Siberian Ibex Capra sibirica Respond to Climate Change by Shifting to Higher Latitudes in Eastern Pamir
Abstract
:1. Introduction
2. Methods and Materials
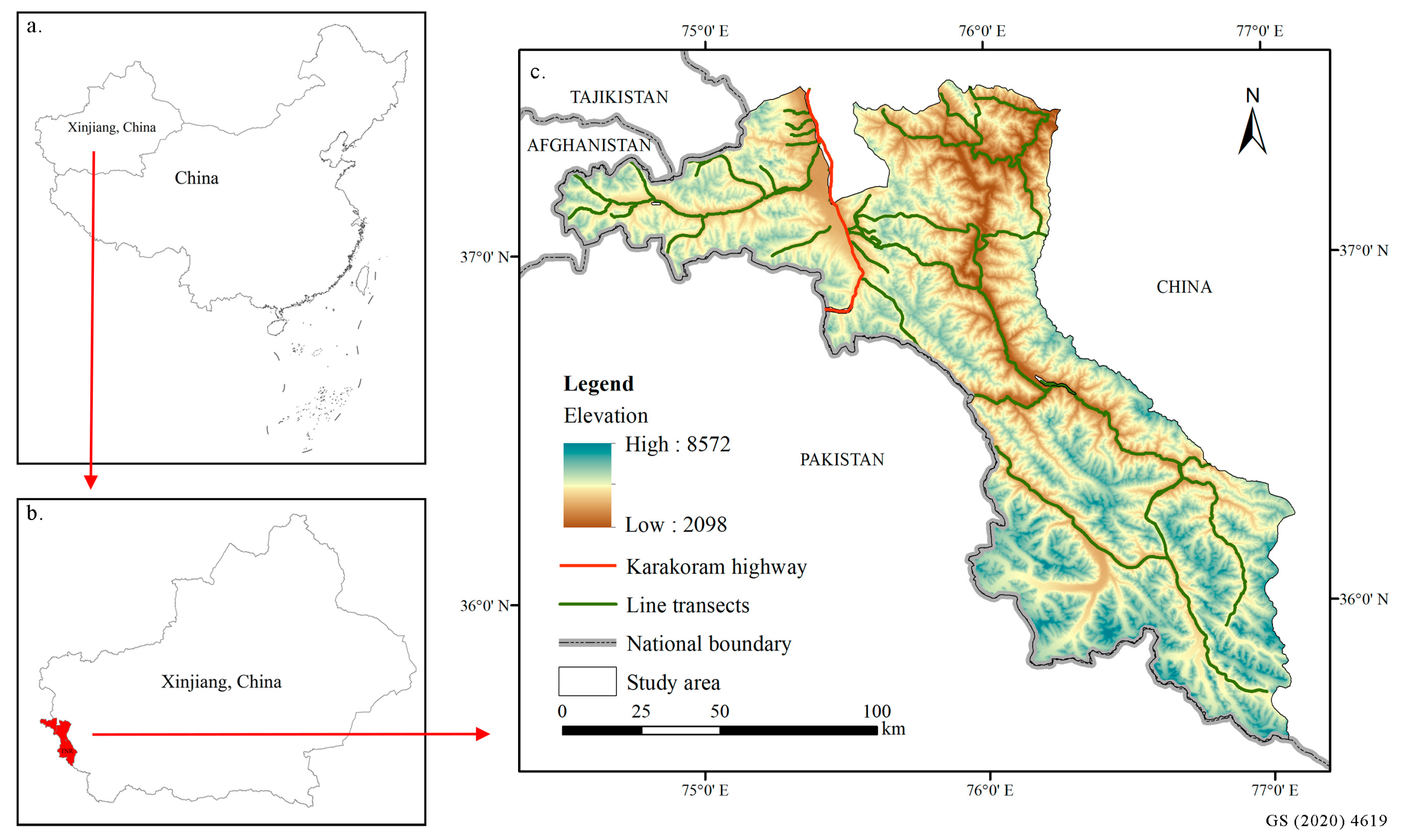
2.1. Study Area
2.2. Occurrence Data
2.3. Environmental Variables
2.4. Species Distribution Model
3. Results
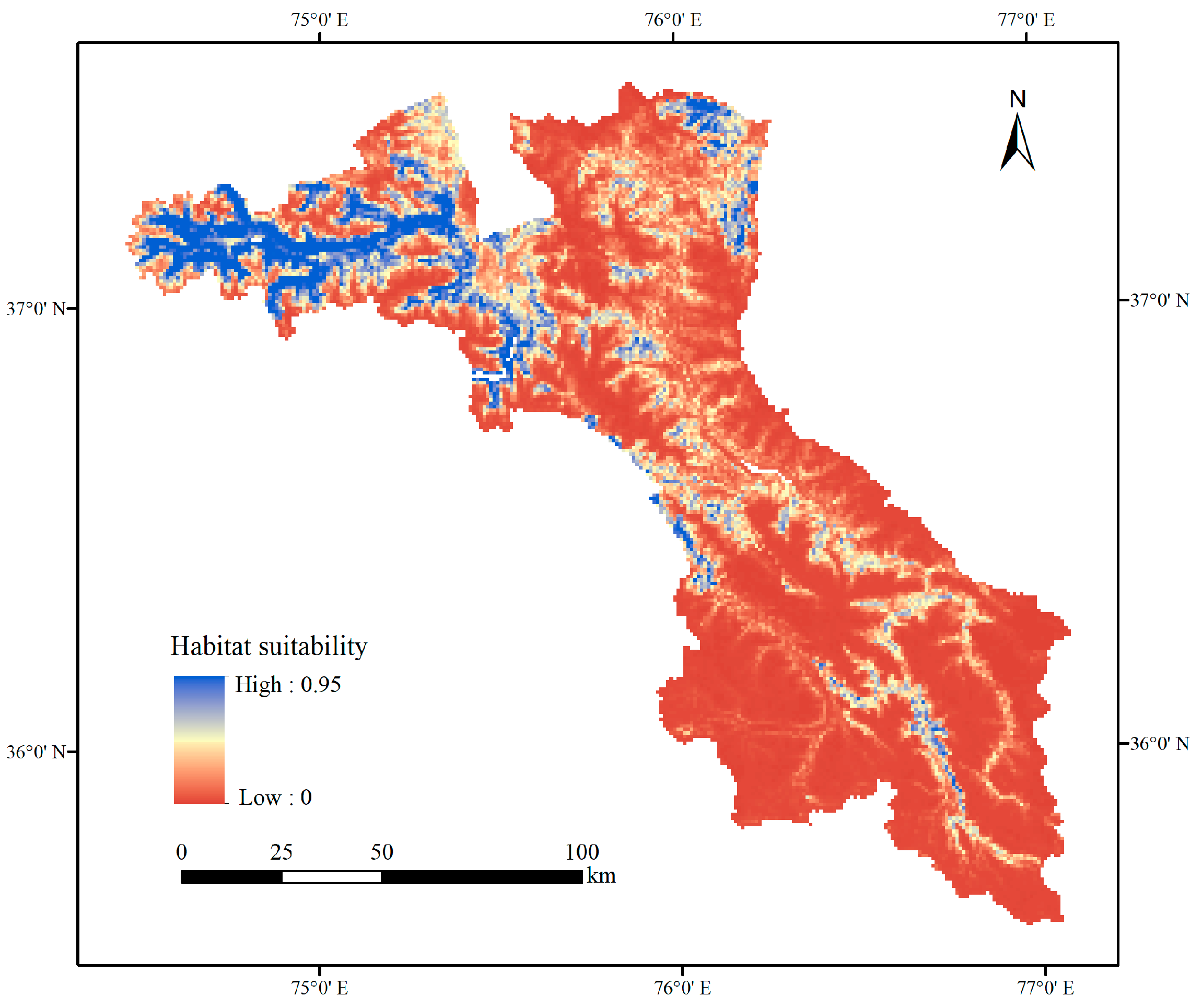
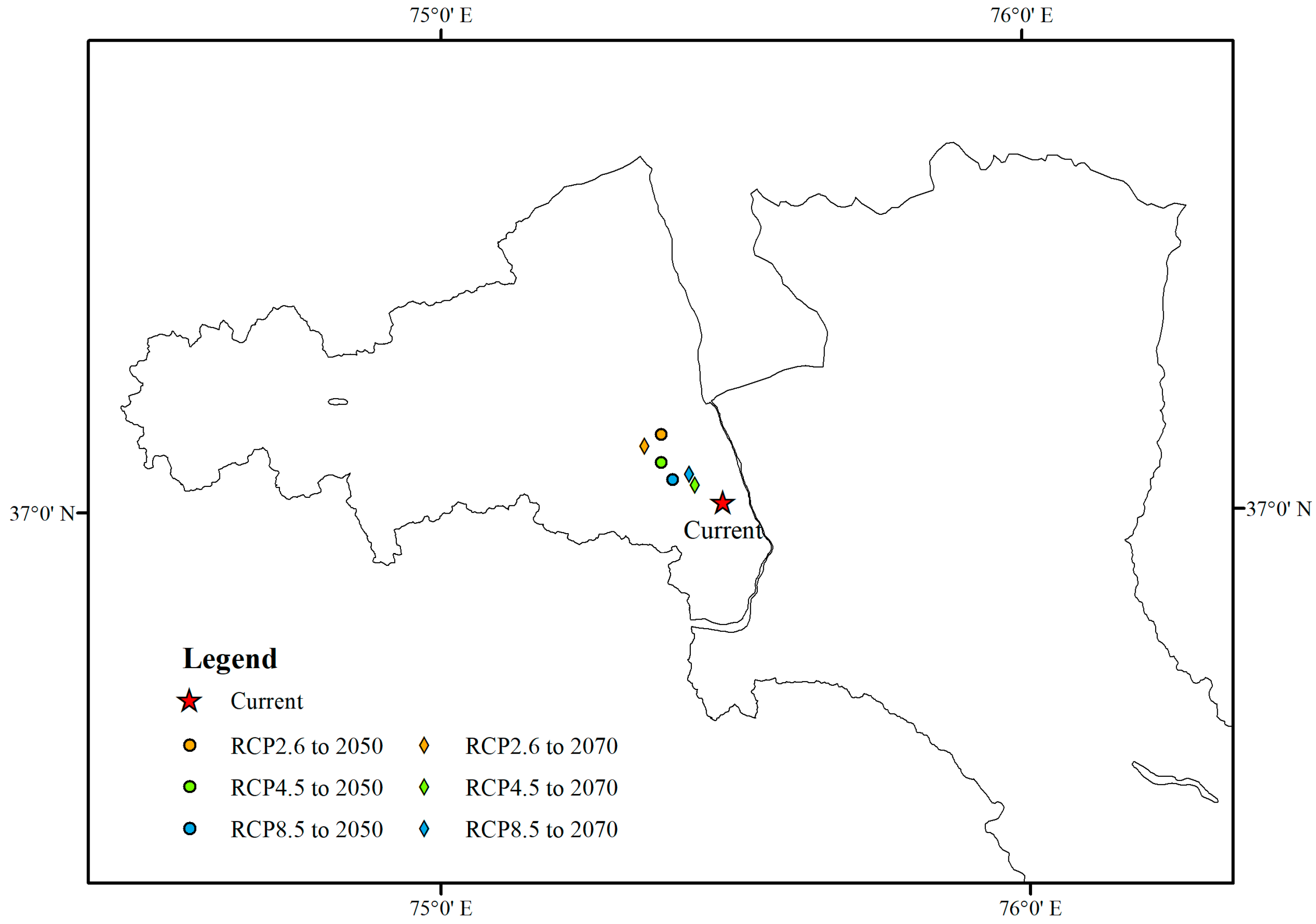
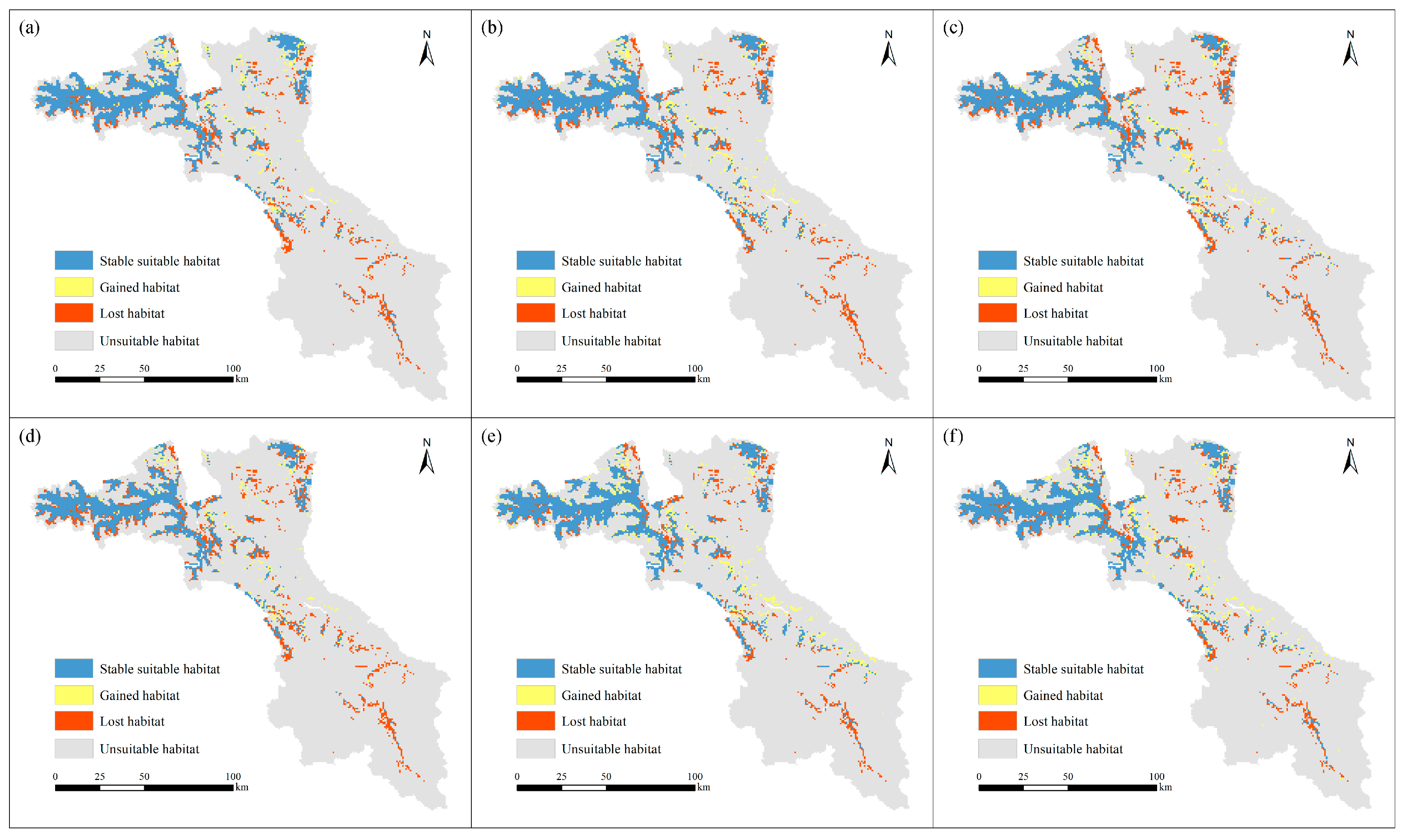
3.1. Current Habitat Distribution
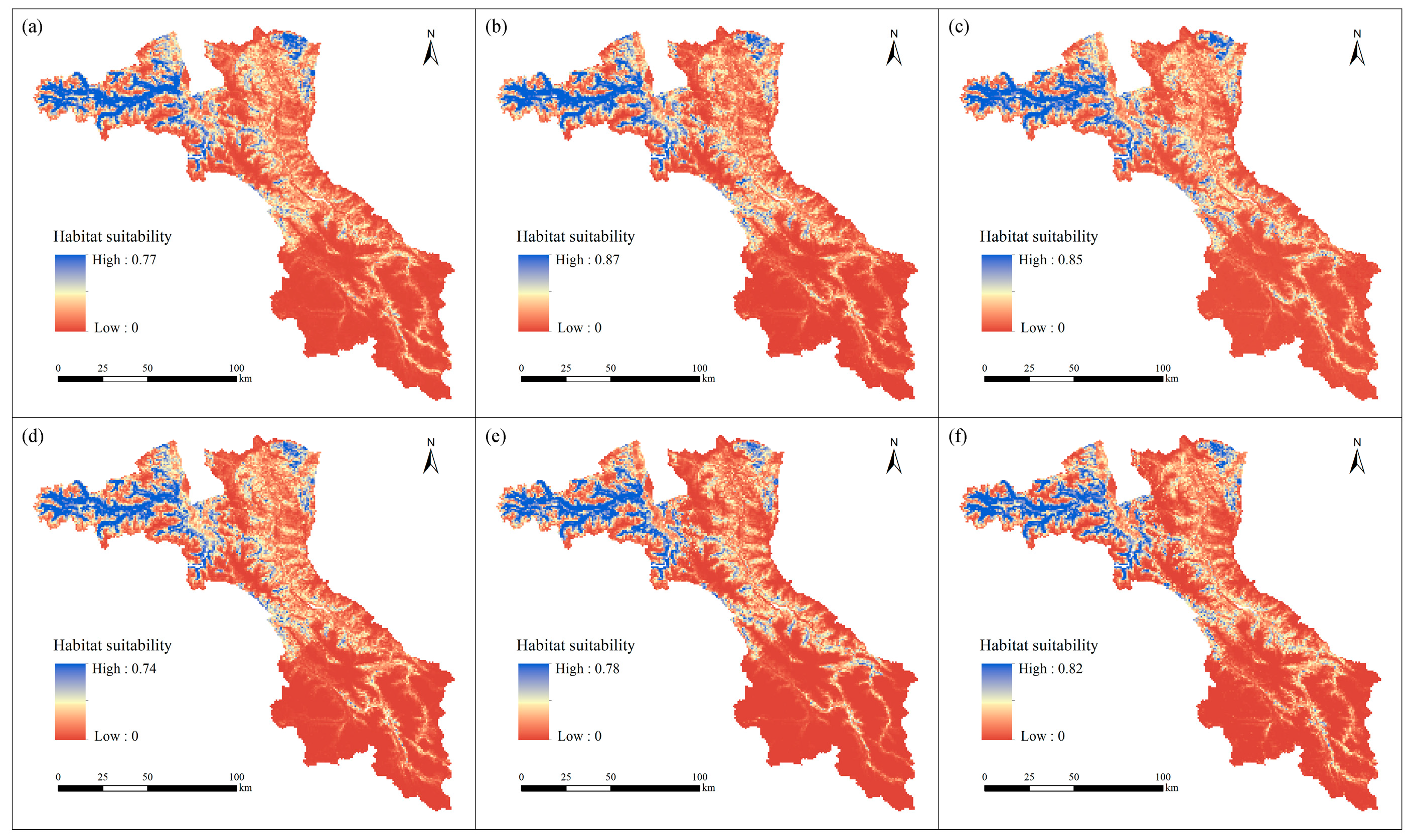
3.2. Future Habitat Distribution
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parmesan, C.; Yohe, G. A globally coherent fingerprint of climate change impacts across natural systems. Nature 2003, 421, 37–42. [Google Scholar] [CrossRef]
- Walther, G.R.; Post, E.; Convey, P.; Menzel, A.; Parmesan, C.; Beebee, T.J.; Fromentin, J.M.; Hoegh-Guldberg, O.; Bairlein, F. Ecological responses to recent climate change. Nature 2002, 416, 389–395. [Google Scholar] [CrossRef]
- Karl, T.R.; Trenberth, K.E. Modern global climate change. Science 2003, 302, 1719–1723. [Google Scholar] [CrossRef]
- Spooner, F.E.B.; Pearson, R.G.; Freeman, R. Rapid warming is associated with population decline among terrestrial birds and mammals globally. Glob. Chang. Biol. 2018, 24, 4521–4531. [Google Scholar] [CrossRef]
- Hetem, R.S.; Fuller, A.; Maloney, S.K.; Mitchell, D. Responses of large mammals to climate change. Temperature 2014, 1, 115–127. [Google Scholar] [CrossRef]
- Bellard, C.; Bertelsmeier, C.; Leadley, P.; Thuiller, W.; Courchamp, F. Impacts of climate change on the future of biodiversity. Ecol. Lett. 2012, 15, 365–377. [Google Scholar] [CrossRef]
