Effect of Depth across a Latitudinal Gradient in the Structure of Rhodolith Seabeds and Associated Biota across the Eastern Atlantic Ocean
Abstract
1. Introduction
2. Material and Methods
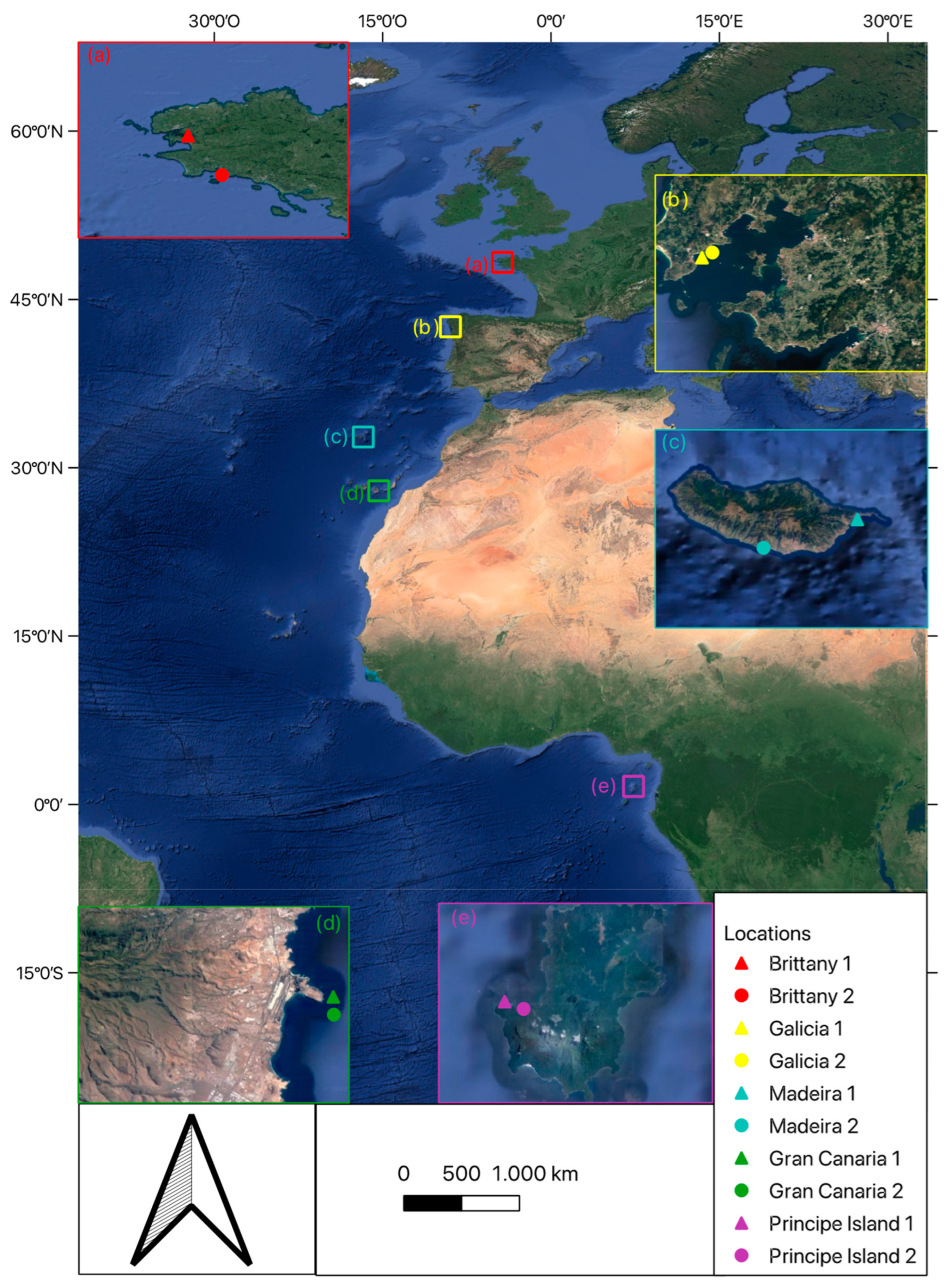
2.1. Study Regions
2.2. Sampling Design and Collection of Samples
2.3. Samples Processing
2.4. Data Analysis
3. Results
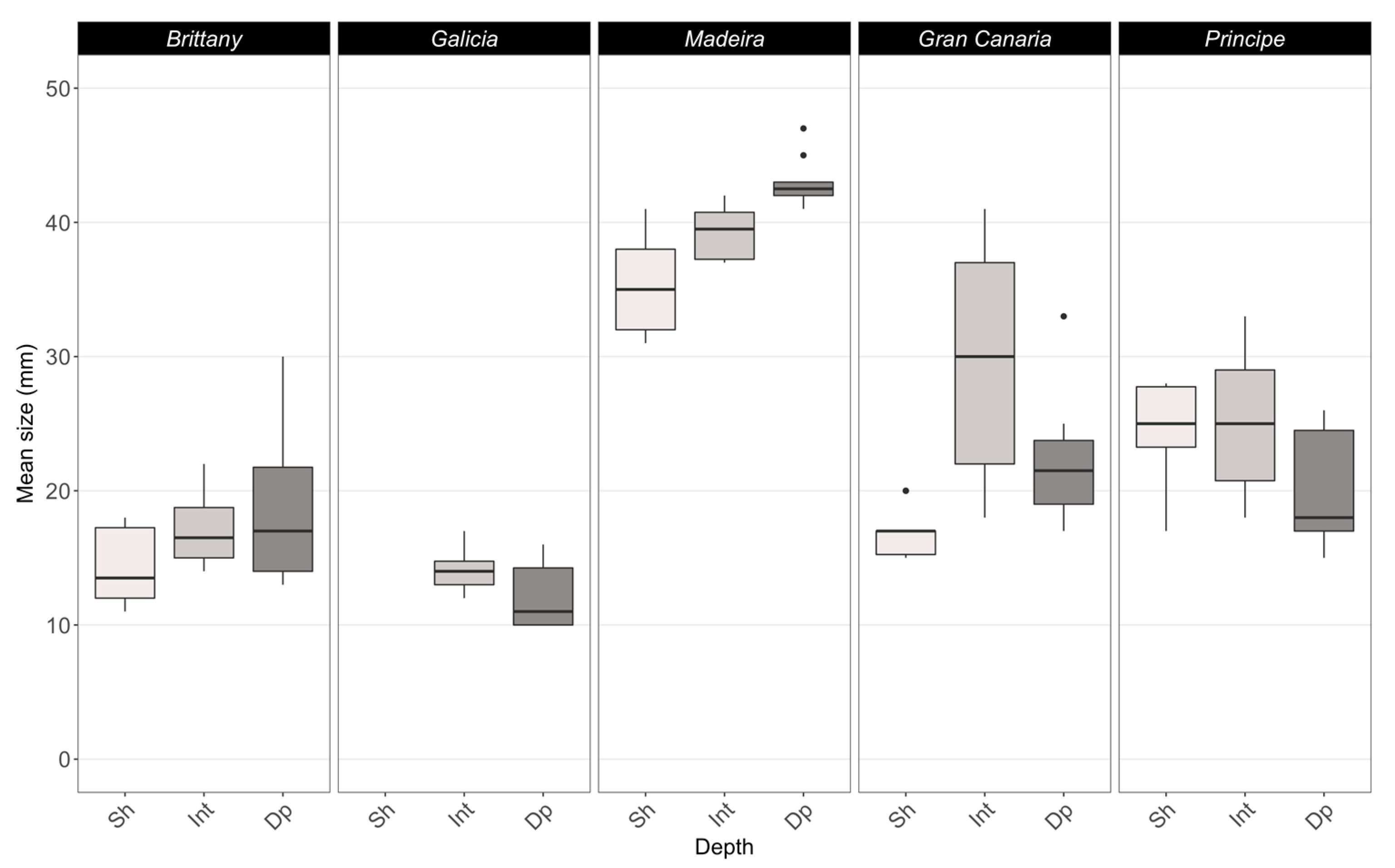
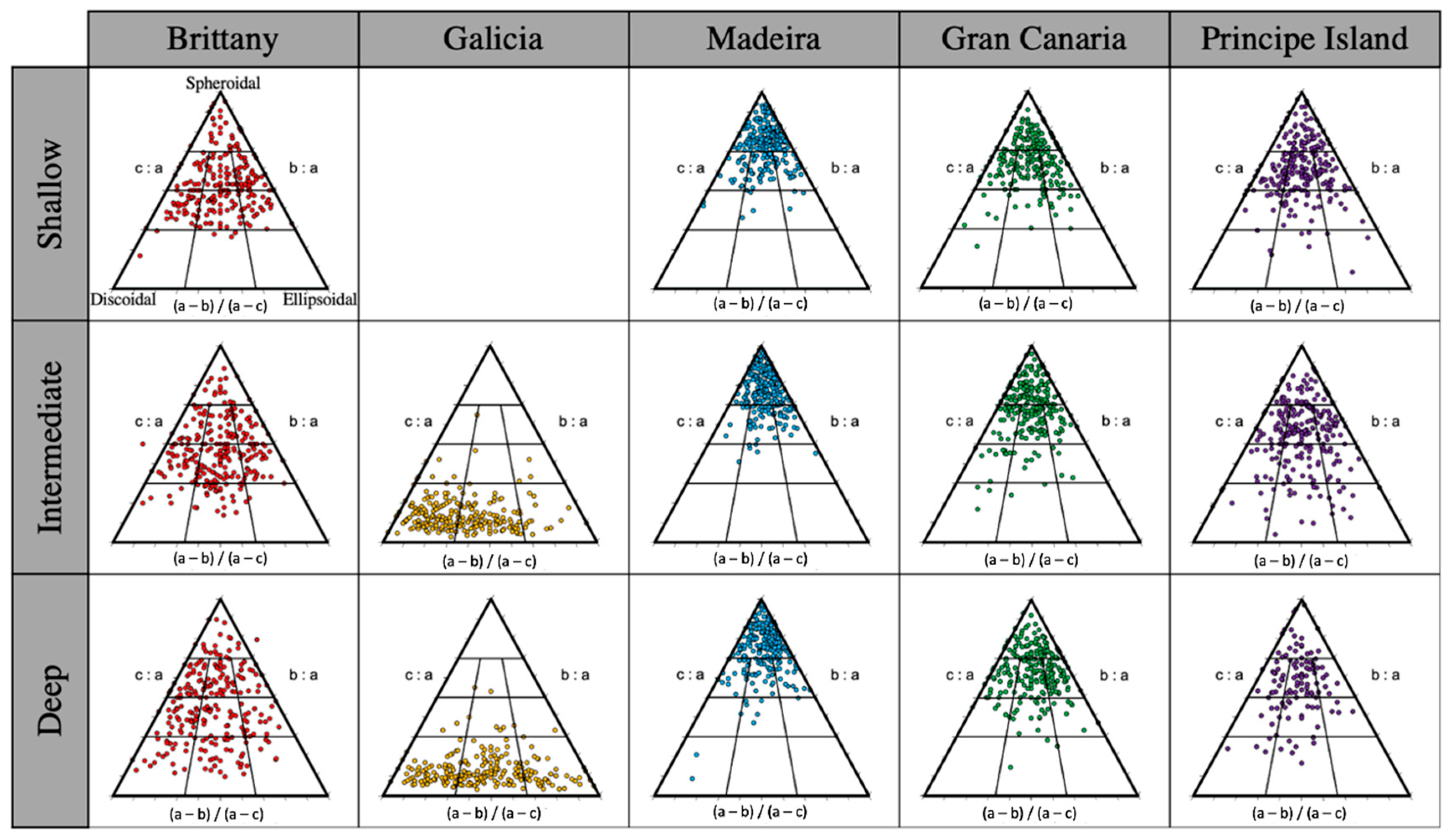
3.1. Rhodolith Structural Attributes
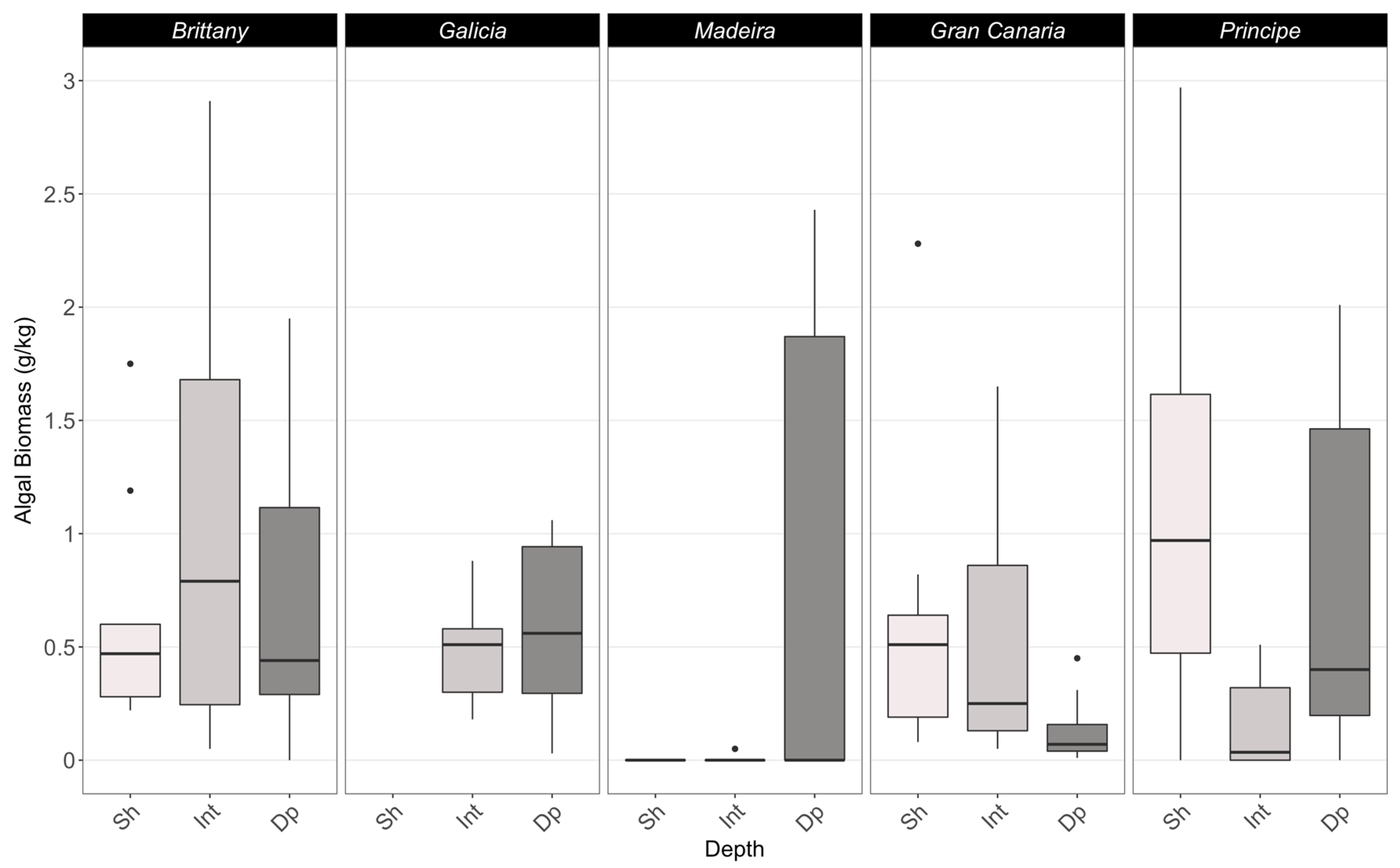
3.2. Epiflora
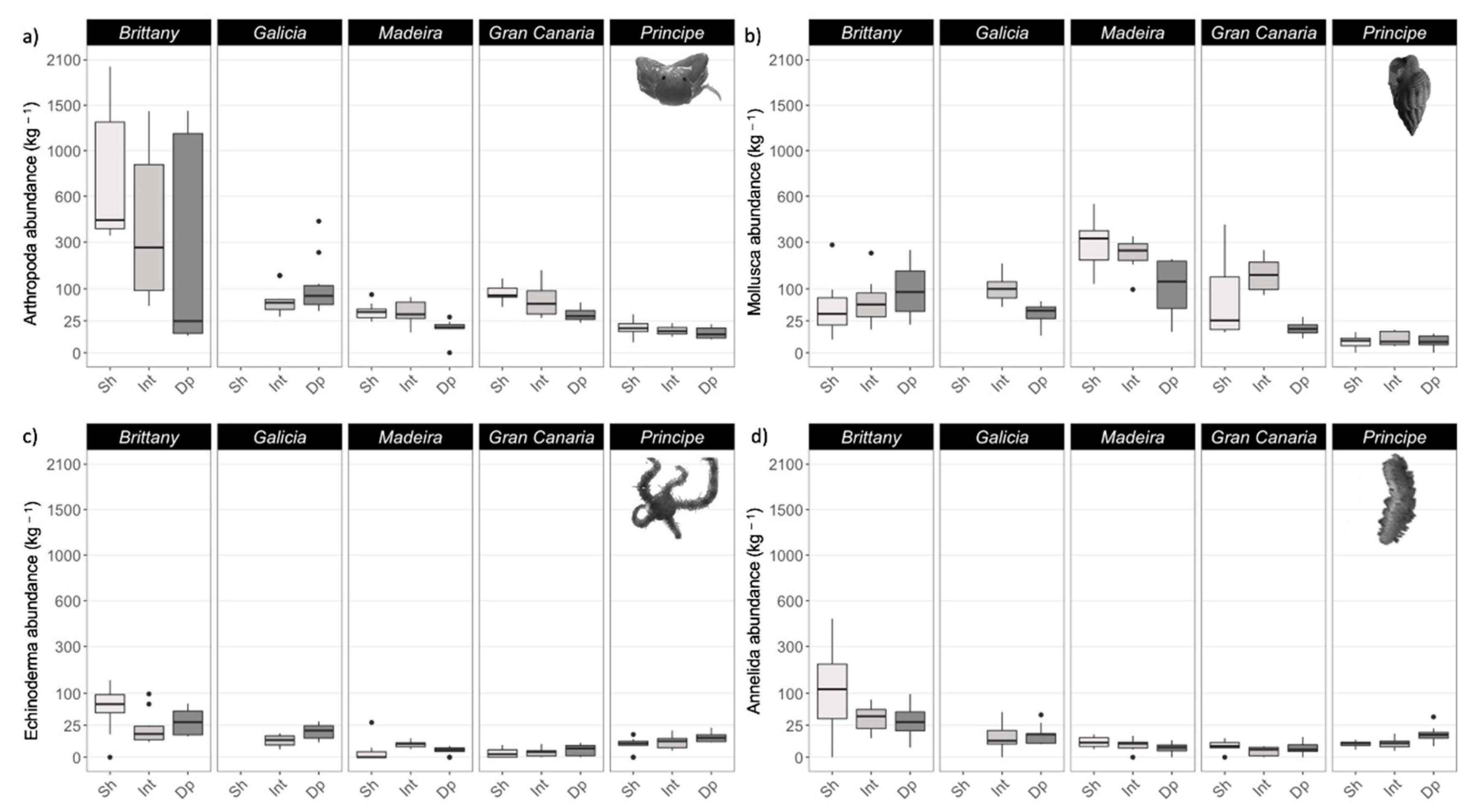
3.3. Epifauna
4. Discussion
4.1. Rhodolith Attributes
4.2. Epiflora
4.3. Epifauna
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jones, C.G.; Lawton, J.H.; Shachak, M. Organisms as Ecosystem Engineers. Oikos 1994, 69, 373–386. [Google Scholar] [CrossRef]
- Berke, S.K. Functional Groups of Ecosystem Engineers: A Proposed Classification with Comments on Current Issues. Integr. Comp. Biol. 2010, 50, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Burel, T.; Schaal, G.; Grall, J.; Le Duff, M.; Chapalain, G.; Schmitt, B.; Gemin, M.; Boucher, O.; Ar Gall, E. Small-Scale Effects of Hydrodynamics on the Structure of Intertidal Macroalgal Communities: A Novel Approach. Estuar. Coast. Shelf Sci. 2019, 226, 106290. [Google Scholar] [CrossRef]
- Madin, J.S.; Connolly, S.R. Ecological Consequences of Major Hydrodynamic Disturbances on Coral Reefs. Nature 2006, 444, 477–480. [Google Scholar] [CrossRef]
- Williams, G.J.; Smith, J.E.; Conklin, E.J.; Gove, J.M.; Sala, E.; Sandin, S.A. Benthic Communities at Two Remote Pacific Coral Reefs: Effects of Reef Habitat, Depth, and Wave Energy Gradients on Spatial Patterns. PeerJ 2013, 1, e81. [Google Scholar] [CrossRef]
- Adams, M.P.; Hovey, R.K.; Hipsey, M.R.; Bruce, L.C.; Ghisalberti, M.; Lowe, R.J.; Gruber, R.K.; Ruiz-Montoya, L.; Maxwell, P.S.; Callaghan, D.P.; et al. Feedback between Sediment and Light for Seagrass: Where Is It Important? Limnol. Oceanogr. 2016, 61, 1937–1955. [Google Scholar] [CrossRef]
- Granata, T.; Serra, T.; Colomer, J.; Casamitjana, X.; Duarte, C.; Gacia, E. Flow and Particle Distributions in a Nearshore Seagrass Meadow before and after a Storm. Mar. Ecol. Prog. Ser. 2001, 218, 95–106. [Google Scholar] [CrossRef]
- Andradi-Brown, D.A.; Gress, E.; Wright, G.; Exton, D.A.; Rogers, A.D. Reef Fish Community Biomass and Trophic Structure Changes across Shallow to Upper-Mesophotic Reefs in the Mesoamerican Barrier Reef, Caribbean. PLoS ONE 2016, 11, e0156641. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.R.; Copus, J.M.; Coffey, D.M.; Whitton, R.K.; Bowen, B.W. Shifting Reef Fish Assemblages along a Depth Gradient in Pohnpei, Micronesia. PeerJ 2018, 6, e4650. [Google Scholar] [CrossRef]
- Otero-Ferrer, F.; Cosme, M.; Tuya, F.; Espino, F.; Haroun, R. Effect of Depth and Seasonality on the Functioning of Rhodolith Seabeds. Estuar. Coast. Shelf Sci. 2020, 235, 106579. [Google Scholar] [CrossRef]
- Tuya, F.; Herrero-Barrencua, A.; Bosch, N.; Abreu, A.; Haroun, R. Reef Fish at a Remote Tropical Island (Principe Island, Gulf of Guinea): Disentangling Taxonomic, Functional and Phylogenetic Diversity Patterns with Depth. Mar. Freshw. Res. 2017, 69, 395–402. [Google Scholar] [CrossRef]
- de Boyer Montégut, C.; Mignot, J.; Lazar, A.; Cravatte, S. Control of Salinity on the Mixed Layer Depth in the World Ocean: 1. General Description. J. Geophys. Res. Oceans 2007, 112. [Google Scholar] [CrossRef]
- Falcon, E.; Laroche, C. Observation of Depth-Induced Properties in Wave Turbulence on the Surface of a Fluid. EPL Europhys. Lett. 2011, 95, 34003. [Google Scholar] [CrossRef]
- Huybers, P.; Wunsch, C. A Depth-Derived Pleistocene Age Model: Uncertainty Estimates, Sedimentation Variability, and Nonlinear Climate Change. Paleoceanography 2004, 19. [Google Scholar] [CrossRef]
