Abstract
Plant growth-promoting bacteria (PGPB) enhance plant growth, as well as protect plants from several biotic and abiotic stresses through a variety of mechanisms. Therefore, the exploitation of PGPB in agriculture is feasible as it offers sustainable and eco-friendly approaches to maintaining soil health while increasing crop productivity. The vital key of PGPB application in agriculture is its effectiveness in colonizing plant roots and the phyllosphere, and in developing a protective umbrella through the formation of microcolonies and biofilms. Biofilms offer several benefits to PGPB, such as enhancing resistance to adverse environmental conditions, protecting against pathogens, improving the acquisition of nutrients released in the plant environment, and facilitating beneficial bacteria–plant interactions. Therefore, bacterial biofilms can successfully compete with other microorganisms found on plant surfaces. In addition, plant-associated PGPB biofilms are capable of protecting colonization sites, cycling nutrients, enhancing pathogen defenses, and increasing tolerance to abiotic stresses, thereby increasing agricultural productivity and crop yields. This review highlights the role of biofilms in bacterial colonization of plant surfaces and the strategies used by biofilm-forming PGPB. Moreover, the factors influencing PGPB biofilm formation at plant root and shoot interfaces are critically discussed. This will pave the role of PGPB biofilms in developing bacterial formulations and addressing the challenges related to their efficacy and competence in agriculture for sustainability.
1. Introduction
Elevating food production to feed a growing population, which is projected to increase to 9.15 billion in 2050, under exacerbated climate change is one of the greatest challenges facing the agricultural sector [1]. Intensive use of agrochemicals such as fertilizers and pesticides has successfully increased yield of crop production [2,3]. However, the increase in agricultural production is not proportional to the increase in the mentioned input [4,5]. Moreover, the long-term application of agrochemicals causes water-holding capacity depletion, soil fertility reduction, and disparity in soil nutrients [6,7,8,9,10]. Therefore, this leads to undesirable problems such as increasing pollution, land degradation, depletion of natural resources, loss of biodiversity, and rising production costs [3,10].
Widespread adoption of sustainable agricultural practices is urgently needed to address soil health degradation due to injudicious application of chemical inputs and loss of soil microbial diversity. Such practices ensure an integrated system of crop and livestock production in the long term by producing sufficient quantities of high-quality food, minimizing waste and environmental impacts, and using nonrenewable resources efficiently and profitably [11,12]. This environmentally friendly approach has recently gained considerable attention. The application of sustainable agriculture can help preserve ecosystems, promote economic stability of farms, and improve the quality of life of farmers [10]. For these reasons, the focus of a sustainable agriculture must be on balancing social, economic, and ecological goals while managing limited resources [3,13].
Soil is not only an essential tool for crop production, but also a complex living medium [14,15], which must be considered holistically. Soil maintains biological productivity, supports the quality of the air and water environment, and sustains the health of plants, animals, and humans [16]. Therefore, healthy soil must be protected and conserved to ensure long-term productivity and stability [17]. Microorganisms, as biological components of soil, are important key players in nutrient cycling, organic matter decomposition, and soil structure maintenance [18]. Hence, they are widely known as “natural soil engineers” [19] and can be an eco-friendly alternative to maintain soil health and improve crop productivity.
Among the plant-associated microbial communities in soil, PGPB can be formulated as biobased organic biofertilizers and biopesticides due to their ability to maintain a nutrient rich soil environment, improve abiotic stress tolerance, and act as antagonists against various pathogens [2]. Moreover, the use of PGPB in agriculture has no negative impact on the ecosystem, ensures food safety, and creates sustainable crop production [12].
This review highlights the importance of biofilm formation as an essential component of plant–PGPB interactions. Understanding the process, mechanisms, and factors influencing biofilm formation by PGPB will lead to its applications and innovative future prospects.
2. PGPB as Multipotent Bioagents
The rhizosphere can be divided into three zones: the endorhizosphere is the interior of the root, the rhizoplane is the surface of the root, and the ectorhizosphere is the zone that extends from the rhizoplane to the bulk soil and consists of soil that adheres to the root [20,21], in addition to the volume of soil that is not part of the rhizosphere and is not influenced by the root is referred to as bulk soil [22]. Plant growth-promoting bacteria are able to colonize plants through different types of plant–microbe interactions and forming associations on root and shoot surfaces as complex, interactive microbial communities [23,24]. These beneficial bacteria can make an important contribution to numerous ecological processes in the soil, including nutrient transformation and fixation, organic matter decomposition, and mitigation of abiotic and biotic stresses [25,26]. Soil in the rhizosphere surrounding plant roots has a microbial population 100 to 1000 times higher than the rest of the soil, which is influenced by substances excreted by the plant roots [21,27]. PGPB colonizing the rhizospheric region interact with other taxa such as fungi, protozoa, nematodes, and plant viruses.
Roots release mainly high-molecular-weight compounds such as enzymes, proteins, and polysaccharides from the root tips, which are called root exudates. This mixture, which consists of polysaccharide-rich secretions, often forms a sheath of slime on the outer surface of the root called mucilage [28]. Other substances such as sugars, amino acids, hormones, vitamins, phenolics, and other secondary metabolites in the form of low-molecular-weight components are also secreted [29]. These various compounds can enrich the rhizosphere with nutrients, make the environment more comfortable for microorganisms, and help maintain stable soil aggregates [6,30]. In addition, the release of compounds by plant roots that affect the physical and chemical properties of the rhizosphere can attract beneficial microbes in the soil, enriching the microbial community [31].
PGPB play an essential role in regulating soil fertility, nutrient cycling, and promoting plant growth [32]. The major regions where plant–microbe interactions occur include the surface and apoplast of leaf tissue (phyllosphere), the rhizosphere, the inner regions of plant tissue (endosphere), and the bulk soil [33]. The microorganisms that originate from the seed are the first to colonize plants. This seed-derived microbiota is eventually supplemented and partially replaced by rhizosphere microbes that enter the plant through the roots. Partnerships between plants and microbes can vary in intricacy. The plant responds to the presence of a microbe and its metabolites; vice versa, the microbe is affected by the plant environment and reacts to plant metabolism and physiology. Plant exudates attract microbes in the soil toward the root zone. In turn, plant development and health are significantly impacted by the microbiota’s activities in the root zone (Figure 1).
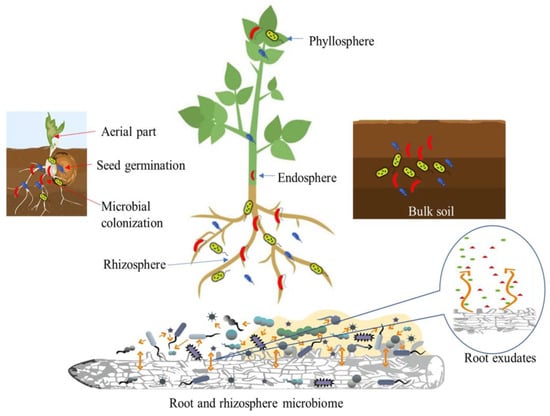
Figure 1.
Plant–microbe interaction: regions involved in the development of plant microbiome.
PGPB generally support plant growth through two types of mechanisms: direct and indirect (Figure 2). The direct mechanisms relate to increasing nutrient availability and phytohormone production. Indirect mechanisms of PGPB, on the other hand, include inhibiting various pathogens or preventing negative effects on plant health. Increasing nutrient availability in the soil can occur through various processes such as nitrogen fixation, solubilization of mineral nutrients, and mineralization of organic compounds [27,32,34,35]. One of the limiting and most important factors in agriculture is the supply of plant nutrients, especially nitrogen, phosphorus, and potassium [27]. Nitrogen fixation is considered an essential trait of PGPB to provide nitrogen through symbiotic and nonsymbiotic mechanisms [27,32]. Phosphate solubilization of mineral forms is possible through secretion of organic acid, release of protons, or production of chelating substances [36]. In contrast, mineralization of organic phosphorus occurs through the synthesis of phosphomonoesterase, phosphodiesterase, and phosphotriesterase, which catalyze the hydrolysis of the phosphoric ester [37]. Potassium in soil is usually deficient as it could be in the form of insoluble stones or silicate minerals [20,38]. Furthermore, phytohormone-producing PGPB can stimulate plant growth and development stages, such as cell elongation, cell division, root development, root hair formation, shoot initiation, and tissue differentiation by providing and regulating various plant hormones, including gibberellins (GAs), cytokinins, abscisic acid (ABA), ethylene, and indole-3-acetic acid (IAA) [39,40].
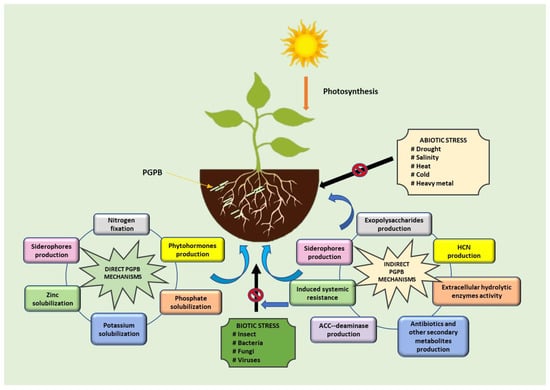
Figure 2.
Direct and indirect mechanisms of PGPB to support the plant development.
PGPB can produce various metabolites, such as antibiotics, siderophores, volatile organic compounds (VOCs), hydrolytic enzymes, hydrogen cyanide (HCN), and 1-aminocyclopropane-1-carboxylic acid deaminase (ACC deaminase), that reduce or prevent pathogenic diseases and protect against environmental stress [35,41,42,43]. Synthesis of antibiotics, which are low-molecular-weight and toxic organic compounds, is a strategy that microorganisms use to compete with other microorganisms. Different microorganisms have the ability to produce different antibiotics. Bacillus can produce several antibiotics such as iturins, mycosubtilin, bacillomycin D, surfactin, fengycin, and zwittermicin A [44]. On the other hand, Pseudomonas protegens FD6 produces a variety of antibiotics including 2,4-diacetylphloroglucinol, pyoluteorin, and pyrrolnitrin to suppress plant pathogens. Zhang et al. [45] conducted a study on the efficacy of FD6 in controlling gray mold on tomato fruit. The results showed that there were no visible disease lesions at 5 days after inoculation. Siderophores are iron chelators that can sequester iron from the environment. Siderophores not only provide essential metal nutrients for the plants but also inhibit the growth of pathogenic microorganisms and trigger the induction of systemic resistance (ISR) in plants [39,42]. Pseudomonas putida B2017 can act negatively against Fusarium oxysporum, Rhizoctonia solani, and Sclerotinia sclerotiorum by secreting siderophores [46]. It has been demonstrated that VOCs produced by microorganisms have antimicrobial effects on plant pathogens by deforming phytopathogenic fungi hyphae and spores [47]. Several VOCs such as 1-undecene, dimethyldisulfide, 2,5-dimethylpyrazine, benzothiazole, 4-chloro-3-methyl, and phenol-2,4-bis(1,1-dimethylethyl) can penetrate the soil and rhizosphere [47,48]. Ossowicki et al. [49] reported that VOCs from Pseudomonas donghuensis P482 significantly inhibited the growth of R. solani AG2.2IIIB and F. culmorum PV. The hydrolytic enzymes, such as chitinases, glucanases, proteases, and lipases are involved in biotic stress mitigation caused by fungi due to their ability to degrade the fungal cell wall [50,51]. Studies by Rasul et al. [52] showed that inoculation of rice seedlings with Pseudomonas sp. MR11 and MR34, and Bacillus sp. MR42 increased plant seed phosphate content, improved plant root, shoot, and grain weight, and suppressed the causal agent of rice bacterial leaf blight. These bacteria increased the enzymes phenylalanine ammonia lyase, catalase, peroxidase, β, 1–3 glucanase, and polyphenol oxidase. Several PGPB can also protect plants from adverse environmental conditions by synthesizing ACC deaminase. Lowering ethylene levels is mediated by the conversion of ACC, the precursor of ethylene, into α-ketobutyrate and ammonia [35,53]. Application of two isolates Aneurinibacillus aneurinilyticus and Paenibacillus sp. to French bean seedlings reduced the negative effects of salt stress and increased root length, root fresh weight, shoot length, shoot fresh weight, root and shoot biomass, and total chlorophyll content [54]. These two isolates showed ACC deaminase activity, as well as several plant growth-promoting traits including production of IAA, siderophores, ammonia, and HCN, and solubilization of P and zinc (Zn). Another indirect mechanism is the ability of PGPB to produce exopolysaccharides and form biofilms [6,55]. Exopolysaccharides are responsible for bacterial adhesion to soil particles and root surfaces; therefore, they are important for bacterial biofilm development [55,56]. Plant-associated biofilms are able of support host plant growth, reduce microbial competition, and protect against pathogens and external stresses [57,58,59,60].
