Increased Abundance Coincides with Range Expansions and Phenology Shifts: A Long-Term Case Study of Two Noctuid Moths in Sweden
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Design
2.2. Study Species
2.3. Citizen Science Data on Spatial Distribution and Variation in Flight Period
2.4. Light Trap Data on Abundance
2.5. Statistical Analysis
2.6. Occupied Grid Modelling
2.7. Species Abundance Modelling
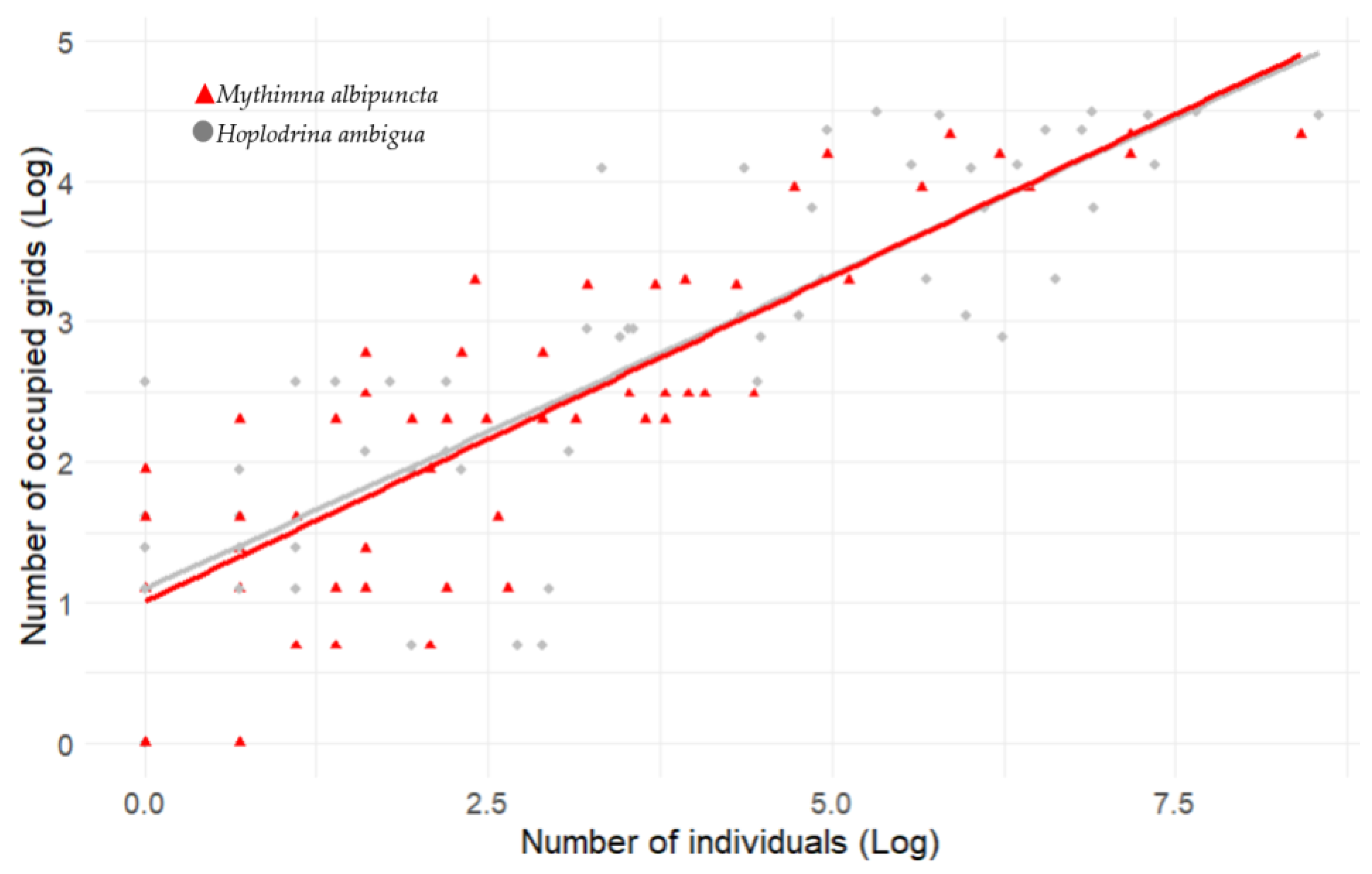
2.8. GLMM for Occupied Grids and Light Trap Abundance
2.9. Phenology
3. Results
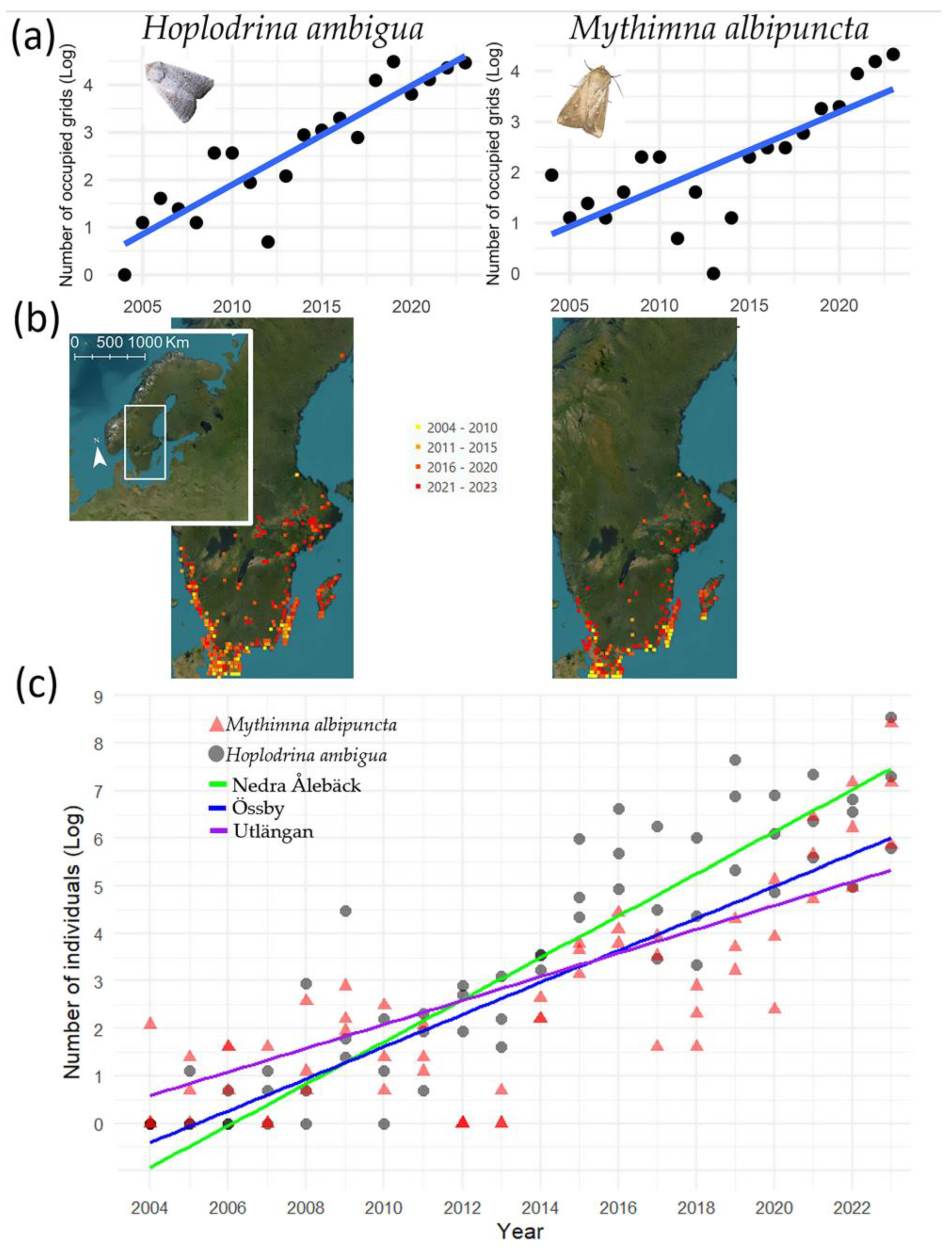
3.1. Spatial Distribution Shifts
3.2. Abundance Shifts
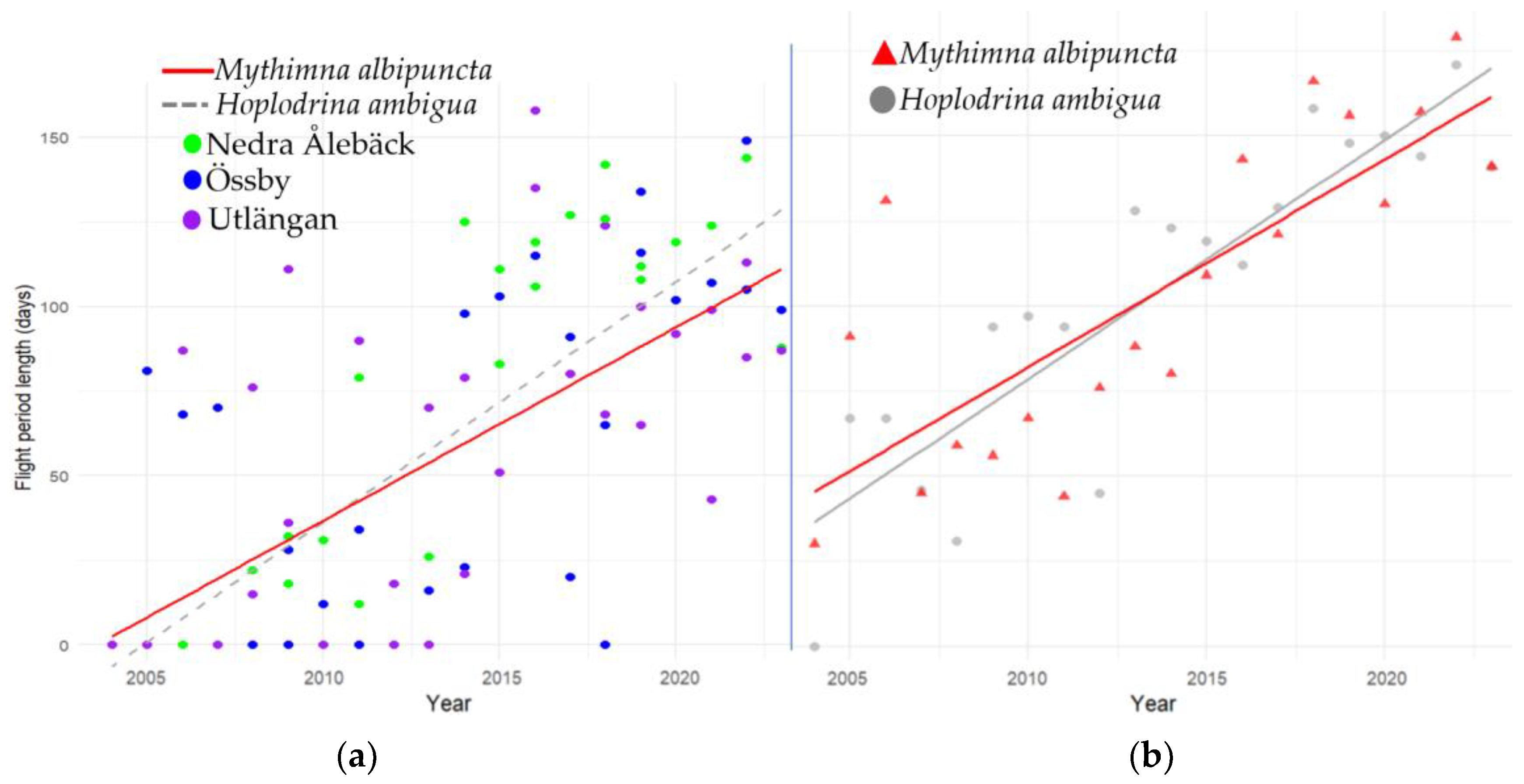
3.3. Phenology Shifts
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wagner, D.L.; Grames, E.M.; Forister, M.L.; Berenbaum, M.R.; Stopak, D. Insect decline in the Anthropocene: Death by a thousand cuts. Proc. Natl. Acad. Sci. USA 2021, 118, e2023989118. [Google Scholar] [CrossRef]
- Sunde, J.; Franzén, M.; Betzholtz, P.-E.; Francioli, Y.; Pettersson, L.B.; Pöyry, J.; Ryrholm, N.; Forsman, A. Century-long butterfly range expansions in northern Europe depend on climate, land use and species traits. Commun. Biol. 2023, 6, 601. [Google Scholar] [CrossRef] [PubMed]
