Abstract
Similar to plants in many other families, members of the Hymenophyllaceae use numerous substrates for growth, e.g., soil, rocks or tree bark. However, substrate preference does not only differ among species but can also vary among members of the same species. There have been several attempts in the past to appropriately capture this variation, but none proved feasible or was replicated in any subsequent work. In our approach, we use textual information from numerous sources like checklists, floras and species descriptions to come up with a quantitative index of the preference of 450 species of filmy ferns (=c. 75% of all species of the family) for epiphytic, lithophytic or terrestrial growth. We show that the majority of species have clear habitat preferences, while strict habitat specificity is rather uncommon. Our compilation will be an important input for future ecological and phylogenetic studies in this family, but the presented approach is of much more general interest: it is immediately applicable to other taxonomic groups and should eventually allow us to replace the current approach of assigning species to distinct categories (epiphyte, lithophyte or terrestrial) by one that finally reflects biological variability more appropriately.
1. Introduction
An epiphyte is commonly defined as a non-parasitic plant that grows on another plant throughout its life, without contact to the ground [1]. It has been acknowledged since early on, e.g., [2] that it is impossible to show that this is really true for all individuals of a given species; hence, there is some inevitable ambiguity in the terms “epiphytic species” vs. “terrestrial species”. Benzing [3] proposed a classification system that distinguished ‘true epiphytes’, or ‘holoepiphytes’, from ‘facultative epiphytes’ and ‘accidental epiphytes’. According to this system, epiphytic species are species that (almost) always grow epiphytically and possess specialized characteristics enabling these species to thrive in tree canopies. ‘Facultative epiphytes’, in turn, are species that occur regularly both on the ground and in the canopy, using sites where terrestrial and arboreal conditions converge, and ‘accidental epiphytes’ are those that only rarely grow as epiphytes, without any conspicuous modifications related to canopy life.
As an elaboration of this scheme, Ibisch [4] proposed a more quantitative variant in which true epiphytes are defined by the fact that >95% of all members of a species grow epiphytically, with a corresponding proportion of 5–95% for facultative epiphytes and < 5% for accidental epiphytes. Later, Burns [5] developed another quantitative approach to classify epiphytes. Although seemingly clear, both approaches face prominent practical problems. First, there were hardly any easily accessible field observations in the literature that could be used. Second, as pointed out by Hoeber and Zotz [6], the spatial scale at which terrestrial and epiphytic occurrence are compared may define the result. As a case in point, they described the observation of a large epiphytic individual of the ornithochorous tree species Sorbus aucuparia growing without any terrestrial conspecifics within a forest area of 1 ha in the Harz mountains in Germany. In this country with a well-studied flora, there is no doubt that this is an accidental epiphytic occurrence of a terrestrial species. However, in areas with more limited botanical exploration, such an observation could be the basis of a publication of an “epiphytic species”. This is what happened in the case of Podocarpus epiphyticus, originally described based on an epiphytic herbarium specimen in Myanmar [7] but later corrected as an epiphytic individual of the terrestrial species P. teysmannii [8].
Yet another problem when trying to quantify the degree of epiphytism of a given species is regional variation. For instance, ferns of the Polypodium vulgare complex are usually terrestrial but may occur as obligate epiphytes with high arboreal abundances in certain regions, e.g., [9,10,11]. Similarly, the bromeliad Aechmea distichantha occurs almost entirely epiphytically at the moist end of its distribution but terrestrially or lithophytically at the dry end [12].
Biological variation is often relatively continuous and the outlined problem with the term “epiphyte” is clearly not idiosyncratic to this group of plants. “Halophytes” [13], “CAM plants” [14] or “succulents” are just three of many other cases of similar nature: “Succulence is not a binary trait” [15]. Any study attempting to produce a list of species belonging to such a category has to acknowledge this ambiguity. We have recently compiled a global list of vascular epiphyte species [16]. For each species, at least one reference was given to justify its inclusion. The large majority of the more than 31,000 species are clearly in the category “holoepiphytes”. There may be the rare occurrence of an individual plant on other substrates than a tree or a shrub, e.g., a rock, soil or substrates provided by humans such as roofs or power lines [17], but the majority of these plants are (almost) entirely restricted to the epiphytic habitat. There are, however, some taxonomic groups, in which many species seem to be more flexible in their propensity to grow as an epiphyte. For example, of the species categorized as “epiphytes” in the Catálogo de las Plantas Vasculares de Panamá [18] in some families a considerable proportion of species was also listed as terrestrials: Araceae (22%), Gesneriaceae (20%), Ericaceae (25%) or Hymenophyllaceae (23%).
This study focuses on one of these families, Hymenophyllaceae, for which Zotz, Weigelt, Kessler, Kreft and Taylor [16] listed 433 epiphytic species globally, i.e., 72% of an estimated total of c. 600 species. Intraspecific variation was discussed in their study but not addressed in the actual published species list (EpiList 1.0). Some previous treatises with filmy ferns went further in highlighting variation in regard to life form. For example, Ebihara et al. [19] gave detailed life form information for each genus and subgenus of the family, Dubuisson et al. [20] did the same for 193 species of the genus Trichomanes s.l. and Lehnert and Krug [21] compiled information for a similar number of species (167) but covered the entire family. However, none of these studies treated the propensity of epiphytic, lithophytic or terrestrial growth as a continuous variable with a truly numeric approach. We did this in the current study, in which we collated life form information for 450 species from numerous sources.
2. Materials and Methods
We scanned the taxononomic literature (species descriptions, checklists, floras), the ecological literature (community studies, vegetation descriptions) and numerous web sites and online data bases, e.g., [22] for information on the life form of filmy ferns. Specifically, we collected all available information on the growing sites (tree, rock, soil) of as many species of filmy ferns as possible. However, only sources that did acknowledge that a species may occur on different substrates were considered. Examples of sources that were checked but excluded are Freitas et al. [23], Carvajal-Hernández et al. [24] or Hennequin et al. [25]. In all these papers species were only assigned to a single category. In contrast, e.g., Werner et al. [26] studied epiphytes on remnant trees in Southern Ecuador. Although all species of filmy fern were listed as “epiphytic”, we accepted this information as valid because these authors clearly did distinguish obligate, facultative and accidental epiphytes in their study.
We note that one may miss valid information on species that never occur on the ground with this conservative approach, but including species as 100% epiphytic would inevitably inflate our estimate of “epiphytes” in cases where authors simply found observations of occasional terrestrial occurrence of epiphytes or occasional epiphytic occurrence of terrestrials not worth mentioning. In the end, we could use data from a total of 181 sources (Table S1). Most sources that fulfilled our requirement were annotated checklists and floras. All species names were standardized against Hassler [27] but the original names of the publications can still be found in Table S1.
The translation of literal descriptions of epiphytic, lithophytic or terrestrial occurrences of a given species in proportions is detailed in Table 1.

Table 1.
Numerical translation of literal descriptions of species occurrences in the original sources as epiphytes, lithophytes or terrestrials. Special cases: with explicit information on life form of specimens we used the proportions, e.g., [28]; “on rotten logs” or “dead wood” counts as “terrestrial” Trichomanes accedens in [29], while “epiphytic and on fallen logs” counts as “epiphytic” assuming plants were already on the tree before the fall. We acknowledge that this may obscure possible preferences for rotting logs as growing sites, e.g., [30,31].
This procedure produced three values per entry, an Epiphyte Value (EV), a Lithophyte Value (LV) and a Terrestrial Value (TV), which add up to unity (100%). While straightforward in those cases in which only occurrences in one of the three habitats were mentioned, it is a major issue to translate vague descriptions like “occasionally epiphytic” or “rarely terrestrial” into a numeric system. This translation is unavoidably arbitrary, but the approach allows a coarse ranking of preferences for epiphytic vs. non-epiphytic growth and is certainly preferable to the usual assignment to just one of several categories, e.g., [16]. Moreover, the consistent use of the rules outlined in Table 1 allows a rapid adjustment of the absolute numbers if there were objective arguments against the used scheme in the future.
Whenever there was information for a given species available in several sources, all sources were considered as equivalent and used without weighting to calculate a single species average of EV, LV and TV (Table S2). Such redundancy should improve the quality of the final estimate, although it is unclear whether different sources really report independent observations or whether life form information may have been copied from another source. The list compiled in this study encompasses 450 species (c. 75% of the family), with an average number of 4.7 sources with occurrence information per species (range 1–45).
For the biological reasons given in the introduction, any number must be considered as a rough estimate. In addition, there are other issues that should be kept in mind when interpreting any single number reported in this study. For example, an entry of 50% epiphyte/50% terrestrial for a species in the final results (Table S2) may have a number of reasons. For one, there may be a single source available that describes the species as “growing epiphytically and terrestrially”. Alternatively, there may be two or more studies, half of which describe the species as “growing epiphytically”, the others as “terrestrial” or many alternative combinations. This could reflect an error in some of these sources or rather be based on biological diversity when sources describe varying occurrence patterns in different regions, as described for Aechmea distichantha [12]. Since we document all entries in the Supplementary Materials, divergent information about occurrences of a given species can be used as a starting point to study the reasons behind such differences.
We performed two types of numerical analyses. First, we produced a histogram of epiphytic (EV) vs. non-epiphytic (LV + TV) occurrences and compared this distribution to a uniform distribution with a Chi2-test. In a second type of analysis, we followed the approach used by Franco and Silvertown [32] to ordinate demographic components in a triangular space. Similar to the elasticity values from matrix analyses in population biology, the Epiphyte Value, Lithophyte Value and the Terrestrial Value of each species sum to unity, which makes it possible to explore the distribution of filmy ferns in the epiphyte-lithophyte-terrestrial space. For these ordinations, which were done both for the family as a whole and for selected genera, we used the plotrix library version 3.8-1 [33] in R version 4.2.0 [34].