- Thomas, C.D.; Cameron, A.; Green, R.E.; Bakkenes, M.; Beaumont, L.J.; Collingham, Y.C.; Erasmus, B.F.; De Siqueira, M.F.; Grainger, A.; Hannah, L.; et al. Extinction risk from climate change. Nature 2004, 427, 145–148. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2022: Impacts, Adaptation, and Vulnerability. Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2022. [Google Scholar]
- Chen, I.C.; Hill, J.K.; Ohlemuller, R.; Roy, D.B.; Thomas, C.D. Rapid range shifts of species associated with high levels of climate warming. Science 2011, 333, 1024–1026. [Google Scholar] [CrossRef]
- Thomas, C.D. Climate, climate change and range boundaries. Divers. Distrib. 2010, 16, 488–495. [Google Scholar] [CrossRef]
- Wilson, R.J.; Gutierrez, D.; Gutierrez, J.; Martinez, D.; Agudo, R.; Monserrat, V.J. Changes to the elevational limits and extent of species ranges associated with climate change. Ecol. Lett. 2005, 8, 1138–1146. [Google Scholar] [CrossRef]
- Dirnbock, T.; Essl, F.; Rabitsch, W. Disproportional risk for habitat loss of high-altitude endemic species under climate change. Glob. Chang. Biol. 2011, 17, 990–996. [Google Scholar] [CrossRef]
- Ye, X.; Yu, X.; Yu, C.; Tayibazhaer, A.; Xu, F.; Skidmore, A.K.; Wang, T. Impacts of future climate and land cover changes on threatened mammals in the semi-arid Chinese Altai Mountains. Sci. Total Environ. 2018, 612, 775–787. [Google Scholar] [CrossRef] [PubMed]