- Lee, Z.; Hu, C.; Shang, S.; Du, K.; Lewis, M.; Arnone, R.; Brewin, R. Penetration of UV-Visible Solar Radiation in the Global Oceans: Insights from Ocean Color Remote Sensing. J. Geophys. Res. Oceans 2013, 118, 4241–4255. [Google Scholar] [CrossRef]
- Liu, Q.; Xie, S.-P.; Li, L.; Maximenko, N.A. Ocean Thermal Advective Effect on the Annual Range of Sea Surface Temperature. Geophys. Res. Lett. 2005, 32, L24604. [Google Scholar] [CrossRef]
- Montgomery, R.B. Water Characteristics of Atlantic Ocean and of World Ocean. Deep Sea Res. 1953 1958, 5, 134–148. [Google Scholar] [CrossRef]
- Bayly, I.A.E. Salinity Tolerance and Osmotic Behavior of Animals in Athalassic Saline and Marine Hypersaline Waters. Annu. Rev. Ecol. Syst. 1972, 3, 233–268. [Google Scholar] [CrossRef]
- Grillas, P.; van Wijck, C.; Bonis, A. The Effect of Salinity on the Dominance-Diversity Relations of Experimental Coastal Macrophyte Communities. J. Veg. Sci. 1993, 4, 453–460. [Google Scholar] [CrossRef]
- Jorda, G.; Marbà, N.; Bennett, S.; Santana-Garcon, J.; Agusti, S.; Duarte, C.M. Ocean Warming Compresses the Three-Dimensional Habitat of Marine Life. Nat. Ecol. Evol. 2020, 4, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Klöser, H.; Quartino, M.L.; Wiencke, C. Distribution of Macroalgae and Macroalgal Communities in Gradients of Physical Conditions in Potter Cove, King George Island, Antarctica. Hydrobiologia 1996, 333, 1–17. [Google Scholar] [CrossRef]
- Philipp, E.; Fabricius, K. Photophysiological Stress in Scleractinian Corals in Response to Short-Term Sedimentation. J. Exp. Mar. Biol. Ecol. 2003, 287, 57–78. [Google Scholar] [CrossRef]
- Nugues, M.M.; Roberts, C.M. Partial Mortality in Massive Reef Corals as an Indicator of Sediment Stress on Coral Reefs. Mar. Pollut. Bull. 2003, 46, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Risk, M.J. Assessing the Effects of Sediments and Nutrients on Coral Reefs. Curr. Opin. Environ. Sustain. 2014, 7, 108–117. [Google Scholar] [CrossRef]
- Rogers, C. Responses of Coral Reefs and Reef Organisms to Sedimentation. Mar. Ecol. Prog. Ser. 1990, 62, 185–202. [Google Scholar] [CrossRef]
- Balata, D.; Piazzi, L. Patterns of Diversity in Rocky Subtidal Macroalgal Assemblages in Relation to Depth. Bot. Mar. 2008, 51, 464–471. [Google Scholar] [CrossRef]
- Connell, S. Assembly and Maintenance of Subtidal Habitat Heterogeneity: Synergistic Effects of Light Penetration and Sedimentation. Mar. Ecol. Prog. Ser. 2005, 289, 53–61. [Google Scholar] [CrossRef]
- Ryan, D.A.; Brooke, B.P.; Collins, L.B.; Kendrick, G.A.; Baxter, K.J.; Bickers, A.N.; Siwabessy, P.J.W.; Pattiaratchi, C.B. The Influence of Geomorphology and Sedimentary Processes on Shallow-Water Benthic Habitat Distribution: Esperance Bay, Western Australia. Estuar. Coast. Shelf Sci. 2007, 72, 379–386. [Google Scholar] [CrossRef]
- Bosellini, A.; Ginsburg, R.N. Form and Internal Structure of Recent Algal Nodules (Rhodolites) from Bermuda. J. Geol. 1971, 79, 669–682. [Google Scholar] [CrossRef]
- Foster, M.S.; Gilberto Filho, M.A.; Kamenos, N.A.; Riosmena-Rodríguez, R.; Steller, D.L. Rhodoliths and rhodolith beds. In Research and Dscoveries: The Revolution of Science through SCUBA; Smithsonian contributions to the marine sciences number 39; Lang, M.A., Marinelli, R.L., Roberts, S.J., Taylor, P.R., Eds.; Smithsonian Institution Scholarly Press: Washington, DC, USA, 2013; pp. 143–155. [Google Scholar]
- Schubert, N.; Peña, V.; Salazar, V.W.; Horta, P.A.; Neves, P.; Ribeiro, C.; Otero-Ferrer, F.; Tuya, F.; Espino, F.; Schoenrock, K. Rhodolith Physiology Across the Atlantic: Towards a Better Mechanistic Understanding of Intra-and Interspecific Differences. Front. Mar. Sci. 2022, 9, 921639. [Google Scholar] [CrossRef]