- Dirzo, R.; Young, H.S.; Galetti, M.; Ceballos, G.; Isaac, N.J.B.; Collen, B. Defaunation in the Anthropocene. Science 2014, 345, 401. [Google Scholar] [CrossRef] [PubMed]
- Hallmann, C.A.; Sorg, M.; Jongejans, E.; Siepel, H.; Hofland, N.; Schwan, H.; Stenmans, W.; Müller, A.; Sumser, H.; Hörren, T.; et al. More than 75 percent decline over 27 years in total flying insect biomass in protected areas. PLoS ONE 2017, 12, e0185809. [Google Scholar] [CrossRef]
- Cardoso, P.; Barton, P.S.; Birkhofer, K.; Chichorro, F.; Deacon, C.; Fartmann, T.; Fukushima, C.S.; Gaigher, R.; Habel, J.C.; Hallmann, C.A. Scientists’ warning to humanity on insect extinctions. Biol. Conserv. 2020, 242, 108426. [Google Scholar] [CrossRef]
- Wilson, R.J.; Fox, R. Insect responses to global change offer signposts for biodiversity and conservation. Ecol. Entomol. 2021, 46, 699–717. [Google Scholar] [CrossRef]
- Antão, L.H.; Pöyry, J.; Leinonen, R.; Roslin, T. Contrasting latitudinal patterns in diversity and stability in a high-latitude species-rich moth community. Glob. Ecol. Biogeogr. 2020, 29, 896–907. [Google Scholar] [CrossRef]
- Sánchez-Bayo, F.; Wyckhuys, K.A.G. Worldwide decline of the entomofauna: A review of its drivers. Biol. Conserv. 2019, 232, 8–27. [Google Scholar] [CrossRef]
