Abstract
Circadian rhythms of Neotropical lizards have been poorly studied, which represents a problem when designing ecological studies or monitoring such species based on direct observations. In this work, 1000 m-long transects were established in an agroecosystem area of the Ecuadorian coast region to study the activity patterns of Stenocercus iridescens. The number of individuals (as a response variable) was correlated with local weather conditions: temperature, precipitation, and heliophany (duration of the solar brightness). We carried out the study in both dry and rainy seasons, and during different time ranges. The time range in which the transects were performed was the most important predictor, and the activity peak was established between 16:00 h and 18:00 h. Heliophany negatively affected lizard activity, but only on the days with higher heliophany during the dry season, whereas temperature was not a significant predictor. Our results suggest that in an area where temperature is relatively constant and the solar radiation is high, particularly during the dry season, the heliophany (an indirect measure of solar radiation) can affect ectotherm activity patterns more than temperature, particularly in open habitats such as agroecosystems.
1. Introduction
Many reptile populations are declining worldwide due to habitat loss, climate change, invasive species, emerging diseases, or agriculture intensification [1,2,3]. In the Neotropical region, the ecology of reptiles is poorly known, and most parameters of the ecology of many species (including circadian rhythms) are unknown [4].
Western Ecuador is one of the most threatened and less studied regions of the Neotropical ecoregion, which includes tropical moist forests and tropical seasonal dry forests. It is part of the biodiversity hotspot called the Chocó–Darién–Western Ecuador Hotspot [5]. This region shows high endemism [6] and has high herpetofauna biodiversity [7]. This hotspot is one of the most endangered areas within the Neotropics because of habitat loss and fragmentation due to agricultural practices [8,9,10], and with the available data it is difficult to assess the impact of agricultural intensification on reptile populations because only a few studies have been conducted to determinate their abundance [11].
Sampling methodologies are frequently developed in temperate regions and later applied in Neotropical environments even though Neotropical lizards could respond differently to environmental conditions than Nearctic or Palearctic lizards, whose environments are where the sampling methodologies were developed. Since lizards are ectotherms, their detectability strongly depends on local environmental conditions, thus affecting their daily activity pattern. Therefore, sampling methodologies based on direct observation should be adapted to local conditions and consider specific behavioral patterns and environmental factors rather than generic ones.
Meteorological stations of the National Institute of Meteorology and Hydrology (INAMHI) have been recording global insolation (Wh/m2/day) and heliophany, or the duration of solar brightness that corresponds to direct solar radiation (h/day), since 1962. There is, therefore, a direct relationship between global insolation and heliophany [12]. Solar radiation is, in theory, an unlimited resource on Earth, but it can be locally limited. Ectotherm organisms use solar radiation to elevate their body temperatures [13]. Many works have shown how the environmental temperature affects reptile activity patterns, but other variables such as solar radiation have been less explored [13,14,15]. Reptiles can attain a higher body temperature with a higher solar radiation (measured as Wh/m2), and therefore, this variable should be also considered in studies on activity patterns. However, in a subtropical environment with a relative constant high temperature, a high insulation level could be a stressful factor.
Behavioral thermoregulation, which occurs mainly by location in the sun or in the shade, enables many ectotherm vertebrates to take advantage of thermal diverse environments and control temperature-sensitive physiological processes [15]. Ectotherm species compensate for changes in their body temperature through behavioral thermoregulation and/or physiological adaptations [16]. For some animals, changing posture or moving to a new location is an adequate method of thermoregulation. For reptiles, sunning or basking is a typical behavior by which the animal exposes its body to as much solar radiation as possible, leading to internal heat gain [13].