- Fredericq, S.; Krayesky-Self, S.; Sauvage, T.; Richards, J.; Kittle, R.; Arakaki, N.; Schmidt, W.E. The critical importance of rhodoliths in the life cycle completion of both macro-and microalgae, and as holobionts for the establishment and maintenance of marine biodiversity. Front. Mar. Sci. 2019, 5, 502. [Google Scholar] [CrossRef]
- Navarro-Mayoral, S.; Fernandez-Gonzalez, V.; Otero-Ferrer, F.; Tuya, F. Spatio-Temporal Variability of Amphipod Assemblages Associated with Rhodolith Seabeds. Mar. Freshw. Res. 2020, 72, 76–83. [Google Scholar] [CrossRef]
- Amado-Filho, G.M.; Pereira-Filho, G.H. Rhodolith Beds in Brazil: A New Potential Habitat for Marine Bioprospection. Rev. Bras. Farmacogn. 2012, 22, 782–788. [Google Scholar] [CrossRef]
- Foster, M.S. Rhodoliths: Between Rocks and Soft Places. J. Phycol. 2001, 37, 659–667. [Google Scholar] [CrossRef]
- Amado-Filho, G.M.; Maneveldt, G.; Manso, R.C.C.; Marins-Rosa, B.V.; Pacheco, M.R.; Guimarães, S. Structure of Rhodolith Beds from 4 to 55 Meters Deep along the Southern Coast of Espírito Santo State, Brazil. Cienc. Mar. 2007, 33, 399–410. [Google Scholar] [CrossRef]
- Steller, D.L.; Foster, M.S. Environmental Factors Influencing Distribution and Morphology of Rhodoliths in Bahía Concepción, B.C.S., México. J. Exp. Mar. Biol. Ecol. 1995, 194, 201–212. [Google Scholar] [CrossRef]
- Wilson, S.; Blake, C.; Berges, J.A.; Maggs, C.A. Environmental Tolerances of Free-Living Coralline Algae (Maerl): Implications for European Marine Conservation. Biol. Conserv. 2004, 120, 279–289. [Google Scholar] [CrossRef]
- Bosence, D.W.J. Ecological Studies on Two Unattached Coralline Algae from Western Ireland. Palaeontology 1976, 19, 365–395. [Google Scholar]
- Georgiadis, M.; Papatheodorou, G.; Tzanatos, E.; Geraga, M.; Ramfos, A.; Koutsikopoulos, C.; Ferentinos, G. Coralligène formations in the eastern Mediterranean Sea: Morphology, distribution, mapping and relation to fisheries in the southern Aegean Sea (Greece) based on high-resolution acoustics. J. Exp. Mar. Biol. Ecol. 2009, 368, 44–58. [Google Scholar] [CrossRef]
- Rendina, F.; Kaleb, S.; Caragnano, A.; Ferrigno, F.; Appolloni, L.; Donnarumma, L.; Falace, A. Distribution and characterization of deep rhodolith beds off the Campania coast (SW Italy, Mediterranean Sea). Plants 2020, 9, 985. [Google Scholar] [CrossRef]
- Bracchi, V.A.; Angeletti, L.; Marchese, F.; Taviani, M.; Cardone, F.; Hajdas, I.; Basso, D. A resilient deep-water rhodolith bed off the Egadi Archipelago (Mediterranean Sea) and its actuopaleontological significance. Alp. Mediterr. Quat. 2019, 32, 131–150. [Google Scholar]
- Bahia, R.G.; Abrantes, D.P.; Brasileiro, P.S.; Pereira Filho, G.H.; Amado Filho, G.M. Rhodolith Bed Structure along a Depth Gradient on the Northern Coast of Bahia State, Brazil. Braz. J. Oceanogr. 2010, 58, 323–337. [Google Scholar] [CrossRef]
- Pascelli, C.; Riul, P.; Riosmena-Rodríguez, R.; Scherner, F.; Nunes, M.; Hall-Spencer, J.M.; de Oliveira, E.C.; Horta, P. Seasonal and Depth-Driven Changes in Rhodolith Bed Structure and Associated Macroalgae off Arvoredo Island (Southeastern Brazil). Aquat. Bot. 2013, 111, 62–65. [Google Scholar] [CrossRef]
- Amado-Filho, G.M.; Bahia, R.G.; Pereira-Filho, G.H.; Longo, L.L. South Atlantic Rhodolith Beds: Latitudinal Distribution, Species Composition, Structure and Ecosystem Functions, Threats and Conservation Status. In Rhodolith/Maërl Beds: A Global Perspective; Springer: Berlin/Heidelberg, Germany, 2017; pp. 299–317. [Google Scholar]
- Hernandez-Kantun, J.J.; Hall-Spencer, J.M.; Grall, J.; Adey, W.; Rindi, F.; Maggs, C.A.; Bárbara, I.; Peña, V. North Atlantic Rhodolith Beds. In Rhodolith/Maërl Beds: A Global Perspective; Riosmena-Rodríguez, R., Nelson, W., Aguirre, J., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 265–279. ISBN 978-3-319-29315-8. [Google Scholar]
