Seasonal Occurrence and Relative Abundance of Marine Fish Larval Families over Healthy and Degraded Seagrass Beds in Coastal Kenya
Abstract
:1. Introduction
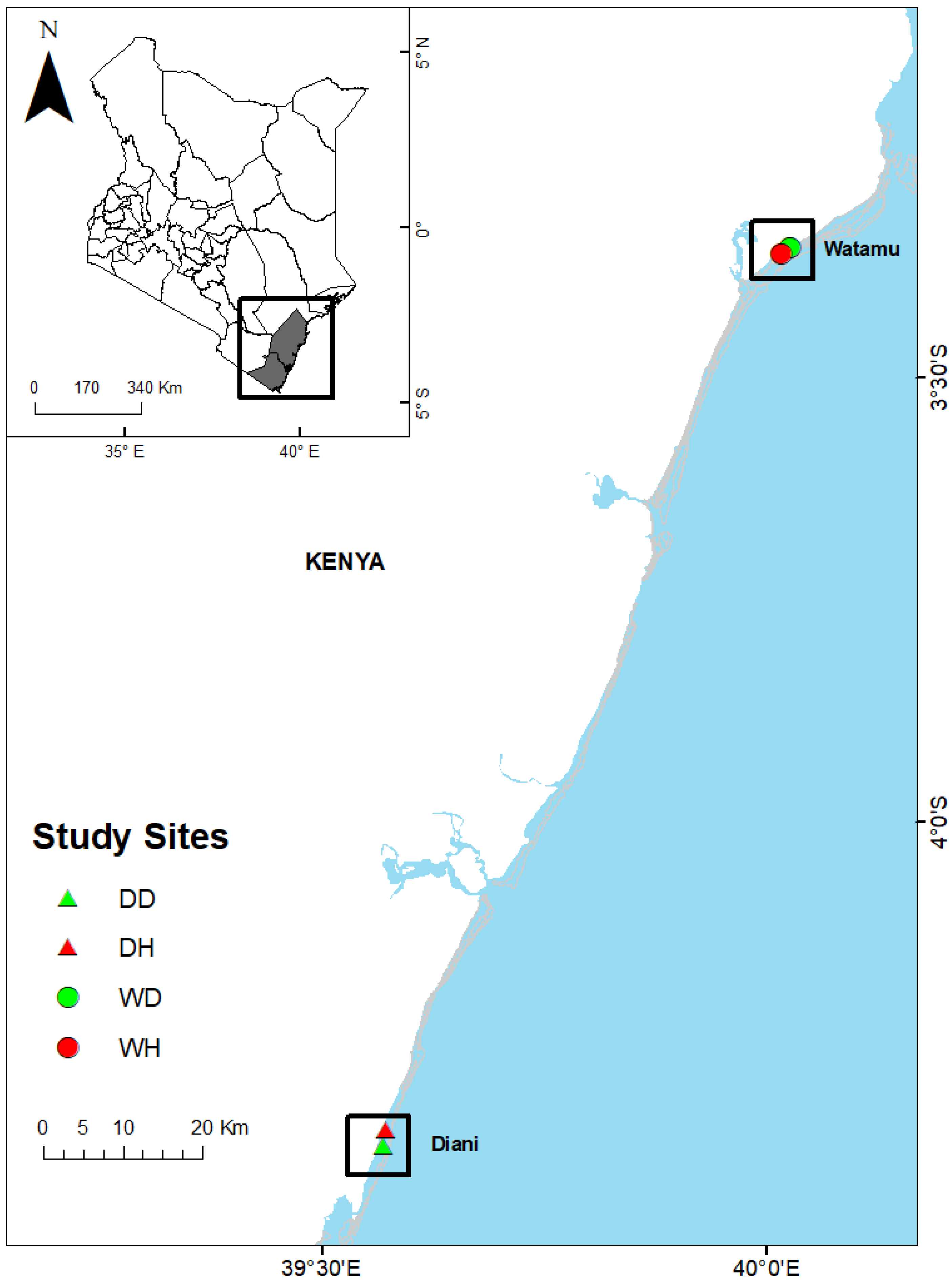
2. Materials and Methods
2.1. Environmental Variables
2.2. Plankton Sampling
2.3. Data Analysis
3. Results
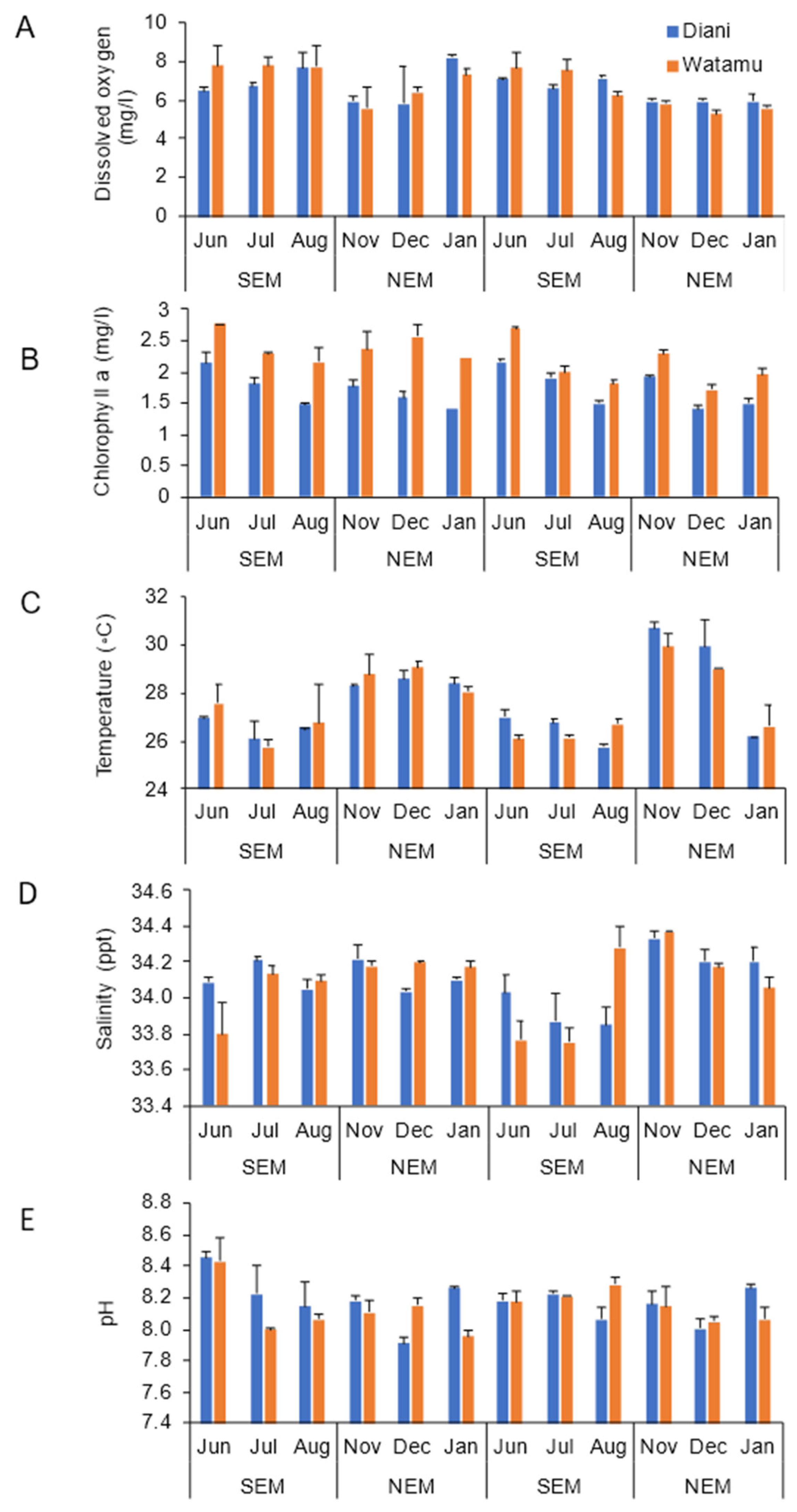
3.1. Environmental Variables
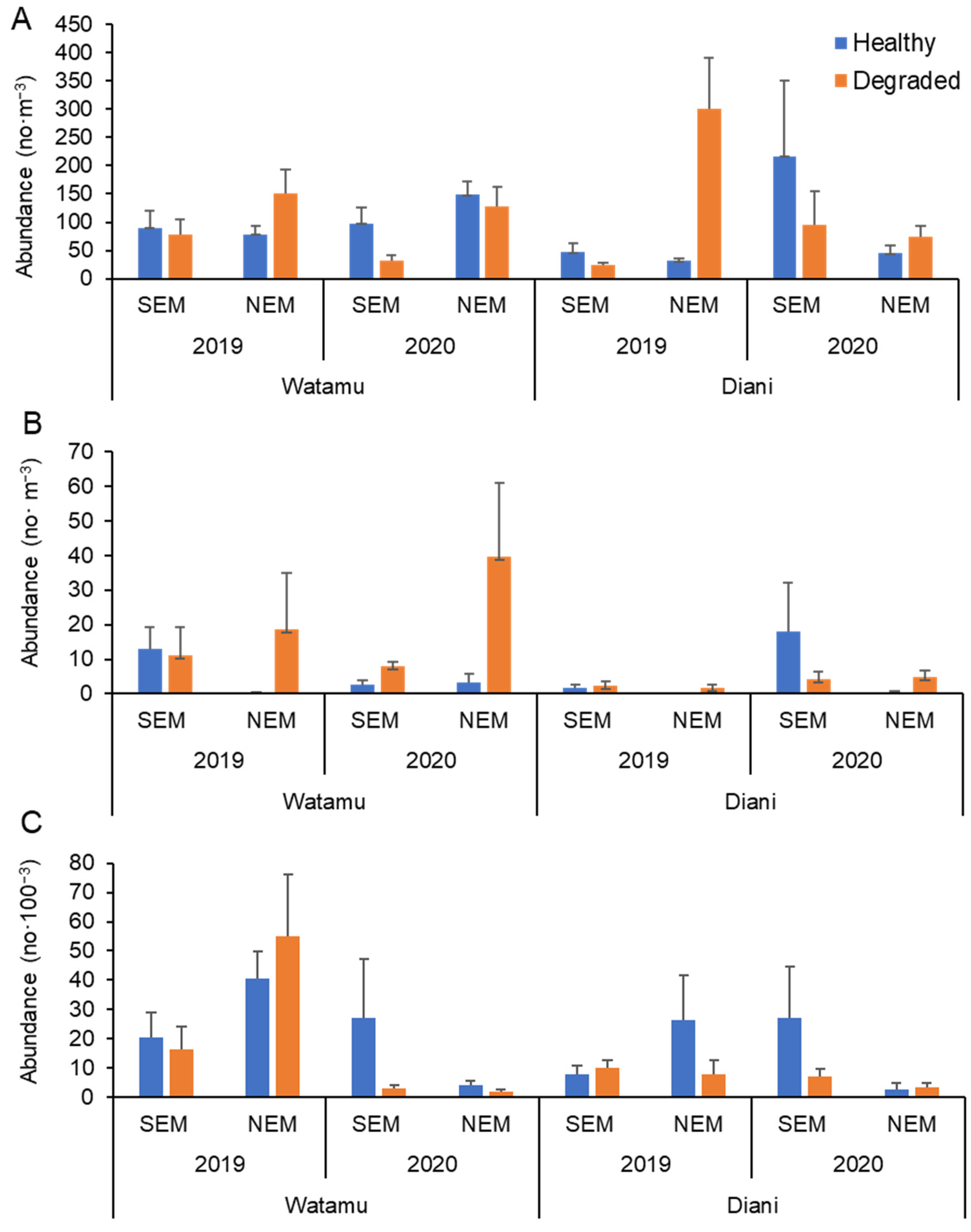
3.2. Zooplankton, Fish Eggs and Larval Fish Abundance
3.3. Fish Larvae
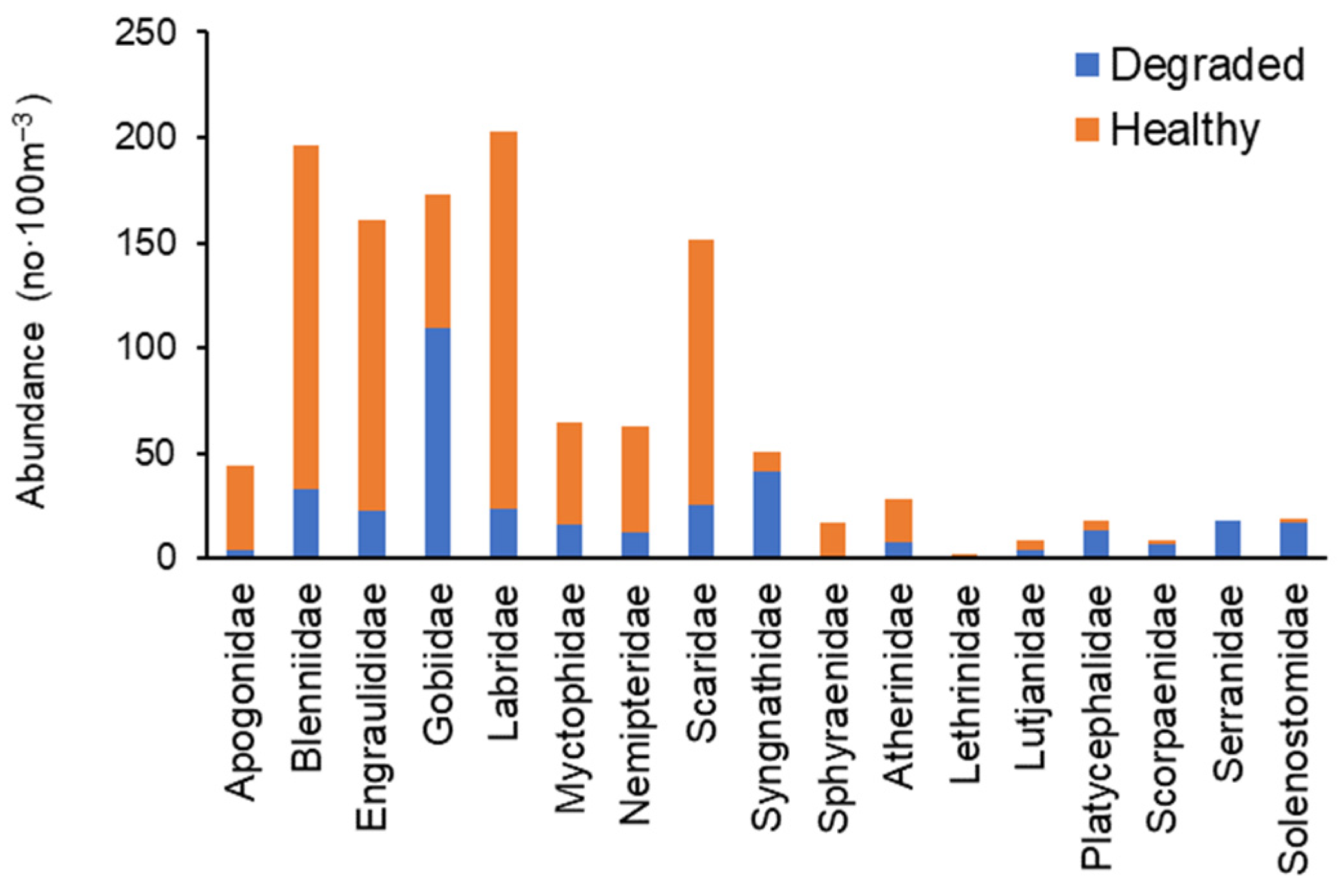
3.3.1. Species Composition and Abundance
3.3.2. Distribution of Larval Fish Families
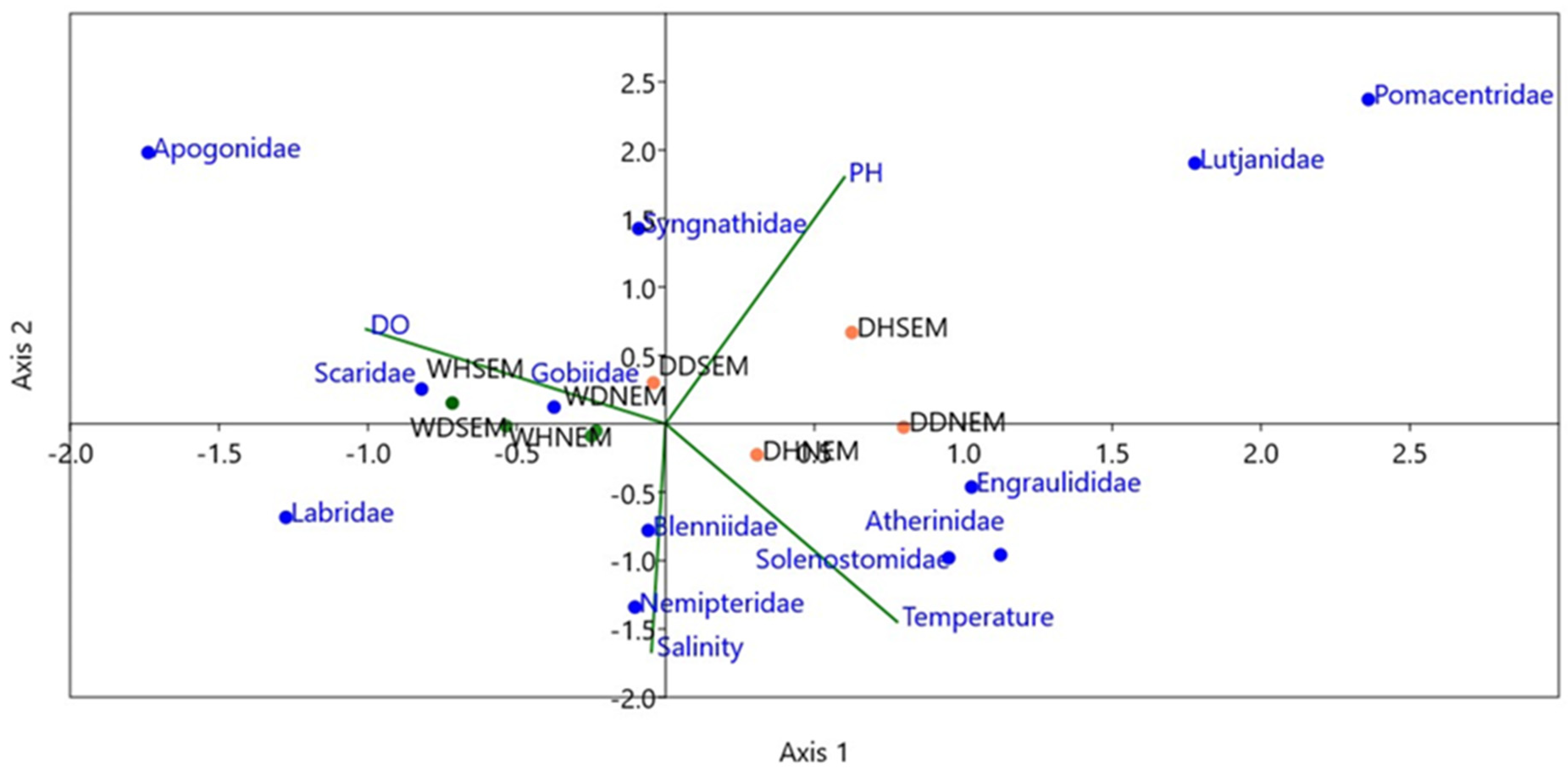
3.4. Influence of Biophysical Variables and Seasonality
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- MacUra, B.; Lönnstedt, O.M.; Byström, P.; Airoldi, L.; Eriksson, B.K.; Rudstam, L.; Støttrup, J. What is the impact on fish recruitment of anthropogenic physical and structural habitat change in shallow nearshore areas in temperate systems? A systematic review protocol. Environ. Evid. 2016, 5, 1–8. [Google Scholar] [CrossRef]
