Biogeographic Patterns and Richness of the Meconopsis Species and Their Influence Factors across the Pan-Himalaya and Adjacent Regions
Abstract
:1. Introduction
2. Materials and Methods
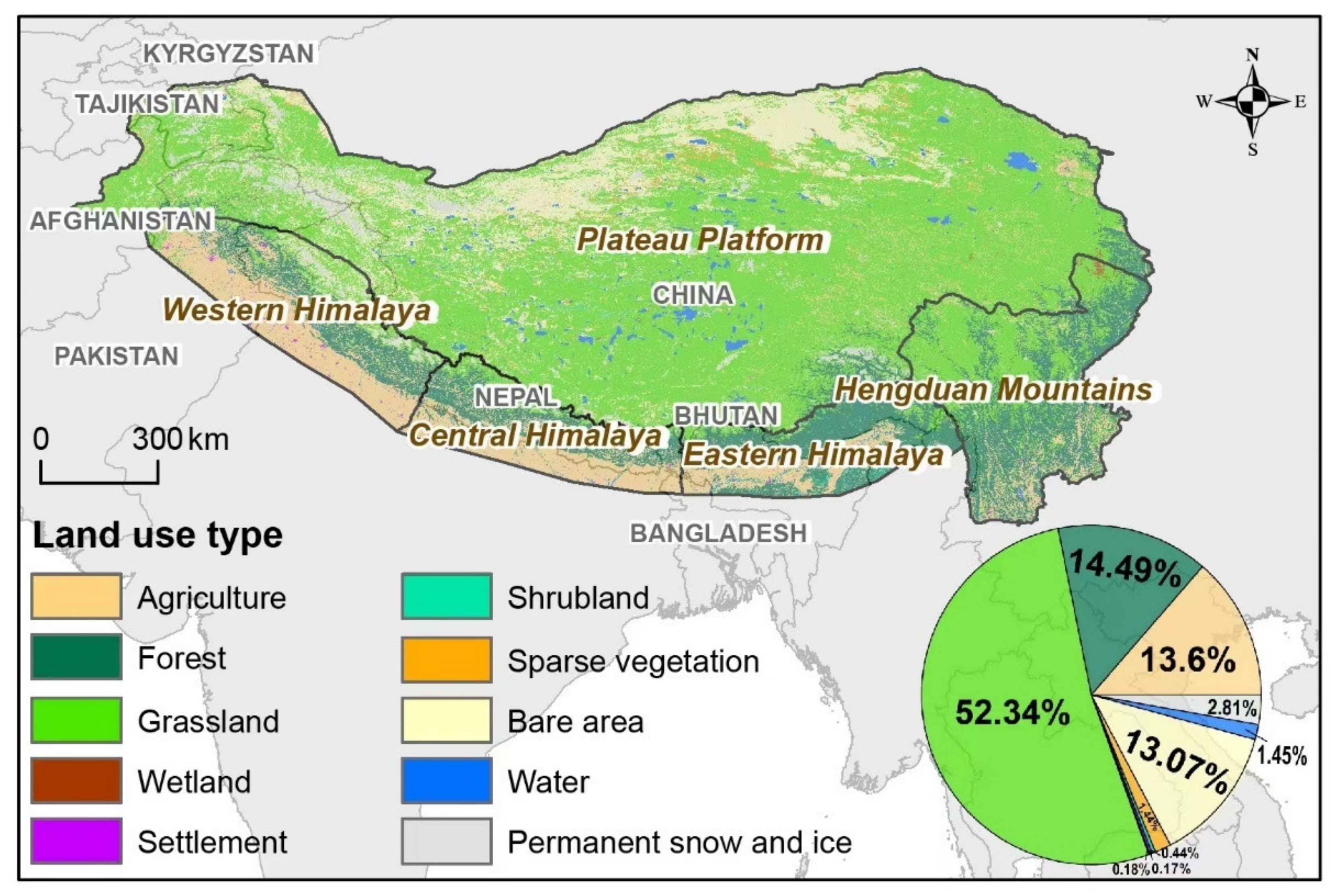
2.1. Study Area
2.2. Data Sources and Preprocessing
2.2.1. Species Occurrence Data
2.2.2. Bioclimatic and Topographic Data
2.2.3. Land Type Data
2.3. Methods
2.3.1. MaxEnt Modeling
2.3.2. Spatial Pattern Distribution of Species Richness
2.3.3. Distribution Pattern of Species Richness along Environmental Gradients
2.3.4. Landscape Fragmentation
3. Results
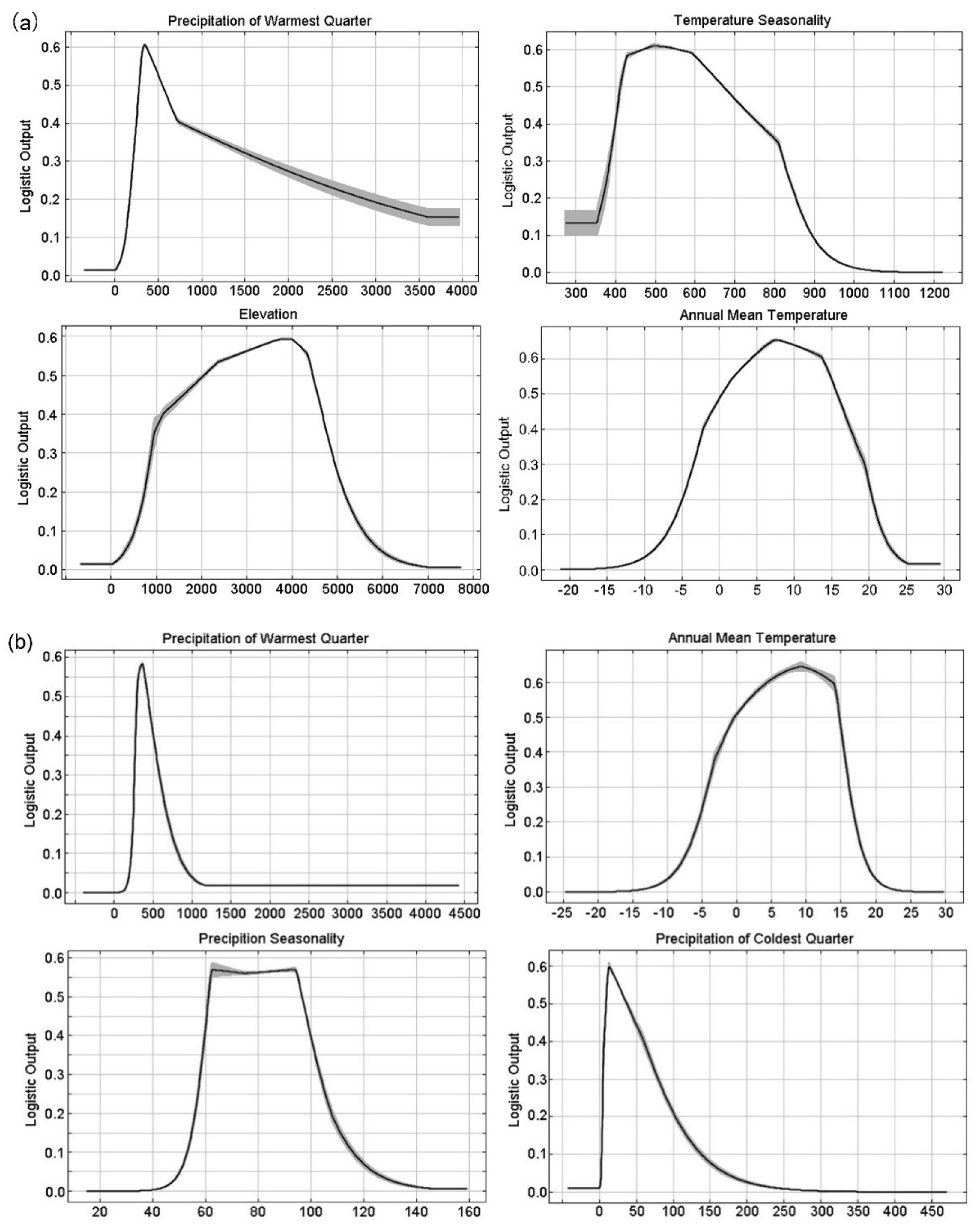
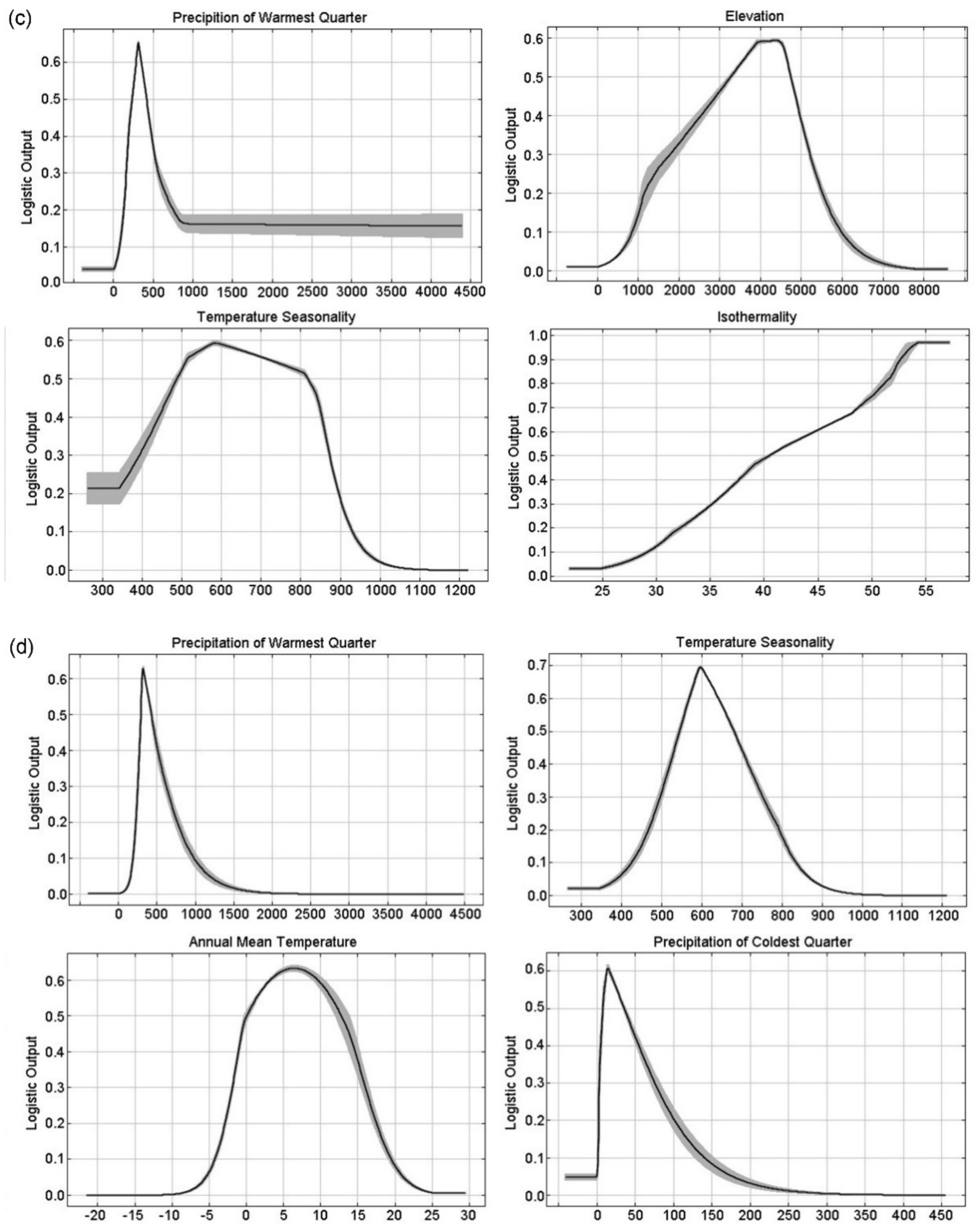
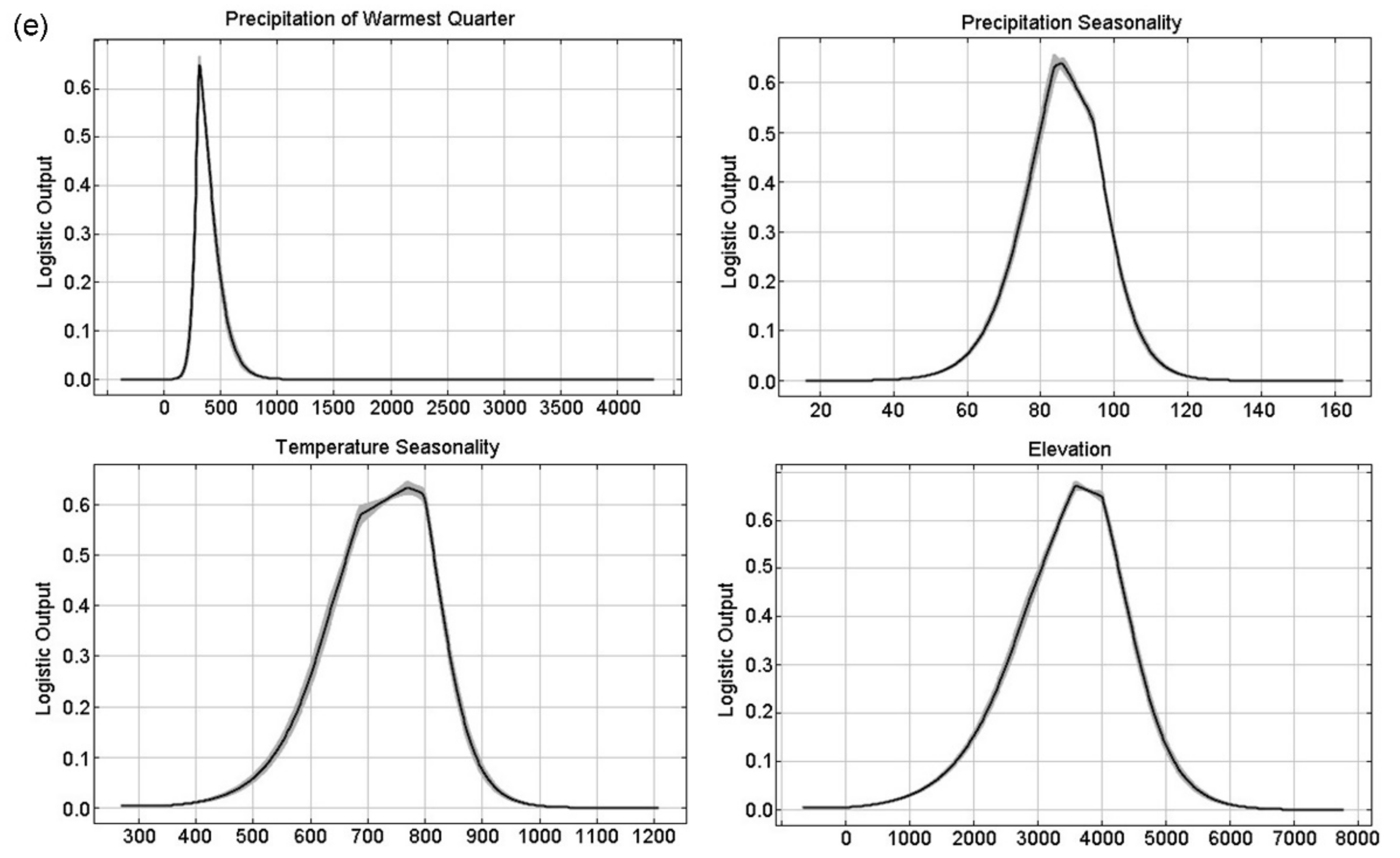
3.1. Model Performance and Key Variables to Predict Typical Meconopsis Species
3.2. Current Potential Geographical Distribution Patterns of Species
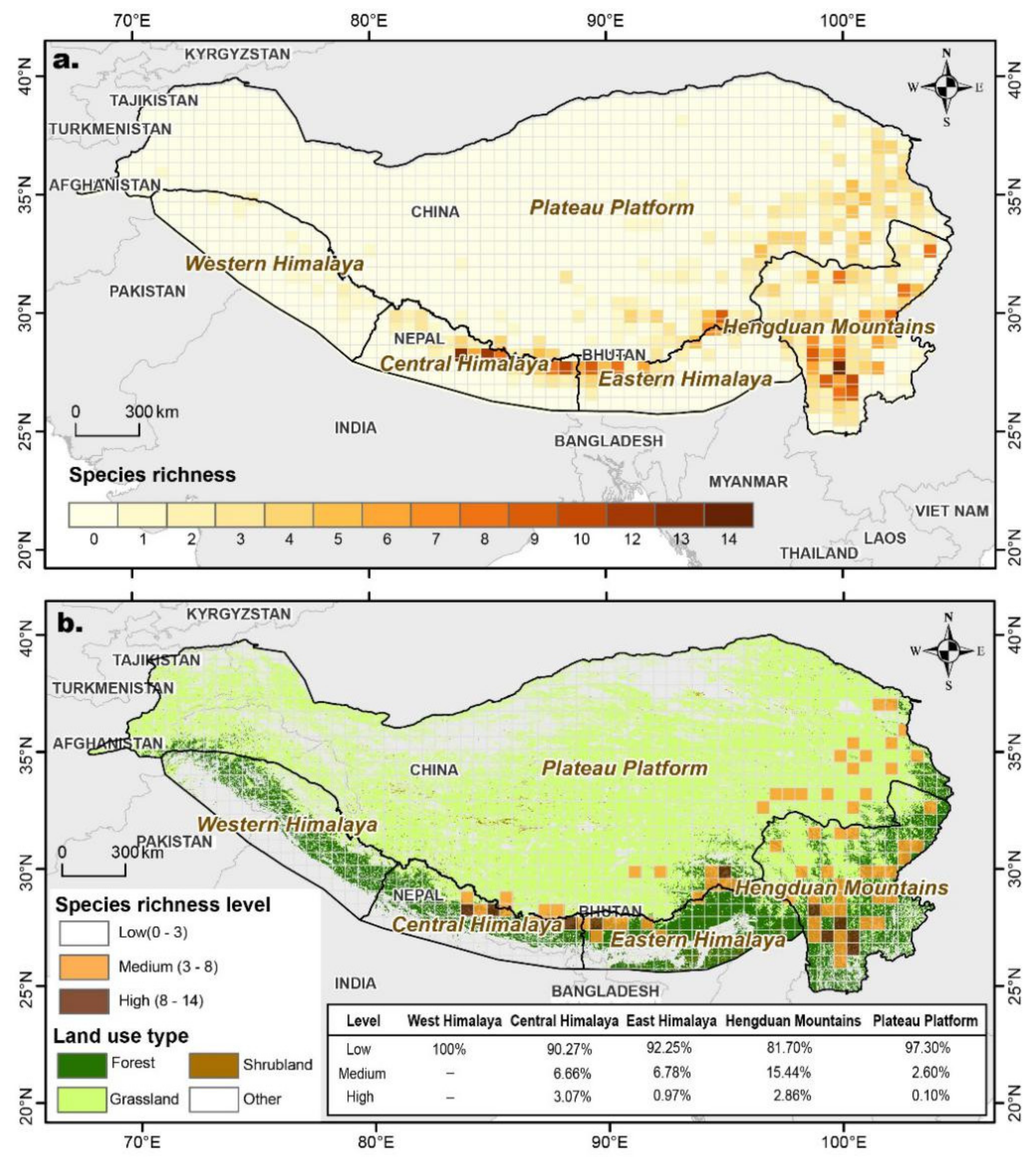
3.3. Species Richness Geospatial Patterns of Meconopsis
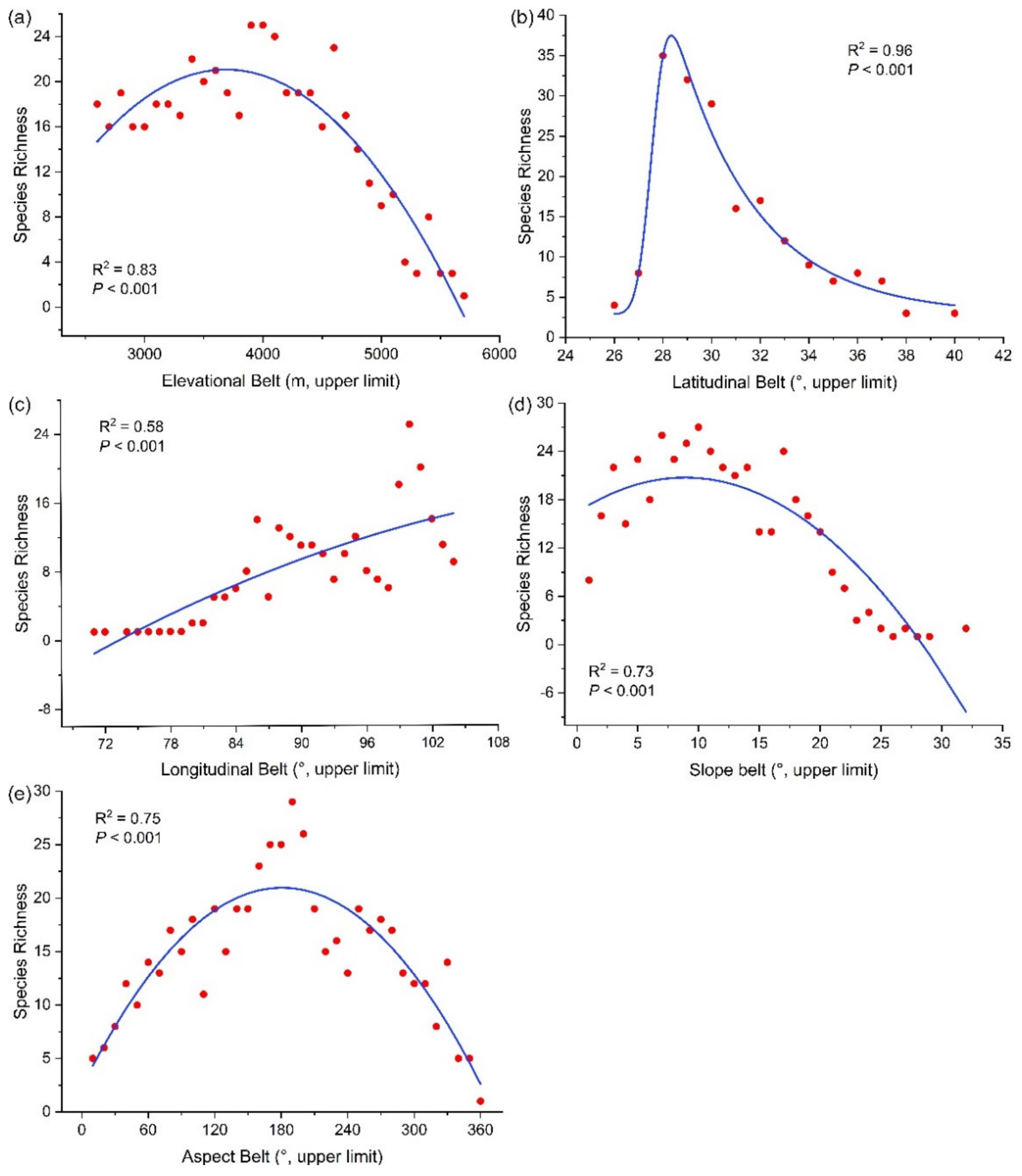
3.4. Species Richness Pattern along Environmental Gradients
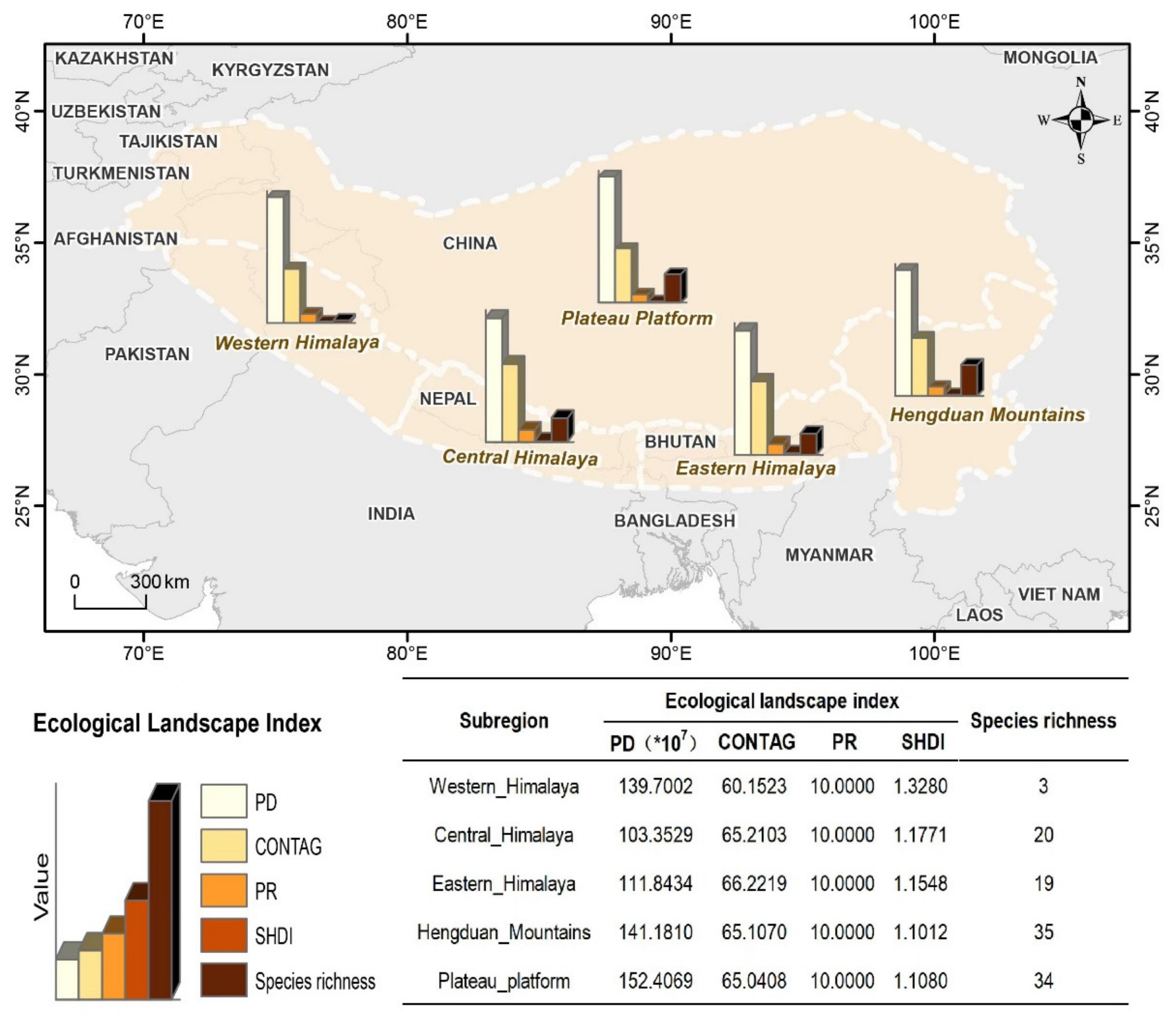
3.5. Ecological Landscape Fragmentation and Species Richness
4. Discussion
4.1. Divergent Environmental Factors Affect the Spatial Distribution of Meconopsis Species
4.2. The Richness of Meconopsis Species Varies along Geospatial Gradients
4.3. Linkage and Relations between Landscape Heterogeneity and Species Richness
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huss, M.; Bookhagen, B.; Huggel, C.; Jacobsen, D.; Bradley, R.S.; Clague, J.J.; Vuille, M.; Buytaert, W.; Cayan, D.R.; Greenwood, G.; et al. Toward mountains without permanent snow and ice. Earth’s Future 2017, 5, 418–435. [Google Scholar] [CrossRef]
- Nie, Y.; Pritchard, H.D.; Liu, Q.; Hennig, T.; Wang, W.; Wang, X.; Liu, S.; Nepal, S.; Samyn, D.; Hewitt, K.; et al. Glacial change and hydrological implications in the Himalaya and Karakoram. Nat. Rev. Earth Environ. 2021, 2, 91–106. [Google Scholar] [CrossRef]
- Shi, H.; Zhou, Q.; He, R.; Zhang, Q.; Dang, H. Climate warming will widen the lagging gap of global treeline shift relative to densification. Agric. For. Meteorol. 2022, 318, 108917. [Google Scholar] [CrossRef]
- Pepin, N.; Bradley, R.S.; Diaz, H.F.; Baraer, M.; Caceres, E.B.; Forsythe, N.; Fowler, H.; Greenwood, G.; Hashmi, M.Z.; Liu, X.D.; et al. Elevation-dependent warming in mountain regions of the world. Nat. Clim. Chang. 2015, 5, 424–430. [Google Scholar] [CrossRef]
- Rogora, M.; Frate, L.; Carranza, M.; Freppaz, M.; Stanisci, A.; Bertani, I.; Bottarin, R.; Brambilla, A.; Canullo, R.; Carbognani, M.; et al. Assessment of climate change effects on mountain ecosystems through a cross-site analysis in the Alps and Apennines. Sci. Total Environ. 2018, 624, 1429–1442. [Google Scholar] [CrossRef]
- Beniston, M. Climatic Change in Mountain Regions: A Review of Possible Impacts. Clim. Chang. 2003, 59, 5–31. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Y.; Wang, Z.; Li, L. Mapping human influence intensity in the Tibetan Plateau for conservation of ecological service functions. Ecosyst. Serv. 2018, 30, 276–286. [Google Scholar] [CrossRef]
- Zhang, Y.; Qian, L.; Spalink, D.; Sun, L.; Chen, J.; Sun, H. Spatial phylogenetics of two topographic extremes of the Hengduan Mountains in southwestern China and its implications for biodiversity conservation. Plant Divers. 2020, 43, 181–191. [Google Scholar] [CrossRef]
- Liang, Q.; Xu, X.; Mao, K.; Wang, M.; Wang, K.; Xi, Z.; Liu, J. Shifts in plant distributions in response to climate warming in a biodiversity hotspot, the Hengduan Mountains. J. Biogeogr. 2018, 45, 1334–1344. [Google Scholar] [CrossRef]
