Abstract
The aquatic microfauna of Africa is poorly characterized, especially in the case of planktonic rotifers inhabiting waterbodies other than large lakes. In addition, little is known about factors that structure these communities. Here, we assessed the roles of climatic region, habitat type, macrophyte abundance, and a suite of abiotic environmental factors in determining rotifer species’ richness and composition in waterbodies located across a 2300 m altitudinal gradient in Kenya. Plankton samples were obtained from 33 sites in 23 waterbodies. From these, 93 rotifer taxa were identified from 18 families comprising 31 genera. About one fourth (25 taxa) were new records for Kenya, from which 4 species were new for Africa. Species richness was the highest in permanent as compared to temporary habitats. Richness was strongly positively correlated with all environmental factors and strongly influenced by macrophyte abundance. When spatial structure was added to the GLM model, species richness was no longer significantly correlated with macrophytes. Unconstrained detrended correspondence analysis conducted at the species level indicated four suites of species associated with either (1) longitude, (2) elevation, (3) latitude, temperature, and hydroperiod, or (4) macrophytes. This study contributes to our knowledge of the patterns of rotifer biogeography and species richness in Africa.
1. Introduction
Understanding species distributions and biogeographic patterns are important for interpreting ecological and evolutionary processes, as well as providing baseline data for potential impacts of climate change [1,2,3,4]. Of freshwater habitats, the Palearctic and Nearctic biogeographic realms have received the most attention. In contrast, important components of zooplankton such as rotifers are understudied in the Afrotropical zone. The most recent checklist of non-marine rotifers from African inland waters comprises 321 taxa, including 41 taxa found in Kenya, while the Afrotropical zone is characterized by 591 species in 85 genera [5]. Of all biogeographical realms, this is the lowest species richness recorded, despite the abundance and variety of habitats appropriate for rotifers. In comparison the Palearctic, the best-known and most-studied region, has a reported 1348 species representing 112 genera. Thus, as currently understood, the Afrotropical zone has less than half the rotifer diversity of the Palearctic zone. This disproportional situation is likely partially attributable to the “rotiferologist” effect [6], i.e., the distribution of rotifers seems to reflect more the distribution of rotifer scientists than that of rotifers themselves [7,8,9]. In addition, tropical Africa is notable for its relatively few endemic rotifer species [10]; however, Luo and Segers [11] recently described eight new species of Lepadellidae from the Congo basin. Not only are African freshwater habitats understudied, there also is little information on how abiotic and biotic factors influence community composition.
The known rotifer fauna of Kenya stems from surveys of large lakes of the Eastern Rift Valley conducted during the mid to late 1900s [5,12,13,14,15,16,17,18,19,20]. More recently, a few additional studies have taken place, but again these mainly focused on the plankton of large lakes including Lake Victoria, Lake Nakuru, and Lake Baringo [21,22,23,24]. In these studies, rotifer species richness varied from 13 to 42, with most large lakes being dominated by the genus Brachionus. The potential importance of including small waterbodies in studies of biogeography and in assessing species diversity in African aquatic ecosystems is illustrated in study of waterbodies in the Lake Victoria basin by Mutune et al. [21], where 63 rotifer taxa were identified. Of these taxa, 32 were found in samples from Lake Victoria, and 56 were found in smaller waterbodies.
Despite their ubiquity and ecological significance, shallow waterbodies are typically overlooked in limnological research. Recently, interest in small temporary waters including rock pools [25,26], freshwater puddles [27,28], and anthropogenic basins [29] has grown. Collectively they may support considerably more species than permanent waterbodies, because they often constitute biodiversity hotspots within a region and have endemic species inhabiting them (e.g., [30,31,32,33,34]). Except for a few specialist groups, many taxa inhabiting temporary waters appear to be habitat generalists that opportunistically exploit these waterbodies. Colonists from nearby large, permanent waterbodies often re-supply smaller basins that dry [35,36,37] through passive dispersal [38] or periodical hydrological connections. Small waterbodies are also often more sensitive to human impacts than larger ones, as they can easily be drained to provide space for agriculture or urban development [39]. Such waterbodies are widely distributed across the African continent and are commonly used for providing water supplies to rural communities, irrigation, as well as serving as watering stations for domestic animals [40].
There are some studies of the biota of temporary habitats in Africa, including investigations of ephemeral rock pools (Botswana: [33,41], South Africa: [41]), shallow waters (Ivory Coast: [42], Nigeria: [43,44,45]) and temporary ponds (e.g., Senegal: [46], Kenya: [47], South Africa: [48,49], Tunisia: [50]). However, these studies typically focused on crustaceans. Recently, Bird et al. [51] reviewed the faunistic composition of southern African shallow waters, including several studies that include some records of rotifers (e.g., [48,52]). While these studies and those on large lakes have increased our knowledge of the biogeography of rotifers, there are few investigations focused on rotifer community composition and none investigating the determinants of their community structure in African temporary waters.
Rotifers are found in a wide variety of freshwater habitats from large permanent lakes to damp mosses and are well adapted to life in temporary habitats [53]. They are known as opportunistic species that can persist under extreme conditions, mainly because of their high tolerance to changes in environmental conditions, high reproductive rates, and ability to produce diapausing stages [54,55]. Diapause, when combined with their ability to adapt to short-term environmental variability [56,57], allows rotifers to be successful in highly dynamic ephemeral ecosystems. Compared with other zooplankton rotifers have shorter generation times and faster population growth rates; however, they are regarded as relatively vulnerable to biotic interactions (i.e., predation [57,58], competition [59,60]). Thus, both abiotic and biotic conditions can be important determinants of rotifer community composition and structure (e.g., [58,59,60,61,62]).
The most common abiotic environmental factors correlated with rotifer richness and abundance in large waterbodies are temperature [63,64], dissolved oxygen [65,66], conductivity [67,68], and pH [69,70]. In temporary systems, species richness is often correlated with hydroperiod (reviewed by Walsh et al. [34]). Hydroperiod duration can be a critical factor for diversity in temporary waters as it determines the ability of organisms to complete their life cycles and/or the strength of biotic interactions [71]. However, Sahuquillo and Miracle [72] found that rotifers were more diverse and more abundant in a drier year with a short, interrupted hydroperiod than in a wetter year. Biotic factors strongly affect the physical structure of habitats [73,74,75] and species’ occurrences [76]. These factors can be particularly important in waterbodies that are small and shallow [77]. For instance, habitats that contain littoral vegetation generally have higher rotifer richness (e.g., [78,79,80]). High diversity in macrophyte zones has been attributed to elevated spatial heterogeneity [81] and food availability [82] as well as providing a stable environment [83]. Aquatic vegetation may also provide a refuge from predation, which can be a major factor influencing rotifer diversity and abundance [84,85]. Nevertheless, no general consensus has emerged regarding when a factor or combination of factors is most important in determining rotifer community structure.
The objective of our study was to provide additional insights into rotifer community composition in a diverse variety of waterbodies in Kenya by investigating how selected geographic and habitat characteristics are correlated with rotifer species richness. We accomplished this by conducting an extensive survey of waterbodies, intentionally focused on less studied waters such as small ponds (including man-made reservoirs) and temporary pools. We predicted that the highest rotifer species richness would be found in large, permanent lakes where habitat heterogeneity is the greatest. We also hypothesized that rotifer assemblages are more affected by biotic factors (e.g., presence or absence of aquatic vegetation) than by biogeographic (e.g., climatic region, elevation) or abiotic conditions (e.g., temperature, salinity) in permanent habitats, while hydroperiod would dominate in temporary systems.
2. Materials and Methods
2.1. Study Area
We collected 112 samples from 33 sites in 23 waterbodies representing a wide spectrum of aquatic habitat types in over a 2300 m altitudinal range (from 45 m a.s.l. in the Arabuko Sokoke Forest to >2375 m a.s.l. at Kibindo reservoir located in the Nyahururu district) in north, central southwest, and southern coast of Kenya during January–February 2014 and February 2015. The sites (Figure 1) were located in diverse climate regions (based on the Köppen-Geiger climate classification [86]; see Table 1). In addition to five large waterbodies (Lake Turkana, Lake Ol’Bolossat, Lake Naivasha, Lake Oloiden, and Kibindo reservoir), we sampled mostly temporary ponds including shallow puddles, rock pools, streams, oases, and man-made systems.
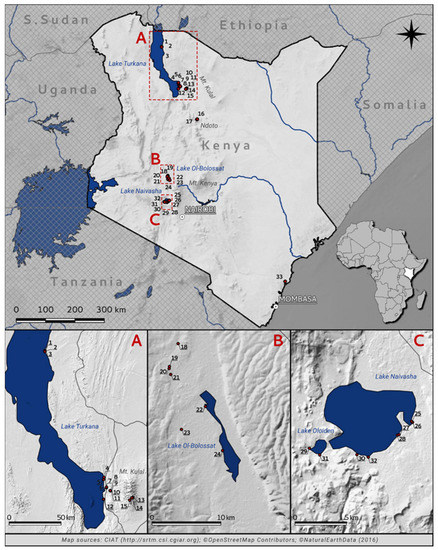
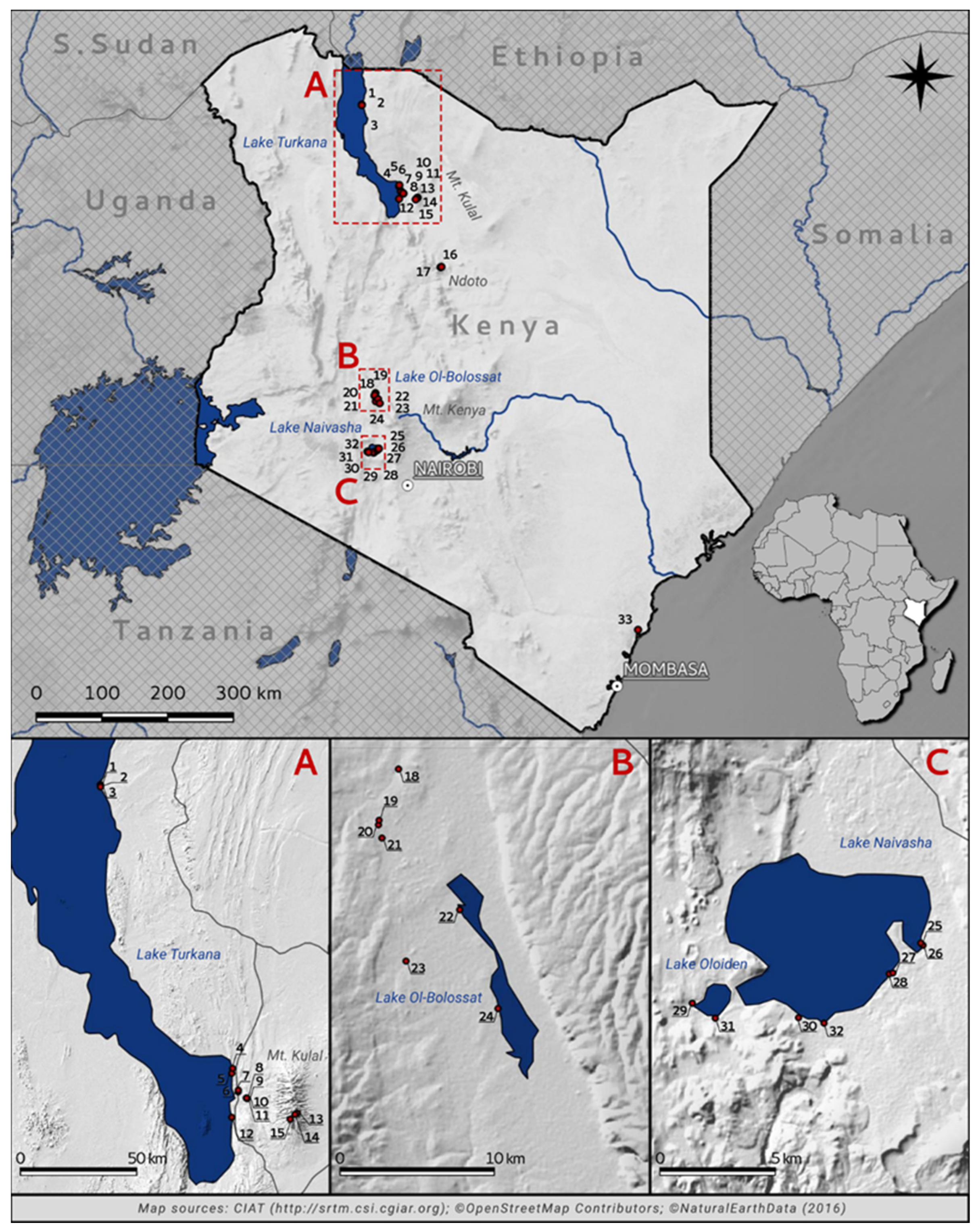
Figure 1.
