Quantifying the Potential Water Filtration Capacity of a Constructed Shellfish Reef in a Temperate Hypereutrophic Estuary
Abstract
1. Introduction
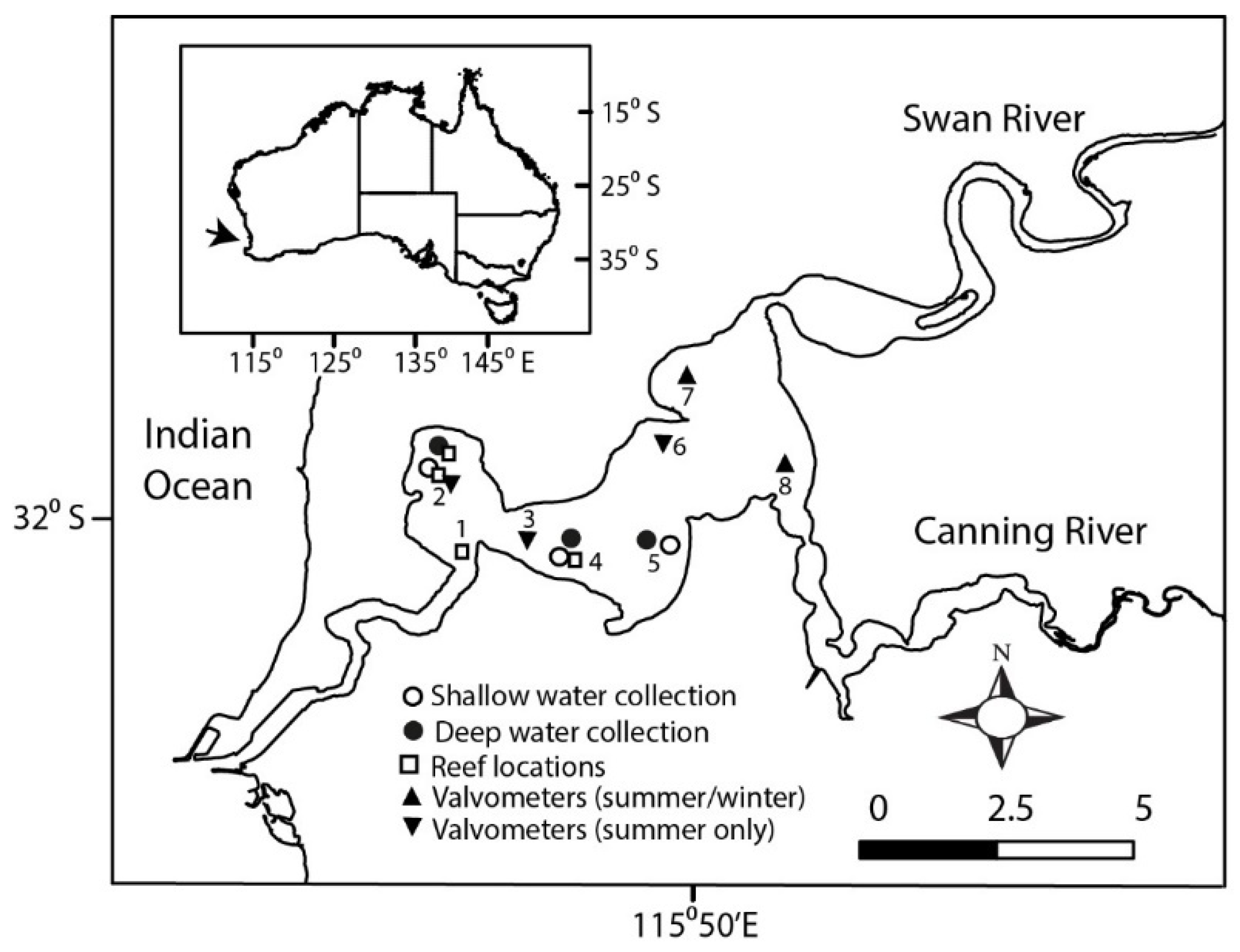
2. Materials and Methods
2.1. Feeding Experiments
2.2. Valve Gape Recordings
2.3. Filtration Capacity of a Shellfish Reef
3. Results
3.1. Characteristics of the Estuary Water
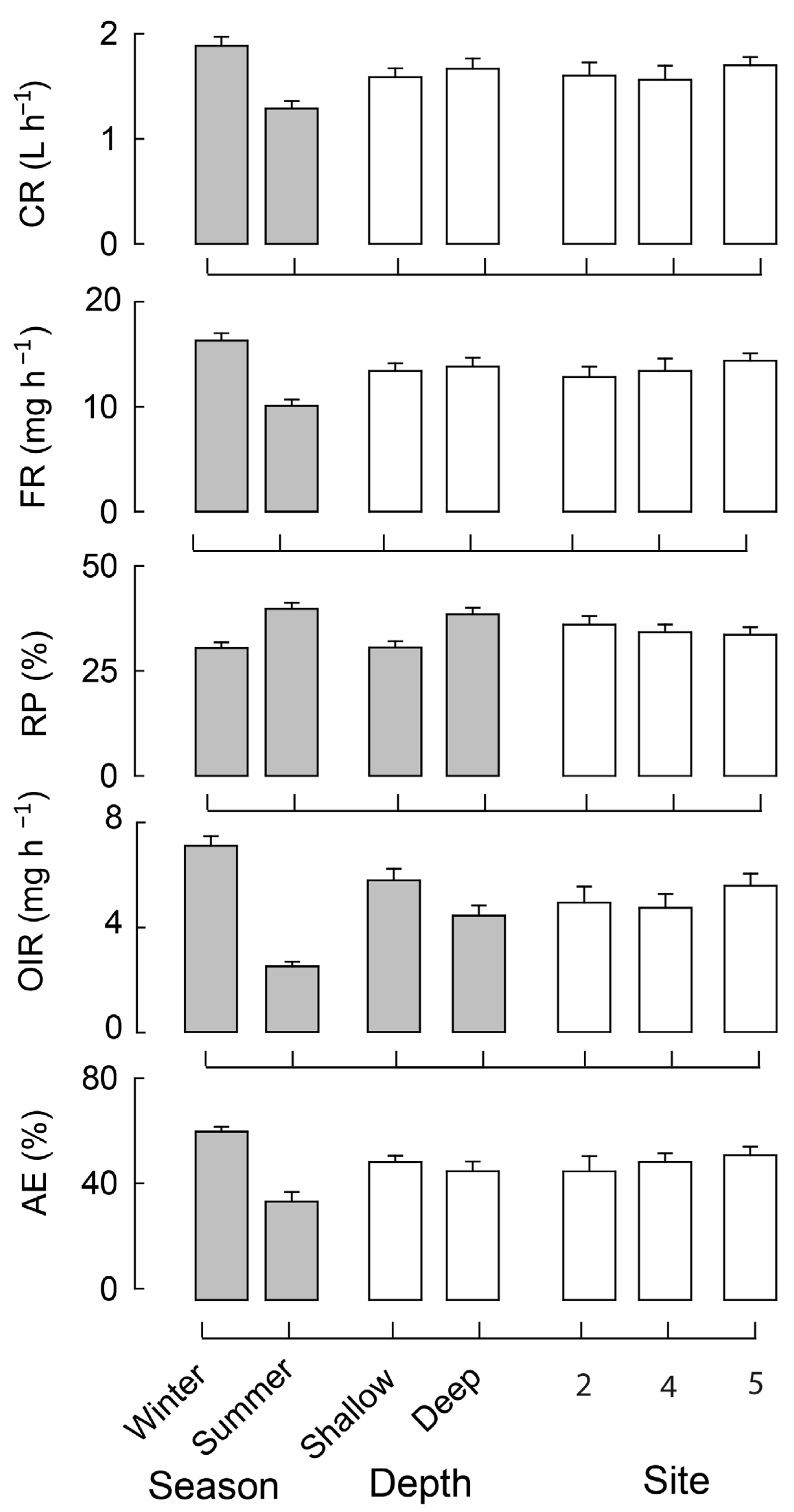
3.2. Mussel Feeding Parameters
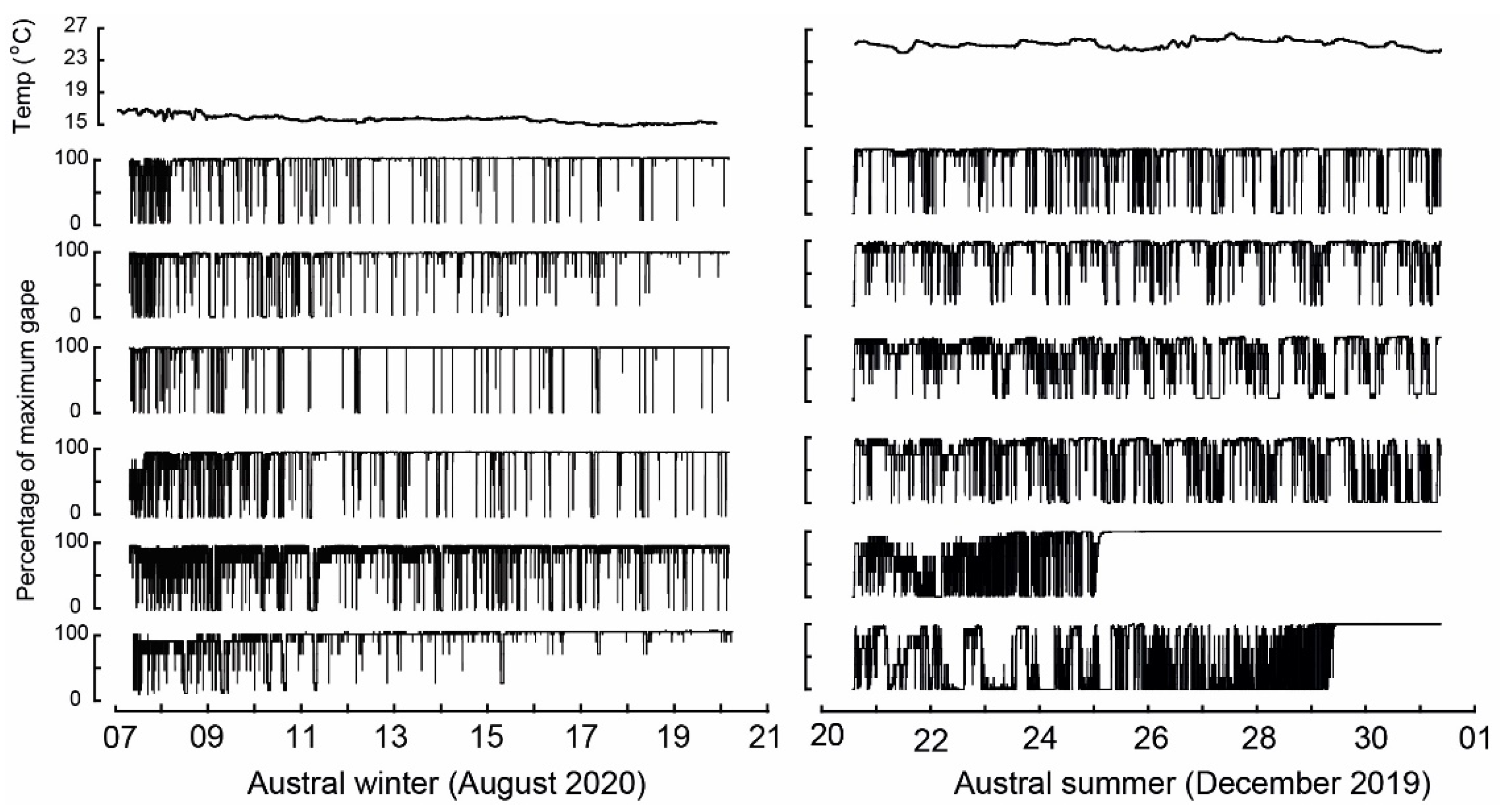
3.3. Water Temperature and Valve Gape Activity
3.4. Potential Filtration
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Region | TPM | POM | PIM | f | Chl a | CR | FR | RP | AE | Temp (°C) | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Swan-Canning | 7.9–8.7 | 2.8–4.8 | 3.9–5.1 | 35–55 | 1.0–5.3 | 0.6–0.9 | 4.5–8.1 | 33–40 | 16–63 | 17–25 | Current study |
| Alfacs Bay (Med.) | 1.0–2.3 | 0.7–1.4 | 0.5–1.2 | 48–73 | 0.9–4.8 | 1.5–7.2 | 0.2–16 | 22–88 | 10–26 | [26] | |
| Milford * (Atlantic) | 3.4–9.6 | 1.6–4.1 | 1.3–5.5 | 37–63 | 1.0–3.1 | 9.0–17.5 | 25–45 | 18–25 | [62] | ||
| Hunts * (Atlantic) | 4.1–12.7 | 1.1–2.3 | 2.9–10.5 | 18–27 | 0.8–2.3 | 7.8–22.4 | 36–73 | 17–24 | [62] | ||
| Wellington (Pacific) | 9.2–14.0 | 2.4–7.0 | 21–60 | 2.8–5.0 | 0–80 | 10–16 | [63] | ||||
| Galicia (Atlantic) | 0.4–2.4 | 1.7–2.0 | 0.02–1.9 | 21–97 | 35–98 | [51] | |||||
| Bay of Fundy (Atlantic) | 1.7–2.0 | 0.5–0.7 | 0.9–1.5 | 27–45 | 55–69 | [51] | |||||
| Gulf of Gaeta (Tyrr.) | 4.3 | 0.2 | 4.1 | 5.5 | 0.5–2.1 | 2.2 | 17 | [64] | |||
| Gulf of Castellammare (Tyrr.) | 4.4 | 0.3 | 4.1 | 7 | 0.02–0.06 | 3.2 | 16 | [64] | |||
| Ria de Arousa (Atlantic) | 0.9–2.7 | 0.3–1.1 | 35–55 | 1.3–3.2 | [65] | ||||||
| Little Swanport (Tas.) | 4.5–22.0 | 1.9–6.0 | 2.5–14.0 | 25–48 | 0.1–6.0 | [46] | |||||
| Pipeclay Lagoon (Tas.) | 6.0–42.5 | 2.0–10.6 | 3.8–33.5 | 18–49 | 0.2–8.0 | [46] |
References
- Coen, L.D.; Brumbaugh, R.D.; Bushek, D.; Grizzle, R.; Luckenbach, M.W.; Posey, M.H.; Powers, S.P.; Tolley, S.G. Ecosystem services related to oyster restoration. Mar. Ecol. Prog. Ser. 2007, 341, 303–307. [Google Scholar] [CrossRef]
- Grabowski, J.H.; Peterson, C.H. Restoring oyster reefs to recover ecosystem services. In Ecosystem Engineers: Plants to Protists; Cuddington, K., Byers, J., Wilson, W., Hastings, A., Eds.; Academic Press: Cambridge, MA, USA, 2007; pp. 281–298. [Google Scholar]