The study of species’ responses to varying environmental conditions has a long history in biological research [17], but the reptile’s ability to compensate has gained particular attention in the face of climate change [18,19]. For example, the upper thermal tolerance limits of many organisms increase (within individuals) as mean body temperatures rise, meaning that physiological adjustments can potentially compensate for the negative consequences of rising habitat temperatures [15]. We therefore expect that lizards synchronize their daily activity patterns to optimize their body temperature according to local conditions [20]. However, daily patterns are influenced not only by temperature but also by water balance, since temperature and hydration are critical factors to optimize metabolism [21,22]. Therefore, not only environmental temperature, but also water and other factors such as heliophany (average sunshine hours), can influence the activity patterns of reptiles.
The aim of this work is to study the activity patterns of Stenocercus iridescens in an agroecosystem of Western Ecuador. The climate of Ecuador is characterized by no marked annual changes in temperature, where the temperature does not reach high values, there are two well-defined seasons (rainy season and dry season), and high solar radiation is due to the perpendicular incidence of sunlight [12,23]. Under these climatic conditions, we hypothesize that temperature is not an important factor, and some factors such as time range or heliophany are more significant.
2. Materials and Methods
2.1. Study Area
The fieldwork was carried out from September 2015 to June 2016, near Calceta in the province of Manabí in the coastal region of Ecuador (0°49′10″ S, 80°10′40″ W; WGS-84 and 15 m above mean sea level), in the ecotone between seasonal dry forests and tropical rain forests of the Chocó-Darién-Western Ecuador Hotspot (Figure 1). The climate has an average annual temperature of 26 °C. During the dry season the temperature ranges between 21 °C and 32 °C, whereas in the rainy season it ranges from 21 °C to 30 °C. The wet season approximately extends from January to April, and the dry season from May to December. The month with the highest rainfall is February, with a monthly average precipitation of 196 mm, whereas in the dry season rainfall ranges from 6 mm to 13 mm. These conditions determinate a significant variation in relative humidity, which reaches 100% in March and 48% in September [14,23].
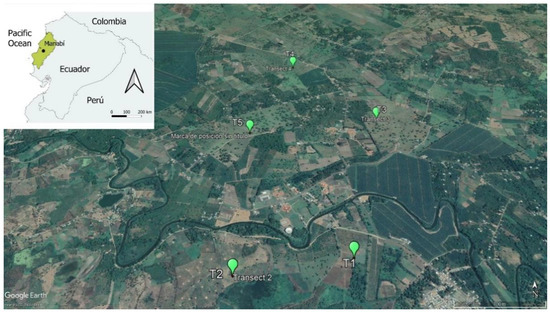
Figure 1.
The location of the study area (black spot) in the province of Manabí (green area) in Ecuador. The location of the five transects are represented by the green spots. Map showing the location of Calceta (Ecuador). Google Earth, earth.google.com/web/ (20 March 2022).
The sampling effort was equal in both dry and rainy seasons. In the study area, 5 transects were established. They were separated to be at least 400 m from one another to ensure independence of the samples. The study area was characterized by a homogeneous agroecosystem with small corn crops and remains of grasslands. Additionally, there were small patches of native shrubby vegetation and a few scattered trees.
2.2. Lizard Sampling
The diurnal activity of Stenocercus iridescens was assessed by walking transects three times per week [24] during the dry season and the rainy season. Transects were 1000 m long and 4 m wide, spaced at least 400 m apart [24]. Lizards were counted by direct observation in each transect on each side of the survey line. Potential reptile microhabitats were searched, including rocks and leaf litter, woody debris, and tree trunks [25,26]. To determinate activity pattern, we established four different time ranges, from 7:00 h to 10:00 h, from 10:00 h to 13:00 h, from 13:00 h to 16:00 h, and from 16:00 h to 18:00 h. Transects were surveyed for one hour within this time range.
Air temperature (°C), daily precipitation (mm), and heliophany were taken hourly from the ESPAM MFL meteorological station, which is connected to the official Ecuadorian network of meteorological stations of the National Institute of Meteorology and Hydrology. This station is located less than a kilometer from the study area.