3. Biofilm Formation by PGPB Communities in Varied Agroecosystems
Biofilm is a structured community of microbial cells in which the cells are often embedded in an extracellular matrix (ECM) composed of extracellular polymeric substances (EPSs) and bound to a surface [61,62]. The ECM is the body of the biofilm and is composed of a conglomerate of different types of EPSs [63]. Microorganisms release EPSs to promote the attachment process on biotic or abiotic surfaces, followed by the formation of the ECM that surrounds and hold cells together [62,64]. Thus, the ECM ensures the structural integrity of a biofilm [65]. Cell density of the biofilm is high, ranging from 108 to 1011 cells·g−1 wet weight [66]. The bacteria can form either a single-layered or multilayered biofilm on the surface with single or multiple bacterial species within the ECM [60]. The ECM in the biofilm provides the mechanical stability of the biofilm, mediates cell–cell communication, and induces the formation of synergistic microconsortia, making the biofilm lifestyle different from the planktonic state [66,67]. Therefore, microbial cells in biofilms provide several advantages over their planktonic counterparts, namely, protection, enhanced cell-to-cell adhesiveness and cohesion between microbial cells, improved nutrient accumulation potential, increased gene change rate, increased tolerance to antimicrobial agents, and better survival in adverse environments [62,66].
3.1. Biofilm Structure
The vast majority of a biofilm is formed by the ECM, while the cells of the microorganisms represent less than 10% of the dry mass [64,68]. The EPS contains mainly polysaccharides, proteins including extracellular enzymes, lipids, and nucleic acids (eDNA and eRNA) [62,66]. The main components of EPSs are exopolysaccharides, long linear or branched chain molecules with a high molecular weight of 500–2000 kDa [69]. There are several types of exopolysaccharides that have been isolated and characterized from a wide variety of bacterial species. Most of them are heteropolysaccharides consisting of two or more different monosaccharides. On the other hand, some of them are homopolysaccharides that are made up of single sugars such as sucrose-derived glucans, fructans, and cellulose [69,70]. Cellulose is one of the most abundant homopolysaccharides in nature, consisting of repeating chains of β-1,4 linked D-glucose that form fibrils [71]. Several bacterial genera such as Agrobacterium, Acetobacter, Azotobacter, Rhizobium, Sarcina, Alcaligenes, and various species from the Enterobacteriaceae and Pseudomonadaceae families can synthesize cellulose [71,72]. One of the best-studied models for biofilm formation is Pseudomonas aeruginosa, which produces at least three different exopolysaccharides, including alginate, polysaccharide synthesis locus (Psl) polysaccharide, and pellicle (Pel) [73,74]. Alginate is composed of anionic polymers, such as β-D-mannuronic acid and α-L-guluronic acid [63]. Alginate has multiple functions, including adhesion, scaffold formation, water/nutrient retention, protection from harsh environments, and antimicrobial agents, and it is also responsible for the mechanical stability of mature biofilms [62]. Overproduction of this exopolysaccharide is characteristic of mucoid strains [75]. Psl polysaccharide is a repeating penta-saccharide containing D-mannose, D-glucose, and L-rhamnose [76]. In addition, Psl polysaccharide plays a role in cell surface attachment and maintenance of biofilm architecture [77]. Pel, on the other hand, is a glucose-rich exopolysaccharide, required for pellicle formation at air–liquid interfaces [71]. Several proteins that constitute the EPS exhibit enzymatic properties, such as protein-degrading enzymes, polysaccharide-degrading enzymes, and lipid-degrading enzymes, are produced in the biofilm mode. The degrading enzymes break down biopolymers into low-molecular-mass products that can be used as carbon and energy sources. Therefore, enzyme proteins have functions in either biofilm reorganization or degradation [78]. In addition, nonenzymatic proteins, such as lectins associated with the cell surface and extracellular carbohydrate-binding proteins involved in the formation and stabilization of the polysaccharide matrix network are also found in the EPS [62].
One component of EPS is extracellular DNA (eDNA), which is either self-secreted or derived from lysed bacteria within the community and serves to strengthen the biofilm infrastructure [74]. The presence of eDNA contributes to the structural integrity of the ECM [79]. It also facilitates the exchange of genetic information between bacterial cells within the biofilm, and can increase the biofilm’s tolerance to antimicrobial agents [67,74].
3.2. Biofilm Formation
Development of microbial cells from a free-living planktonic life form into biofilms lifestyle is divided into five main phases. It consists of reversible attachment of bacteria on a surface, irreversible attachment by adhesion to the surface, development of microcolonies, maturation with a three-dimensional structure, and dispersion by the release of bacterial cells to initiate to form a new biofilm [59,80]. Figure 3 shows the phase of biofilm formation.
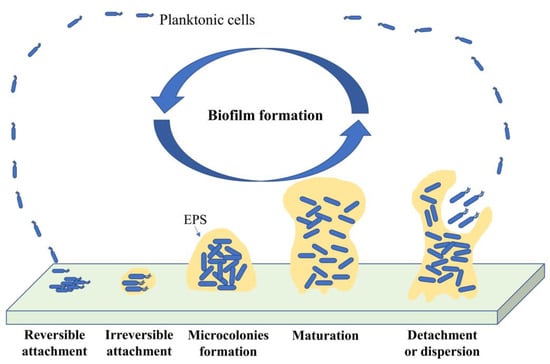
Figure 3.
Schematic diagram of different stages in biofilm development.
3.2.1. Bacterial Attachment to a Surface
The first step of biofilm formation is reversible bacterial attachment to the surface. This initial attachment can be easily dislodged and reassembled, either by bacterial mobility or by the action of fluid shear forces [59,81]. Planktonic cells interact with a substrate surface as a result of a random process mediated by Brownian motion and bacterial surface appendages such as pili, fimbriae, and flagella. Another mechanism for surface transport of bacterial cells is sedimentation due to gravitational forces or convection, in which cells are physically transported to the surface by the movement of the bulk fluid [58,59]. Motile bacteria are more competent to overcome hydrodynamic and repulsive forces by utilizing flagella [82]. Flagellum-based motility can be either forward propelling motion in liquids (swimming) or on solid moist surfaces (swarming) [74,83]. On the other hand, bacteria can exhibit twitching that allows bacterial movement along the surface, driven by type IV pili. Type IV pili play a role in motility, cell–cell adhesion, cell–surface adhesion, and horizontal gene transfer [84,85].
Bacteria constantly change their movement in response to their environment [74]. The ability of motile bacteria to monitor changes in environmental conditions and direct their movement toward a favorable environment in nutrient gradients is called chemotaxis [29,86]. The process of chemotaxis is initiated when a nutrient source or chemoattractants in the environment bind to membrane-anchored receptors and trigger phosphorylation of the associated cytoplasmic histidine kinase CheA. Phosphorylated CheA (CheA-P) interacts with phosphorylated CheY. In addition, CheY-P diffuses through the cytoplasm and then interacts with the flagellar motor, changing the direction of the flagellar motor rotation of the cell toward a favorable environment through a tumble [87,88]. Plants release various complex organic compounds including sugars, amino acids, organic acids, and other small molecules from the roots. Therefore, the chemotaxis response of bacteria to chemical gradients depends on the plant host and root region [29]. O’neal et al. [88] reported that motile cells of A. brasilense accumulated in root hairs and elongation zones of wheat but were shed in the root tip. The increased accumulation of A. brasilense in the root hairs and elongation zones of wheat was caused by attractant organic acids such as pyruvate, malic acid, and succinate, whereas the low accumulation of A. brasilense at the root tips of wheat was due to the production of reactive oxygen species such as hydrogen peroxide.
3.2.2. Adhesion to Surface
The next step in biofilm development is the irreversible attachment of bacterial cells through a permanent bond in the presence of EPSs [80]. The EPSs play an essential role in the host attachment process, acting as a “molecular glue” responsible for the firm anchoring of bacteria to the surface and nurturing contact between cells [59,89,90]. Diazotrophs, such as Rhizobium leguminosarum, Azospirillium brasilense, and Gluconacetobacter diazotrophicus attach to the surface of roots using cellulose microfibrils with agglutinating activity [89]. P. aeruginosa produces at least three types of exopolysaccharides, including Psl polysaccharide, Pel, and alginate, which play an important role in biofilm formation [73,74]. Ghafoor et al. [91] reported that P. aeruginosa mutants deficient in Psl polysaccharide exhibited decreased crystal violet staining of static biofilms at an initial stage of biofilm development. In contrast, mutants deficient in Pel lacked the formation of pellicle at the air–liquid interphase in inert culture medium. At this stage, it becomes more difficult to break the adhesion of bacterial cells to a surface by treatments such as shear force or chemical treatment with enzymes, detergents, disinfectants, and surfactants [80,92].
3.2.3. Microcolony Formation
The production of EPS matrix leads to the growth and aggregation of microorganisms to form microcolonies [80]. Adhesion of bacterial cells to the substrate and between bacterial cells has a major impact on microcolony formation. Microcolony structures form due to the downregulation of the type IV pili-dependent twitch motility and the production of matrix components that adhere tightly to cells within the biofilm, stopping motility [93,94]. The production of biosurfactants such as rhamnolipids from P. aeruginosa plays an additional role in promoting the formation of microcolonies in the initial phase and facilitates the migration of cells to form a mushroom cap in the maturation phase of the biofilm [95]. Several bacteria can form microcolonies on different roots from the tip to the elongation zone [96]. The advantages of forming microcolonies are that they allow substrate exchange between species and remove end products from bacterial cells [59,90].
3.2.4. Biofilm Maturation
After microcolony formation and EPS accumulation, the biofilm community grows by simultaneous cell proliferation [74]. The biofilm expands from a thin layer to up to 100 layers [97]. Formation of the mushroom shape occurs in a sequential process in which a nonmotile bacterial subpopulation forms the initial microcolonies and the mushroom stalk. The nonmotile bacterial subpopulation may form in a subpopulation due to downregulation of motility [59]. Moreover, mature cells supported by type IV pili and guided by a chemotaxis gradient climb up the mushroom stalk due to nutrients and subsequently form the mushroom caps. As expected, bacteria in the biofilm move toward the nutrient-rich zone to maintain their activity and biofilm growth [93,98,99]. Sheraton et al. [99] revealed that the development of mushroom-shape in P. aeruginosa biofilm was influenced by chemotactic motility. Their study showed that chemotaxis-deficient mutants lacking chemotactic motility formed large stalks without caps. In addition, Ghanbari et al. [100] found that the formation of mushroom-shaped structures in the biofilm of P. aeruginosa also depends on the nutrient content and initial density of cells on the stalk.
3.2.5. Biofilm Dispersal
During dispersion, the final step of the biofilm life cycle, individual cells leave the biofilm to resume a planktonic lifestyle. Dispersion is the final stage of a biofilm’s life cycle, during which individual cells detach from the biofilm and resume a planktonic lifestyle. The detachment process is a strategy used by bacterial cells to leave biofilms and begin a new life cycle under suitable environmental conditions [59]. It is a complex process in response to changes in their environment, such as nutrient starvation, oxygen starvation, production of matrix-degrading enzymes, accumulation of toxic products, and passive behaviors such as detachment and erosion by external forces such as shear forces [77]. In addition, bacterial cells within a mature biofilm can also spread when the number of cells reaches a threshold [74]. During the spreading process, genes responsible for cell motility and EPS degradation are usually upregulated, while genes related to EPS production are often downregulated [59]. Quorum sensing (QS) is the intercellular signaling among bacteria. This process has been shown to play a special role in biofilm spreading in response to cell population density through the cell–cell communication system [59]. Insufficient carbon and nitrogen sources may be the critical factors leading to the bacterial dispersal process. In addition, EPS-degrading enzymes, such as the glycoside hydrolases PelA and PslG in P. aeruginosa, also contribute to the detachment of bacteria from the matrix [101]. Another mechanism to escape from mature biofilms is the release of a mixture of D-amino acids, such as D-tyrosine, D-leucine, D-tryptophan, and D-methionine. This mechanism is controlled by racemase enzymes, which catalyze the stereochemical conversion of L- to D-amino acids. Studies by Guilhen et al. [102] have shown that dispersed biofilm cells are more efficient at attaching to plant roots, forming microcolonies, and surviving better than their planktonic counterparts. Dispersion is considered an important stage for biofilm control as the planktonic state becomes more susceptible to immune responses and antimicrobial agents.
