Abstract
Traditional crop varieties are receiving increasing attention in sustainable agriculture, conservation genetics, and plant science because they offer significant and largely unexplored diversity. The DNA profiling of landraces is being applied to numerous crops, yet a detailed knowledge of morphological diversity is often needed to increase the efficiency of both the conservation and exploitation of local germplasm. In this work, morphological, pomological, and fruit-quality traits (16 qualitative and 16 quantitative) were collected from 44 traditional apricot landraces cultivated in Campania, the Italian region with the highest number of traditional varieties. The aim was to assess varietal diversity and to highlight possible trends and phenotypes that may have driven the morphological differentiation. All traits were polymorphic, and each variety had a distinctive phenotype. The qualitative and quantitative traits provided different classifications of the varieties. Nonetheless, the Factorial Analysis of Mixed data indicated that, for both categories of variables, the fruit traits were the most influential for landrace classification. Interestingly, some easily discernible color phenotypes of the fruits mostly contributed to the discrimination of the analyzed apricot germplasm. We conclude that these specific, commercially relevant features of the fruit were important drivers of the differentiation of the cultivated apricot material at regional scale.
1. Introduction
Apricot (Prunus armeniaca L.) is a deciduous stone fruit tree of the Rosaceae’s family that is cultivated for the sweet, aromatic, vividly colored, fleshy fruits [1]. Although cosmopolitan, this species thrives in temperate areas. In particular, the countries around the Mediterranean Basin provide approximately half of the world’s total production, with Turkey being the top producer. In the EU, the cultivated area and fruit production rank apricot as the fourth stone fruit, following cherry, plum and peach. Nonetheless, the EU production of apricots in the last ten years has fallen less in than peaches and plumes, fluctuating around 680,000 tons (2011–2021; source: EUROSTAT). Italy has a leading role in apricot production, ranking first in terms of total fruit production and yield in the EU. This position is the concurrent result of different factors, which include favorable growing conditions, importance in gastronomy, and more recently, a strong varietal renewal. Moreover, apricot has a long history of cultivation in Italy, being popular in Southern Italy since the Roman times [2]. For instance, in the Campania region (Southern Italy), which supplies about a third of the Italian production (2019; source: ISTAT), apricot cultivation has traditionally been, and still is today, more important than cherry and plum. It has been estimated that the Campania has over a third of the Italian local varieties of apricot [3].
Considering the ancient introduction, the different oro-geographic characteristics of Italy, and the societal and cultural differences that characterized the Italian history after the fall of the Western Roman Empire, it is not surprising that Italy is considered a secondary centre of diversity [4], also because this country arguably has the most abundant germplasm in terms of accessions [1]. These varieties often have a regional diffusion and are linked to specific geographic areas and traditions and therefore, they can be considered landraces today [5]. Apricot landraces have lost competitiveness and are cultivated mainly in small farms. Since 2000, the apricot sector in Italy has experienced a fast transition towards mostly self-compatible, Sharka-resistant cultivars bred for the fresh market, which typically share common esthetic features (i.e., round-shaped, fully strongly over-colored fruits of medium-large size). This renewal was also the result of the concomitant fall of the apricot industrial transformation and the decline of the peach cultivation.
Traditional germplasm is a largely unexplored resource of diversity, for both plant science research and breeding [6,7]. For instance, it is generally believed that landraces are a source of trait variation for local adaptation and resilience to extreme climate conditions [8,9]. Landraces of fruit trees are also appreciated because they provide fruits with superior aroma and taste [10,11] and this is also related to the fruiting habit of the variety [12]. It is likely that this adaptive diversity is not largely considered in apricot breeding programs, currently carried out in a limited number of nations. Regrettably, the exploitation of genetic resources in farms or public collections (e.g., in academia or research centers) is strongly limited by the challenge of obtaining good quality, consistent and homogenous phenotypic description [10,13]. For apricot, this is also aggravated by the need of large and intensive resources for the time-consuming characterization of clonally propagated material with a long juvenile phase and the seasonal production of easily perishable fleshy fruits. To date, it is still not fully understood if the numerous old varietal names do correspond to phenotypically distinct entities. It is known that the variety richness for agamically propagated species is higher than that of other breeding systems [14].Yet, it is unclear whether the differentiation among the large number of varieties present in a territory is due to some adaptive and/or commercially related forces, or it is predominantly due to other processes deriving, for instance, by socio-cultural dynamics, linguistic issues in folk taxonomy, or inconsistency in ethnobotanical nomenclature [15].
In the last decades, a considerable effort has been carried out to characterize the molecular diversity of traditional varieties in several countries [16,17,18,19,20], under the principle that germplasm erosion and loss of diversity in the apricot germplasm are worldwide concerns [21,22]. While the number of molecular markers-based screenings of traditional apricot varieties is continuously increasing due to the lowering price and accessibility of DNA typing tools [21], the phenotypic characterization of this material is lagging. With the aim of going further from providing information on the origin, identity, and molecular relationship of apricot genetic resources, in this work we thoroughly characterized morphological, phenological, and pomological traits (also including fruit-quality traits) of a collection of traditional apricot varieties once diffused in cultivation in the Campania region of Italy. Our intent was to make evident the level of phenotypic diversity from different perspectives, illustrate possible trait-correlations, establish phenotypic relationships among genotypes considering both quantitative and qualitative traits, and highlight the contribution of variables and traits to their discrimination, with the final goal to make more efficient the screening, conservation, promotion, and exploitation of apricot genetic resources.
2. Materials and Methods
The work was carried out at the experimental facilities managed by the Department of Agricultural Sciences (Federico II University of Naples) using 44 apricot (Prunus armeniaca L.) varieties listed in Table S1. The morphological evaluation of the trees was carried out in two independent growing cycles using standard cultural practices to ensure satisfactory growth and fruit production. Flowering of the various landraces lasted from the 3rd week of February to the 4th week of March. Fruits were harvested at commercial maturity for each landrace and overall, the fruit production calendar spanned from the 2nd week of June up to the 1st decade of July. The following traits were categorically scored: tree vigor (assigned to one of the following categories: very weak, weak, medium, strong, very strong); one-year-old shoot color on sunny side (yellow brown, red brown, purple brown); shape of base of the leaf blade (acute, obtuse, truncate, cordate); incisions of margin of the leaf blade (crenate, bicrenate, serrate, biserrate); anthocyanin coloration of upper side of the petiole (weak, medium, strong); color on lower side of the petal (white, light pink, dark pink); fruit shape in lateral view (triangular, ovate, oblong, elliptic, circular, oblate, obovate, oblique rhombic); depth of stalk cavity of the fruit (shallow, medium, deep); fruit suture (raised, slightly sunken, moderately sunken, deeply sunken); presence of mucron of the fruit (absent, present); ground color of fruit skin (not visible, white, yellowish, yellow green, light orange, medium orange, dark orange); hue of over color of the fruit (orange red, red, pink, purple); intensity of over color of fruit skin (light, medium, dark); color of fruit flesh (whitish green cream, light orange, medium orange, dark orange); stone adherence to flesh (absent or very weak, weak, medium, strong); kernel bitterness (absent/weak, present), according to the International Union For The Protection Of New Varieties Of Plants (UPOV) instructions (document TG/70/4; available at: www.upov.int/edocs/tgdocs/en/tg070_04_rev.pdf, accessed on 1 July 2022). For the plant (respectively, part of plant) trait(s) to be examined, observations were made on three plants (resp., three parts for each of the three plants). Leaf, fruit, and stone traits were individually measured on a total of 60 samples per variety. We also evaluated pollen germinability because it is one of the key parameters in controlling cropping in fruit trees [23] and it is genotype-dependent [24]. To determine pollen germinability, flowers were harvested just before anthesis and transferred to the laboratory in a portable cooler. Anthers were extracted and incubated at 24 °C for one day. Pollen was scattered on a 15% (w/v) sucrose solution solidified with 1% microbiological agar (Sigma Aldrich, Milan, Italy) by tapping a pollen-coated soft brush. Petri dishes were incubated in the darkness at 20 °C for one day. Observations were made with a dissecting microscope (×100 magnification) in three different fields per Petri dish. Pollen was considered germinated if the tube reached a length at least comparable to that of the grain. Each variety was analyzed in duplicate. Results were categorized in high (>60%), medium (60–50%), and weak (<50%) germinability.
For the traits that were quantitatively expressed (namely, blade length, width, and area of the leaf; petiole length; weight, height, lateral width, and ventral width of the fruit; weight, longitudinal width, transversal width, and ventral width of the stone) data are reported as mean ± standard error of the mean. These continuously scored parameters were measured on the same fruit, stone, and leaf samples used for the evaluation of the previously described categorical traits. Leaf blade area was calculated with a leaf area meter (LI-3100; LI-COR, Inc., Lincoln, NE, USA). Masses were measured with a digital scale (PBP423-1S VWR International, Radnor, PA, USA) while metric measurements were carried out with a digital caliper. Flesh firmness (N) was evaluated with a digital fruit firmness tester (model #53205, TR, Forlì, Italy) fitted with an 8-mm diameter plunger. The soluble solids content in fruit (SSC) was measured with a digital refractometer (HI96811, Hanna Instruments, TX, USA) and expressed in °Brix. Titratable acidity (TA) was calculated by adding a 0.1 M NaOH solution to the filtered fruit juice until reaching a pH value of 8.2. The pH was continuously monitored with a laboratory pH-meter (GLP 21; Crison, Alella, Barcelona, Spain) and expressed in grams per liter of malic acid (g/L) as described [25]. The SSC and TA were measured because they are major fruit qualitative traits that directly affect consumer’s acceptance of stone fruits [26], and they are regarded as suitable fruit quality descriptors to discriminate apricot cultivars [27].
