et Perspectives. Ph.D. Thesis, University of Nice, Nice, France, 1974; pp. 1–176, Annexe A1–A164. [Google Scholar]
- Lucas, M. Les polyplacophores des cotes europeennes. Bull. Mens. Ass. Belg. Malac. Conchyl. Paleont. 1 1968. 1(2): 9–10; 1(3): 11–14; 1(4): 13–14; 1(5): 13–14; 1(6): 13–14; 1(7): 15–16; 2 (1969): 2(1): 17–18; 2(2): 17–18; 2(3): 13–14; 2(4): 15–16. [Google Scholar]
- Mifsud, C.; Ovalis, P. On the presence of Leptochiton asellus (Gmelin, 1791) (Polyplacophora: Leptochitonidae) in the Mediterranean Sea. Boll. Malacol. 2008, 44, 133–134. [Google Scholar]
- Wood, S.V. A Catalogue of Shells from the Crag. Ann. Mag. Nat. Hist. 1842, 9, 455–462, 527–544. [Google Scholar] [CrossRef]
- Wood, S.V. A Monograph of the Crag Mollusca, with Descriptions of Shells from the Upper Tertiaries of the British Isles. Vol. I. Univalves; Paleontographical Society: London, UK, 1848; pp. xii + 208. [Google Scholar]
- Reid, C. Pliocene Deposits of Britain. Mem. Geolog. Surv. UK. 1890, 326. Available online: https://www.biodiversitylibrary.org/bibliography/153206 (accessed on 15 January 2023).
- Marquet, R. A remarkable molluscan fauna from the Kattendijk Formation (Lower Pliocene) at Kallo (Oost-Vlaanderen, Belgium). Bull. Soc. Belge Géol. Paléontol. Hydrol. 1984, 93, 335–345. [Google Scholar]
- Marquet, R. The Neogene Amphineura and Bivalvia (Protobranchia and Pteriomorphia) from Kallo and Doel (Oost-Vlaanderen, Belgium). Palaeontos 2002, 2, 1–100. [Google Scholar]
- Brøgger, W.C. Om de senglaciale og postglaciale nivåforandringer i Kristiania-feltet (Mollukfaunan). Norges Geol. Unders. 1901, 31, 1–731. [Google Scholar]
- Antevs, E. Post-glacial marine shell-beds in Bohuslän. Geolol. Fören. Stockholm Förhand. 1917, 39, 247–425. [Google Scholar] [CrossRef]
- Antevs, E. Shell Beds on the Skagerack. Geolol. Fören. Stockholm Förhand. 1928, 50, 479–748. [Google Scholar] [CrossRef]
- Bellomo, E.; Sabelli, B. A new addition to the Mediterranean Pleistocene “Boreal Guests”: Hanleya nagelfar (Lovén, 1864) (Mollusca, Polyplacophora) from Calabria (S. Italy). Boll. Soc. Paleontol. Ital. 1995, 34, 201–204. [Google Scholar]
- Dons, C. Norges strandfauna. V. Chitonidae. Det Kong. Norske Vidensk. Selsk. 1934, 7, 4–7. [Google Scholar]
- Hansson, H.G. NEAT (North East Atlantic Taxa): Scandinavian marine Mollusca Check-List. Internet Ed. Aug. 1998. Available online: http://www.tmbl.gu.se (accessed on 15 January 2023).
