Effects of Fire on Pyrodiversity of Terricolous Non-Tracheophytes Photoautotrophs in a Páramo of Southern Ecuador
Abstract
1. Introduction
2. Materials and Methods
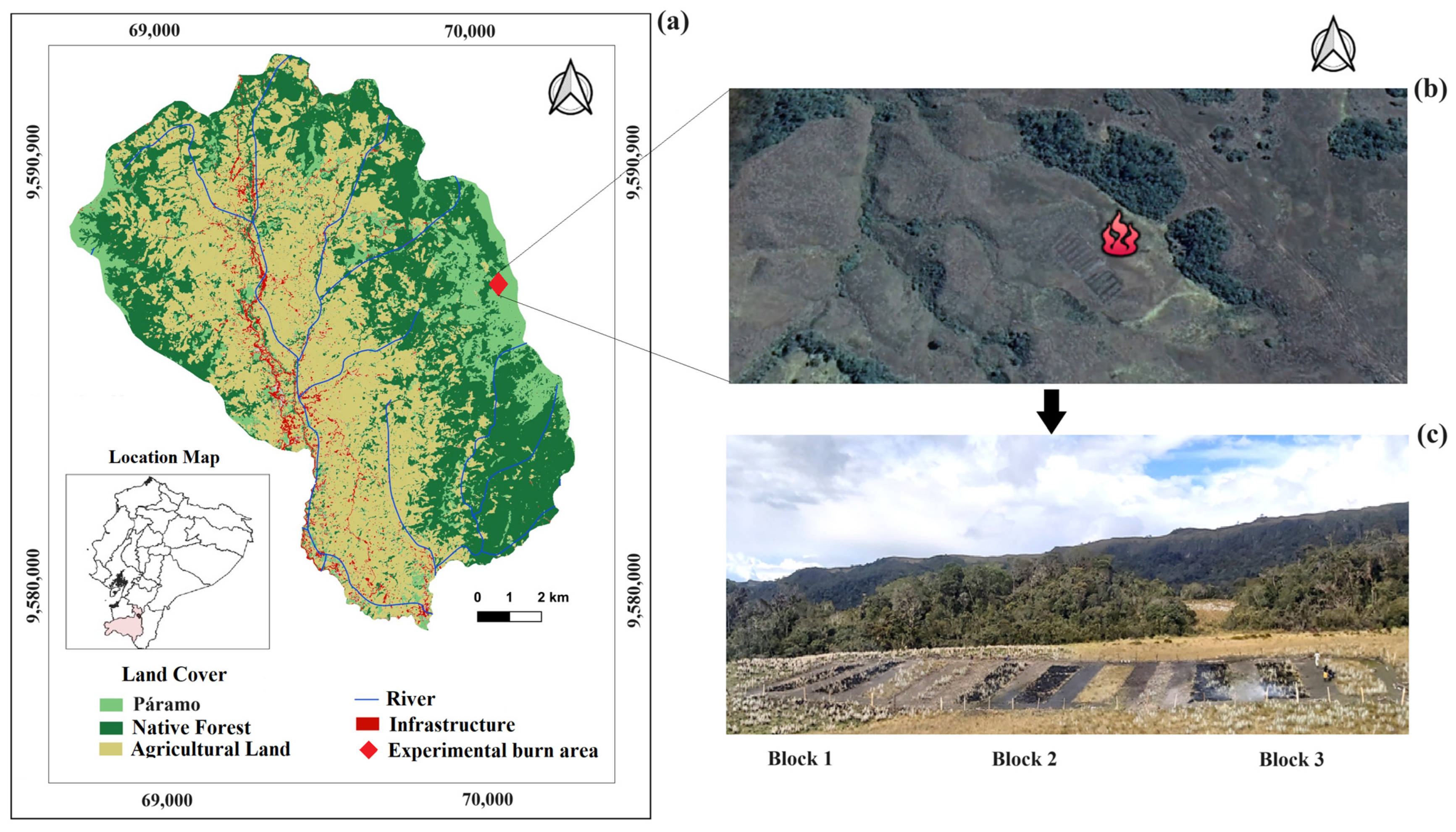
2.1. Study Area
2.2. Design and Data Collection
2.2.1. Experimental Burns and Determination of Fire Severity
2.2.2. Sampling of Terricolous Non-tracheophyte Photoautotrophs
2.2.3. Statistical Analysis
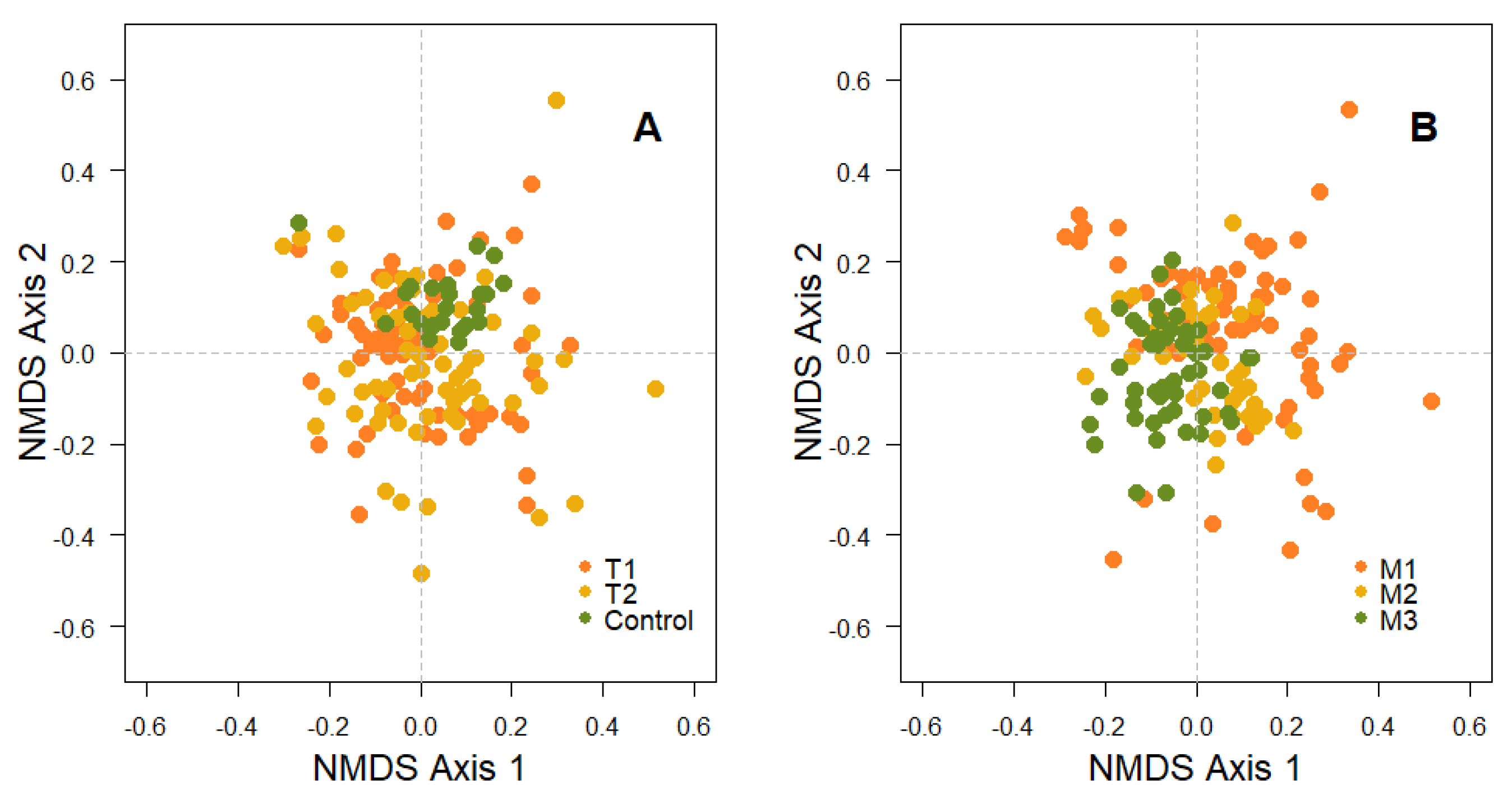
3. Results
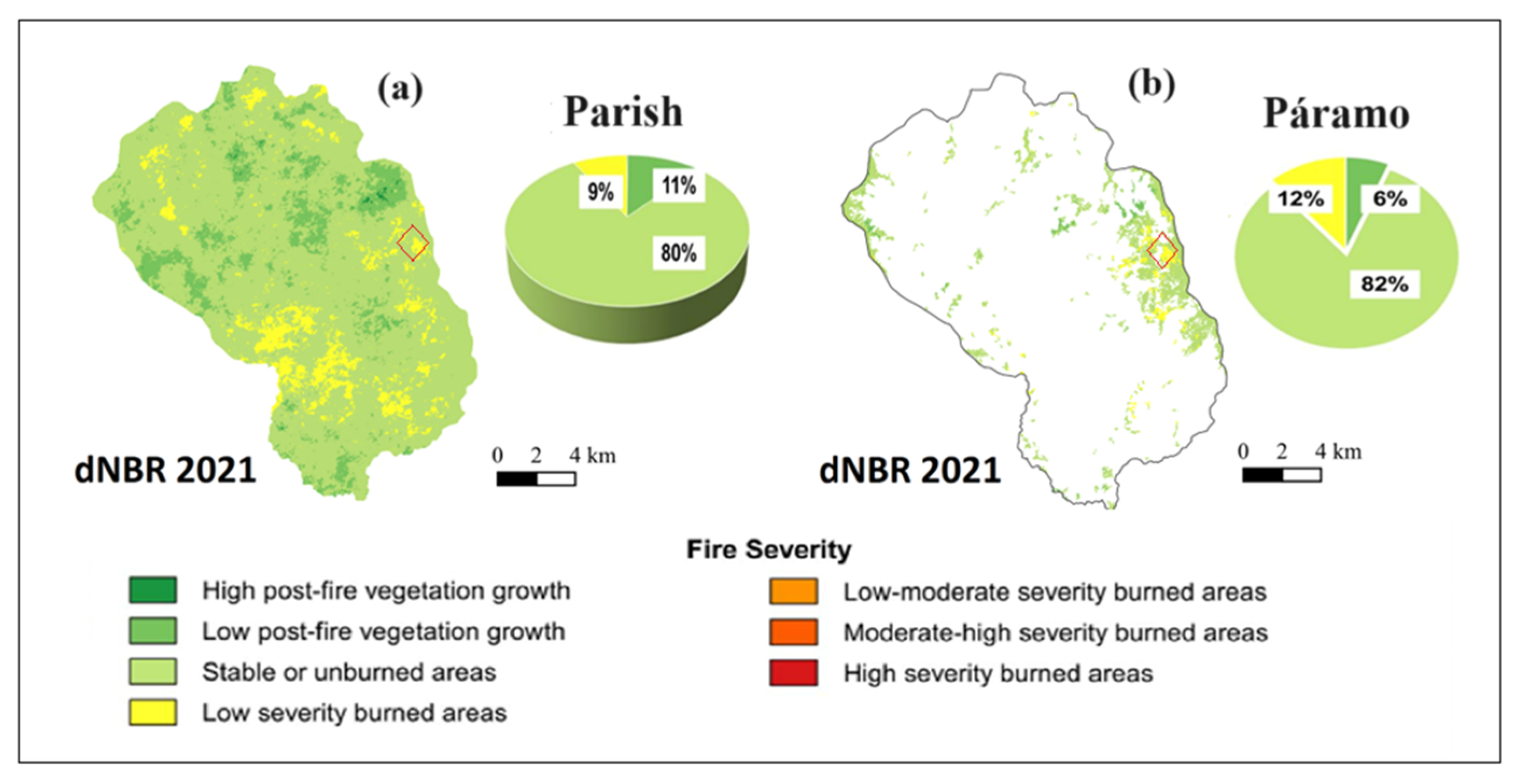
3.1. Fire Severity of Experimental Burns
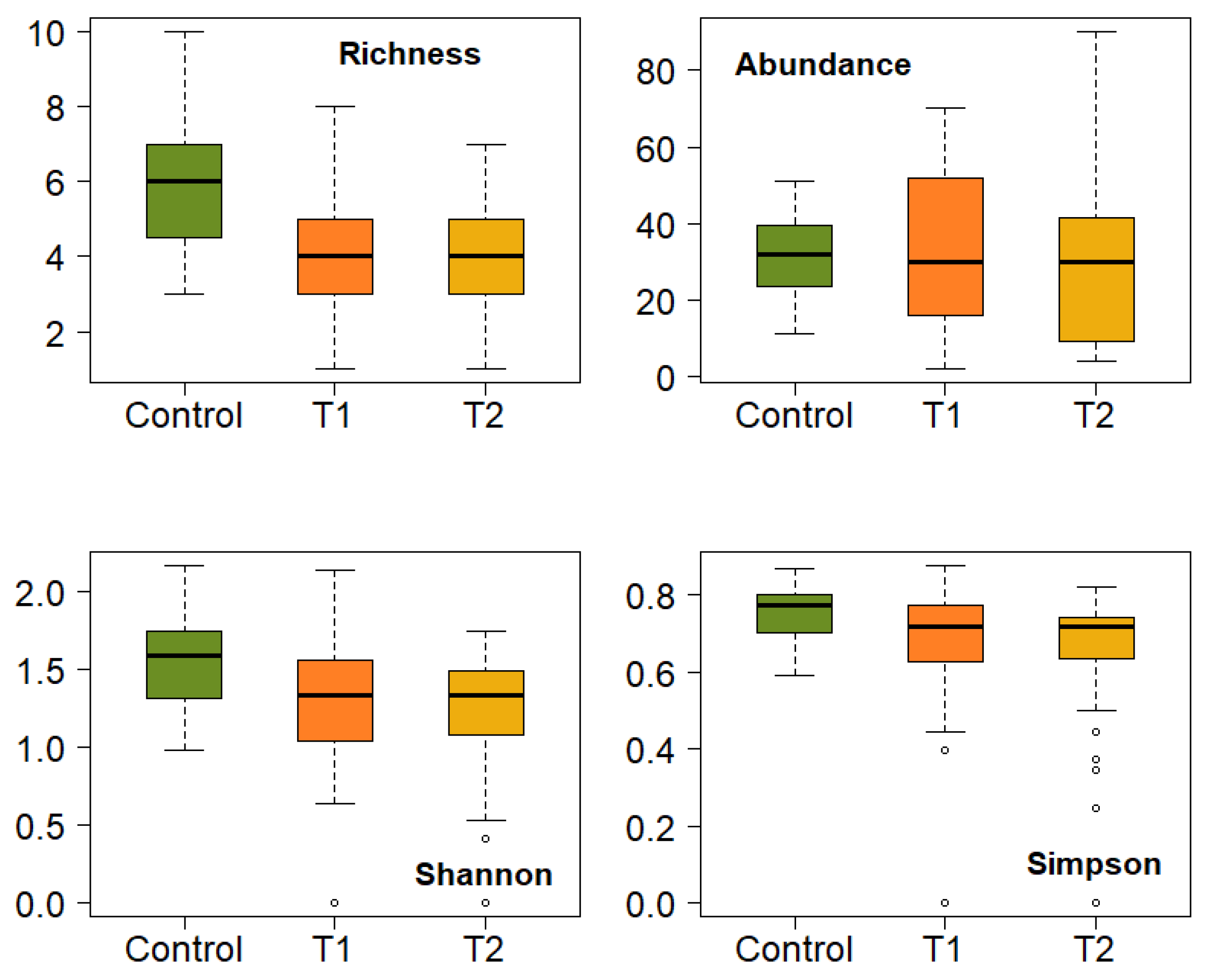
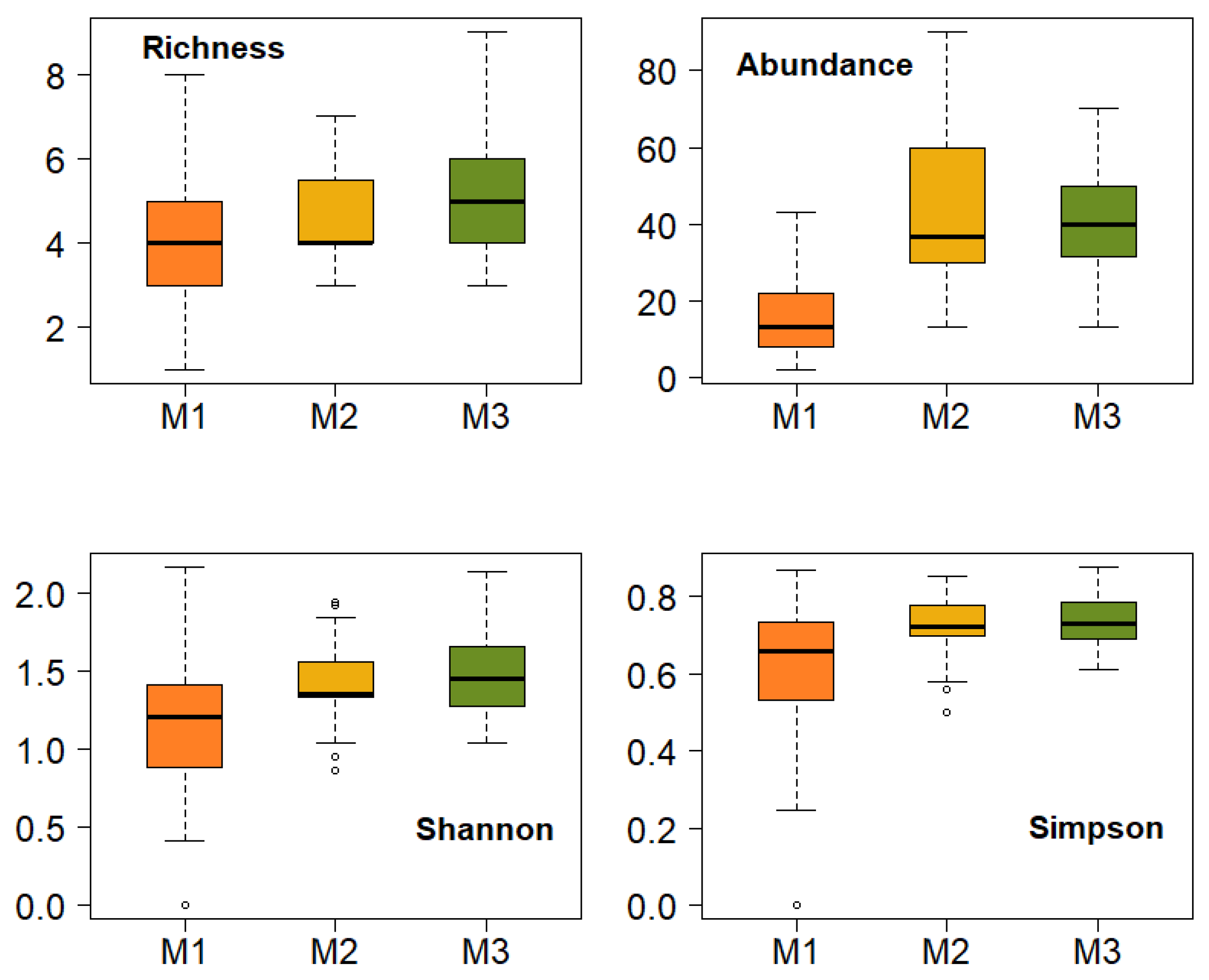
3.2. Effect of Fire on the Diversity of Bryophytes and Lichens
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rada, F.; Azócar, A.; García-Núñez, C. Plant functional diversity in tropical Andean páramos. Plant Ecol. Divers. 2019, 12, 539–553. [Google Scholar] [CrossRef]
- González, Y.; Aragón, G.; Benítez, A.; Prieto, M. Changes in soil cryptogamic communities in tropical Ecuadorean páramos. Community Ecol. 2017, 18, 11–20. [Google Scholar] [CrossRef]
- Morocho, C.C.; Chuncho, G. Páramos of Ecuador, importance and affectations: A review. Bosques Latid. Cero 2019, 9, 71–83. [Google Scholar]
- Buytaert, W.; Célleri, R.; De Bièvre, B.; Cisneros, F.; Wyseure, G.; Deckers, J.; Hofstede, R. Human impact on the hydrology of the Andean páramos. Earth Sci. Rev. 2006, 79, 53–72. [Google Scholar] [CrossRef]
- Espinosa, J.; Rivera, D. Variations in water resources availability at the Ecuadorian páramo due to land-use changes. Environ. Earth Sci. 2016, 75, 1173. [Google Scholar] [CrossRef]
- Keating, P.L. Effects of anthropogenic disturbances on páramo vegetation in Podocarpus National Park, Ecuador. Phys. Geogr. 1998, 19, 221–238. [Google Scholar] [CrossRef]
- Keating, P.L. Chronically disturbed páramo vegetation at a site in southern Ecuador. J. Torrey Bot. Soc. 2000, 127, 162–171. [Google Scholar] [CrossRef]
- Keating, P.L. Fire ecology and conservation in the high tropical Andes: Observations from northern Ecuador. J. Lat. Am. Geogr. 2007, 6, 43–62. [Google Scholar] [CrossRef]