3. Results
In a first analysis of the final data set including 2134 entries of 450 species, we lumped terrestrials and lithophytes as non-epiphytes and quantified the degree of epiphytism of each species. If the propensity to grow as an epiphyte were a continuous trait, we would expect the bins of the histogram not to differ much in frequency, while the existence of strong preferences for growth on trees, or not, would lead to a pronounced bimodal distribution with peaks at the extremes. “Obligate” epiphytes (EV > 0.9) clearly make up the single largest bin (accounting for 30% of all species), followed by terrestrial/lithophytic species (EV < 0.1) with some 14% of the total (Figure 1), the distribution deviating significantly from uniformity (χ² = 261.8, df = 9, p < 0.001). Whether facultative epiphytes cover the whole range from rarely to mostly epiphytic more or less evenly, or whether there may be a third mode of facultative epiphytes with about even probability to occur epiphytically or not is doubtful. Two thirds of the species that fell into the category of even probability to occur epiphytically or not were single entry cases, and thus the existence of a third peak is not strongly supported.
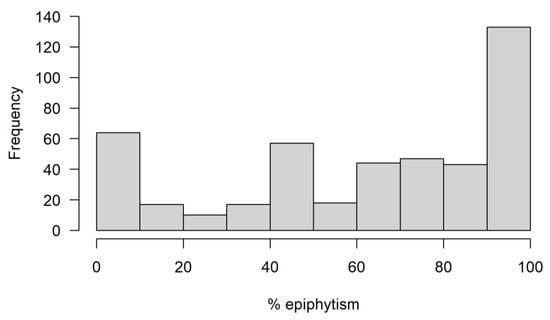
Figure 1.
Histogram showing the tendency of 450 species of Hymenophyllaceae to occur epiphytically.
In a second analysis, we used a triangular ordination to include preferences for epiphytic, lithophytic and terrestrial growth in a single analysis (Figure 2). While obligate epiphytes (EV > 0.9) make up just about 30% of all species of filmy ferns, there is clearly a preference for epiphytic growth in Hymenophyllaceae. About 68% of all taxa grow primarily as epiphytes, i.e., can be found in the lower left triangle of the epiphyte-lithophyte-terrestrial space, i.e., EV ≥ 0.5, whereas species with LV and TV ≥ 0.5 made up about 11% and 16%, respectively (note that in these calculations we assigned half of the species at the border, e.g., EV = LV = 50% to either E or L). Obligate lithophytes (LV > 0.9) and obligate terrestrial taxa (TV > 0.9) make up less than, respectively, 4% and 6% of all species. A similarly small number of species could be called “generalists” with <50% preference for any of the three growing sites (6%).
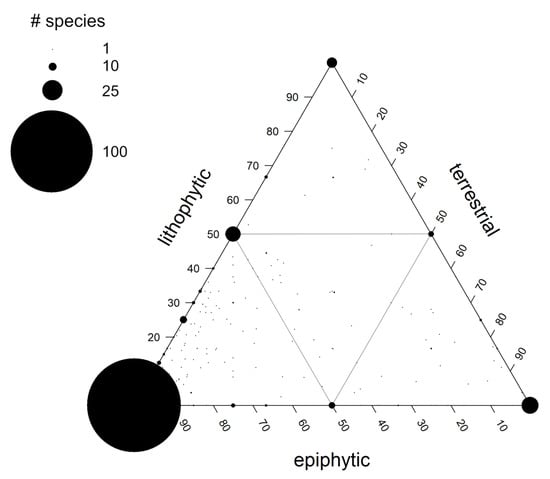
Figure 2.
Distribution of 450 species of filmy ferns including members of all nine accepted genera in the epiphyte-lithophyte-terrestrial space. The number of species with a preference for epiphytic growth, i.e., E ≥ 50, was 304 (note that in this calculation we assigned half of the species at the border, e.g., EV = LV = 50% to E and L). Symbol size varies with the number of species that share the same values. Individual species values are based on 1–45 sources. The grey lines separate three zones of preference for epiphytic, lithophytic or terrestrial growth, with the central triangle being occupied by generalists. The full data set is given in Tables S1 and S2.
A third analysis explored the distribution of four species-rich genera of the Hymenophyllaceae in the epiphyte-lithophyte-terrestrial space (Figure 3). The different genera differ strongly in their habitat preferences, but invariably the majority of species are not restricted to one habitat. On average, however, Abrodictyum species show a clear preference for terrestrial growth, while Didymoglossum and Hymenophyllum species use mostly epiphytic growing sites and, to a lesser extent, rocks, while preferences are most evenly distributed among Trichomanes species.
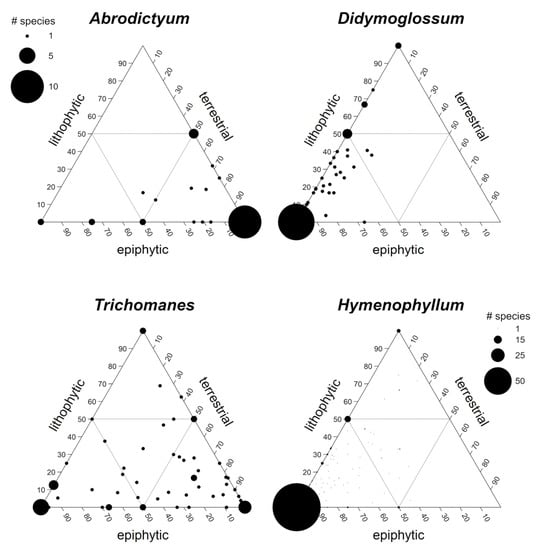
Figure 3.
Distribution of 28 Abrodictyum, 47 Didymoglossum, 61 Trichomanes and 233 Hymenophyllum species in the epiphyte-lithophyte-terrestrial space. Symbol size varies with the number of species with the same values of EV, LV and TV. Scaling is identical for Abrodictyum, Didymoglossum and Trichomanes but differs for Hymenophyllum.
4. Discussion
Both histogram (Figure 1) and the two ordinations (Figure 2 and Figure 3) are highly informative visualisations of the preference of the family Hymenophyllaceae as a whole or individual genera for epiphytic, lithophytic or terrestrial growth, which goes far beyond the typical categorisation as either epiphyte or terrestrial/epiphytic [16,35] or the verbal descriptions in, e.g., Ebihara, Dubuisson, Iwatsuk, Hennequin and Ito [19]. While each individual data point in our data set is certainly questionable, the overall patterns are probably quite robust. Most importantly, by collating and presenting these data, we expose them to further scrutiny. Using the figures together with Tables S1 and S2, it is possible to critically analyse the intraspecific variation in habitat preference of hundreds of species. This should either lead to the correction of erroneous descriptions in the literature or allow the interesting study of the nature of the real biological variation.
How good is the match of the current analysis, EpiList 1.0 and previous descriptions of individual genera?
Typically, the tendency for epiphytism in a particular genus or family has been assessed by the relative number of “epiphytic species”, e.g., [16,35] or verbal characterisations, e.g., [19]. The current study introduces a new method. The results of all three approaches, which are summarized in Table 2, are generally numerically consistent, particularly in the more species-rich genera.

Table 2.
Average values of the Epiphyte value (EV), lithophyte value (LV) and terrestrial value (TV) in the current analysis, the proportion of all species in the nine accepted genera or the family as a whole with an EV ≥ 0.5 and the proportion of epiphyte species per genus or entire family in EpiList 1.0 [16].
For example, most Abrodictyum species prefer a terrestrial habitat as described by Ebihara, Dubuisson, Iwatsuk, Hennequin and Ito [19]; the genus has also the highest TV and the lowest proportion of epiphyte species in EpiList 1.0, the opposite being true for members of the genus Didymoglossum. However, a strict preference of a Didymoglossum species for growth on a tree or on a rock is relatively rare: for the majority of species, growth on more than one substrate has been reported. Assuming that rare occurrences of, e.g., a typically epiphytic species on a rock or epiphytic occurrence of a typical terrestrial or lithophytic species probably remain unreported, strict preference may even be less frequent than reflected in our data set. Noteworthy, the number of “epiphyte species” in EpiList 1.0 is always higher than the average EV of the studied genera or the family as a whole (Table 2). This consistent difference is easily explained by the dissimilar approaches in the two studies. In the former [16], species were included when at least one reference in the scientific literature described that species as epiphytic or primarily epiphytic, while in the current study we actually quantified habitat preference using as many sources as possible. For example, Trichomanes egleri and T. pinnatum were described as facultative epiphytes in Hokche et al. [36] and Steyermark et al. [37], respectively, which led to their inclusion in EpiList 1.0, but the current analysis based on numerous sources suggests that epiphytic occurrences are too rare overall as to justify their inclusion. However, any discrepancy depends on the definition of “epiphyte species”. Confronted with a similar problem in the case of plants that show some degree of nocturnal acidification, Winter [38] defined “CAM species” as those that obtain the majority of their carbon through the CAM pathway throughout their lives, typically deduced from a δ13C values of leaf tissue > −20 ‰. Using a similar rationale, we may define “epiphyte species” as those taxa with an EV ≥ 0.5, i.e., species that occur primarily in tree crowns. In Figure 2 and Figure 3, this would be equivalent to all species in the lower left of the four triangles within the epiphyte-lithophyte-terrestrial space. For the family as whole, this would yield 71%, which is remarkably close to the 72% of epiphyte “species” in EpiList 1.0 (Table 2). For individual genera, the outcome of such a comparison is more diverse, with very similar numbers in Hymenophyllum and Didymoglossum but quite different outcomes in smaller genera like Callistopteris or Cephalomanes (Table 2).
Caveats
The quality of an analysis as the present one depends on the quality of the sources. A major problem is the inconsistent use of terminology, even within a single paper. For example, Ebihara, Dubuisson, Iwatsuk, Hennequin and Ito [19] state that “all species in Didymoglossum are dwarf epiphytes, mainly in tropical regions”, only to specify a few lines later that their habitat is “epilithic or low-epiphytic”. Similarly, Polyphlebium endlicherianum is called “epiphyte” in the Flora of the Marquesas Islands [39], but the habitat is described as “trees and rocks”. In such cases, we used the habitat details to score a species. Apart from such inconsistencies, there is a tendency among many researchers to prefer neat categories over “noise”. If explicitly mentioned, one can at least reject such data in an analysis like the current one. As an example, Mellado-Mansilla et al. [40], noted that a large proportion of the species described as “epiphytes” by Moreno et al. [41], were actually found primarily on rocks or slopes of river beds at their study site but classified them as epiphytes as the information in Moreno, Le Quesne, Díaz and Rodríguez [41] was taken as “the most common growth forms for Andean temperate forest species”. Similarly, Chen et al. [42], used the “most common description” if there was a conflict between published studies, with an aim to avoid scoring taxa as polymorphic. Such approaches produce neat categories but obscure biological variation. In many cases, however, such an approach is not explicitly mentioned. For that reason, we did not include the life form information of numerous studies.