Location of the studied waterbodies in Kenya, with a close-up of four large lakes ((A) Lake Turkana, (B) Lake Ol’Bolossat, (C) Lake Naivasha and Lake Oloiden) (source data available in Jarvis et al. [87]). For a key to numbered sites, see Table 1.

Table 1.
List of the 33 sampled sites from 23 Kenyan waterbodies and their main environmental features. Sites ordered as in map (Figure 1). Temp = temperature; Sal. = salinity; artif. = artificial; d. = downstream; t. Mediterranean = temperate Mediterranean.
Due to the large differences in the surface areas of the studied waterbodies, we classified them in two groups according to size. The five large lakes (Lake Turkana, Lake Ol’Bolossat, Lake Naivasha, Lake Oloiden, and Kibindo reservoir) were designated as large waterbodies. The surface area of the five large lakes ranged from ~6000 m2 (Kibindo reservoir) to ~7560 km2 (Lake Turkana) [88]. All others, mostly temporary ponds, were assigned to the category small waterbodies. Samples were taken from the 11 permanent sites including the five large lakes, a group of four interconnected basins at Loiyangalani oasis (pools H1-H4 [See Table 1], all connected during the rainy season), a permanent oasis at the Molo camp in Loiyangalani village, and Amina fishpond in the Nyahururu district. Permanent waterbodies had maximum depths between 0.4–30 m (mean ± standard error [SE]: 6.14 m ± 3.72) and surface area ranged from 3.15 m2 (Molo camp oasis) to ~7560 km2 (Lake Turkana). The second category included 12 temporary habitats. (1) A large clay-lined puddle located in Loiyangalani village near Lake Turkana. This site was situated in the warm desert climatic region. (2–4) Three additional temporary puddles (Nanta Mesi, Lelekan, and Lolkujita) were sampled on Mt. Kulal. These three sites have an elevational gradient from 1470 to 2149 m a.s.l., but all have a humid subtropical climate. (5–6) Three isolated rockpools in the upper part of Darawell stream that formed during the rainy season in the bed of the perennial stream in the Ndoto Mountains, ~100 km south of Mt. Kulal. (7) A small, isolated water basin connected to temporary stream (Bridge Hill). (8–9) A two temporary water channels designated as Ahiti farm and an artificial concrete cattle tank in the Nyahururu district. (10) An artificial concrete circular pond (diam. ~3 m; depth: 0.4 m) in the Arabuko Sokoke Forest; this site had the lowest elevation (45 m a.s.l.). (11) Lake Turkana Koobi Fora Pond (~50 m2; depth: 0.45 m) and (12) a metal bucket near Lake Naivasha (0.12 m2; depth: 0.6 m). Temporary ponds had maximum depths ranging from 0.1 to 1.2 m (0.38 ± 0.11 m) and surface areas from 0.12 m2 (Lake Naivasha—metal bucket) to 60 m2 (Loiyangalani village—clay puddle). Although Lake Turkana Koobi Fora Pond and the metal bucket near Lake Naivasha are temporary habitats due to their small water volume and high potential to dry, their rotifer species compositions are likely derived from the adjacent lake. Lake Turkana Koobi Fora Pond was separated from the main lake by a 5 m wide sandy mound created after water level of the lake decreased. Water in the metal bucket was taken from Lake Naivasha and left for an unknown period of time.
2.2. Sampling
Qualitative, open water samples were collected using a plankton net (25 cm diameter; 25 µm mesh) attached to 0.5 m long handle from the shoreline. Samples and water chemistry measurements were taken from the surface (10–30 cm depth). From the temporary ponds and waterbodies with poorly developed or no littoral vegetation, samples were taken only from the water column near the shore. In the permanent large lakes, samples consisted of 10 plankton tows. Each tow consisted of a smooth pulling motion for 30 s. In the small temporary ponds where plankton tows could not be performed, the minimum volume of sampled water was 4 L. All samples were fixed immediately in 96% ethanol. Presence of rotifer taxa in these samples was recorded. Electrical conductivity, pH, temperature, dissolved oxygen, and salinity were measured in situ using a Multi 3401i WTW probe. We determined depth, surface area, macrophyte cover, GPS coordinates, elevation, climatic region, hydroperiod, and categorized sites by habitat type (Table 1). When measurements were not possible, depth and surface area were retrieved from published works (e.g., [17,89,90]). Due to the substantial differences in surface area among sites, for statistical evaluation they were placed into 5 categories (1: <5; 2: 5–20; 3: 21–100; 4: 101–1000; 5: >1001 m2). Macrophyte percent cover was estimated by visual inspection at each sampling site. Macrophyte cover of Lake Naivasha differed among sampling sites from extensive (>50%), to very sparse (<5%), or littoral vegetation absent [17,90,91]. Preserved specimens were identified to the lowest possible taxonomic level using the Guides to the Identification of the Microinvertebrates of the Continental Waters of the World [89,90,91,92,93] and other appropriate keys [94,95]. As the samples were immediately preserved, this precluded identification to species level in several cases (e.g., bdelloids and other species whose identification rely on features seen only in live specimens). The taxonomic validity of each taxon follows the requirements established by the International Code of Zoological Nomenclature: i.e., the List of Available Names in Zoology, Candidate Part Phylum Rotifera (LAN) [96].
2.3. QB/T and QB/L Quotients
For a comparison of trophic status among the habitat types surveyed in our study, we calculated Sládeček’s [97] trophic condition quotient, QB/T, where B is the number of Brachionus species and T is the number of Trichocerca species. Brachionus are generally associated with eutrophic waters, while members the genus Trichocerca are found primarily in oligotrophic habitats. Thus, the higher the index, the more eutrophic the system. Similarly, Brachionus is typically considered a planktonic or semi-planktonic species found in open waters in the pelagic and littoral zones of waterbodies, while members of the genus Lecane are closely associated with substrata such as submerged macrophytes or terrestrial plants [89]. As many of our systems were dominated by Brachionus, we also constructed a QB/L quotient (QB/L = #Brachionus spp./#Lecane spp.) to further categorize the sampled waterbodies. For this index, values of <1.0 represents lakes, ponds, or wetlands with a well-developed littoral zone with abundant macrophytes, values between 1.0 and 2.0 represent those with poorly developed littoral vegetation, and >2.0 represent waterbodies without a littoral zone (typically temporary puddles, rock pools, or man-made small waterbodies).
2.4. Statistical Analyses
For statistical analyses, sites were categorized by climatic region and habitat type. Four climatic regions were represented: tropical savanna (code = 1), warm desert (2), humid subtropical (3), and temperate Mediterranean (4; Table 1). Sites were also assigned a habitat type from: large lake (1), oasis (2), stream basin (3), puddle (4), rock pool (5), and anthropogenic habitats (6; e.g., metal bucket, waterhole).
To analyze species richness, we first did pairwise correlations to which environmental factors were correlated with species richness. We then used generalized linear model (GLM) forward and backward selection to determine which environmental parameters were associated with richness (S). Log (S) was used for all analysis and models were chosen based on AIC values. Further, because we are using geospatial data, we wanted to account for spatial autocorrelation in our statistical models. Thus, we used two methods: generalized least squares (GLS) with Gaussian spatial correlation to remove spatial effects and, as an alternate method to investigate the spatial effects, we used a Moran eigenvector spatial filtering approach. The second approach allows for spatial dependence present in the residuals to be included into the model. For climate and habitat types, we investigated whether there were significant differences among categories based on Tukey-adjusted p values. These analyses were conducted using R version 4.2.1 in the nlMS package.
To assess the similarities in the species composition among these waterbodies, we used a cluster analysis based on the Jaccard index and the unweighted pair group method with the arithmetic mean (UPGMA) algorithm. For hierarchical cluster analysis, we included 12 locations: six with the highest species richness from permanent sites (Lake Turkana, Lake Ol’Bolossat, Lake Naivasha, Lake Oloiden, Loiyangalani oasis, and Nyahururu Amina) and six temporary ponds (Loiyangalani clay puddle [Loiya CP]; Lelekan, Lolkujita, Nanta Mesi, Ndoto Mts.—Darawell rock pools [Darawell RP]; and Arabuko Sokoke Forest [Arabuko SF]). Some individual waterbodies included several sampling sites, so they were combined for this analysis to provide a comparison of waterbodies as opposed to sites. Further, sites with poor diversity (i.e., species richness of 1; sites: #6, 18, 19, 20) were not included. Box plots were computed to investigate patterns among species richness and hydroperiod classes (permanent versus temporary). We used an unconstrained detrended correspondence analysis (DCA) with supplementary variables to determine relationships of species occurrences with environmental factors using R version 4.1.2 [98]. This method was selected because response data were compositional, and the gradient is one dimensional. As many preserved specimens could not be identified to species, we conducted two analyses: one at the species level (subspecies were subsumed) and a second at the genus level. Unidentified bdelloids were not included in the species level analysis. We used emmeans to test for differences in richness among habitat types.
3. Results
3.1. QB/T and QB/L Quotients
Using trophic indices, we found that the QB/T value was over 2.0 for both permanent and temporary habitat types, and thus the waterbodies we sampled were deemed as eutrophic. For permanent waterbodies, which mostly had abundant littoral vegetation, QB/L was 0.92, while in temporary waterbodies, where littoral vegetation was generally reduced or lacking, it was 2.0.
3.2. Water Chemistry Parameters
During the survey, water temperature ranged from 16.6° C (Mt. Kulal—Lelekan, Table 1, #14) to 32.2° C (Loiyangalani—Oasis H4, #11), pH ranged from 6.68 (Darawell upstream site, #16) to 9.46 (Lake Turkana—Koobi Fora pond, #2), and electrical conductivity from 88 μ Scm−1 (Kibindo reservoir, #23) to 6550 μ Scm−1 (Lake Turkana—Koobi Fora pond, #2). Dissolved oxygen ranged from 0.06 mg L−1 (0.9%) (Nyahururu—watering hole, #20) to 6.84 mg L−1 (80%) (Loiyangalani—Oasis H2, #9).
3.3. Species Richness
In the 112 samples, we found 18 rotifer families (1 bdelloid, 17 monogonont) and four orders (Collothecaceae, Flosculariacea, Philodinida, and Ploima) comprising 31 genera and 93 taxa (74 were identified to species or subspecies, the remaining were identified to genus). Of these, 25 species were new reports for Kenya and four species (Dipleuchlanis elegans, Cephalodella forficula, Cephalodella tenuiseta, and Lecane elsa) were also new reports for Africa (Table 2). The most widespread taxa were unidentified bdelloids that were found in 16 of all 23 waterbodies and in all hydroperiod classes, followed by Lecane bulla (15 waterbodies) and Brachionus calyciflorus species complex (13 waterbodies). In addition, when compared to large habitats (large lakes) all other habitat types had significantly lower species richness, with the exception of the smallest habitats (anthropogenic watering holes).

Table 2.
Rotifer taxa found in aquatic habitats surveyed in Kenya 2014–2015.