- Barbera, C.; Bordehore, C.; Borg, J.A.; Glémarec, M.; Grall, J.; Hall-Spencer, J.M.; De La Huz, C.H.; Lanfranco, E.; Lastra, M.; Moore, P.G. Conservation and Management of Northeast Atlantic and Mediterranean Maerl Beds. Aquat. Conserv. Mar. Freshw. Ecosyst. 2003, 13, S65–S76. [Google Scholar] [CrossRef]
- Grall, J.; Hall-Spencer, J.M. Problems Facing Maerl Conservation in Brittany. Aquat. Conserv. Mar. Freshw. Ecosyst. 2003, 13, S55–S64. [Google Scholar] [CrossRef]
- Wehrmann, A.; Freiwald, A.; Zankl, H. Formation of Cold-Temperate Water Multispecies Rhodoliths in Intertidal Gravel Pools from Northern Brittany, France. Oceanogr. Lit. Rev. 1997, 11, 1331. [Google Scholar]
- Dutertre, M.; Grall, J.; Ehrhold, A.; Hamon, D. Environmental Factors Affecting Maerl Bed Structure in Brittany (France). Eur. J. Phycol. 2015, 50, 371–383. [Google Scholar] [CrossRef]
- Peña, V.; Criado, I. Distribution of the Galician Maerl Beds and Their Shape Classes (Atlantic Iberian Peninsula): Proposal of Areas in Future Conservation Actions. Cah. Biol. Mar. 2009, 50, 353. [Google Scholar]
- Teichert, S.; Woelkerling, W.; Rüggeberg, A.; Wisshak, M.; Piepenburg, D.; Meyerhöfer, M.; Form, A.; Büdenbender, J.; Freiwald, A. Rhodolith Beds (Corallinales, Rhodophyta) and Their Physical and Biological Environment at 80 31′ N in Nordkappbukta (Nordaustlandet, Svalbard Archipelago, Norway). Phycologia 2012, 51, 371–390. [Google Scholar] [CrossRef]
- Haroun, R.J.; Gil-Rodríguez, M.C.; de Castro, J.D.; Reine, W.P.H.V. A Checklist of the Marine Plants from the Canary Islands (Central Eastern Atlantic Ocean). Bot. Mar. 2002, 45, 139–169. [Google Scholar] [CrossRef]
- Neves, P.A.; Silva, J.; Peña, V.; Ribeiro, C. “Pink Round Stones”—Rhodolith Beds: An Overlooked Habitat in Madeira Archipelago. Biodivers. Conserv. 2021, 30, 3359–3383. [Google Scholar] [CrossRef]
- Johnson, M.E.; Ledesma-Vázquez, J.; Ramalho, R.S.; da Silva, C.M.; Rebelo, A.C.; Santos, A.; Baarli, B.G.; Mayoral, E.; Cachão, M. Taphonomic Range and Sedimentary Dynamics of Modern and Fossil Rhodolith Beds: Macaronesian Realm (North Atlantic Ocean). In Rhodolith/Maërl Beds: A Global Perspective; Riosmena-Rodríguez, R., Nelson, W., Aguirre, J., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 221–261. ISBN 978-3-319-29315-8. [Google Scholar]
- Cosme De Esteban, M.; Otero Ferrer, F.; Tuya, F.; Espino Rodríguez, F.; Abreu, A.D.; Haroun, R. Mapping Subtropical and Tropical Rhodolith Seabeds Using Side Scan Sonar Technology. In Proceedings of the VI International Rhodolith Workshop 2018, Roscoff, France, 25–29 June 2018. [Google Scholar]
- Steentoft, M. A Revision of the Marine Algae of São Tomé and Principe (Gulf of Guinea). Bot. J. Linn. Soc. 1967, 60, 99–146. [Google Scholar] [CrossRef]
- Cabioch, J. Les fonds de maerl de la Baie de Moriaix et leurpeuplement vegetal. Cah. Biol. Mar. 1969, 9, 139–161. [Google Scholar]
- Hernandez-Kantun, J.J.; Rindi, F.; Adey, W.H.; Heesch, S.; Peña, V.; Le Gall, L.; Gabrielson, P.W. Sequencing Type Material Resolves the Identity and Distribution of the Generitype Lithophyllum incrustans, and Related European Species L. hibernicum and L. bathyporum (Corallinales, Rhodophyta). J. Phycol. 2015, 51, 791–807. [Google Scholar] [CrossRef]
- Braga-Henriques, A.; Buhl-Mortensen, P.; Tokat, E.; Martins, A.; Silva, T.; Jakobsen, J.; Canning-Clode, J.; Jakobsen, K.; Delgado, J.; Voirand, T.; et al. Benthic Community Zonation from Mesophotic to Deep Sea: Description of First Deep-Water Kelp Forest and Coral Gardens in the Madeira Archipelago (Central NE Atlantic). Front. Mar. Sci. 2022, 9, 1680. [Google Scholar] [CrossRef]
- Pardo, C.; Bárbara, I.; Barreiro, R.; Peña, V. Insights into Species Diversity of Associated Crustose Coralline Algae (Corallinophycidae, Rhodophyta) with Atlantic European Maerl Beds Using DNA Barcoding. An. Jardín Botánico Madr. 2017, 74, e059. [Google Scholar] [CrossRef]