- Betzholtz, P.-E.; Forsman, A.; Franzén, M. Associations of 16-year population dynamics in range-expanding moths with temperature and years since establishment. Insects 2023, 14, 55. [Google Scholar] [CrossRef]
- Betzholtz, P.-E.; Pettersson, L.B.; Ryrholm, N.; Franzén, M. With that diet, you will go far: Trait-based analysis reveals a link between rapid range expansion and a nitrogen-favoured diet. Proc. R. Soc. B Biol. Sci. 2013, 280, 1–6. [Google Scholar] [CrossRef]
- Forsman, A.; Betzholtz, P.-E.; Franzén, M. Faster poleward range shifts in moths with more variable colour patterns. Sci. Rep. 2016, 6, 36265. [Google Scholar] [CrossRef] [PubMed]
- Fox, R.; Oliver, T.H.; Harrower, C.; Parsons, M.S.; Thomas, C.D.; Roy, D.B. Long-term changes to the frequency of occurrence of British moths are consistent with opposing and synergistic effects of climate and land-use changes. J. Appl. Ecol. 2014, 51, 949–957. [Google Scholar] [CrossRef] [PubMed]
- Boyes, D.H.; Fox, R.; Shortall, C.R.; Whittaker, R. Bucking the trend: The diversity of Anthropocene ‘winners’ among British moths. Front. Biogeogr. 2019, 11, e43862. [Google Scholar] [CrossRef]
- Inouye, D.W. Climate change and phenology. Wiley Interdiscip. Rev. Clim. Chang. 2022, 13, e764. [Google Scholar] [CrossRef]