The full list of abbreviations, traits, type, and unit of measurement is reported in Table S2.
Data Analysis
Cluster analysis based on the qualitative traits was carried out using the Jaccard similarity index and the UPGMA agglomerative cluster algorithm implemented in the ade4 package [28]. To this purpose, qualitative states were dummified in 53 binary variables using the spatstat package [29]. The Shannon Index of Diversity (SDI) for each trait was calculated as: −∑[(pi) × ln(pi)], where pi is the ratio between the number of individuals with the phenotype i and the total number of scored individuals. For each quantitative trait, total variability was analyzed with a one-way ANOVA approach followed by mean separation with the Fisher Least Significant Difference (LSD) test at α = 0.05, using the SPSS 27 software (IBM, Armonk, NY, USA). Pairwise correlation between quantitative traits were calculated using the Pearson correlation coefficient (Pearson’s r) in R and visualized using the corrplot package [30]. Cluster analysis of the landraces according to the quantitative traits was obtained based on Euclidean distances and the UPGMA algorithm, using ade4. For each trait, values were standardized (Z-score) considering the different units of measurement. The correlation (Pearson’s r) between the cophenetic matrices of the two dendrograms was verified applying the Mantel test with 9999 permutations at default settings, using the vegan package [31]. To analyse the similarity among landraces using a combined dataset of quantitative and qualitative traits we used the Factor Analysis of Mixed Data (FAMD) principal component method using the factoMineR and factoextra packages [32,33]. All calculations were performed in R 4.02.3.
3. Results
3.1. Analysis of Qualitative Traits
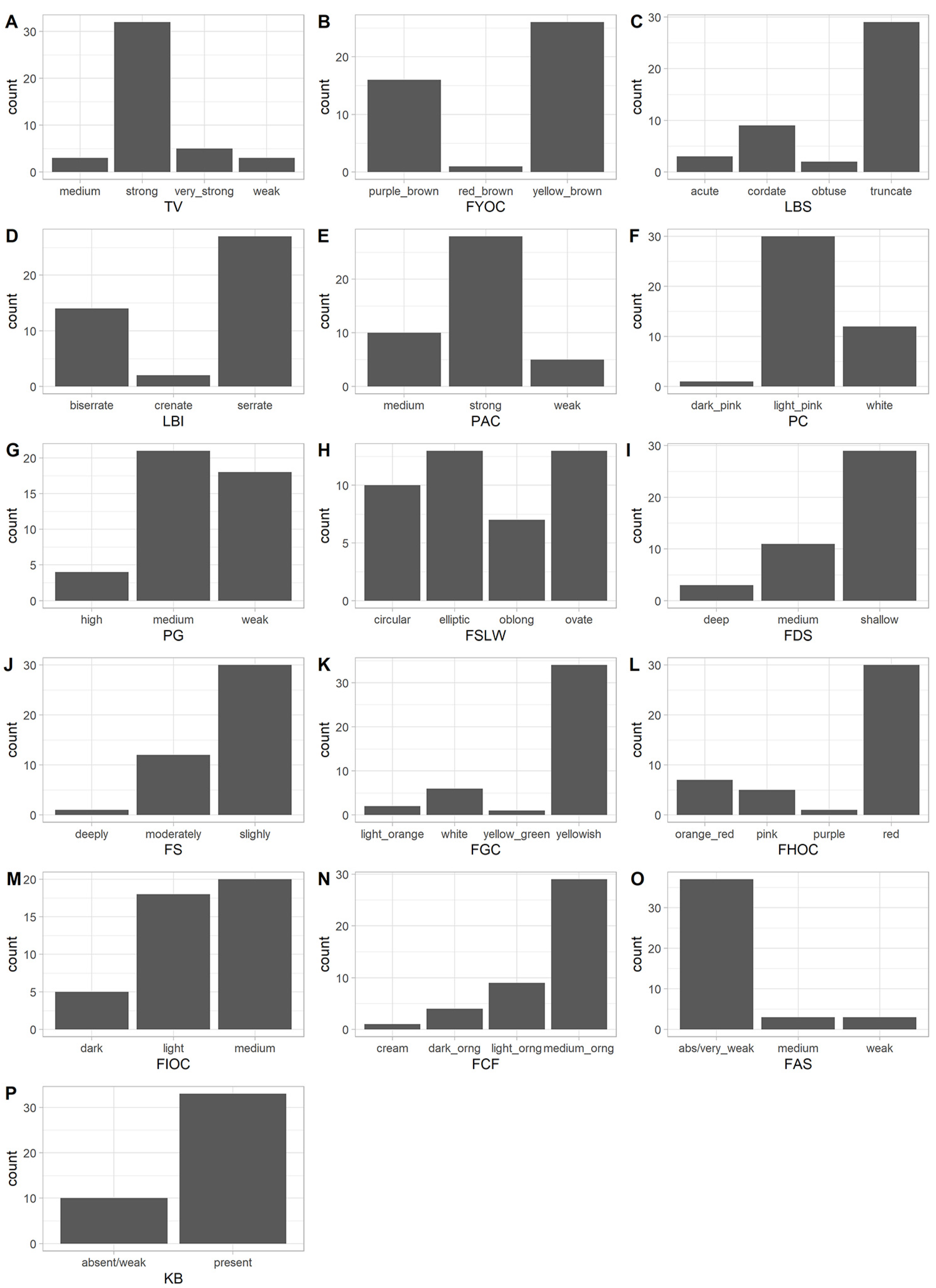
The diversity in the apricot germplasm was first assessed using the 15 qualitatively traits scored according to UPOV document TG/70/4 and pollen germinability, which was expressed in three discontinuous ordinal states. The frequency of each phenotype is illustrated in Figure 1 and results are detailed in Table S3. All qualitative traits were polymorphic, presenting two or more different phenotypes. A predominant phenotype, that is with a relative frequency (rf) higher than 75%, was recorded for four traits (Table S3). Specifically, an “absent/very weak” adherence of the stone to the fruit flesh was the most common one (rf: 86%). Other predominant phenotypes were a yellowish background color of the skin (rf: 80%) and a medium orange pulp (rf: 68%), along with a bitter kernel (rf: 77%). On the other hand, the highest level of diversity, as measured by the Shannon Diversity Index (Table S3), was recorded for the fruit shape (SDI = 1.35). Similarly, two other aesthetically and commercially important traits (the intensity of the over color and the flesh color) were also highly diverse (SDI of 0.97 and 0.89, respectively). The average SDI of the 16 traits was 0.830 indicating a good distribution of the different phenotypes in the apricot population.
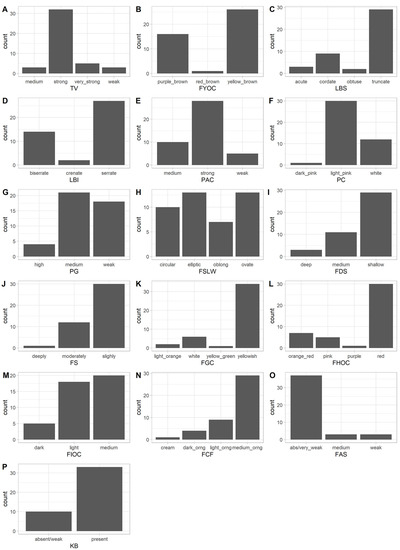
Figure 1.
Distribution of the phenotypes for the 16 qualitative traits in the apricot population. (A) TV: tree vigor; (B) FYOC: one-year-old shoot color on sunny side; (C) LBS: leaf blade shape of base; (D) LBI: leaf blade incisions of margin; (E) PAC: petiole anthocyanin coloration of upper side; (F) PC: petal color on lower side; (G) PG: pollen germinability; (H) FSLW: fruit shape in lateral view; (I) FDS: fruit depth of stalk cavity; (J) FS: fruit suture; (K) FGC: fruit ground color of skin; (L) FHOV: fruit hue of over color; (M) FIOC: fruit intensity of over color; (N) FCF: fruit color of the flesh; (O) FAS: fruit adherence of stone to flesh; (P) KB: kernel bitterness.