- Hickling, R.; Roy, D.B.; Hill, J.K.; Fox, R.; Thomas, C.D. The distributions of a wide range of taxonomic groups are expanding polewards. Glob. Chang. Biol. 2006, 12, 450–455. [Google Scholar] [CrossRef]
- White, K.S.; Gregovich, D.P.; Levi, T. Projecting the future of an alpine ungulate under climate change scenarios. Glob. Chang. Biol. 2017, 24, 1136–1149. [Google Scholar] [CrossRef] [PubMed]
- Büntgen, U.; Greuter, L.; Bollmann, K.; Jenny, H.; Liebhold, A.; Galván, J.D.; Stenseth, N.C.; Andrew, C.; Mysterud, A. Elevational range shifts in four mountain ungulate species from the Swiss Alps. Ecosphere 2017, 8, e01761. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, F.; Li, G.; Qin, W.; Wu, T.; Xu, F.; Hou, Y.; Song, P.; Cai, Z.; Zhang, T. The four antelope species on the Qinghai-Tibet plateau face habitat loss and redistribution to higher latitudes under climate change. Ecol. Indic. 2021, 123, 107337. [Google Scholar] [CrossRef]
- Brivio, F.; Zurmuhl, M.; Grignolio, S.; von Hardenberg, J.; Apollonio, M.; Ciuti, S. Forecasting the response to global warming in a heat-sensitive species. Sci. Rep. 2019, 9, 3048. [Google Scholar] [CrossRef]
- Mason, T.H.; Stephens, P.A.; Apollonio, M.; Willis, S.G. Predicting potential responses to future climate in an alpine ungulate: Interspecific interactions exceed climate effects. Glob. Chang. Biol. 2014, 20, 3872–3882. [Google Scholar] [CrossRef]
- Yao, T.; Liu, X.; Wang, N.; Shi, Y. Amplitude of climatic changes in Qinghai-Tibetan Plateau. Chin. Sci. Bull. 2000, 45, 1236–1243. [Google Scholar] [CrossRef]
- Ma, R.; Jiang, Z. Impact of global climate change on wildlife. Acta Ecol. Sin. 2005, 25, 3061–3066. [Google Scholar]