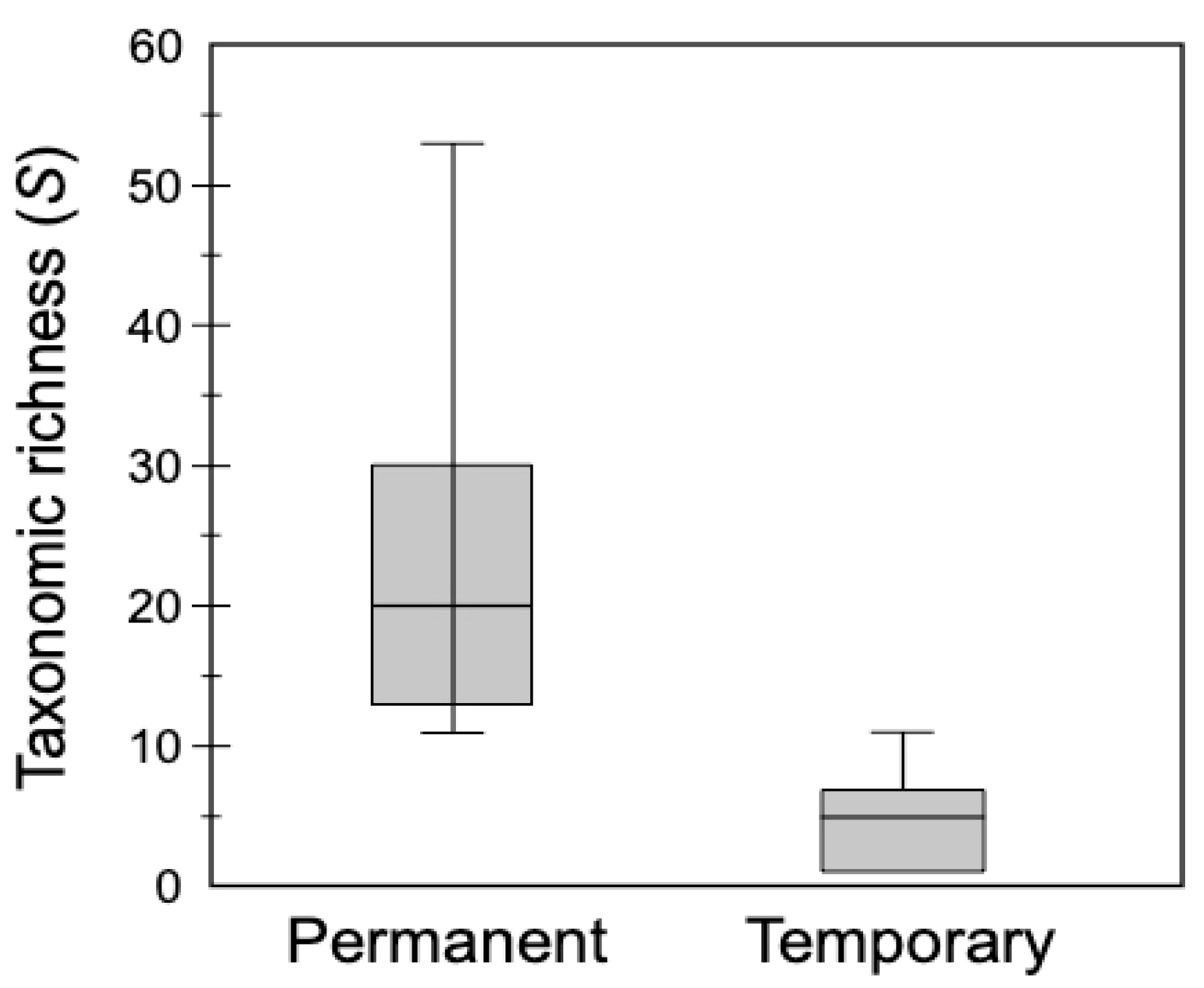
Box plots of species richness (S) in permanent (S = 88; mean = 23.6, SE: ±6.0) versus temporary (S = 26; mean = 5.1, SE: ±1.5) waterbodies indicated that the highest richness occurred in permanent habitats (Figure 2). The most frequent taxon in permanent waterbodies was L. bulla, which occurred in all habitats; this was followed by unidentified bdelloids (75% of habitats). The most frequently found genus was Brachionus. From brachionids, B. calyciflorus, a thermophilic species and bioindicator of eutrophication, had the highest occurence (87.5%). Brachionus angularis, Brachionus caudatus, Brachionus plicatilis, Brachionus quadridentatus, and Keratella tropica were all found in 62.5% of the permanent habitats. The most frequently occurring taxa found in temporary habitats were unidentified bdelloids (57.1%), followed by L. bulla (42.9%) and B. angularis, B. caudatus, Brachionus bidentatus, and Euchlanis dilatata (28.6%).
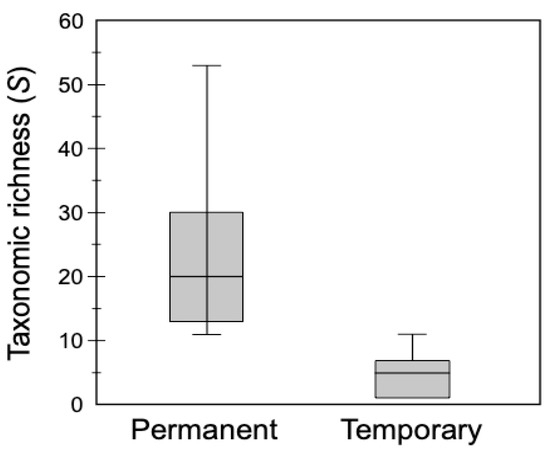
Figure 2.
Box plots comparing rotifer taxonomic richness between permanent and temporary waterbodies surveyed in Kenya in 2014 and 2015. Horizontal lines are the median, boxes represent the interquartile range (25–75%), and whiskers represent minimum and maximum values.
Lecane bulla was found in all five sampled large lakes (Table 3). Brachionus calyciflorus, Filinia sp., Trichocerca sp., and unidentified bdelloids were found in 60% of large lakes. In small waterbodies, again unidentified bdelloids were most commonly encountered (69.2%). For monogononts, L. bulla was found in 61.5% while B. calyciflorus occurred in 53.9% of them. Two species, B. caudatus and K. tropica, were found only in four of the large lakes, while two species, Lecane papuana and E. dilatata, were found in six and four small waterbodies, respectively, but none of the large lakes.

Table 3.
Rotifer taxa found in large lakes surveyed in Kenya 2014–2015. § see [99].
The highest number of species found at a given locality was 53 in Lake Naivasha (including species found in the nearby metal bucket filled with lake water). Waterbodies of the Loiyangalani Oasis (H1–H4) had 30 species, the Nyahururu Amina fishpond had 25 species, and Lake Turkana (including the adjacent pond) had 20 species. The highest species richness (S = 11) in temporary ponds was found in an artificial concrete pond in Arabuko Sokoke Forest, which serves primarily as watering station for elephants. In the Darawell riverbed rock pools and in Loiyangalani Clay puddle, we found seven species. Several species were found only in permanent waterbodies (e.g., Lecane luna was found in five permanent ponds but no temporary waterbodies; B. quadridentatus, B. caudatus, K. tropica, and Lecane hamata were found in four permanent waterbodies and no temporary ones). Alternatively, some species were found in waterbodies with variable hydroperiods, but were more frequent in permanent waterbodies (e.g., B. calyciflorus [8 permanent; 2 temporary], B. plicatilis [6; 1]). The taxa found only in temporary waterbodies were E. dilatata (0; 3) and Proales sp. (0; 1).
Based on area, we classified the studied habitats into (1) large lakes (Lake Turkana, Lake Ol’Bolossat, Lake Naivasha, Lake Oloiden, and Kibindo reservoir) where rotifer community composition has been studied in the past, and (2) small waterbodies (mostly temporary waters) that have not been previously studied. According to classification, of the 25 newly identified taxa for Kenya, 64% (16 species) were found in small waterbodies. This despite the higher richness typically found in larger waterbodies. Of the four new records for Africa, three (75%) were found in small waterbodies.
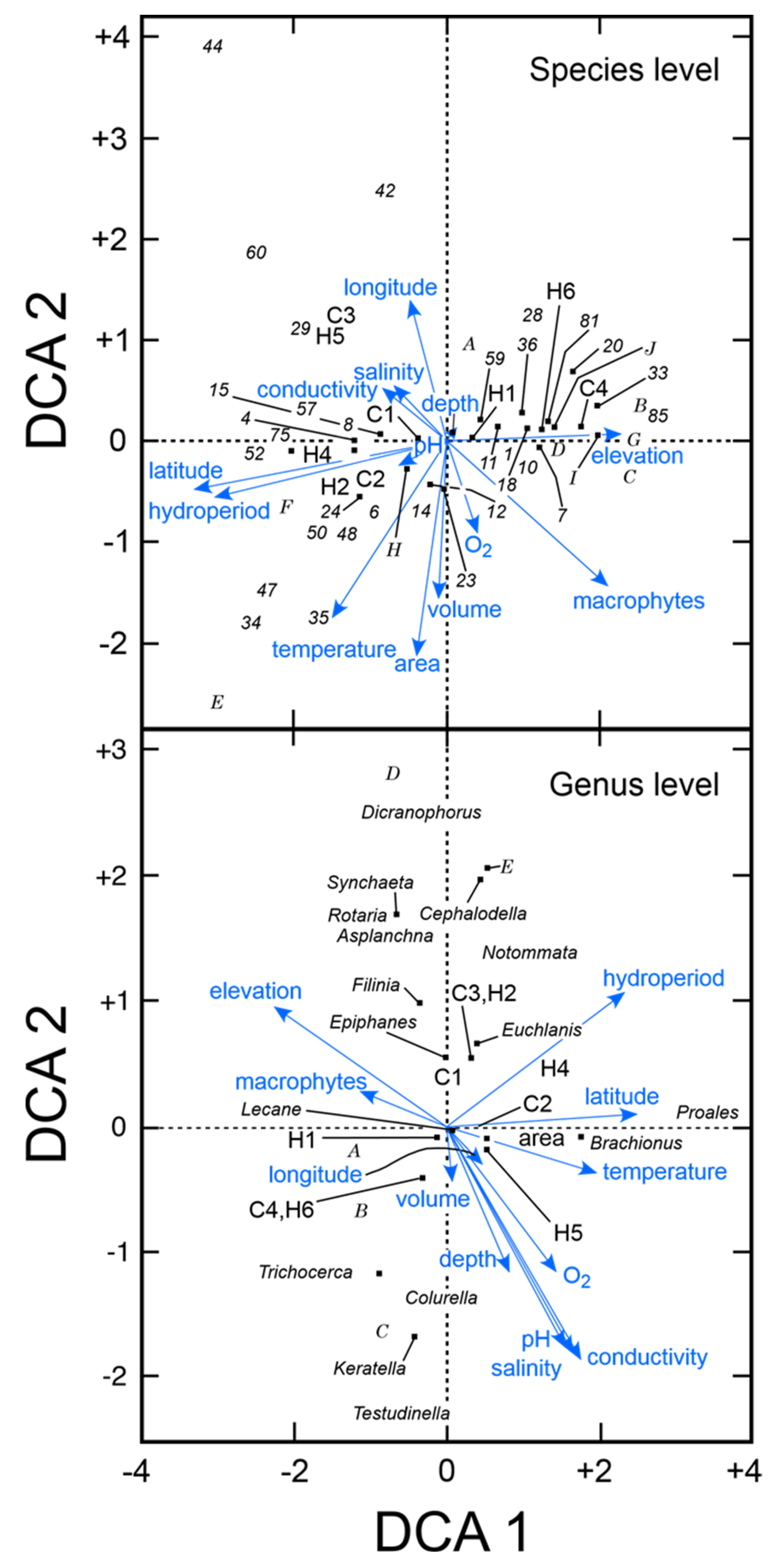
Unconstrained detrended correspondence analysis demonstrated the importance of latitude in determining species composition at both the species and generic level (Figure 3). When the analysis was conducted using the taxonomic dataset at the species level (n = 72) with 8 environmental variables including 21 sites, elevation, macrophytes, oxygen concentration, large lake, and anthropogenic habitats were positively associated with Axis 1. In this analysis, >30 species, including littoral species Trichocerca cylindrica, Testudinella parva semiparva, Cephalodella gibba, and sessile Floscularia ringens and Limnias sp., were positively associated with the presence of macrophytes along Axis 1 (Figure 3, upper panel). The geographic features of elevation and climatic region 4 (temperate Mediterranean) were also associated with Axis 1. When the analysis was done on the taxonomic dataset collapsed to genus level (n = 31), hydroperiod, latitude, temperature, conductivity, salinity, pH, and depth were positively associated with Axis 1. Elevation and macrophytes were associated with Axis 2 (Figure 3, lower panel).
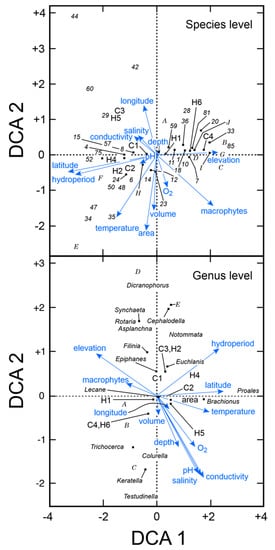
Figure 3.
Unconstrained detrended correspondence analysis (DCA) of rotifers and key environmental factors for 21 Kenyan waterbodies. A. Analysis of taxa at species level. Numbers correspond to those given for species in Table 2. B. Analysis of taxa reduced to genus level. Numbers correspond to habitat types (H1–6) and climatic regions (C1–4) given in Table 1. Letters A–I include multiple species that overlapped. Upper panel: A = 32, 40; B = 19, 21, 38, 39, 49, 53, 54, 61–63, 65, 68, 69, 79, 83; C = 5, 66; D = 46, 55; E = 27, 30, 31, 45, 67, 72; F = 25, 26, 73, G = 17, 22, 58, 76, 82; H = 3, 13, 51; I = 11, 18; J = 46, 55. Lower panel: A = Nolthoca, Floscularia, Limnias, Plationus; B = Platyias, Mytilina; C = Polyarthra, Hexarthra; D = Encentrum, Kostea; E = Pleurotrocha, Dipleuchlanis.