- Kaas, P. On a collection of Polyplacophora (Mollusca, Amphineura) from the Bay of Biscay. Bull. Mus. Nat. Hist. Natur. 1979, 1, 13–31. [Google Scholar]
- Rolan Mosquera, E.; Otero Schmitt, J.; Rolan Alvarez, E. Moluscos de la Ria de Vigo. II. Poliplacoforos, Bivalvos, Escafopodos, Cefalopodos. Thalassas 1990, 2, 276. [Google Scholar]
- Consolado Macedo, M.C.; Macedo, M.I.; Borges, J.P. Conchas Marinhas de Portugal; Editorial Verbo: Lisbon, Portugal, 1999; p. 516. [Google Scholar]
- Dell’Angelo, B.; Forli, M. I Polyplacophora del Pleistocene inferiore di Riparbella (Pisa), con elenco dei molluschi rinvenuti. Boll. Malacol. 1995, 30, 221–252. [Google Scholar]
- Garilli, V.; Dell’Angelo, B.; Vardala-Theodorou, E. Polyplacophora from the Pleistocene of Kyllini (NW Peloponnese, Greece). Boll. Soc. Paleontol. Ital. 2005, 44, 117–134. [Google Scholar]
- Koskeridou, E.; Vardala-Theodorou, E.; Moissette, P. Pliocene and Pleistocene shallow-water chitons (Mollusca) from Rhodes Island, Greece. N. Jahrb. Geol. Paläont—Abh. 2009, 251, 303–330. [Google Scholar] [CrossRef]
- Dell’Angelo, B.; Sosso, M.; Prudenza, M.; Bonfitto, A. Notes on Fossil Chitons. 5. Polyplacophora from the Pliocene of Western Liguria, Northwest Italy. Riv. Ital. Paleont. Strat. 2013, 119, 65–107. [Google Scholar]
- Dell’Angelo, B.; Sosso, M.; Tavano, M. Chitons (Mollusca, Polyplacophora) from the Lower Pliocene of Borzoli (Genova). Ann. Mus. Civ. St. Nat. G. Doria 2021, 114, 403–449. [Google Scholar]
- Lovén, S.L. Index molluscorum litora Scandinaviae Occidentalia habitantium. Öfversigt Af K.Sven. Vetensk.-Akad. Förhandlingar 1846, 3, 134–160. [Google Scholar]
- Ferreira, A.J. The family Lepidopleuridae (Mollusca: Polyplacophora) in the Eastern Pacific. Veliger 1979, 22, 145–165. [Google Scholar]
- Wu, S.-K.; Okutani, T. The deepsea chitons (Mollusca: Polyplacophora) collected by the R/V Soyo-Maru from Japan: I. Lepidopleuridae. Venus 1984, 43, 1–31. [Google Scholar]
- Dell’Angelo, B.; Bonfitto, A. Notes on fossil chitons. 1. A new species of Lepidopleurus (Mollusca: Polyplacophora) from the Pleistocene of Salice (Sicily, Italy). Zootaxa 2005, 821, 1–6. [Google Scholar] [CrossRef]
- Squires, R.L.; Goedert, J.L. An Extant Species of Leptochiton (Mollusca: Polyplacophora) in Eocene and Oligocene cold-seep limestones, Olympic Peninsula, Washington. Veliger 1995, 38, 47–53. [Google Scholar]
- Dell’Angelo, B.; Bonfitto, A.; Taviani, M. Chitons (Polyplacophora) from Paleogene strata in Western Washington State, U.S.A. J. Paleontol. 2011, 85, 936–954. [Google Scholar] [CrossRef]
- Schwabe, E.; Sellanes, J. Revision of Chilean bathyal chitons (Mollusca: Polyplacophora) associated with cold-seeps, including description of a n. sp. of Leptochiton (Leptochitonidae). Org. Divers. Evol. 2010, 10, 31–55. [Google Scholar] [CrossRef]
- Bertolaso, L.; Garilli, V.; Parrinello, D.; Sosso, M.; Dell’Angelo, B. A new Miocene deep-sea chiton and early evidence for Teredinidae-sustained wood-fall communities. Palaeont. Electr. 2015, 18, 1–15. [Google Scholar] [CrossRef]