- Christianen, M.J.A.; van der Heide, T.; Holthuijsen, S.J.; van der Reijden, K.J.; Borst, A.C.W.; Olff, H. Biodiversity and food web indicators of community recovery in intertidal shellfish reefs. Biol. Coserv. 2017, 213, 317–324. [Google Scholar] [CrossRef]
- Harding, J.M.; Mann, R. Diet and habitat use by bluefish, Pomatomus saltatrix, in a Chesapeake Bay estuary. Environ. Biol. Fish. 2001, 60, 401–409. [Google Scholar] [CrossRef]
- Lenihan, H.S.; Peterson, C.H.; Byers, J.E.; Grabowski, J.H.; Thayer, G.W.; Colby, D.R. Cascading of habitat degradation: Oyster reefs invaded by refugee fishes escaping stress. Ecol. Appl. 2001, 11, 764–782. [Google Scholar] [CrossRef]
- Tolley, S.G.; Volety, A.K. The role of oysters in habitat use of oyster reefs by resident fishes and decapod crustaceans. J. Shellfish Res. 2005, 24, 1007–1012. [Google Scholar]
- Beck, M.W.; Brumbaugh, R.D.; Airoldi, L.; Carranza, A.; Coen, L.D.; Crawford, C.; Defeo, O.; Edgar, G.J.; Hancock, B.; Kay, M.C.; et al. Oyster reefs at risk and recommendations for conservation, restoration, and management. Bioscience 2011, 61, 107–116. [Google Scholar] [CrossRef]
- Gillies, C.L.; McLeod, I.M.; Alleway, H.K.; Cook, P.; Crawford, C.; Creighton, C.; Diggles, B.; Ford, J.; Hamer, P.; Heller-Wagner, G.; et al. Australian shellfish ecosystems: Past distribution, current status and future direction. PLoS ONE 2018, 13, e0190914. [Google Scholar] [CrossRef]
- Christensen, J.; Martin, D.J.; Bossie, A.; Valesini, F. Subfossil shell assemblages and the shifting role of history in ecological restoration: How a dynamic past informs shellfish ecosystem reconstruc-tion at an Australian urban estuary. Glob. Environ. 2023, accepted. [Google Scholar]
- Alder, A.; Jeffs, A.; Hillman, J.R. Considering the use of subadult and juvenile mussels for mussel reef restoration. Rest Ecol. 2020, 29, e13322. [Google Scholar] [CrossRef]
- Reeves, S.E.; Renzi, J.J.; Fobert, E.K.; Silliman, B.R.; Hancock, B.; Gillies, C.L. Facilitating better outcomes: How positive species interactions can improve oyster reef restoration. Front. Mar. Sci. 2020, 7, 656. [Google Scholar] [CrossRef]
- McAfee, D.; McLeod, I.M.; Alleway, H.K.; Bishop, M.J.; Branigan, S.; Connell, S.D.; Copeland, C.; Crawford, C.M.; Diggles, B.K.; Fitzsimons, J.A.; et al. Turning a lost reef ecosystem into a national restoration program. Biol. Coserv. 2022, 36, e13958. [Google Scholar] [CrossRef]