3.3. Factors Responsible for Biofilm Formation
Several factors are largely responsible for the formation of biofilms. Abiotic factors are involved in biofilm formation, such as nutrient availability, temperature, pH, oxygen, and surface physicochemical properties. Biotic factors such as microbial cells and metabolites produced by bacteria also influence biofilm formation [89,103]. Nutrient-depleted conditions stress microorganisms but stimulate biofilm formation. However, prolonged nutrient deprivation prevents biofilms from advancing their maturation [104,105]. Savijoki et al. [106] reported that culturing Lactobacillus rhamnosus GG in a medium containing 2% fructose increased biofilm formation twofold compared to 2% glucose and that planktonic cells cultured on fructose exhibited higher adherence levels compared to glucose. The concentration of carbon sources also affects biofilm formation. In a study by Zou and Liu [107], different carbon sources such as glucose, maltose, lactose, and sucrose were used at different concentrations (from 0–10 mg/L) to induce biofilm formation. The results showed that the optimal concentration for Staphylococcus epidermidis biofilm production was 2.5 mg/mL for all carbon sources. As mentioned earlier, biofilms are stimulated under stress conditions. Wang et al. [108] demonstrated that P. stutzeri A1501 formed a biofilm with the highest biomass in lactate-containing K (LK) medium without added nitrogen, compared to LK medium supplemented with ammonia chloride as a nitrogen source.
Extreme temperatures and pH negatively affect biofilm formation [103]. The optimal growth temperature for Listeria monocytogenes was 37 °C. Moreover, biofilm production of 58 isolates of L. monocytogenes was five times higher at 37 °C than at 10 °C [104]. Citrobacter werkmanii BF-6 has been shown to grow in a wide pH range. Interestingly, biofilm formation increased with decreasing pH at 30 °C in LB medium, although it inhibited bacterial growth [109]. A study by Haque et al. [110] showed that Enterobacter asburiae ENSD102, Enterobacter ludwigii ENSH201, Vitreoscilla sp. ENSG301, Acinetobacter lwoffii ENSG302, and Bacillus thuringiensis ENSW401 developed thick and robust air–liquid biofilms at pH 7. In addition, E. asburiae ENSD102 and Vitreoscilla sp. ENSG301 produced optimal solid–air–liquid biofilms at pH 4. Restriction of oxygen availability also affects bacterial biofilm metabolic activity and usually triggers active dispersal [111].
Cell adhesion to the surface is a prerequisite for colonization [103]. However, the physicochemical properties of the surface between the substrate and the bacteria influence each other [59]. For example, surface roughness may promote bacterial attachment and biofilm development due to lower shear forces and greater surface area. However, bacteria prefer to adhere to hydrophobic and nonpolar surfaces rather than hydrophilic surfaces because hydrophobicity reduces the repulsive forces between the surface of the bacteria and the substrate [59,89].
QS is an intercellular signaling in bacteria that regulates gene expression in response to cell population density via a cell–cell communication system. It is mediated by the production and detection of signaling molecules called autoinducers, such as N-acylhomoserine lactones (AHL) in Gram-negative bacteria, oligopeptides in Gram-positive bacteria, and autoinducers-2 (AI-2) in both Gram-negative and Gram-positive bacteria [20,75,112,113]. QS contributes to several key ecological and interdependent properties of bacteria, such as secretion of antibiotics, siderophores, or enzymes, virulence factors of phytopathogens, and communication between plants and microbes [58,114]. QS in Aeromonas regulates the expression of biofilm formation, motility, and virulence [96,115].
Lipopeptides such as serawettins and rubiwettins were produced by Serratia marcescens and Serratia rubidaea, respectively, indicating wetting activity involved in surface colonization [116]. In contrast, B. subtilis produced antibacterial agents such as surfactin and bafilomycin L, which are important for swarm motility. Moreover, the inability of B. subtilis mutants to produce surfactin, bacillomycin L, or both surfactin and bafilomycin L indicated a significant reduction in biofilm formation [117]. Zhu et al. [118] also reported that the expression of genes encoding the mutants was lower than wildtype B. pumilus HR10, affecting swarm motility performance, polysaccharide content, and biofilm formation. Surfactin reduces surface tension and acts as a wetting agent, thereby affecting bacterial swarm motility [117].
EPSs are responsible for facilitating cell-to-surface and cell-to-cell interactions, aggregation or adhesion of cells to the surface, diffusion barrier to protect the cell, and regulation of biofilm formation and structure [60]. EPSs can suppress cell adhesion through electrostatic forces, while large amounts enhance adhesion through interactions between functional groups in EPS [90]. In addition to EPSs, eDNA plays an important role in biofilm formation, including mediating cell–cell interactions, and it is mainly found in the stalk region of microcolonies [74].
Bacteria produce siderophores to chelate iron under low iron availability [119,120]. Under Fe-limiting conditions, microbial surface hydrophobicity and biofilm formation decrease [121]. Guo et al. [122] used E. coli UTI89 as a model organism to study the effect of different iron concentrations. They found that biofilm formation increased under an iron concentration of 0 to 10 μM, but was completely inhibited in the presence of 2000 μM iron. Thus, the iron siderophore plays a role in the formation of structured biofilms. Siderophore-deficient mutants of P. aeruginosa showed a decreased ability to form biofilms [123].
4. Role of Biofilm-Forming PGPB in Sustainable Agriculture
PGPB biofilms are found in various niches within agroecosystems. Rhizosphere and plant roots are hotspots for PGPB interactions as significant amounts of nutrients are released from plant roots [29]. Diverse PGPB are able to form microcolonies on different parts of roots, from the tip to the elongation zone. They also grow into large populations and form mature biofilms [124]. Most studies on root colonization patterns have shown that PGPB colonization in the rhizosphere forms microcolonies or aggregates on root surfaces. The distribution of these colonies is patchy and nonuniform [58]. Living in biofilms provides several benefits to PGPB, including facilitating bacterial interactions with plants, improving bacterial resistance to adverse environments, protecting against pathogens, and enhancing uptake of nutrients released in the plant environment [118]. Many beneficial bacterial biofilms associated with various plants have been studied (Table 1).

Table 1.
Role of PGPB biofilms associated with various plant species.
4.1. Biocontrol Activity against Plant Pathogens
Evidence that the survival, persistence, and virulence mechanisms of microorganisms with biocontrol potential are largely related to their biofilm lifestyle has been well established since the early 1980s [137,138]. Various PGPB efficiently occupy, colonize, and form biofilms in/on their rhizospheric or phyllospheric niches as their own survival strategy, as well as to mediate plant control activities [103,139]. Plant-associated biofilms are able to protect plants from pathogenic microorganisms by competing with and inhibiting their colonization, stimulating ISR, and producing antimicrobials [34,45,58,131,134].
Several research groups [140,141,142,143] have demonstrated that a lipopeptide “surfactin” triggers bacillus biofilm formation via KinC activation of the Spo0A pathway. Zeriouh et al. [144] demonstrated that surfactin produced by B. subtilis strain UMAF6614 triggers biofilm formation on melon phylloplane and contributes to its biocontrol activity against plant pathogens (Pectobacterium carotovorum, Xanthomonas campestris, and Podosphaera fusca). Biofilm formation by biopesticides can be stimulated by plant root exudates or through exposure of the microorganisms to antimicrobial products or stress [125,145,146,147]. Haggag and Timmusk [125] demonstrated that biofilm formation by Paenibacillus polymyxa B5 on peanut plant inhibits colonization of the pathogenic fungus, Aspergillus niger, as well as helps in reducing 96.7% crown rot disease and successfully promoting 43.5% of plant yield. Furthermore, Timmusk et al. [148] demonstrated the antagonistic abilities of two P. polymyxa strains against Phytophthora palmivora and Pythium aphanidermatum in an A. thaliana model system. Biofilm-forming PGPB significantly protected the plants from various pathogens, resulting in increased plant productivity. However, there were only a few reports that included plant yield information as a consequence of biocontrol agents of biofilm-forming PGPB.
4.1.1. Root Colonization
Colonization of the rhizosphere or roots by PGPB plays essential roles in eliminating pathogenic microorganisms by reducing the availability of root exudates and triggering an innate immune response [139]. As a result, horizontal filtering of soil bacteria occurs, leading to a reduction in bacterial diversity in the soil and greater bacterial specialization on the root. Subsequently, interactions among soil bacteria increase, leading to higher bacterial persistence and colonization of roots through processes such as metabolic exchanges, secretion of antimicrobial substances, and other processes. The ability of PGPB to colonize faster, as well as compete with nutrients and niches, represents the basic mechanisms that protect plants from phytopathogens [149]. Fan et al. [150] reported that the Gram-positive rhizobacterium Bacillus amyloliquefaciens FZB42 can colonize different plants with a specific region on the roots. FZB42 preferentially colonized the tips of the primary roots of Arabidopsis, along the furrows between the epidermal cells of the roots, and in the concave spaces on the ventral sides of the fronds of Lemna. This is dependent on the availability of plant tissue, water, and nutrients [103]. A study by Harting et al. [126] showed that inoculation of P. synxantha or P. brassicacearum on A. thaliana seedlings successfully protected the roots from Verticillium dahliae colonization. The roots of A. thaliana treated with the bacterial culture were examined under a fluorescence microscope. Fewer fungal hyphae were detected on the roots, and large portions of the root were free of fungal hyphae. This suggests that those Pseudomonas isolates may be an effective biocontrol agent. In addition, Haggag and Timmusk [125] used P. polymyxa to colonize and form a biofilm on the root of A. thaliana to inhibit crown root rot caused by A. niger. Their study showed that P. polymyxa B5 significantly colonized the root, and the density reached 109 bacteria per gram of soil after 30 days. The results may indicate that the higher population densities of strain B5 in the rhizosphere effectively control A. niger.
4.1.2. Triggering Induced Systemic Resistance (ISR)
Induced systemic resistance is one of the plant defense strategies initiated by beneficial microbes such as PGPB and mycorrhizal fungi against pathogenic attack [151,152,153]. The density of PGPB must to reach 105–107 colony-forming units (CFU) per gram of root to elicit ISR [151]. Many plants such as bean, cucumber, tobacco, tomato, carnation, radish, and A. thaliana can use ISR as a protective mechanism against a variety of plant pathogens, including bacteria, fungi, viruses, and insects [154,155].
Phytohormone signaling pathways such as salicylic acid (SA), jasmonic acid (JA), and ethylene (ET) are required as primary regulators in the plant immune signaling network [131,152,154]. In general, SA is effective against biotrophic pathogens, whereas JA/ET is effective against necrotrophic pathogens and insects [131,153]. B. cereus AR156 was involved in both the SA and JA/ET pathways and triggered ISR against the biotrophic pathogen Pseudomonas syringe DC3000 on A. thaliana [156]. In contrast, B. cereus AR156 activated only JA/ET signaling pathways and stimulated ISR against necrotrophic Botrytis cinerea on Arabidopsis. Activation of signal transduction pathways during ISR depends on ISR-inducing strains, host plants, and pathogens [154]. According to Zhu et al. [118], the wildtype B. pumilus HR10 was able to colonize the roots of Pinus massoniana seedlings and inhibit R. solani better than the biofilm-deficient mutant MA15. Their study also showed that the expression of PR2, which regulates the salicylic acid-related gene of B. pumilus HR10, was higher than that of the biofilm-deficient mutants and the control. This suggests that B. pumilus HR10 contributes to the initiation of ISR. Zebelo et al. [157] demonstrated biocontrol activity of Bacillus spp. against Spodoptera exigua larvae when larvae were fed cotton (Gossypium hirsutum) inoculated with Bacillus spp.