- Sirenko, B.; Saito, H.; Schwabe, E. A redescription of Leptochiton belknapi Dall, 1878 (Mollusca: Polyplacophora: Leptochitonidae), the type species of the new genus Belknapchiton. Zootaxa 2022, 5205, 101–124. [Google Scholar] [CrossRef]
- Vayssière, A. Mollusques de la France et des Régions Voisines; Amphineures, Gastéropodes, Opisthobranches, Hétéropodes, Marséniades et Oncidiidés; Octave Doin et Fils: Paris, France, 1913; Volume 1, p. 420. [Google Scholar]
- Van Belle, R.A. Sur la presence en Mediterranée de Lepidopleurus alveolus (Lovén, 1846). Inform. Soc. Belge Malacol. 1975, 4, 57–58. [Google Scholar]
- Sirenko, B.I.; Sigwart, J.; Dell’Angelo, B. Hanleya hanleyi (Bean in Thorpe, 1844) (Mollusca, Polyplacophora) and the influence of the Gulf Stream System on its distribution. Ruthenica 2016, 26, 57–70. [Google Scholar]
- Janssen, R. Revision der Polyplacophora des Oligozäns in Mitteleuropa. Arch. Mollusk. 1978, 108, 215–235. [Google Scholar]
- Šulc, J. Studien über die fossilen Chitonen. I. Die fossilen Chitonen im Neogen des Wiener Beckens und der angrenzenden Gebiete. Ann. Des Nat. Mus. Wien 1934, 47, 1–31. [Google Scholar]
- Ruman, A.; Hudácková, N.H. Middle Miocene chitons (Polyplacophora) from the Slovak part of the Vienna Basin and the Danube Basin (Central Paratethys). Acta Geol. Slovaca 2015, 7, 155–173. [Google Scholar]
- Dell’Angelo, B.; Giuntelli, P.; Sosso, M.; Zunino, M. Polyplacophora from the Miocene of North Italy. Part 1: Leptochitonidae, Hanleyidae, Ischnochitonidae and Callistoplacidae. Riv. Ital. Paleont. Strat. 2015, 121, 217–242. [Google Scholar]
- Dell’Angelo, B.; Landau, B.; Van Dingenen, F.; Ceulemans, F. The upper Miocene chitons of northwest France (Mollusca: Polyplacophora). Zootaxa 2018, 4447, 62. [Google Scholar] [CrossRef] [PubMed]
- Bean, W. A supplement of new species. In British Marine Conchology; Being a Descriptive Catalogue, Arranged According to the Lamarckian System, of The Salt Water Shells in Great Britain; Thorpe, C., Ed.; Edward Lumley: London, UK, 1844; pp. 263–267. [Google Scholar]
- Sabelli, B. Sulla presenza e distribuzione di Hanleya hanleyi in Mediterraneo. Conchiglie 1972, 8, 97–100. [Google Scholar]
- Sabelli, B. Hanleya hanleyi (Bean, 1844). Schede Malacol. del Mediterr. 1974, 6. Società Malacologica Italiana, scheda n° 44. Available online: https://www.societaitalianadimalacologia.it/Schede/Hanleya%20hanleyi.pdf (accessed on 15 January 2023).
- Dell’Angelo, B.; Lesport, J.-F.; Cluzaud, A.; Sosso, M. The Oligocene to Miocene chitons (Mollusca: Polyplacophora) of the Aquitaine Basin, southwestern France, and Ligerian Basin, western France. Part 2: Lepidochitonidae, Tonicellidae, Acanthochitonidae, Cryptoplacidae and Additions to Part 1. Boll. Malacol. 2020, 56, 1–58. [Google Scholar]
- Dell’Angelo, B.; Renda, W.; Sirenko, B.I.; Sosso, M.; Giacobbe, S. The Mediterranean distribution of Hanleya hanleyi (Bean in Thorpe, 1844) and H. mediterranea Sirenko, 2014 (Polyplacophora). Boll. Malacol. 2021, 57, 124–135. [Google Scholar]