- Vásquez, D.L.A.; Balslev, H.; Sklenář, P. Human impact on tropical-alpine plant diversity in the northern Andes. Biodivers. Conserv. 2015, 24, 2673–2683. [Google Scholar] [CrossRef]
- Augustine, D.J.; Brewer, P.; Blumenthal, D.M.; Derner, J.D.; von Fischer, J.C. Prescribed fire, soil inorganic nitrogen dynamics, and plant responses in a semiarid grassland. J. Arid Environ. 2014, 104, 59–66. [Google Scholar] [CrossRef]
- Díaz, S.C.; Quezada, L.C.; Álvarez, L.J.; Loján-Córdova, J.; Carrión-Paladines, V. Indigenous use of fire in the paramo ecosystem of southern Ecuador: A case study using remote sensing methods and ancestral knowledge of the Kichwa Saraguro people. Fire Ecol. 2023, 19, 5. [Google Scholar] [CrossRef]
- Barreal, J.; Loureiro, M.; Picos, J. Estudio de la causalidad de los incendios forestales en Galicia Study of the causality of forest fires in Galicia. Econ. Agraria Recur. Nat. 2012, 12, 99–114. [Google Scholar]
- Pausas, J.G.; Ribeiro, E. Fire and plant diversity at the global scale. Glob. Ecol. Biogeogr. 2017, 26, 889–897. [Google Scholar] [CrossRef]
- He, T.; Lamont, B.B.; Pausas, J.G. Fire as a key driver of Earth’s biodiversity. Biol. Rev. 2019, 94, 1983–2010. [Google Scholar] [CrossRef] [PubMed]
- McLauchlan, K.K.; Higuera, P.E.; Miesel, J.; Rogers, B.M.; Schweitzer, J.; Shuman, J.K.; Tepley, A.J.; Varner, J.M.; Veblen, T.T.; Adalsteinsson, S.A.; et al. Fire as a fundamental ecological process: Research advances and frontiers. J. Ecol. 2020, 108, 2047–2069. [Google Scholar] [CrossRef]
- Jones, G.M.; Tingley, M.W. Pyrodiversity and biodiversity: A history, synthesis, and outlook. Divers. Distrib. 2022, 28, 386–403. [Google Scholar] [CrossRef]
- Porada, P.; Bader, M.Y.; Berdugo, M.B.; Colesie, C.; Ellis, C.J.; Giordani, P.; Herzschuh, U.; Ma, Y.; Launiainen, S.; Nascimbene, J.; et al. A research agenda for non-vascular photoautotrophs under climate change. New Phytol. 2022, 237, 1495–1504. [Google Scholar] [CrossRef] [PubMed]