- Mao, K.; Wang, Y.; Liu, J. Evolutionary origin of species diversity on the Qinghai–Tibet Plateau. J. Syst. Evol. 2021, 59, 1142–1158. [Google Scholar] [CrossRef]
- Liu, Y.; Ye, J.; Hu, H.; Peng, D.; Zhao, L.; Lu, L.; Zaman, W.; Chen, Z.; Ebach, M. Influence of elevation on bioregionalisation: A case study of the Sino-Himalayan flora. J. Biogeogr. 2021, 48, 2578–2587. [Google Scholar] [CrossRef]
- Körner, C.; Jetz, W.; Paulsen, J.; Payne, D.; Rudmann-Maurer, K.; Spehn, E.M. A global inventory of mountains for bio-geographical applications. Alp. Bot. 2016, 127, 1–15. [Google Scholar] [CrossRef]
- Liu, J.; Li, J.; Lai, Y. Plant diversity and ecology on the Qinghai–Tibet Plateau. J. Syst. Evol. 2021, 59, 1139–1141. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, J.; Deng, T.; Boufford, D.E. Origins and evolution of plant diversity in the Hengduan Mountains, China. Plant Divers. 2017, 39, 161–166. [Google Scholar] [CrossRef]
- Chen, S.; Wang, W.; Xu, W.; Wang, Y.; Wan, H.; Chen, D.; Tang, Z.; Tang, X.; Zhou, G.; Xie, Z.; et al. Plant diversity enhances productivity and soil carbon storage. Proc. Natl. Acad. Sci. USA 2018, 115, 4027–4032. [Google Scholar] [CrossRef]
- Quijas, S.; Schmid, B.; Balvanera, P. Plant diversity enhances provision of ecosystem services: A new synthesis. Basic Appl. Ecol. 2010, 11, 582–593. [Google Scholar] [CrossRef]
- Guo, F.; Lenoir, J.; Bonebrake, T.C. Land-use change interacts with climate to determine elevational species redistribution. Nat. Commun. 2018, 9, 1–7. [Google Scholar] [CrossRef]
- Wu, J.; Liu, Z.M. Effect of habitat fragmentation on biodiversity: A review. Chinese J. Ecol. 2014, 33, 1946–1952. [Google Scholar]
- Bálint, M.; Domisch, S.; Engelhardt, C.H.M.; Haase, P.; Lehrian, S.; Sauer, J.; Theissinger, K.; Pauls, S.U.; Nowak, C. Cryptic biodiversity loss linked to global climate change. Nat. Clim. Chang. 2011, 1, 313–318. [Google Scholar] [CrossRef]
- Boddy, L.; Büntgen, U.; Egli, S.; Gange, A.C.; Heegaard, E.; Kirk, P.M.; Mohammad, A.; Kauserud, H. Climate variation effects on fungal fruiting. Fungal Ecol. 2014, 10, 20–33. [Google Scholar] [CrossRef]
- Hooper, D.U.; Adair, E.C.; Cardinale, B.J.; Byrnes, J.; Hungate, B.A.; Matulich, K.L.; Gonzalez, A.; Duffy, J.E.; Gamfeldt, L.; O’Connor, M.I. A global synthesis reveals biodiversity loss as a major driver of ecosystem change. Nature 2012, 486, 105–108. [Google Scholar] [CrossRef]
- Zhang, K.; Yao, L.; Meng, J.; Tao, J. Maxent modeling for predicting the potential geographical distribution of two peony species under climate change. Sci. Total Environ. 2018, 634, 1326–1334. [Google Scholar] [CrossRef]
- Lenoir, J.; Gégout, J.-C.; Guisan, A.; Vittoz, P.; Wohlgemuth, T.; Zimmermann, N.E.; Dullinger, S.; Pauli, H.; Willner, W.; Svenning, J.-C. Going against the flow: Potential mechanisms for unexpected downslope range shifts in a warming climate. Ecography 2010, 33, 295–303. [Google Scholar] [CrossRef]
- Alatalo, J.M.; Jägerbrand, A.K.; Molau, U. Impacts of different climate change regimes and extreme climatic events on an alpine meadow community. Sci. Rep. 2016, 6, 21720. [Google Scholar] [CrossRef]
- Ceballos, G.; Ehrlich, P.R.; Barnosky, A.D.; García, A.; Pringle, R.M.; Palmer, T.M. Accelerated modern human–induced species losses: Entering the sixth mass extinction. Sci. Adv. 2015, 1, e1400253. [Google Scholar] [CrossRef]
- Di Giulio, M.; Holderegger, R.; Tobias, S. Effects of habitat and landscape fragmentation on humans and biodiversity in densely populated landscapes. J. Environ. Manag. 2009, 90, 2959–2968. [Google Scholar] [CrossRef]
- Fahrig, L. Effects of Habitat Fragmentation on Biodiversity. Annu. Rev. Ecol. Evol. Syst. 2003, 34, 487–515. [Google Scholar] [CrossRef]
