Soil Seed Bank of Alien and Native Cornus (Cornaceae) Taxa in Lithuania: What Determines Seed Density and Vertical Distribution in Soil?
Abstract
1. Introduction
2. Materials and Methods
2.1. Studied Taxa
2.2. Study Sites
2.3. Sampling Procedures
2.4. Seed Bank Analysis
2.5. Tests of Seed Viability
2.6. Statistical Analyses
3. Results
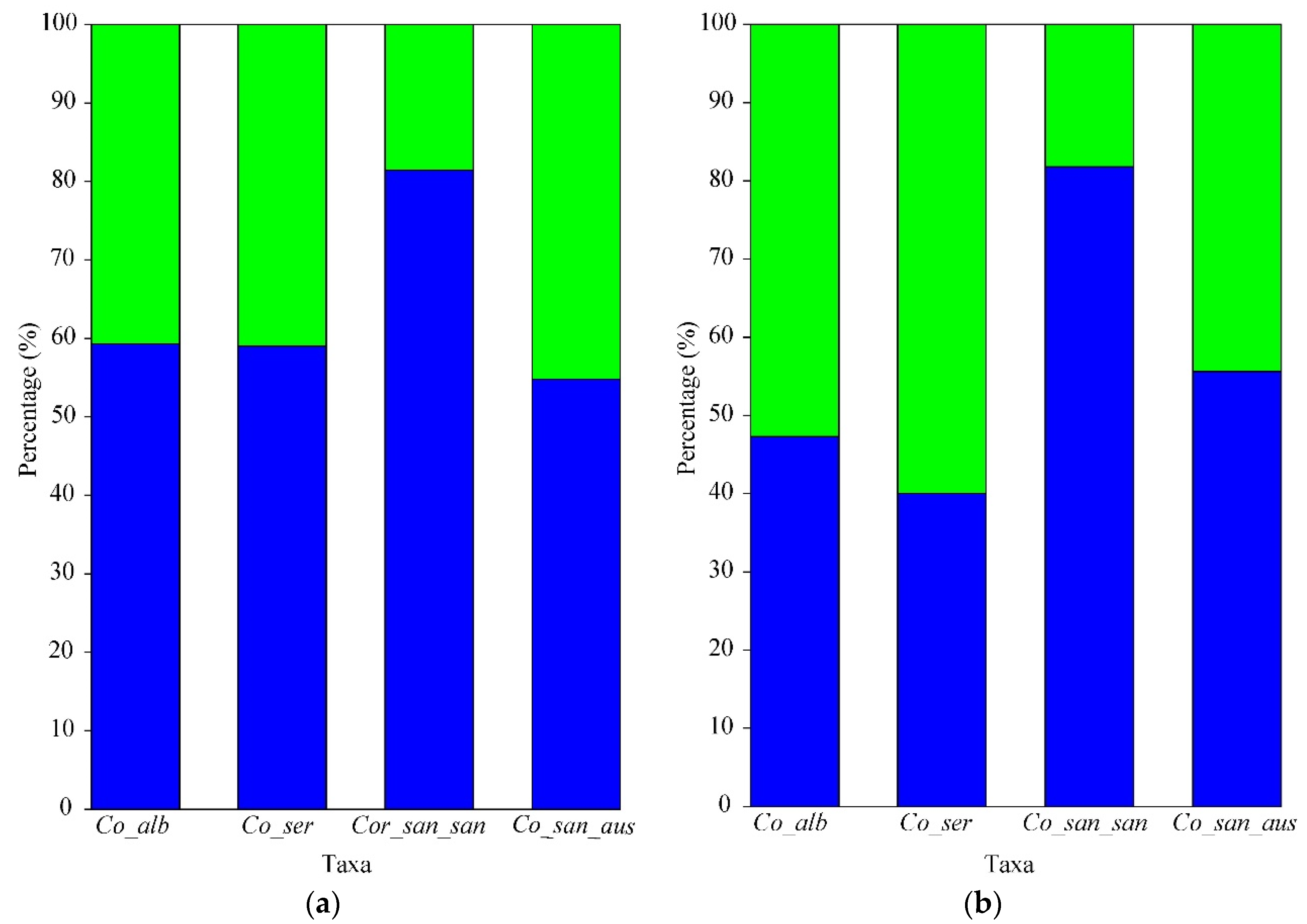
3.1. Vertical Seed Distribution
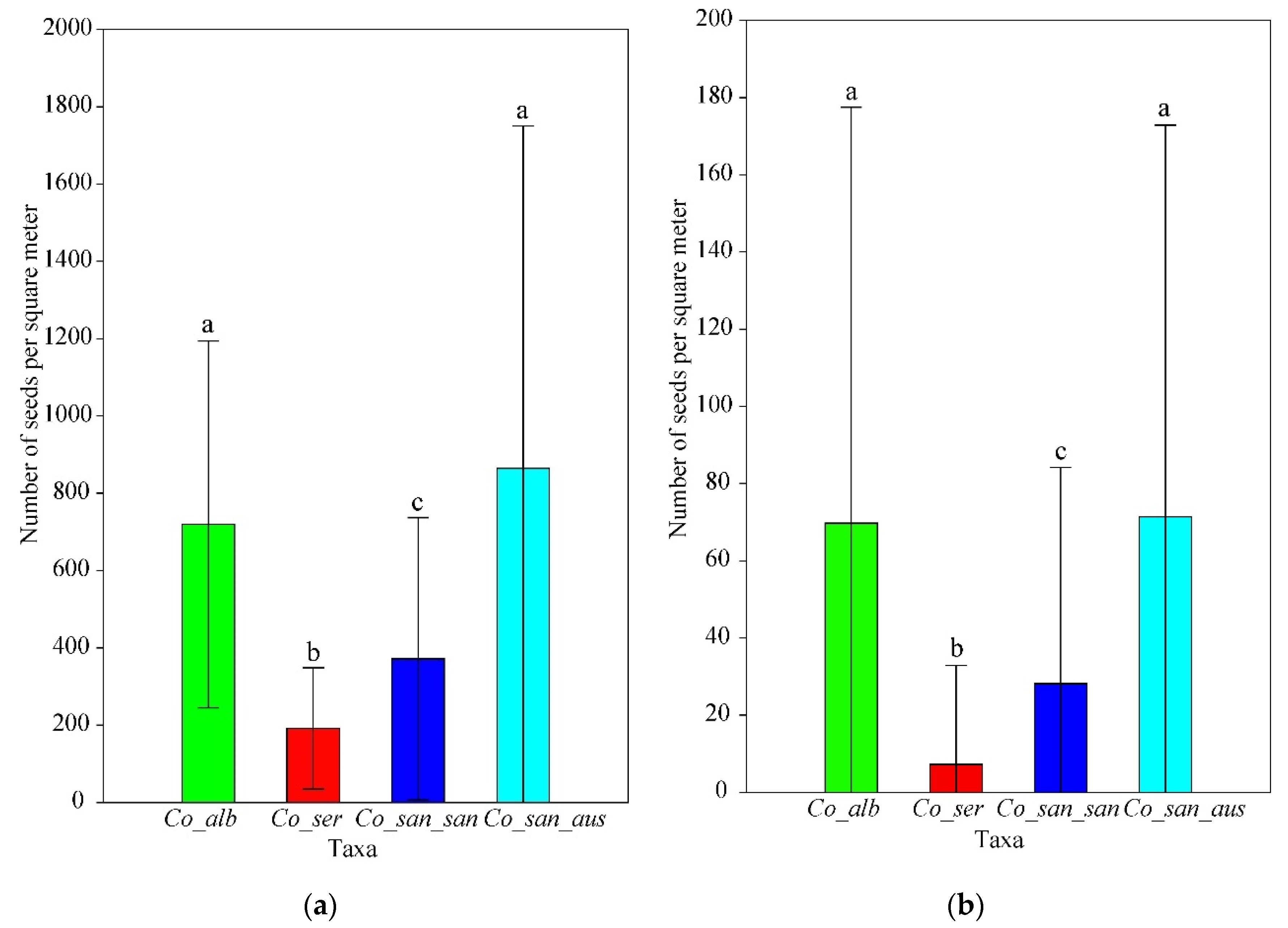
3.2. Seed Bank Density
3.3. Seed Viability
4. Discussion
4.1. Vertical Seed Distribution
4.2. Density of the Seed Bank
4.3. Viable Seeds
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Richardson, D.M.; Kluge, R.L. Seed banks of invasive Australian Acacia species in South Africa: Role in invasiveness and options for management. Perspect. Plant Ecol. Evol. Syst. 2008, 10, 161–177. [Google Scholar] [CrossRef]
- Berge, G.; Hestmark, G. Composition of seed banks of roadsides, stream verges and agricultural fields in southern Norway. Ann. Bot. Fennici 1997, 34, 77–90. [Google Scholar]
- Thompson, K.; Bakker, J.P.; Bekker, R.M. The Soil Seed Banks of North West Europe: Methodology, Density and Longevity; Cambridge University Press: Cambridge, UK, 1997. [Google Scholar]