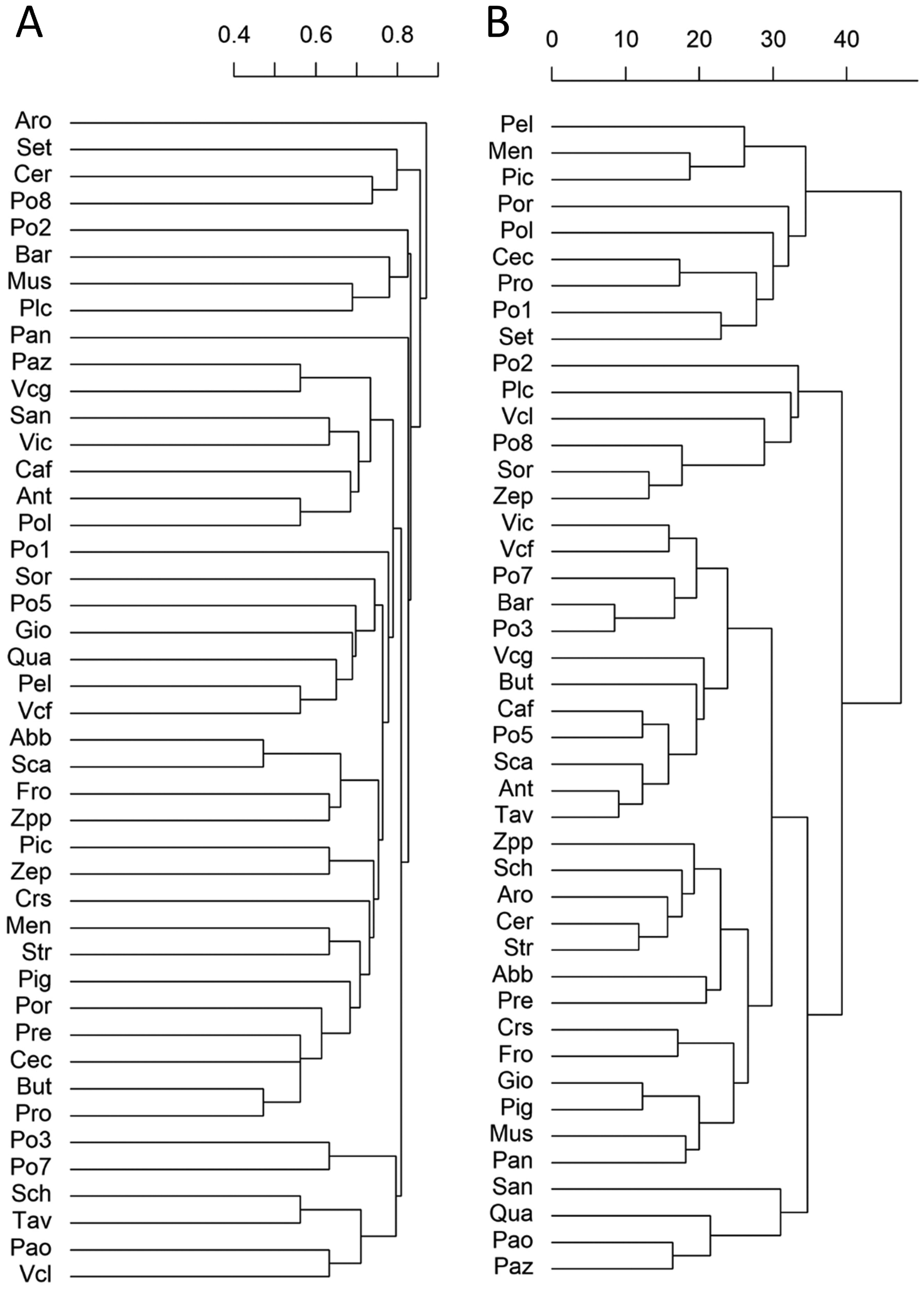
All the 44 landraces could be distinguished by the qualitative traits, without the presence of a duplicate phenotype. To visualize the relationships among the apricot landraces, we performed a multivariate cluster analysis using dummified qualitative traits (Figure 2). A clear clustering according to traits that may be subject to farmers’ selection (e.g., fruit shape, flesh, and skin color, etc.) was not evident. This suggests that the ample morphological diversity is distributed well among the landraces and that the presence of derived clones or accessions should be limited.
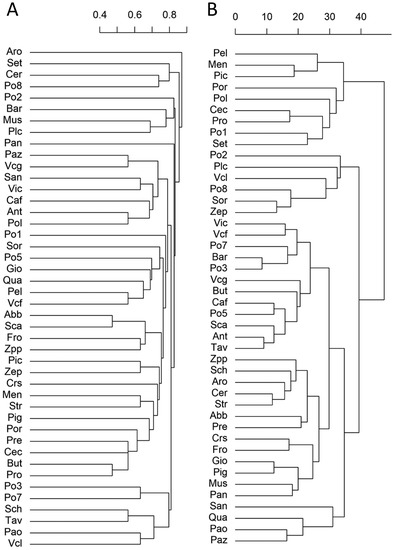
Figure 2.
Cluster analysis of the landraces based on the phenotypic traits. (A) Dendrogram of the 44 apricot landraces based on 16 qualitative traits. (B) Dendrogram of the 44 apricot landraces based on 16 quantitative traits. The full name of the landraces is reported in Table S1.
3.2. Analysis of Quantitative Traits
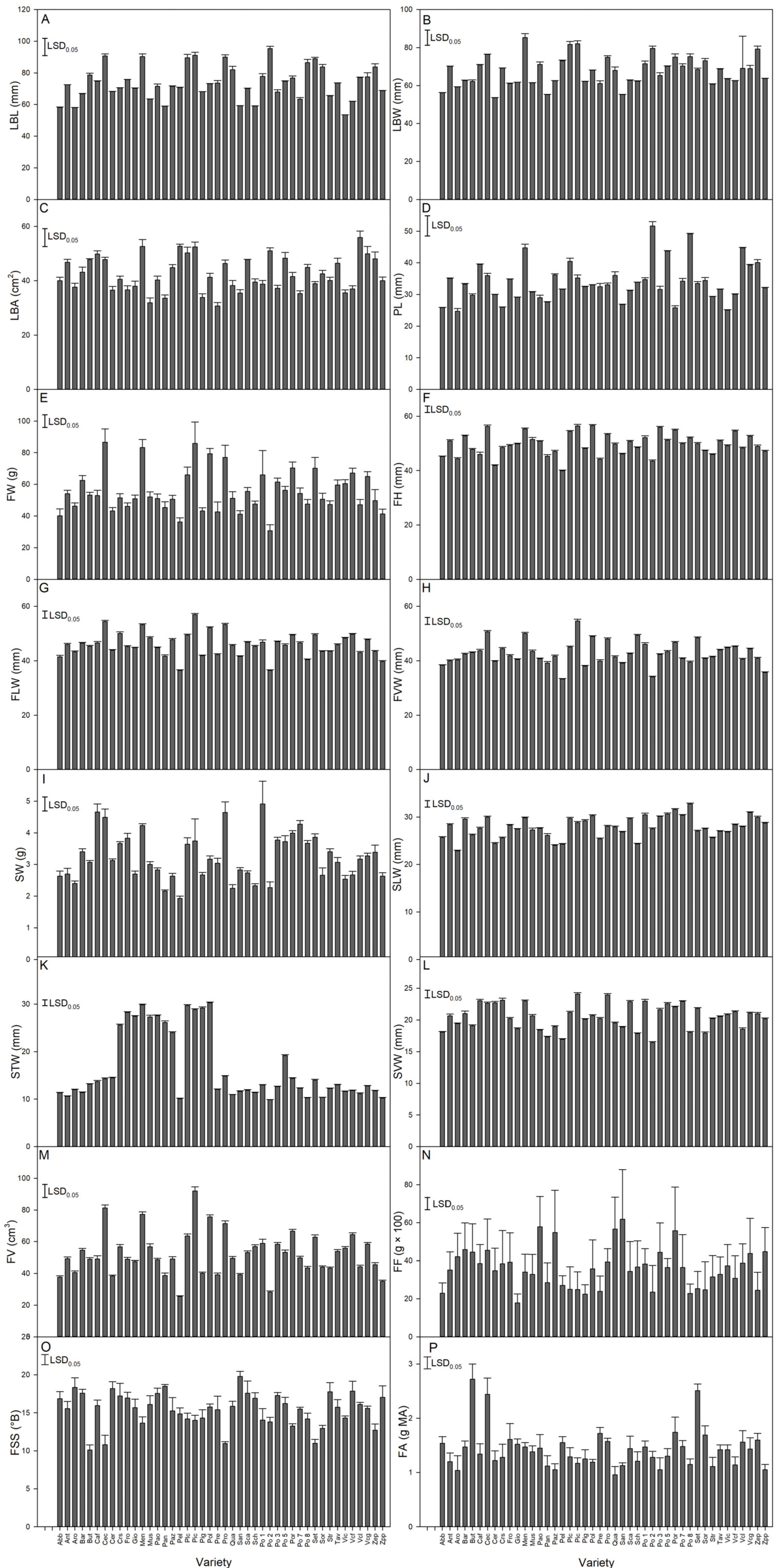
The analysis of the 16 quantitatively scored variables indicated significant differences among the landraces for all traits (Figure 3). Considering the coefficient of variation (CV) (Table S4), the higher extent of variability was observed for the stone transversal width (CV = 43.7%) followed by the fruit firmness (CV = 29.3%). While the fruit (CV = 24%) and the stone (CV = 22.8%) weights were highly variable, the three metric expressions of fruit size (i.e., height, lateral, ventral width) were the traits with the lowest CVs (8.1%, 9.4%, and 10.1, respectively).