- Fischer, J.; Lindenmayer, D.B. Landscape modification and habitat fragmentation: A synthesis. Glob. Ecol. Biogeogr. 2007, 16, 265–280. [Google Scholar] [CrossRef]
- Reino, L.; Beja, P.; Araújo, M.B.; Dray, S.; Segurado, P. Does local habitat fragmentation affect large-scale distributions? The case of a specialist grassland bird. Divers. Distrib. 2013, 19, 423–432. [Google Scholar] [CrossRef]
- Xu, C.; Huang, Z.Y.X.; Chi, T.; Chen, B.J.W.; Zhang, M.; Liu, M. Can local landscape attributes explain species richness patterns at macroecological scales? Glob. Ecol. Biogeogr. 2013, 23, 436–445. [Google Scholar] [CrossRef]
- Huang, H. Plant diversity and conservation in China: Planning a strategic bioresource for a sustainable future. Bot. J. Linn. Soc. 2011, 166, 282–300. [Google Scholar] [CrossRef] [PubMed]
- Brooks, T.M.; Mittermeier, R.A.; Da Fonseca, G.A.B.; Gerlach, J.; Hoffmann, M.; Lamoreux, J.F.; Mittermeier, C.G.; Pilgrim, J.D.; Rodrigues, A.S.L. Global Biodiversity Conservation Priorities. Science 2006, 313, 58–61. [Google Scholar] [CrossRef] [PubMed]
- Xin, L.; Zhi-Heng, W.; Zhi-Yao, T.; Shu-Qing, Z.; Jing-Yun, F. Geographic patterns and environmental correlates of terrestrial mammal species richness in China. Biodivers. Sci. 2009, 17, 652–663. [Google Scholar] [CrossRef]
- Stiles, A.; Scheiner, S.M. A multi-scale analysis of fragmentation effects on remnant plant species richness in Phoenix, Arizona. J. Biogeogr. 2010, 37, 1721–1729. [Google Scholar] [CrossRef]
- Giladi, I.; Ziv, Y.; May, F.; Jeltsch, F. Scale-dependent determinants of plant species richness in a semi-arid fragmented agro-ecosystem. J. Veg. Sci. 2011, 22, 983–996. [Google Scholar] [CrossRef]
- Xiao, W.; Simpson, B.B. A New Infrageneric Classification of Meconopsis (Papaveraceae) Based on a Well-supported Molecular Phylogeny. Syst. Bot. 2017, 42, 226–233. [Google Scholar] [CrossRef]
- Xie, H.; Ash, J.E.; Linde, C.; Cunningham, S.; Nicotra, A. Himalayan-Tibetan Plateau Uplift Drives Divergence of Polyploid Poppies: Meconopsis Viguier (Papaveraceae). PLoS ONE 2014, 9, e99177. [Google Scholar] [CrossRef]
- Egan, P.A. Meconopsis autumnalis and M. manasluensis (Papaveraceae), two new species of Himalayan poppy endemic to central Nepal with sympatric congeners. Phytotaxa 2011, 20, 47–56. [Google Scholar] [CrossRef]
- Duffy, J.E.; Godwin, C.M.; Cardinale, B.J. Biodiversity effects in the wild are common and as strong as key drivers of productivity. Nature 2017, 549, 261–264. [Google Scholar] [CrossRef]
- Li, X.X.; Tan, W.; Sun, J.Q.; Du, J.H.; Zheng, C.G.; Tian, X.X.; Zheng, M.; Xiang, B.B.; Wang, Y. Comparison of four complete chloroplast genomes of medicinal and ornamental Meconopsis species: Genome organization and species discrimination. Sci. Rep. 2019, 9, 112. [Google Scholar] [CrossRef]
- Guo, Q.; Bai, R.; Zhao, B.; Feng, X.; Zhao, Y.; Tu, P.; Chai, X. An Ethnopharmacological, Phytochemical and Pharmacological Review of the Genus Meconopsis. Am. J. Chin. Med. 2016, 44, 439–462. [Google Scholar] [CrossRef]
- Shi, N.; Naudiyal, N.; Wang, J.; Gaire, N.P.; Wu, Y.; Wei, Y.; He, J.; Wang, C. Assessing the Impact of Climate Change on Potential Distribution of Meconopsis punicea and Its Influence on Ecosystem Services Supply in the Southeastern Margin of Qinghai-Tibet Plateau. Front. Plant Sci. 2022, 12, 3338. [Google Scholar] [CrossRef]
- Xiao, W.; Simpson, B.B. The Role of Allotriploidy in the Evolution of Meconopsis (Papaveraceae): A Preliminary Study of Ancient Polyploid and Hybrid Speciation. Lundellia 2014, 17, 5–17. [Google Scholar] [CrossRef]
- Forester, B.R.; DeChaine, E.G.; Bunn, A.G. Integrating ensemble species distribution modelling and statistical phylogeography to inform projections of climate change impacts on species distributions. Divers. Distrib. 2013, 19, 1480–1495. [Google Scholar] [CrossRef]