- Salas, E.A.L.; Valdez, R.; Michel, S.; Boykin, K.G. Habitat assessment of Marco Polo sheep (Ovis ammon polii) in Eastern Tajikistan: Modeling the effects of climate change. Ecol. Evol. 2018, 8, 5124–5138. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Din, J.U.; Bosso, L.; Hameed, S.; Kabir, M.; Younas, M.; Nawaz, M.A. Expanding or shrinking? range shifts in wild ungulates under climate change in Pamir-Karakoram mountains, Pakistan. PLoS ONE 2021, 16, e0260031. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhang, C.; Mi, C.; Han, L.; Li, M.; Xu, W.; Yang, W. Potential impacts of climate change on suitable habitats of Marco Polo sheep in China. Chin. J. Appl. Ecol. 2021, 32, 3127–3135. [Google Scholar]
- Reading, R.; Michel, S.; Suryawanshi, K.; Bhatnagar, Y.V. Capra sibirica in e.T42398A22148720; The IUCN Red List of Threatened Species. 2020. Available online: https://dx.doi.org/10.2305/IUCN.UK.2020-2.RLTS.T42398A22148720.en (accessed on 3 July 2022).
- Fedosenko, A.; Blank, D. Capra sibirica. Mamm. Species 2001, 2011, 1–13. [Google Scholar] [CrossRef]
- Otgonbayar, B.; Buyandelger, S.; Amgalanbaatar, S.; Reading, R.P. Siberian Ibex (Capra sibirica) Neonatal Kid Survival and Morphometric Measurements in Ikh Nart Nature Reserve, Mongolia. Mong. J. Biol. Sci. 2017, 15, 23–30. [Google Scholar]
- National Forestry and Grassland Administration. National Key Protected Wildlife List. 2021. Available online: http://www.forestry.gov.cn/main/5461/20210205/122418860831352.html (accessed on 3 July 2022).