2.3. Statistical Analysis
Two generalized linear mixed models (GLMMs) were applied separately for the dry and wet season using the number of individuals of Stenocercus iridescens in each transect as a response variable, which fitted a Poisson distribution with a log-link function. In the initial full model, the time range, precipitation, temperature, heliophany (all of them calculated hourly), and the double interactions among them were included as independent variables, whereas the transect site was included as random factor. The full arrangement of models (all possible combinations) was performed, and model selection was selected by the best subset approach using the Akaike information criterion corrected for a small sample size (AICc) [27]. The generated models were ranked according to AICc values, where the model with the lowest AICc is the best one. The variance inflation factor (VIF) was used to check for collinearity among the predictors, and those models including predictors with VIF > 3 were discarded. Finally, a post hoc test within a mixed analysis was carried out to check for differences among the levels of categorical variables. Statistical analyses were performed using InfoStat software [28].
3. Results
In the study area, 847 individuals of Stenocercus iridescens (Günther, 1859) were registered; 438 in the dry season, (mean per transect ± SD = 3.62 ± 1.33); and 409 in the wet season (3.12 ± 3.01). Heliophany and air temperature were not correlated in our study site (ρ = −0.01; p-value = 0.783).
The time range was the most important predictor of the number of Stenocercus iridescens individuals, since this variable was included in all the best candidate models (Table 1 and Table 2), both in the dry and wet season. The post hoc test within the mixed model showed that the highest number of individuals was detected between 13:00 h and 16:00 h and between 16:00 h and 18:00 h (Figure 2). The heliophany was also an important predictor since it was included in many candidate models (Table 1 and Table 2). However, the heliophany had a greater effect in the dry season models (Table 2), showing a negative association between the heliophany and the number of recorded individuals in the dry season, whereas in the wet season there was a slightly positive effect (Figure 3).

Table 1.
Best candidate models explaining the number of individuals according to the AIC criteria for the dry and wet season separately. Only candidate models (∆AIC < 2) are shown. k = number of parameters.

Table 2.
Results of the best candidate models explaining the number of individuals of Stenocercus iridescens for the dry and the wet season separately.
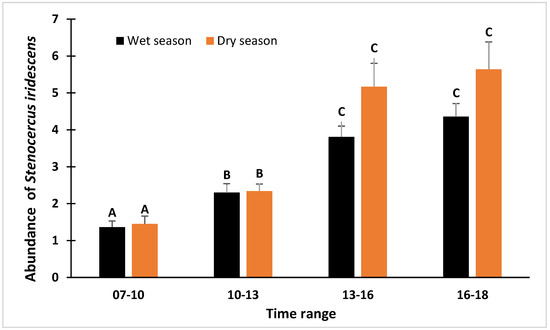
Figure 2.
Abundance of Stenocercus iridescens expressed as the number of individuals observed per transect during the four time ranges for the dry and wet season separately. Error bars represent the standard error. Different uppercase letters indicate significant differences among time ranges according to the post hoc tests (p-value < 0.05) for the wet and dry season separately.
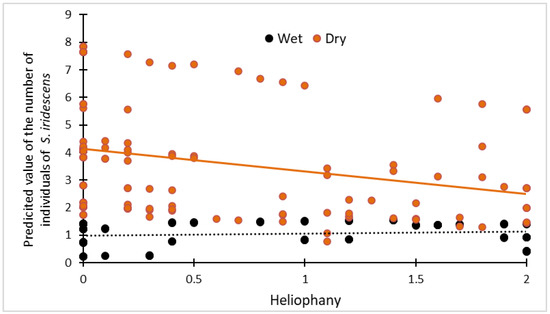
Figure 3.
Scatterplot showing the relationship between the heliophany and the predicted number of individuals of Stenocercus iridescens in dry (orange spots) and wet (dark spots) seasons separately, obtained from the best candidate model.