- Okemwa, G.M.; Kaunda-Arara, B.; Kimani, E.N. Patterns of juvenile reef-fish recruitment in Kenya’s shallow fringing- lagoon reefs. Afr. J. Mar. Sci. 2019, 41, 291–304. [Google Scholar] [CrossRef]
- Madi Moussa, R.; Bertucci, F.; Jorissen, H.; Gache, C.; Waqalevu, V.P.; Parravicini, V.; Lecchini, D.; Galzin, R. Importance of intertidal seagrass beds as nursery area for coral reef fish juveniles (Mayotte, Indian Ocean): Nursery areas in a tropical island. Reg. Stud. Mar. Sci. 2020, 33, 100965. [Google Scholar] [CrossRef]
- Tarimo, B.; Winder, M.; Mtolera, M.S.; Muhando, C.A.; Gullström, M. Seasonal distribution of fish larvae in mangrove-seagrass seascapes of Zanzibar (Tanzania). Sci. Rep. 2022, 12, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Gullström, M.; de la Torre Castro, M.; Bandeira, S.O.; Björk, M.; Dahlberg, M.; Kautsky, N.; Rönnbäck, P.; Öhman, M.C. Seagrass ecosystems in the western Indian Ocean. AMBIO A J. Hum. Environ. 2002, 31, 588–596. [Google Scholar] [CrossRef]