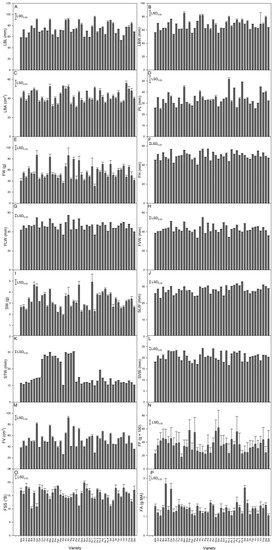
Figure 3.
Histograms of the 16 quantitative traits scored in the apricot germplasm. For each trait, the graph reports the mean + standard error. The Least Significant Difference (LSD; one-way ANOVA, p < 0.05) is reported in the top left corner. (A) LBL: leaf blade length; (B) LBW: leaf blade width; (C) LBA: leaf blade area: (D) PL: leaf petiole length; (E) FW: fruit weight; (F) FH: fruit height; (G) FLW: fruit lateral width; (H) FVW: fruit ventral width; (I) SW: stone weight; (J) SLW: stone longitudinal width; (K) STW: stone transversal width; (L) SVW: stone ventral width; (M) FV: fruit volume; (N) FF: fruit firmness; (O) FSS: fruit solute soluble content; (P) FA: fruit acidity.
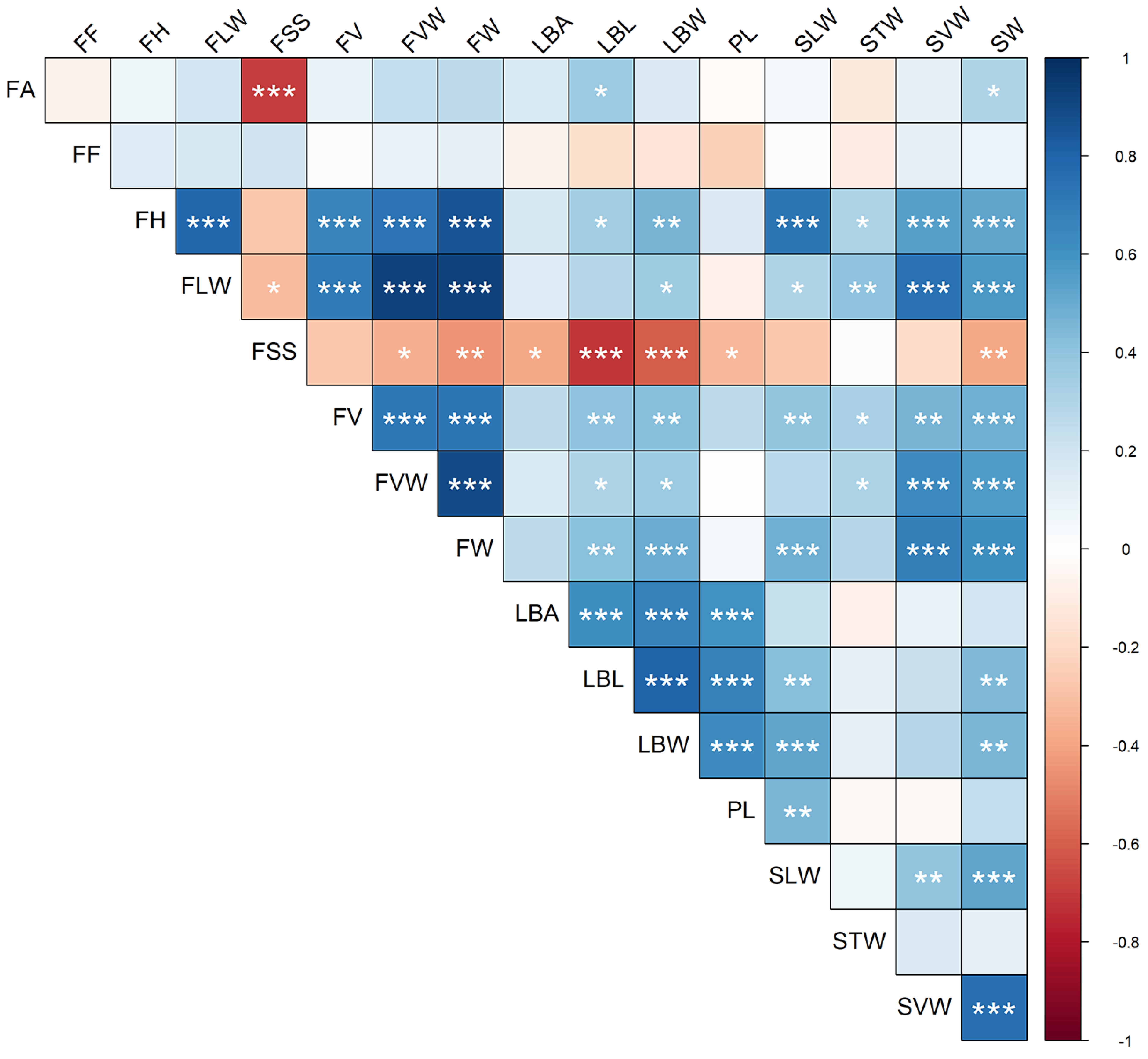
The analysis of the pairwise linear correlation between quantitative traits indicated, as expected for morphometric traits, that the majority (78%) were positive (i.e., higher than 0) (Figure 4). Overall, 63 correlations (respectively, 49) were significant at p < 0.05 (resp. at 0.01) out of the possible 120. The traits with higher significant correlation coefficient were FV and FW (r = 0.98; p < 0.001). Overall, higher correlations were found for the dimensions of the fruits and of the leaf. Among the stone metric traits, the transversal width was the more independent from the others. For the leaf traits, the area did not significantly correlate with the fruit dimensions. The traits that mostly correlated with others were LBL, LBW, SW, and FV, with 11 significant correlations. Negative correlations were mostly found for the soluble content of the fruit (FSS), with ten significant correlations with leaf and fruit traits. The strongest negative correlation was between FSS and FA (r = −0.70; p < 0.001). The latter mildly correlated also with LBL (r = 0.36; p = 0.015) and SW (r = 0.31; p = 0.04). Fruit firmness was the only characteristic with low (max r value: 0.21; min r value: −0.23) and non-significant correlations with all the other traits.
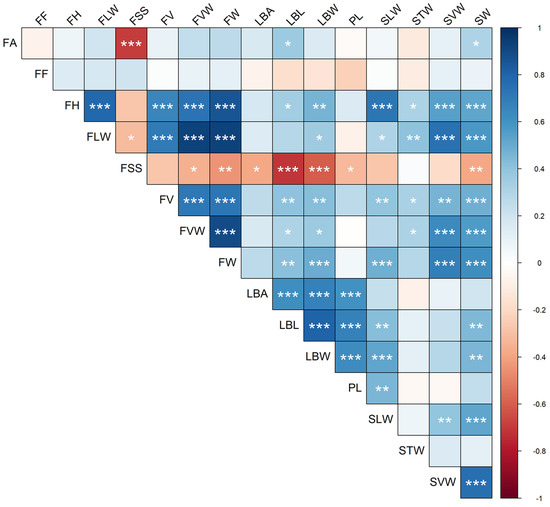
Figure 4.
Correlogram (Pearson) of the 16 quantitative traits. Pairwise correlations between variables are color-mapped according to the scale bar on the right-hand side. Asterisks indicate statistically significant correlations (*: p < 0.05; **: p < 0.01; ***: p < 0.001). Trait codes are reported in Table S2.
To illustrate the relation between landraces according to the quantitative traits, we built a dendrogram calculating Euclidean distances of scaled variables (Figure 2B). As also noticed for the qualitative traits, it was not possible to identify clusters having similar phenotypes. Moreover, the classifications obtained with either the quantitative or the qualitative traits did not show a similarity. Specifically, the Mantel statistic based on Pearson’s product-moment correlation of the cophenetic matrix of the two dendrograms showed a very low and non-significant correlation (r = 0.0036; p = 0.478), indicating that the morphological qualitative and quantitative traits provided different classifications.