4. Discussion
Most studies on daily activity patterns have been carried out in temperate regions where environmental conditions strongly change between seasons and where a strong daily temperature variation also occurs with a maximum during midday when the activity peak of reptiles occurs [29,30]. However, there are not many studies on Neotropical lizards’ activity patterns and habitat selection [29,31,32]. Most of the studies have been carried out in high latitudes, where the environmental temperature range is radically different during the year. In the tropics, the temperature is very stable during the day and year. Studies in tropical zones seems to show that tropical lizards have unimodal or bimodal activity patterns. Those with a unimodal activity pattern are active around noon, whereas those lizards with bimodal activity patterns are more active during the morning and afternoon, avoiding the hours with the highest solar radiation [33].
Here, we found that the variable with the highest relative importance predictor (ωp) was the time range in which the transects were performed. In our study, the activity pattern of Stenocercus iridescens was higher in the range from 13:00 h to 16:00 h and even higher from 16:00 h to 18:00 h (Figure 2). This could be explained as at least some Neotropical lizards avoid the hours of highest radiation (noon) to thermoregulate [15]. The independent role played by heliophany and seasonality in the activity pattern of Stenocercus iridescens is not clear. However, the interaction between heliophany and seasonality seems to have an impact (Figure 3, Table 2). Our results suggest that heliophany (sunshine) has a different influence during the dry or wet season (Figure 3). The relationship between the heliophany and the number of individuals of Stenocercus iridescens counted in dry and wet seasons separately showed that the predicted value is not affected by heliophany during the wet season. Interestingly, during the dry season, the heliophany showed a negative relationship with observed individuals and Stenocercus iridescens avoided the maximum heliophany periods (Figure 3). The lack of the effect of the air temperature and the effect of the heliophany on activity patterns could be due to the high solar radiation in Ecuador owing to the perpendicularity of the sun rays, whereas the daily thermal variation is low. Thus, heliophany is more important than temperature.
Recently, ref. [34] pointed out the trade-off between water and thermal regulation in terrestrial ectotherms, suggesting that they should be considered as two integrated systems. Our finding on the negative effect of heliophany during the dry season might be explained by the water-specific heat capacity and its relationship with ectotherm vertebrates [35]. It is possible that Stenocercus iridescens found refuge in small puddles (rainy season), when water could help individuals to lose heat, or even air saturation during the wet season might keep a more stable environmental temperature range [36], and therefore, the activity pattern does not depend on the heliophany. Environmental characteristics in Palearctic regions exert little influence on thermal preference with the exception that females from habitats with permanent access to water had lower thermal preferences [34. However, sexual dimorphism was not considered in the analysis of our data.
During the dry season, Stenocercus iridescens would need behavioral strategies (similar to what many lizards do in high latitudes) to face a quick loss or earn corporal heat because of a lack of water or environmental humidity [35,36]. Likewise, [37] suggested that lizards exposed to dehydration thermoregulate less precisely than hydrated lizards, and if dehydrated lizards are less active, they can change the daily activity pattern according to their thermal preference. Dehydration negatively affected thermoregulation and dehydrated lizards reduced their preferred body temperature and showed a species-specific pattern of hourly change in thermal preference. Furthermore, they more frequently used the colder parts of the gradients and spent more time hidden [36,38]. Something similar could explain Stenocercus iridescens’ low records when heliophany was higher (noon) during the dry season (Figure 3). Additionally, since our study was performed in a disturbed area with low tree coverage, the heliophany could have a more marked effect owing to the lack of shadow, making the thermoregulation of lizard species particularly difficult [19].
5. Conclusions
In conclusion, the activity pattern of Stenocercus iridescens could be influenced by heliophany and not by air temperature as other studies suggest for Neotropical lizards with different ecological preferences [31,36]. Heliophany could also explain the change in Stenocercus iridescens’ daily activity patterns between the rainy and dry season. Therefore, we suggest further studies of reptiles to perform a brief pilot survey in order to check the peak activity patterns in the study area at that moment. Deforestation opens the canopy, increasing the solar radiation penetration, reducing the humidity, and increasing wind speed, and thus affecting the ectotherms’ sensitivity to desiccation [39]. Moreover, ecological studies on Neotropical lizard activity patterns are scarce, and further studies are needed in the face of climate change.