4.1.3. Antimicrobial-Producing Biofilm
Microbes produce biosurfactants, such as polysaccharide–protein complexes, lipopeptides, glycolipids, phospholipids, fatty acids, and natural lipids. Lipopeptides have high surface activity and antibiotic potential that can be used as biocontrol agents [158]. Bacillus subtilis is a recognized biocontrol agent due to the production of lipopeptides such as surfactin, iturin, and fengicin. Hazarika et al. [159] found that B. subtilis SCB-1 produces iturin and exhibits strong antagonistic activity against several phytopathogenic fungi belonging to the genera Alternaria, Cochliobolus, Curvularia, Fusarium, Neodeightonia, Phomopsis, and Saccharicola. Luo et al. [117] also reported that the surfactin-deficient mutant and bafilomycin-deficient mutant of B. subtilis 916 altered swarming motility, reduced biofilm formation, and reduced antagonistic activity of Rhizoctonia solans-infected rice scales. Moreover, Stoll et al. [127] concluded that surfactin production by Bacillus velezensis is involved in biofilm formation and stable colonization to further activate ISR. The lipopeptide syringomycin produced by Pseudomonas spp. was able to inhibit the growth of saprophytic Aspergillus nidulans and Verticillium spp. Moreover, the inhibitory effect depends on the presence of the gene encoding the transcriptional regulator LuxR or genes involved in the synthesis of syringomycin [126]. In addition to antibiotics, PGPB also produce exoproteases, HCN, or phenazines [27].
4.2. Promoting Plant Growth by Biofilm-Forming PGPB
Beneficial biofilms attached to plant roots aid in nutrient cycling, provide essential macro- and micronutrients, and produce growth-promoting substances, including auxins (indolyl-3-acetic acid), gibberellins, and cytokinins [40]. A study conducted by Haque et al. [132] showed that 26.9% of rhizobacterial strains isolated from drought ecosystems were able to form biofilms in a NaCl-containing liquid culture and exhibit several plant growth-promoting properties such as nitrogen fixation, solubilization of nutrients (P, K, and Zn), and production of IAA, ammonia, siderophores, ACC deaminase, catalases, lipases, cellulases, and proteases. Inoculation of Xanthomonas, Stenotrophomonas, and Microbacterium isolates that formed a biofilm in the soil significantly increased the fresh weight of Arabidopsis plants and resulted in systemic resistance to downy mildew [131]. Biofilms of Rhizobium miluonense Rm3 and Burkholderia anthina Ba8 were able to dissolve inorganic phosphates, lower pH, and release organic acids [160]. In addition, consortium biofilms of PGPB and Aspergillus were able to dissolve phosphorus, produce IAA, and exhibit higher nitrogenase activity than their bacterial and fungal counterparts [161]. Further work by Hazarika et al. [129] found that Stenotrophomonas sp. K96 and Pseudomonas sp. M45 exhibited strong biofilm formation and colonization on tea roots, as well as synergistic plant growth-promoting properties, resulting in significant increases in several plant growth-promoting vegetative parameters. Bandara et al. [162] reported that the production of indole acetic acid-like substances (IAAS) and the acidity of biofilms composed of endophytic bacteria and fungi were higher than those of mixed cultures, fungi, or bacteria. The acidity was higher because biofilms release H+, which is reflected in IAA production and solubilization of minerals. In addition, microbial acid production is important for the suppression of plant pathogens [163].
Naturally, biofilm formation in the rhizosphere is composed of several bacterial species [29,164]. Several studies have shown that mixed biofilm-based inoculants form stronger biofilms than single species, suggesting collaboration among strains [29]. A four-species consortium consisting of Stenotrophomonas rhizophila, Xanthomonas retroflexus, Microbacterium oxydans, and Paenibacillus amylolyticus produced greater biofilm biomass than individual species, suggesting that all individual strains benefit from inclusion in the multispecies community [164]. A multispecies consortium of Bacillus spp. and Microbacterium sp. isolated from the rhizosphere showed high synergy in biofilm formation [29]. Biofilm formation of PGPB on plant roots helped to enhance photosynthesis and leaf growth. Co-inoculation of 50% recommended fertilizers with PGPB biofilm biofertilizers helps to increase leaf growth [20]. Conventional application of monocrops or inoculation with mixed crops may not result in the highest microbial effects than application of biofilm consortia [60]. Prasanna et al. [165] showed that inoculation with cyanobacteria induces a plant defense response and increased Zn mobilization in corn hybrids. They also confirmed that cyanobacterial treatment resulted in significant changes in glomalin-related soil proteins and polysaccharides in the soil and the activity of defense enzymes in plant roots and shoots.
4.3. Mitigating Abiotic Stress in Plants by Biofilm-Producing PGPB
Biofilm formation is considered a primary protective strategy against unpredictable and adverse environmental conditions. Therefore, PGPB can survive in biofilms in the rhizosphere and interact with plants better than planktonic cells. Cells within the biofilm matrix have been shown to be more resistant to antimicrobial compounds, desiccation, and UV light [166]. Environmental stresses such as nutrients and osmotic stress lead to increased competition among bacteria for available nutrients. Therefore, planktonic bacteria congregate and form biofilms to protect them in the rhizosphere. In addition, increased production of exopolysaccharides can support biofilms and improve tolerance to abiotic stressors [57].
Studies by Kasim et al. [57], showed that 20 of the PGPB tested had the ability to form a biofilm under 0, 250, 500, and 1000 mM NaCl, which increased with increasing salt concentration. In addition, barley grains coated with nine selected biofilm-forming PGPB isolates showed that they attenuated the deleterious effect of salinity and had higher shoot length by 10–15%, fresh mass of shoots and roots by 64% and 35%, respectively, dry mass of shoots and roots by 41–43% and 8–27%, respectively, and relative water content of shoots and roots by ±7% and 12–15%, respectively, compared to the control. A similar result was also reported by [167]. Inoculation of salt-tolerant strains Pseudomonas plecoglossicida PB5 and Bacillus licheniformis AP6, which had the ability to form a biofilm, withstood salt stress better than non-inoculated plants, significantly promoted dry mass (89–96%), and improved photosynthetic pigments (10–67%), gas exchange activities (42–67%), and nutrient uptake (9–14%) in sunflower. Some PGPB that form EPS can bind cations such as Na+, suggesting a role in alleviating salt stress by reducing Na+ availability, increasing K+ absorption, and improving water uptake [20,133].
According to Wang et al. [134], biofilms of Bacillus amyloliquefaciens 54 improved root colonization and drought tolerance in tomato plants. In addition, plants inoculated with hyper-robust biofilm (ΔabrB and ΔywcC) of mutant B. amyloliquefaciens 54 were better able to withstand drought stress. In another study conducted by Timmusk et al. [168], Bacillus thuringiensis AZP2 formed a biofilm and produced an EPS matrix around wheat root hairs, which is an important strategy to improve tolerance to drought stress. Their study also found that AZP2 produced small amounts of alginate, which has the property of retaining water. Thus, it may increase tolerance to drought stress. In addition, Ansari et al. [135] reported that the strain Pseudomonas azotoforman FAP5 effectively alleviated drought stress in wheat plants through enhanced biofilm development, photosynthetic pigment efficiency, antioxidant enzymatic activities, and root colonization. Moreover, plant inoculated with strain FAP5 had higher root adhering soil per root tissue (RAS/RT) by 29% compared to the control. Enhancement of RAS/RT indicates an increase in soil aggregation and higher water-holding capacity around the roots, which improves nutrient uptake in plants. A consortium of four species, Stenotrophomonas rhizophila, Paenibacillus amylolyticus, Microbacterium oxydans, and Xanthomonas retroflexus, had the strongest synergy in biofilm formation and induced drought tolerance through increased ABA biosynthesis and chlorophyll content [136]. It has also been reported that some soil bacteria form biofilms in response to heavy metal pollution and scavenge heavy-metal ions such as arsenic, lead, mercury, and cadmium, leading to attenuation of heavy-metal contamination of plants in polluted areas [169,170].
5. Conclusions and Future Prospects
PGPB biofilms are considered to have a fundamental role in future agriculture to achieve sustainable development goals. They can provide an alternative to agrochemicals in the agricultural sector as biofertilizers and biocontrol agents. Beneficial biofilms on plant roots help in nutrient cycling, provide inorganic nutrients (N, P, and K), and mitigate abiotic stress. They protect plants from pathogens by competing with and colonizing pathogenic microorganisms, stimulating ISR, and producing antimicrobials. Moreover, the beneficial effects of the interaction between plants and PGPB are enhanced by the formation of a biofilm toward the planktonic form. PGPB as a bioinoculant sometimes fails to competitively colonize plant roots and rhizosphere. Therefore, PGPB biofilm formulation is one of the strategies in bioinoculant development to overcome conflicting in vivo effects. However, significant efforts are still required to develop effective formulations for sustainable agriculture based on biofilms producing PGPB. The most important steps will be (a) the selection of biofilms producing PGPB over planktonic PGPB, (b) crop-specific plant/biofilm-producing PGPB interaction studies before preparing them for commercial scale, (c) the formulation’s physical form (e.g., liquid, solid, wettable powder), (d) carrier material selection for the formulation, etc. In addition, it is necessary to understand the communication of bacteria within the biofilm with other microorganisms and plants through a variety of molecular signals. This will help stabilize the effect of biofilm–plant interactions and provide insight into microbial ecology.