- Merckx, T.; Nielsen, M.E.; Heliölä, J.; Kuussaari, M.; Pettersson, L.B.; Pöyry, J.; Tiainen, J.; Gotthard, K.; Kivelä, S.M. Urbanization extends flight phenology and leads to local adaptation of seasonal plasticity in Lepidoptera. Proc. Natl. Acad. Sci. USA 2021, 118, e2106006118. [Google Scholar] [CrossRef] [PubMed]
- Parmesan, C. Ecological and Evolutionary Responses to Recent Climate Change. Annu. Rev. Ecol. Evol. Syst. 2006, 37, 637–669. [Google Scholar] [CrossRef]
- Ettinger, A.; Buonaiuto, D.; Chamberlain, C.; Morales-Castilla, I.; Wolkovich, E. Spatial and temporal shifts in photoperiod with climate change. New Phytol. 2021, 230, 462–474. [Google Scholar] [CrossRef]
- Forsman, A.; Polic, D.; Sunde, J.; Betzholtz, P.-E.; Franzén, M. Variable colour patterns indicate multidimensional, intraspecific trait variation and ecological generalization in moths. Ecography 2020, 43, 1–11. [Google Scholar] [CrossRef]
- Pocock, M.J.; Chandler, M.; Bonney, R.; Thornhill, I.; Albin, A.; August, T.; Bachman, S.; Brown, P.M.; Cunha, D.G.F.; Grez, A. A vision for global biodiversity monitoring with citizen science. In Advances in Ecological Research; Elsevier: Amsterdam, The Netherlands, 2018; Volume 59, pp. 169–223. [Google Scholar]