Author Contributions
Conceptualization, F.S.T. and R.H.Z.; data collection, R.H.Z. and V.A.C.; statistical analysis, J.G.-C.; writing—original draft preparation, R.H.Z.; writing—review and editing, F.S.T. and J.G.-C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was founded in part by the Prometeo Project of the Secretariat for Higher Education, Science, Technology and Innovation of the Republic of Ecuador (SENESCYT). José Guerrero-Casado is supported by the European Regional Development Fund (ERDF) and the Consejería de Economía, Conocimiento, Empresas y Universidad de la Junta de Andalucía (project reference: 1264483-R).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data of this study are available by request to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Foley, J.A.; DeFries, R.; Asner, G.P.; Barford, C.; Bonan, G.; Carpenter, S.R.; Chapin, F.S.; Coe, M.T.; Daily, G.C.; Gibbs, H.K.; et al. Global consequences of land use. Science 2005, 309, 570–574. [Google Scholar] [CrossRef] [PubMed]
- Stuart, S.N.; Chanson, J.S.; Cox, N.A.; Young, B.E.; Rodrigues, A.S.L.; Fischman, D.L.; Waller, R.W. Status and trends of amphibian declines and extinctions worldwide. Science 2004, 306, 1783–1786. [Google Scholar] [CrossRef] [PubMed]
- Doherty, T.S.; Balouch, S.; Bell, K.; Burns, T.J.; Feldman, A.; Fist, C.; Garvey, T.F.; Jessop, T.S.; Meiri, S.; Driscoll, D.A. Reptile responses to anthropogenic habitat modification: A global meta-analysis. Glob. Ecol. Biogeogr. 2020, 29, 1265–1279. [Google Scholar] [CrossRef]
- Stoate, C.; Baldi, A.; Beja, P.; Boatman, N.D.; Herzon, I.; van Doorn, A.; de Snoo, G.R.; Rakosy, L.; Ramwell, C. Ecological impacts of early 21st century agricultural change in Europe—A review. J. Environ. Manag. 2009, 91, 22–46. [Google Scholar] [CrossRef] [PubMed]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; da Fonseca, G.A.B.; Kent, J. Biodiversity otspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, C.I.; de la Cruz, M.; Luzuriaga, A.; Escudero, A. Bosques tropicales secos de la región Pacífico Ecuatorial: Diversidad, estructura, funcionamiento e implicaciones para la conservación. Rev. Ecosistemas 2012, 21, 167–179. [Google Scholar]
- Reyes-Puig, C.; Almendáriz, A.; Torres-Carvajal, O. Diversity, threat, and conservation of reptiles from continental Ecuador. Amphib. Reptile Conserv. 2017, 11, 51–58. [Google Scholar]
- Ferrer-Paris, J.R.; Zager, I.; Keith, D.A.; Oliveira-Miranda, M.A.; Rodríguez, J.P.; Barrow, E. An ecosystem risk assessment of temperate and tropical forests of the Americas with an outlook on future conservation strategies. Conserv. Lett. 2019, 12, e12623. [Google Scholar] [CrossRef]
- Rivas, C.A.; Navarro-Cerillo, R.M.; Johnston, J.C.; Guerrero-Casado, J. Dry forest is more threatened but less protected than evergreen forest in Ecuador’s coastal region. Environ. Conserv. 2020, 47, 79–83. [Google Scholar] [CrossRef]
- Rivas, C.A.; Guerrero-Casado, J.; Navarro-Cerillo, R.M. Deforestation and fragmentation trends of seasonal dry tropical forest in Ecuador: Impact on conservation. For. Ecosyst. 2021, 8, 46. [Google Scholar] [CrossRef]