- Peña, V.; Bárbara, I. Los fondos marinos de maërl del Parque Nacional de las Islas Atlánticas (Galicia, España): Distribución, abundancia y flora asociada. Nova Acta Ci. Compostel. Biol. 2006, 15, 7–25. [Google Scholar]
- Peña, V.; Bárbara, I. Maërl Community in the North-Western Iberian Peninsula: A Review of Floristic Studies and Long-Term Changes. Aquat. Conserv. Mar. Freshw. Ecosyst. 2008, 18, 339–366. [Google Scholar] [CrossRef]
- Pardo, C.; Peña, V.; Barreiro, R.; Bárbara, I. A Molecular and Morphological Study of Corallina Sensu Lato (Corallinales, Rhodophyta) in the Atlantic Iberian Peninsula. Cryptogam. Algol. 2015, 36, 31–54. [Google Scholar] [CrossRef]
- Adey, W.H.; McKibbin, D.L. Studies on the maerl species Phymatolithon calcareum (Pallas) nov. comb. and Lithothamnium coralloides Crouan in the Ria de Vigo. Bot. Mar. 1970, 13, 100–106. [Google Scholar] [CrossRef]
- Sauriau, P.-G.; Curti, C.; Jourde, J.; Aubert, F.; Cajeri, P.; Lavesque, N.; Dubois, S.; Lepareur, F.; Gouesbier, C.; Sauriau, F. Le Maerl Algues Corallinacées Marines Dans Les Pertuis Charentais. Ann. Soc. Sci. Nat. Charente-Marit. 2012, 10, 281–300. [Google Scholar]
- Sneed, E.D.; Folk, R.L. Pebbles in the Lower Colorado River, Texas a Study in Particle Morphogenesis. J. Geol. 1958, 66, 114–150. [Google Scholar] [CrossRef]
- Graham, D.J.; Midgley, N.G. Graphical Representation of Particle Shape Using Triangular Diagrams: An Excel Spreadsheet Method. Earth Surf. Process. Landf. 2000, 25, 1473–1477. [Google Scholar] [CrossRef]
- Gagnon, P.; Matheson, K.; Stapleton, M. Variation in Rhodolith Morphology and Biogenic Potential of Newly Discovered Rhodolith Beds in Newfoundland and Labrador (Canada). Bot. Mar. 2012, 55, 85–99. [Google Scholar] [CrossRef]
- Otero-Ferrer, F.; Mannarà, E.; Cosme, M.; Falace, A.; Montiel-Nelson, J.A.; Espino, F.; Haroun, R.; Tuya, F. Early-Faunal Colonization Patterns of Discrete Habitat Units: A Case Study with Rhodolith-Associated Vagile Macrofauna. Estuar. Coast. Shelf Sci. 2019, 218, 9–22. [Google Scholar] [CrossRef]
- Warwick, R. Analysis of Community Attributes of the Macrobenthos of Frierfjord/Langesundfjord at Taxonomic Levels Higher than Species. Mar. Ecol. Prog. Ser. 1988, 46, 167–170. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using Lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Bosence, D.W.J. Description and Classification of Rhodoliths (Rhodoids, Rhodolites). In Coated Grains; Peryt, T.M., Ed.; Springer: Berlin, Heidelberg, 1983; pp. 217–224. [Google Scholar]
- Sciberras, M.; Rizzo, M.; Mifsud, J.R.; Camilleri, K.; Borg, J.A.; Lanfranco, E.; Schembri, P.J. Habitat Structure and Biological Characteristics of a Maerl Bed off the Northeastern Coast of the Maltese Islands (Central Mediterranean). Mar. Biodivers. 2009, 39, 251–264. [Google Scholar] [CrossRef]
- Littler, M.M.; Littler, D.S.; Hanisak, M.D. Deep-water rhodolith distribution, productivity, and growth history at sites of formation and subsequent degradation. J. Exp. Mar. Biol. Ecol. 1991, 150, 163–182. [Google Scholar] [CrossRef]
- Marrack, E.C. The Relationship between Water Motion and Living Rhodolith Beds in the Southwestern Gulf of California, Mexico. Palaios 1999, 14, 159–171. [Google Scholar] [CrossRef]
- Basso, D. Deep rhodolith distribution in the Pontian Islands, Italy: A model for the paleoecology of a temperate sea. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1998, 137, 173–187. [Google Scholar] [CrossRef]
- Bassoulet, P. Etude de La Dynamique Des Sédiments En Suspension Dans l’estuaire de l’Aulne (Rade de Brest); Université de Bretagne Occidentale: Brest, France, 1979. [Google Scholar]
- Gregoire, G.; Ehrhold, A.; Le Roy, P.; Jouet, G.; Garlan, T. Modern Morpho-Sedimentological Patterns in a Tide-Dominated Estuary System: The Bay of Brest (West Britanny, France). J. Maps 2016, 12, 1152–1159. [Google Scholar] [CrossRef]