- Jackson, J.B.C.; Kirby, M.X.; Berger, W.H.; Bjorndal, K.A.; Botsford, L.W.; Bourque, B.J.; Bradbury, R.H.; Cooke, R.G.; Erlandson, J.; Estes, J.A.; et al. Historical overfishing and the recent collapse of coastal ecosystems. Science 2001, 293, 629–638. [Google Scholar] [CrossRef] [PubMed]
- Cottingham, A.; Hesp, S.A.; Hall, N.G.; Hipsey, M.R.; Potter, I.C. Marked deleterious changes in the condition, growth and maturity schedules of Acanthopagrus butcheri (Sparidae) in an estuary reflect environmental degradation. Estuar. Coast. Shelf Sci. 2014, 149, 109–119. [Google Scholar] [CrossRef]
- Tweedley, J.R.; Warwick, R.M.; Potter, I.C. The contrasting ecology of temperate macrotidal and microtidal estuaries. In Oceanography and Marine Biology: An Annual Review; Hughes, R.N., Hughes, D.J., Smith, I.P., Dale, A.C., Eds.; CRC Press: Boca Raton, FL, USA, 2016; pp. 73–171. [Google Scholar]
- Petrone, K.C.; Hughes, J.D.; Van Niel, T.G.; Silberstein, R.P. Streamflow decline in southwestern Australia, 1950–2008. Geophys. Res. Lett. 2010, 37, 11. [Google Scholar] [CrossRef]
- Silberstein, R.P.; Aryal, S.K.; Durrant, J.; Pearcey, M.; Braccia, M.; Charles, S.P.; McFarlane, D.J. Climate change and runoff in south-western Australia. J. Hydrol. 2012, 475, 441–455. [Google Scholar] [CrossRef]
- Smith, I.; Power, S. Past and future changes to inflows into Perth (Western Australia) dams. J. Hydrol. Reg. Stud. 2014, 2, 84–96. [Google Scholar] [CrossRef]
- Cottingham, A.; Huang, P.; Hipsey, M.R.; Hall, N.G.; Ashworth, E.; Williams, J.; Potter, I.C. Growth, condition, and maturity schedules of an estuarine fish species change in estuaries following increased hypoxia due to climate change. Ecol. Evol. 2018, 8, 7111–7130. [Google Scholar] [CrossRef]
- Cloern, J.E.; Foster, S.Q.; Kleckner, A.E. Phytoplankton primary production in the world’s estuarine-coastal ecosystems. Biogeosciences 2014, 11, 2477–2501. [Google Scholar] [CrossRef]
- Ehrich, M.K.; Harris, L.A. A review of existing eastern oyster filtration rate models. Ecol. Model. 2015, 297, 201–212. [Google Scholar] [CrossRef]
- Riisgård, H.U.; Larsen, P.S.; Pleissner, D. Allometric equations for maximum filtration rate in blue mussels Mytilus edulis and importance of condition index. Helgol. Mar. Res. 2014, 68, 193–198. [Google Scholar] [CrossRef]