- Macgregor, C.J.; Williams, J.H.; Bell, J.R.; Thomas, C.D. Moth biomass increases and decreases over 50 years in Britain. Nat. Ecol. Evol. 2019, 3, 1645–1649. [Google Scholar] [CrossRef]
- Pöyry, J.; Leinonen, R.; Söderman, G.; Nieminen, M.; Heikkinen, R.K.; Carter, T.R. Climate-induced increase of moth multivoltinism in boreal regions. Glob. Ecol. Biogeogr. 2011, 20, 289–298. [Google Scholar] [CrossRef]
- Hällfors, M.H.; Heikkinen, R.K.; Kuussaari, M.; Lehikoinen, A.; Luoto, M.; Pöyry, J.; Virkkala, R.; Saastamoinen, M.; Kujala, H. Recent range shifts of moths, butterflies, and birds are driven by the breadth of their climatic niche. Evol. Lett. 2023, qrad004. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical, R version 4.3.0; R Foundation for Statistical Computing: Vienna, Austria, 2023.
- Fox, J.; Weisberg, S.; Price, B.; Adler, D.; Bates, D.; Baud-Bovy, G.; Bolker, B. car: Companion to Applied Regression. R Package Version 3.0-2. Available online: https://cran.r-project.org/web/packages/car/index.html (accessed on 1 June 2019).
- Bolker, B. Getting Started with the ’glmmTMB’ Package. Vers. 1.1.8 2023. Available online: https://cran.uni-muenster.de/web/packages/glmmTMB/glmmTMB.pdf (accessed on 1 June 2019).