- Pérez, F.L. Microbiotic crusts in the high equatorial Andes, and their influence on paramo soils. Catena 1997, 31, 173–198. [Google Scholar] [CrossRef]
- Gradstein, S.R.; Holz, I. Diversity of Bryophytes in the Paramos of Costa Rica. Páramos de Costa Rica; INBio: Santo Domingo, Costa Rica, 2005; pp. 361–374. [Google Scholar]
- Benítez, Á.; Gradstein, S.R.; Cevallos, P.; Medina, J.; Aguirre, N. Terrestrial bryophyte communities related to climatic and topographic factors in a páramo of southern Ecuador. Caldasia 2019, 41, 370–379. [Google Scholar] [CrossRef]
- Belnap, J.; Phillips, S.L.; Troxler, T. Soil lichen and moss cover and species richness can be highly dynamic: The effects of invasion by the annual exotic grass Bromus tectorum, precipitation, and temperature on biological soil crusts in SE Utah. Appl. Soil Ecol. 2006, 32, 63–76. [Google Scholar] [CrossRef]
- Li, X.R.; Song, G.; Hui, R.; Wang, Z.R. Precipitation and topsoil attributes determine the species diversity and distribution patterns of crustal communities in desert ecosystems. Plant Soil 2017, 420, 163–175. [Google Scholar] [CrossRef]
- Gradstein, S.R.; Churchill, S.P.; Salazar-Allen, N. Guide to the bryophytes of tropical America. Mem. N. Y. Bot. Gard. 2001, 86, 1–577. [Google Scholar]
- Johansen, J.R.; Clair, L.L.S.; Webb, B.L.; Nebeker, G.T. Recovery patterns of cryptogamic soil crusts in desert rangelands following fire disturbance. Bryologist 1984, 87, 238–243. [Google Scholar] [CrossRef]
- Salinas, P.; Mazon, M.; Carrion-Paladines, V.; Cumbicus, N.; Guzman, P.; Giordani, P.; Benítez, A. Influence of soil and elevation on roadside cryptogam diversity in the tropical Andes. Forest Ecosys. 2022, 9, 100061. [Google Scholar] [CrossRef]
- Calabria, L.M.; Petersen, K.; Hamman, S.T.; Smith, R.J. Prescribed Fire Decreases Lichen and Bryophyte Biomass and Alters Functional Group Composition in Pacific Northwest Prairies. Northwest Sci. 2016, 90, 470–483. [Google Scholar] [CrossRef]
- Wienskoski, M.B.; Santos, N.D.D. Post-fire effects on bryophytes in High-Altitude Fields. Acta Bot. Bras. 2022, 36, e021abb0250. [Google Scholar] [CrossRef]