To analyze species richness, a stepwise process was conducted to select the GLM model with the lowest AIC value. Macrophytes had a very strong effect and other variables had to be forced into the model. The resulting model indicated that species richness was positively correlated to all environmental variables, with stream basin (habitat type 3), temperate Mediterranean and humid subtropical climates (climate types 5 and 4, respectively), and pH being highly significant variables (Table 4A). When correcting for spatial autocorrelation and using the same model, macrophytes and many other environmental factors were no longer a significant predictor of species richness while pH and several habit types remained significant contributors (Table 4B). We found no significant differences when making comparisons among habitat types or between climatic zones. Using spatial regression with eigenvector spatial filtering, only macrophyte abundance (p < 0.009) was a significant predictor of species composition among sites (Table 4).

Table 4.
Importance of environmental features in determining species richness (S) in 21 Kenyan waterbodies. A. Generalized linear model (GLM): S = macrophytes + habitat type + climatic region + depth + temperature + pH + conductivity. B. Generalized least squares fit (GLS) with Gaussian spatial correlations using the same model but accounting for longitude, latitude, and elevation. AIC = 117.9. C. Moran’s eigenvector with spatial filtering. Habitat types and climatic regions are defined in the text.
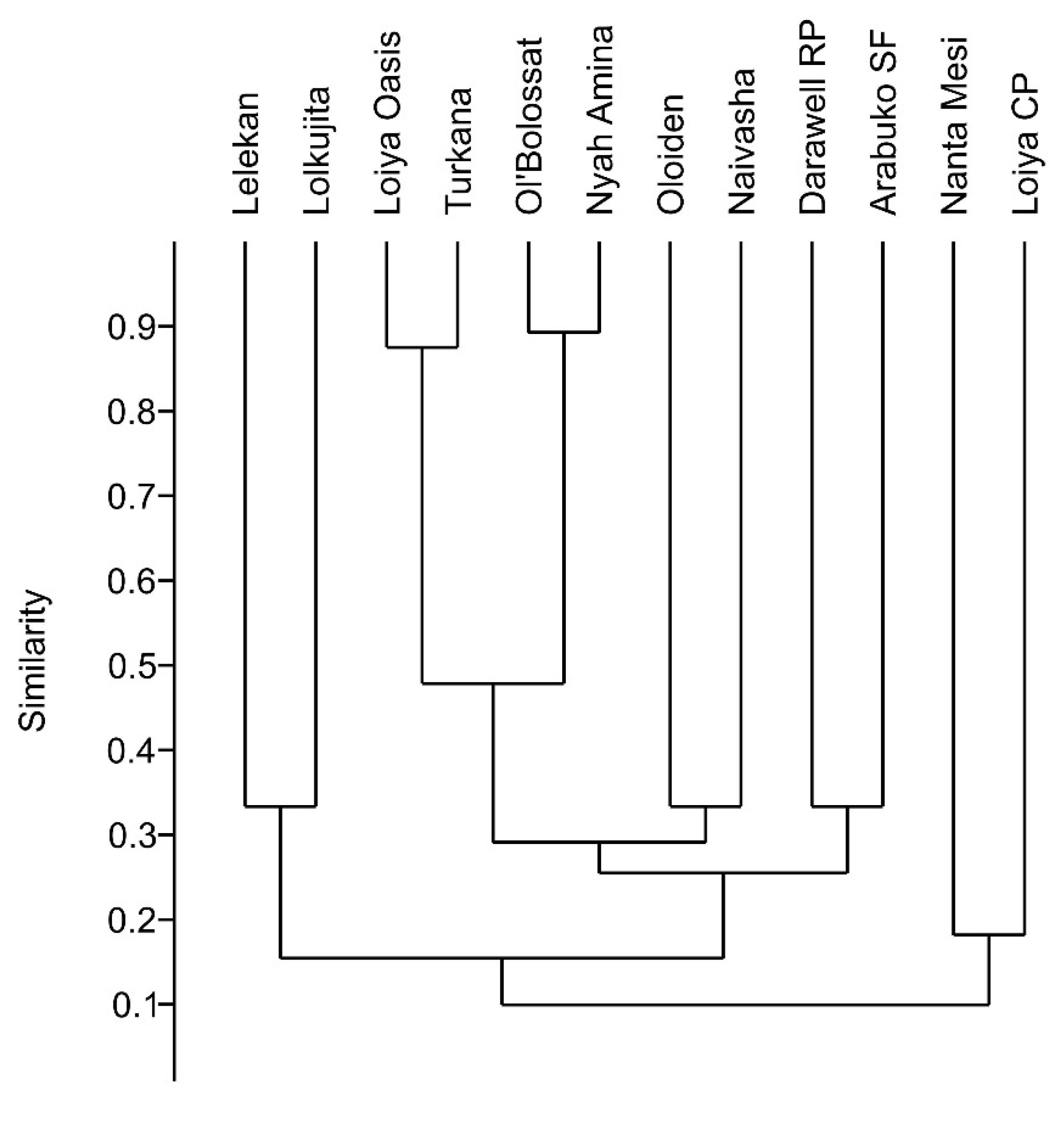
Cluster analysis showed high similarities in species composition in closely situated waterbodies (Figure 4). However, the closely situated (within 12 km) and highly turbid Loiyangalani CP (clay puddle) and Nanta Mesi systems formed a cluster even though they are in adjacent climatic regions. Rotifer assemblage similarity between Lake Turkana and Loiyangalani Oasis (H1–H4) is likely a consequence of their hydrologic connection. The lake was fed by a stream flowing through the oasis. Lake Ol’Bolossat and Nayhururu Amina lie in close proximity to one another, are in the same climate region, and are both permanent and used for fish production. Lake Naivasha and Lake Oloiden are connected by a narrow channel.
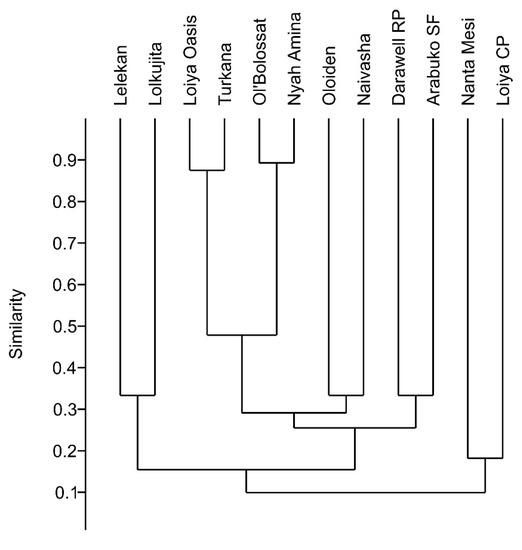
Figure 4.
Hierarchical cluster analysis based on unpaired group (UPGMA) Jaccard similarity index. Abbreviations: Turkana (including species from: Lake Turkana—Koobi Fora north, Lake Turkana—Koobi Fora pond, Lake Turkana—Koobi Fora south, Lake Turkana—Loiyangalani, Lake Turkana—Elmolo village, and Lake Turkana—south); Ol’Bolossat (including species from: Lake Ol’Bolossat—north, and Lake Ol’Bolossat—south); Nyah Amina (Nyahururu—Amina); Naivasha (including species from: Lake Naivasha—Kijabe camp, Naivasha—NINI Farm #1, and #2, Naivasha—Valley Breeze camp, and Naivasha—Cray Fish camp); Oloiden (Lake Oloiden #1, and #2); Loiya CP (Loiyangalani—Clay puddle); Loiya Oasis (Loiyangalani—Oasis H1, H2, H3, and H4); Nanta Mesi (Mt. Kulal—Nanta Mesi); Lelekan (Mt. Kulal—Lelekan); Lolkujita (Mt. Kulal—Lolkujita); Darawell RP (Ndoto Mts.—Darawell upstream rock pools); Arabuko SF (Arabuko Sokoke Forest).
4. Discussion
Here we provide the first compiled species list for rotifers in Kenya. We expanded the known biogeography for 4 taxa in Africa and 25 in Kenya and improved the understanding of how abiotic and biotic conditions may influence species distributions. We found that both biogeographic (e.g., climatic region, elevation) and local habitat conditions (e.g., abundance of macrophytes, hydroperiod, and temperature) can influence species richness in temporary and permanent waterbodies. Species richness was highest in permanent habitats and lowest in those with shorter hydroperiods. However, many unique species were found only in temporary or artificial habitats.
Lake Turkana is a unique ecosystem, distinguished as the world’s largest permanent desert lake and the largest alkaline water body with a surface area of about 7560 km2 [88]. This lake is a sodium carbonate lake possessing a high pH and high dissolved salts, and with an ionic composition typical of East African saline lakes [100]. During our sampling period, conductivity ranged from 3150–3660 μS cm−1 and salinity ranged 1.5–1.8 g kg−1. Thus, it was not surprising that of the 20 species we found in Lake Turkana, half are brachionids, many of which are tolerant to high levels of salinity. Our finding confirmed those of De Ridder [5] with the exceptions of Brachionus dorcas and B. calyciflorus, which were not present in our samples. In addition, we recently reported that a new cryptic species of the B. plicatilis species was found in Lake Turkana, designated as B. plicatilis “(SM9) Turkana” [99]. We found several additional specimens that likely represent new species. Additional studies of their genetics and ecology are needed to substantiate their status.
Lake Naivasha, an endorheic freshwater lake that lies in the Eastern Rift Valley, is the second largest freshwater lake in Kenya. It was formed as a result of tectonic faulting and volcanic activity associated with the formation of the Rift Valley [17]. In this study, Lake Naivasha had the highest rotifer species richness with 53 taxa (Table 3). This is greater than previously reported (Pejler, [14]: 12 taxa, in 1967–1968; Nogrady, [15]: 18 taxa, 1980; Mavuti, [19]: 16 taxa, 1978, 1980; Uku and Mavuti, [17]: 13 taxa, 1990, 1991). Brachionids predominated and were most abundant in our samples in terms of number of species but also anecdotally in terms of number of individuals. However, we were not able to determine absolute numbers of individuals due to the sampling method. We confirmed the dominance of three Brachionus species: B. angularis, B. caudatus, and B. calyciflorus as was observed by Nogrady [15] and Uku and Mavuti [17]. Brachionus falcatus and Plationus patulus were also abundant in the survey by Mavuti [19]. Nogrady [15] and Uku and Mavuti [17] found a few individuals of B. falcatus in their surveys. We did not find this species in the main lake, but it did occur in the nearby metal bucket. The relatively high abundance of macrophytes in some sampling locations within Lake Naivasha likely contributed to the high number of rotifer taxa found. Interestingly, the contribution of species (9 taxa) from the bucket resulted in this site having the highest species richness. The bucket had been left unattended in a shaded area and may have provided rotifers with a refugium from predators or provided another unknown factor that contributed to the success of the rotifer community.
Lake Oloiden, a saline-alkaline water body with a surface area ranging from 4 to 7.5 km2 [101], is a remnant of Lake Naivasha that lies to the southwest of the main lake. Until 1955, Oloiden was a bay of Lake Naivasha during high water. A boat canal was built in the 1960s to connect it to the main lake, but the canal is now blocked by water hyacinth (Eichhornia crassipes (Mart.) Solms—Laub.) and papyrus (Cyperus papyrus L.). Currently, there is little water inflow from the main lake, and consequently the waters in Lake Oloiden are highly saline [17]. Uku and Mavuti [17] found 11 rotifer taxa, while Pejler [14] reported 7, and Nogrady [15] found 19 in their surveys of this lake. Our observations increased the total number of taxa to 22, with the additions of Epiphanes clavulata, Hexarthra intermedia, and Rotaria sp. Overall, Lake Oloiden had relatively low species richness consisting of taxa that are tolerant to high salinity levels. Brachionus calyciforus, B. falcatus, K. tropica, Filinia sp., Hexarthra sp., Polyarthra sp., Trichocerca sp., Euchlanis sp., and Asplanchna sp. were found in Lake Oloiden and Lake Naivasha. The most common genus found in Lake Oloiden was Brachionus. This is similar to the findings of Pejler [14] and Uku and Mavuti [17]. Plationus patulus, B. calyciflorus, Filinia opoliensis, and Collotheca sp. were dominant, followed by B. quadridentatus and B. caudatus in Pejler’s [14] study. While in the survey of Uku and Mavuti [17] the dominant rotifer species was B. angularis, followed by B. caudatus and B. calyciflorus. Nogrady [15] noted high numbers of Anuraeopsis coelata, which he stated reflected eutrophic conditions at the time of sampling.
The rotifer community of Lake Ol‘Bolossat has not been previously characterized to our knowledge. This shallow freshwater body that covers an area of 43 km2 [101] is situated in the valley between the northwestern slopes of the Aberdares Mountains and the Dundori Ridge. This drainage basin, Ewaso Ngiro North Basin covering 210.2 km2, is Kenya’s largest. The altitude ranges from 2340 to 2400 m a.s.l. The area has an average precipitation of 975 to 1100 mm/year. The 13 rotifer taxa we identified from the lake are widespread and typical of those found in other lakes in our survey.