- Salas, E.A.L.; Valdez, R.; Michel, S.; Boykin, K.G.; Salas, E.A.L.; Valdez, R.; Michel, S.; Boykin, K.G. Response of Asiatic ibex (Capra sibirica) under Climate Change Scenarios. J. Resour. Ecol. 2020, 11, 27–37. [Google Scholar] [CrossRef]
- Odonjavkhlan, C.; Alexsander, J.S.; Mishra, C.; Samelius, G.; Sharma, K.; Lkhagvajav, P.; Suryawanshi, K.R. Factors affecting the spatial distribution and co-occurrence of two sympatric mountain ungulates in southern Mongolia. J. Zool. 2021, 314, 266–274. [Google Scholar] [CrossRef]
- Han, L.; Blanks, D.; Wang, M.Y.; Yang, W.K.; Alves Da Silva, A.; Alves, J. Grouping patterns and social organization in Siberian ibex (Capra sibirica): Feeding strategy matters. Folia Zool. 2019, 68, 35–42. [Google Scholar]
- Schaller, G.B. Mountain Monarchs: Wild Sheep and Goats of the Himalaya; University of Chicago Press: Chicago, IL, USA, 1979. [Google Scholar]
- Bhatnagar, Y.V.; Manjrekar, N.; Stuewe, M.; Rawat, G.S.; Johnsingh, A.J.T. Grouping patterns of Asiatic ibex, Capra ibex sibirica in Pin Valley National Park, India. In Proceedings of the the 2nd World Conference on Mountain Ungulates, Saint Vincent, Italy, 5–7 May 1997. [Google Scholar]
- Marmion, M.; Parviainen, M.; Luoto, M.; Heikkinen, R.K.; Thuiller, W. Evaluation of consensus methods in predictive species distribution modelling. Divers. Distrib. 2008, 15, 59–69. [Google Scholar] [CrossRef]
- Hao, T.; Elith, J.; Guillera-Arroita, G.; Lahoz-Monfort, J.J.; Serra-Diaz, J. A review of evidence about use and performance of species distribution modelling ensembles like BIOMOD. Divers. Distrib. 2019, 25, 839–852. [Google Scholar] [CrossRef]
- Li, M.; Chen, Q.; Wang, M.; Yang, W.; Zhang, C.; Luo, G.; Ding, J.; Lin, Y. Assessment of habitat suitability of Ovis ammon polii based on MaxEnt modeling in Taxkorgan Wildlife Nature Reserve. Chin. J. Ecol. 2019, 38, 594–603. [Google Scholar]
- Schaller, G.B.; Kang, A. Status of Marco Polo sheep Ovis ammon polii in China and adjacent countries: Conservation of a Vulnerable subspecies. Oryx 2008, 42, 100–106. [Google Scholar] [CrossRef]
- Wang, M.; Blank, D.; Wang, Y.; Xu, W.; Yang, W.; Alves, J. Seasonal changes in the sexual segregation patterns of Marco Polo sheep in Taxkorgan Nature Reserve. J. Ethol. 2019, 37, 203–211. [Google Scholar] [CrossRef]
- Li, M.; Chen, Q.; Han, L.; Wang, P.; Yang, J.; Wang, M.; Yang, W. Habitat suitability assessment of Marco Polo sheep in Taxkorgan Nature Reserve in Xinjiang. Acta Ecol. Sin. 2020, 40, 3549–3559. [Google Scholar]
- Wang, M.; Blank, D.; Liu, W.; Wang, Y.; Yang, W. The group pattern of Marco Polo sheep in the Chinese Pamir plateau. Eur. J. Wildl. Res. 2018, 64, 75. [Google Scholar] [CrossRef]
- Syfert, M.M.; Smith, M.J.; Coomes, D.A. The effects of sampling bias and model complexity on the predictive performance of MaxEnt species distribution models. PLoS ONE 2013, 8, e55158. [Google Scholar] [CrossRef]
- Kiedrzyński, M.; Zielińska, K.M.; Rewicz, A.; Kiedrzyńska, E. Habitat and spatial thinning improve the Maxent models performed with incomplete data. J. Geophys. Res. Biogeosci. 2017, 122, 1359–1370. [Google Scholar] [CrossRef]
- Fourcade, Y.; Besnard, A.G.; Secondi, J. Paintings predict the distribution of species, or the challenge of selecting environmental predictors and evaluation statistics. Glob. Ecol. Biogeogr. 2018, 27, 245–256. [Google Scholar] [CrossRef]
- Aiello-Lammens, M.E.; Boria, R.A.; Radosavljevic, A.; Vilela, B.; Anderson, R.P. spThin: An R package for spatial thinning of species occurrence records for use in ecological niche models. Ecography 2015, 38, 541–545. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 19 April 2022).