- McCloskey, R.M.; Unsworth, R.K.F. Decreasing seagrass density negatively influences associated fauna. PeerJ 2015, 2015, 1053. [Google Scholar] [CrossRef]
- Unsworth, R.K.; McKenzie, L.J.; Collier, C.J.; Cullen-Unsworth, L.C.; Duarte, C.M.; Eklöf, J.S.; Jarvis, J.C.; Jones, B.L.; Nordlund, L.M. Global challenges for seagrass conservation. Ambio 2019, 48, 801–815. [Google Scholar] [CrossRef]
- Yoshikawa, K.; Tomiyama, T.; Shoji, J. Temporal changes of the fish community in a seagrass bed after disappearance of vegetation caused by disturbance of the sea bottom and sediment deposition. J. Mar. Biol. Assoc. U. K. 2019, 99, 1857–1864. [Google Scholar] [CrossRef]
- Carter, A.B.; McKenna, S.A.; Rasheed, M.A.; Collier, C.; McKenzie, L.; Pitcher, R.; Coles, R. Synthesizing 35 years of seagrass spatial data from the Great Barrier Reef World Heritage Area, Queensland, Australia. Limnol. Oceanogr. Lett. 2021, 6, 216–226. [Google Scholar] [CrossRef]
- Beaugrand, G.; Brander, K.M.; Alistair Lindley, J.; Souissi, S.; Reid, P.C. Plankton effect on cod recruitment in the North Sea. Nature 2003, 426, 661–664. [Google Scholar] [CrossRef]