- Fernandes, P.M.; Davies, G.M.; Ascoli, D.; Fernández, C.; Moreira, F.; Rigolot, E.; Stoof, C.R.; Vega, J.A.; Molina, D. Prescribed burning in southern Europe: Developing fire management in a dynamic landscape. Front. Ecol. Environ. 2013, 11, E1–E14. [Google Scholar] [CrossRef]
- Alcañiz, M.; Outeiro, L.; Francos, M.; Úbeda, X. Effects of Prescribed Fires on Soil Properties: A Review. Sci. Total Environ. 2018, 613, 944–957. [Google Scholar] [CrossRef]
- Brockway, D.G.; Lewis, C.E. Long-Term Effects of Dormant-Season Prescribed Fire on Plant Community Diversity, Structure and Productivity in a Longleaf Pine Wiregrass Ecosystem. For. Ecol. Manag. 1997, 96, 167–183. [Google Scholar] [CrossRef]
- Kral, K.; Limb, R.; Ganguli, A.; Hovick, T.; Sedivec, K. Seasonal prescribed fire variation decreases inhibitory ability of Poa pratensis L. and promotes native plant diversity. J. Environ. Manag. 2018, 223, 908–916. [Google Scholar] [CrossRef]
- Barefoot, C.R.; Willson, K.G.; Hart, J.L.; Schweitzer, C.J.; Dey, D.C. Effects of thinning and prescribed fire frequency on ground flora in mixed pinus-hardwood stands. For. Ecol. Manag. 2019, 432, 729–740. [Google Scholar] [CrossRef]
- Durigan, G.; Pilon, N.A.; Abreu, R.C.; Hoffmann, W.A.; Martins, M.; Fiorillo, B.F.; Antunes, A.Z.; Carmignotto, A.P.; Maravalhas, J.B.; Vieira, J. No Net Loss of Species Diversity after Prescribed Fires in the Brazilian Savanna. Front. For. Glob. Chang. 2020, 3, 13. [Google Scholar] [CrossRef]
- Sonnier, G.; Boughton, E.H.; Whittington, R. Long-term Response of Wetland Plant Communities to Management Intensity, Grazing Abandonment, and Prescribed Fire. Ecol. Appl. 2023, 33, e2732. [Google Scholar] [CrossRef] [PubMed]
- Úbeda, X.; Lorca, M.; Outeiro, L.R.; Bernia, S.; Castellnou, M. The effects of prescribed fire on soil quality (Prades Mountains, North East Spain). Int. J. Wildland Fire 2005, 14, 379–384. [Google Scholar] [CrossRef]
- Fonseca, F.; de Figueiredo, T.; Nogueira, C.; Queirós, A. Effect of prescribed fire on soil properties and soil erosion in a Mediterranean mountain area. Geoderma 2017, 307, 172–180. [Google Scholar] [CrossRef]