Kibindo reservoir is a natural lake that has been partially modified to raise water levels and to support fish production. The reservoir had an area of ~6000 m2, low turbidity, and the lowest conductivity (88 μS cm−1) of the 33 sites sampled. Due to its high elevation (2372 m a.s.l.), water temperature is generally <20 °C. This reservoir has not been previously surveyed for zooplankton. We identified 11 rotifer taxa: four brachionids, four lecanids, an unidentified Collotheca, Dicranophorus grandis, and Filinia pejleri.
In contrast to large lakes, little is known regarding smaller and more temporary systems. Riato et al. [48] provides a description of the zooplankton communities of 19 permanent and temporary freshwater pans in the Mpumalanga Highveld region of South Africa. Freshwater temporary pans included one rotifer species (Platyias quadricornis), while two rotifer species (Brachionus dimidiatus, and B. plicatilis) were found in permanent pans. Similarly, B. plicatilis was frequently encountered in ephemeral salt pans of the Makgadikgadi Basin in the northeast Botswana [102]. In Zimbabwe, Anusa et al. [52] found nutrient status and community diversity in rock pools are determined by pool area and depth, a proxy for habitat duration. They reported eight rotifer species (belonging to the genera Asplanchna, Brachionus, Conochilus, Epiphanes, Lepadella, Rotaria, and Synchaeta) as common inhabitants across a range of hydroperiods in temporary rock pools. Overall, the number of species present increased as pool area increased.
To our knowledge, there is only one published study that includes small waterbodies in Kenya. Masai et al. [21] investigated rotifer diversity of Lake Victoria (Kenya) and adjacent small waterbodies. They found that the small systems had the highest rotifer species richness. This may have been due to varied biological and chemical characteristics found in the large number of sampled sites. These are generally shallow waterbodies with heavy macrophyte growth. With 17 and 9 species respectively, Lecanidae and Brachionidae were the genera with the highest richness. The low species diversity in Lake Victoria may be attributed to the relatively harsh conditions and a relative lack of macrophytes. In our study, the species richness of permanent lakes was more than three times that of temporary waters (permanent lakes: 88; temporary waterbodies: 26), similar to the findings of other authors (e.g., [103,104,105]).
Geographic distance between aquatic habitats can play an important role in determining zooplankton distribution [106,107]. The size of the diapausing stage is a factor related to dispersal capacity [108], with larger, more dense stages less likely to be transported over long distances by hydrochory or anemochory. Consequently, the community composition of closely situated waterbodies often consists of nested subsets of taxa (e.g., [109,110]). Our cluster analysis indicated similarities in species composition in closely situated ponds (Figure 4), indicating an influence of geographic proximity and the climatic region on community composition. Sites that are in close geographical proximity were clustered based on species composition. For example, Lake Turkana (Table 1 sites #1–5, 12) and Loiyangalani—Oasis (H1–H4) (# 8–11) form a cluster, as do Lake Ol’Bolossat (#22, 24) and Nayhururu—Amina (#21). Lake Naivasha (#25, 27–28, 30, 32) shows species similarity to geographically close Lake Oloiden (#29, 31), similarly as Lelekan (#14) to Lolkujita (#15) (Figure 4). As noted above, one group comprised two sites: Loiyangalani—clay puddle (#7) and Nanta Mesi (#13), which are in close geographic proximity (12 km); these belong to two different climatic regions and differ in altitude. In addition, both sites are shallow, temporary puddles with high turbidity. The reason for the close association of taxa from the geographically separated Darawell stream sites (#16, 17) and Arabuko Sokoke Forest site (#33) is unknown.
Rotifer species composition can be used as an indicator of trophic conditions [111,112]. For comparison among the habitat types surveyed in our study, we calculated Sládeček’s [97] trophic condition quotient and found that the QB/T value was over 2.0 for both habitat types and thus the waterbodies were eutrophic during our survey. This is not surprising given that past studies of several of these lakes found eutrophic conditions (e.g., [15,19,113]) and many of the temporary habitats we sampled are highly impacted by human activities (livestock use, etc.). Interestingly, while water quality variables (e.g., temperature, pH, conductivity) were significant in determining species richness using a general linear model, those effects were removed after accounting for spatial autocorrelation in our study. Rather, our results indicated that climatic region, which can be considered as a combination of temperature, elevation, and relative distance between ponds, and macrophyte presence as important drivers of community composition.
One suite of species was associated with high elevation, while another large group correlated with permanent habitats, latitude, area, and temperature. In addition, several species were associated with a high abundance of macrophytes. Not surprisingly, sessile (e.g., Floscularia, Collotheca) and littoral (e.g., Cephalodella gibba, Lecane lunaris) species were highly associated with macrophytes. In addition, conductivity was associated with the occurrence of some species (e.g., Brachionus plicatilis, Hexarthra mira). While species of the genus Lecane are typically considered as freshwater (conductivity < 1000 μS cm−1) or subsaline (1000–6000 μS cm−1 [68]; we confirmed their tolerance to high conductivity (272–6550 μS cm−1), similar to that reported for saline waters (200 to over 7000 μS cm−1) in the Chihuahuan Desert of México by Walsh et al. [67].
Macrophytes are known to modify habitat conditions [57,114] and strongly affect zooplankton occurrence [115,116]. The surface area of the waterbodies and presence or absence of specific macrophyte species [117] can affect the ability of rotifers to sustain significant populations [118]. In the permanent waterbodies in our survey, which are mostly large and have abundant littoral vegetation, QB/L was 0.92, while in temporary waterbodies, where littoral vegetation was generally reduced or lacking, it was 2.0. For instance, Lake Naivasha had the highest species richness (S = 53) of our sampling sites and had a QB/L = 1.14. This reflected that although most of the lake lacks submerged vegetation, a few of the sampled substations had macrophyte coverages of 70–90%. These findings support the results of our multivariate analyses and give additional evidence that macrophytes are important in structuring these rotifer communities.
Hydroperiod length is often the most influential hydrological parameter in temporary waters and is a major driver of community structure (e.g., [34,119,120]). The influence of hydroperiod has been evaluated for various faunal groups, with variable results depending on the taxa considered and the study area. Most of these studies show that invertebrate species richness increases with the length of hydroperiod [119,120,121,122,123]. In this study, hydroperiod played an important role in determining species richness. The most temporary habitats typically had few species. Although the species richness of individual habitats was low, they made a substantial contribution to overall rotifer diversity in the regions we sampled. In this study we did not find a strong relationship between hydroperiod and species richness. This may be a consequence of the relatively few samples in some of the hydroperiod classes.
While we identified 93 taxa in our survey, we acknowledge that this study underrepresents the true diversity of the Rotifera in these waterbodies. Undoubtedly, sampling additional sites and over longer temporal scales would yield additional taxa. However, our results yielded rotifer diversity similar to other survey-based studies such as those from the Upper Parana River floodplain (from 2 sites where samples were collected with a motorized pump daily for 14 days, S = 143 [124]; from 28 locations, S = 100 [125]; and from 36 environments, S = 104 [126]), aquatic systems in Costa Rica (40 habitats, S = 105 [127]), and the Salado River in Argentina (15 locations, S = 63 [128]). In the Salado River system, associated shallow lakes and tributaries were an important source of species, similar to our results showing that smaller, temporary habitats make important contributions to γ diversity. A final example is a survey of 19 sites in the Upper Tietê basin of Brazil (S = 109) with Lecanidae, Brachionidae, Trichocercidae, Notommatidae, and Lepadellidae as the predominant families [129]. Likewise in our study, we found Lecane (with 14 species) and Brachionus (12 sp.) to be the most common genera, as is typical for tropical and sub-tropical waters.
We worked with preserved material due to logistical constraints and many species of rotifers can only be identified while alive. This is particularly true for bdelloids [130]. Another limitation of this study is that samples were collected only in January and February following the rainy season. In larger habitats it is well known that there is seasonal succession of species [76,131]. Further, many temporary habitats had dried before the sampling effort. For some monogonont taxa we had only one or a few specimens and could not observe critical features of the body and trophi; these remain unidentified. DNA barcoding may be helpful in identifying some species [132] and this study is currently underway. It is also well documented that many traditional rotifer species are in fact complexes of morphologically cryptic species (e.g., [133,134,135,136]). We found a new species within the B. plicatilis species complex in Lake Turkana [99] (see also Table 2 and Table 3); this species co-occurred with individuals representing other lineages within the complex. Other species we isolated may also represent new cryptic lineages, in particular B. calyciflorus, E. dilatata, and Testudinella patina.
5. Conclusions
Traditionally, estimates of aquatic biodiversity have focused on lakes, rivers, and other permanent water sources. It is becoming increasingly clear that temporary waters can make significant contributions to rotifer species richness. The results of this study indicted the highest species richness in permanent habitats compared to temporary habitats, but small, temporary systems made important contributions to regional diversity. Rotifer diversity was affected by macrophyte abundance, but also by some habitat types and climatic regions, and the geographic proximity of ponds. From an ecological perspective, permanent lakes with dense macrophyte beds were the habitats with the highest rotifer richness. Temporary waterbodies are typical mostly widespread or cosmopolitan rotifer species. However, the highest potential to discover new records or species is in small, unexplored isolated waterbodies. The diversity of climatic conditions and aquatic biotopes makes Kenya an interesting location for further investigations, including surveying additional waterbodies, repeated sampling over longer time periods, determining the ecological relevance of rotifer diversity in these systems, and genetic analyses of isolated populations to better understand evolutionary processes in rotifers. Our study enhanced research efforts in Eastern Africa by contributing 34 previously undocumented taxa for this region. The number of rotifer species identified in this study is the highest reported from Kenya. This study also supports the contention that small freshwater habitats such as ponds and pools are important for the conservation of aquatic biodiversity [137,138] by contributing to metapopulation and metacommunity dynamics and regional species diversity [139].
Author Contributions
Conceptualization, R.S. and E.J.W.; methodology, R.S. and E.J.W.; validation, R.S. and E.J.W.; formal analysis, R.S. and E.J.W.; investigation, R.S. and E.J.W.; resources, R.S. and E.J.W.; data curation, R.S.; writing—original draft preparation, R.S. and E.J.W.; writing—review and editing, R.S. and E.J.W.; visualization, R.S. and E.J.W.; supervision, R.S. and E.J.W.; project administration, R.S; funding acquisition, R.S. and E.J.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Slovak Scientific Grant Agency (VEGA-1/0012/20). Fieldwork was funded by the Agency of the Ministry of Education, Science, Research and Sport of the Slovak Republic (ITMS: 26110230119) and by Grant Agency of Presov University (29/2018). Laboratory identifications and some statistical analyses were supported by grant 5G12MD007592 from the National Institutes on Minority Health and Health Disparities (NIMHD), a component of the National Institutes of Health (NIH).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Source data for creating the map of the location of the studied waterbodies in Kenya (Figure 1) are available in Jarvis et al. [87].