- Booth, T.H.; Nix, H.A.; Busby, J.R.; Hutchinson, M.F.; Franklin, J. Bioclim: The first species distribution modelling package, its early applications and relevance to most current MaxEnt studies. Divers. Distrib. 2014, 20, 1–9. [Google Scholar] [CrossRef]
- Tang, Y.; Winkler, J.A.; Vina, A.; Liu, J.; Zhang, Y.; Zhang, X.; Li, X.; Wang, F.; Zhang, J.; Zhao, Z. Uncertainty of future projections of species distributions in mountainous regions. PLoS ONE 2018, 13, e0189496. [Google Scholar] [CrossRef]
- Liao, Z.; Nobis, M.P.; Xiong, Q.; Tian, X.; Wu, X.; Pan, K.; Zhang, A.; Wang, Y.; Zhang, L. Potential distributions of seven sympatric sclerophyllous oak species in Southwest China depend on climatic, non-climatic, and independent spatial drivers. Ann. For. Sci. 2021, 78, 5. [Google Scholar] [CrossRef]
- Liu, X.-T.; Yuan, Q.; Ni, J. Research advances in modelling plant species distribution in China. Chin. J. Plant Ecol. 2019, 43, 273–283. [Google Scholar] [CrossRef]
- Naudiyal, N.; Wang, J.; Ning, W.; Gaire, N.P.; Peili, S.; Wei, Y.; He, J.; Ning, S. Potential distribution of Abies, Picea, and Juniperus species in the sub-alpine forest of Minjiang headwater region under current and future climate scenarios and its implications on ecosystem services supply. Ecol. Indic. 2021, 121, 107131. [Google Scholar] [CrossRef]
- Du, Z.; He, Y.; Wang, H.; Wang, C.; Duan, Y. Potential geographical distribution and habitat shift of the genus Ammopiptanthus in China under current and future climate change based on the MaxEnt model. J. Arid Environ. 2020, 184, 104328. [Google Scholar] [CrossRef]
- Wu, X.; Dong, S.; Liu, S.; Liu, Q.; Han, Y.; Zhang, X.; Su, X.; Zhao, H.; Feng, J. Identifying priority areas for grassland endangered plant species in the Sanjiangyuan Nature Reserve based on the MaxEnt model. Biodivers. Sci. 2018, 26, 138–148. [Google Scholar] [CrossRef]
- Li, J.; Fan, G.; He, Y. Predicting the current and future distribution of three Coptis herbs in China under climate change conditions, using the MaxEnt model and chemical analysis. Sci. Total Environ. 2019, 698, 134141. [Google Scholar] [CrossRef]
- Saunders, D.A.; Hobbs, R.J.; Margules, C.R. Biological Consequences of Ecosystem Fragmentation: A Review. Conserv. Biol. 1991, 5, 18–32. [Google Scholar] [CrossRef]
- McGarigal, K.; Marks, B.J. FragStats: Spatial Pattern Analysis Program for Quantifying Landscape Structure; PNW-GTR-351; U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station: Portland, OR, USA, 1995; p. 122. [Google Scholar]
- Zhang, Y.; Li, B.; Liu, L.; Zheng, D. Redetermine the region and boundaries of Tibetan Plateau. Geogr. Res. 2021, 40, 1543–1553. [Google Scholar]
- Ding, W.-N.; Ree, R.H.; Spicer, R.A.; Xing, Y.-W. Ancient orogenic and monsoon-driven assembly of the world’s richest temperate alpine flora. Science 2020, 369, 578–581. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Zhang, J.-Q.; Nie, Z.-L.; Zhong, Y.; Sun, H. Evolutionary diversifications of plants on the Qinghai-Tibetan Plateau. Front. Genet. 2014, 5, 4. [Google Scholar] [CrossRef]
- Merow, C.; Smith, M.J.; Silander, J.A., Jr. A practical guide to MaxEnt for modeling species’ distributions: What it does, and why inputs and settings matter. Ecography 2013, 36, 1058–1069. [Google Scholar] [CrossRef]
- Pearson, R.G.; Raxworthy, C.J.; Nakamura, M.; Peterson, A.T. Predicting species distributions from small numbers of occurrence records: A test case using cryptic geckos in Madagascar. J. Biogeogr. 2007, 34, 102–117. [Google Scholar] [CrossRef]