We are also aware that our numerical approach is not without problems. First, we obviously cannot assume that terms like “commonly” or “rarely” are written with the same numerical equivalent in mind in the many sources included in this study. Second, to assign a 50% preference for E and L for “on trees and rocks” was simply the most parsimonious approach for want of a more convincing alternative. Due to the fact that we typically had several entries per species with at least slightly divergent information, there were only 7% of all species with 50%/50% averages for EV, LV and TV (Figure 1). We also realize that life form categorisations in a large flora, e.g., [43], a checklist, e.g., [36], or a large review, e.g., [44] integrate much more information than a report about one particular locality, e.g., [45,46], but we were not sure how exactly to weigh sources and again used the most parsimonious option. In the end, our analysis should simply be taken as semi-quantitative.
The hemiepiphyte issue
So-called “hemiepiphytic” species and climbers pose a special problem in a study that distinguishes between epiphytic, lithophytic and terrestrial growth. As discussed in detail in Zotz et al. [47], hemiepiphytic vascular plants are defined by their ontogeny. In the case of hemiepiphytic ferns, gametophytes start epiphytically on a tree and the sporophyte, which is initially epiphytic as well, later establishes root contact with the soil, i.e., the sporophyte would be categorized as terrestrial. Thus, hemiepiphytes do not neatly fall in any of the three basic categories of our study.
Hemiepiphytic growth in ferns has been originally demonstrated for Vandenboschia collariata by Nitta and Epps [48] and subsequently for a number of other fern taxa in several families [47,49,50]. Although other Vandenboschia or Trichomanes species have been given the label hemiepiphyte repeatedly, e.g., [19,29], we are not aware that this has actually been shown unambiguously for these and any other species in the genus or the family. Unfortunately, researchers routinely deduce ontogeny from single observations, which is bound to produce dubious results. Moreover, the labels “hemiepiphyte”, “climber”, “epiphyte” are very inconsistently used in the different sources, particularly for Vandenboschia. However, the genus is relatively species-poor, and thus including/excluding Vandenboschia does hardly change the overall result—we show the ordination without the genus in the Supplementary Materials as Figure S1. Species in other genera, e.g., Trichomanes tanaicum (subgenus Lacostea) are also sometimes labelled hemiepiphyte, e.g., [29], again without evidence. A better understanding of the occurrence of hemiepiphytism in this and other families is needed and is particularly important in the context of evolutionary studies. Frequently, hemiepiphytes seem to be intermediates in the transition from terrestrial to epiphytic growth and vice versa [42,51], but such an analysis depends on the correct identification of the life form of the studied species.
Towards a more realistic view of epiphytes vs. non-epiphytes
Clearly, if we want to understand the ecology of Hymenophyllaceae and other ferns and the mechanistic basis of habitat preferences, the correct assessment of their growing sites is an indispensable requisite. Acknowledging variation is also critical for analyses of trait evolution. Many such analyses use simple dichotomies, e.g., [25], but there are others which at least use a “mixed” category [52] or even more elaborate schemes [21]. We argue that treating evolutionary transitions from terrestrial/lithophytic to epiphytic growth [25] or vice versa [42] as a step change instead of a gradual change is probably not an appropriate description of the actual developments. Acknowledging facultative life forms should enrich any such analysis. Similarly, treating, e.g., lithophytic, terrestrial and epiphytic habitats as inherently distinct is also an oversimplification. On the one hand, there can be much overlap in growth conditions; on the other hand, there is much variation within each of these three habitat types, e.g., [53]. For example, the actual growth conditions on moss-covered rocks in the understory or on the lower portions of similarly moss-covered tree trunks may vary little for a fern, but bark and rock do differ in many other aspects, e.g., in thermal properties or in stability [1]. In contrast, the growth conditions of two lithophytes may be quite distinct, one growing in the open may experience rather dry conditions with high temperature peaks, while conditions on a shaded rock near a creek may resemble those of a rheophyte.
The range of different environmental conditions of epiphytic filmy ferns is probably not as large as in other taxa with many epiphytic members, e.g., in bromeliads or orchids, which may be found from the shaded understory to very exposed sites in the uppermost crown of trees. In general, filmy ferns are restricted to more shaded and humid growing sites in the understory, but there are reports of at least occasional growth in higher parts of the forest canopy [54,55].
In summary, we present a new semi-quantitative approach to assessing preferences of filmy ferns for particular growing sites. Consistent with conclusions of Lehnert and Krug [21], we find that a majority of species show clear preferences to either epiphytic, lithophytic or terrestrial growth, while strict specialisation is not as common as may be deduced from species lists such as EpiList 1.0. Our approach should be very useful in phylogenetic and ecological studies in this particular family, but the method we used is of much more general interest. It is straightforward to apply it to other genera or families, which will allow us to replace the current approach of classifying species into distinct categories (epiphytic, lithophytic or terrestrial) with a much more realistic one that reflects the more continuous variation observed in nature.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d15020270/s1, Figure S1: Distribution of filmy ferns in the epiphyte-lithophyte-terrestrial space excluding Vandenboschia; Table S1: Original data from 181 sources; Table S2: Mean growth preference values for 450 species [56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176,177,178,179,180,181,182,183,184,185,186,187,188,189,190,191,192,193,194,195,196,197,198,199,200,201,202,203,204,205,206,207,208,209,210,211,212,213,214,215,216,217,218,219,220,221,222,223].
Author Contributions
Conceptualization, G.Z.; Methodology, G.Z. and H.J.R.E.; Formal Analysis, G.Z. and H.J.R.E.; Data Curation, G.Z.; Writing—Original Draft Preparation, G.Z.; Writing—Review & Editing, G.Z. and H.J.R.E.; Visualization, G.Z. and H.J.R.E. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
All data are given in the Supplementary Materials.
Acknowledgments
Thanks for help with the data input to Norbert Wagner (Oldenburg), Ibrahim Elias (Oldenburg), Sarah Petrasz (Oldenburg).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zotz, G. Plants on Plants. The Biology of Vascular Epiphytes; Springer International Publishing Switzerland: Cham, Switzerland, 2016; p. 282. [Google Scholar]
- Schimper, A.F.W. Die Epiphytische Vegetation Amerikas; Gustav Fischer: Jena, Germany, 1888; Volume 2, p. 162. [Google Scholar]
- Benzing, D.H. The evolution of epiphytism. In Vascular Plants as Epiphytes: Evolution and Ecophysiology, 76th ed.; Lüttge, U., Ed.; Ecological Studies; Springer: Heidelberg, Germany, 1989; pp. 15–41. [Google Scholar]
- Ibisch, P.L. Neotropische Epiphytendiversität-das Beispiel Bolivien; Martina Galunder-Verlag: Wiehl, Germany, 1996; Volume 1, p. 357. [Google Scholar]
- Burns, K.C. How arboreal are epiphytes? A null model for Benzing’s classifications. N. Z. J. Bot. 2010, 48, 185–191. [Google Scholar] [CrossRef]
- Hoeber, V.; Zotz, G. Accidental epiphytes: Ecological insights and evolutionary implications. Ecol. Monogr. 2022, 92, e1527. [Google Scholar] [CrossRef]
- de Laubenfels, D.J.; Silba, J. Notes on Asian-Pacific Podocarpaceae I (Podocarpus). Phytologia 1988, 64, 290–292. [Google Scholar]
- de Laubenfels, D.J. New sections and species of Podocarpus based on the taxonomic status of P. neriifolius (Podocarpaceae) in tropical Asia. Novon A J. Bot. Nomencl. 2015, 24, 133–152. [Google Scholar] [CrossRef]
- Zotz, G. Gefässepiphyten in temperaten Wäldern. Bauhinia 2002, 16, 13–22. [Google Scholar]
- Johnson, D.S. Polypodium vulgare as an epiphyte. Bot. Gaz. 1921, 72, 237–244. [Google Scholar] [CrossRef]
- Klinghardt, M.; Zotz, G. Abundance and seasonal growth of epiphytic ferns at three sites along a rainfall gradient in Western Europe. Flora 2021, 274, 151749. [Google Scholar] [CrossRef]
- Barberis, I.M.; Mogni, V.Y.; Oakley, L.J.; Vogt, C.; Prado, D.E. Biogeography of different life forms of the southernmost neotropical tank bromeliad. J. Biogeogr. 2021, 48, 2085–2097. [Google Scholar] [CrossRef]
- Grigore, M.-N.; Ivanescu, L.; Toma, C. Halophytes: An. Integrative Anatomical Study; Springer International Publishing Switzerland: Cham, Switzerland, 2014. [Google Scholar]
- Winter, K.; Smith, J.A.C. CAM photosynthesis: The acid test. New Phytol. 2022, 233, 599–609. [Google Scholar] [CrossRef]
- Ogburn, R.M.; Edwards, E.J. The ecological water-use strategies of succulent plants. Adv. Bot. Res. 2010, 55, 179–225. [Google Scholar]
- Zotz, G.; Weigelt, P.; Kessler, M.; Kreft, H.; Taylor, A. EpiList 1.0-A global checklist of vascular epiphytes. Ecology 2021, 102, e03326. [Google Scholar] [CrossRef]
- Wester, S.; Zotz, G. Growth and survival of Tillandsia flexuosa on electricity cables in Panama. J. Trop. Ecol. 2010, 26, 123–126. [Google Scholar] [CrossRef]
- Correa, M.D.; Galdames, C.; de Stapf, M.S. Catálogo de las Plantas Vasculares de Panamá; Universidad de Panamá & Instituto Smithsonian de Investigaciones Tropicales: Panama City, Panama, 2004. [Google Scholar]
- Ebihara, A.; Dubuisson, J.-Y.; Iwatsuk, K.; Hennequin, S.; Ito, M. A taxonomic revision of Hymenophyllaceae. Blumea 2006, 51, 221–280. [Google Scholar] [CrossRef]
- Dubuisson, J.Y.; Hennequin, S.; Rakotondrainibe, F.; Schneider, H. Ecological diversity and adaptive tendencies in the tropical fern Trichomanes L. (Hymenophyllaceae) with special reference to climbing and epiphytic habits. Bot. J. Linn. Soc. 2003, 142, 41–63. [Google Scholar] [CrossRef]
- Lehnert, M.; Krug, M. Evolution of substrate specificity and fungal symbiosis in filmy ferns (Hymenophyllaceae): A Bayesian approach for ambiguous character state reconstruction. Symbiosis 2019, 78, 141–147. [Google Scholar] [CrossRef]
- Brazilian Flora 2020 in Construction. Available online: http://floradobrasil.jbrj.gov.br/ (accessed on 15 May 2022).