- Palmer, G.; Platts, P.J.; Brereton, T.; Chapman, J.W.; Dytham, C.; Fox, R.; Pearce-Higgins, J.W.; Roy, D.B.; Hill, J.K.; Thomas, C.D. Climate change, climatic variation and extreme biological responses. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20160144. [Google Scholar] [CrossRef] [PubMed]
- Harvey, J.A.; Tougeron, K.; Gols, R.; Heinen, R.; Abarca, M.; Abram, P.K.; Basset, Y.; Berg, M.; Boggs, C.; Brodeur, J.; et al. Scientists’ warning on climate change and insects. Ecol. Monogr. 2023, 93, e1553. [Google Scholar] [CrossRef]
- Franzén, M.; Francioli, Y.; Sjöberg, G.; Forsman, A. Positive shifts in species richness and abundance of moths over five decades coincide with community-wide phenotypic trait homogenisation. J. Insect Conserv. 2023, 27, 323–333. [Google Scholar] [CrossRef]
- Hampe, A.; Jump, A.S. Climate relicts: Past, present, future. Annu. Rev. Ecol. Evol. Syst. 2011, 42, 313–333. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Masson-Delmotte, V., Zhai, P., Pirani, A., Connors, S.L., Péan, C., Berger, S., Caud, N., Chen, Y., Goldfarb, L., Gomis, M.I., et al., Eds.; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
- Peters, W.; Bastos, A.; Ciais, P.; Vermeulen, A. A historical, geographical and ecological perspective on the 2018 European summer drought. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2020, 375, 20190505. [Google Scholar] [CrossRef] [PubMed]
- Lande, R.; Engen, S.; Saether, B.-E. Stochastic Population Dynamics in Ecology and Conservation; Oxford University Press: New York, NY, USA, 2003. [Google Scholar]
- Miller, T.E.; Angert, A.L.; Brown, C.D.; Lee-Yaw, J.A.; Lewis, M.; Lutscher, F.; Marculis, N.G.; Melbourne, B.A.; Shaw, A.K.; Szűcs, M. Eco-evolutionary dynamics of range expansion. Ecology 2020, 101, e03139. [Google Scholar] [CrossRef] [PubMed]
- Hunter, A.F.; Dwyer, G. Outbreaks and interacting factors: Insect population explosions synthesized and dissected. Integr. Biol. Issues News Rev. Publ. Assoc. Soc. Integr. Comp. Biol. 1998, 1, 166–177. [Google Scholar] [CrossRef]
- Morin, X.; Thuiller, W. Comparing niche-and process-based models to reduce prediction uncertainty in species range shifts under climate change. Ecology 2009, 90, 1301–1313. [Google Scholar] [CrossRef]
- Phillips, B.L.; Brown, G.P.; Shine, R. Life-history evolution in range-shifting populations. Ecology 2010, 91, 1617–1627. [Google Scholar] [CrossRef]
- Parmesan, C.; Ryrholm, N.; Stefanescu, C.; Hill, J.K.; Thomas, C.D.; Descimon, H.; Huntley, B.; Kaitila, L.; Kullberg, J.; Tammaru, T.; et al. Poleward shifts in geographical ranges of butterfly species associated with regional warming. Nature 1999, 399, 579–584. [Google Scholar] [CrossRef]
- Betzholtz, P.E.; Franzén, M. Mobility is related to species traits in noctuid moths. Ecol. Entomol. 2011, 136, 369–376. [Google Scholar] [CrossRef]
- Franzén, M.; Betzholtz, P.-E.; Pettersson, L.B.; Forsman, A.J. Urban moth communities suggest that life in the city favours thermophilic multi-dimensional generalists. Proc. R. Soc. B Biol. Sci. 2020, 287, 20193014. [Google Scholar] [CrossRef] [PubMed]
- Tylianakis, J.M.; Didham, R.K.; Bascompte, J.; Wardle, D.A. Global change and species interactions in terrestrial ecosystems. Ecol. Lett. 2008, 11, 1351–1363. [Google Scholar] [CrossRef] [PubMed]
- van Dyck, H.; van Strien, A.J.; Maes, D.; van Swaay, C.A.M. Declines in common, widespread butterflies in a landscape under intense human use. Conserv. Biol. 2009, 23, 957–965. [Google Scholar] [CrossRef] [PubMed]