- Lambers, H.; Chapin, F.S.; Pons, T.L. Plant Physiology Ecology, 2nd ed.; Springer-Verlag: New York, NY, USA, 2008. [Google Scholar]
- Peng, J.; Gou, X.; Chen, F.; Li, J.; Liu, P.; Zhang, Y.; Fang, K. Difference in Tree Growth Responses to Climate at the Upper Treeline: Qilian Juniper in the Anyemaqen Mountains. J. Integr. Plant Biol. 2008, 50, 982–990. [Google Scholar] [CrossRef]
- Chmielewski, F.-M.; Rötzer, T. Response of tree phenology to climate change across Europe. Agric. For. Meteorol. 2001, 108, 101–112. [Google Scholar] [CrossRef]
- Molina-Montenegro, M.A.; Atala, C.; Gianoli, E. Phenotypic plasticity and performance of Taraxacum officinale (dandelion) in habitats of contrasting environmental heterogeneity. Biol. Invasions 2010, 12, 2277–2284. [Google Scholar] [CrossRef]
- Muhanguzi, H.D.R.; Obua, J.; Oryem-Origa, H.; Vetaas, O.R. Tree fruiting phenology in Kalinzu Forest, Uganda. Afr. J. Ecol. 2003, 41, 171–178. [Google Scholar] [CrossRef]
- Pol, R.G.; Pirk, G.I.; Marone, L. Grass seed production in the central Monte desert during successive wet and dry years. Plant Ecol. 2010, 208, 65–75. [Google Scholar] [CrossRef]
- Zhang, F.; Quan, Q.; Song, B.; Sun, J.; Chen, Y.; Zhou, Q.; Niu, S. Net primary productivity and its partitioning in response to precipitation gradient in an alpine meadow. Sci. Rep. 2017, 7, 15193. [Google Scholar] [CrossRef]
- Perring, M.P.; Hovenden, M.J. Seedling survivorship of temperate grassland perennials is remarkably resistant to projected changes in rainfall. Aust. J. Bot. 2012, 60, 328–339. [Google Scholar] [CrossRef]
- Koster, R.D.; Dirmeyer, P.A.; Guo, Z.; Bonan, G.; Chan, E.; Cox, P.; Gordon, C.T.; Kanae, S.; Kowalczyk, E.; Lawrence, D.; et al. Regions of Strong Coupling Between Soil Moisture and Precipitation. Science 2004, 305, 1138–1140. [Google Scholar] [CrossRef]
- Hopkins, F.; Gonzalez-Meler, M.A.; Flower, C.E.; Lynch, D.J.; Czimczik, C.; Tang, J.; Subke, J.-A. Ecosystem-level controls on root-rhizosphere respiration. New Phytol. 2013, 199, 339–351. [Google Scholar] [CrossRef]
- Brant, A.N.; Chen, H.Y. Patterns and Mechanisms of Nutrient Resorption in Plants. Crit. Rev. Plant Sci. 2015, 34, 471–486. [Google Scholar] [CrossRef]
- Yan, X.; Wang, S.; Duan, Y.; Han, J.; Huang, D.; Zhou, J. Current and future distribution of the deciduous shrub Hydrangea macrophylla in China estimated by MaxEnt. Ecol. Evol. 2021, 11, 16099–16112. [Google Scholar] [CrossRef]
- Xin, X.-F.; Nomura, K.; Aung, K.; Velásquez, A.C.; Yao, J.; Boutrot, F.; Chang, J.; Zipfel, C.; He, S.Y. Bacteria establish an aqueous living space in plants crucial for virulence. Nature 2016, 539, 524–529. [Google Scholar] [CrossRef]
- Zhang, K.; Sun, L.; Tao, J. Impact of Climate Change on the Distribution of Euscaphis japonica (Staphyleaceae) Trees. Forests 2020, 11, 525. [Google Scholar] [CrossRef]
- Li, Z.; Han, H.; You, H.; Cheng, X.; Wang, T. Effects of local characteristics and landscape patterns on plant richness: A multi-scale investigation of multiple dispersal traits. Ecol. Indic. 2020, 117, 106584. [Google Scholar] [CrossRef]
- Swenson, N.G.; Anglada-Cordero, P.; Barone, J.A. Deterministic tropical tree community turnover: Evidence from patterns of functional beta diversity along an elevational gradient. Proc. R. Soc. B Biol. Sci. 2011, 278, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, L.; Luo, T. Rainy season onset mainly drives the spatiotemporal variability of spring vegetation green-up across alpine dry ecosystems on the Tibetan Plateau. Sci. Rep. 2020, 10, 8797. [Google Scholar] [CrossRef]
- Ren, Z.S. The effects of climate on the growth of Meconopsis seedlings in Kunming. Acta Bot. Yunnanica 1993, 15, 110–112. [Google Scholar]