- Ghosh, D.; Basu, P. Factors influencing herpetofauna abundance and diversity in a tropical agricultural landscape mosaic. Biotropica 2020, 52, 927–937. [Google Scholar] [CrossRef]
- Rodriguez, A.C.Z.; Gamez, M.R.; Faure, L.G. Design, construction, and energy of sustainable solar dryers in Jipijapa Canton. Int. J. Phys. Sci. Eng. 2018, 2, 88–100. [Google Scholar] [CrossRef][Green Version]
- Žagar, A.; Carretero, M.A.; Osojnik, N.; Sillero, N.; Vrezec, A. A place in the sun: Interspecific interference affects thermoregulation in coexisting lizards. Behav. Ecol. Sociobiol. 2015, 69, 1127–1137. [Google Scholar] [CrossRef]
- Méndez-Galeano, M.A.; Calderón-Espinosa, M.L. Thermoregulation in the Andean lizard Anolis heterodermus (Squamata: Dactyloidae) at high elevation in the Eastern Cordillera of Colombia. Iheringia. Série Zool. 2017, 107, e2017018. [Google Scholar] [CrossRef]
- Templeton, J.R.; Whittow, G.C. (Eds.) Comparative Physiology of Thermoregulation: Invertebrates and Nonmammalian Vertebrates; Academic Press, Inc.: London, UK, 1970; Volume 1, pp. 167–218. [Google Scholar]
- Gunderson, A.R.; Stillman, J.H. Plasticity in thermal tolerance has limited potential to buffer ectotherms from global warming. Proc. R. Soc. B Biol. Sci. 2015, 282, 20150401. [Google Scholar] [CrossRef]
- Abram, P.K.; Boivin, G.; Moiroux, J.; Brodeur, J. Behavioural effects of temperature on ectothermic animals: Unifying thermal physiology and behavioural plasticity. Biol. Rev. 2017, 92, 1859–1876. [Google Scholar] [CrossRef]
- Huey, R.; Stevenson, R. Integrating Thermal Physiology and Ecology of Ectotherms: A Discussion of Approaches. Am. Zool. 1979, 19, 357–366. [Google Scholar] [CrossRef]
- Kearney, M.; Shine, R.; Porter, W.P. The potential for behavioral thermoregulation to buffer “cold-blooded” animals against climate warming. Proc. Natl. Acad. Sci. USA 2009, 106, 3835–3840. [Google Scholar] [CrossRef]
- Huey, R.B.; Deutsch, C.A.; Tewksbury, J.J.; Vitt, L.J.; Hertz, P.E.; Alvarez Pérez, H.J.; Garland, T., Jr. Why tropical forest lizards are vulnerable to climate warming. Proc. R. Soc. B Biol. Sci. 2009, 276, 1939–1948. [Google Scholar] [CrossRef]
- Ibargüengoytía, N. Field, selected body temperature and thermal tolerance of the syntopic lizards Phymaturus patagonicus and Liolaemus elongatus (Iguania: Liolaemidae). J. Arid Environ. 2005, 62, 435–448. [Google Scholar] [CrossRef]
- Rozen-Rechels, D.; Dupoué, A.; Lourdais, O.; Chamaillé-Jammes, S.; Meylan, S.; Clobert, J.; Le Galliard, J.F. When water interacts with temperature: Ecological and evolutionary implications of thermo-hydroregulation in terrestrial ectotherms. Ecol. Evol. 2019, 9, 10029–10043. [Google Scholar] [CrossRef]
- Pawson, S.; Gass, J. Modern Era Retrospective Analysis Research and Applications, Version 2 (MERA-2); NASA: Washington, DC, USA, 2019. Available online: https://gmao.gsfc.nasa.gov/reanalysis/MERRA-2/ (accessed on 2 January 2019).