- Galimany, E.; Lunt, J.; Domingos, A.; Paul, V.J. Feeding behavior of the native mussel Ischadium recurvum and the invasive mussels Mytella charruana and Perna viridis in FL, USA, across a salinity gradient. Estuar Coast. 2018, 41, 2378–2388. [Google Scholar] [CrossRef]
- Moody, J.; Kreeger, D. Ribbed mussel (Geukensia demissa) filtration services are driven by seasonal temperature and site-specific seston variability. J. Exp. Mar. Biol Ecol. 2020, 522, 151237. [Google Scholar] [CrossRef]
- Bayne, B.L.; Hawkins, A.J.S.; Navarro, E.; Iglesias, J.I.P. Effects of seston concentration on feeding, digestion and growth in the mussel Mytilus edulis. Mar. Ecol. Prog Ser. 1989, 55, 47–54. [Google Scholar] [CrossRef]
- Galimany, E.; Ramón, M.; Ibarrola, I. Feeding behavior of the mussel Mytilus galloprovincialis (L.) in a Mediterranean estuary: A field study. Aquaculture 2011, 314, 236–243. [Google Scholar] [CrossRef]
- Maire, O.; Amouroux, J.M.; Duchêne, J.C.; Grémare, A. Relationship between filtration activity and food availability in the Mediterranean mussel Mytilus galloprovincialis. Mar. Biol. 2007, 152, 1293–1307. [Google Scholar] [CrossRef]
- Jørgensen, C.B.; Famme, P.; Kristensen, H.S.; Larsen, P.S.; Møhlenberg, F.; Riisgård, H.U. The bivalve pump. Mar. Ecol. Prog. Ser. 1986, 34, 69–77. [Google Scholar] [CrossRef]
- Comeau, L.A.; Babarro, J.M.F.; Riobó, P.; Scarratt, M.; Starr, M. PSP-producing dinoflagellate Alexandrium minutum induces valve microclosures in the mussel Mytilus galloprovincialis. Aquaculture 2019, 500, 407–413. [Google Scholar] [CrossRef]
- Filgueira, R.; Fernández-Reiriz, M.J.; Labarta, U. Clearance rate of the mussel Mytilus galloprovincialis. I. Response to extreme chlorophyll ranges. Cienc. Mar. 2009, 35, 405–417. [Google Scholar] [CrossRef]
- Anestis, A.; Lazou, A.; Pörtner, H.O.; Michaelidis, B. Behavioural, metabolic and molecular stress responses of marine bivalve Mytilus galloprovincialis during long-term acclimation at increasing ambient temperature. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 293, R911–R921. [Google Scholar] [CrossRef]
- Lucas, L.V.; Thompson, J.K. Changing restoration rules: Exotic bivalves in-teract with residence time and depth to control phytoplankton productivity. Ecosphere 2012, 3, 1–26. [Google Scholar] [CrossRef]
- Fernández-Reiriz, M.J.; Irisarri, J.; Labarta, U. Feeding behaviour and differential absorption of nutrients in mussel Mytilus galloprovincialis: Responses to three microalgae diets. Aquaculture 2015, 446, 42–47. [Google Scholar] [CrossRef]