- Still, S.; Kitto, S.; Swasey, J.; Harbage, J. Influence of Temperature on Growth and Flowering of Four Meconopsis Genotypes. Acta Hortic. 2003, 620, 289–298. [Google Scholar] [CrossRef]
- Willig, M.R.; Kaufman, D.M.; Stevens, R.D. Latitudinal Gradients of Biodiversity: Pattern, Process, Scale, and Synthesis. Annu. Rev. Ecol. Evol. Syst. 2003, 34, 273–309. [Google Scholar] [CrossRef]
- Behera, M.D.; Roy, P.S.; Panda, R.M. Plant species richness pattern across India’s longest longitudinal extent. Curr. Sci. India 2016, 111, 1220–1225. [Google Scholar] [CrossRef]
- O’Brien, E.M. Climatic Gradients in Woody Plant Species Richness: Towards an Explanation Based on an Analysis of Southern Africa’s Woody Flora. J. Biogeogr. 1993, 20, 181–198. [Google Scholar] [CrossRef]
- Chou, C. Land–sea heating contrast in an idealized Asian summer monsoon. Clim. Dyn. 2003, 21, 11–25. [Google Scholar] [CrossRef]
- Carpenter, C. The environmental control of plant species density on a Himalayan elevation gradient. J. Biogeogr. 2005, 32, 999–1018. [Google Scholar] [CrossRef]
- Zhang, Y.-Z.; Qian, L.-S.; Chen, X.-F.; Sun, L.; Sun, H.; Chen, J.-G. Diversity patterns of cushion plants on the Qinghai-Tibet Plateau: A basic study for future conservation efforts on alpine ecosystems. Plant Divers. 2021, 44, 231–242. [Google Scholar] [CrossRef]
- Shrestha, N.; Su, X.; Xu, X.; Wang, Z. The drivers of high Rhododendron diversity in south-west China: Does seasonality matter? J. Biogeogr. 2018, 45, 438–447. [Google Scholar] [CrossRef]
- Dong, S.K. Views on distinguishing the concepts of rangeland and grassland and proposing proper use of their terminology. Chinese J. Ecol. 2022, 41, 992–1000. [Google Scholar] [CrossRef]
- Nagamatsu, D.; Hirabuki, Y.; Mochida, Y. Influence of micro-landforms on forest structure, tree death and recruitment in a Japanese temperate mixed forest. Ecol. Res. 2003, 18, 533–547. [Google Scholar] [CrossRef]
- Palmer, M.W.; Dixon, P.M. Small-scale environmental heterogeneity and the analysis of species distributions along gradients. J. Veg. Sci. 1990, 1, 57–65. [Google Scholar] [CrossRef]
- Guo, Q.; Kelt, D.A.; Sun, Z.; Liu, H.; Hu, L.-J.; Ren, H.; Wen, J. Global variation in elevational diversity patterns. Sci. Rep. 2013, 3, 03007. [Google Scholar] [CrossRef]
- Manish, K.; Pandit, M.K.; Telwala, Y.; Nautiyal, D.C.; Koh, L.P.; Tiwari, S. Elevational plant species richness patterns and their drivers across non-endemics, endemics and growth forms in the Eastern Himalaya. J. Plant Res. 2017, 130, 829–844. [Google Scholar] [CrossRef]
- Pandey, B.; Nepal, N.; Tripathi, S.; Pan, K.; Dakhil, M.A.; Timilsina, A.; Justine, M.F.; Koirala, S.; Nepali, K.B. Distribution Pattern of Gymnosperms’ Richness in Nepal: Effect of Environmental Constrains along Elevational Gradients. Plants 2020, 9, 625. [Google Scholar] [CrossRef]
- Zhang, W.; Huang, D.; Wang, R.; Liu, J.; Du, N. Altitudinal Patterns of Species Diversity and Phylogenetic Diversity across Temperate Mountain Forests of Northern China. PLoS ONE 2016, 11, e0159995. [Google Scholar] [CrossRef]
- Miehe, G. Vegetation patterns on Mount Everest as influenced by monsoon and föhn. Vegetatio 1988, 79, 21–32. [Google Scholar] [CrossRef]
- Gao, J.; Liu, Y. Climate stability is more important than water–energy variables in shaping the elevational variation in species richness. Ecol. Evol. 2018, 8, 6872–6879. [Google Scholar] [CrossRef]
- Rai, H.; Upreti, D.K.; Gupta, R.K.; Rai, H. Diversity and distribution of terricolous lichens as indicator of habitat heterogeneity and grazing induced trampling in a temperate-alpine shrub and meadow. Biodivers. Conserv. 2012, 21, 97–113. [Google Scholar] [CrossRef]