- Dell’Angelo, B.; Landau, B.M.; da Silva, C.M.; Sosso, M. Biogeography of northeastern Atlantic Neogene chitons (Mollusca, Polyplacophora): New data from the Pliocene of Portugal. J. Paleont. 2022, 96, 814–838. [Google Scholar] [CrossRef]
- Hernández, J.M.; Rolán, E. Clase Polyplacophora. In Moluscos y Conchas Marinas de Canarias; Rolán, E., Ed.; ConchBooks: Vigo, Spain, 2011; pp. 46–53, 407–411. [Google Scholar]
- Segers, W.; Swinnen, F.; de Prins, R. Marine molluscs of Madeira: The Living Marine Molluscs of the Province of Madeira (Madeira and Selvagens Archipelago); Snoeck Publishers: Zwijndrecht, Belgium, 2009; p. 612. [Google Scholar]
- Sabelli, B.; Taviani, M. The making of the Mediterranean molluscan biodiversity. In The Mediterranean Sea; Goffredo, S., Dubinsky, Z., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 285–306. [Google Scholar]
- Rosso, A.; Vertino, A.; Di Geronimo, I.; Sanfilippo, R.; Sciuto, F.; Di Geronimo, R.; Violanti, D.; Corselli, C.; Taviani, M.; Mastrototaro, F.; et al. Hard- and soft-bottom thanatofacies from the Santa Maria di Leuca deep-water coral province, Mediterranean. Deep-Sea Res. II 2010, 57, 360–379. [Google Scholar] [CrossRef]
- Panetta, P.; Mastrototaro, F.; Matarrese, A.; Tursi, A. Tanatocenosi a molluschi presenti nelle acque adriatiche albanesi. Biol. Mar. Mediter. 2003, 10, 597–601. [Google Scholar]
- Panetta, P.; Mastrototaro, F.; D’Onghia, G. Tanatocenosi a molluschi della provincia a coralli di Santa Maria di Leuca (Mar Ionio). Biol. Mar. Mediter. 2012, 19, 186–187. [Google Scholar]
- Negri, M.P.; Corselli, C. Bathyal Mollusca from the cold-water coral biotope of Santa Maria di Leuca (Apulian margin, southern Italy). Zootaxa 2016, 4186, 1–97. [Google Scholar] [CrossRef] [PubMed]
- Nasto, I.; Cardone, F.; Mastrototaro, F.; Panetta, P.; Rosso, A.; Sanfilippo, R.; Taviani, M.; Tursi, A. Benthic invertebrates associated with subfossil cold-water coral frames and hardgrounds in the Albanian deep waters (Adriatic Sea). Turk. J. Zool. 2018, 42, 360–371. [Google Scholar] [CrossRef]
- Bouchet, P.; Taviani, M. Atlantic deep sea gastropods in the Mediterranean: New findings. Boll. Malacol. 1989, 25, 137–148. [Google Scholar]
- WoRMS. World Register of Marine Species. Available online: https://www.marinespecies.org (accessed on 25 December 2022).
| Station | Depth (m) | Gear | Latitude N | Longitude E |
|---|---|---|---|---|
| 10 | 335 | rock dredge | 4133.67 | 1728.70 |
| 13 | 423 | rock dredge | 4122.03 | 1706.72 |
| 18 | 341 | box-corer | 4119.07 | 1705.16 |
| 19 | 308 | grab | 4118.97 | 1705.10 |
| 22 | 722 | rock dredge | 4117.73 | 1715.83 |
| 24 | 199 | box-corer | 4121.31 | 1705.98 |
| 25 | 376 | grab | 4117.47 | 1709.29 |
| 35 | 878 | grab | 4113.46 | 1734.35 |
| 40 | 764 | grab | 4143.03 | 1703.95 |
| 48 | 728 | epibenthic dredge | 4143.31 | 1703.39 |
| 50 | 755 | grab | 4144.91 | 1703.31 |
| Family | Genus | Species | Author | 10 | 13 | 18 | 19 | 22 | 24 | 25 | 35 | 40 | 48 | 50 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lepetidae | Propilidium | exiguum | (Thompson, 1844) | X | X | X | X | |||||||