- Jardim, V.L.; Gauthier, O.; Toumi, C.; Grall, J. Quantifying Maerl (Rhodolith) Habitat Complexity along an Environmental Gradient at Regional Scale in the Northeast Atlantic. Mar. Environ. Res. 2022, 181, 105768. [Google Scholar] [CrossRef]
- Scoffin, T.P.; Stoddart, D.R.; Tudhope, A.W.; Woodroffe, C. Rhodoliths and Coralliths of Muri Lagoon, Rarotonga, Cook Islands. Coral Reefs 1985, 4, 71–80. [Google Scholar] [CrossRef]
- Seoane-Camba, J.; Campo Sancho, J. Resultados de una primera exploración algológica con escafandra autónoma en la Ría de Vigo. Publ. Técnicas Junta Estud. Pesca 1968, 7, 333–344. [Google Scholar]
- Chimienti, G.; Rizzo, L.; Kaleb, S.; Falace, A.; Fraschetti, S.; Giosa, F.D.; Tursi, A.; Barbone, E.; Ungaro, N.; Mastrototaro, F. Rhodolith Beds Heterogeneity along the Apulian Continental Shelf (Mediterranean Sea). J. Mar. Sci. Eng. 2020, 8, 813. [Google Scholar] [CrossRef]
- Schlüter, M.; Pyko, I.; Wisshak, M.; Schulbert, C.; Teichert, S. Growth Interruptions in Arctic Rhodoliths Correspond to Water Depth and Rhodolith Morphology. Minerals 2021, 11, 538. [Google Scholar] [CrossRef]
- Hurd, C.L. Water Motion, Marine Macroalgal Physiology, and Production. J. Phycol. 2000, 36, 453–472. [Google Scholar] [CrossRef]
- Duarte, C.M. Seagrass Depth Limits. Aquat. Bot. 1991, 40, 363–377. [Google Scholar] [CrossRef]
- Frade, P.R.; Bongaerts, P.; Winkelhagen, A.J.S.; Tonk, L.; Bak, R.P.M. In Situ Photobiology of Corals over Large Depth Ranges: A Multivariate Analysis on the Roles of Environment, Host, and Algal Symbiont. Limnol. Oceanogr. 2008, 53, 2711–2723. [Google Scholar] [CrossRef]
- Bessell-Browne, P.; Negri, A.P.; Fisher, R.; Clode, P.L.; Duckworth, A.; Jones, R. Impacts of Turbidity on Corals: The Relative Importance of Light Limitation and Suspended Sediments. Mar. Pollut. Bull. 2017, 117, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Davies-Colley, R.J.; Smith, D.G. Turbidity Suspeni) Ed Sediment, and Water Clarity: A Review 1. JAWRA J. Am. Water Resour. Assoc. 2001, 37, 1085–1101. [Google Scholar] [CrossRef]
- Steinman, A.D.; McIntire, C.D. Effects of Irradiance on the Community Structure and Biomass of Algal Assemblages in Laboratory Streams. Can. J. Fish. Aquat. Sci. 1987, 44, 1640–1648. [Google Scholar] [CrossRef]
- Grall, J.; Le Loc’h, F.; Guyonnet, B.; Riera, P. Community Structure and Food Web Based on Stable Isotopes (Δ15N and Δ13C) Analysis of a North Eastern Atlantic Maerl Bed. J. Exp. Mar. Biol. Ecol. 2006, 338, 1–15. [Google Scholar] [CrossRef]
- Peña, V.; Bárbara, I.; Grall, J.; Maggs, C.A.; Hall-Spencer, J.M. The Diversity of Seaweeds on Maerl in the NE Atlantic. Mar. Biodivers. 2014, 44, 533–551. [Google Scholar] [CrossRef]
- Hellio, C.; Marechal, J.-P.; Véron, B.; Bremer, G.; Clare, A.S.; Le Gal, Y. Seasonal Variation of Antifouling Activities of Marine Algae from the Brittany Coast (France). Mar. Biotechnol. 2004, 6, 67–82. [Google Scholar] [CrossRef]
- Gómez, M.; Barreiro, F.; López, J.; Lastra, M.; de la Huz, R. Deposition Patterns of Algal Wrack Species on Estuarine Beaches. Aquat. Bot. 2013, 105, 25–33. [Google Scholar] [CrossRef]
- Qui-Minet, Z.N.; Delaunay, C.; Grall, J.; Six, C.; Cariou, T.; Bohner, O.; Legrand, E.; Davoult, D.; Martin, S. The Role of Local Environmental Changes on Maerl and Its Associated Non-Calcareous Epiphytic Flora in the Bay of Brest. Estuar. Coast. Shelf Sci. 2018, 208, 140–152. [Google Scholar] [CrossRef]
- McConnico, L.A.; Carmona, G.H.; Morales JS, M.; Rodríguez, R.R. Temporal variation in seaweed and invertebrate assemblages in shallow rhodolith beds of Baja California Sur, México. Aquat. Bot. 2017, 139, 37–47. [Google Scholar] [CrossRef]
- Steller, D.L.; Riosmena-Rodríguez, R.; Foster, M.S.; Roberts, C.A. Rhodolith Bed Diversity in the Gulf of California: The Importance of Rhodolith Structure and Consequences of Disturbance. Aquat. Conserv. Mar. Freshw. Ecosyst. 2003, 13, S5–S20. [Google Scholar] [CrossRef]