Author Contributions
Conceptualization, K.P.; writing—original draft preparation, N.A. and A.F.; writing—review and editing, A.F., A.K.P., A.R. and K.P.; supervision, K.P.; funding acquisition, K.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a grant from the “Fly ash as the precursor of functionalized materials for applications in environmental engineering, civil engineering, and agriculture” project (no. POIR.04.04.00-00-14E6/18-00), carried out within the TEAM-NET program of the Foundation for Polish Science, co-financed by the European Union under the European Regional Development Fund.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Calicioglu, O.; Flammini, A.; Bracco, S.; Bellù, L.; Sims, R. The Future Challenges of Food and Agriculture: An Integrated Analysis of Trends and Solutions. Sustainability 2019, 11, 222. [Google Scholar] [CrossRef]
- Dhir, B. Biofertilizers and Biopesticides: Eco-Friendly Biological Agents. In Advances in Environmental Biotechnology; Kumar, R., Sharma, A.K., Ahluwalia, S.S., Eds.; Springer Nature Singapore: Singapore, 2017; pp. 1–288. ISBN 9789811040412. [Google Scholar]
- Siebrecht, N. Sustainable Agriculture and Its Implementation Gap—Overcoming Obstacles to Implementation. Sustainability 2020, 12, 3853. [Google Scholar] [CrossRef]
- Zhang, F.; Shen, J.; Zhang, J.; Zuo, Y.; Li, L.; Chen, X. Rhizosphere Processes and Management for Improving Nutrient Use Efficiency and Crop Productivity. Implications for China. In Advances in Agronomy: Volume 107; Sparks, D.L., Ed.; Elsevier Inc.: Oxford, UK, 2010; pp. 1–222. ISBN 9780123810335. [Google Scholar]
- Pii, Y.; Mimmo, T.; Tomasi, N.; Terzano, R.; Cesco, S.; Crecchio, C. Microbial Interactions in the Rhizosphere: Beneficial Influences of Plant Growth-Promoting Rhizobacteria on Nutrient Acquisition Process. A Review. Biol. Fertil. Soils 2015, 51, 403–415. [Google Scholar] [CrossRef]
- Gupta, G.; Parihar, S.S.; Ahirwar, N.; Snehi, S.; Singh, V. Plant Growth Promoting Rhizobacteria (PGPR): Current and Future Prospects for Development of Sustainable Agriculture. J. Microb. Biochem. Technol. 2015, 7, 96–102. [Google Scholar] [CrossRef]
- Mahmood, I.; Imadi, S.; Shazadi, K.; Gul, A.; Hakeem, K. Plant, Soil and Microbes: Volume 1: Implications in Crop Science. In Plant, Soil and Microbes: Volume 1: Implications in Crop Science; Hakeem, K.R., Akhtar, M.S., Abdullah, S.N.A., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 1–366. ISBN 9783319274553. [Google Scholar]
- Nicolopoulou-Stamati, P.; Maipas, S.; Kotampasi, C.; Stamatis, P.; Hens, L. Chemical Pesticides and Human Health: The Urgent Need for a New Concept in Agriculture. Front. Public Health 2016, 4, 148. [Google Scholar] [CrossRef] [PubMed]
- Haskett, T.L.; Tkacz, A.; Poole, P.S. Engineering Rhizobacteria for Sustainable Agriculture. ISME J. 2020, 15, 949–964. [Google Scholar] [CrossRef]
- Gebska, M.; Grontkowska, A.; Swiderek, W.; Golebiewska, B. Farmer Awareness and Implementation of Sustainable Agriculture Practices in Different Types of Farms in Poland. Sustainability 2020, 12, 8022. [Google Scholar] [CrossRef]
- Reganold, J.P.; Papendick, R.I.; Parr, J.F. Sustainable Agriculture. Sci. Am. 1990, 262, 112–120. [Google Scholar] [CrossRef]
- Velten, S.; Leventon, J.; Jager, N.; Newig, J. What Is Sustainable Agriculture? A Systematic Review. Sustainability 2015, 7, 7833–7865. [Google Scholar] [CrossRef]
- Farrell, A.; Hart, M. What Does Sustainability Really Mean?: The Search for Useful Indicators. Environment 1998, 40, 4–31. [Google Scholar] [CrossRef]
- Wong, W.S.; Tan, S.N.; Ge, L.; Chen, X.; Yong, J.W.H. The Importance of Phytohormones and Microbes in Biofertilizers. In Bacterial Metabolites in Sustainable Agroecosystem; Sustainable Development and Biodiversity; Maheshwari, D., Ed.; Springer: Cham, Switzerland, 2015; Volume 12, pp. 105–158. ISBN 9783319246543. [Google Scholar]
- Lehman, R.M.; Cambardella, C.A.; Stott, D.E.; Acosta-Martinez, V.; Manter, D.K.; Buyer, J.S.; Maul, J.E.; Smith, J.L.; Collins, H.P.; Halvorson, J.J.; et al. Understanding and Enhancing Soil Biological Health: The Solution for Reversing Soil Degradation. Sustainability 2015, 7, 988–1027. [Google Scholar] [CrossRef]
- Bünemann, E.K.; Bongiorno, G.; Bai, Z.; Creamer, R.E.; De Deyn, G.; de Goede, R.; Fleskens, L.; Geissen, V.; Kuyper, T.W.; Mäder, P.; et al. Soil Quality—A Critical Review. Soil Biol. Biochem. 2018, 120, 105–125. [Google Scholar] [CrossRef]
- Szczepanek, M.; Piotrowska-Dlugosz, A.; Kanopka, I. Sustainable Crop Production Protects the Quality of Soil and Plant Raw Materials. Agronomy 2021, 11, 1178. [Google Scholar] [CrossRef]
- Mumtaz, M.; Ahmad, M.; Jamil, M.; Hussain, T. Zinc Solubilizing Bacillus spp. Potential Candidates for Biofortification in Maize. Microbiol. Res. 2017, 202, 51–60. [Google Scholar] [CrossRef]
- Varma, A.; Choudhary, D.K. Mycorrhizosphere and Pedogenesis; Varma, A., Choudary, D., Eds.; Springer: Singapore, 2019; ISBN 9789811364792. [Google Scholar]
- Gupta, G.; Snehi, S.K.; Singh, V. Role of PGPR in Biofilm Formations and Its Importance in Plant Health. In Biofilms in Plant and Soil Health; Ahmad, I., Husain, F.M., Eds.; John Wiley & Sons Ltd.: London, UK, 2017; ISBN 9781119246329. [Google Scholar]
- Hakim, S.; Naqqash, T.; Nawaz, M.S.; Laraib, I.; Siddique, M.J.; Zia, R.; Mirza, M.S.; Imran, A. Rhizosphere Engineering With Plant Growth-Promoting Microorganisms for Agriculture and Ecological Sustainability. Front. Sustain. Food Syst. 2021, 5, 617157. [Google Scholar] [CrossRef]
- York, L.M.; Carminati, A.; Mooney, S.J.; Ritz, K.; Bennett, M.J. The Holistic Rhizosphere: Integrating Zones, Processes, and Semantics in the Soil Influenced by Roots. J. Exp. Bot. 2016, 67, 3629–3643. [Google Scholar] [CrossRef] [PubMed]
- Sasse, J.; Martinoia, E.; Northen, T. Feed Your Friends: Do Plant Exudates Shape the Root Microbiome? Trends Plant Sci. 2018, 23, 25–41. [Google Scholar] [CrossRef] [PubMed]
- Xiong, C.; Singh, B.K.; He, J.Z.; Han, Y.L.; Li, P.P.; Wan, L.H.; Meng, G.Z.; Liu, S.Y.; Wang, J.T.; Wu, C.F.; et al. Plant Developmental Stage Drives the Differentiation in Ecological Role of the Maize Microbiome. Microbiome 2021, 9, 171. [Google Scholar] [CrossRef]
- Suman, J.; Rakshit, A.; Ogireddy, S.D.; Singh, S.; Gupta, C.; Chandrakala, J. Microbiome as a Key Player in Sustainable Agriculture and Human Health. Front. Soil Sci. 2022, 2, 821589. [Google Scholar] [CrossRef]
- Sehrawat, A.; Sindhu, S.S. Potential of Biocontrol Agents in Plant Disease Control for Improving Food Safety. Def. Life Sci. J. 2019, 4, 220–225. [Google Scholar] [CrossRef]
- Goswami, D.; Thakker, J.N.; Dhandhukia, P.C. Portraying Mechanics of Plant Growth Promoting Rhizobacteria (PGPR): A Review. Cogent Food Agric. 2016, 2, 1127500. [Google Scholar] [CrossRef]
- Galloway, A.F.; Knox, P.; Krause, K. Sticky Mucilages and Exudates of Plants: Putative Microenvironmental Design Elements with Biotechnological Value. New Phytol. 2020, 225, 1461–1469. [Google Scholar] [CrossRef] [PubMed]
- Saleh, D.; Sharma, M.; Seguin, P.; Jabaji, S. Organic Acids and Root Exudates of Brachypodium distachyon: Effects on Chemotaxis and Biofilm Formation of Endophytic Bacteria. Can. J. Microbiol. 2020, 66, 562–575. [Google Scholar] [CrossRef] [PubMed]
- Ahkami, A.; White, R.; Handakumbura, P.; Jansson, C. Rhizosphere Engineering: Enhancing Sustainable Plant Ecosystem Productivity. Rhizosphere 2017, 3, 233–243. [Google Scholar] [CrossRef]
- Zuluaga, M.Y.A.; Milani, K.M.L.; Gonçalves, L.S.A.; De Oliveira, A.L.M. Diversity and Plant Growth-Promoting Functions of Diazotrophic/N-Scavenging Bacteria Isolated from the Soils and Rhizospheres of Two Species of Solanum. PLoS ONE 2020, 15, e0227422. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishna, W.; Yadav, R.; Li, K. Plant Growth Promoting Bacteria in Agriculture: Two Sides of a Coin. Appl. Soil Ecol. 2019, 138, 10–18. [Google Scholar] [CrossRef]
- del Carmen Orozco-Mosqueda, M.; del Carmen Rocha-Granados, M.; Glick, B.R.; Santoyo, G. Microbiome Engineering to Improve Biocontrol and Plant Growth-Promoting Mechanisms. Microbiol. Res. 2018, 208, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Salomon, M.; Pinter, I.; Piccoli, P.; Bottini, R. Use of Plant Growth-Promoting Rhizobacteria as Biocontrol Agents: Induced Systemic Resistance Against Biotic Stress in Plants. In Microbial Applications; Kalia, V., Ed.; Springer: Cham, Switzerland, 2017; Volume 2, pp. 1–336. ISBN 9783319526690. [Google Scholar]
- Glick, B.R.; Gamalero, E. Recent Developments in the Study of Plant Microbiomes. Microorganisms 2021, 9, 1533. [Google Scholar] [CrossRef]
- Gamalero, E.; Bona, E.; Todeschini, V.; Lingua, G. Saline and Arid Soils: Impact on Bacteria, Plants, and Their Interaction. Biology 2020, 9, 116. [Google Scholar] [CrossRef]
- Jarosch, K.A.; Kandeler, E.; Frossard, E.; Bünemann, E.K. Is the Enzymatic Hydrolysis of Soil Organic Phosphorus Compounds Limited by Enzyme or Substrate Availability? Soil Biol. Biochem. 2019, 139, 107628. [Google Scholar] [CrossRef]
- Samantray, J.; Anand, A.; Dash, B.; Ghosh, M.; Behera, A. Silicate Minerals—Potential Source of Potash—A Review. Miner. Eng. 2022, 179, 107463. [Google Scholar] [CrossRef]
- Santoyo, G.; Sánchez-Yáñez, J.M.; de los Santos-Villalobos, S. Methods for Detecting Biocontrol and Plant Growth-Promoting Traits in Rhizobacteria. In Methods in Rhizosphere Biology Research; Springer Nature Singapore: Singapore, 2019; pp. 133–149. [Google Scholar] [CrossRef]
- Kang, S.M.; Khan, A.L.; Waqas, M.; Asaf, S.; Lee, K.E.; Park, Y.G.; Kim, A.Y.; Khan, M.A.; You, Y.H.; Lee, I.J. Integrated Phytohormone Production by the Plant Growth-Promoting Rhizobacterium Bacillus tequilensis SSB07 Induced Thermotolerance in Soybean. J. Plant Interact. 2019, 14, 416–423. [Google Scholar] [CrossRef]
- Olanrewaju, O.S.; Glick, B.R.; Babalola, O.O. Mechanisms of Action of Plant Growth Promoting Bacteria. World J. Microbiol. Biotechnol. 2017, 33, 197. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.; Prasad, V.; Lata, C. Bacillus: Plant Growth Promoting Bacteria for Sustainable Agriculture and Environment. In New and Future Developments in Microbial Biotechnology and Bioengineering; Singh, J.S., Singh, D.P., Eds.; Elsevier B.V.: Amsterdam, The Netherlands, 2019; pp. 43–55. ISBN 9780444641915. [Google Scholar]