| Lepetidae | Iothia | fulva | (Mueller, 1776) | X | X | X | ||||||||
| Fissurellidae | Puncturella | noachina | (Linnaeus, 1771) | X | X | X | X | X | X | |||||
| Fissurellidae | Emarginula | adriatica | O.G.Costa, 1829 | X | ||||||||||
| Fissurellidae | Emarginula | fissura | (Linnaeus, 1758) | X | ||||||||||
| Fissurellidae | Emarginula | rosea | Bell, 1824 | X | ||||||||||
| Fissurellidae | Emarginula | punctulum | Piani, 1980 | X | ||||||||||
| Fissurellidae | Fissurisepta | granulosa | Jeffreys, 1883 | X | ||||||||||
| Scissurellidae | Anatoma | crispata | Fleming, 1828 | X | X | X | X | X | X | X | ||||
| Scissurellidae | Anatoma | umbilicata | (Jeffreys, 1883) | X | ||||||||||
| Lepetellidae | Bogia | labronica | (Bogi, 1984) | |||||||||||
| Trochidae | Clelandella | miliaris | (Brocchi, 1814) | X | ||||||||||
| Chilodontaidae | Putzeysia | wiseri | (Calcara, 1842) | X | X | |||||||||
| Chilodontaidae | Danilia | tinei | (Calcara, 1839) | X | X | X | X | X | ||||||
| Skeneidae | Cirsonella | romettensis | (Granata Grillo, 1877) | X | X | X | X | X | ||||||
| Skeneidae | gen. | sp. | X | |||||||||||
| Bathysciadiidae | Pilus | conicus | (Verrill, 1884) | X | X | |||||||||
| Rissoidae | Alvania | cimicoides | (Forbes, 1844) | X | X | X | X | X | X | X | ||||
| Rissoidae | Alvania | testae | (Aradas e Maggiore, 1844) | X | X | X | X | X | X | X | ||||
| Rissoidae | Alvania | zetlandica | (Montagu, 1815) | X | X | X | X | |||||||
| Rissoidae | Benthonella | tenella | (Jeffreys, 1869) | X | X | X | X | X | ||||||
| Capulidae | Capulis | ungaricus | (Linnaeus, 1758) | X | X | X | ||||||||
| Capulidae | Torellia | delicata | (Philippi, 1844) | X | ||||||||||
| Ovulidae | Simnia | nicaeensis | Risso, 1826 | X | ||||||||||
| Naticidae | Euspira | cf. fusca | (Blainville, 1825) | X | ||||||||||
| Cerithiidae | Cerithidium | submammillatum | (De Rayneval & Ponzi,1854) | X | X | X | ||||||||
| Turritellidae | Turriterinella | tricarinata | (Brocchi, 1814) | X | ||||||||||
| Triphoridae | Marshallora | adversa | (Montagu, 1893) | X | ||||||||||
| Triphoridae | gen. | sp. | X | |||||||||||
| Cerithiopsidae | Cerithiopsis | atalaya | R. B. Watson, 1885 | X | X | |||||||||
| Cerithiopsidae | Cerithiopsis | sp. | X | |||||||||||
| Newtonielliidae | Cerithiella | metula | (Loven, 1846) | X | X | X | ||||||||
| Epitoniidae | Epitonium | algerianum | (Weinkauff, 1866) | X | X | |||||||||
| Epitoniidae | Epitonium | cf. algerianum | (Weinkauff, 1866) | X | ||||||||||
| Eulimidae | Aclis | attenuans | Jeffreys, 1883 | X | X | X | ||||||||
| Eulimidae | Haliella | stenostoma | (Jeffreys, 1858) | X | X | X | ||||||||
| Eulimidae | Melanella | compactilis | (Locard, 1891) | X | X | |||||||||
| Eulimidae | Melanella | petitiana | (Brusina, 1879) | X | X | |||||||||
| Eulimidae | Melanella | sp. | X | |||||||||||
| Atlantidae | Atlanta | peroni | Lesueur, 1817 | X | ||||||||||
| Atlantidae | Atlanta | sp. | X | X | ||||||||||
| Muricidae | Ocinebrina | helleri | (Brusina, 1865) | X | ||||||||||