Acknowledgments
We thank Robert L. Wallace, Diego Fontaneto, and four anonymous reviewers for their helpful comments that improved the manuscript. We thank Amy Wagler and Patrick D. Brown for their help with statistical analyses. The research in Kenya was performed under a permit from the National Commission for Science, Technology and Innovation (NACOSTI/P/14/4653/660). Special thanks to Miloslav Michalko for constructing the map. We thank Lina Hamdan for her participation in the project.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Daufresne, M.; Lengfellner, K.; Sommer, U. Global Warming Benefits the Small in Aquatic Ecosystems. Proc. Natl. Acad. Sci. USA 2009, 106, 12788–12793. [Google Scholar] [CrossRef] [PubMed]
- Heino, J. A Macroecological Perspective of Diversity Patterns in the Freshwater Realm. Freshw. Biol. 2011, 56, 1703–1722. [Google Scholar] [CrossRef]
- Pecl, G.T.; Araújo, M.B.; Bell, J.D.; Blanchard, J.; Bonebrake, T.C.; Chen, I.-C.; Clark, T.D.; Colwell, R.K.; Danielsen, F.; Evengård, B.; et al. Biodiversity Redistribution under Climate Change: Impacts on Ecosystems and Human Well-Being. Science 2017, 355. [Google Scholar] [CrossRef] [PubMed]
- Chase, J.M.; McGill, B.J.; Thompson, P.L.; Antão, L.H.; Bates, A.E.; Blowes, S.A.; Dornelas, M.; Gonzalez, A.; Magurran, A.E.; Supp, S.R.; et al. Species Richness Change across Spatial Scales. Oikos 2019, 128, 1079–1091. [Google Scholar] [CrossRef]
- De Ridder, M. Additions to the “Annotated Checklist of Nonmarine Rotifers from African Inland Waters”. Rev. Hydrobiol. Trop. 1991, 24, 25–46. [Google Scholar]
- Fontaneto, D.; Barbosa, A.M.; Segers, H.; Pautasso, M. The ‘Rotiferologist’ Effect and Other Global Correlates of Species Richness in Monogonont Rotifers. Ecography 2012, 35, 174–182. [Google Scholar] [CrossRef]
- Dumont, H.J. Biogeography of Rotifers. Hydrobiologia 1983, 104, 19–30. [Google Scholar] [CrossRef]
- Segers, H.; De Smet, W.H. Diversity and Endemism in Rotifera: A Review, and Keratella Bory de St Vincent. In Protist Diversity and Geographical Distribution; Springer: Dordrecht, The Netherlands, 2007; pp. 69–82. ISBN 9789048128006. [Google Scholar]
- Ejsmont-Karabin, J. Does the World Need Faunists? Based on Rotifer (Rotifera) Occurrence Reflections on the Role of Faunistic Research in Ecology. Int. Rev. Hydrobiol. 2019, 104, 49–56. [Google Scholar] [CrossRef]
- Segers, H. Global Diversity of Rotifers (Rotifera) in Freshwater. In Freshwater Animal Diversity Assessment; Balian, E.V., Lévêque, C., Segers, H., Martens, K., Eds.; Springer: Dordrecht, The Netherlands, 2008; pp. 49–59. ISBN 9781402082597. [Google Scholar]
- Luo, Y.; Segers, H. Eight New Lepadellidae (Rotifera, Monogononta) from the Congo Bring to Level Endemism in Africa’s Rotifers. Zootaxa 2020, 4731, 371–387. [Google Scholar] [CrossRef]
- Ahlstrom, E.H. A Revision of the Rotatorian Genera Brachionus and Platyias, with Descriptions of One New Species and Two New Varieties. Bulletin of the AMNH; v. 77, Article 3; Bulletin of The American Museum of Natural History: New York, NY, USA, 1940; pp. 143–184. [Google Scholar]
- Ahlstrom, E.H. A Revision of the Rotatorian Genus Keratella, with Descriptions of Three New Species and Five New Varieties. Bulletin of the AMNH; v. 80, Article 12; Bulletin of The American Museum of Natural History: New York, NY, USA, 1943; pp. 411–457. [Google Scholar]
- Pejler, B. On the Rotifer Plankton of Some East African Lakes. Hydrobiologia 1974, 44, 389–396. [Google Scholar] [CrossRef]
- Nogrady, T. Succession of Planktonic Rotifer Populations in Some Lakes of the Eastern Rift Valley, Kenya. Hydrobiologia 1983, 98, 45–54. [Google Scholar] [CrossRef]
- Green, J. Keratella Cochlearis (Gosse) in Africa. In Proceedings of the Fourth Rotifer Symposium; May, L., Wallace, R., Herzig, A., Eds.; Springer: Dordrecht, The Netherlands, 1987; pp. 3–8. [Google Scholar]
- Uku, J.N.; Mavuti, K.M. Comparative Limnology, Species Diversity and Biomass Relationship of Zooplankton and Phytoplankton in Five Freshwater Lakes in Kenya. Hydrobiologia 1994, 272, 251–258. [Google Scholar] [CrossRef]
- Gophen, M.; Ochumba, P.B.O.; Kaufman, L.S. Some Aspects of Perturbation in the Structure and Biodiversity of the Ecosystem of Lake Victoria (East Africa). Aquat. Living Resour. 1995, 8, 27–41. [Google Scholar] [CrossRef]
- Mavuti, K.M. Ecology and Role of Zooplankton in the Fishery of Lake Naivasha. Hydrobiologia 1990, 208, 131–140. [Google Scholar] [CrossRef]
- Segers, H.; Mbogo, D.K.; Dumont, H.J. New Rotifera from Kenya, with a Revision of the Ituridae. Zool. J. Linn. Soc. 1994, 110, 193–206. [Google Scholar] [CrossRef]
- Masai, D.M.; Omondi, R.; Owili, M. Systematics and Distribution of Zooplankton in Lake Victoria Basin, Kenya. In Proceedings of the 11th World Lakes Conference, Nairobi, Kenya, 31 October–11 November 2005; Odada, E., Olago, D.O., Eds.; Aqua Docs: Nairobi, Kenya, 2006; Volume 2, pp. 230–235. [Google Scholar]
- Omondi, R.; Yasindi, A.W.; Magana, A.M. Diel Vertical Distribution of Zooplankton in Lake Baringo, Kenya. JLS 2014, 8, 447–460. [Google Scholar]
- Omondi, R.; Yasindi, A.W.; Magana, A. Spatial and Temporal Variations of Zooplankton in Relation to Some Environmental Factors in Lake Baringo, Kenya. Egerton J. Sci. Technol. 2015, 11. [Google Scholar]
- Oyoo-Okoth, E.; Muchiri, M.; Ngugi, C.C.; Njenga, E.W.; Ngure, V.; Orina, P.S.; Chemoiwa, E.C.; Wanjohi, B.K. Zooplankton Partitioning in a Tropical Alkaline–Saline Endorheic Lake Nakuru, Kenya: Spatial and Temporal Trends in Relation to the Environment. Lakes Reserv. 2011, 16, 35–47. [Google Scholar] [CrossRef]
- Wallace, R.L.; Walsh, E.J.; Arroyo, M.L.; Starkweather, P.L. Life on the Edge: Rotifers From Springs and Ephemeral Waters in the Chihuahuan Desert, Big Bend National Park (Texas, USA). Hydrobiologia 2005, 546, 147–157. [Google Scholar] [CrossRef]
- Vale, C.G.; Pimm, S.L.; Brito, J.C. Overlooked Mountain Rock Pools in Deserts Are Critical Local Hotspots of Biodiversity. PLoS One 2015, 10, e0118367. [Google Scholar] [CrossRef]
- Olmo, C.; Armengol, X.; Ortells, R. Re-Establishment of Zooplankton Communities in Temporary Ponds after Autumn Flooding: Does Restoration Age Matter? Limnologica 2012, 42, 310–319. [Google Scholar] [CrossRef]
- Smolak, R. What Do Forest Wells and Temporary Puddles Hide? Nat. Sci. Biol.-Ecol. 2013, 17, 36–41. [Google Scholar]
- Obona, J.; Demkova, L.; Smolak, R.; Dominiak, P.; Scerbakova, S. Invertebrates in Overlooked Aquatic Ecosystem in the Middle of the Town. Period. Biol. 2017, 119. [Google Scholar] [CrossRef]
- Thiéry, A. Multispecies Coexistence of Branchiopods (Anostraca, Notostraca & Spinicaudata) in Temporary Ponds of Chaouia Plain (Western Morocco): Sympatry or Syntopy between Usually Allopatric Species. In Developments in Hydrobiology: Studies on Large Branchiopod Biology and Aquaculture; Springer: Dordrecht, The Netherlands, 1991; pp. 117–136. ISBN 9789401054881. [Google Scholar]
- Williams, D.D. Temporary Ponds and Their Invertebrate Communities. Aquat. Conserv. Mar. Freshw. Ecosyst. 1997, 7, 105–117. [Google Scholar] [CrossRef]
- Bayly, I.A.E. Invertebrate Occurrence and Succession after Episodic Flooding of a Central Australian Rock-Hole. J. R. Soc. West. Aust. 2001, 84, 29–32. [Google Scholar]
- Jocqué, M.; Martens, K.; Riddoch, B.; Brendonck, L. Faunistics of Ephemeral Rock Pools in Southeastern Botswana. Arch. Hydrobiol. 2006, 165, 415–431. [Google Scholar] [CrossRef]
- Walsh, E.J.; Smith, H.A.; Wallace, R.L. Rotifers of Temporary Waters. Int. Rev. Hydrobiol. 2014, 99, 3–19. [Google Scholar] [CrossRef]
- Sharma, B.K. Rotifer Communities of Deepor Beel, Assam, India: Richness, Abundance and Ecology. J. Threat. Taxa 2010, 2, 1077–1086. [Google Scholar] [CrossRef]
- Sharma, B.K. Zooplankton Diversity of Two Floodplain Lakes (Pats) of Manipur, Northeast India. Opusc. Zool. Bp. 2011, 42, 185–197. [Google Scholar]
- Sharma, B.K.; Sharma, S. Faunal Diversity of Rotifers (Rotifera: Eurotatoria) of Nokrek Biosphere Reserve, Meghalaya, India. J. Threat. Taxa 2011, 3, 1535–1541. [Google Scholar] [CrossRef]
- Fontaneto, D. Long-Distance Passive Dispersal in Microscopic Aquatic Animals. Mov. Ecol. 2019, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Wood, P.J.; Greenwood, M.T.; Agnew, M.D. Pond Biodiversity and Habitat Loss in the UK. Area 2003, 35, 206–216. [Google Scholar] [CrossRef]
- Straubinger-Gansberger, N.; Kaggwa, M.N.; Schagerl, M. Phytoplankton Patterns along a Series of Small Man-Made Reservoirs in Kenya. Environ. Monit. Assess. 2014, 186, 5153–5166. [Google Scholar] [CrossRef] [PubMed]
- Brendonck, L.; Lanfranco, S.; Timms, B.; Vanschoenwinkel, B. Invertebrates in Rock Pools. In Invertebrates in Freshwater Wetlands; Batzer, D., Boix, D., Eds.; Springer Nature: Cham, Switzerland, 2016; pp. 25–53. [Google Scholar]
- Aka, M.; Pagano, M.; Saint-Jean, L.; Arfi, R.; Bouvy, M.; Cecchi, P.; Corbin, D.; Thomas, S. Zooplankton Variability in 49 Shallow Tropical Reservoirs of Ivory Coast (West Africa). Internat. Rev. Hydrobiol. 2000, 85, 491–504. [Google Scholar] [CrossRef]
- Edema, C.U.; Ayeni, J.O.; Aruoture, A. Some Observations on the Zooplankton and Macrobenthos of the Okhuo River, Nigeria. J. Aquat. Sci. 2002, 17, 145–149. [Google Scholar] [CrossRef]
- Mustapha, M.K. Zooplankton Assemblage of Oyun Reservoir, Offa, Nigeria. Rev. Biol. Trop. 2009, 57, 1027–1047. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Okogwu, O.I. Seasonal Variations of Species Composition and Abundance of Zooplankton in Ehoma Lake, a Floodplain Lake in Nigeria. Rev. Biol. Trop. 2010, 58, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Lahr, J. An Ecological Assessment of the Hazard of Eight Insecticides Used in Desert Locust Control, to Invertebrates in Temporary Ponds in the Sahel. Aquat. Ecol. 1998, 32, 153–162. [Google Scholar] [CrossRef]
- Osore, M.K.W. Zooplankton of the Kenya Coast: Ecology and Systematics. Ph.D. Thesis, Vrije Universiteit Brussel, Brussel, Belgium, 2003. [Google Scholar]
- Riato, L.; Van Ginkel, C.; Taylor, J.C. Zooplankton and Diatoms of Temporary and Permanent Freshwater Pans in the Mpumalanga Highveld Region, South Africa. Afr. Zool. 2014, 49, 113–127. [Google Scholar] [CrossRef][Green Version]
- Dalu, T.; Weyl, O.L.F.; Froneman, P.W.; Wasserman, R.J. Trophic Interactions in an Austral Temperate Ephemeral Pond Inferred Using Stable Isotope Analysis. Hydrobiologia 2016, 768, 81–94. [Google Scholar] [CrossRef]
- Stoch, F.; Korn, M.; Turki, S.; Naselli-Flores, L.; Marrone, F. The Role of Spatial Environmental Factors as Determinants of Large Branchiopod Distribution in Tunisian Temporary Ponds. Hydrobiologia 2016, 782, 37–51. [Google Scholar] [CrossRef]