- González-Tagle, M.A.; Schwendenmann, L.; Pérez, J.J.; Schulz, R. Forest structure and woody plant species composition along a fire chronosequence in mixed pine–oak forest in the Sierra Madre Oriental, Northeast Mexico. For. Ecol. Manag. 2008, 256, 161–167. [Google Scholar] [CrossRef]
- Zhang, Q.-P.; Wang, J.; Wang, Q. Effects of abiotic factors on plant diversity and species distribution of alpine meadow plants. Ecol. Inform. 2021, 61, 101210. [Google Scholar] [CrossRef]
- Burt, T.P.; Butcher, D.P. Topographic controls of soil moisture distributions. J. Soil Sci. 1985, 36, 469–486. [Google Scholar] [CrossRef]
- Dauber, J.; Hirsch, M.; Simmering, D.; Waldhardt, R.; Otte, A.; Wolters, V. Landscape structure as an indicator of biodiversity: Matrix effects on species richness. Agric. Ecosyst. Environ. 2003, 98, 321–329. [Google Scholar] [CrossRef]
- Marden, M.; Rowan, D.; Phillips, C. Stabilising Characteristics of New Zealand Indigenous Riparian Colonising Plants. Plant Soil 2005, 278, 95–105. [Google Scholar] [CrossRef]
- Gaston, K.J. Global patterns in biodiversity. Nature 2000, 405, 220–227. [Google Scholar] [CrossRef]
- Tsianou, M.A.; Koutsias, N.; Mazaris, A.D.; Kallimanis, A.S. Climate and landscape explain richness patterns depending on the type of species’ distribution data. Acta Oecologica 2016, 74, 19–27. [Google Scholar] [CrossRef]
- Katayama, N.; Amano, T.; Naoe, S.; Yamakita, T.; Komatsu, I.; Takagawa, S.-I.; Sato, N.; Ueta, M.; Miyashita, T. Landscape Heterogeneity–Biodiversity Relationship: Effect of Range Size. PLoS ONE 2014, 9, e93359. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Luo, J.; Qian, L.; Deng, T.; Sun, H. The relationship between elevation and seed-plant species richness in the Mt. Namjagbarwa region (Eastern Himalayas) and its underlying determinants. Glob. Ecol. Conserv. 2020, 23, e01053. [Google Scholar] [CrossRef]
- Li, J.J. The Seed Plants Differentiation and It’s Response to the Uplifts in Himalayans. Ph.D. Thesis, Chengdu University of Technology, Chengdu, China, 2014. [Google Scholar]
- Boral, D.; Moktan, S. Predictive distribution modeling of Swertia bimaculata in Darjeeling-Sikkim Eastern Himalaya using MaxEnt: Current and future scenarios. Ecol. Process. 2021, 10, 26. [Google Scholar] [CrossRef]



| Type | Variable Name | Code | Data Source | Unit | Resolution |
|---|---|---|---|---|---|
| Bio-Climatic | Annual Mean Temperature | Bio1 | WorldClim | °C | 30″ |
| Mean Diurnal Range | Bio2 | WorldClim | °C | 30″ | |
| Isothermality (Bio2/Bio7) (×100) | Bio3 | WorldClim | °C | 30″ | |
| Temperature Seasonality (Standard Deviation × 100) | Bio4 | WorldClim | °C | 30″ | |
| Precipitation of Driest Month | Bio14 | WorldClim | mm | 30″ | |
| Precipitation Seasonality (Coefficient of Variation) | Bio15 | WorldClim | 1 | 30″ | |
| Precipitation of Warmest Quarter | Bio18 | WorldClim | mm | 30″ | |
| Precipitation of Coldest Quarter | Bio19 | WorldClim | mm | 30″ | |
| Topographic | Elevation | Elevation | WorldClim | m | 30″ |
| Slope | Slope | DEM | ° | 30″ | |
| Aspect | Aspect | DEM | ° | 30″ |
| Landscape Indices | Range of Value | Ecological Significance |
|---|---|---|
| Patch density index (PD) | PD > 0 | It can reflect the degree of fragmentation of the landscape. The higher the value, the higher the degree of fragmentation. |
| Patch richness index (PR) | PR >= 1 | It indicates the total number of all patch types in the landscape and is one of the key indicators of landscape components, as well as spatial heterogeneity, and has implications for many ecological processes. |