| Muricidae | Trophon | muricatus | (Montagu, 1803) | X | X | X | X | X | X | X | X | |||
| Muricidae | Pagodula | echinata | (Kiener, 1839) | X | X | X | X | X | X | X | X | X | ||
| Nassariidae | Tyitia | lima | (Dillwyn, 1817) | X | X | X | ||||||||
| Columbellidae | Mitrella | minor | (Scacchi, 1836) | X | ||||||||||
| Columbellidae | Amphissa | acutecostata | (Philippi, 1844) | X | X | X | ||||||||
| Fasciolaridae | Pseudofusus | rostratus | (Olivi, 1792) | X | X | X | ||||||||
| Fasciolaridae | Pseudofusus | sp. | X | |||||||||||
| Raphitomidae | Cyrillia | aequalis | (Jeffreys, 1867) | X | ||||||||||
| Raphitomidae | Cyrillia | linearis | (Montagu, 1803) | X | ||||||||||
| Raphitomidae | Leufroya | sp.1 | X | |||||||||||
| Raphitomidae | Leufroya | sp.2 | X | |||||||||||
| Raphitomidae | Leufroya | concinna | (Scacchi, 1836) | X | ||||||||||
| Raphitomidae | Leufroyia | erronea | Monterosato | X | ||||||||||
| Raphitomidae | Teretia | teres | (Reeve, 1844) | X | X | X | X | X | ||||||
| Borsoniidae | Typhlomangelia | nivalis | (Lovén, 1846) | X | ||||||||||
| Borsoniidae | Drilliola | emendata | (Monterosato, 1872) | X | X | |||||||||
| Borsoniidae | Drilliola | loprestiana | (Calcara, 1841) | X | X | X | ||||||||
| Mangeliidae | Mangelia | costata | (Pennat, 1777) | X | ||||||||||
| Mangeliidae | Mangelia | sp. | X | |||||||||||
| Mangeliidae | Mangelia | costulata | Risso, 1826 | X | ||||||||||
| Architectonicidae | Heliacus | fallaciosus | (Tiberi, 1872) | X | ||||||||||
| Mathildidae | Mathilda | cochlaeformis | Brugnone, 1873 | X | ||||||||||
| Mathildidae | Mathilda | coronata | Monterosato, 1875 | X | ||||||||||
| Mathildidae | Mathilda | retusa | Brugnone, 1873 | X | ||||||||||
| Pyramidellidae | Turbonilla | micans | (Monterosato, 1865) | X | ||||||||||
| Pyramidellidae | Tibersyrnola | unifasciata | (Forbes, 1844) | X | ||||||||||
| Pyramidellidae | Parthenina | flexuosa | (Monterosato, 1874) | X | X | |||||||||
| Pyramidellidae | Tragula | fenestrata | (Jeffreys, 1848) | X | ||||||||||
| Pyramidellidae | Eulimella | scillae | (Scacchi, 1835) | X | X | X | ||||||||
| Pyramidellidae | Eulimella | unifasciata | (Forbes, 1844) | X | ||||||||||
| Pyramidellidae | Megastomia | conoidea | (Brocchi, 1814) | X | ||||||||||
| Pyramidellidae | Odostomia | acuta | Jeffreys, 1848 | X | ||||||||||
| Pyramidellidae | Odostomia | carrozzai | Van Aartsen, 1987 | X | ||||||||||
| Pyramidellidae | Ondina | cf. crystallina | Locard, 1891 | X | X | |||||||||
| Pyramidellidae | Ondina | sp. | X | |||||||||||
| Acteonidae | Crenilabium | exile | (Jeffreys, 1870) | X | ||||||||||
| Retusidae | Retusa | nitidula | (Lovén, 1846) | X | X | |||||||||
| Retusidae | Retusa | umbilicata | (Montagu, 1803) | X | ||||||||||
| Retusidae | Retusa | sp. | X | X | X | |||||||||
| Ringiculidae | Ringicula | gianninii | F. Nordsieck, 1974 | X | ||||||||||
| Ringiculidae | Ringicula | cf. gianninii | F. Nordsieck, 1974 | X | ||||||||||
| Philinidae | Hermania | scabra | (O. F. Muller, 1784) | X | ||||||||||
| Philinidae | Philine | striatula | Monterosato, 1874 | X | X | |||||||||