3.3. Factorial Analysis of Mixed Data
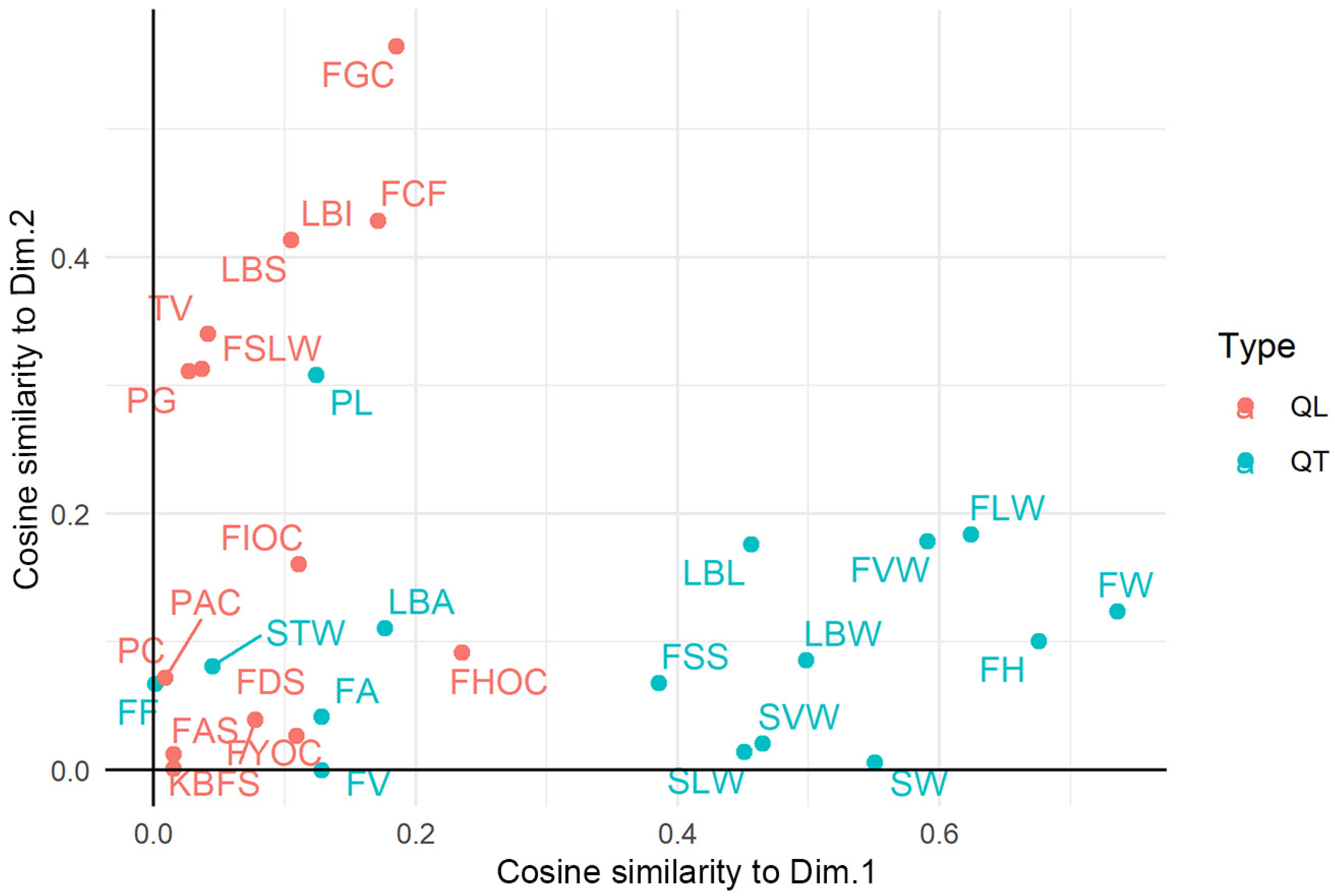
Considering the equal and high number of quantitative and qualitative traits and the lack of correlation between the classifications provided by the two types of variables, we summarized and visualized the morphological resemblance among landraces using a Factorial Analysis of Mixed Data procedure. The first five factors cumulatively explained 44.6% of the total variance, suggesting that multiple factors are needed to model the systematic variation of the morphological data set. We retained for graphical representation the first two principal components (Dim). The correlation of each trait to the two Dims is illustrated in Figure 5. The traits that mostly contributed to the first principal component (Dim. 1) were quantitative, while the contribution of qualitative traits was mainly related to the Dim. 2. Considering that variables group together when contributing similar information, the analysis indirectly proved that the contribution of quantitative and qualitative traits to the varietal classification wasnot correlated. Interestingly, the fruit weight (FW) and the three fruit dimensions (FH, FLW, and FVW) were the most influential quantitative traits, while the fruit quality characteristics, like juice acidity and flesh firmness, were the least influential (Figure S1). A distinction among the qualitative traits according to their nature or organ was not evident in relation to their contribution to the two first factors (Figure S2). Nonetheless, two fruit traits (ground-skin and flesh color) had the highest correlations to Dim 2. The kernel bitterness was the least correlated with both principal components among the whole set of traits.
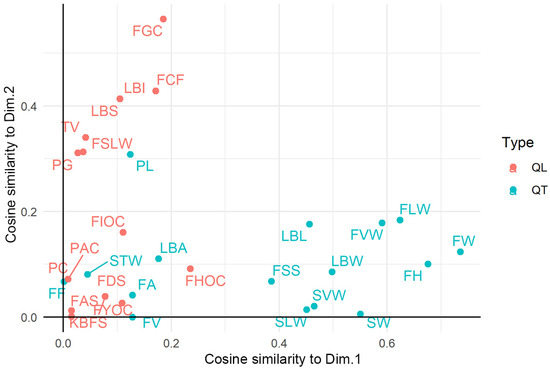
Figure 5.
Scatterplot of the traits along the two main classification axes according to cosine similarity. The coordinates are the product of the factor/component loading and the (absolute) standard deviation of the component loading. A larger positive (respectively, negative) loading indicates a larger positive (resp., negative) correlation between a variable and the principal component. Qualitative (QL) (resp., quantitative, QT) traits are colored in salmon (resp. azure) color. Variable code names (and their classification) are reported in Table S2.
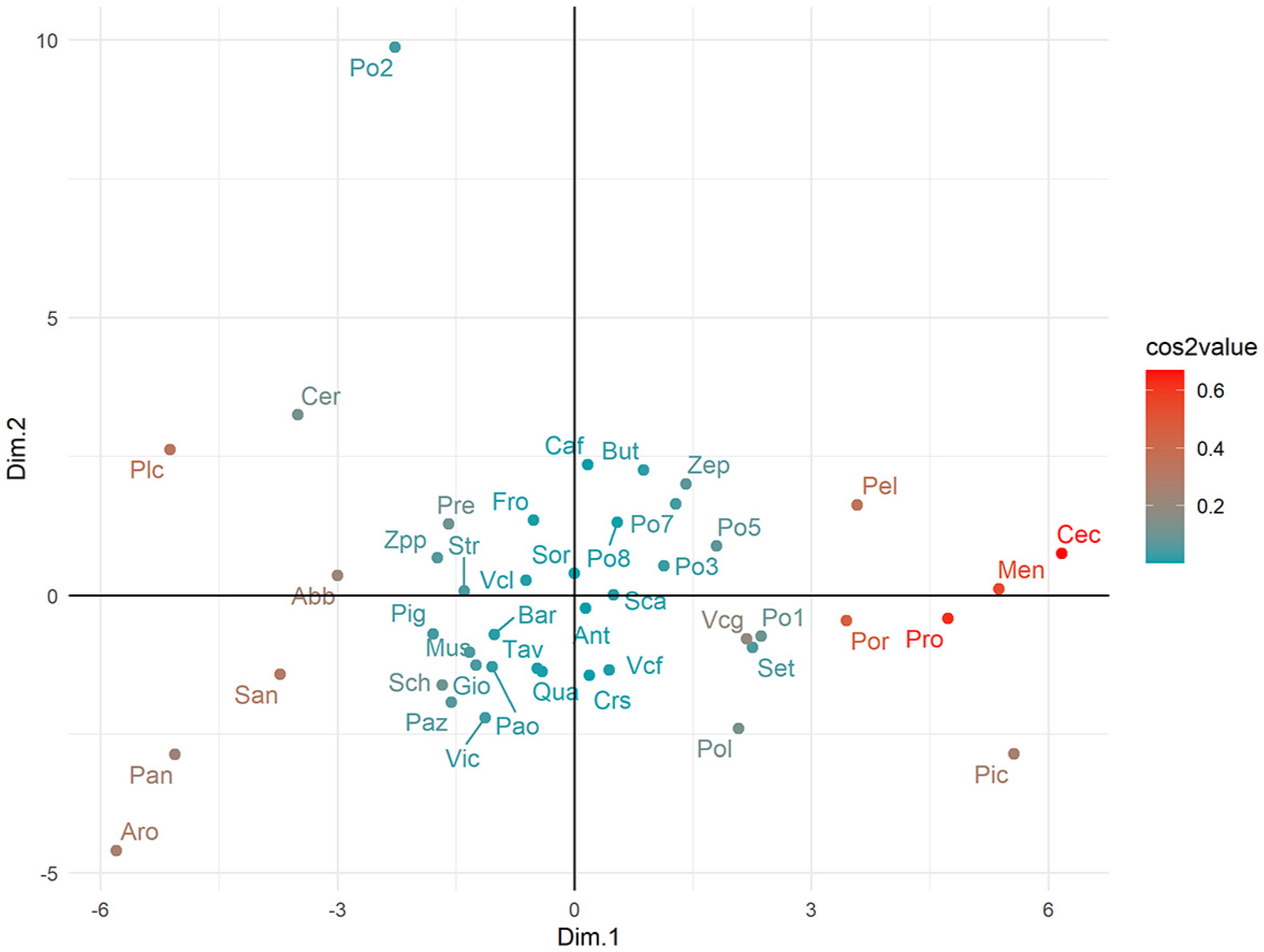
The clusters of apricots based on their overall phenotypic similarity are illustrated in Figure 6. Landraces were mostly distributed along the Dim. 1, and only for some cases (e.g., Po2, Aro, and Pan) was a distinctive position present. A clear agglomeration according to specific fruit types, colors or the place of origin was not evident, being many landraces rather uniformly scattered in the bidimensional representation of the variance.