- Lahlali, R.; Ezrari, S.; Radouane, N.; Kenfaoui, J.; Esmaeel, Q.; El Hamss, H.; Belabess, Z.; Barka, E.A. Biological Control of Plant Pathogens: A Global Perspective. Microorganisms 2022, 10, 596. [Google Scholar] [CrossRef] [PubMed]
- Kenawy, A.; Dailin, D.; Abo-Zaid, G.; Malek, R.; Ambehabati, K.; Zakaria, K.; Sayyed, R.; El Enshasy, H. Biosynthesis of Antibiotics by PGPR and Their Roles in Biocontrol of Plant Diseases. In Plant Growth Promoting Rhizobacteria for Sustainable Stress Management; Sayyed, R., Ed.; Springer Nature Singapore: Singapore, 2019; ISBN 9789811369865. [Google Scholar]
- Zhang, Q.X.; Kong, X.W.; Li, S.Y.; Chen, X.J.; Chen, X.J. Antibiotics of Pseudomonas protegens FD6 Are Essential for Biocontrol Activity. Australas. Plant Pathol. 2020, 49, 307–317. [Google Scholar] [CrossRef]
- Daura-Pich, O.; Hernández, I.; Pinyol-Escala, L.; Lara, J.M.; Martínez-Servat, S.; Fernández, C.; López-García, B. No Antibiotic and Toxic Metabolites Produced by the Biocontrol Agent Pseudomonas putida Strain B2017. FEMS Microbiol. Lett. 2020, 367, fnaa075. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.H.; Xiang, W.; Cao, K.X.; Lu, X.; Yao, S.C.; Hung, D.; Huang, R.S.; Li, L.B. Characterization of Volatile Organic Compounds Emitted from Endophytic Burkholderia cenocepacia ETR-B22 by SPME-GC-MS and Their Inhibitory Activity against Various Plant Fungal Pathogens. Molecules 2020, 25, 3765. [Google Scholar] [CrossRef]
- Prigigallo, M.I.; De Stradis, A.; Anand, A.; Mannerucci, F.; L’Haridon, F.; Weisskopf, L.; Bubici, G. Basidiomycetes Are Particularly Sensitive to Bacterial Volatile Compounds: Mechanistic Insight Into the Case Study of Pseudomonas protegens Volatilome Against Heterobasidion Abietinum. Front. Microbiol. 2021, 12, 684664. [Google Scholar] [CrossRef]
- Ossowicki, A.; Jafra, S.; Garbeva, P. The Antimicrobial Volatile Power of the Rhizospheric Isolate Pseudomonas donghuensis P482. PLoS ONE 2017, 12, e0174362. [Google Scholar] [CrossRef]
- Bonaterra, A.; Badosa, E.; Daranas, N.; Francés, J.; Roselló, G.; Montesinos, E. Bacteria as Biological Control Agents of Plant Diseases. Microorganisms 2022, 10, 1759. [Google Scholar] [CrossRef]
- Jadhav, H.; Shaikh, S.S.; Sayyed, R.Z. Rhizotrophs: Plant Growth Promotion to Bioremediation. In Rhizotrophs Plant Growth Promot. to Bioremediation; Mehnaz, S., Ed.; Springer Nature Singapore: Singapore, 2017. [Google Scholar] [CrossRef]
- Rasul, M.; Yasmin, S.; Zubair, M.; Mahreen, N.; Yousaf, S.; Arif, M.; Iqbal, Z.; Sajjad, M. Phosphate Solubilizers as Antagonists for Bacterial Leaf Blight with Improved Rice Growth in Phosphorus Deficit Soil. Biol. Control 2019, 136, 103997. [Google Scholar] [CrossRef]
- Oleńska, E.; Małek, W.; Wójcik, M.; Swiecicka, I.; Thijs, S.; Vangronsveld, J. Beneficial Features of Plant Growth-Promoting Rhizobacteria for Improving Plant Growth and Health in Challenging Conditions: A Methodical Review. Sci. Total Environ. 2020, 743, 140682. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Pandey, S. ACC Deaminase Producing Bacteria with Multifarious Plant Growth Promoting Traits Alleviates Salinity Stress in French Bean (Phaseolus vulgaris) Plants. Front. Microbiol. 2019, 10, 1506. [Google Scholar] [CrossRef]
- Singh, P.; Singh, R.K.; Zhou, Y.; Wang, J.; Jiang, Y.; Wang, Y.; Yang, L.; Jiang, M. Unlocking the Strength of Plant Growth Promoting Pseudomonas in Improving Crop Productivity in Normal and Challenging Environments: A Review. J. Plant Interact. 2022, 17, 220–238. [Google Scholar] [CrossRef]
- Abdelaal, K.; Alkahtani, M.; Attia, K.; Hafez, Y. The Role of Plant Growth-Promoting Bacteria in Alleviating the Adverse Effecys of Drought on Plants. Biology 2021, 10, 520. [Google Scholar] [CrossRef]
- Kasim, W.A.; Gaafar, R.M.; Abou-Ali, R.M.; Omar, M.N.; Hewait, H.M. Effect of Biofilm Forming Plant Growth Promoting Rhizobacteria on Salinity Tolerance in Barley. Ann. Agric. Sci. 2016, 61, 217–227. [Google Scholar] [CrossRef]
- Altaf, M.M.; Khan, M.; Abulreesh, H.; Ahmad, I. Quorum Sensing in Plant Growth-Promoting Rhizobacteria and Its Impact on Plant-Microbe Interaction. In Plant-Microbe Interactions in Agro-Ecological Perspectives; Singh, D.P., Singh, H.B., Prabha, R., Eds.; Springer Nature Singapore: Singapore, 2017; Volume 1, pp. 1–657. ISBN 9789811058134. [Google Scholar]
- Muhammad, M.H.; Idris, A.L.; Fan, X.; Guo, Y.; Yu, Y.; Jin, X.; Qiu, J.; Guan, X.; Huang, T. Beyond Risk: Bacterial Biofilms and Their Regulating Approaches. Front. Microbiol. 2020, 11, 928. [Google Scholar] [CrossRef]
- Pandit, A.; Adholeya, A.; Cahill, D.; Brau, L.; Kochar, M. Microbial Biofilms in Nature: Unlocking Their Potential for Agricultural Applications. J. Appl. Microbiol. 2020, 129, 199–211. [Google Scholar] [CrossRef]
- Azulay, D.N.; Spaeker, O.; Ghrayeb, M.; Wilsch-Bräuninger, M.; Scoppola, E.; Burghammer, M.; Zizak, I.; Bertinetti, L.; Politi, Y.; Chai, L. Multiscale X-Ray Study of Bacillus subtilis Biofilms Reveals Interlinked Structural Hierarchy and Elemental Heterogeneity. Proc. Natl. Acad. Sci. USA 2022, 119, e2118107119. [Google Scholar] [CrossRef] [PubMed]
- Karygianni, L.; Ren, Z.; Koo, H.; Thurnheer, T. Biofilm Matrixome: Extracellular Components in Structured Microbial Communities. Trends Microbiol. 2020, 28, 668–681. [Google Scholar] [CrossRef]
- del Mar Cendra, M.; Torrents, E. Pseudomonas aeruginosa Biofilms and Their Partners in Crime. Biotechnol. Adv. 2021, 49, 107734. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.C.; Wingender, J. The Biofilm Matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Berlanga, M.; Guerrero, R. Living Together in Biofilms: The Microbial Cell Factory and Its Biotechnological Implications. Microb. Cell Fact. 2016, 15, 165. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An Emergent Form of Bacterial Life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Pinto, R.M.; Soares, F.A.; Reis, S.; Nunes, C.; Van Dijck, P. Innovative Strategies Toward the Disassembly of the EPS Matrix in Bacterial Biofilms. Front. Microbiol. 2020, 11, 952. [Google Scholar] [CrossRef] [PubMed]
- Soler-Arango, J.; Figoli, C.; Muraca, G.; Bosch, A.; Brelles-Mariño, G. The Pseudomonas aeruginosa Biofilm Matrix and Cells Are Drastically Impacted by Gas Discharge Plasma Treatment: A Comprehensive Model Explaining Plasma-Mediated Biofilm Eradication. PLoS ONE 2019, 14, e0216817. [Google Scholar] [CrossRef]
- Kungwani, N.; Shukla, S.K.; Rao, T.; Das, S. Biofilm-Mediated Bioremediation of Polycyclic Aromatic Hydrocarbons: Current Status and Future Perspectives. In Microbial Biodegradation and Bioremediation, 2nd ed.; Das, S., Dash, H.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 547–570. [Google Scholar]
- Bhagat, N.; Raghav, M.; Dubey, S.; Bedi, N. Bacterial Exopolysaccharides: Insight into Their Role in Plant Abiotic Stress Tolerance. J. Microbiol. Biotechnol. 2021, 31, 1045–1059. [Google Scholar] [CrossRef] [PubMed]
- Limoli, D.H.; Jones, C.J.; Wozniak, D.J. Bacterial Extracellular Polysaccharides in Biofilm Formation and Function. Microbiol. Spectr. 2015, 3, 1–30. [Google Scholar] [CrossRef]
- Lahiri, D.; Nag, M.; Dutta, B.; Dey, A.; Sarkar, T.; Pati, S.; Edinur, H.A.; Kari, Z.A.; Noor, N.H.M.; Ray, R.R. Bacterial Cellulose: Production, Characterization and Application as Antimicrobial Agent. Int. J. Mol. Sci. 2021, 22, 12984. [Google Scholar] [CrossRef]
- Ryder, C.; Byrd, M.; Wozniak, D.J. Role of Polysaccharides in Pseudomonas aeruginosa Biofilm Development. Curr. Opin. Microbiol. 2016, 10, 644–648. [Google Scholar] [CrossRef] [PubMed]
- Floyd, K.A.; Eberly, A.R.; Hadjifrangiskou, M. Adhesion of Bacteria to Surfaces and Biofilm Formation on Medical Devices. In Biofilms and Implantable Medical Devices: Infection and Control; Deng, Y., Lv, W., Eds.; Elsevier Ltd.: Amsterdam, The Netherlands, 2017; pp. 47–95. ISBN 9780081003985. [Google Scholar]
- Molina-Santiago, C.; de Vicente, A.; Romero, D. Bacterial Extracellular Matrix as a Natural Source of Biotechnologically Multivalent Materials. Comput. Struct. Biotechnol. J. 2021, 19, 2796–2805. [Google Scholar] [CrossRef] [PubMed]
- Chin, J.S.F.; Sinha, S.; Nalaparaju, A.; Yam, J.K.H.; Qin, Z.; Ma, L.; Liang, Z.X.; Lu, L.; Bhattacharjya, S.; Yang, L. Pseudomonas aeruginosa Psl Exopolysaccharide Interacts with the Antimicrobial Peptide LG21. Water 2017, 9, 681. [Google Scholar] [CrossRef]
- Lee, K.; Yoon, S.S. Pseudomonas aeruginosa Biofilm, a Programmed Bacterial Life for Fitness. J. Microbiol. Biotechnol. 2017, 27, 1053–1064. [Google Scholar] [CrossRef]
- Fong, J.N.C.; Yildiz, F.H. Biofilm Matrix Proteins. Microb. Biofilms 2015, 3, 201–222. [Google Scholar] [CrossRef]
- Fulaz, S.; Vitale, S.; Quinn, L.; Casey, E. Nanoparticle–Biofilm Interactions: The Role of the EPS Matrix. Trends Microbiol. 2019, 27, 915–926. [Google Scholar] [CrossRef]
- Altaf, M.; Ahmad, I. Biofilm Formation on Plant Surfaces by Rhizobacteria: Impact on Plant Growth and Ecological Significance. Handb. Microb. Bioresour. 2016, 5, 81–95. [Google Scholar] [CrossRef]
- Carniello, V.; Peterson, B.W.; van der Mei, H.C.; Busscher, H.J. Physico-Chemistry from Initial Bacterial Adhesion to Surface-Programmed Biofilm Growth. Adv. Colloid Interface Sci. 2018, 261, 1–14. [Google Scholar] [CrossRef]
- Bennett, R.R.; Lee, C.K.; De Anda, J.; Nealson, K.H.; Yildiz, F.H.; O’Toole, G.A.; Wong, G.C.L.; Golestanian, R. Species-Dependent Hydrodynamics of Flagellum-Tethered Bacteria in Early Biofilm Development. J. R. Soc. Interface 2016, 13, 20150966. [Google Scholar] [CrossRef] [PubMed]
- Conrad, J.C.; Gibiansky, M.L.; Jin, F.; Gordon, V.D.; Motto, D.A.; Mathewson, M.A.; Stopka, W.G.; Zelasko, D.C.; Shrout, J.D.; Wong, G.C.L. Flagella and Pili-Mediated near-Surface Single-Cell Motility Mechanisms in P. aeruginosa. Biophys. J. 2011, 100, 1608–1616. [Google Scholar] [CrossRef]
- Ligthart, K.; Belzer, C.; de Vos, W.M.; Tytgat, H.L.P. Bridging Bacteria and the Gut: Functional Aspects of Type IV Pili. Trends Microbiol. 2020, 28, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Gibiansky, M.L.; Wang, J.; Wang, C.; Lux, R.; Li, Y.; Wong, G.C.L.; Shi, W. Interplay between Type IV Pili Activity and Exopolysaccharides Secretion Controls Motility Patterns in Single Cells of Myxococcus xanthus. Sci. Rep. 2016, 6, 17790. [Google Scholar] [CrossRef]
- Colin, R.; Ni, B.; Laganenka, L.; Sourjik, V. Multiple Functions of Flagellar Motility and Chemotaxis in Bacterial Physiology. FEMS Microbiol. Rev. 2021, 45, fuab038. [Google Scholar] [CrossRef]