| Laonidae | Laona | quadrata | S.V. Wood, 1839) | X | X | |||||||||
| Laonidae | Laona | sp. | X | |||||||||||
| Cylichnidae | Scaphander | clavus | Dall, 1889 | X | ||||||||||
| Alacuppidae | Roxania | sp. | X | |||||||||||
| Cavoliniidae | Cavolinia | gibbosa | (d’Orbigny, 1835) | X | ||||||||||
| Cavoliniidae | Cavolinia | inflexa | (Lesueur, 1813) | X | X | X | X | |||||||
| Cavoliniidae | Diacria | trispinosa | (Blainville, 1821) | X | X | |||||||||
| Cliidae | Clio | pyramidata | Linnaeus, 1767 | X | X | X | X | X | X | X | X | |||
| Hyalocylidae | Hyalocylis | striata | (Rang, 1828) | X | X | |||||||||
| Creseidae | Styliola | subula | (Quoy & Gaimard, 1827) | X | X | X | X | |||||||
| Creseidae | Creseis | acicula | (Rang, 1828) | X | X | |||||||||
| Limacinidae | Limacina | bulimoides | (d’Orbigny, 1836) | X | X | X | X | X | ||||||
| Limacinidae | Limacina | retroversa | (J. Fleming, 1823) | X | X | X | ||||||||
| Heliconoididae | Heliconoides | inflatus | (d’Orbigny, 1835) | X | X | |||||||||
| Tylodinidae | Tylodina | perversa | (Gmelin, 1791) | X | ||||||||||
| Nuculidae | Ennucula | aegeensis | (Forbes, 1844) | X | X | X | X | X | X | X | ||||
| Nuculanidae | Saccella | commutata | (Philippi, 1844) | X | X | |||||||||
| Yoldiidae | Yoldiella | lucida | (Loven, 1846) | X | X | X | X | X | X | X | ||||
| Yoldiidae | Yoldiella | messanensis | (Jeffreys, 1870) | X | X | X | X | X | ||||||
| Yoldiidae | Yoldiella | philippiana | (Nyst, 1845) | X | X | X | X | X | ||||||
| Yoldiidae | Yoldiella | striolata | (Brugnone, 1876) | X | X | X | ||||||||
| Yoldiidae | Yoldiella | wareni | La Perna, 2004 | X | X | |||||||||
| Arcidae | Asperarca | nodulosa | (O.F. Muller, 1776) | X | X | X | X | X | X | X | X | |||
| Arcidae | Bathyarca | pectunculoides | (Scacchi, 1835) | X | X | X | X | X | X | X | X | X | ||
| Arcidae | Bathyarca | philippiana | (Nyst, 1848) | X | X | X | X | X | X | |||||
| Propeamussiidae | Cyclopecten | hoskynsi | (Forbes, 1844) | X | ||||||||||
| Pectinidae | Pseudamussium | clavatum | (Poli, 1795) | X | X | |||||||||
| Pectinidae | Pseudamussium | peslutrae | (Linnaeus, 1771) | X | X | X | X | |||||||
| Pectinidae | Deletopecten | vitreus | (Gmelin, 1791) | X | X | X | X | X | ||||||
| Propeamussiidae | Similipecten | similis | (Laskey, 1811) | X | X | X | ||||||||
| Propeamussiidae | Mesopeplum | fenestratum | (Forbes, 1844) | X | X | X | X | X | X | X | X | |||
| Mytiliidae | Modiolula | phaseolina | (Philippi, 1844) | X | X | X | X | X | X | X | X | |||
| Anomiidae | Heteranomia | squamula | (Linnaeus, 1758) | X | X | X | X | X | X | X | ||||
| Anomiidae | Pododesmus | patelliformis | (Linnaeus, 1761) | X | X | X | ||||||||
| Limidae | Limaea | crassa | (Forbes, 1844) | X | X | X | X | X | X | X | ||||
| Limidae | Limatula | gwyni | (Sikes, 1903) | X | X | X | X | X | X | X | X | |||
| Thyasiridae | Leptaxinus | incrassatus | (Jeffreys, 1876) | X | X | |||||||||
| Ungulinidae | Microstagon | trigonum | (Scacchi, 1835) | X | ||||||||||