- Williamson, G.B.; Schatz, G.E.; Alvarado-Hernandez, A.; Redhead, C.S.; Stam, A.C.; Sterner, R.W. Effects of repeated fires on tropical paramo vegetation. Effects of repeated fires on tropical paramo vegetation. Trop. Ecol. 1986, 27, 62–69. [Google Scholar]
- Ramsay, P.M.; Oxley, E.R.B. Fire temperatures and postfire plant community dynamics in Ecuadorian grass páramo. Vegetatio 1996, 124, 129–144. [Google Scholar] [CrossRef]
- Suárez, E.; Medina, G. Vegetation structure and soil properties in Ecuadorian páramo grasslands with different histories of burning and grazing. Arct. Antarct. Alp. Res. 2001, 33, 158–164. [Google Scholar]
- Sklenár, P.; Ramsay, P.M. Diversity of zonal páramo plant communities in Ecuador. Divers. Distrib. 2001, 7, 113–124. [Google Scholar] [CrossRef]
- Ramsay, P.M. Giant rosette plant morphology as an indicator of recent fire history in Andean paramo grasslands. Ecol. Indic. 2014, 45, 37–44. [Google Scholar] [CrossRef]
- Ray, D.G.; Barton, J.W.; Lendemer, J.C. Lichen community response to prescribed burning and thinning in southern pine forests of the Mid-Atlantic coastal plain, USA. Fire Ecol. 2015, 11, 14–33. [Google Scholar] [CrossRef]
- Greene, R.S.B.; Chartres, C.J.; Hodgkinson, K.C. The effects of fire on the soil in a degraded semiarid woodland. I. Cryptogam cover and physical and micromorphological properties. Soil Res. 1990, 28, 755–777. [Google Scholar] [CrossRef]
- Perazzo, A.; Rodríguez, J. Impact of fire on soil non-vascular vegetation: A case study in Polylepis australis (Rosaceae) forests of central Argentina. Lilloa 2019, 56, 67–80. [Google Scholar] [CrossRef][Green Version]
- Hueso-González, P.; Martínez-Murillo, J.F.; Ruiz-Sinoga, J.D. Prescribed fire impacts on soil properties, overland flow and sediment transport in a Mediterranean forest: A 5 year study. Sci. Total Environ. 2018, 636, 1480–1489. [Google Scholar] [CrossRef] [PubMed]
- Manals-Cutiño, E.M.; Penedo-Medina, M.; Salas-Tort, D. Caracterización del bagazo de caña como biomasa vegetal. Tecnol. Química. 2015, 35, 244–255. [Google Scholar]