- López-Farfán, D.; Reyes-Darias, J.A.; Matilla, M.A.; Krell, T. Concentration Dependent Effect of Plant Root Exudates on the Chemosensory Systems of Pseudomonas putida KT2440. Front. Microbiol. 2019, 10, 78. [Google Scholar] [CrossRef] [PubMed]
- O’Neal, L.; Vo, L.; Alexandre, G. Specific Root Exudate Compounds Sensed by Dedicated Chemoreceptors Shape Azospirillum brasilense Chemotaxis in the Rhizosphere. Appl. Environ. Microbiol. 2020, 86, e01026-20. [Google Scholar] [CrossRef] [PubMed]
- Velmourougane, K.; Prasanna, R.; Saxena, A.K. Agriculturally Important Microbial Biofilms: Present Status and Future Prospects. J. Basic Microbiol. 2017, 57, 548–573. [Google Scholar] [CrossRef] [PubMed]
- Costa, O.Y.A.; Raaijmakers, J.M.; Kuramae, E.E. Microbial Extracellular Polymeric Substances: Ecological Function and Impact on Soil Aggregation. Front. Microbiol. 2018, 9, 1636. [Google Scholar] [CrossRef] [PubMed]
- Ghafoor, A.; Hay, I.D.; Rehm, B.H.A. Role of Exopolysaccharides in Pseudomonas aeruginosa Biofilm Formation and Architecture. Appl. Environ. Microbiol. 2011, 77, 5238–5246. [Google Scholar] [CrossRef]
- Jahid, I.K.; Ha, S. Do Inactivation Kinetics of Various Chemical Disinfectants on Aeromonas hydrophila Planktonic Cells and Biofilms. Foodborne Pathog. Dis. 2014, 11, 346–353. [Google Scholar] [CrossRef]
- Klausen, M.; Aaes-Jørgensen, A.; Molin, S.; Tolker-Nielsen, T. Involvement of Bacterial Migration in the Development of Complex Multicellular Structures in Pseudomonas aeruginosa Biofilms. Mol. Microbiol. 2003, 50, 61–68. [Google Scholar] [CrossRef]
- Barken, K.B.; Pamp, S.J.; Yang, L.; Gjermansen, M.; Bertrand, J.J.; Klausen, M.; Givskov, M.; Whitchurch, C.B.; Engel, J.N.; Tolker-Nielsen, T. Roles of Type IV Pili, Flagellum-Mediated Motility and Extracellular DNA in the Formation of Mature Multicellular Structures in Pseudomonas aeruginosa Biofilms. Environ. Microbiol. 2008, 10, 2331–2343. [Google Scholar] [CrossRef]
- Otzen, D.E. Biosurfactants and Surfactants Interacting with Membranes and Proteins: Same but Different? Biochim. Biophys. Acta Biomembr. 2017, 1859, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Źuñiga, A.; Donoso, R.A.; Ruiz, D.; Ruz, G.A.; Gonźalez, B. Quorum-Sensing Systems in the Plant Growth-Promoting Bacterium Paraburkholderia phytofirmans PsJN Exhibit Cross-Regulation and Are Involved in Biofilm Formation. Mol. Plant-Microbe Interact. 2017, 30, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Rabin, N.; Zheng, Y.; Opoku-Temeng, C.; Du, Y.; Bonsu, E.; Sintim, H.O. Biofilm Formation Mechanisms and Targets for Developing Antibiofilm Agents. Futur. Med. Chem. 2015, 7, 493–512. [Google Scholar] [CrossRef] [PubMed]
- Pamp, S.; Tolker-Nielsen, T. Multiple Roles of Biosurfactants in Structural Biofilm Development by Pseudomonas aeruginosa. J. Bacteriol. 2007, 189, 2531–2539. [Google Scholar] [CrossRef] [PubMed]
- Sheraton, M.V.; Yam, J.K.H.; Tan, C.H.; Oh, H.S.; Mancini, E.; Yang, L.; Sloot, P.M.A. Mesoscopic Energy Minimization Drives Pseudomonas stratification of Antibiotic Activity Based on Cell Metabolism. Antimicrob. Agents Chemother. 2018, 62, e02544–e17. [Google Scholar] [CrossRef] [PubMed]
- Ghanbari, A.; Dehghany, J.; Schwebs, T.; Müsken, M.; Häussler, S.; Meyer-Hermann, M. Inoculation Density and Nutrient Level Determine the Formation of Mushroom-Shaped Structures in Pseudomonas aeruginosa Biofilms. Sci. Rep. 2016, 6, 32097. [Google Scholar] [CrossRef]
- Wille, J.; Coenye, T. Biofilm Dispersion: The Key to Biofilm Eradication or Opening Pandora’s Box? Biofilm 2020, 2, 100027. [Google Scholar] [CrossRef]
- Guilhen, C.; Miquel, S.; Charbonnel, N.; Joseph, L.; Carrier, G.; Forestier, C.; Balestrino, D. Colonization and Immune Modulation Properties of Klebsiella pneumoniae Biofilm-Dispersed Cells. npj Biofilms Microbiomes 2019, 5, 25. [Google Scholar] [CrossRef] [PubMed]
- Ansari, F.A.; Jafri, H.; Ahmad, I.; Abulreesh, H.H. Factors Affecting Biofilm Formation in in Vitro and in the Rhizosphere. In Biofilms in Plant and Soil Health; Ahmad, I., Husain, F.M., Eds.; John Wiley & Sons Ltd: London, UK, 2017; pp. 275–290. ISBN 9781119246343. [Google Scholar] [CrossRef]
- Lee, B.H.; Cole, S.; Badel-Berchoux, S.; Guillier, L.; Felix, B.; Krezdorn, N.; Hébraud, M.; Bernardi, T.; Sultan, I.; Piveteau, P. Biofilm Formation of Listeria monocytogenes Strains Under Food Processing Environments and Pan-Genome-Wide Association Study. Front. Microbiol. 2019, 10, 2698. [Google Scholar] [CrossRef] [PubMed]
- Samrot, A.V.; Mohamed, A.A.; Faradjeva, E.; Jie, L.S.; Sze, C.H.; Arif, A.; Sean, T.C.; Michael, E.N.; Mun, C.Y.; Qi, N.X.; et al. Mechanisms and Impact of Biofilms and Targeting of Biofilms Using Bioactive Compounds—A Review. Medicina 2021, 57, 839. [Google Scholar] [CrossRef] [PubMed]
- Savijoki, K.; Nyman, T.A.; Kainulainen, V.; Miettinen, I.; Siljamäki, P.; Fallarero, A.; Sandholm, J.; Satokari, R.; Varmanen, P. Growth Mode and Carbon Source Impact the Surfaceome Dynamics of Lactobacillus rhamnosus GG. Front. Microbiol. 2019, 10, 1272. [Google Scholar] [CrossRef] [PubMed]
- Zou, M.; Liu, D. Effects of Carbon Sources and Temperature on the Formation and Structural Characteristics of Food-Related Staphylococcus epidermidis Biofilms. Food Sci. Hum. Wellness 2020, 9, 370–376. [Google Scholar] [CrossRef]
- Wang, D.; Xu, A.; Elmerich, C.; Ma, L.Z. Biofilm Formation Enables Free-Living Nitrogen-Fixing Rhizobacteria to Fix Nitrogen under Aerobic Conditions. ISME J. 2017, 11, 1602–1613. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Li, L.J.; Shi, Q.S.; Ouyang, Y.S.; Chen, Y.B.; Hu, W.F. Effects of Nutritional and Environmental Conditions on Planktonic Growth and Biofilm Formation of Citrobacter werkmanii BF-6. J. Microbiol. Biotechnol. 2013, 23, 1673–1682. [Google Scholar] [CrossRef]
- Haque, M.M.; Mosharaf, M.K.; Haque, M.A.; Tanvir, M.Z.H.; Alam, M.K. Biofilm Formation, Production of Matrix Compounds and Biosorption of Copper, Nickel and Lead by Different Bacterial Strains. Front. Microbiol. 2021, 12, 615113. [Google Scholar] [CrossRef] [PubMed]
- Alotaibi, G.F.; Bukhari, M.A. Factors Influencing Bacterial Biofilm Formation and Development. Am. J. Biomed. Sci. Res. 2021, 12, 617–626. [Google Scholar] [CrossRef]
- Helman, Y.; Chernin, L. Silencing the Mob: Disrupting Quorum Sensing as a Means to Fight Plant Disease. Mol. Plant Pathol. 2015, 16, 316–329. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Singh, S.K.; Chowdhury, I.; Singh, R. Understanding the Mechanism of Bacterial Biofilms Resistance to Antimicrobial Agents. Open Microbiol. J. 2017, 11, 53–62. [Google Scholar] [CrossRef]
- Harjai, K.; Sabharwal, N. Biofilm Formation and Quorum Sensing in Rhizosphere. In Biofilms in Plant and Soil Health; Ahmad, I., Husain, F.M., Eds.; John Wiley & Sons Ltd: London, UK, 2017; pp. 111–130. ISBN 9781119246343. [Google Scholar] [CrossRef]
- Talagrand-Reboul, E.; Jumas-Bilak, E.; Lamy, B. The Social Life of Aeromonas through Biofilm and Quorum Sensing Systems. Front. Microbiol. 2017, 8, 37. [Google Scholar] [CrossRef]
- Clements, T.; Ndlovu, T.; Khan, S.; Khan, W. Biosurfactants Produced by Serratia Species: Classification, Biosynthesis, Production and Application. Appl. Microbiol. Biotechnol. 2019, 103, 589–602. [Google Scholar] [CrossRef]
- Luo, C.; Zhou, H.; Zou, J.; Wang, X.; Zhang, R.; Xiang, Y.; Chen, Z. Bacillomycin L and Surfactin Contribute Synergistically to the Phenotypic Features of Bacillus subtilis 916 and the Biocontrol of Rice Sheath Blight Induced by Rhizoctonia solani. Appl. Microbiol. Biotechnol. 2015, 99, 1897–1910. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Wu, X.; Wang, Y.; Dai, Y. Role of Biofilm Formation by Bacillus pumilus HR10 in Biocontrol against Pine Seedling Damping-Off Disease Caused by Rhizoctonia solani. Forests 2020, 11, 652. [Google Scholar] [CrossRef]
- Sayyed, R.; Seifi, S.; Patel, P.R.; Shaikh, S.S.; Jadhav, H.P.; Enshasy, H. El Siderophore Production in Groundnut Rhizosphere Isolate, Achromobacter sp. RZS2 Influenced by Physicochemical Factors and Metal Ions. Environ. Sustain. 2019, 2, 117–124. [Google Scholar] [CrossRef]
- Mishra, P.; Mishra, J.; Dwivedi, S.K.; Arora, N. Microbial Enzymes in Biocontrol of Phytopathogens. In Microbial Enzymes: Roles and Applications in Industries; Arora, N., Mishra, J., Mishra, V., Eds.; Springer: Singapore, 2020; pp. 259–285. ISBN 9789811517105. [Google Scholar] [CrossRef]
- Ahmed, E.; Holmström, S.J.M. Minireview Siderophores in Environmental Research: Roles and Applications. Microb. Biotechnol. 2014, 7, 196–208. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Luo, X.; Liu, J.; Lu, H. Novel Functional Metabolites That Affect Biofilm Formation Are Regulated by Bioavailable Iron with Siderophore-Dependent Pathway. SSRN Electron. J. 2020. [Google Scholar] [CrossRef]
- Harrison, F.; Buckling, A. Siderophore Production and Biofilm Formation as Linked Social Traits. ISME J. 2009, 3, 632–634. [Google Scholar] [CrossRef]
- Tovi, N.; Frenk, S.; Hadar, Y.; Minz, D. Host Specificity and Spatial Distribution Preference of Three Pseudomonas Isolates. Front. Microbiol. 2019, 10, 3263. [Google Scholar] [CrossRef]
- Haggag, W.M.; Timmusk, S. Colonization of Peanut Roots by Biofilm-Forming Paenibacillus polymyxa Initiates Biocontrol against Crown Rot Disease. J. Appl. Microbiol. 2008, 104, 961–969. [Google Scholar] [CrossRef]
- Harting, R.; Nagel, A.; Nesemann, K.; Höfer, A.M.; Bastakis, E.; Kusch, H.; Stanley, C.E.; Stöckli, M.; Kaever, A.; Hoff, K.J.; et al. Pseudomonas Strains Induce Transcriptional and Morphological Changes and Reduce Root Colonization of Verticillium spp. Front. Microbiol. 2021, 12, 652468. [Google Scholar] [CrossRef]
- Stoll, A.; Salvatierra-Martínez, R.; González, M.; Araya, M. The Role of Surfactin Production by Bacillus velezensis on Colonization, Biofilm Formation on Tomato Root and Leaf Surfaces and Subsequent Protection (ISR) against Botrytis cinerea. Microorganisms 2021, 9, 2251. [Google Scholar] [CrossRef]
- Azri, M.H.; Ismail, S.; Abdullah, R. An Endophytic Bacillus Strain Promotes Growth of Oil Palm Seedling by Fine Root Biofilm Formation. Rhizosphere 2018, 5, 1–7. [Google Scholar] [CrossRef]