- Freitas, L.; Salino, A.; Menini Neto, L.; Almeida, T.; Mortara, S.; Stehmann, J.; Amorim, A.M.; Guimaraes, E.; Nadruz Coelho, M.; Zanin, A.; et al. A comprehensive checklist of vascular epiphytes of the Atlantic Forest reveals outstanding endemic rates. PhytoKeys 2016, 58, 65–79. [Google Scholar] [CrossRef]
- Carvajal-Hernández, C.I.; Silva-Mijangos, L.; Kessler, M.; Lehnert, M. Additions to the pteridoflora of Tabasco, Mexico: The importance of the humid montane forest. Acta Bot. Mex. 2018, 124, 7–18. [Google Scholar] [CrossRef]
- Hennequin, S.; Schuettpelz, E.; Pryer, K.M.; Ebihara, A.; Dubuisson, J.Y. Divergence times and the evolution of epiphytism in filmy ferns (Hymenophyllaceae) revisited. Int. J. Plant Sci. 2008, 169, 1278–1287. [Google Scholar] [CrossRef]
- Werner, F.; Homeier, J.; Gradstein, S.R. Diversity of vascular epiphytes on isolated remnant trees in the montane forest belt of southern Ecuador. Ecotropica 2005, 11, 21–40. [Google Scholar]
- Hassler, M. World Ferns. Synonymic Checklist and Distribution of Ferns and Lycophytes of the World. Version 14.4; Last Update December 4th, 2022. Available online: www.worldplants.de/ferns/ (accessed on 12 December 2022).
- Bidin, A. A preliminary survey of the fern flora of Langkawi Islands. Gard. ´S Bull. Singap. 1987, 40, 77–102. [Google Scholar]
- Lellinger, D.B. Hymenophyllaceae. In Flora of the Guianas; Rijn, A.R.A.G.-v., Ed.; Fasc. 3; Koeltz Scientific Books: Königstein, Germany, 1994; Volume Ser. B, pp. 1–66. [Google Scholar]
- Khanina, L.; Bobrovsky, M. Value of large Quercus robur fallen logs in enhancing the species diversity of vascular plants in an old-growth mesic broad-leaved forest in the Central Russian Upland. For. Ecol. Manag. 2021, 491, 119172. [Google Scholar] [CrossRef]
- Ough, K. Regeneration of wet forest flora a decade after clear-felling or wildfire-is there a difference? Austr. J. Bot. 2001, 49, 645–664. [Google Scholar] [CrossRef]
- Franco, M.; Silvertown, J. Life history variation in plants: An exploration of the fast-slow continuum hypothesis. Phil. Trans. R. Soc. Lond. B Biol. Sci. 1996, 351, 1341–1348. [Google Scholar]
- Lemon, J. Plotrix: A package in the red light district of R. R-News 2006, 6, 8–12. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing Vienna: Vienna, Austria, 2013; Available online: http://www.R-project.org (accessed on 12 December 2022).
- Kress, W.J. The systematic occurrence of vascular epiphytes. In Vascular Plants as Epiphytes: Evolution and Ecophysiology, 76th ed.; Lüttge, U., Ed.; Ecological Studies; Springer: Heidelberg, Germany, 1989; pp. 234–261. [Google Scholar]
- Hokche, O.; Berry, P.E.; Huber, O. (Eds.) Nuevo Catálogo de la Flora Vascular de Venezuela; Fundación Instituto Botánico de Venezuela: Caracas, Venezuela, 2008; p. 859. [Google Scholar]
- Steyermark, J.A.; Berry, P.E.; Holst, B.K.; Yatskievych, K. (Eds.) Flora of the Venezuelan Guayana: Pteridophytes, Spermatophytes Acanthaceae-Araceae; Timber Press: Portland, OR, USA, 1995. [Google Scholar]
- Winter, K. Ecophysiology of constitutive and facultative CAM photosynthesis. J. Exp. Bot. 2019, 70, 6495–6508. [Google Scholar] [CrossRef]
- Flora of the Marquesas Islands. Available online: https://naturalhistory2.si.edu/botany/marquesasflora/index.htm. (accessed on 12 December 2022).
- Mellado-Mansilla, D.; Diaz, I.A.; Godoy-Guinao, J.; Ortega-Solis, G.; Moreno-Gonzalez, R. Bosque Pehuen Park’s Flora: A contribution to the knowledge of the Andean montane forests in the Araucania region, Chile. Nat. Areas J. 2018, 38, 298–311. [Google Scholar] [CrossRef]
- Moreno, R.; le Quesne, C.; Díaz, I.; Rodríguez, R. Flora vascular del Parque Futangue, Region de Los Rios (Chile). Gayana Bot. 2013, 70, 121–135. [Google Scholar] [CrossRef]
- Chen, C.-C.; Hyvönen, J.; Schneider, H. Re-terrestrialization in the phylogeny of epiphytic plant lineages: Microsoroid ferns as a case study. J. Syst. Evol. 2022. [Google Scholar] [CrossRef]
- Kessler, M.; Smith, A.R. Prodromus of a fern flora for Bolivia. X. Hymenophyllaceae. Phytotaxa 2017, 328, 201–226. [Google Scholar] [CrossRef]
- Espejo-Serna, A.; López-Ferrari, A.R.; Mendoza-Ruiz, A.; García-Cruz, J.; Ceja-Romero, J.; Pérez-García, B. Mexican vascular epiphytes: Richness and distribution. Phytotaxa 2021, 503, 1–124. [Google Scholar] [CrossRef]
- Acebey, A.R.; Krömer, T.; Kessler, M. Species richness and vertical distribution of ferns and lycophytes along an elevational gradient in Los Tuxtlas, Veracruz, Mexico. Flora 2017, 235, 83–91. [Google Scholar] [CrossRef]
- Bøgh, A. Composition and distribution of the vascular epiphyte flora of an Ecuadorian montane rain forest. Selbyana 1992, 13, 25–34. [Google Scholar]
- Zotz, G.; Almeda, F.; Bautista-Bello, A.P.; Eskov, A.K.; Giraldo-Cañas, D.; Hammel, B.E.; Harrison, R.D.; Köster, N.; Krömer, T.; Lowry, P.P., II; et al. Hemiepiphytes revisited. Perspect. Plant Ecol. Evol. Syst. 2021, 51, 125620. [Google Scholar] [CrossRef]
- Nitta, J.H.; Epps, M.J. Hemi-epiphytism in Vandenboschia collariata (Hymenophyllaceae). Brittonia 2009, 61, 392–397. [Google Scholar] [CrossRef]
- Watts, J.L.; Moran, R.C.; Watkins, J.E., Jr. Hymenasplenium volubile: Documentation of its gametophytes and the first record of a hemiepiphyte in the Aspleniaceae. Ann. Bot. 2019, 124, 829–835. [Google Scholar] [CrossRef]
- Watkins, J., Jr. E.; Moran, R.C. Gametophytes of the fern genera Dracoglossum and Lomariopsis (Lomariopsidaceae) and their phylogenetic significance. Int. J. Pl. Sci. 2019, 180, 1004–1015. [Google Scholar] [CrossRef]
- Testo, W.; Sundue, M. Primary hemiepiphytism in Colysis ampla (Polypodiaceae) provides new insight into the evolution of growth habit in ferns. Int. J. Plant Sci. 2014, 175, 526–536. [Google Scholar] [CrossRef]
- Zizka, A.; Azevedo, J.; Leme, E.; Neves, B.; da Costa, A.F.; Caceres, D.; Zizka, G. Biogeography and conservation status of the pineapple family (Bromeliaceae). Divers. Distrib. 2020, 26, 183–195. [Google Scholar] [CrossRef]
- Khine, P.K.; Fraser-Jenkins, C.; Lindsay, S.; Middleton, D.; Miehe, G.; Thomas, P.; Kluge, J. A contribution toward the knowledge of ferns and lycophytes from Northern and Northwestern Myanmar. Am. Fern J. 2017, 107, 219–256. [Google Scholar] [CrossRef]
- Krömer, T.; Kessler, M. Filmy ferns (Hymenophyllaceae) as high-canopy epiphytes. Ecotropica 2006, 12, 57–63. [Google Scholar]
- Zotz, G.; Büche, M. The epiphytic filmy ferns of a tropical lowland forest-species occurrence and habitat preferences. Ecotropica 2000, 6, 203–206. [Google Scholar]
- Roux, J.P. Synopsis of the Lycopodiophyta and Pteridophyta of Africa, Madagascar and neighbouring islands. Strelitzia 2009, 23, 227–255. [Google Scholar]
- Brownlie, G. The pteridophyte flora of Fiji. Nova Hedwig Beih. 1977, 55, 1–397. [Google Scholar]
- Iwatsuki, K. Taxonomic studies of pteridophyta II. Acta Phytotax. Geobot. 1958, 17, 161–166. [Google Scholar]
- Game, J.C.; Fawcett, S.E.; Smith, A.R. New pteridophyte records for Taveuni (Fiji) and a new species of Chingia (Thelypteridaceae). N. Z. J. Bot. 2018, 56, 26–37. [Google Scholar] [CrossRef]
- Nitta, J.H. Distribution, Ecology, and Systematics of the Filmy Ferns (Hymenophyllaceae) of Moorea, French Polynesia. Water Resources Center Archives. Biology and Geomorphology of Tropical Islands. 2006. Available online: https://escholarship.org/uc/item/6vt6p2w8 (accessed on 12 December 2022).