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Van Vuuren, D.P.; Stehfest, E.; den Elzen, M.G.J.; Kram, T.; van Vliet, J.; Deetman, S.; Isaac, M.; Klein Goldewijk, K.; Hof, A.; Mendoza Beltran, A.; et al. RCP2.6: Exploring the possibility to keep global mean temperature increase below 2 °C. Clim. Chang. 2011, 109, 95–116. [Google Scholar] [CrossRef]
- Thomson, A.M.; Calvin, K.V.; Smith, S.J.; Kyle, G.P.; Volke, A.; Patel, P.; Delgado-Arias, S.; Bond-Lamberty, B.; Wise, M.A.; Clarke, L.E.; et al. RCP4.5: A pathway for stabilization of radiative forcing by 2100. Clim. Chang. 2011, 109, 77–94. [Google Scholar] [CrossRef]
- Riahi, K.; Rao, S.; Krey, V.; Cho, C.; Chirkov, V.; Fischer, G.; Kindermann, G.; Nakicenovic, N.; Rafaj, P. RCP 8.5—A scenario of comparatively high greenhouse gas emissions. Clim. Chang. 2011, 109, 33–57. [Google Scholar] [CrossRef]
- Wu, T.W.; Song, L.C.; Li, W.P.; Wang, Z.Z.; Zhang, H.; Xin, X.G.; Zhang, Y.W.; Zhang, L.; Li, J.L.; Wu, F.H.; et al. An Overview of BCC Climate System Model Development and Application for Climate Change Studies. J. Meteorol. Res. 2014, 28, 34–56. [Google Scholar] [CrossRef]
- WCS. Last of the Wild Project, Version 2, 2005 (LWP-2): Global Human Influence Index (HII) Dataset (Geographic); NASA Socioeconomic Data and Applications Center (SEDAC): Palisades, NY, USA, 2005. [Google Scholar] [CrossRef]
- Peng, Y.; He, G.J.; Zhang, Z.M.; Yin, R.Y. Landsat Spectral Indices Products over China; China Scientific Data: Beijing, China, 2020. [Google Scholar]
- Naimi, B.; Hamm, N.A.S.; Groen, T.A.; Skidmore, A.K.; Toxopeus, A.G. Where is positional uncertainty a problem for species distribution modelling? Ecography 2014, 37, 191–203. [Google Scholar] [CrossRef]
- Chatterjee, S.; Hadi, A.S. Regression Analysis by Example, 5th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2006. [Google Scholar]
- Thuiller, W.; Lafourcade, B.; Engler, R.; Araújo, M.B. BIOMOD—A platform for ensemble forecasting of species distributions. Ecography 2009, 32, 369–373. [Google Scholar] [CrossRef]
- Wisz, M.S.; Guisan, A. Do pseudo-absence selection strategies influence species distribution models and their predictions? An information-theoretic approach based on simulated data. BMC Ecol. 2009, 9, 8. [Google Scholar] [CrossRef]
- Lobo, J.M.; Tognelli, M.F. Exploring the effects of quantity and location of pseudo-absences and sampling biases on the performance of distribution models with limited point occurrence data. J. Nat. Conserv. 2011, 19, 1–7. [Google Scholar] [CrossRef]
- Jiménez-Valverde, A.; Lobo, J.M. Threshold criteria for conversion of probability of species presence to either–or presence–absence. Acta Oecologica 2007, 31, 361–369. [Google Scholar] [CrossRef]
- Liu, C.; White, M.; Newell, G.; Pearson, R. Selecting thresholds for the prediction of species occurrence with presence-only data. J. Biogeogr. 2013, 40, 778–789. [Google Scholar] [CrossRef]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the accuracy of species distribution models: Prevalence, kappa and the true skill statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- Swets, J.A. Measuring the accuracy of diagnostic systems. Science 1988, 240, 1285–1293. [Google Scholar] [CrossRef] [PubMed]
- Thuiller, W.; Georges, D.; Gueguen, M.; Engler, R.; Breiner, F. biomod2: Ensemble Platform for Species Distribution Modeling. R Package Version 3.5.1. 2021. Available online: https://CRAN.R-project.org/package=biomod2 (accessed on 20 September 2021).
- Luo, Z.; Jiang, Z.; Tang, S. Impacts of climate change on distributions and diversity of ungulates on the Tibetan Plateau. Ecol. Appl. 2015, 25, 24–38. [Google Scholar] [CrossRef]
- Wu, X.; Dong, S.; Liu, S.; Su, X.; Han, Y.; Shi, J.; Zhang, Y.; Zhao, Z.; Sha, W.; Zhang, X.; et al. Predicting the shift of threatened ungulates’ habitats with climate change in Altun Mountain National Nature Reserve of the Northwestern Qinghai-Tibetan Plateau. Clim. Chang. 2017, 142, 331–344. [Google Scholar] [CrossRef]
- Halpin, P.N. Global climate change and natural-area protection: Management responses and research directions. Ecol. Appl. 1997, 7, 828–843. [Google Scholar] [CrossRef]
- Lannoo, M. Amphibian Declines: The Conservation Status of United States Species; University of California Press: Berkeley, CA, USA, 2005. [Google Scholar]
- Lovari, S.; Franceschi, S.; Chiatante, G.; Fattorini, L.; Fattorini, N.; Ferretti, F. Climatic changes and the fate of mountain herbivores. Clim. Chang. 2020, 162, 2319–2337. [Google Scholar] [CrossRef]