- Friedlander, A.M.; Ballesteros, E.; Clemente, S.; Gonçalves, E.J.; Estep, A.; Rose, P.; Sala, E. Contrasts in the Marine Ecosystem of Two Macaronesian Islands: A Comparison between the Remote Selvagens Reserve and Madeira Island. PLoS ONE 2017, 12, e0187935. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, S.; José, R.; Neves, P.; Góis, A.; Cordeiro, N.; Andrade, C.; Ribeiro, C. Population Density, Reproduction Cycle and Nutritional Value of Sphaerechinus granularis (Echinodermata: Echinoidea) in an Oceanic Insular Ecosystem. Front. Mar. Sci. 2022, 8, 2040. [Google Scholar] [CrossRef]
- Maia, H.A.; Morais, R.A.; Quimbayo, J.P.; Dias, M.S.; Sampaio, C.L.S.; Horta, P.A.; Ferreira, C.E.L.; Floeter, S.R. Spatial Patterns and Drivers of Fish and Benthic Reef Communities at São Tomé Island, Tropical Eastern Atlantic. Mar. Ecol. 2018, 39, e12520. [Google Scholar] [CrossRef]
- Ganf, G.G.; Oliver, R.L. Vertical Separation of Light and Available Nutrients as a Factor Causing Replacement of Green Algae by Blue-Green Algae in the Plankton of a Stratified Lake. J. Ecol. 1982, 70, 829–844. [Google Scholar] [CrossRef]
- Emilio Sánchez-Moyano, J.; García-Asencio, I.; Carlos García-Gómez, J. Effects of Temporal Variation of the Seaweed Caulerpa Prolifera Cover on the Associated Crustacean Community. Mar. Ecol. 2007, 28, 324–337. [Google Scholar] [CrossRef]
- Thomsen, M.S.; Wernberg, T.; Altieri, A.; Tuya, F.; Gulbransen, D.; McGlathery, K.J.; Holmer, M.; Silliman, B.R. Habitat Cascades: The Conceptual Context and Global Relevance of Facilitation Cascades via Habitat Formation and Modification. Integr. Comp. Biol. 2010, 50, 158–175. [Google Scholar] [CrossRef]
- Riera, R.; Delgado, J.D.; Rodríguez, M.; Monterroso, O.; Ramos, E. Macrofaunal Communities of Threatened Subtidal Maërl Seabeds on Tenerife (Canary Islands, North-East Atlantic Ocean) in Summer. Acta Oceanol. Sin. 2012, 31, 98–105. [Google Scholar] [CrossRef]
- Bergen, M.; Weisberg, S.B.; Smith, R.W.; Cadien, D.B.; Dalkey, A.; Montagne, D.E.; Stull, J.K.; Velarde, R.G.; Ranasinghe, J.A. Relationship between Depth, Sediment, Latitude, and the Structure of Benthic Infaunal Assemblages on the Mainland Shelf of Southern California. Mar. Biol. 2001, 138, 637–647. [Google Scholar] [CrossRef]
- De Grave, S.; Myers, A.A. Records of Macrobenthic Crustacea from Maërl Habitats in Irish Waters. Bull.-Ir. Biogeogr. Soc. 1999, 23, 101–123. [Google Scholar]
- Deidun, A.; Marrone, A.; Gauci, A.; Galdies, J.; Lorenti, M.; Mangano, M.C.; Cutajar, K.; Mirto, S.; Sarà, G. Structure and Biodiversity of a Maltese Maerl Bed: New Insight into the Associated Assemblage 24 Years after the First Investigation. Reg. Stud. Mar. Sci. 2022, 52, 102262. [Google Scholar] [CrossRef]
- Sánchez-Latorre, C.; Triay-Portella, R.; Cosme, M.; Tuya, F.; Otero-Ferrer, F. Brachyuran Crabs (Decapoda) Associated with Rhodolith Beds: Spatio-Temporal Variability at Gran Canaria Island. Diversity 2020, 12, 223. [Google Scholar] [CrossRef]
- Hall-Spencer, J.M. Conservation issues relating to maerl beds as habitats for molluscs. J. Conchol. Sp. Publ. 1998, 2, 271–286. [Google Scholar]
- Muths, D.; Jollivet, D.; Davoult, D. Population Dynamics Disparities of the Common Brittle-Star Ophiothrix Fragilis between Three Localities of the English Channel. In Proceedings of the 12th International Echinoderm Conference, Durham, NH, USA, 7–11 August 2006; pp. 7–11. [Google Scholar]
- Muths, D.; Jollivet, D.; Gentil, F.; Davoult, D. Large-Scale Genetic Patchiness among NE Atlantic Populations of the Brittle Star Ophiothrix Fragilis. Aquat. Biol. 2009, 5, 117–132. [Google Scholar] [CrossRef]