- Liu, J.X.; Zhang, Q.Y.; Ebihara, A.; Iwatsuki, K. Hymenophyllaceae. In Flora of China, Vol. 2–3 (Pteridophytes); Wu, Z.Y., Raven, P.H., Hong, D.Y., Eds.; Science Press: Beijing, China; Missouri Botanical Garden Press: St. Louis, MO, USA, 2013; pp. 93–109. [Google Scholar]
- Cámara-Leret, R.; Frodin, D.G.; Adema, F.; Anderson, C.; Appelhans, M.S.; Argent, G.; Arias Guerrero, S.; Ashton, P.; Baker, W.J.; Barfod, A.S.; et al. New Guinea has the world’s richest island flora. Nature 2020, 584, 579–583. [Google Scholar] [CrossRef]
- Christensen, C. Botanical results of the Archbold Expedition No. 8: New and noteworthy Papuan ferns. Brittonia 1937, 2, 265–317. [Google Scholar] [CrossRef]
- Murdock, A.G.; Smith, A.R. Pteridophytes of Moorea, French Polynesia, with a new species, Tmesipteris gracilis (Psilotaceae). Pacific Sci. 2003, 57, 253–265. [Google Scholar] [CrossRef]
- Pichi Sermolli, R.E.G. A contribution to the knowledge of the Pteridophyta of Rwanda, Burundi, and Kivu (Zaire). I. Bull. Jard. Bot. Natl. Belg. Bull. Van Natl. Plantentuin Van Belg. 1983, 53, 177–284. [Google Scholar] [CrossRef]
- Roux, J.P. Conspectus of Southern African Pteridophyta: An Enumeration of the Pteridophyta of Angola, Botswana, Lesotho, Malawi, Mozambique, Namibia, South. Africa (Including the Marion Island Group), Swaziland, Zambia and Zimbabwe; Project Coordinator, Southern African Botanical Diversity Network; c/o National Botanical Institute: Pretoria, South Africa, 2001. [Google Scholar]
- Roux, J.P.; Network, S.A.B.D. Swaziland Ferns and Fern Allies; National Botanical Institute, SABONET: Pretoria, Pretoria, 2003; Volume 19. [Google Scholar]
- Clarkson, B.D. The vegetation of the Kaitake Range Egmont National Park, New Zealand. N. Z. J. Bot. 1985, 23, 15–31. [Google Scholar] [CrossRef]
- Ebihara, A.; Matsumoto, S.; Iwashina, T.; Sugimura, K.; Iwatsuki, K. Hymenophyllaceae (Pteridophytes) of Vanuatu. Ann. Tsukuba Bot. Gard. 2002, 21, 61–72. [Google Scholar]
- Dubuisson, J.Y.; Bauret, L.; Grall, A.; Li, T.; Ebihara, A.; Hennequin, S. Discussion on the taxonomy of African fern Abrodictyum rigidum (Sw.) Ebihara & Dubuisson and description of two new Abrodictyum C.Presl species (Hymenophyllaceae, Polypodiidae) for the Afro-Malagasy region. Phytotaxa 2016, 284, 151–168. [Google Scholar] [CrossRef]
- Benl, G. The pteridophyta of Bioko (Fernando Po):(Contributions to a Flora of the island): II: Marattiaceae, Hymenophyllaceae, Adiantaceae, Hemionitidaceae. Acta Bot. Barcinonensia 1980, 32, 3–34. [Google Scholar]
- Harley, W.J. The ferns of Liberia. Contrib. Gray Herb. Harv. Univ. 1955, 177, 58–101. [Google Scholar] [CrossRef]
- Velayos, M.; Aedo, C.; Cabezas, F.; Estrella, M. XIII-Hymenophyllaceae. In Flora de Guinea Ecuatorial, I; Velayos, M., Aedo, C., Cabezas, F., Estrella, M., Eds.; 2008; p. 28. Available online: https://digital.csic.es/bitstream/10261/117484/3/013-HYMENOPHYLLACEAE.pdf (accessed on 12 December 2022).
- Iwatsuki, K.; Ebihara, A.; Kato, M. Taxonomic studies of pteridophytes of Ambon and Seram (Moluccas) collected on Indonesian-Japanese botanical expeditions 1983-1986. XIII. Hymenophyllaceae. PhytoKeys 2019, 119, 107–115. [Google Scholar] [CrossRef]
- Turner, I.M. A Catalogue of the vascular plants of Malaya. Gard. Bull. Singap. 1995, 47, 1–757. Available online: https://books.google.com.au/books/about/A_Catalogue_of_the_Vascular_Plants_of_Ma.html?id=4zXgswEACAAJ&redir_esc=y (accessed on 12 December 2022).
- Piggott, A.G. Ferns of Malaysia in Colour; Tropical Press: Kuala Lumpur, Malaysia, 1988. [Google Scholar]
- Becker, M. A preliminary survey of the fern flora of Penang Hill. Flora Males. Bull. 1994, 11, 293–300. [Google Scholar]
- Iwatsuki, K.; Price, M.G. The pteridophytes of Mt Burnay and vicinity, Northern Luzon. Southeast. Asian Stud. 1977, 14, 540–572. [Google Scholar]
- Lindsay, S.; Middleton, D.J. Ferns of Thailand, Laos and Cambodia. Available online: http://rbg-web2.rbge.org.uk/thaiferns/ (accessed on 12 December 2022).
- Popelka, O.; Dančák, M.; Sukri, R.S.; Metali, F. Novitates Bruneienses, 10. Filmy ferns (Hymenophyllaceae) of Kuala Belalong. Brunei Darussalam 2018, 70, 123–154. [Google Scholar]
- Sledge, W.A. The Hymenophyllaceae of Ceylon. J. Linn. Soc. Lond. Bot. 1968, 60, 289–308. [Google Scholar] [CrossRef]
- Saïd, A.H.; Hennequin, S.; Rouhan, G.; Dubuisson, J.Y. Disentangling the diversity and taxonomy of Hymenophyllaceae (Hymenophyllales, Polypodiidae) in the Comoros. Eur. J. Taxon. 2017, 313. [Google Scholar] [CrossRef]
- Beentje, H.J. Hymenophyllaceae; Royal Botanic Gardens: Kew, Thailand, 2008. [Google Scholar]
- Blum, C.T.; Roderjan, C.V.; Galvao, F. Composição florística e distribuição altitudinal de epífitas vasculares da Floresta Ombrófila Densa na Serra da Prata, Morretes, Paraná, Brasil. Biota Neotrop. 2011, 11, 141–159. [Google Scholar] [CrossRef]
- Cetzal-Ix, W.; Noguera-Savelli, E.; Ramirez-Marcial, N. New records of ferns for Tabasco, Mexico. Rev. Mex. Biodiv. 2013, 84, 977–982. [Google Scholar] [CrossRef]
- Christenhusz, M.J.M. Index Pteridophytorum Guadalupensium or a revised checklist to the ferns and club mosses of Guadeloupe (French West Indies). Bot. J. Linn. Soc. 2009, 161, 213–277. [Google Scholar] [CrossRef]
- Dittrich, V.A.d.O.; Waechter, J.L.; Salino, A. Species richness of pteridophytes in a montane Atlantic rain forest plot of Southern Brazil. Acta Bot. Bras. 2005, 19, 519–525. [Google Scholar] [CrossRef]
- Ferreira de Lima, R.A.; de Oliveira Dittrich, V.A.; de Souza, V.C.; Salino, A.; Breier, T.B.; de Aguiar, O.T. Flora vascular do Parque Estadual Carlos Botelho, São Paulo, Brasil. Biota Neotrop. 2011, 11, 173–214. [Google Scholar] [CrossRef]
- Gasper, A.L.d.; Sevegnani, L. Lycophyta e samambaias do Parque Nacional da Serra do Itajaí, Vale do Itajaí, SC, Brasil. Hoehnea 2010, 37, 755–767. [Google Scholar] [CrossRef]
- Grayum, M.H.; Churchill, H.W. The vascular flora of La Selva Biological Station, Costa Rica- Polypodiophyta. Selbyana 1989, 11, 66–118. [Google Scholar]
- Haber, W. Appendix 1-Vascular plants of Monteverde. In Monteverde: Ecology and Conservation of a Tropical Cloud Forest; Nadkarni, N.M., Whellwright, N., Eds.; Oxford University Press: New York, NY, USA, 2000; pp. 457–518. [Google Scholar]
- Hyde, M.A.; Wursten, B.T.; Ballings, P.; Coates Palgrave, M. Flora of Zimbabwe: Home page. 2019. Available online: https://www.zimbabweflora.co.zw/index.php (accessed on 12 December 2022).