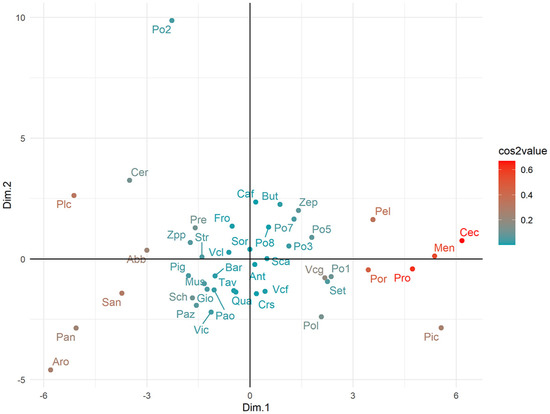
Figure 6.
Factor Analysis of Mixed Data to represent the relationship among apricot landraces. Landraces are colored according to the squared coordinates (cos 2) following the color scale bar in the right-hand side of the scatterplot. The cos2 value, which ranges between 0 and 1, is indicative of the quality of representation on the factor map. Landraces abbreviations are reported in Table S1.
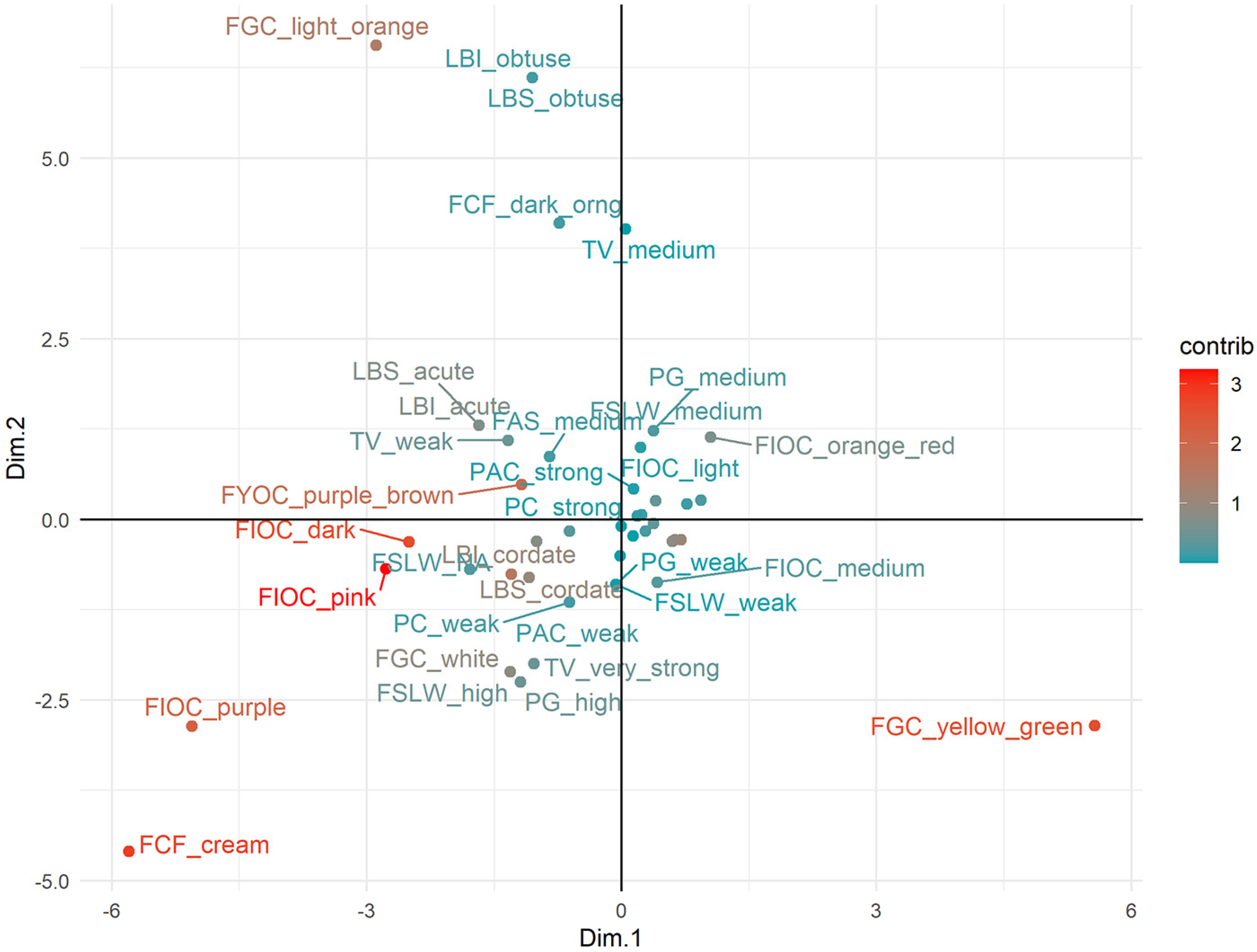
Finally, we attempted to reveal the most important qualitative phenotypes to interpret the observed variation. According to the contribution to the two principal dimensions, the most influential phenotype was the light orange ground color of the skin (Figure 7). Similarly, also the yellow-green ground color was associated with a high value and as expected, these two alternative phenotypes lied in opposite quadrants. Other important phenotypes were the cream color of the flesh, along with its alternative dark orange. It is interesting that also another visually discernible phenotype, the purple over color of the skin, was also among the most important. Some leaf phenotypes of the blade (an obtuse shape of the base, and the incision of the margin) were mostly influential relative to the second dimension.
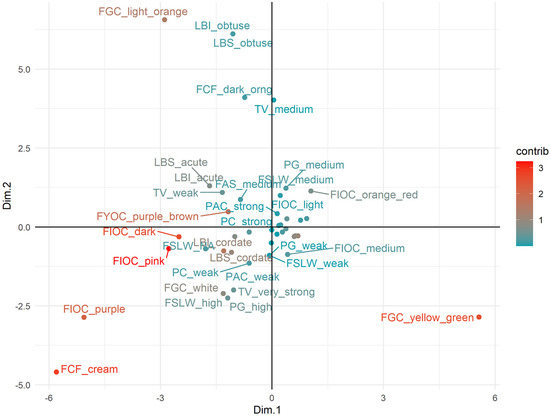
Figure 7.
Distribution of the qualitative phenotypes (categories of the qualitatively scored traits) in the bidimensional factorial plain. For graphical reasons, the label of18 phenotypes in the central area is omitted. Phenotypes are colored according to their relative contribution to the principal dimensions following the scale bar reported on the right-hand side of the plot. Variable code names and phenotypes are reported in Table S3.
4. Discussion
Morphological descriptors are useful for landraces’ characterization because they are technically undemanding, applicable in a variety of cultural contexts, and translatable in information that is easily understood by stakeholders [34]. In this work, we characterized an ample collection of apricot landraces using agro-morphological and pomological traits as well as fruit-quality parameters. The qualitative traits were sufficient to discriminate all the landraces. This is likely due to their relatively high number and discriminating ability since we used UPOV descriptors. The average Shannon Index of Diversity indicated a good distribution of the evaluated traits yet there were some notable and highly present phenotypes. Specifically, the “absent/very weak” adherence of the stone to the fruit flesh was the most common. This phenotype is important for industrial transformation. Starting from the 1970’, different efforts were carried out to strengthen the agro-industrial sector in Southern Italy, which in the Campania region included the selection from the local germplasm of varieties more suitable to produce apricots in syrup (the so-called “spiccagnole” varieties) [35,36]. Other frequent qualitative phenotypes were also related to commercially important attributes, such as a yellowish fruit skin and a medium orange pulp. These phenotypes are associated with the apricot ideotype in the Campania region and could be considered the basis for maintenance breeding [37]. This hypothesis is reinforced by the fact that, on the contrary, other color traits (e.g., color of the pulp; hue and intensity of overcolor) and fruit shapes were among the most variable characters. It is also worth mentioning that most of the varieties had a bitter kernel, differently from other reports [38]. For Mediterranean gastronomy, the apricot kernel has very little use (if any), being largely overshadowed by almonds, hazelnuts, and pistachios, and it is unlikely that this trait is under scrutiny by farmers and consumers [39].
Significant variability was also found regarding the quantitatively scored traits, including those related to fruit quality. Although far less practical, the landraces could be distinguished using only the quantitative traits yet, the use of quantitative and qualitative variables provided different and complementary information, as indicated by the cluster and the factorial analyses [40,41,42]. Moreover, as quantitative traits are sensitive to environmental conditions, it remains to be seen whether a similar discrimination may be achieved if the plants are in different environments.