- Bird, M.S.; Mlambo, M.C.; Wasserman, R.J.; Dalu, T.; Holland, A.J.; Day, J.A.; Villet, M.H.; Bilton, D.T.; Barber-James, H.M.; Brendonck, L. Deeper Knowledge of Shallow Waters: Reviewing the Invertebrate Fauna of Southern African Temporary Wetlands. Hydrobiologia 2019, 827, 89–121. [Google Scholar] [CrossRef]
- Anusa, A.; Ndagurwa, H.G.T.; Magadza, C.H.D. The Influence of Pool Size on Species Diversity and Water Chemistry in Temporary Rock Pools on Domboshawa Mountain, Northern Zimbabwe. Afr. J. Aquat. Sci. 2012, 37, 89–99. [Google Scholar] [CrossRef]
- Wallace, R.L.; Snell, T.W.; Ricci, C.; Nogrady, T. Rotifera 1: Biology, Ecology and Systematics, 2nd ed.; Guides to the Identification of the Microinvertebrates of the Continental Waters of the World; Segers, H., Ed.; 23; Backhuys Publishers: Leiden, The Netherlands, 2006; Volume 1, ISBN 9789057821783. [Google Scholar]
- Allan, J.D. Life History Patterns in Zooplankton. Am. Nat. 1976, 110, 165–180. [Google Scholar] [CrossRef]
- Wallace, R.L.; Snell, T.W.; Smith, H.A. Chapter 13—Phylum Rotifera. In Thorp and Covich’s Freshwater Invertebrates, 4th ed.; Thorp, J.H., Covich, A.P., Eds.; Academic Press: Cambridge, MA, USA, 2015; pp. 225–271. [Google Scholar]
- Moore, M.; Folt, C. Zooplankton Body Size and Community Structure: Effects of Thermal and Toxicant Stress. Trends Ecol. Evol. 1993, 8, 178–183. [Google Scholar] [CrossRef]
- Špoljar, M.; Dražina, T.; Šargač, J.; Borojević, K.K.; Žutinić, P. Submerged Macrophytes as a Habitat for Zooplankton Development in Two Reservoirs of a Flow-through System (Papuk Nature Park, Croatia). Ann. Limnol.-Int. J. Lim. 2012, 48, 161–175. [Google Scholar] [CrossRef]
- Pejler, B.; Bērziņš, B. On Relation to Substrate in Sessile Rotifers. Hydrobiologia 1993, 259, 121–124. [Google Scholar] [CrossRef]
- Branco, C.W.C.; Rocha, M.-I.A.; Pinto, G.F.S.; Gômara, G.A.; Filippo, R.D. Limnological Features of Funil Reservoir (R.J., Brazil) and Indicator Properties of Rotifers and Cladocerans of the Zooplankton Community. Lakes Reserv. 2002, 7, 87–92. [Google Scholar] [CrossRef]
- Bini, L.M.; Galli Vieira, L.C.; Machado, J.; Machado Velho, L.F. Concordance of Species Composition Patterns among Microcrustaceans, Rotifers and Testate Amoebae in a Shallow Pond. Int. Rev. Hydrobiol. 2007, 92, 9–22. [Google Scholar] [CrossRef]
- Angeler, D.G.; Alvarez-Cobelas, M.; Sánchez-Carrillo, S. Evaluating Environmental Conditions of a Temporary Pond Complex Using Rotifer Emergence from Dry Soils. Ecol. Indic. 2010, 10, 545–549. [Google Scholar] [CrossRef]
- Stefanidis, K.; Papastergiadou, E. Influence of Hydrophyte Abundance on the Spatial Distribution of Zooplankton in Selected Lakes in Greece. Hydrobiologia 2010, 656, 55–65. [Google Scholar] [CrossRef]
- Ferreira, M.; Wepener, V.; Van Vuren, J.H.J. Aquatic Invertebrate Communities of Perennial Pans in Mpumalanga, South Africa: A Diversity and Functional Approach. Afr. Invertebr. 2012, 53, 751–768. [Google Scholar] [CrossRef]
- Gürbüzer, P.; Buyurgan, Ö.; Tekatli, Ç.; Altindağ, A. Species Diversity and Community Structure of Zooplankton in Three Different Types of Water Body within the Sakarya River Basin, Turkey. Turk. J. Zool. 2017, 41, 848–859. [Google Scholar] [CrossRef]
- Bērziņš, B.; Pejler, B. Rotifer Occurrence in Relation to Oxygen Content. Hydrobiologia 1989, 183, 165–172. [Google Scholar] [CrossRef]
- Armengol, X.; Esparcia, A.; Miracle, M.R. Rotifer Vertical Distribution in a Strongly Stratified Lake: A Multivariate Analysis. In Proceedings of the Rotifera VIII: A Comparative Approach; Wurdak, E., Wallace, R., Segers, H., Eds.; Springer: Dordrecht, The Netherlands, 1998; pp. 161–170. [Google Scholar]
- Walsh, E.J.; Schröder, T.; Wallace, R.L.; Ríos-Arana, J.V.; Rico-Martínez, R. Rotifers from Selected Inland Saline Waters in the Chihuahuan Desert of México. Aquat. Biosyst. 2008, 4, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kaya, M.; Fontaneto, D.; Segers, H.; Altindağ, A. Temperature and Salinity as Interacting Drivers of Species Richness of Planktonic Rotifers in Turkish Continental Waters. J. Limnol. 2010, 69, 297–304. [Google Scholar] [CrossRef]
- Hessen, D.O.; Andersen, T.; Faafeng, B.A. Replacement of Herbivore Zooplankton Species along Gradients of Ecosystem Productivity and Fish Predation Pressure. Can. J. Fish. Aquat. Sci. 1995, 52, 733–742. [Google Scholar] [CrossRef]
- Pinel-Alloul, B.; Niyonsenga, T.; Legendre, P.; Gril, G. Spatial and Environmental Components of Freshwater Zooplankton Structure. Écoscience 1995, 2, 1–19. [Google Scholar] [CrossRef]
- Anton-Pardo, M.; Ortega, J.C.G.; Melo, A.S.; Bini, L.M. Global Meta-Analysis Reveals That Invertebrate Diversity Is Higher in Permanent than in Temporary Lentic Water Bodies. Freshw. Biol. 2019, 64, 2234–2246. [Google Scholar] [CrossRef]
- Sahuquillo, M.; Miracle, M.R. Crustacean and Rotifer Seasonality in a Mediterranean Temporary Pond with High Biodiversity (Lavajo de Abajo de Sinarcas, Eastern Spain). Limnetica 2010, 29, 75–92. [Google Scholar] [CrossRef]
- Habdija, I.; Primc-Habdija, B.; Špoljar, M.; Perić, M.S. Ecological Determinants of Rotifer Vertical Distribution in a Coastal Karst Lake (Vrana Lake, Cres Island, Croatia). Biologia 2011, 66, 130–137. [Google Scholar] [CrossRef]
- Basińska, A.M.; Świdnicki, K.; Kuczyńska-Kippen, N. Effect of Surrounding Trees and Dry Rush Presence on Spring Zooplankton Community in an Urban Pond Complex. Ann. Limnol.-Int. J. Lim. 2014, 50, 315–323. [Google Scholar] [CrossRef]
- Meksuwan, P.; Pholpunthin, P.; Walsh, E.J.; Segers, H.; Wallace, R.L. Nestedness in Sessile and Periphytic Rotifer Communities: A Meta-Analysis. Int. Rev. Hydrobiol. 2014, 99, 48–57. [Google Scholar] [CrossRef]
- Gabaldón, C.; Devetter, M.; Hejzlar, J.; Šimek, K.; Znachor, P.; Nedoma, J.; Seďa, J. Seasonal Strengths of the Abiotic and Biotic Drivers of a Zooplankton Community. Freshw. Biol. 2019, 64, 1326–1341. [Google Scholar] [CrossRef]
- Kuczyńska-Kippen, N.; Pronin, M. Diversity and Zooplankton Species Associated with Certain Hydroperiods and Fish State in Field Ponds. Ecol. Indic. 2018, 90, 171–178. [Google Scholar] [CrossRef]
- Zimmermann-Timm, H.; Holst, H.; Kausch, H. Spatial Dynamics of Rotifers in a Large Lowland River, the Elbe, Germany: How Important Are Retentive Shoreline Habitats for the Plankton Community? Hydrobiologia 2007, 593, 49–58. [Google Scholar] [CrossRef]
- Malekzadeh Viayeh, R.; Špoljar, M. Structure of Rotifer Assemblages in Shallow Waterbodies of Semi-Arid Northwest Iran Differing in Salinity and Vegetation Cover. Hydrobiologia 2012, 686, 73–89. [Google Scholar] [CrossRef]
- Kuczyńska-Kippen, N.; Joniak, T. Zooplankton Diversity and Macrophyte Biometry in Shallow Water Bodies of Various Trophic State. Hydrobiologia 2016, 774, 39–51. [Google Scholar] [CrossRef]
- Castro, B.B.; Antunes, S.C.; Pereira, R.; Soares, A.M.V.M.; Gonçalves, F. Rotifer Community Structure in Three Shallow Lakes: Seasonal Fluctuations and Explanatory Factors. Hydrobiologia 2005, 543, 221–232. [Google Scholar] [CrossRef]
- Kuczynska-Kippen, N. The Species Diversity of Rotifers (Rotifera) of Differentiated Macrophyte Habitats of Lake Budzynskie. Rocz. Ak. Rol. Poz. Bot.-Steciana 2005, 9, 171–176. [Google Scholar]
- Basu, B.K.; Kalff, J.; Pinel-Alloul, B. The Influence of Macrophyte Beds on Plankton Communities and Their Export from Fluvial Lakes in the St Lawrence River. Freshw. Biol. 2000, 45, 373–382. [Google Scholar] [CrossRef]
- Walsh, E.J. Habitat-Specific Predation Susceptibilities of a Littoral Rotifer to Two Invertebrate Predators. Hydrobiologia 1995, 313, 205–211. [Google Scholar] [CrossRef]
- Enríquez García, C.; Nandini, S.; Sarma, S.S.S. Seasonal Dynamics of Zooplankton in Lake Huetzalin, Xochimilco (Mexico City, Mexico). Limnologica 2009, 39, 283–291. [Google Scholar] [CrossRef]
- Peel, M.C.; Finlayson, B.L.; McMahon, T.A. Updated World Map of the Köppen-Geiger Climate Classification. Hydrol. Earth Syst. Sci. Discuss. 2007, 4, 439–473. [Google Scholar] [CrossRef]
- Jarvis, A.; Reuter, H.I.; Nelson, A.; Guevara, E. Hole-Filled Seamless SRTM Data V4, International Centre for Tropical Agriculture (CIAT). Available online: https://ci.nii.ac.jp/naid/10027883137/ (accessed on 15 December 2021).
- Ojwang, W.O.; Obiero, K.O.; Donde, O.O.; Gownaris, N.; Pikitch, E.K.; Omondi, R.; Agembe, S.; Malala, J.; Avery, S.T. Lake Turkana: World’s Largest Permanent Desert Lake (Kenya); Springer: Heidelberg, Germany, 2016; Volume 1, ISBN 9789400761735. [Google Scholar]
- Segers, H. Rotifera 2: The Lecanidae (Monogononta). Guides to the Identification of the Microinvertebrates of the Continental Waters of the World; Nogrady, T., Ed.; 6; SPB Academic Publishing: The Hague, The Netherlands, 1995; Volume 2, ISBN 9789051030914. [Google Scholar]
- De Smet, W.H. Rotifera 4: The Proalidae (Monogomonta). Guides to the Identifications of Microinvertebrates of the Continental Continental Waters of the World; Nogrady, T., Ed.; 9; SPB Academic Publishing: The Hague, The Netherlands, 1996; Volume 4, ISBN 9789051031195. [Google Scholar]
- Nogrady, T.; Pourriot, R.; Segers, H. Rotifera 3: The Notommatidae (Monogononta) and the Scaridiidae (Monogononta). Guides to the Identification of the Microinvertebrates of the Continental Waters of the World; Nogrady, T., Ed.; 8; SPB Academic Publishing: The Hague, The Netherlands, 1995; Volume 3, ISBN 9789051031034. [Google Scholar]
- De Smet, W.H.; Pourriot, R. Rotifera 5: The Dicranophoridae (Monogononta) and The Ituridae (Monogononta). Guides to the Identification of the Microinvertebrates of the Continental Waters of the World; Nogrardy, T., Ed.; 12; SPB Academic Publishing: The Hague, The Netherlands, 1997; Volume 5, ISBN 9789051031355. [Google Scholar]
- Segers, H. Rotifera 6: Asplanchnidae, Gastropodidae, Lindiidae, Microcodidae, Synchaetidae, Trochosphaeridae and Filinia. Guides to the Identification of the Microinvertebrates of the Continental Waters of the World; Nogrady, T., Ed.; 18; Backhuys Publishers: Leiden, The Netherlands, 2002; Volume 6, ISBN 9780009282447. [Google Scholar]
- Ruttner-Kolisko, A. Plankton Rotifers: Biology and Taxonomy; Stuttgarut E. Schweizerbart’sche Verlagsbuchhandlung: Stuttgart, Germany, 1974; Volume 26, p. 146. [Google Scholar]
- Koste, W. Die Radertiere Mitteleuropas I; Borntraeger, Gebruder: Berlin and Stuttgart, Germany, 1978; Volume 1, p. 673. [Google Scholar]
- Jersabek, C.D.; De Smet, W.H.; Hinz, C.; Fontaneto, D.; Hussey, C.G.; Michaloudi, E.; Wallace, R.L.; Segers, H. List of Available Names in Zoology, Candidate Part Phylum Rotifera, Species-Group Names Established before 1 January 2000. Available online: https://archive.org/details/LANCandidatePartSpeciesRotifera (accessed on 22 September 2021).