- Kornás, J. Life-forms and seasonal patterns in the pteridophytes in Zambia. Acta Soc. Bot. Pol. 1977, 46, 669–690. [Google Scholar] [CrossRef]
- Lellinger, D.B. The Ferns and Fern-Allies of Costa Rica, Panama, and the Chocó (Part. I: Psilotaceae through Dicksoniaceae); American Fern Society: Washington, DC, USA, 1989; Volume 2a, p. 364. [Google Scholar]
- Mori, S.A.; Cremers, G.; Gracie, C.; de Granville, J.-J.; Hoff, M.; Mitchell, J.D. Pteridophytes, Gymnosperms, and Monocotyledons; New York Botanical Garden: New York, NY, USA, 1997; Volume 1, p. 422 S. [Google Scholar]
- Nitta, J.H.; Ebihara, A.; Smith, A.R. A taxonomic and molecular survey of the pteridophytes of the Nectandra Cloud Forest Reserve, Costa Rica. PLoS ONE 2020, 15. [Google Scholar] [CrossRef]
- Nóbrega, G.A.; Aidar, M.P.M.; Paciencia, M.; Prado, J. Identification key for lycophytes and ferns from the Picinguaba and Santa Virginia Nuclei, Parque Estadual da Serra do Mar, Ubatuba, SP, Brazil. Biota Neotrop. 2016, 16. [Google Scholar] [CrossRef]
- Shreve, F. Studies on Jamaican Hymenophyllaceae. Bot. Gaz. 1911, 51, 184–209. [Google Scholar] [CrossRef]
- Windisch, P.G. Pteridófitas do Estado de Mato Grosso: Hymenophyllaceae. Bradea 1996, 6, 400–423. [Google Scholar]
- Schippers, R.R. Pteridophytes of Tanzania with special reference to Pare and Usambara Mountains. Fern Gaz. 1993, 14, 171–192. [Google Scholar]
- Windisch, P.G. Hymenophyllaceae (Polypodiopsida) no estado do Rio Grande do Sul. Pesqui. Botânica 2014, 65, 15–48. [Google Scholar]
- Graham, B.M.; Rickard, M.H. A revised list of the pteridophytes of Nevis. Fern Gaz. 1992, 14, 85–95. [Google Scholar]
- Peña-Chocorro, M.C.; Jiménez, B.; Marín, G.; Knapp, S. Checklist of the pteridophytes of the Mbaracayú Forest Nature Reserve, Paraguay. Fern Gaz. 1999, 15, 221–258. [Google Scholar]
- Peña-Chocarro, M.; Espada-Mateos, C.; Vera, M.; Céspedes, G.; Knapp, S. Updated checklist of vascular plants of the Mbaracayú Forest Nature Reserve (Reserva Natural del Bosque Mbaracayú), Paraguay. Phytotaxa 2010, 12, 1–224. [Google Scholar] [CrossRef]
- Monro, A.K.; Santamaria-Aguilar, D.; Gonzalez, F.; Chacon, O.; Solano, D.; Rodriguez, A.; Zamora, N.; Fedele, E.; Correa, M. A first checklist to the vascular plants of La Amistad International Park (PILA), Costa Rica-Panama. Phytotaxa 2017, 322, 1–283. [Google Scholar] [CrossRef]
- Wilson, H.D. Field Guide Stewart Island Plants; Manuka Press: Christchurch, New Zealand, 1994. [Google Scholar]
- Lellinger, D.B. Notes on neotropical Hymenophyllaceae. Am. Fern J. 1991, 81, 24–36. [Google Scholar] [CrossRef]
- Mildawati; Sobir, S.; Sulistijorini, S.; Chikmawati, T. The diversity of pteridophytes in Siberut National Park, Mentawai Islands, West Sumatra, Indonesia. Biodiversitas 2020, 21, 3200–3208. [Google Scholar] [CrossRef]
- Chambers, T.C.; Jermy, A.C.; Crabbe, J.A. A collection of ferns from Espiritu Santo, New Hebrides. Fern Gaz. 1971, 10, 175. [Google Scholar]
- Flora of the Hawaiian Islands. Available online: https://naturalhistory2.si.edu/botany/hawaiianflora/index.htm (accessed on 12 November 2022).
- Barcelona, J.F. The taxonomy and ecology of the pteridophytes of Mt. Iraya and vicinity, Batan Island, Batanes Province, Northern Philippines. In Pteridology in the New Millenium; Springer: Dordrecht, The Netherlands, 2003; pp. 299–325. [Google Scholar]
- Cadet, T. La Végétation de l’Île de la Réunion. Etude Phytoécologique et Phytosociologique. Ph.D. Thesis, Université d’Aix-Marseille, St. Denis, La Rèunion, 1977. [Google Scholar]
- Dubuisson, J.Y.; Rouhan, G.; Grall, A.; Hennequin, S.; Senterre, B.; Pynee, K.; Ebihara, A. New insights into the systematics and evolution of the filmy fern genus Crepidomanes (Hymenophyllaceae) in the Mascarene Archipelago with a focus on dwarf species. Acta Bot. Gall. 2013, 160, 173–194. [Google Scholar] [CrossRef]
- Yuyen, Y.; Boonkerd, T. Pteridophyte flora of Huai Yang waterfall National Park, Prachuap Khiri Khan province, Thailand. Nat. Hist. J. Chulalongkorn Univ. 2002, 2, 39–49. [Google Scholar]
- Chapman, J.D.; Chapman, H.M. The forests of Taraba and Adamawa States, Nigeria. An. Ecological Account and Plant Species Checklist; University of Canterbury: Christchurch, New Zealand, 2001; pp. 146–175. [Google Scholar]
- Fischer, E.; Rembold, K.; Althof, A.; Obholzer, J.; Malombe, I.; Mwachala, G.; Onyango, J.C.; Dumbo, B.; Theisen, I. Annotated checklist of the vascular plants of Kakamega Forest, Western Province, Kenya. J. East. African Nat. Hist. 2010, 99, 129–226. [Google Scholar] [CrossRef]
- Flora of Australia. Australian Biological Resources Study, Canberra. Available online: http://www.ausflora.org.au (accessed on 12 December 2022).
- Game, J.C.; Pene, S.; Smith, A.R. A new specimen-based checklist of ferns and lycophytes from Rotuma (Fiji). N. Z. J. Bot. 2021, 59, 137–153. [Google Scholar] [CrossRef]
- Hameed, C.A.; Madhusoodanan, P.V. A new species of filmy fern (Hymenophyllaceae: Pteridophyta) from South India. SIDA Contr. Bot. 1998, 18, 519–522. [Google Scholar]
- Madhusoodanan, P.V.; Hameed, C.A. Crepidomanes agasthianum, a new filmy fern species (Hymenophyllaceae—Pteridophyta) from India. Nord. J. Bot. 1998, 18, 169–170. [Google Scholar] [CrossRef]
- Farrar, D.R. Trichomanes intricatum: The independent trichomanes gametophyte in the Eastern United States. Am. Fern J. 1992, 82, 68–74. [Google Scholar] [CrossRef]
- Trigoboff, N.; Li, F.W. Crepidomanes intricatum (Hymenophyllaceae), a sporophyte-less filmy fern new to Central New York. Am. Fern J. 2022, 112, 139–141. [Google Scholar]
- Patil, S.M.; Hande, P.R.; Dongare, M.M. A filmy fern: Crepidomanes latealatum (Bosch) Copel. new to Northern Western Ghats of India. Indian For. 2014, 140, 939–940. [Google Scholar]
- Chen, C.W.; Hsu, T.C.; Chao, Y.S.; Lu, P.F.; Li, C.W.; Tram, N.K.T.; Nguyen, I.; Dang, M.T.; Luu, H.T.; Parris, B. A newly recorded genus and twelve newly recorded species of ferns for Vietnam from Lang Biang Plateau. Phytotaxa 2020, 443, 121–143. [Google Scholar] [CrossRef]
- Grieve, G.R.H.; Downs, C.T. A checklist of the plants of the forests and grasslands in the Weza district, southern KwaZulu-Natal and a review of their status in the Red Data List. Koedoe 2015, 57, 1237. [Google Scholar] [CrossRef]
- Pynee, K.; Hennequin, S.; Echternacht, L.; Dubuisson, J.-Y. A new local variety of Crepidomanes minutum (Hymenophyllaceae) in the Mascarene Archipelago (Indian Ocean) and a new record for Mauritius. Phytotaxa 2012, 62, 25–30. [Google Scholar] [CrossRef]
- Faden, R.B. A new species of Trichomanes from Eastern Africa. Am. Fern J. 1977, 67, 5–10. [Google Scholar] [CrossRef]
- Price, M.G. Notes on Philippine ferns. Fern Gaz. 1972, 10, 253–262. [Google Scholar]
- Joseph, J.; Singh, S. Trichomanes saxifragoides Presl (Hymenophyllaceae)-A new record of fern for North Eastern India from Tirap District, Arunachal Pradesh. Bull. Bot. Surv. India 1981, 21, 213–214. [Google Scholar]
- Lee, C.S.; Lee, K.; Hwang, Y. First record of Crepidomanes schmidtianum (Zenker ex Tasch.) K. Iwats. (Hymenophyllaceae) from Korea. Korean J. Plant Tax. 2014, 44, 1–5. [Google Scholar] [CrossRef]
- Bir, S.S.; Irudayaraj, V.; Manickam, V.S. Cytology of some ferns from the Nilgiris, South India III. Fern Gaz. 1996, 15, 141–149. [Google Scholar]
- Da Silva, W.R.; Ferreira, A.W.C.; Ilkiu-Borges, A.L.; Fernandes, R.S. Ferns and lycophytes of remnants in Amazonia Maranhense, Brazil. Biota Neotrop. 2020, 20. Available online: https://www.scielo.br/j/bn/a/nDDPNQNktNz8VhpQyFGrLxx/?lang=en (accessed on 12 December 2022). [CrossRef]
- Matos, F.B.d. Samambaias e Licófitas da RPPN Serra Bonita, Município de Camacan, sul da Banhia, Brasil. 2009. Available online: https://www.researchgate.net/publication/26980966_Samambaias_e_licofitas_da_RPPN_Serra_Bonita_municipio_de_Camacan_sul_da_Bahia_Brasil (accessed on 12 December 2022).