- Kaunda-Arara, B.; Mwaluma, J.M.; Locham, G.A.; Øresland, V.; Osore, M.K. Temporal variability in fish larval supply to Malindi Marine Park, coastal Kenya. Aquat. Conserv. Mar. Freshw. Ecosyst. 2009, 19, S10–S18. [Google Scholar] [CrossRef]
- Mwaluma, M.J. Community Structure and Spatio-Temporal Variability of Ichthyoplankton in Kenyan Coastal Waters; University of Nairobi: Nairobi, Kenya, 2010; pp. 1–137. [Google Scholar]
- Mwaluma, J.M.; Kaunda-Arara, B.; Rasowo, J.; Osore, M.K.; Øresland, V. Seasonality in fish larval assemblage structure within marine reef National Parks in coastal Kenya. Environ. Biol. Fishes 2011, 90, 393–404. [Google Scholar] [CrossRef]
- Mwaluma, J.M.; Kaunda-Arara, B.; Rasowo, J. Diel and lunar variations in larval supply to Malindi Marine Park, Kenya. West. Indian Ocean J. Mar. Sci. 2014, 13, 57–67. [Google Scholar]
- Crochelet, E.; Roberts, J.; Lagabrielle, E.; Obura, D.; Petit, M.; Chabanet, P. A model-based assessment of reef larvae dispersal in the Western Indian Ocean reveals regional connectivity patterns—Potential implications for conservation policies. Reg. Stud. Mar. Sci. 2016, 7, 159–167. [Google Scholar] [CrossRef]
- Zanre, R.; Kithi, E. Preliminary Sea Urchin Study and Kill Report; Local Ocean Trust Watamu Turtle Watch: Watamu, Kenya, 2004. [Google Scholar]
- Uku, J.; Ndirangu, S.; Muthama, C.; Kimathi, A. An Evaluation of the Effect of Sea Urchin Herbivory in the Diani-Chale Lagoon (No. 1); Preliminary Report; KMFRI: Mombasa, Kenya, 2005. [Google Scholar]
- Daudi, L.N. The Role of Food Availability and Presence of Predators on Population Trends of the Sea Urchin Tripneustes gratilla (L.) in Seagrass Beds of Watamu Marine National Park and Reserve, Kenya; WIOMSA/MARG;-I/2010-06; Western Indian Ocean Marine Science Association: Zanzibar, Tanzania, 2010. [Google Scholar]
- UNEP-Nairobi Convention/WIOMSA. Guidelines for Seagrass Ecosystem Restoration in the Western Indian Ocean Region; UNEP: Nairobi, Kenya, 2020; p. 63. Available online: www.nairobiconvention.org/ (accessed on 22 August 2022).