- Pérez-Portela, R.; Almada, V.; Turon, X. Cryptic Speciation and Genetic Structure of Widely Distributed Brittle Stars (Ophiuroidea) in Europe. Zool. Scr. 2013, 42, 151–169. [Google Scholar] [CrossRef]
- Bilyard, G.R.; Carey, A.G. Distribution of Western Beaufort Sea Polychaetous Annelids. Mar. Biol. 1979, 54, 329–339. [Google Scholar] [CrossRef]
- Pamungkas, J.; Glasby, C.J.; Costello, M.J. Biogeography of Polychaete Worms (Annelida) of the World. Mar. Ecol. Prog. Ser. 2021, 657, 147–159. [Google Scholar] [CrossRef]

| Species | Region | ||||
|---|---|---|---|---|---|
| B | G | M | C | P | |
| Lithophyllum | X | X | |||
| L. africanum (Foslie) | X [57] | ||||
| L. fasciculatum (Lamarck) Foslie | X [58] | ||||
| L. incrustans (Philippi) | X [59] | X [60] | |||
| L. hibernicum (Foslie) | X [61] | ||||
| L. retusum (Foslie) | X [57] | ||||
| L. sp. | X [61] | ||||
| Lithothamnion | X | X | X | X | |
| L. corallioides (P. Crouan and H. Crouan) | X [41] | X [61] | X [54] | X [53] | |
| L. sp. 2 | X [61] | ||||
| Mesophyllum | X | ||||
| M. sphaericum (Peña et al., 2011) | X [62] | ||||
| M. sp. 3 | X [61] | ||||
| M. sp. 4 | X [61] | ||||
| Neogoniolithon | X | X | |||
| N. hirtum (Me. Lemoine) Alfonso-Carrillo | X [53] | ||||
| N. sp. | X [57] | ||||
| Phymatolithon | X | X | X | X | |
| P. calcareum (Pallas) W. H. Adey and D. L. McKibbin | X [46] | X [63] | X [54] | X [53] | |
| P. lamii (Me. Lemoine) Y. Chamberlain | X [48] | ||||
| P. lusitanicum (V. Peña) | X [64] | ||||
| P. purpureum (P. Crouan and H. Crouan) Woelkerling and L. M. Irvine | X [46] | ||||
| Spongites | X | ||||
| S. fruticulosus(Kützing) | X [54] | ||||
| Sporolithon | X | ||||
| S. sp. | X [54] |
| Location | Depth (m) | ||||||
|---|---|---|---|---|---|---|---|
| Date | Region | Site | Latitude | Longitude | Shallow | Intermediate | Deep |
| August-21 | Brittany | 1 | 47°44′30″ N | 3°47′48″ W | 2 | 8 | 16 |
| 2 | 48°18′13″ N | 4°20′07″ W | |||||
| April-17 | Galicia | 1 | 42°33′56″ N | 8°57′55″ W | - | 10 | 17 |
| 2 | 42°34′07″ N | 8°57′45″ W | |||||
| June-16 | Madeira | 1 | 32°43′56″ N | 16°44′19″ W | 16 | 22 | 35 |
| 2 | 32°39′07″ N | 17°00′10″ W | |||||
| January-16 | Gran Canaria | 1 | 27°55′44″ N | 15°21′10″ W | 18 | 25 | 40 |
| 2 | 27°55′44″ N | 15°21′10″ W | |||||
| November-16 | Principe Island | 1 | 1°36′35″ N | 7°20′08″ E | 5 | 10 | 20 |
| 2 | 1°36′13″ N | 7°21′01″ E | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Peris, I.; Navarro-Mayoral, S.; de Esteban, M.C.; Tuya, F.; Peña, V.; Barbara, I.; Neves, P.; Ribeiro, C.; Abreu, A.; Grall, J.; et al. Effect of Depth across a Latitudinal Gradient in the Structure of Rhodolith Seabeds and Associated Biota across the Eastern Atlantic Ocean. Diversity 2023, 15, 103. https://doi.org/10.3390/d15010103
Pérez-Peris I, Navarro-Mayoral S, de Esteban MC, Tuya F, Peña V, Barbara I, Neves P, Ribeiro C, Abreu A, Grall J, et al. Effect of Depth across a Latitudinal Gradient in the Structure of Rhodolith Seabeds and Associated Biota across the Eastern Atlantic Ocean. Diversity. 2023; 15(1):103. https://doi.org/10.3390/d15010103
Chicago/Turabian StylePérez-Peris, Inés, Sandra Navarro-Mayoral, Marcial Cosme de Esteban, Fernando Tuya, Viviana Peña, Ignacio Barbara, Pedro Neves, Claudia Ribeiro, Antonio Abreu, Jacques Grall, and et al. 2023. "Effect of Depth across a Latitudinal Gradient in the Structure of Rhodolith Seabeds and Associated Biota across the Eastern Atlantic Ocean" Diversity 15, no. 1: 103. https://doi.org/10.3390/d15010103
APA StylePérez-Peris, I., Navarro-Mayoral, S., de Esteban, M. C., Tuya, F., Peña, V., Barbara, I., Neves, P., Ribeiro, C., Abreu, A., Grall, J., Espino, F., Bosch, N. E., Haroun, R., & Otero-Ferrer, F. (2023). Effect of Depth across a Latitudinal Gradient in the Structure of Rhodolith Seabeds and Associated Biota across the Eastern Atlantic Ocean. Diversity, 15(1), 103. https://doi.org/10.3390/d15010103