- Wessels Boer, J.G. The new world species of Trichomanes sect. Didymoglossum and Microgonium. Acta Bot. Neerl. 1962, 11, 277–330. [Google Scholar] [CrossRef]
- Pichi Sermolli, R.E.G. Microgonium erosum and related species (Hymenophyllaceae) in continental Africa. Webbia 1982, 35, 241–260. [Google Scholar] [CrossRef]
- Senterre, B.; Rouhan, G.; Morel, C.; Dubuisson, J.Y. New species and records in the fern genus Didymoglossum for the flora of Seychelles, with notes on the Southeast Asian D. motleyi complex (Hymenophyllaceae). Phytotaxa 2017, 292, 201–217. [Google Scholar] [CrossRef]
- Croxall, J.P. Microgonium (Hymenophyllaceae) in Malesia, with special reference to Peninsular Malaysia. Kew Bull. 1986, 41, 519–531. [Google Scholar] [CrossRef]
- Palacios-Rios, M. Pteridophytes of the state of Veracruz, Mexico: New records. Fern Gaz. 1992, 14, 119–122. [Google Scholar] [CrossRef]
- Sánchez, C. A new filmy fern species and new unispecific section of Trichomanes (Hymenophyllaceae) ("filmy ferns") from Cuba. Willdenowia 2001, 31, 125–127. [Google Scholar] [CrossRef]
- Croat, T.B. Flora of Barro Colorado Island; Stanford University Press: Stanford, CA, USA, 1978; p. 943. [Google Scholar]
- Christensen, C. XIX.-On the ferns of the Seychelles and the Aldabra Group. Trans. Linn. Soc. Lond. -Second. Ser. Bot. 1912, VII, 409–424. [Google Scholar] [CrossRef]
- Senterre, B.; Gerlach, J.; Mougal, J.; Matatiken, D. Old growth mature forest types and their floristic composition along the altitudinal gradient on Silhouette Island (Seychelles)-the telescoping effect on a continental mid-oceanic island. Phytocoen 2009, 39, 157–174. [Google Scholar] [CrossRef]
- Cascante-Marín, A.; Estrada-Chavarría, A. Las plantas vasculares de El Rodeo, Costa Rica. Brenesia 2012, 77, 71–128. [Google Scholar]
- Hameed, C.A.; Madhusoodanan, P.V. Trichomanes vamana, a new filmy fern species (Hymenophyllaceae-Pteridophyta) from India. Nord. J. Bot. 2005, 23, 437–439. [Google Scholar] [CrossRef]
- Acebey, A.R.; Lopez-Acosta, J.C.; Tejero-Diez, J.D.; Krömer, T. Richness and composition of ferns and lycophytes in three areas of humid montane forest in Los Tuxtlas, Veracruz, Mexico. Rev. Mex. Biodiv. 2017, 88, 625–635. [Google Scholar] [CrossRef]
- Arana, M.D.; Larsen, C.; Ponce, M.M. Revisión y análisis panbiogeográfico de las Hymenophyllaceae de las Yungas meridionales de Argentina (Selva Tucumano-Boliviana). Rodriguésia 2016, 67, 55–76. [Google Scholar] [CrossRef]
- Trusty, J.L.; Kesler, H.C.; Delgado, G.H. Vascular flora of Isla del Coco, Costa Rica. Proc. Calif. Acad. Sci. 2006, 57, 247–355. [Google Scholar]
- Kornás, J. Notes on African Hymenophyllaceae 2. Trichomanes lenormandii v. d. Bosch New to Continental Africa. Bull. Jard. Bot. Natl. Belg. Bull. Van Natl. Plantentuin Van Belg. 1976, 46, 393–397. [Google Scholar] [CrossRef]
- Bragança Guarnier, G.; da Silva Sylvestre, L.; Abraão Araújo da Silva, I. Checklist preliminar da biodiversidade de pteridófitas do Parque Natural Municipal do Curió. In Parque do Curió, Frago, M.E., Ed.; Missão Asa Editorial: 2020; pp. 43–54. Available online: https://www.researchgate.net/profile/Eduardo-Silva-Neto/publication/348390297_Solos_e_Fragilidade_Ambiental_do_Parque_Natural_Municipal_do_Curio_Paracambi_RJ/links/5ffc58bd299bf1408888fb2c/Solos-e-Fragilidade-Ambiental-do-Parque-Natural-Municipal-do-Curio-Paracambi-RJ.pdf (accessed on 12 December 2022).
- Lellinger, D.B. A Field Manual to the Ferns and Fern-Allies of the United States and Canada; Smithsonian Institution Press: Washington, DC, USA, 1985; p. 389. [Google Scholar]
- Prado, J.; Moran, R.C. Checklist of the ferns and lycophytes of Acre State, Brazil. Fern Gaz. 2009, 18, 230–263. [Google Scholar]
- Tardieu-Blot, M.L. Sur quelques Hymenophyllaceae des îles Mascareignes. Adansonia sér. 2 1977, 17, 147–150. [Google Scholar]
- Farrar, D.R.; Parks, J.C.; McAlpin, B.W. The fern genera Vittaria and Trichomanes in the northeastern United States. Rhodora 1983, 85, 83–92. [Google Scholar]
- Pinson, J.B.; Chambers, S.M.; Sessa, E.B. Vittaria graminifolia (Pteridaceae) and Didymoglossum petersii (Hymenophyllaceae) in Broxton Rocks, GA. Am. Fern J. 2017, 107, 257–264. [Google Scholar] [CrossRef]
- Martínez-Meléndez, N.; Perez-Farrera, M.A.; Martinez-Camilo, R. The vascular epiphyte flora of El Triunfo biosphere reserve, Chiapas, México. Rhodora 2009, 111, 503–535. [Google Scholar] [CrossRef]
- Fosberg, F.R.; Sachet, M.-H. Systematic studies of Micronesian plants. Smiths. Contr. Bot. 1980, 1–40. Available online: https://repository.si.edu/bitstream/handle/10088/6955/scb-0045.pdf?sequence=1&isAllowed=y (accessed on 12 December 2022).
- León, B. Hymenophyllaceae endémicas del Perú. Rev. Peru. Biol. 2006, 13, 903–904. [Google Scholar] [CrossRef]
- Kessler, M.; Smith, A.R.; Sundue, M. Three new Andean species of Hymenophyllum (Hymenophyllaceae–Pteridophyta). Blumea 2005, 50, 555–559. [Google Scholar] [CrossRef]
- Kirby, C. Field Guide to New Zealand’s Epiphytes, Vines & Mistletoes; Environmental Research Institute, University of Waikato: Hamilton, NZ, USA, 2014. [Google Scholar]
- Oliver, W.R.B. New Zealand epiphytes. J. Ecol. 1930, 18, 1–50. [Google Scholar] [CrossRef]
- Gonzatti, F.; Windisch, P.G. Flora of Espírito Santo: Hymenophyllum (Hymenophyllaceae). Rodriguésia 2018, 69, 611–629. [Google Scholar] [CrossRef]
- Benson, D.; McDougall, L. Ecology of Sydney plant species Part 1: Ferns, fern-allies, cycads, conifers and dicotyledon families Acanthaceae to Asclepiadaceae. Cunninghamia 1993, 3, 257–422. [Google Scholar]
- Jarman, S.J.; Kantvilas, G.; Brown, M.J. The ecology of pteridophytes in Tasmanian cool temperate rainforest. Fern Gaz. 1986, 13, 77–86. [Google Scholar]
- Brass, L.J. Ferns of a New Guinea gully. Am. Fern J. 1953, 43, 150–158. [Google Scholar] [CrossRef]
- Nwe, T.Y.; Zhang, X.-C. Taxonomic studies of lycophytes and ferns from the Pan-Himalaya (I): Hymenophyllum (Hymenophyllaceae). Turczaninowia 2017, 20, 75–96. [Google Scholar]
- Chen, C.W.; Ebihara, A.; Cheng, K.Y.; Hsu, T.C.; Huang, Y.M.; Dang, V.D.; Luu, H.T.; Le, V.S.; Li, C.W. Studies of Vietnamese pteridophyte flora 1. Syst. Bot. 2021, 46, 573–581. [Google Scholar] [CrossRef]
- Larsen, C.; Ponce, M.M.; Scataglini, M.A. Revisión de las especies de Hymenophyllum (Hymenophyllaceae) del sur de Argentina y Chile. Gayana. Bot. 2013, 70, 275–330. [Google Scholar] [CrossRef]
- Carvalho, F.A.; Salino, A.; Zartman, C.E. New country and regional records from the Brazilian side of Neblina Massif. Am. Fern J. 2012, 102, 228–232. [Google Scholar] [CrossRef]
- Gonzatti, F.; Windisch, P.G.; Scariot, F.J.; Echeverrigaray, S.; Ritter, M.R. Revision of Hymenophyllum subg. Sphaerocionium (Hymenophyllaceae) in the Atlantic Forest domain (Brazil), based on molecular and morphological evidence. Syst. Bot. 2020, 45, 707–748. [Google Scholar] [CrossRef]
- Kornás, J. Filmy ferns (Hymenophyllaceae) of Central Africa (Zaire, Rwanda, Burundi). 1. Hymenophyllum. Fragmenta Flor. Geobot. Polonica 1993, 38, 3–19. [Google Scholar]
- Diem, J.; Lichtenstein, J.S.D. Las Himenofiláceas del área argentino-chilena del sur. Darwiniana 1959, 11, 611–760. [Google Scholar] [CrossRef]
- Larsen, C.; Gonzatti, F.; Acosta, J.M.; Ponce, M.M. Morphological and molecular evidence to segregate a disjunct species of Hymenophyllum (Hymenophyllaceae) from Southern South America. Syst. Bot. 2020, 45, 439–449. [Google Scholar] [CrossRef]
- Pincheira-Ulbrich, J.; Zambrano, U.; Urrutia-Estrada, J. New record of Hymenophyllum caudatum Bosch (Polypodiopsida, Hymenophyllaceae) extends the mainland distribution in the coastal Mediterranean Forest of South America. Biodiv. Data J. 2022, 10. [Google Scholar] [CrossRef]
- Larsen, C.; Arana, M.D.; Acosta, J.M.; Ponce, M.M. Two new species segregated from Hymenophyllum tunbrigense (Hymenophyllaceae) in southern South America, based on morphological, anatomical, molecular and distributional evidence. Phytotaxa 2017, 303, 218–232. [Google Scholar] [CrossRef]
- Carvajal-Hernández, C.I.; Krömer, T. Riqueza y distribución de helechos y licófitos en el gradiente altitudinal del Cofre de Perote, centro de Veracruz, México. Bot. Sci. 2015, 93, 601–614. [Google Scholar] [CrossRef]
- Christenhusz, M.J.; Schwartsburd, P.B.; Labiak, P.H. Hymenophyllum filmenofilicum (Hymenophyllaceae, Pteridophyta): A new epipetric filmy fern from Paraná, southern Brazil. Kew Bull. 2009, 64, 175–178. [Google Scholar] [CrossRef]
- Manickam, V.S. Rare and endangered ferns of the Western Ghats of South India. Fern Gaz. 1995, 15, 1–10. [Google Scholar]