Apricot quality is a complex attribute, whose definition and requirements may be different according to specific perspectives (e.g., commercial, nutritional, flavorsome, etc.) [43,44]. High variability was observed in firmness and acidity, and to a lower degree, also in the total soluble content. Considering that also the fruit weight and volume had high CVs (24% and 28%, respectevely), the variability in fruit shapes, and the number of detected UPOV classes, it is possible to conclude that the germplasm under investigation represents a suitable panel of apricot diversity. Meaningful significant and positive correlations were found among agronomically important traits, such as those related to the measurement of the fruits and stones, and as expected, between the fruit and stone dimensions [45]. Fruit firmness did not correlate with other characteristics, as previously reported [46]. A strong negative correlation was observed between FSS and FA. It is well established that during fruit ripening the organic acids decrease while soluble sugars (mainly fructose, sucrose, and glucose) increase because of the metabolic interconnection between these two classes of compounds [47].
We used the FAMD technique to characterize the germplasm using quantitative and qualitative variables simultaneously and to understand which traits and phenotypes are important for classification purposes. Similar to studies in other species, the dimensionality reduction by FAMD indicated that various factors are needed to capture most of the variance [48,49]. This can be explained by considering that the dataset is complex (being made of an equal and substantial number of qualitative and quantitative variables) and that the relationships between the two types of traits are non-linear, bearing in mind the observed significant relationships between quantitative traits. This was also evident considering that the two types of traits were orthogonally distributed in relation to the two main components. It should also be added that it would not be fully appropriate to numerically compare the contribution of quantitative and qualitative traits because for the latter their influence on the factorial analysis is also related to the number of scored categories per trait. Nonetheless, it is interesting that the fruit traits were the most influential for both categories, suggesting that the process of the diversification of the apricot germplasm should have been mainly driven by the features of the fruits [50].
The distribution of the landraces on a two-dimensional map did not allow us to identify groups according to one specific trait or to some trends, aside from the wider spreading of the varieties along the first dimension, which was consistent with the explained variance. It is also necessary to say that the germplasm under investigation did not have an a priori classification into classes (also in relation to the various phenotypes) and originated from the same regional area. Therefore, the unsupervised FAMD technique could be mainly aimed at highlighting the multidimensional relationship among varieties. This information is useful for selecting a more limited group of highly divergent landraces to be preserved and exploited. Under this perspective, we identified the most discriminating categories of the qualitative traits. Interestingly, the phenotypes that mostly contribute to the discrimination of the analyzed apricot germplasm were related to the color attributes of the fruits (the yellow-green and the light-orange skin ground color; the cream and the dark orange color of the flesh; the dark orange of the flesh). This indicates that these visual characteristics are probably main drivers of the variation of the landraces at a regional scale. Therefore, our work has two main implications. Firstly, it is likely that farmers’ practices and consumer perception significantly influence the diversity of agamically propagated cultivated material [51]. Moreover, the abundance of apricot landraces should be related to a conscious selection of easily discernible traits. This may include not only farmers’ maintenance breeding but also the wish to preserve less-common phenotypes [52]. Before the advent of contemporary cultivars, none of the local varieties predominated at a regional level. Since distinctiveness is at the core of both conservation selection and the adoption of new types, the strong influence of specific visual phenotypes on the multivariate classification of the germplasm suggests that farmers selected a high number of varieties to deliberately diversify fruit production. Consequently, the large number of small farms, typical of the traditional apricot sector of the Campania region, was almost certainly essential to develop and maintain a large apricot diversity in situ [53].
5. Conclusions
Our work provides insights into the variability of traditional apricot varieties based on morphological, pomological, and fruit-quality traits. Variability was present for all the traits under investigation and therefore, they all contribute to the observed ample diversity of the landraces. Our work also allowed us to underline that, among the analyzed traits, some specific color features of the fruits can be considered key elements that drove the differentiation at a local scale of cultivated apricot genetic resources.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d14080608/s1, Figure S1: Contributions of the traits to the Dim. 1, Figure S2: Contributions of the traits to the Dim. 2, Table S1: List of the traditional varieties/landraces under investigation, Table S2: List of the traits under investigation, Table S3: Frequency of the qualitative scored traits in the apricot germplasm and SDI, Table S4: Coefficient of Variation (CV) of the 16 quantitative traits. See Table S2 for the abbreviations.
Author Contributions
Conceptualization, G.C.; methodology, B.B. and G.C.; formal analysis, B.B., A.M. and G.C.; investigation, B.B., A.M. and M.F.; writing—original draft preparation, G.C.; writing—review and editing, B.B. and G.C. All authors have read and agreed to the published version of the manuscript.
Funding
We thank the SALVE project (Salvaguardia della Biodiversità Vegetale della Campania-Regione Campania, PSR 2007–2013, misura 214 az. f2).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data of this study that are not already presented in the article are available on request from the corresponding author upon a data sharing agreement between the parties.
Acknowledgments
We thank the staff of the Agricultural Experimental Facilities of the Department of Agricultural Sciences of the Federico II University of Naples.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ledbetter, C.A. Apricots. In Temperate Fruit Crop Breeding: Germplasm to Genomics; Hancock, J.F., Ed.; Springer: Dordrecht, The Netherlands, 2008; pp. 39–82. [Google Scholar]
- Jashemski, W.F. Ancient Roman gardens in Campania and Tunisia: A comparison of the evidence. J. Gard. Hist. 1996, 16, 231–243. [Google Scholar] [CrossRef]
- Guerriero, R. Albicocco. In Atlante dei Fruttiferi Autoctoni Italiani; Fideghelli, C., Ed.; MIPAF-CREA: Rome, Italy, 2016; Volume 2, pp. 477–552. [Google Scholar]
- Zhebentyayeva, T.; Ledbetter, C.; Burgos, L.; Llácer, G. Apricot. In Fruit Breeding; Springer: New York, NY, USA, 2012; pp. 415–458. [Google Scholar]
- Zeven, A.C. Landraces: A review of definitions and classifications. Euphytica 1998, 104, 127–139. [Google Scholar] [CrossRef]
- Migicovsky, Z.; Warschefsky, E.; Klein, L.L.; Miller, A.J. Using living germplasm collections to characterize, improve, and conserve woody perennials. Crop Sci. 2019, 59, 2365–2380. [Google Scholar]
- Furman, B.; Noorani, A.; Mba, C. On-Farm Crop Diversity for Advancing Food Security and Nutrition. In Landraces-Traditional Variety and Natural Breed; IntechOpen: London, UK, 2021. [Google Scholar]
- Corrado, G.; Rao, R. Towards the genomic basis of local adaptation in landraces. Diversity 2017, 9, 51. [Google Scholar] [CrossRef] [Green Version]
- Van Wallendael, A.; Soltani, A.; Emery, N.C.; Peixoto, M.M.; Olsen, J.; Lowry, D.B. A molecular view of plant local adaptation: Incorporating stress-response networks. Annu. Rev. Plant Biol. 2019, 70, 559–583. [Google Scholar]
- Krška, B. Genetic apricot resources and their utilisation in breeding. In Breeding and Health Benefits of Fruit and Nut Crops; IntechOpen: London, UK, 2018. [Google Scholar]
- Fratianni, F.; Cozzolino, R.; d’Acierno, A.; Ombra, M.N.; Spigno, P.; Riccardi, R.; Malorni, L.; Stocchero, M.; Nazzaro, F. Biochemical Characterization of Some Varieties of Apricot Present in the Vesuvius Area, Southern Italy. Front. Nutr. 2022, 9, 4868. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, C.; Basile, B.; Hernandez, G.; Pannico, A.; Giaccone, M.; Forlani, M. Influence of fruiting shoot on flowering pattern and fruit quality of Vesuvian apricot cultivars. Acta Hortic. 2010, 862, 557–564. [Google Scholar] [CrossRef]