| Carditidae | Centrocardita | aculeata | (Poli, 1795) | X | X | X | X | |||||||
| Astartidae | Goodallia | triangularis | (Montagu, 1803) | X | X | X | ||||||||
| Astartidae | Astarte | sulcata | (da Costa, 1778) | X | X | X | X | X | X | |||||
| Carditidae | Centrocardita | aculeata | (Poli, 1795) | X | X | X | ||||||||
| Cardiidae | Papillicardium | minimum | (Philippi, 1836) | X | X | X | X | X | X | X | ||||
| Semelidae | Abra | longicallus | (Scacchi, 1835) | X | X | X | X | X | ||||||
| Kellielliidae | Kelliella | miliaris | (Philippi, 1844) | X | X | X | X | X | X | |||||
| Trapezidae | Coralliophaga | lithophagella | (Lamarck, 1819) | X | X | |||||||||
| Veneridae | Timoclea | ovata | (Pennant, 1777) | X | X | X | X | X | X | |||||
| Corbulidae | Corbula | sp. | X | |||||||||||
| Hiatellidae | Hiatella | arctica | (Linnaeus, 1767) | X | X | X | X | X | X | X | ||||
| Xylophagaidae | Xylophaga | dorsalis | (W. Turton, 1819) | X | ||||||||||
| Cuspidariidae | Cuspidaria | jugosa | (S. V. Wood, 1857 | X | ||||||||||
| Dentaliidae | Antalis | agilis | (M. Sars, 1872) | X | X | X | X | X | X | |||||
| Dentaliidae | Antalis | dentalis | (Linnaeus, 1758) | X | ||||||||||
| Fustariidae | Fustiaria | rubescens | (Deshayes, 1826) | X | ||||||||||
| Entalinidae | Entalina | tetragona | (Brocchi, 1814) | X | X | X | X | X | X | |||||
| Pulselliidae | Pulsellum | lofotense | (M. Sars, 1865) | X | ||||||||||
| Gadilidae | Cadulus | jeffreysi | Monterosato, 1875 | X | X | X | X | X | X | |||||
| Gadilidae | Cadulus | ovulum | (Philippi, 1844) | X | ||||||||||
| Gadilidae | Cadulus | cf. subfusiformia | (M. Sars, 1865) | X | X | |||||||||
| Valloniidae | Vallonia | pulchella | (O. F. Muller, 1774) | X | ||||||||||
| Lauriidae | Lauria | sp. | X | |||||||||||
| Leptochitonidae | Leptochiton | asellus | (Gmelin, 1791) | X | X | X | X | X | X | X | X | X | X | X |
| Leptochitonidae | Leptochiton | antondohrni | new species | X | X | X | X | X | ||||||
| Leptochitonidae | Belknapchiton | alveolus | (M. Sars MS, Lovén, 1846) | X | X | X | ||||||||
| Hanleyidae | Hanleya | hanleyi | (Bean in Thorpe, 1844) | X |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taviani, M.; Sosso, M.; Dell’Angelo, B. Chitons from Deep-Water Mollusk-Rich Deposits in the Southwestern Adriatic Sea (Mollusca, Polyplacophora). Diversity 2023, 15, 359. https://doi.org/10.3390/d15030359
Taviani M, Sosso M, Dell’Angelo B. Chitons from Deep-Water Mollusk-Rich Deposits in the Southwestern Adriatic Sea (Mollusca, Polyplacophora). Diversity. 2023; 15(3):359. https://doi.org/10.3390/d15030359
Chicago/Turabian StyleTaviani, Marco, Maurizio Sosso, and Bruno Dell’Angelo. 2023. "Chitons from Deep-Water Mollusk-Rich Deposits in the Southwestern Adriatic Sea (Mollusca, Polyplacophora)" Diversity 15, no. 3: 359. https://doi.org/10.3390/d15030359
APA StyleTaviani, M., Sosso, M., & Dell’Angelo, B. (2023). Chitons from Deep-Water Mollusk-Rich Deposits in the Southwestern Adriatic Sea (Mollusca, Polyplacophora). Diversity, 15(3), 359. https://doi.org/10.3390/d15030359