- Radić, V.; Hock, R. Glaciers in the Earth’s Hydrological Cycle: Assessments of Glacier Mass and Runoff Changes on Global and Regional Scales. Surv. Geophys. 2013, 35, 813–837. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, J.-L.; Liu, S.-Y.; Guo, W.-Q.; Wei, J.-F.; Feng, T. Glacier changes since the early 1960s, eastern Pamir, China. J. Mt. Sci. 2016, 13, 276–291. [Google Scholar] [CrossRef]
- Huaming, S.; Yuting, F.; Ruibo, Z.; Shulong, Y.; Tongwen, Z.; Wenshou, W.; Weiping, L. Streamflow variation in the eastern Pamirs and its response to climate change. Progress. Inquisitiones De Mutat. Clim. 2021, 17, 352–360. [Google Scholar]
- Payne, J.C.; Buuveibaatar, B.; Bowler, D.E.; Olson, K.A.; Walzer, C.; Kaczensky, P. Hidden treasure of the Gobi: Understanding how water limits range use of khulan in the Mongolian Gobi. Sci. Rep. 2020, 10, 2989. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, Q.S.; Rubenstein, D.I.; Zang, S.; Songer, M.; Leimgruber, P.; Chu, H.; Cao, J.; Li, K.; Hu, D. Water Use Patterns of Sympatric Przewalski’s Horse and Khulan: Interspecific Comparison Reveals Niche Differences. PLoS ONE 2015, 10, e0132094. [Google Scholar] [CrossRef] [PubMed]
- Khan, B.; Ablimit, A.; Khan, G.; Jasra, A.W.; Ali, H.; Ali, R.; Ahmad, E.; Ismail, M. Abundance, distribution and conservation status of Siberian ibex, Marco Polo and Blue sheep in Karakoram-Pamir mountain area. J. King Saud Univ.-Sci. 2016, 28, 216–225. [Google Scholar] [CrossRef]
- Pettorelli, N.; Pelletier, F.; Von Hardenberg, A.; Festa-Bianchet, M.; Cote, S.D. Early onset of vegetation growth vs. rapid green-up: Impacts on juvenile mountain ungulates. Ecology 2007, 88, 381–390. [Google Scholar] [CrossRef]
- Scillitani, L.; Sturaro, E.; Monaco, A.; Rossi, L.; Ramanzin, M. Factors affecting home range size of male Alpine ibex (Capra ibex ibex) in the Marmolada massif. Hystrix 2012, 23, 19–27. [Google Scholar]
- Han, L.; Wang, Z.; Blank, D.; Wang, M.; Yang, W. Different environmental requirements of female and male Siberian ibex, Capra sibirica. Sci. Rep. 2021, 11, 6064. [Google Scholar] [CrossRef]
- Forero-Medina, G.; Joppa, L.; Pimm, S.L. Constraints to species’ elevational range shifts as climate changes. Conserv. Biol. 2011, 25, 163–171. [Google Scholar] [CrossRef]
- Martinez-Lopez, O.; Koch, J.B.; Martinez-Morales, M.A.; Navarrete-Gutierrez, D.; Enriquez, E.; Vandame, R. Reduction in the potential distribution of bumble bees (Apidae: Bombus) in Mesoamerica under different climate change scenarios: Conservation implications. Glob. Chang. Biol. 2021, 27, 1772–1787. [Google Scholar] [CrossRef]
- Moritz, C.; Patton, J.L.; Conroy, C.J.; Parra, J.L.; White, G.C.; Beissinger, S.R. Impact of a century of climate change on small-mammal communities in Yosemite National Park, USA. Science 2008, 322, 261–264. [Google Scholar] [CrossRef]
- Hickling, R.; Roy, D.B.; Hill, J.K.; Thomas, C.D. A northward shift of range margins in British Odonata. Glob. Chang. Biol. 2005, 11, 502–506. [Google Scholar] [CrossRef]
- MacLean, S.A.; Beissinger, S.R. Species’ traits as predictors of range shifts under contemporary climate change: A review and meta-analysis. Glob. Chang. Biol. 2017, 23, 4094–4105. [Google Scholar] [CrossRef]
- Walther, G.-R.; Beißner, S.; Burga, C.A. Trends in the upward shift of alpine plants. J. Veg. Sci. 2005, 16, 541–548. [Google Scholar] [CrossRef]
- Elsen, P.R.; Tingley, M.W. Global mountain topography and the fate of montane species under climate change. Nat. Clim. Chang. 2015, 5, 772–776. [Google Scholar] [CrossRef]
- Han, L. Sexual Segregation of Siberian ibex (Capra sibirica) in Middle Tianshan Mountains, Xinjiang, China; University of Chinese Academy of Sciences: Beijing, China, 2021. [Google Scholar]
- Gebremedhin, B.; Chala, D.; Flagstad, Ø.; Bekele, A.; Bakkestuen, V.; van Moorter, B.; Ficetola, G.F.; Zimmermann, N.E.; Brochmann, C.; Stenseth, N.C. Quest for New Space for Restricted Range Mammals: The Case of the Endangered Walia Ibex. Front. Ecol. Evol. 2021, 9, 611632. [Google Scholar] [CrossRef]
- Huang, P.Z.; Bian, K.; Huang, Z.P.; Li, Q.; Dunn, D.W.; Fang, G.; Liu, J.H.; Wang, M.Y.; Yang, X.F.; Pan, R.L.; et al. Human activities and elevational constraints restrict ranging patterns of snub-nosed monkeys in a mountainous refuge. Integr. Zool. 2020, 16, 202–213. [Google Scholar] [CrossRef]
- Yang, L.; Shi, K.C.; Ma, C.; Ren, G.P.; Fan, P.F. Mechanisms underlying altitudinal and horizontal range contraction: The western black crested gibbon. J. Biogeogr. 2020, 48, 321–331. [Google Scholar] [CrossRef]
- Root, T.L.; Price, J.T.; Hall, K.R.; Schneider, S.H.; Rosenzweig, C.; Pounds, J.A. Fingerprints of global warming on wild animals and plants. Nature 2003, 421, 57–60. [Google Scholar] [CrossRef]