- His, E.; Robert, R.; Dinet, A. Combined effects of temperature and salinity on fed and starved larvae of the Mediterranean mussel Mytilus galloprovincialis and the Japanese oyster Crassostrea gigas. Mar. Biol 1989, 100, 455–463. [Google Scholar] [CrossRef]
- Lee, H., II; Reusser, D.A. Atlas of Nonindigenous Marine and Estuarine Species in the North Pacific; Office of Research and Development, National Health and Environmental Effects Research Laboratory: Washington, DC, USA, 2012.
- Westfall, K.M.; Gardner, J.P.A. Genetic diversity of Southern Hemisphere blue mussels (Bivalvia: Mytilidae) and the non-indigenous taxa. Biol. J. Linn. Soc. 2010, 101, 898–909. [Google Scholar] [CrossRef]
- Galimany, E.; Rose, J.M.; Dixon, M.S.; Alix, R.; Li, Y.; Wikfors, G.H. Design and use of an apparatus for quantifying bivalve suspension feeding at sea. J. Vis. Exp. 2018, 139, e58213. [Google Scholar] [CrossRef] [PubMed]
- Baird, R.B. Chlorophyll sociation. In Standard Methods for the Examination of Water and Wastewater, 23rd ed.; Rice, E.W., Baird, R.B., Eaton, A.D., Eds.; American Public Health Association, American Water Works Association, Water Environment Federation: Washington, DC, USA, 2017; pp. 10–22. [Google Scholar]
- Comeau, L.A.; Babarro, J.M.F. Narrow valve gaping in the invasive mussel Limnoperna securis: Implications for competition with the indigenous mussel Mytilus galloprovincialis in NW Spain. Aquac. Int. 2014, 22, 1215–1227. [Google Scholar] [CrossRef]
- Comeau, L.A.; Babarro, J.M.F.; Longa, A.; Padin, X.A. Valve-gaping behavior of raft-cultivated mussels in the Ría de Arousa, Spain. Aquac. Rep. 2018, 9, 68–73. [Google Scholar] [CrossRef]
- Gnyubkin, V.F. The circadian rhythms of valve movements in the mussel Mytilus galloprovincialis. Russ. J. Mar. Biol. 2010, 36, 419–428. [Google Scholar] [CrossRef]
- Nagai, K.; Honjo, T.; Go, J.; Yamashita, H.; Oh, S.J. Detecting the shellfish killer Heterocapsa circularisquama (Dinophyceae) by measuring bivalve valve activity with a Hall element sensor. Aquaculture 2006, 255, 395–401. [Google Scholar] [CrossRef]
- Cho, H.J. Effects of prevailing winds on turbidity of a shallow estuary. Int. J. Environ. Res. Public Health 2007, 4, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Dame, R.F.; Prins, T.C. Bivalve carrying capacity in coastal ecosystems. Aquac. Ecol. 1998, 31, 409–421. [Google Scholar] [CrossRef]