- Geron, C.; Hays, M. Air emissions from organic soil burning on the coastal plain of North Carolina. Atmos. Environ. 2013, 64, 192–199. [Google Scholar] [CrossRef]
- National Wildfire Coordinating Group. Interagency Fire Use Module Field Guide. NWCG Guide for Wildland Fire Modules, PMS 431, Boise, Idaho, USA. 2014. Available online: https://training.nwcg.gov/pre-courses/s490/s490pcw/Interagency%20Fire%20Use%20Module.pdf (accessed on 1 January 2019).
- Bodí, M.; Martin, D.A.; Santín, C.; Balfour, V.; Doerr, S.H.; Pereira, P.; Cerdà, A.; MataixSolera, J. Wildland fire ash: Production, composition, and eco-hydro-geomorphic effects. Earth Sci. Rev. 2014, 130, 103–127. [Google Scholar] [CrossRef]
- Pereira, P.; Jordan, A.; Cerda, A.; Martin, D. Editorial: The role of ash in fire-affected ecosystems. Catena 2015, 135, 337–339. [Google Scholar] [CrossRef]
- Carrión-Paladines, V.; Hinojosa, M.B.; Álvarez, L.J.; Reyes-Bueno, F.; Quezada, L.C.; García-Ruiz, R. Effects of the Severity of Wildfires on Some Physical-Chemical Soil Properties in a Humid Montane Scrublands Ecosystem in Southern Ecuador. Fire 2022, 5, 66. [Google Scholar] [CrossRef]
- Van Dijk, D.; Shoaie, S.; van Leeuwen, T.; Veraverbeke, S. Spectral signature analysis of false positive burned area detection from agricultural harvests using Sentinel-2 data. Int. J. Appl. Earth Obs. Geoinf. 2021, 97, 102296. [Google Scholar] [CrossRef]
- Parker, B.M.; Lewis, T.; Srivastava, S.K. Estimation and evaluation of multi-decadal fire severity patterns using Landsat sensors. Remote Sens. Environ. 2015, 170, 340–349. [Google Scholar] [CrossRef]
- Dos Santos, S.M.B.; Bento-Gonçalves, A.; Franca-Rocha, W.; Baptista, G. Assessment of burned forest area severity and postfire regrowth in chapada diamantina national park (Bahia, Brazil) using dnbr and rdnbr spectral indices. Geosciences 2020, 10, 106. [Google Scholar] [CrossRef]
- Dinno, A. Package ‘Dunn.Test’. Version 1.3.5. 2017. Available online: https://cran.r-project.org/web/packages/dunn.test/dunn.test.pdf (accessed on 7 February 2021).