- La Sorte, F.A.; Jetz, W. Avian distributions under climate change: Towards improved projections. J. Exp. Biol. 2010, 213, 862–869. [Google Scholar] [CrossRef] [Green Version]
- Zuckerberg, B.; Woods, A.M.; Porter, W.F. Poleward shifts in breeding bird distributions in New York State. Glob. Chang. Biol. 2009, 15, 1866–1883. [Google Scholar] [CrossRef]
- Thomas, C.D.; Lennon, J.J. Birds extend their ranges northwards. Nature 1999, 399, 213. [Google Scholar] [CrossRef]
- Hitch, A.T.; Leberg, P.L. Breeding distributions of north American bird species moving north as a result of climate change. Conserv. Biol. 2007, 21, 534–539. [Google Scholar] [CrossRef]
- Peh, K.S.H. Potential Effects of Climate Change on Elevational Distributions of Tropical Birds in Southeast Asia. Condor 2007, 109, 437–441. [Google Scholar] [CrossRef]
- Colwell, R.K.; Brehm, G.; Cardelus, C.L.; Gilman, A.C.; Longino, J.T. Global warming, elevational range shifts, and lowland biotic attrition in the wet tropics. Science 2008, 322, 258–261. [Google Scholar] [CrossRef]
- Manes, S.; Costello, M.J.; Beckett, H.; Debnath, A.; Devenish-Nelson, E.; Grey, K.-A.; Jenkins, R.; Khan, T.M.; Kiessling, W.; Krause, C.; et al. Endemism increases species’ climate change risk in areas of global biodiversity importance. Biol. Conserv. 2021, 257, 109070. [Google Scholar] [CrossRef]
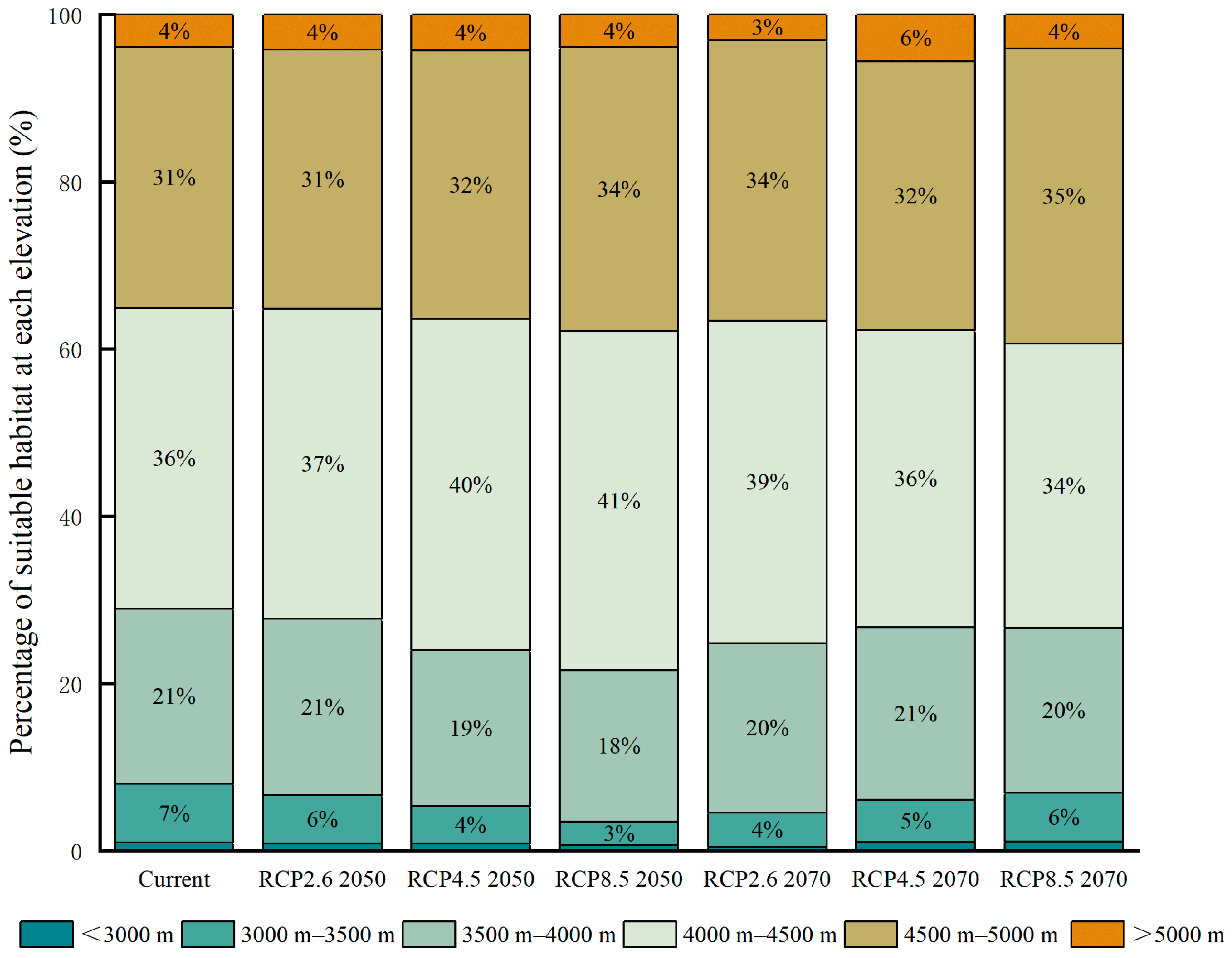
| Variable Type | Variable Name | Description ** | Unit |
|---|---|---|---|
| Climate | Bio1 | Annual mean temperature | ℃ |
| Bio2 | Mean diurnal range | ℃ | |
| Bio3 * | Isothermality | - | |
| Bio4 * | Temperature seasonality | - | |
| Bio5 | Max temperature of warmest month | ℃ | |
| Bio6 | Min temperature of coldest month | ℃ | |
| Bio7 | Temperature annual range | ℃ | |
| Bio8 * | Mean temperature of wettest quarter | ℃ | |
| Bio9 * | Mean temperature of driest quarter | ℃ | |
| Bio10 | Mean temperature of warmest quarter | ℃ | |
| Bio11 | Mean temperature of coldest quarter | ℃ | |
| Bio12 | Annual precipitation | mm | |
| Bio13 * | Precipitation of wettest month | mm | |
| Bio14 * | Precipitation of driest month | mm | |
| Bio15 * | Precipitation seasonality | - | |
| Bio16 | Precipitation of wettest quarter | mm | |
| Bio17 | Precipitation of driest quarter | mm | |
| Bio18 | Precipitation of warmest quarter | mm | |
| Bio19 | Precipitation of coldest quarter | mm | |
| Topographic | Elevation * | The height above sea level | m |
| Slope * | The degree of steepness | ° | |
| Aspect * | Orientation of the topographic slope | - | |
| Ruggedness * | The difference between the highest and lowest elevations in a given area | m | |
| Human distribution | Human Influence Index * | The extent of human activity | - |
| Food resources | Land cover * | Land cover type | - |
| Distance to water * | Distance from the nearest water source | m | |
| NDVI * | Normalized Difference Vegetation Index | - |
| Period | Future Scenarios | Suitable Habitat (km2) | The Change of Suitable Habitat (km2) | The Change of Suitable Habitat (%) | |||
|---|---|---|---|---|---|---|---|
| Stable | Gain | Loss | Gain | Loss | |||
| Current | - | 2702.15 | - | - | - | - | - |
| 2050 | RCP2.6 | 2290.33 | 1963.76 | 326.57 | 738.40 | 12.09 | 27.33 |
| RCP4.5 | 2268.65 | 1911.01 | 357.64 | 791.14 | 13.24 | 29.28 | |
| RCP8.5 | 2234.70 | 1868.39 | 366.31 | 833.77 | 13.56 | 30.86 | |
| 2070 | RCP2.6 | 1984.71 | 1752.79 | 231.92 | 948.64 | 8.58 | 35.11 |
| RCP4.5 | 2577.88 | 2068.52 | 509.36 | 632.91 | 18.85 | 23.42 | |
| RCP8.5 | 2478.90 | 2015.05 | 463.85 | 687.10 | 17.17 | 25.43 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhuo, Y.; Wang, M.; Zhang, B.; Ruckstuhl, K.E.; Alves da Silva, A.; Yang, W.; Alves, J. Siberian Ibex Capra sibirica Respond to Climate Change by Shifting to Higher Latitudes in Eastern Pamir. Diversity 2022, 14, 750. https://doi.org/10.3390/d14090750
Zhuo Y, Wang M, Zhang B, Ruckstuhl KE, Alves da Silva A, Yang W, Alves J. Siberian Ibex Capra sibirica Respond to Climate Change by Shifting to Higher Latitudes in Eastern Pamir. Diversity. 2022; 14(9):750. https://doi.org/10.3390/d14090750
Chicago/Turabian StyleZhuo, Yingying, Muyang Wang, Baolin Zhang, Kathreen E. Ruckstuhl, António Alves da Silva, Weikang Yang, and Joana Alves. 2022. "Siberian Ibex Capra sibirica Respond to Climate Change by Shifting to Higher Latitudes in Eastern Pamir" Diversity 14, no. 9: 750. https://doi.org/10.3390/d14090750
APA StyleZhuo, Y., Wang, M., Zhang, B., Ruckstuhl, K. E., Alves da Silva, A., Yang, W., & Alves, J. (2022). Siberian Ibex Capra sibirica Respond to Climate Change by Shifting to Higher Latitudes in Eastern Pamir. Diversity, 14(9), 750. https://doi.org/10.3390/d14090750