- Gallien, L.; Münkemüller, T.; Albert, C.H.; Boulangeat, I.; Thuiller, W. Predicting potential distributions of invasive species: Where to go from here? Divers. Distrib. 2010, 16, 331–342. [Google Scholar] [CrossRef]
- Mooney, H.A.; Cleland, E.E. The evolutionary impact of invasive species. Proc. Natl. Acad. Sci. USA 2001, 98, 5446–5451. [Google Scholar] [CrossRef]
- Zehnder, G.; Gurr, G.M.; Kühne, S.; Wade, M.R.; Wratten, S.D.; Wyss, E. Arthropod pest management in organic crops. Annu. Rev. Entomol. 2007, 52, 57–80. [Google Scholar] [CrossRef]
- Hufnagel, L.; Kocsis, M. Impacts of climate change on Lepidoptera species and communities. Appl. Ecol. Environ. Res. 2011, 9, 43–72. [Google Scholar]
- Polis, G.A.; Anderson, W.B.; Holt, R.D. Toward an integration of landscape and food web ecology: The dynamics of spatially subsidized food webs. Annu. Rev. Ecol. Syst. 1997, 28, 289–316. [Google Scholar] [CrossRef]
- Altermatt, F. Climatic warming increases voltinism in European butterflies and moths. Proc.—R. Soc. Biol. Sci. 2010, 277, 1281–1287. [Google Scholar] [CrossRef] [PubMed]
- Teder, T. Phenological responses to climate warming in temperate moths and butterflies: Species traits predict future changes in voltinism. Oikos 2020, 129, 1051–1060. [Google Scholar] [CrossRef]
- Werner, E.E.; Peacor, S.D. A review of trait-mediated indirect interactions in ecological communities. Ecology 2003, 84, 1083–1100. [Google Scholar] [CrossRef]
- Russo, D.; Bosso, L.; Ancillotto, L. Novel perspectives on bat insectivory highlight the value of this ecosystem service in farmland: Research frontiers and management implications. Agric. Ecosyst. Environ. 2018, 266, 31–38. [Google Scholar] [CrossRef]
- Barbaro, L.; Dulaurent, A.-M.; Payet, K.; Blache, S.; Vetillard, F.; Battisti, A. Winter bird numerical responses to a key defoliator in mountain pine forests. For. Ecol. Manag. 2013, 296, 90–97. [Google Scholar] [CrossRef]
- Van Noordwijk, A.; McCleery, R.; Perrins, C. Selection for the timing of great tit breeding in relation to caterpillar growth and temperature. J. Anim. Ecol. 1995, 64, 451–458. [Google Scholar] [CrossRef]
- Power, M.E. Top-down and bottom-up forces in food webs: Do plants have primacy. Ecology 1992, 73, 733–746. [Google Scholar] [CrossRef]
- Menzel, A.; Fabian, P. Growing season extended in Europe. Nature 1999, 397, 659. [Google Scholar] [CrossRef]
- Hällfors, M.H.; Antão, L.H.; Itter, M.; Lehikoinen, A.; Lindholm, T.; Roslin, T.; Saastamoinen, M. Shifts in timing and duration of breeding for 73 boreal bird species over four decades. Proc. Natl. Acad. Sci. USA 2020, 117, 18557–18565. [Google Scholar] [CrossRef]
- Parmesan, C.; Hanley, M.E. Plants and climate change: Complexities and surprises. Ann. Bot. 2015, 116, 849–864. [Google Scholar] [CrossRef]
- Gaston, K.J. Geographic range limits: Achieving synthesis. Proc. R. Soc. B Biol. Sci. 2009, 276, 1395–1406. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.H. On the relationship between abundance and distribution of species. Am. Nat. 1984, 124, 255–279. [Google Scholar] [CrossRef]
- Sporbert, M.; Keil, P.; Seidler, G.; Bruelheide, H.; Jandt, U.; Aćić, S.; Biurrun, I.; Campos, J.A.; Čarni, A.; Chytrý, M. Testing macroecological abundance patterns: The relationship between local abundance and range size, range position and climatic suitability among European vascular plants. J. Biogeogr. 2020, 47, 2210–2222. [Google Scholar] [CrossRef]