- Carpio, A.J.; Cabrera, M.; Tortosa, F.S. Evaluation of Methods for Estimating Species Richness and Abundance of Reptiles in Olive Groves. Herpetol. Conserv. Biol. 2015, 10, 54–63. [Google Scholar]
- Hutchens, S.J.; DePerno, C.S. Efficacy of sampling techniques for determining species richness estimates of reptiles and amphibians. Wild. Biol. 2009, 15, 113–122. [Google Scholar] [CrossRef]
- Sung, Y.H.; Karraker, N.E.; Hau, B.C. Evaluation of the effectiveness of three survey methods for sampling terrestrial herpetofauna in South China. Herpetol. Conserv. Biol. 2011, 6, 479–489. [Google Scholar]
- Burnham, K.P.; Anderson, D.R.; Huyvaert, K.P. AIC model selection and multimodel inference in behavioral ecology: Some background, observations, and comparisons. Behav. Ecol. Sociobiol. 2011, 65, 23–35. [Google Scholar] [CrossRef]
- Di Rienzo, J.A.; Casanoves, F.; Balzarini, M.G.; González, L.; Tablada, M.; Robledo, C.W. InfoStat Version; Universidad Nacional de Córdoba: Córdoba, Argentina, 2020. [Google Scholar]
- Radder, R.S.; Saidapur, S.K.; Shanbhag, B.A. Population density, microhabitat use and activity pattern of the Indian rock lizard, Psammophilus dorsalis (Agamidae). Curr. Sci. 2005, 89, 560–565. [Google Scholar]
- Mukherjee, R.K.; Parida, P. Behavioral ecology, breeding period, sexual dimorphism and ovipositional behavior of Psammophilus blanfordanus (Family: Agamidae): Case study. Indian J. Appl. Res. Biol. 2014, 4, 28–32. [Google Scholar] [CrossRef]
- Bauwens, D.; Castilla, A.M.; Mounton, F.N. Field body temperatures, activity levels and opportunities for thermoregulation in an extreme microhabitat specialist, the girdled lizard (Cordylus macropholis). J. Zool. 1999, 249, 11–18. [Google Scholar] [CrossRef]
- Winne, C.T.; Keck, M.B. Daily activity patterns of whiptail lizards (Squamata: Teiidae: Aspidoscelis): A proximate response to environmental conditions or an endogenous rhythm? Funct. Ecol. 2004, 18, 314–321. [Google Scholar] [CrossRef]
- Atencia, P.L.; Castillo, C.J.; Montes, L.F. Use of microhabitat and activity patterns of two lizard species from a seasonal dry forest in northern Colombia. Neotrop. Biol. Conserv. 2020, 15, 153. [Google Scholar] [CrossRef]
- Rozen-Rechels, D.; Rutschmann, A.; Dupoué, A.; Blaimont, P.; Chauveau, V.; Miles, D.B.; Guillon, M.; Richard, M.; Badiane, A.; Meylan, S.; et al. Interaction of hydric and thermal conditions drive geographic variation in thermoregulation in a widespread lizard. Ecol. Monogr. 2021, 91, e01440. [Google Scholar] [CrossRef]
- Dupoué, A.; Rutschmann, A.; Le Galliard, J.F.; Miles, D.B.; Clobert, J.; DeNardo, D.F.; Meylan, S. Water availability and environmental temperature correlate with geographic variation in water balance in common lizards. Oecologia 2017, 185, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, D.; Mautz, W.J.; Nagy, K.A. Evaporative Water Loss: Humidity Acclimation in Anolis carolinensis Lizards. Copeia 1983, 1983, 701–704. [Google Scholar] [CrossRef]
- Sannolo, M.; Carretero, M.A. Dehydration constrains thermoregulation and space use in lizards. PLoS ONE 2019, 14, e0220384. [Google Scholar]
- Wei, X.; Yan, L.; Zhao, C.; Zhang, Y.; Xu, Y.; Cai, B.; Jiang, N.; Huang, Y. Geographic variation in body size and its relationship with environmental gradients in the Oriental Garden Lizard, Calotes versicolor. Ecol. Evol. 2018, 8, 4443–4454. [Google Scholar] [CrossRef]
- Tewksbury, J.J.; Huey, R.B.; Deutsch, C.A. Putting the heat on tropical animals. Science 2008, 320, 1296–1297. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).