- Sládeček, V. Rotifers as Indicators of Water Quality. Hydrobiologia 1983, 100, 169–201. [Google Scholar] [CrossRef]
- Šmilauer, P.; Lepš, J. Multivariate Analysis of Ecological Data Using CANOCO 5; Cambridge University Press: New York, NY, USA, 2014; p. 361. ISBN 9781107694408. [Google Scholar]
- Mills, S.; Alcántara-Rodríguez, J.A.; Ciros-Pérez, J.; Gómez, A.; Hagiwara, A.; Galindo, K.H.; Jersabek, C.D.; Malekzadeh-Viayeh, R.; Leasi, F.; Lee, J.-S.; et al. Fifteen Species in One: Deciphering the Brachionus Plicatilis Species Complex (Rotifera, Monogononta) through DNA Taxonomy. Hydrobiologia 2017, 796, 39–58. [Google Scholar] [CrossRef]
- Avery, S.T. What Future for Lake Turkana? The Impact of Hydropower and Irrigation Development on the World’s Large Desert Lake; African Studies Centre, University of Oxford: Oxford, UK, 2013; p. 64. [Google Scholar]
- Kiama, C.W.; Njire, M.M.; Kambura, A.K.; Mugweru, J.N.; Matiru, V.N.; Wafula, E.N.; Kagali, R.N.; Kuja, J.O. Prokaryotic Diversity and Composition within Equatorial Lakes Olbolosat and Oloiden in Kenya (Africa). Curr. Res. Microb. Sci. 2021, 2, 100066. [Google Scholar] [CrossRef]
- Seaman, M.T.; Ashton, P.J.; Williams, W.D. Inland Salt Waters of Southern Africa. Hydrobiologia 1991, 210, 75. [Google Scholar] [CrossRef]
- Williams, D.D. Introduction to Temporary Waters. In The Ecology of Temporary Waters; Williams, D.D., Ed.; 1; Croom Helm: London, UK, 1987; pp. 1–3. ISBN 9789401160841. [Google Scholar]
- Collinson, N.H.; Biggs, J.; Corfield, A.; Hodson, M.J.; Walker, D.; Whitfield, M.; Williams, P.J. Temporary and Permanent Ponds: An Assessment of the Effects of Drying out on the Conservation Value of Aquatic Macroinvertebrate Communities. Biol. Conserv. 1995, 74, 125–133. [Google Scholar] [CrossRef]
- Brucet, S.; Boix, D.; López-Flores, R.; Badosa, A.; Moreno-Amich, R.; Quintana, X.D. Zooplankton Structure and Dynamics in Permanent and Temporary Mediterranean Salt Marshes: Taxon-Based and Size-Based Approaches. Arch. Hydrobiol. 2005, 162, 535–555. [Google Scholar] [CrossRef]
- Florencio, M.; Díaz-Paniagua, C.; Serrano, L. Relationships between Hydroperiod Length, and Seasonal and Spatial Patterns of Beta-Diversity of the Microcrustacean Assemblages in Mediterranean Ponds. Hydrobiologia 2016, 774, 109–121. [Google Scholar] [CrossRef]
- Santos, J.B.O.; Silva, L.H.S.; Branco, C.W.C.; Huszar, V.L.M. The Roles of Environmental Conditions and Geographical Distances on the Species Turnover of the Whole Phytoplankton and Zooplankton Communities and Their Subsets in Tropical Reservoirs. Hydrobiologia 2016, 764, 171–186. [Google Scholar] [CrossRef]
- Fontaneto, D.; Ricci, C. Spatial Gradients in Species Diversity of Microscopic Animals: The Case of Bdelloid Rotifers at High Altitude. J. Biogeogr. 2006, 33, 1305–1313. [Google Scholar] [CrossRef]
- Fontaneto, D.; Ficetola, G.F.; Ambrosini, R.; Ricci, C. Patterns of Diversity in Microscopic Animals: Are They Comparable to Those in Protists or in Larger Animals? Glob. Ecol. Biogeogr. 2006, 15, 153–162. [Google Scholar] [CrossRef]
- Ríos-Arana, J.V.; Agüero-Reyes, L.d.C.; Wallace, R.L.; Walsh, E.J. Limnological Characteristics and Rotifer Community Composition of Northern Mexico Chihuahuan Desert Springs. J. Arid Environ. 2019, 160, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Ejsmont-Karabin, J. The Usefulness of Zooplankton as Lake Ecosystem Indicators: Rotifer Trophic State Index. Pol. J. Ecol. 2012, 60, 339–350. [Google Scholar]
- Liang, D.; Wang, Q.; Wei, N.; Tang, C.; Sun, X.; Yang, Y. Biological Indicators of Ecological Quality in Typical Urban River-Lake Ecosystems: The Planktonic Rotifer Community and Its Response to Environmental Factors. Ecol. Indic. 2020, 112, 106127. [Google Scholar] [CrossRef]
- Harper, D.M.; Phillips, G.; Chilvers, A.; Kitaka, N.; Mavuti, K. Eutrophication Prognosis for Lake Naivasha, Kenya. Verh. Int. Ver. Theor. Angew. Limnol. 1993, 25, 861–865. [Google Scholar] [CrossRef]
- Takamura, N.; Kadono, Y.; Fukushima, M.; Nakagawa, M.; Kim, B.-H.O. Effects of Aquatic Macrophytes on Water Quality and Phytoplankton Communities in Shallow Lakes. Ecol. Res. 2003, 18, 381–395. [Google Scholar] [CrossRef]
- Habdija, I.; Radanović, I.; Maria, P.-H.B.Š. Vegetation Cover and Substrate Type as Factors Influencing the Spatial Distribution of Trichopterans along a Karstic River. Internat. Rev. Hydrobiol. 2002, 97, 423–437. [Google Scholar] [CrossRef]
- Basińska, A.; Kuczyńska-Kippen, N.; Świdnicki, K. The Body Size Distribution of Filinia Longiseta (Ehrenberg) in Different Types of Small Water Bodies in the Wielkoposka Region. Limnetica 2010, 29, 171–182. [Google Scholar] [CrossRef]
- Braghin, L.d.S.M.; Simões, N.R.; Bonecker, C.C. Hierarchical Effects of Local Factors on Zooplankton Species Diversity. Inland Waters 2016, 6, 645–654. [Google Scholar] [CrossRef][Green Version]
- Balkić, A.G.; Ternjej, I.; Špoljar, M. Hydrology Driven Changes in the Rotifer Trophic Structure and Implications for Food Web Interactions. Ecohydrology 2018, 11, e1917. [Google Scholar] [CrossRef]
- Fahd, K.; Arechederra, A.; Florencio, M.; León, D.; Serrano, L. Copepods and Branchiopods of Temporary Ponds in the Doñana Natural Area (SW Spain): A Four-Decade Record (1964–2007). In Pond Conservation in Europe; Springer: Dordrecht, The Netherlands, 2009; pp. 375–386. ISBN 9789048190874. [Google Scholar]
- Florencio, M.; Serrano, L.; Gómez-Rodríguez, C.; Millán, A.; Díaz-Paniagua, C. Inter- and Intra-Annual Variations of Macroinvertebrate Assemblages Are Related to the Hydroperiod in Mediterranean Temporary Ponds. In Pond Conservation in Europe. Developments in Hydrobiology 210; Oertli, B., Céréghino, R., Biggs, J., Declerck, S., Hull, A., Miracle, M.R., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 323–339. [Google Scholar]
- Serrano, L.; Fahd, K. Zooplankton Communities across a Hydroperiod Gradient of Temporary Ponds in the Donana National Park (SW Spain). Wetlands 2005, 20, 101–111. [Google Scholar] [CrossRef]
- Tavernini, S.; Mura, G.; Rossetti, G. Factors Influencing the Seasonal Phenology and Composition of Zooplankton Communities in Mountain Temporary Pools. Int. Rev. Hydrobiol. 2005, 90, 358–375. [Google Scholar] [CrossRef]
- Waterkeyn, A.; Grillas, P.; Vanschoenwinkel, B.; Brendonck, L. Invertebrate Community Patterns in Mediterranean Temporary Wetlands along Hydroperiod and Salinity Gradients. Freshw. Biol. 2008, 53, 1808–1822. [Google Scholar] [CrossRef]
- Bonecker, C.C.; Lansac-Tôha, F.A.; Rossa, D.C. Planktonic and Non-Planktonic Rotifers in Two Environments of the Upper Paraná River Floodplain, State of Mato Grosso Do Sul, Brazil. Braz. Arch. Biol. Technol. 1998, 41, 447–456. [Google Scholar] [CrossRef]
- Aoyagui, A.S.M.; Bonecker, C.C. Rotifers in Different Environments of the Upper Paraná River Floodplain (Brazil): Richness, Abundance and the Relationship with Connectivity. Hydrobiologia 2004, 522, 281–290. [Google Scholar] [CrossRef]
- Bonecker, C.C.; Costa, C.L.D.; Velho, L.F.M.; Lansac-Tôha, F.A. Diversity and Abundance of the Planktonic Rotifers in Different Environments of the Upper Paraná River Floodplain (Paraná State—Mato Grosso Do Sul State, Brazil). Hydrobiologia 2005, 546, 405–414. [Google Scholar] [CrossRef]
- Kuczyńska-Kippen, N.; Ejsmont-Karabin, J. Rotifera of Various Aquatic Environments of Costa Rica in Reference to Central American Rotifer Fauna. Turk. J. Zool. 2020, 44, 238–247. [Google Scholar] [CrossRef]
- Neschuk, N.; Claps, M.; Gabellone, N. Planktonic Rotifers of a Saline-Lowland River: The Salado River (Argentina). Ann. Limnol.-Int. J. Lim. 2002, 38, 191–198. [Google Scholar] [CrossRef]
- Lucinda, I.; Moreno, I.H.; Melão, M.G.G.; Matsumura-Tundisi, T. Rotifers in Freshwater Habitats in the Upper Tietê River Basin, São Paulo State, Brazil. Acta Limnol. Brasil. 2004, 16, 203–224. [Google Scholar]
- Ricci, C.; Melone, G. Key to the Identification of the Genera of Bdelloid Rotifers. Hydrobiologia 2000, 418, 73–80. [Google Scholar] [CrossRef]
- Stemberger, R.S.; Evans, M.S. Rotifer Seasonal Succession and Copepod Predation in Lake Michigan. J. Great Lakes Res. 1984, 10, 417–428. [Google Scholar] [CrossRef]
- Fontaneto, D.; Eckert, E.M.; Aninic, N.; Lara, E.; Mitchell, E.A.D. We Are Ready for Faunistic Surveys of Bdelloid Rotifers through DNA Barcoding: The Example of Sphagnum Bogs of the Swiss Jura Mountains. Limnetica 2019, 38, 213–225. [Google Scholar] [CrossRef]
- Fontaneto, D.; Giordani, I.; Melone, G.; Serra, M. Disentangling the Morphological Stasis in Two Rotifer Species of the Brachionus Plicatilis Species Complex. Hydrobiologia 2007, 583, 297–307. [Google Scholar] [CrossRef]
- Fontaneto, D.; Herniou, E.A.; Boschetti, C.; Caprioli, M.; Melone, G.; Ricci, C.; Barraclough, T.G. Independently Evolving Species in Asexual Bdelloid Rotifers. PLoS Biol. 2007, 5, e87. [Google Scholar] [CrossRef]
- Kordbacheh, A.; Garbalena, G.; Walsh, E.J. Population Structure and Cryptic Species in the Cosmopolitan Rotifer Euchlanis Dilatata. Zool. J. Linn. Soc. 2017, 181, 757–777. [Google Scholar] [CrossRef]
- Michaloudi, E.; Papakostas, S.; Stamou, G.; Neděla, V.; Tihlaříková, E.; Zhang, W.; Declerck, S.A.J. Reverse Taxonomy Applied to the Brachionus Calyciflorus Cryptic Species Complex: Morphometric Analysis Confirms Species Delimitations Revealed by Molecular Phylogenetic Analysis and Allows the (Re)Description of Four Species. PLoS One 2018, 13, e0203168. [Google Scholar] [CrossRef]
- Blaustein, L.; Schwartz, S.S. Why Study Ecology in Temporary Pools? Isr. J. Zool. 2001, 47, 303–312. [Google Scholar] [CrossRef]
- De Meester, L.; Declerck, S.; Stoks, R.; Louette, G.; Van De Meutter, F.; De Bie, T.; Michels, E.; Brendonck, L. Ponds and Pools as Model Systems in Conservation Biology, Ecology and Evolutionary Biology. Aquat. Conserv. 2005, 15, 715–725. [Google Scholar] [CrossRef]
- Semlitsch, R.D.; Bodie, J.R. Are Small, Isolated Wetlands Expendable? Conserv. Biol. 1998, 12, 1129–1133. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).