- Smaal, A.C.; Verhagen, J.H.G.; Coosen, J.; Haas, H.A. Interaction between seston quantity and quality and benthic suspension feeders in the Oosterschelde, The Netherlands. Ophelia 1986, 26, 385–399. [Google Scholar] [CrossRef]
- Mitchell, I.M. Relationship between Water Quality Parameters (Nutrients, Seston, Chlorophyll a), Hydrodynamics and Oyster Growth in Three Major Pacific Oyster (Crassostrea gigas) Growing Areas in Southern Tasmania (Australia); University of Tasmania: Hobarts, Australia, 2001. [Google Scholar]
- Ward, J.E.; Shumway, S.E. Separating the grain from the chaff: Particle selection in suspension- and deposit-feeding bivalves. J. Exp. Mar. Biol. Ecol. 2004, 300, 83–130. [Google Scholar] [CrossRef]
- González, J.G.; Yevich, P. Responses of an estuarine population of the blue mussel Mytilus edulis to heated water from a steam generating plant. Mar. Biol. 1976, 34, 177–189. [Google Scholar] [CrossRef]
- Bayne, B.L.; Bayne, J.C.; Carefoot, C.T.; Thompson, J.R. The physiological ecology of Mytilus californianus Conrad: Metabolism and energy balance. Oecologia 1976, 22, 211–228. [Google Scholar] [CrossRef]
- Anestis, A.; Pörtner, H.O.; Karagiannis, D.; Angelidis, P.; Staikou, A.; Michaelidis, B. Response of Mytilus galloprovincialis (L.) to increasing seawater temperature and to marteliosis: Metabolic and physiological parameters. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2010, 156, 57–66. [Google Scholar] [CrossRef]
- Irisarri, J.; Fernández-Reiriz, M.J.; Robinson, S.M.C.; Cranford, P.J.; Labarta, U. Absorption efficiency of mussels Mytilus edulis and Mytilus galloprovincialis cultured under Integrated Multi-Trophic Aquaculture conditions in the Bay of Fundy (Canada) and Ría Ares-Betanzos (Spain). Aquaculture 2013, 388–391, 182–192. [Google Scholar] [CrossRef]
- Incze, L.S.; Lutz, R.A.; Watling, L. Relationships between effects of environmental temperature and seston on growth and mortality on Mytilus edulis in a temperate northern estuary. Mar. Biol. 1980, 57, 147–156. [Google Scholar] [CrossRef]
- Mallet, A.L.; Carver, C.E.A.; Freeman, K.R. Summer mortality of the blue mussel in eastern Canada: Spatial, temporal, stock and age variation. Mar. Ecol. Prog. Ser. 1990, 67, 35–41. [Google Scholar] [CrossRef]