| Contagion index (CONTAG) | 0 < CONTAG <= 100 | It describes the degree of clustering or extension trend of different patch types in the landscape. Since this indicator contains spatial information, it is one of the most important indices to describe the landscape pattern. Generally, high spreading values indicate that some dominant patch types in the landscape form a good connectivity; conversely, it indicates that the landscape is a dense pattern with multiple elements and a high degree of fragmentation in the landscape. |
| Shannon’s diversity index (SHDI) | SHDI >= 0 | An increase in SHDI indicates an increase in patch types or an equalizing trend in the distribution of each patch type in the landscape. |
| Variable | Code | a | b | c | d | e |
|---|---|---|---|---|---|---|
| Precipitation of Warmest Quarter | Bio18 | 43.8 | 59.2 | 36 | 37.7 | 43.6 |
| Temperature Seasonality | Bio4 | 20 | 2.7 | 11.5 | 24.4 | 8.6 |
| Elevation | Elevation | 13.5 | 1.8 | 11.8 | 4.4 | 8.5 |
| Annual Mean Temperature | Bio1 | 7.8 | 18.9 | 10 | 16.7 | 7.5 |
| Precipitation Seasonality | Bio15 | 5.3 | 8.3 | 4.9 | 1.4 | 15.8 |
| Isothermality | Bio3 | 3.9 | 0.2 | 10.2 | 4.3 | 1.4 |
| Slope | Slope | 2.6 | 2.3 | 5.4 | 2.4 | 2.3 |
| Mean Diurnal Range | Bio2 | 1.5 | 0.6 | 3.7 | 1.6 | 3.2 |
| Precipitation of Driest Month | Bio14 | 1.1 | 0.4 | 4 | 0.3 | 4.9 |
| Precipitation of Coldest Quarter | Bio19 | 0.3 | 5.5 | 1.6 | 4.4 | 2.6 |
| Aspect | Aspect | 0.1 | 0.3 | 1 | 2.4 | 1.6 |
| Probability of Occurrence | Meconopsis | M. integrifolia | M. horridula | M. racemosa | M. punicea |
|---|---|---|---|---|---|
| Unsuitable (<25%) | 3049.07 | 3361.65 | 3038.57 | 3761.98 | 4059.79 |
| Low suitable (25–50%) | 944.89 | 672.12 | 920.74 | 453.50 | 231.63 |
| Medium suitable (50–75%) | 462.92 | 424.07 | 486.94 | 226.99 | 152.11 |
| High suitable (>75%) | 10.11 | 9.14 | 20.72 | 24.51 | 23.45 |
| Species | Low Suitable (25–50%) | Medium Suitable (50–75%) | High Suitable (>75%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Min | Max | Mean ± SD | Min | Max | Mean ± SD | Min | Max | |
| a | 4111 ± 791 | 2014 | 5983 | 3586± 737 | 2049 | 4988 | 4194 ± 142 | 4043 | 4424 |
| b | 4139 ± 760 | 2033 | 5983 | 3628 ± 713 | 2049 | 4925 | 3620 ± 367 | 3279 | 4122 |
| c | 4265 ± 807 | 2049 | 5953 | 4027 ± 643 | 2126 | 5406 | 4118 ± 540 | 2599 | 4617 |
| d | 3877 ± 766 | 2049 | 5142 | 3718 ± 647 | 2058 | 4707 | 3697 ± 391 | 2928 | 4259 |
| e | 4084 ± 565 | 2180 | 4958 | 3817 ± 454 | 2326 | 4646 | 3234 ± 484 | 2237 | 3677 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, N.; Wang, C.; Wang, J.; Wu, N.; Naudiyal, N.; Zhang, L.; Wang, L.; Sun, J.; Du, W.; Wei, Y.; et al. Biogeographic Patterns and Richness of the Meconopsis Species and Their Influence Factors across the Pan-Himalaya and Adjacent Regions. Diversity 2022, 14, 661. https://doi.org/10.3390/d14080661
Shi N, Wang C, Wang J, Wu N, Naudiyal N, Zhang L, Wang L, Sun J, Du W, Wei Y, et al. Biogeographic Patterns and Richness of the Meconopsis Species and Their Influence Factors across the Pan-Himalaya and Adjacent Regions. Diversity. 2022; 14(8):661. https://doi.org/10.3390/d14080661
Chicago/Turabian StyleShi, Ning, Chunya Wang, Jinniu Wang, Ning Wu, Niyati Naudiyal, Lin Zhang, Lihua Wang, Jian Sun, Wentao Du, Yanqiang Wei, and et al. 2022. "Biogeographic Patterns and Richness of the Meconopsis Species and Their Influence Factors across the Pan-Himalaya and Adjacent Regions" Diversity 14, no. 8: 661. https://doi.org/10.3390/d14080661
APA StyleShi, N., Wang, C., Wang, J., Wu, N., Naudiyal, N., Zhang, L., Wang, L., Sun, J., Du, W., Wei, Y., Chen, W., & Wu, Y. (2022). Biogeographic Patterns and Richness of the Meconopsis Species and Their Influence Factors across the Pan-Himalaya and Adjacent Regions. Diversity, 14(8), 661. https://doi.org/10.3390/d14080661