- Mwaluma, J.; Kaunda-Arara, B.; Strydom, N. A Guide to Commonly Occurring Larval Stages of Fishes in Kenyan Coastal Waters; Western Indian Ocean Marine Science Association: Zanzibar, Tanzania, 2014. [Google Scholar]
- Leis, J.M.; Rennis, D.S. The Larvae of Indo-Pacific Coral Reef Fishes; New South Wales University Press: Sydney, Australia, 1983; 269p. [Google Scholar]
- Leis, J.M.; Trnski, T. The Larvae of Indo-Pacific Shore Fishes; University of Hawaii Press: Honolulu, HI, USA, 1989; 371p. [Google Scholar]
- Leis, J.M.; Carson-Ewart, B.M. (Eds.) The Larvae of Indo-Pacific Coastal Fishes: An Identification Guide to Marine Fish Larvae; Brill: Amsterdam, The Netherlands, 2000; Volume 2. [Google Scholar]
- Giesbrecht, W. Systematik and faunistik der pelagischen copepoden des Golfes Von Naepal und der angrenzenden meeresabschinitte. Fauna Flora Golf. Naepal 1892, 19, 1–830. [Google Scholar]
- Sars, G.O. An Account of Crustacea of Norway. Copepoda Calanoida; Bergen Museum: Bergen, Norway, 1901; Volume 4, pp. 1–28. [Google Scholar]
- Scott, A. The Copepoda of the Siboga Expedition. Siboga Expedite Monogr. 1909, 29A, 1–28. [Google Scholar]
- Sewell, R.B.S. Notes on the surface-living Copepoda of the Bay of Bengal. Rec. Ind. Mus. 1929, 8, 313–382. [Google Scholar]
- Sewell, R.B.S. The copepod fauna of the Indian seas. Calanoida. Mem. Ind. Mus. 1932, 10, 223–407. [Google Scholar]
- Sewell, R.B.S. The free-swimming planktonic Copepoda. Systematic account. Sci. Rep. John Murray Exped. 1947, 8, 1–303. [Google Scholar]
- Sewell, R.B.S. The free-swimming planktonic Copepoda. Systematic account. Sci. Rep. John Murray Exped. 1948, 3, 317–592. [Google Scholar]
- Wickstead, J.H. An Introduction to the Study of Tropical Plankton; Hutchinson, Co.: London, UK, 1965; p. 160. [Google Scholar]
- Wickstead, J.H. Marine Zooplankton; The Camelot Press Ltd.: Southampton, UK, 1976; p. 59. [Google Scholar]
- Owre, H.B.; Foyo, M. Copepods of the Florida current Fauna. Caribbea 1967, 1, 1–37. [Google Scholar]
- Mwaluma, J.M. Distribution and Abundance of Zooplankton off the Kenya Coast during the Monsoons. Master’s Thesis, University of Nairobi, Nairobi, Kenya, 1997; p. 90. [Google Scholar]
- Osore, M.K.W.; Mwaluma, J.M.; Fiers, F.; Daro, M.H. Zooplankton composition and abundance in Mida Creek, Kenya. Zool. Stud. 2004, 43, 415–424. [Google Scholar]
- Aboud, S.A.; Kannah, J.F. Abundance, Distribution and Diversity of Seagrass Species in Lagoonal Reefs on the Kenyan Coast. Am. Acad. Sci. Res. J. Eng. Technol. Sci. 2017, 37, 52–67. Available online: https://www.asrjetsjournal.org/index.php/American_Scientific_Journal/article/view/3484 (accessed on 18 August 2022).