- Tremblay, R.; Myrand, B.; Sevigny, J.M.; Guderley, H. Bioenergetic and genetic parameters in relation to susceptibility of blue mussels, Mytilus edulis (L) to summer mortality. J. Exp. Mar. Biol. Ecol 1998, 221, 27–58. [Google Scholar] [CrossRef]
- Baker, J.D.; Cosgrove, J.J. Swan Canning Catchment Data Report January–December 2019; Department of Biodiversity, Conservation and Attractions: Perth, Australia, 2021. [Google Scholar]
- Bricker, S.B.; Grizzle, R.E.; Trowbridge, P.; Rose, J.M.; Ferreira, J.G.; Wellman, K.; Zhu, C.; Galimany, E.; Wikfors, G.H.; Saurel, C.; et al. Bioextractive removal of nitrogen by oysters in Great Bay Piscataqua River Estuary, New Hampshire, USA. Estuar. Coast. 2020, 43, 23–38. [Google Scholar] [CrossRef]
- Smaal, A.C.; Ferreira, J.G.; Grant, J.; Petersen, J.K.; Strand, Ø. Goods and Services of Marine Bivalves; Springer Nature: Berlin, Germany, 2019; p. 598. [Google Scholar]
- Ermgassen, P.S.E.; Thurstan, R.H.; Corrales, J.; Alleway, H.; Carranza, A.; Dankers, N.; DeAngelis, B.; Hancock, B.; Kent, F.; McLeod, I.; et al. The benefits of bivalve reef restoration: A global synthesis of underrepresented species. Aquac. Conserv. 2020, 30, 2050–2065. [Google Scholar] [CrossRef]
- Kreeger, D.A.; Gatenby, C.M.; Bergstrom, P.W. Restoration potential of several native species of bivalve molluscs for water quality improvement in Mid-Atlantic watersheds. J. Shellfish Res. 2018, 37, 1121–1157. [Google Scholar] [CrossRef]
- Waltham, N.J.; Elliott, M.; Lee, S.Y.; Lovelock, C.; Duarte, C.M.; Buelow, C.; Simenstad, C.; Nagelkerken, I.; Claassens, L.; Wen, C.K.-C.; et al. UN Decade on Ecosystem Restoration 2021–2030—What Chance for Success in Restoring Coastal Ecosystems? Front. Mar. Sci. 2020, 7, 71. [Google Scholar] [CrossRef]
- Bayne, B.L.; Iglesias, J.I.P.; Hawkins, A.J.S.; Navarro, E.; Heral, M.; Deslous-Pauli, J.M. Feeding behavior of the mussel Mytilus edulis: Responses to variations in quantity and org.ganic content of seston. J. Mar. Biol. Assoc. UK 1993, 73, 813–829. [Google Scholar] [CrossRef]
- Galimany, E.; Rose, J.M.; Dixon, M.S.; Wikfors, G.H. Quantifying feeding behavior of ribbed mussels (Geukensia demissa) in two urban sites (Long Island Sound, USA) with different seston characteristics. Estuar. Coast. 2013, 36, 1265–1273. [Google Scholar] [CrossRef]
- Gardner, J.P.A. Effects of seston variability on the clearance rat and absorption efficiency of the mussel Aulacomya maoriana, Mytilus galloprovincialis and Perna canaliculus from New Zealand. J. Exp. Mar. Biol. Ecol. 2002, 268, 83–101. [Google Scholar] [CrossRef]