- Li, H.; Rasheed, A.; Hickey, L.T.; He, Z. Fast-forwarding genetic gain. Trends Plant Sci. 2018, 23, 184–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jarvis, D.I.; Brown, A.H.; Cuong, P.H.; Collado-Panduro, L.; Latournerie-Moreno, L.; Gyawali, S.; Tanto, T.; Sawadogo, M.; Mar, I.; Sadiki, M. A global perspective of the richness and evenness of traditional crop-variety diversity maintained by farming communities. Proc. Natl. Acad. Sci.USA 2008, 105, 5326–5331. [Google Scholar]
- Loko, L.E.Y.; Toffa, J.; Adjatin, A.; Akpo, A.J.; Orobiyi, A.; Dansi, A. Folk taxonomy and traditional uses of common bean (Phaseolus vulgaris L.) landraces by the sociolinguistic groups in the central region of the Republic of Benin. J. Ethnobiol. Ethnomedicine 2018, 14, 1–15. [Google Scholar] [CrossRef]
- Martínez Mora, C.; Rodríguez, J.; Cenis, J.L.; Ruiz García, L. Genetic variability among local apricots (Prunus armeniaca L.) from the Southeast of Spain. Span. J. Agric. Res. 2009, 7, 855–868. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Z.; Huang, H. SSR fingerprinting Chinese peach cultivars and landraces (Prunus persica) and analysis of their genetic relationships. Sci. Hortic. 2009, 120, 188–193. [Google Scholar] [CrossRef]
- Bourguiba, H.; Khadari, B.; Krichen, L.; Trifi-Farah, N.; Mamouni, A.; Trabelsi, S.; Audergon, J.-M. Genetic relationships between local North African apricot (Prunus armeniaca L.) germplasm and recently introduced varieties. Sci. Hortic. 2013, 152, 61–69. [Google Scholar] [CrossRef]
- Rao, R.; Bencivenni, M.; Mura, L.; Araujo-Burgos, T.; Corrado, G. Molecular characterisation of Vesuvian apricot cultivars: Implications for the certification and authentication of protected plant material. J. Hortic. Sci. Biotechnol. 2010, 85, 42–47. [Google Scholar] [CrossRef]
- Sheikh, Z.N.; Sharma, V.; Shah, R.A.; Raina, S.; Aljabri, M.; Mir, J.I.; AlKenani, N.; Hakeem, K.R. Elucidating Genetic Diversity in Apricot (Prunus armeniaca L.) Cultivated in the North-Western Himalayan Provinces of India Using SSR Markers. Plants 2021, 10, 2668. [Google Scholar] [CrossRef]
- Herrera, S.; Hormaza, J.I.; Lora, J.; Ylla, G.; Rodrigo, J. Molecular Characterization of Genetic Diversity in Apricot Cultivars: Current Situation and Future Perspectives. Agronomy 2021, 11, 1714. [Google Scholar]
- Hagen, L.; Khadari, B.; Lambert, P.; Audergon, J.-M. Genetic diversity in apricot revealed by AFLP markers: Species and cultivar comparisons. Theor. Appl. Genet. 2002, 105, 298–305. [Google Scholar] [CrossRef]
- Sanzol, J.; Herrero, M. The “effective pollination period” in fruit trees. Sci. Hortic. 2001, 90, 1–17. [Google Scholar] [CrossRef]
- Lachkar, A.; Chaar, H.; Mars, M. Reproductive behavior of Tunisian apricot cultivars as related to floral morphometric characteristics and pollen quality. Int. J. Fruit Sci. 2014, 14, 188–204. [Google Scholar] [CrossRef]
- Basile, B.; Cirillo, C.; Santin, A.; Forlani, M. Fruit quality of Vesuvian apricots harvested at different ripening stages after a cold-storage period. Acta Hortic. 2005, 682, 1443–1450. [Google Scholar] [CrossRef]
- Crisosto, C.; Crisosto, G.; Neri, F. Understanding tree fruit quality based on consumer acceptance. In Proceedings of the IV International Conference on Managing Quality in Chains-The Integrated View on Fruits and Vegetables Quality 712, Bangkok, Thailand, 26–30 June 2006; pp. 183–190. [Google Scholar]
- Gurrieri, F.; Audergon, J.-M.; Albagnac, G.; Reich, M. Soluble sugars and carboxylic acids in ripe apricot fruit as parameters for distinguishing different cultivars. Euphytica 2001, 117, 183–189. [Google Scholar] [CrossRef]
- Dray, S.; Dufour, A.-B. The ade4 package: Implementing the duality diagram for ecologists. J. Stat. Softw. 2007, 22, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Baddeley, A.; Turner, R. Spatstat: An R package for analyzing spatial point patterns. J. Stat. Softw. 2005, 12, 1–42. [Google Scholar] [CrossRef] [Green Version]
- Wei, T.; Simko, V.; Levy, M.; Xie, Y.; Jin, Y.; Zemla, J. Package ‘corrplot’. Statistician 2017, 56, 24. [Google Scholar]
- Dixon, P. VEGAN, a package of R functions for community ecology. J. Veg. Sci. 2003, 14, 927–930. [Google Scholar] [CrossRef]
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R package for multivariate analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Kassambara, A. Practical Guide to Principal Component Methods in R: PCA, M (CA), FAMD, MFA, HCPC, Factoextra; Sthda: Montpellier, France, 2017; Volume 2. [Google Scholar]
- Engels, J. A Guide to Effective Management of Germplasm Collections; Bioversity International: Rome, Italy, 2003. [Google Scholar]
- Pennone, F. Costituzione di cultivar di albicocco idonee alla trasformazione industriale. Agric. Ric. 1991, 119, 79–80. [Google Scholar]
- Pugliano, G.; Cirillo, A. Cultivar di albicocche vesuviane che meglio si prestano alla produzione di sciroppati. Inf. Agrar. 1975, 31, 20055–20057. [Google Scholar]
- Corrado, G.; Forlani, M.; Rao, R.; Basile, B. Diversity and Relationships among Neglected Apricot (Prunus armeniaca L.) Landraces Using Morphological Traits and SSR Markers: Implications for Agro-Biodiversity Conservation. Plants 2021, 10, 1341. [Google Scholar] [CrossRef]
- Wani, A.A.; Zargar, S.A.; Malik, A.H.; Kashtwari, M.; Nazir, M.; Khuroo, A.A.; Ahmad, F.; Dar, T.A. Assessment of variability in morphological characters of apricot germplasm of Kashmir, India. Sci. Hortic. 2017, 225, 630–637. [Google Scholar] [CrossRef]
- Salas-Salvadó, J.; Casas-Agustench, P.; Salas-Huetos, A. Cultural and historical aspects of Mediterranean nuts with emphasis on their attributed healthy and nutritional properties. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 1–6. [Google Scholar] [CrossRef]
- Corrado, G.; La Mura, M.; Ambrosino, O.; Pugliano, G.; Varricchio, P.; Rao, R. Relationships of Campanian olive cultivars: Comparative analysis of molecular and phenotypic data. Genome 2009, 52, 692–700. [Google Scholar] [CrossRef] [Green Version]
- Pop, I.F.; Vicol, A.C.; Botu, M.; Raica, P.A.; Vahdati, K.; Pamfil, D. Relationships of walnut cultivars in a germplasm collection: Comparative analysis of phenotypic and molecular data. Sci. Hortic. 2013, 153, 124–135. [Google Scholar] [CrossRef]
- Manco, R.; Basile, B.; Capuozzo, C.; Scognamiglio, P.; Forlani, M.; Rao, R.; Corrado, G. Molecular and Phenotypic Diversity of Traditional European Plum (Prunus domestica L.) Germplasm of Southern Italy. Sustainability 2019, 11, 4112. [Google Scholar] [CrossRef] [Green Version]
- Hormaza, J.; Yamane, H.; Rodrigo, J. Apricot. In Fruits and Nuts; Springer: New York, NY, USA, 2007; pp. 171–187. [Google Scholar]
- Mencarelli, F.; Bellincontro, A.; Forniti, R.; Vizovitis, K.; Botondi, R.; Valentini, M.; Sequi, P.; DiNatale, C.; Basile, B.; Romano, R. Factors affecting the apricot quality for the consumer with special attention to the use of 1-MCP and of NDT for detection of bruising. Acta Hortcult. 2006, 717, 315–320. [Google Scholar] [CrossRef]
- Cirillo, C.; Pannico, A.; Basile, B.; Rivera, C.; Giaccone, M.; Colla, G.; De Pascale, S.; Rouphael, Y. A simple and accurate allometric model to predict single leaf area of twenty-one European apricot cultivars. Eur. J. Hortic. Sci 2017, 82, 65–71. [Google Scholar] [CrossRef]
- Ruiz, D.; Egea, J. Phenotypic diversity and relationships of fruit quality traits in apricot (Prunus armeniaca L.) germplasm. Euphytica 2008, 163, 143–158. [Google Scholar] [CrossRef]
- Bae, H.; Yun, S.K.; Yoon, I.K.; Nam, E.Y.; Kwon, J.H.; Jun, J.H. Assessment of organic acid and sugar composition in apricot, plumcot, plum, and peach during fruit development. J. Appl. Bot. Food Qual. 2014, 87, 24–29. [Google Scholar]
- Zhang, C.; Xie, D.; Bai, T.; Luo, X.; Zhang, F.; Ni, Z.; Chen, Y. Diversity of a large collection of natural populations of mango (Mangifera indica Linn.) revealed by agro-morphological and quality traits. Diversity 2020, 12, 27. [Google Scholar] [CrossRef] [Green Version]
- Krima, S.B.; Slim, A.; Gélisse, S.; Kouki, H.; Nadaud, I.; Sourdille, P.; Yahyaoui, A.; M’barek, S.B.; Suffert, F.; Marcel, T.C. Life story of Tunisian durum wheat landraces revealed by their genetic and phenotypic diversity. bioRxiv 2020. [Google Scholar] [CrossRef]
- Dwivedi, S.; Goldman, I.; Ortiz, R. Pursuing the potential of heirloom cultivars to improve adaptation, nutritional, and culinary features of food crops. Agronomy 2019, 9, 441. [Google Scholar] [CrossRef] [Green Version]
- Testa, R.; Migliore, G.; Schifani, G.; Tinebra, I.; Farina, V. Chemical–physical, sensory analyses and consumers’ quality perception of local vs. imported Loquat fruits: A sustainable development perspective. Agronomy 2020, 10, 870. [Google Scholar] [CrossRef]
- Zeven, A.C. Traditional maintenance breeding of landraces: 2. Practical and theoretical considerations on maintenance of variation of landraces by farmers and gardeners. Euphytica 2002, 123, 147–158. [Google Scholar] [CrossRef]
- Altieri, M.A.; Merrick, L. In situ conservation of crop genetic resources through maintenance of traditional farming systems. Econ. Bot. 1987, 41, 86–96. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).