- Bock, C.E.; Ricklefs, R.E. Range size and local abundance of some North American songbirds: A positive correlation. Am. Nat. 1983, 122, 295–299. [Google Scholar] [CrossRef]
- Betzholtz, P.-E.; Forsman, A.; Franzén, M. Inter-individual variation in colour patterns in noctuid moths characterizes long-distance dispersers and agricultural pests. J. Appl. Entomol. 2019, 143, 992–999. [Google Scholar] [CrossRef]
- Harrison, P.A.; Harmáčková, Z.V.; Karabulut, A.A.; Brotons, L.; Cantele, M.; Claudet, J.; Dunford, R.W.; Guisan, A.; Holman, I.P.; Jacobs, S. Synthesizing plausible futures for biodiversity and ecosystem services in Europe and Central Asia using scenario archetypes. Ecol. Soc. 2019, 24, 27. [Google Scholar] [CrossRef]
- Gonthier, D.J.; Ennis, K.K.; Farinas, S.; Hsieh, H.-Y.; Iverson, A.L.; Batáry, P.; Rudolphi, J.; Tscharntke, T.; Cardinale, B.J.; Perfecto, I. Biodiversity conservation in agriculture requires a multi-scale approach. Proc. R. Soc. B Biol. Sci. 2014, 281, 20141358. [Google Scholar] [CrossRef] [PubMed]
- Theobald, E.J.; Ettinger, A.K.; Burgess, H.K.; DeBey, L.B.; Schmidt, N.R.; Froehlich, H.E.; Wagner, C.; HilleRisLambers, J.; Tewksbury, J.; Harsch, M.A. Global change and local solutions: Tapping the unrealized potential of citizen science for biodiversity research. Biol. Conserv. 2015, 181, 236–244. [Google Scholar] [CrossRef]
- Hecker, S.; Bonney, R.; Haklay, M.; Hölker, F.; Hofer, H.; Goebel, C.; Gold, M.; Makuch, Z.; Ponti, M.; Richter, A. Innovation in citizen science–perspectives on science-policy advances. Citiz. Sci. Theory Pract. 2018, 3, 4. [Google Scholar] [CrossRef]
- Liu, H.-Y.; Dörler, D.; Heigl, F.; Grossberndt, S. Citizen science platforms. Sci. Citiz. Sci. 2021, 22, 439–459. [Google Scholar]
| Variable | Estimate | Std. Error | t-Value | p-Value |
|---|---|---|---|---|
| (Intercept) | 1.26668 | 0.16889 | 7.500 | <0.0001 |
| Year | 0.13986 | 0.01409 | 9.926 | <0.0001 |
| Species (M. albipuncta) | −0.27418 | 0.08134 | −3.371 | <0.0001 |
| Number of records | 0.23756 | 0.05280 | 4.499 | <0.0001 |
| Variable | Estimate | Std. Error | Z-Value | p-Value |
|---|---|---|---|---|
| Intercept | 4.00921 | 0.45907 | 8.73 | <0.0001 |
| Year (scaled) | 1.95315 | 0.01457 | 134.03 | <0.0001 |
| Species (M. albipuncta) | −3.61106 | 0.06546 | −55.17 | <0.0001 |
| Year * Species | 2.15215 | 0.04441 | 48.47 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Betzholtz, P.-E.; Forsman, A.; Franzén, M. Increased Abundance Coincides with Range Expansions and Phenology Shifts: A Long-Term Case Study of Two Noctuid Moths in Sweden. Diversity 2023, 15, 1177. https://doi.org/10.3390/d15121177
Betzholtz P-E, Forsman A, Franzén M. Increased Abundance Coincides with Range Expansions and Phenology Shifts: A Long-Term Case Study of Two Noctuid Moths in Sweden. Diversity. 2023; 15(12):1177. https://doi.org/10.3390/d15121177
Chicago/Turabian StyleBetzholtz, Per-Eric, Anders Forsman, and Markus Franzén. 2023. "Increased Abundance Coincides with Range Expansions and Phenology Shifts: A Long-Term Case Study of Two Noctuid Moths in Sweden" Diversity 15, no. 12: 1177. https://doi.org/10.3390/d15121177
APA StyleBetzholtz, P.-E., Forsman, A., & Franzén, M. (2023). Increased Abundance Coincides with Range Expansions and Phenology Shifts: A Long-Term Case Study of Two Noctuid Moths in Sweden. Diversity, 15(12), 1177. https://doi.org/10.3390/d15121177