- Hsu, T.C.; Huang, Y.F.; Chao, Y.S. Systematics of Hymenophyllum subgenus Mecodium (Hymenophyllaceae) in Taiwan. Syst. Bot. 2019, 44, 753–767. [Google Scholar] [CrossRef]
- Godley, E.J. The flora of Antipodes Island. N. Z. J. Bot. 1989, 27, 531–564. [Google Scholar] [CrossRef]
- Olivera Tarifeño, C.M.; Ramos Sandoval, J.D. Determinación Taxonomica, Distribucion y Descripcion de los Helechos (Div. Pteridophyta) en el Parque Nacional de Cutervo; Cutervo–Region Cajamarca. Lic.: Lambayeque, Peru, 2014. [Google Scholar]
- Singh, B.; Singh, V.N.; Phukan, S.J.; Sinha, B.K.; Borthakur, S.K. Contribution to the pteridophytic flora of India: Nokrek Biosphere Reserve, Meghalaya. J. Threatened Taxa 2012, 4, 2277–2294. [Google Scholar] [CrossRef]
- Fowler, R.L. Annotated list of ferns of the Kilauea-Mauna Loa Section of Hawaii National Park. Am. Fern J. 1940, 30, 9–18. [Google Scholar] [CrossRef]
- Ebihara, A. Hymenophyllaceae collected in the Mt. Kinabalu Area in 2000 and 2007. Mem. Nat. Mus. Nat. Sci. 2008, 45, 105–110. [Google Scholar]
- Copeland, E.B. Papuan pteridophytes collected for the Arnold Arboretum by LJ Brass. J. Arnold Arb. 1929, 10, 174–182. [Google Scholar] [CrossRef]
- Gibby, M.; Lovis, J.D. New ferns of Madeira. Fern Gaz. 1989, 13, 285–290. [Google Scholar]
- Fineran, B.A. A botanical survey of seven mutton-bird islands, south-west Stewart Island. J. R. Soc. N. Z. 1973, 3, 475–525. [Google Scholar] [CrossRef]
- Ríos Saldaña, R.; Rincón, G.; Rafael, R.; Santos Mido, M. Guía de Helechos y Plantas Afines del PARQUE Nacional Volcán Barú; SENACYT: Clayton, Panama, 2016. [Google Scholar]
- Rio, C.D.; Hennequin, S.; Rouhan, G.; Ebihara, A.; Lowry, P.P., II; Dubuisson, J.-Y.; Gaudeul, M. Origins of the fern genus Hymenophyllum (Hymenophyllaceae) in New Caledonia: Multiple independent colonizations from surrounding territories and limited in situ diversification. Taxon 2017, 66, 1041–1064. [Google Scholar] [CrossRef]
- Barcelona, J.F. Preliminary report on the ferns and fern allies (pteridophytes) of Mt. Bali-it, Balbalasang-Balbalan National Park, Kalinga, Northern Luzon, Philippines. Sylvatrop 2003, 13, 81–92. [Google Scholar]
- Ebihara, A.; Iwatsuki, K.; Ohsawa, T.A.; Ito, M. Hymenophyllum paniense (Hymenophyllaceae), a new species of filmy fern from New Caledonia. Syst. Bot. 2003, 28, 228–235. [Google Scholar]
- Lindsay, S.; Middleton, D.J.; Saengrit, S. Hymenophyllum pilosissimum C. Chr.(Hymenophyllaceae), a new record for Thailand. Thai For. Bull. (Bot.) 2014, 42, 48–51. [Google Scholar]
- Moore, S.-J.; Chen, C.-M.; Wang, J.-C. Hymenophyllum pilosissimum C. Chr. (Hymenophyllaceae), a new recorded fern from Taiwan. Am. Fern J. 2010, 100, 180–183. [Google Scholar] [CrossRef]
- Perrie, L.R.; Shepherd, L.D.; de Lange, P.J.; Batty, E.L.; Ohlsen, D.J.; Bayly, M.J.; Brownsey, P.J. Hymenophyllum pluviatile, a new and uncommon fern from New Zealand. N. Z. J. Bot. 2013, 51, 308–320. [Google Scholar] [CrossRef]
- Fall, P.L.; Drezner, T.D. Vascular plant species of the Kingdom of Tonga by vegetation type, species origin, growth form, and dispersal mechanism. Ecology 2020, 101, e02902. [Google Scholar] [CrossRef]
- Janzen, D.H.; Liesner, R. Annotated check-list of plants of lowland Guanacaste Province, Costa Rica, exclusive of grasses and non-vascular cryptogams. Brenesia 1980, 18, 15–90. [Google Scholar]
- Klein, V.P.; Demarchi, L.O.; Quaresma, A.C.; Cruz, J.d.; Piedade, M.T.F. The vascular epiphyte flora in a white-sand ecosystem of the Uatumã Sustainable Development Reserve, Central Amazon. Check List 2022, 18, 157–186. [Google Scholar] [CrossRef]
- Meave, J.A.; Rincon-Gutierrez, A.; Ibarra-Manriquez, G.; Gallardo-Hernandez, C.; Romero-Romero, M.A. Checklist of the vascular flora of a portion of the hyper-humid region of La Chinantla, Northern Oaxaca Range, Mexico. Bot. Sci. 2017, 95, 722–759. [Google Scholar] [CrossRef]
- Sánchez Villaverde, C. Tres nuevas especies de Hymenophyllaceae para Cuba. Rev. Jard. Bot. Nac. 1987, 8, 9–16. [Google Scholar]
- Perrie, L.R.; Shepherd, L.D.; Brownsey, P.J.; Larraín, J.; Shaw, B.; Thouvenot, L.; von Konrat, M. Rediscovery and reinstatement of the New Caledonian endemic filmy fern Hymenophyllum pumilio Rosenst. N. Z. J. Bot. 2016, 54, 1–10. [Google Scholar] [CrossRef]
- Dubuisson, J.Y.; Hennequin, S.; Droissart, V. Hymenophyllum senterreanum Dubuisson & Deblauwe, sp. nov.(Hymenophyllaceae) and its relatives in western Central Africa. Phytotaxa 2016, 257, 287–294. [Google Scholar]
- Rojas-Alvarado, A.F. Una especie nueva de Hymenophyllum y una variedad nueva de Trichomanes collariatum Bosch (Filicales: Hymenophyllaceae) en Costa Rica. Lankesteriana 2004, 4, 143–148. [Google Scholar] [CrossRef]
- Ratcliffe, D.A.; Birks, H.J.B.; BIRKS, H.H. The ecology and conservation of the Killarney fern Trichomanes speciosum WILLD. in Britain and Ireland. Biol. Conserv. 1993, 66, 231–247. [Google Scholar] [CrossRef]
- Richards, P.W.; Evans, G.B. Biological flora of the British Isles: Hymenophyllum. J. Ecol. 1972, 60, 245–268. [Google Scholar] [CrossRef]
- Wilmanns, O.; Rasbach, H. Observations on the pteridophytes of Sao Miguel, Acores. Fern Gaz. 1973, 10, 315–329. [Google Scholar]
- Willmot, A. An ecological survey of the ferns of the Killarney District, Co. Kerry, Ireland. Brit. Fern Gaz. 1983, 12, 249–265. [Google Scholar]
- Aguiar, S.; Herrero, A.; Quintanilla, L.G. Confirmación citológica de la presencia de Hymenophyllum wilsonii en España. Lazaroa 2006, 27, 129–131. [Google Scholar]
- Sánchez Velázquez, T. Hymenopyllum wilsonii Hook. (Pteridophyta, Hymenophyllaceae). Confirmación de su presencia en las Islas Canarias. Bot. Macaronésica 2003, 24, 207–2011. [Google Scholar]
- Rasbach, H.; Rasbach, K.; Bennert, H.W. New records and new cytological results for the fern flora of Madeira. Brit. Fern Gaz. 1990, 13, 391–395. [Google Scholar]
- Lee, C.S.; Lee, K.; Lee, S.G.; Ebihara, A. A new taxon of Hymenophyllum (Hymenophyllaceae): H. wrightii f serratum. Korean J. Plant Taxon. 2014, 44, 233–237. [Google Scholar] [CrossRef]
- Mallmann, I.T.; Schmitt, J.L. Richness and floristic composition of the fern community in riparian forest of the river ‘Cadeia’, in Rio Grande do Sul state, Brazil. Cienc. Florest. 2014, 24, 97–109. [Google Scholar] [CrossRef]
- Ponce, M.M.; del Rio, C.; Ebihara, A.; Dubuisson, J.Y. Discussion on taxonomy of the fern genera Crepidomanes and Polyphlebium (Hymenophyllaceae) in Argentina and south-eastern South America, and description of a new local variety for Crepidomanes pyxidiferum. Bot. Lett. 2017, 164, 5–18. [Google Scholar] [CrossRef]
- Croxall, J.P. The Hymenophyllaceae of Queensland. Austr. J. Bot. 1975, 23, 509–547. [Google Scholar] [CrossRef]
- Windisch, P.G. Trichomanes crispum L. (Pteridophyta, Hymenophyllaceae) and allied species. Bradea 1992, VI, 78–117. [Google Scholar]
- Bauret, L.; Grall, A.; Senterre, B.; Rouhan, G.; Hennequin, S.; Ebihara, A.; Dubuisson, J.-Y. New circumscription of Trichomanes cupressoides Desvaux (Hymenophyllaceae), an endemic filmy fern from the Seychelles (Indian Ocean), and new insights into the genus Abrodictyum C.Presl in the western Indian Ocean. Phytotaxa 2015, 202, 14. [Google Scholar] [CrossRef]
- Rojas-Alvarado, A.F. Novelties in Trichomanes l. From Colombia. Caldasia 2009, 31, 9–11. [Google Scholar]
- Moran, R.C. Trichomanes resinosum (Hymenophyllaceae), a new species from southern Venezuela and adjacent Guyana. Brittonia 2000, 52, 238–240. [Google Scholar] [CrossRef]
- Pacheco, L. Trichomanes ribae (Hymenophyllaceae), a new filmy fern from Costa Rica and Panama. Am. Fern J. 2002, 92, 292, 294–295. [Google Scholar] [CrossRef]
- Ebihara, A.; Matsumoto, S.; Ito, M. Taxonomy of the reticulate Vandenboschia radicans complex (Hymenophyllaceae) in Japan. Acta Phytotax.Geobot. 2009, 60, 26–40. [Google Scholar]
- Bidin, A.A.; Jaman, R. The pteridophytes of Tawau Hills Park, Sabah. ASEAN Rev. Biodiv. Environ. Cons. (ARBEC) 1999, 1, 1–11. [Google Scholar]
- Ward, C.M. The pteridophytes of Flores (Açores): A survey with bibliography. Brit. Fern Gaz. 1970, 10, 119–126. [Google Scholar]
- Vumba Nature. Available online: http://www.vumba-nature.com/ (accessed on 12 October 2022).
- Available online: herbario.zamorano.edu/handle/11036/1677 (accessed on 10 October 2022).
- Available online: https://fm-digital-assets.fieldmuseum.org/1140/158/C0628078F.jpg (accessed on 12 October 2022).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).