- Sarà, G.; Mazzola, A. The carrying capacity for Mediterranean bivalve suspension feeders: Evidence from analysis of food availability and hydrodynamics and their integration into local model. Ecol. Modell. 2004, 179, 281–296. [Google Scholar] [CrossRef]
- Navarro, E.; Iglesias, J.I.P.; Perez Camacho, A.; Labarta, U.; Beiras, R. The physiological energetics of mussels (Mytilus galloprovincialis Lmk) from different cultivation rafts in the Ria de Arosa (Galicia, N.W. Spain). Aquaculture 1991, 94, 197–212. [Google Scholar] [CrossRef]



| Parameter | Units | Calculation |
|---|---|---|
| Clearance Rate (CR) | L h−1 | |
| Filtration Rate (FR) | mg h−1 | |
| Rejection Proportion (RP) | % | |
| Organic Ingestion Rate (OIR) | mg h−1 | |
| Absorption Rate (AR) | mg h−1 | |
| Absorption Efficiency (AE) | na |
| TPM | POM | PIM | ||||
|---|---|---|---|---|---|---|
| p-values | r values | p-values | r values | p-values | r values | |
| CR | 0.488 | - | 0.090 | - | 0.112 | |
| FR | 0.166 | - | 0.026 | 0.64 | 0.113 | |
| RP | 0.073 | - | <0.001 | −0.86 | 0.013 | 0.69 |
| OIR | 0.089 | - | <0.001 | 0.89 | 0.005 | −0.75 |
| AE | 0.011 | 0.70 | <0.001 | 0.84 | 0.085 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cottingham, A.; Bossie, A.; Valesini, F.; Tweedley, J.R.; Galimany, E. Quantifying the Potential Water Filtration Capacity of a Constructed Shellfish Reef in a Temperate Hypereutrophic Estuary. Diversity 2023, 15, 113. https://doi.org/10.3390/d15010113
Cottingham A, Bossie A, Valesini F, Tweedley JR, Galimany E. Quantifying the Potential Water Filtration Capacity of a Constructed Shellfish Reef in a Temperate Hypereutrophic Estuary. Diversity. 2023; 15(1):113. https://doi.org/10.3390/d15010113
Chicago/Turabian StyleCottingham, Alan, Andrew Bossie, Fiona Valesini, James R. Tweedley, and Eve Galimany. 2023. "Quantifying the Potential Water Filtration Capacity of a Constructed Shellfish Reef in a Temperate Hypereutrophic Estuary" Diversity 15, no. 1: 113. https://doi.org/10.3390/d15010113
APA StyleCottingham, A., Bossie, A., Valesini, F., Tweedley, J. R., & Galimany, E. (2023). Quantifying the Potential Water Filtration Capacity of a Constructed Shellfish Reef in a Temperate Hypereutrophic Estuary. Diversity, 15(1), 113. https://doi.org/10.3390/d15010113