- Oksanen, J.; Ovaskainen, O.; de Jonge, M.M.J.; Lehikoinen, A.; Abrego, N.; Opedal, Ø.H.; Tikhonov, G. Joint species distribution modelling with the r-package Hmsc. Methods Ecol. Evol. 2019, 11, 442–447. [Google Scholar] [CrossRef]
- Keeley, J.E. Fire intensity, fire severity and burn severity: A brief review and suggested usage. Int. J. Wildland Fire 2009, 18, 116–126. [Google Scholar] [CrossRef]
- Richter, C.; Rejmanek, M.; Miller, J.E.D.; Welch, K.R.; Weeks, J.; Safford, H. The species diversity x fire severity relationship is hump-shaped in semiarid yellow pine and mixed conifer forests. Ecosphere 2019, 10, e02882. [Google Scholar] [CrossRef]
- Dzwonko, Z.; Loster, S.; Gawronski, S. Impact of fire severity on soil properties and the development of tree and shrub species in a Scots pine moist forest site in southern Poland. For. Ecol. Manag. 2015, 342, 56–63. [Google Scholar] [CrossRef]
- Johansson, P.; Reich, P. Population size and fire intensity determine post-fire abundance in grassland lichens. Appl. Veg. Sci. 2005, 8, 193–198. [Google Scholar] [CrossRef]
- Ward, B.; Cranfield, R.; Wills, A.; Tunsell, V. Influence of fire-age mosaics on macrolichens and 465 bryophytes in southwestern Australia. J. R. Soc. West. Aust. 2017, 100, 32–45. [Google Scholar]
- Hylander, K.; Frisk, C.A.; Nemomissa, S.; Johansson, M.U. Rapid post-fire re-assembly of 356 species-rich bryophyte communities in Afroalpine heathlands. J. Veg. Sci. 2021, 32, 2–11. [Google Scholar] [CrossRef]
- Orumaa, A.; Köster, K.; Tullus, A.; Tullus, T.; Metslaid, M. Forest fires have long-term effects on the composition of vascular plants and bryophytes in Scots pine forests of hemiboreal Estonia. Silva Fenn. 2022, 402, 56. [Google Scholar] [CrossRef]
- Paquette, M.; Boudreault, C.; Fenton, N.; Pothier, D.; Bergeron, Y. Bryophyte species assemblages in fire and clear-cut origin boreal forests. For. Ecol. Manag. 2016, 359, 99–108. [Google Scholar] [CrossRef]
- Ferguson, A.V.; Pharo, E.J.; Kirkpatrick, J.B.; Marsden-Smedley, J.B. The early effects of fire and grazing on bryophytes and lichens in tussock grassland and hummock sedgeland in north-eastern Tasmania. Aust. J. Bot. 2009, 57, 556–561. [Google Scholar] [CrossRef]
- Zabala, C.; Aranibar, J.; Rodriguez, D. Cryptogam communities as potential indicators of post-fire recovery in the piedmont. Ecol. Austral. 2023, 33, 108–123. [Google Scholar] [CrossRef]
- Burton, J.I.; Ares, A.; Olson, D.H.; Puettmann, K.J. Management trade-off between aboveground carbon storage and understory plant species richness in temperate forests. Ecol. Appl. 2013, 23, 1297–1310. [Google Scholar] [CrossRef] [PubMed]
- Cong, W.-F.; van Ruijven, J.; Mommer, L.; De Deyn, G.B.; Berendse, F.; Hoffland, E. Plant species richness promotes soil carbon and nitrogen stocks in grasslands without legumes. J. Ecol. 2014, 102, 1163–1170. [Google Scholar] [CrossRef]
- Roscher, C.; Karlowsky, S.; Milcu, A.; Gessler, A.; Bachmann, D.; Jesch, A.; Gleixner, G. Functional composition has stronger impact than species richness on carbon gain and allocation in experimental grasslands. PLoS ONE 2019, 14, e0204715. [Google Scholar] [CrossRef] [PubMed]
- Sizov, O.; Brodt, L.; Soromotin, A.; Prikhodko, N.; Heim, R. Fire-induced changes in soil and vegetation in the forest-tundra of Western Siberia. E3S Web Conf. 2020, 223, 3001. [Google Scholar] [CrossRef]
- Pharo, E.J.; Meagher, D.A.; Lindenmayer, D.B. Bryophyte persistence following major fire in eucalypt forest of southern Australia. For. Ecol. Manag. 2013, 296, 24–32. [Google Scholar] [CrossRef]
- Dove, N.C.; Safford, H.D.; Bohlman, G.N.; Estes, B.L.; Hart, S.C. High-severity wildfire leads to multi-decadal impacts on soil biogeochemistry in mixed-conifer forests. Ecol. Appl. 2020, 30, e02072. [Google Scholar] [CrossRef]
- Sulwiński, M.; Mętrak, M.; Wilk, M.; Suska-Malawska, M. Smouldering fire in a nutrient-limited wetland ecosystem: Long-lasting changes in water and soil chemistry facilitate shrub expansion into a drained burned fen. Sci. Total Environ. 2020, 746, 141142. [Google Scholar] [CrossRef]
- Mandl, N.A.; Kessler, M.; Robbert Gradstein, S. Effects of environmental heterogeneity on species diversity and composition of terrestrial bryophyte assemblages in tropical montane forests of southern Ecuador. Plant Ecol. Divers. 2009, 2, 313–321. [Google Scholar] [CrossRef]