- Hazarika, S.N.; Saikia, K.; Borah, A.; Thakur, D. Prospecting Endophytic Bacteria Endowed With Plant Growth Promoting Potential Isolated From Camellia sinensis. Front. Microbiol. 2021, 12, 738058. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Yang, D.; Wang, D.; Miao, Y.; Shao, J.; Zhou, X.; Xu, Z.; Li, Q.; Feng, H.; Li, S.; et al. Whole Transcriptomic Analysis of the Plant-Beneficial Rhizobacterium Bacillus amyloliquefaciens SQR9 during Enhanced Biofilm Formation Regulated by Maize Root Exudates. BMC Genom. 2015, 16, 685. [Google Scholar] [CrossRef] [PubMed]
- Berendsen, R.L.; Vismans, G.; Yu, K.; Song, Y.; De Jonge, R.; Burgman, W.P.; Burmølle, M.; Herschend, J.; Bakker, P.A.H.M.; Pieterse, C.M.J. Disease-Induced Assemblage of a Plant-Beneficial Bacterial Consortium. ISME J. 2018, 12, 1496–1507. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.; Mosharaf, K.; Khatun, M.; Shozib, H.B.; Miah, M.U.; Molla, A.H. Biofilm Producing Rhizobacteria With Multiple Plant Growth-Promoting Traits Promote Growth of Tomato Under Water-Deficit Stress. Front. Microbiol. 2020, 11, 542053. [Google Scholar] [CrossRef]
- Mahdi, I.; Fahsi, N.; Hafidi, M.; Allaoui, A.; Biskri, L. Plant Growth Enhancement Using Rhizospheric Halotolerant Phosphate Solubilizing Bacterium Bacillus licheniformis Qa1 and Enterobacter asburiae Qf11 Isolated from Chenopodium quinoa Willd. Microorganisms 2020, 8, 948. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Jiang, C.; Zhang, L.; Chen, L.; Zhang, X. Biofilms Positively Contribute to Bacillus amyloliquefaciens 54-Induced Drought Tolerance in Tomato Plants. Int. J. Mol. Sci. 2019, 20, 6271. [Google Scholar] [CrossRef] [PubMed]
- Ansari, F.A.; Jabeen, M.; Ahmad, I. Pseudomonas azotoformans FAP5, a Novel Biofilm-Forming PGPR Strain, Alleviates Drought Stress in Wheat Plant. Int. J. Environ. Sci. Technol. 2021, 18, 3855–3870. [Google Scholar] [CrossRef]
- Yang, N.; Nesme, J.; Røder, H.L.; Li, X.; Zuo, Z.; Petersen, M.; Burmølle, M.; Sørensen, S.J. Emergent Bacterial Community Properties Induce Enhanced Drought Tolerance in Arabidopsis. NPJ Biofilms Microbiomes 2021, 7, 82. [Google Scholar] [CrossRef]
- Costerton, J.; Geesey, G.; Cheng, K. How Bacteria Stick. Sci. Am. 1978, 238, 86–95. [Google Scholar] [CrossRef]
- Lam, J.; Chan, R.; Lam, K.; Costerton, J. Production of Mucoid Microcolonies by Pseudomonas aeruginosa within Infected Lungs in Cystic Fibrosis. Infect. Immun. 1980, 28, 546–556. [Google Scholar] [CrossRef]
- Ahmad, I.; Khan, M.; Altaf, M.; Qais, A.; Ansari, F.; Rumbaugh, K. Biofilms: An Overview of Their Significance in Plant and Soil Health. In Biofilms in Plant and Soil Health; Ahmad, I., Husain, F., Eds.; John Wiley and Sons Ltd: London, UK, 2017; p. 585. ISBN 9781119246343. [Google Scholar]
- Branda, S.; Gonzalez-Pator, J.; Ben-Yehuda, S.; Losick, R.; Kolter, R. Fruiting Body Formation by Bacillus subtilis. Proc. Natl. Acad. Sci. USA 2001, 98, 11621–11626. [Google Scholar] [CrossRef]
- Kinsinger, R.F.; Shirk, M.C.; Fall, R. Rapid Surface Motility in Bacillus subtilis Is Dependent on Extracellular Surfactin and Potassium Ion. J. Bacteriol. 2003, 185, 5627–5631. [Google Scholar] [CrossRef] [PubMed]
- López, D.; Fischbach, M.A.; Chu, F.; Losick, R.; Kolter, R. Structurally Diverse Natural Products That Cause Potassium Leakage Trigger Multicellularity in Bacillus subtilis. Proc. Natl. Acad. Sci. USA 2009, 106, 280–285. [Google Scholar] [CrossRef] [PubMed]
- McLoon, A.L.; Guttenplan, S.B.; Kearns, D.B.; Kolter, R.; Losick, R. Tracing the Domestication of a Biofilm-Forming Bacterium. J. Bacteriol. 2011, 193, 2027–2034. [Google Scholar] [CrossRef] [PubMed]
- Zeriouh, H.; de Vicente, A.; Pérez-García, A.; Romero, D. Surfactin Triggers Biofilm Formation of Bacillus subtilis in Melon Phylloplane and Contributes to the Biocontrol Activity. Environ. Microbiol. 2014, 16, 2196–2211. [Google Scholar] [CrossRef]
- Bais, H.; Fall, R.; Vivanco, J.M. Biocontrol of Bacillus subtilis against Infection of Arabidopsis Roots by Pseudomonas syringae Is Facilitated by Biofilm Formation and Surfactin Production. Plant Physiol. 2004, 134, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Selin, C.; Habibian, R.; Poritsanos, N.; Athukorala, S.N.P.; Fernando, D.; De Kievit, T.R. Phenazines Are Not Essential for Pseudomonas Chlororaphis PA23 Biocontrol of Sclerotinia sclerotiorum, but Do Play a Role in Biofilm Formation. FEMS Microbiol. Ecol. 2010, 71, 73–83. [Google Scholar] [CrossRef]
- Sang, M.K.; Kim, K.D. Biocontrol Activity and Root Colonization by Pseudomonas corrugata Strains CCR04 and CCR80 against Phytophthora Blight of Pepper. BioControl 2014, 59, 437–448. [Google Scholar] [CrossRef]
- Timmusk, S.; Van West, P.; Gow, N.A.R.; Paul Huffstutler, R. Paenibacillus polymyxa Antagonizes Oomycete Plant Pathogens Phytophthora palmivora and Pythium aphanidermatum. J. Appl. Microbiol. 2009, 106, 1473–1481. [Google Scholar] [CrossRef]
- Ruiu, L. Plant-Growth-Promoting Bacteria (PGPB) against Insects and Other Agricultural Pests. Agronomy 2020, 10, 861. [Google Scholar] [CrossRef]
- Fan, B.; Borriss, R.; Bleiss, W.; Wu, X. Gram-Positive Rhizobacterium Bacillus amyloliquefaciens FZB42 Colonizes Three Types of Plants in Different Patterns. J. Microbiol. 2012, 50, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Romera, F.J.; García, M.J.; Lucena, C.; Martínez-Medina, A.; Aparicio, M.A.; Ramos, J.; Alcántara, E.; Angulo, M.; Pérez-Vicente, R. Induced Systemic Resistance (ISR) and Fe Deficiency Responses in Dicot Plants. Front. Plant Sci. 2019, 10, 287. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Ryu, C. Understanding Plant Social Networking System: Avoiding Deleterious Microbiota but Calling Beneficials. Int. J. Mol. Sci. 2021, 22, 3319. [Google Scholar] [CrossRef] [PubMed]
- Timmermann, T.; Poupin, M.J.; Vega, A.; Urrutia, C.; Ruz, G.A.; González, B. Gene Networks Underlying the Early Regulation of Paraburkholderia phytofirmans PsJN Induced Systemic Resistance in Arabidopsis. PLoS ONE 2019, 14, e0221358. [Google Scholar] [CrossRef] [PubMed]
- Nie, P.; Li, X.; Wang, S.; Guo, J.; Zhao, H.; Niu, D. Induced Systemic Resistance against Botrytis cinerea by Bacillus cereus AR156 through a JA/ET- and NPR1-Dependent Signaling Pathway and Activates PAMP-Triggered Immunity in Arabidopsis. Front. Plant Sci. 2017, 8, 238. [Google Scholar] [CrossRef] [PubMed]
- Pieterse, C.M.J.; Zamioudis, C.; Berendsen, R.L.; Weller, D.M.; Van Wees, S.C.M.; Bakker, P.A.H.M. Induced Systemic Resistance by Beneficial Microbes. Annu. Rev. Phytopathol. 2014, 52, 347–375. [Google Scholar] [CrossRef] [PubMed]
- Niu, D.D.; Liu, H.X.; Jiang, C.H.; Wang, Y.P.; Wang, Q.Y.; Jin, H.L.; Guo, J.H. The Plant Growth-Promoting Rhizobacterium Bacillus cereus AR156 Induces Systemic Resistance in Arabidopsis thaliana by Simultaneously Activating Salicylate- and Jasmonate/Ethylene-Dependent Signaling Pathways. Mol. Plant-Microbe Interact. 2011, 24, 533–542. [Google Scholar] [CrossRef]
- Zebelo, S.; Song, Y.; Kloepper, J.W.; Fadamiro, H. Rhizobacteria Activates (+)-δ-Cadinene Synthase Genes and Induces Systemic Resistance in Cotton against Beet Armyworm (Spodoptera exigua). Plant Cell Environ. 2016, 39, 935–943. [Google Scholar] [CrossRef]
- Fazle Rabbee, M.; Baek, K.H. Antimicrobial Activities of Lipopeptides and Polyketides of Bacillus velezensis for Agricultural Applications. Molecules 2020, 25, 4973. [Google Scholar] [CrossRef] [PubMed]
- Hazarika, D.J.; Goswami, G.; Gautom, T.; Parveen, A.; Das, P.; Barooah, M.; Boro, R.C. Lipopeptide Mediated Biocontrol Activity of Endophytic Bacillus subtilis against Fungal Phytopathogens. BMC Microbiol. 2019, 19, 71. [Google Scholar] [CrossRef]
- Taktek, S.; St-Arnaud, M.; Piché, Y.; Fortin, J.A.; Antoun, H. Igneous Phosphate Rock Solubilization by Biofilm-Forming Mycorrhizobacteria and Hyphobacteria Associated with Rhizoglomus irregulare DAOM 197198. Mycorrhiza 2017, 27, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Hettiarachchi, R.P.; Dharmakeerthi, R.S.; Seneviratne, G.; Jayakody, A.N.; De Silva, K.E.; Gunathilake, T.; Thewarapperuma, A. Determination of Desirable Properties of Bacteria, Fungi and Their Biofilm Associated with Rubber Rhizosphere. Trop. Agric. Res. 2016, 27, 399. [Google Scholar] [CrossRef]
- Bandara, W.M.M.S.; Seneviratne, G.; Kulasooriya, S.A. Interactions among Endophytic Bacteria and Fungi: Effects and Potentials. J. Biosci. 2006, 31, 645–650. [Google Scholar] [CrossRef] [PubMed]
- Browning, M.; Wallace, D.B.; Dawson, C.; Alm, S.R.; Amador, J.A. Potential of Butyric Acid for Control of Soil-Borne Fungal Pathogens and Nematodes Affecting Strawberries. Soil Biol. Biochem. 2006, 38, 401–404. [Google Scholar] [CrossRef]
- Ren, D.; Madsen, J.S.; Sørensen, S.J.; Burmølle, M. High Prevalence of Biofilm Synergy among Bacterial Soil Isolates in Cocultures Indicates Bacterial Interspecific Cooperation. ISME J. 2015, 9, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Prasanna, R.; Bidyarani, N.; Babu, S.; Hossain, F.; Shivay, Y.S.; Nain, L. Cyanobacterial Inoculation Elicits Plant Defense Response and Enhanced Zn Mobilization in Maize Hybrids. Cogent Food Agric. 2015, 1, 998507. [Google Scholar] [CrossRef]
- Amaya-Gómez, C.V.; Porcel, M.; Mesa-Garrig, L.; Gómez-Álvarez, M.I. A Framework for the Selection of Plant Growth-Promoting Rhizobacteria Based on Bacterial Competence Mechanisms. Appl. Environ. Microbiol. 2020, 86, e00760-20. [Google Scholar] [CrossRef]
- Yasmeen, T.; Ahmad, A.; Arif, M.S.; Mubin, M.; Rehman, K.; Shahzad, S.M.; Iqbal, S.; Rizwan, M.; Ali, S.; Alyemeni, M.N.; et al. Biofilm Forming Rhizobacteria Enhance Growth and Salt Tolerance in Sunflower Plants by Stimulating Antioxidant Enzymes Activity. Plant Physiol. Biochem. 2020, 156, 242–256. [Google Scholar] [CrossRef]
- Timmusk, S.; Abd El-Daim, I.A.; Copolovici, L.; Tanilas, T.; Kännaste, A.; Behers, L.; Nevo, E.; Seisenbaeva, G.; Stenström, E.; Niinemets, Ü. Drought-Tolerance of Wheat Improved by Rhizosphere Bacteria from Harsh Environments: Enhanced Biomass Production and Reduced Emissions of Stress Volatiles. PLoS ONE 2014, 9, e96086. [Google Scholar] [CrossRef]
- Nocelli, N.; Bogino, P.C.; Banchio, E.; Giordano, W. Roles of Extracellular Polysaccharides and Biofilm Formation in Heavy Metal Resistance of Rhizobia. Materials 2016, 9, 418. [Google Scholar] [CrossRef]
- Ozdemir, G.; Ceyhan, N.; Manav, E. Utilization in Alginate Beads for Cu(II) and Ni(II) Adsorption of an Exopolysaccharide Produced by Chryseomonas luteola TEM05. World J. Microbiol. Biotechnol. 2005, 21, 163–167. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).