- Cowburn, B.; Musembi, P.M.; Sindorf, V.; Kohlmeier, D.; Raker, C.; Nussbaumer, A.; Hereward, H.F.R.; Van Baelenberghe, B.; Goebbels, D.; Kamire, J.; et al. The Habitats and Biodiversity of Watamu Marine National Park: Evaluating Our Knowledge of One of East Africa’s Oldest Marine Protected Areas; Atoll Research Bulletin; Smithsonian Institution Scholarly Press: Washington, DC, USA, 2018; p. 618. [Google Scholar]
- Nzioka, R.M. Observations on the spawning seasons of East African reef fishes. J. Fish Biol. 1979, 14, 329–342. [Google Scholar] [CrossRef]
- Sanvicente-Anorve, L.; Soto, L.A.; Espinosa-Fuentes, M.A.; Flores-Coto, C. Relationship patterns between ichthyoplankton and zooplankton: A conceptual model. Hydrobiologia 2006, 559, 11–22. [Google Scholar] [CrossRef]
- Mwaluma, J.; Ngisiang’e, N.; Osore, M.; Kamau, J.; Ong’anda, H.; Kilonzi, J.; Roberts, M.; Popova, E.; Painter, S.C. Assemblage structure and distribution of fish larvae on the North Kenya Banks during the Southeast Monsoon season. Ocean Coast. Manag. 2021, 212, 105800. [Google Scholar] [CrossRef]
- Munday, P.L.; Donelson, J.M.; Dixson, D.L.; Endo, G.G. Effects of ocean acidification on the early life history of a tropical marine fish. Proc. R. Soc. B Biol. Sci. 2009, 276, 3275–3283. [Google Scholar] [CrossRef]
- Igulu, M.M.; Nagelkerken, I.; van der Beek, M.; Schippers, M.; van Eck, R.; Mgaya, Y.D. Orientation from open water to settlement habitats by coral reef fish: Behavioral flexibility in the use of multiple reliable cues. Mar. Ecol. Prog. Ser. 2013, 493, 243–257. [Google Scholar] [CrossRef]
- Nonaka, M.; Fushimi, H.; Yamakawa, T. The spiny lobster fishery in Japan and restocking. In Spiny Lobsters Fisheries and Culture; Wiley: Hoboken, NJ, USA, 2000; pp. 221–242. [Google Scholar]
- Tzeng, W.N.; Wang, Y.T. Hydrography and distribution dynamics of larval and juvenile fishes in the coastal waters of the Tanshui River estuary, Taiwan, with reference to estuarine larval transport. Mar. Biol. 1993, 116, 205–217. [Google Scholar] [CrossRef]
- Harris, S.A.; Cyrus, D.P.; Beckley, L.E. The larval fish assemblage in nearshore coastal waters off the St Lucia Estuary, South Africa. Estuar. Coast. Shelf Sci. 1999, 49, 789–811. [Google Scholar] [CrossRef]
- Gross, C.; Donoghue, C.; Pruitt, C.; Trimble, A.C.; Ruesink, J.L. Taxonomic and functional assessment of meso-predator diversity across an estuarine habitat mosaic. Ecosphere 2017, 8, e01792. [Google Scholar] [CrossRef]
- Lefcheck, J.S.; Hughes, B.B.; Johnson, A.J.; Pfirrmann, B.W.; Rasher, D.B.; Smyth, A.R.; Williams, B.L.; Beck, M.W.; Orth, R.J. Are coastal habitats important nurseries? A meta-analysis. Conserv. Lett. 2019, 12, e12645. [Google Scholar] [CrossRef]
| Sites | Diani | Watamu | ||||
|---|---|---|---|---|---|---|
| Family | NEM | SEM | Overall (%) | NEM | SEM | Overall (%) |
| Acanthuridae | 1.0 | 0.0 | 0.1 | 2.0 | 0.0 | 0.3 |
| Atherinidae | 0.0 | 0.0 | 0.0 | 2.6 | 0.0 | 0.4 |
| Apogonidae | 19.2 | 2.5 | 3.1 | 16.2 | 5.8 | 3.3 |
| Atherinidae | 15.4 | 3.4 | 2.7 | 9.2 | 0.0 | 1.4 |
| Belonidae | 0.0 | 0.6 | 0.1 | 0.0 | 0.0 | 0.0 |
| Blenniidae | 124.8 | 4.2 | 18.5 | 55.4 | 12.1 | 10.2 |
| Bothidae | 16.2 | 0.0 | 2.3 | 0.0 | 0.0 | 0.0 |
| Callionymidae | 0.0 | 0.0 | 0.0 | 1.9 | 0.0 | 0.3 |
| Carangidae | 1.9 | 3.4 | 0.8 | 0.0 | 0.0 | 0.0 |
| Diodontidae | 8.1 | 0.0 | 1.2 | 0.9 | 0.0 | 0.1 |
| Eleotrididae | 1.0 | 0.0 | 0.1 | 0.0 | 0.0 | 0.0 |