- Couput, P.; Fernanda, M.; Yelitza, L.V. Bryophytes of the high Andean peatland system of Mifafí, Sierra de la Culata National Park, Venezuela. Cryptogam. Bryol. 2013, 34, 77–87. [Google Scholar] [CrossRef]


| Species | T1 | T2 | Control |
|---|---|---|---|
| Bryophytes | |||
| Anastrophyllum tubulosum (Nees) Grolle | 0 | 5 | 3 |
| Breutelia tomentosa (Brid.) A. Jaeger | 0 | 0 | 1 |
| Campylopus richardii Brid. | 22 | 25 | 6 |
| Campylopus sp. | 31 | 26 | 12 |
| Campylopus sp. 1 | 0 | 0 | 1 |
| Isotachis multiceps (Lindenb. & Gottsche) Gottsche | 3 | 7 | 1 |
| Jensenia spinosa (Lindenb. & Gottsche) Grolle | 29 | 30 | 2 |
| Kurzia capillaris (Sw.) Grolle | 21 | 33 | 3 |
| Odontoschisma longiflorum Steph. | 34 | 25 | 16 |
| Polytrichum juniperinum Hedw. | 2 | 2 | 1 |
| Riccardia regnellii (Ǻngstr.) K.G. Hell | 14 | 8 | 3 |
| Rhacocarpus purpurascens (Brid.) Paris | 0 | 0 | 5 |
| Sphagnum magellanicum Brid. | 0 | 3 | 0 |
| Syzygiella rubricaulis (Nees) Steph. | 0 | 0 | 2 |
| Telaranea nematodes (Austin) M. Howe | 1 | 1 | 0 |
| Lichens | |||
| Cladia aggregata (Sw.) Nyl. | 31 | 18 | 16 |
| Cladia fuliginosa Filson | 7 | 2 | 2 |
| Cladia sp. | 2 | 12 | 0 |
| Cladonia subreticulata Ahti | 14 | 1 | 0 |
| Cladonia arbuscula ssp. boliviana (Ahti) Ahti & DePriest | 7 | 1 | 6 |
| Cladonia rappii A. Evans | 6 | 4 | 10 |
| Cladonia sp. 1 | 10 | 5 | 8 |
| Cladonia sp. 2 | 30 | 32 | 21 |
| Diploschistes diacapsis (Ach.) Lumbsch | 1 | 12 | 7 |
| Phyllobaeis imbricata (Hook.) Kalb & Gierl | 14 | 18 | 11 |
| Peltigera sp. | 4 | 2 | 0 |
| Siphula sp. | 27 | 25 | 0 |
| Treatment | Richness | Chao 1 | Jack1 |
|---|---|---|---|
| T1 | 21 | 21.98 (1.84) | 22.97 (1.39) |
| T2 | 23 | 24.47 (2.26) | 25.95 (1.70) |
| Control | 21 | 23.98 (3.51) | 25.78 (2.13) |
| Monitoring | |||
| M1 | 24 | 28.10 (4.81) | 28.92 (2.20) |
| M2 | 22 | 22.24 (0.71) | 22.97 (0.97) |
| M3 | 21 | 21.16 (0.52) | 21.97 (0.97) |
| Richness | |||
| T1 | T2 | Control | |
| T1 | 0.4254 | 0.0010 | |
| T2 | 0.4254 | 0.0001 | |
| Control | 0.0010 | 0.0001 | |
| Abundance | |||
| T1 | T2 | Control | |
| T1 | <0.0001 | <0.0001 | |
| T2 | <0.0001 | <0.0001 | |
| Control | <0.0001 | <0.0001 | |
| Simpson index | |||
| T1 | T2 | Control | |
| T1 | <0.0001 | <0.0001 | |
| T2 | <0.0001 | 0.2077 | |
| Control | <0.0001 | 0,2077 | |
| Shannon–Weaver index | |||
| T1 | T2 | Control | |
| T1 | <0.0001 | <0.0001 | |
| T2 | <0.0001 | 0.6082 | |
| Control | <0.0001 | 0.6082 | |
| Monitoring | |||
| Richness | |||
| M1 | M2 | M3 | |
| M1 | 0.0047 | 0.0005 | |
| M2 | 0.0047 | 0.3967 | |
| M3 | 0.0005 | 0.3967 | |
| Abundance | |||
| M1 | M2 | M3 | |
| M1 | <0.0001 | <0.0001 | |
| M2 | <0.0001 | <0.0001 | |
| M3 | <0.0001 | <0.0001 | |
| Simpson | |||
| M1 | M2 | M3 | |
| M1 | 0.1725 | <0.0001 | |
| M2 | 0.1725 | <0.0001 | |
| M3 | <0.0001 | <0.0001 | |
| Shannon | |||
| M1 | M2 | M3 | |
| M1 | 0.4935 | <0.0001 | |
| M2 | 0.4935 | <0.0001 | |
| M3 | <0.0001 | <0.0001 |
| Factor | Df | SS | R2 | F | p-Value |
|---|---|---|---|---|---|
| Treatment | 2 | 3.313 | 0.06011 | 61.954 | 0.001 |
| Monitoring | 2 | 8.759 | 0.15890 | 163.781 | 0.001 |
| Residual | 161 | 43.051 | 0.78100 | ||
| Total | 165 | 55.123 | 100.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yangua-Solano, E.; Carrión-Paladines, V.; Benítez, Á. Effects of Fire on Pyrodiversity of Terricolous Non-Tracheophytes Photoautotrophs in a Páramo of Southern Ecuador. Diversity 2023, 15, 1176. https://doi.org/10.3390/d15121176
Yangua-Solano E, Carrión-Paladines V, Benítez Á. Effects of Fire on Pyrodiversity of Terricolous Non-Tracheophytes Photoautotrophs in a Páramo of Southern Ecuador. Diversity. 2023; 15(12):1176. https://doi.org/10.3390/d15121176
Chicago/Turabian StyleYangua-Solano, Erika, Vinicio Carrión-Paladines, and Ángel Benítez. 2023. "Effects of Fire on Pyrodiversity of Terricolous Non-Tracheophytes Photoautotrophs in a Páramo of Southern Ecuador" Diversity 15, no. 12: 1176. https://doi.org/10.3390/d15121176
APA StyleYangua-Solano, E., Carrión-Paladines, V., & Benítez, Á. (2023). Effects of Fire on Pyrodiversity of Terricolous Non-Tracheophytes Photoautotrophs in a Páramo of Southern Ecuador. Diversity, 15(12), 1176. https://doi.org/10.3390/d15121176