| Engraulidae | 136.3 | 4.6 | 20.2 | 18.4 | 1.4 | 3.0 |
| Exocoetidae | 0.0 | 0.0 | 0.0 | 8.1 | 0.0 | 1.2 |
| Gerreidae | 0.0 | 1.3 | 0.2 | 1.5 | 1.1 | 0.4 |
| Gobiidae | 28.4 | 8.3 | 5.3 | 117.5 | 19.2 | 20.7 |
| Haemulidae | 0.0 | 1.1 | 0.2 | 0.0 | 0.0 | 0.0 |
| Hemiramphidae | 8.1 | 2.7 | 1.5 | 0.0 | 0.0 | 0.0 |
| Istiophoridae | 0.0 | 0.0 | 0.0 | 16.2 | 0.0 | 2.4 |
| Labridae | 141.6 | 0.5 | 20.4 | 51.2 | 9.2 | 9.1 |
| Leiognathidae | 1.1 | 0.0 | 0.2 | 1.1 | 0.0 | 0.2 |
| Lethrinidae | 0.7 | 0.5 | 0.2 | 1.1 | 0.0 | 0.2 |
| Lutjanidae | 4.4 | 1.9 | 0.9 | 2.8 | 0.0 | 0.4 |
| Monodactylidae | 0.0 | 0.0 | 0.0 | 8.1 | 5.0 | 2.0 |
| Mullidae | 2.5 | 0.0 | 0.4 | 0.0 | 0.0 | 0.0 |
| Myctophidae | 0.0 | 0.0 | 0.0 | 64.8 | 0.0 | 9.8 |
| Nemipteridae | 9.1 | 0.0 | 1.3 | 50.7 | 3.1 | 8.1 |
| Nomeidae | 0.0 | 0.0 | 0.0 | 8.1 | 0.5 | 1.3 |
| Ostraciidae | 1.6 | 0.0 | 0.2 | 0.0 | 7.8 | 1.2 |
| Platycephalidae | 1.8 | 3.9 | 0.8 | 8.1 | 4.2 | 1.9 |
| Pleuronectidae | 0.0 | 0.5 | 0.1 | 0.0 | 0.0 | 0.0 |
| Pomacentridae | 4.8 | 2.2 | 1.0 | 3.0 | 0.0 | 0.5 |
| Scaridae | 77.0 | 3.6 | 11.6 | 49.4 | 21.1 | 10.6 |
| Scombridae | 0.0 | 0.5 | 0.1 | 0.0 | 0.0 | 0.0 |
| Scorpaenidae | 0.0 | 0.5 | 0.1 | 1.1 | 7.5 | 1.3 |
| Serranidae | 0.0 | 0.9 | 0.1 | 16.2 | 1.0 | 2.6 |
| Sillaginidae | 0.0 | 0.5 | 0.1 | 0.0 | 0.0 | 0.0 |
| Solenostomidae | 7.4 | 0.8 | 1.2 | 2.9 | 8.0 | 1.6 |
| Sphyraenidae | 16.2 | 0.0 | 2.3 | 0.9 | 0.0 | 0.1 |
| Syngnathidae | 9.1 | 5.8 | 2.1 | 34.7 | 0.9 | 5.4 |
| Terapontidae | 1.3 | 0.0 | 0.2 | 0.0 | 0.0 | 0.0 |
| Tetraodontidae | 0.7 | 2.6 | 0.5 | 0.0 | 0.0 | 0.0 |
| Trichonotidae | 0.7 | 0.0 | 0.1 | 0.0 | 0.0 | 0.0 |
| Grand Total | 640.4 | 56.8 | 554.1 | 107.9 | ||
| n = 56 | Beta | S.E. | B | S.E. | T (51) | p-Level |
|---|---|---|---|---|---|---|
| Intercept | 0.2525 | 0.501 | 0.5031 | 0.617 | ||
| Chl-a | 0.571 | 0.0998 | 1.529 | 0.267 | 5.718 | * 0.000 |
| Phy abn | −0.131 | 0.0999 | −0.210 | 0.159 | −1.314 | 0.194 |
| Zoop abn | 0.601 | 0.1242 | 0.687 | 0.141 | 4.844 | * 0.000 |
| Fish eggs | −0.077 | 0.0122 | −0.063 | 0.101 | −0.632 | 0.529 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mwaluma, J.M.; Okemwa, G.M.; Mboga, A.M.; Ngisiange, N.; Winder, M.; Kyewalyanga, M.S.; Kilonzo, J.; Kinyua, I.M. Seasonal Occurrence and Relative Abundance of Marine Fish Larval Families over Healthy and Degraded Seagrass Beds in Coastal Kenya. Diversity 2022, 14, 730. https://doi.org/10.3390/d14090730
Mwaluma JM, Okemwa GM, Mboga AM, Ngisiange N, Winder M, Kyewalyanga MS, Kilonzo J, Kinyua IM. Seasonal Occurrence and Relative Abundance of Marine Fish Larval Families over Healthy and Degraded Seagrass Beds in Coastal Kenya. Diversity. 2022; 14(9):730. https://doi.org/10.3390/d14090730
Chicago/Turabian StyleMwaluma, James M., Gladys M. Okemwa, Alphine M. Mboga, Noah Ngisiange, Monika Winder, Margareth S. Kyewalyanga, Joseph Kilonzo, and Immaculate M. Kinyua. 2022. "Seasonal Occurrence and Relative Abundance of Marine Fish Larval Families over Healthy and Degraded Seagrass Beds in Coastal Kenya" Diversity 14, no. 9: 730. https://doi.org/10.3390/d14090730
APA StyleMwaluma, J. M., Okemwa, G. M., Mboga, A. M., Ngisiange, N., Winder, M., Kyewalyanga, M. S., Kilonzo, J., & Kinyua, I. M. (2022). Seasonal Occurrence and Relative Abundance of Marine Fish Larval Families over Healthy and Degraded Seagrass Beds in Coastal Kenya. Diversity, 14(9), 730. https://doi.org/10.3390/d14090730