- Baskin, C.; Baskin, J.M. Seeds: Ecology, Biogeography, and Evolution of Dormancy and Germination; Academic Press: San Diego, CA, USA, 1998. [Google Scholar]
- Beismann, H.; Kollmann, J.; Bogenrieder, A.; Speck, T. Reconstruction of local vegetation patterns by seed bank analysis-results from three case studies in the Upper Rhine region, Germany. Neues Jahrb. Geol. Palaontol. Abh. 1996, 202, 169–181. [Google Scholar] [CrossRef]
- Faist, A.M.; Ferrenberg, S.; Collinge, S.K. Banking on the past: Seed banks as a reservoir for rare and native species in restored vernal pools. AoB PLANTS 2013, 5, 043. [Google Scholar] [CrossRef]
- Thompson, K.; Grime, J.P. Seasonal variation in the seed banks of herbaceous species in ten contrasting habitats. J. Ecol. 1979, 67, 893–921. [Google Scholar] [CrossRef]
- Walck, J.L.; Baskin, J.M.; Baskin, C.C.; Hidayati, S.N. Defining transient and persistent seed banks in species with pronounced seasonal dormancy and germination patterns. Seed Sci. Res. 2005, 15, 189–196. [Google Scholar] [CrossRef]
- Louda, S.M. Predation in the dynamics of seed regeneration. In Ecology of Soil Seed Banks, 1st ed.; Leck, M.A., Parker, V.T., Simpson, R.L., Eds.; Academic Press: London, UK, 1989; pp. 25–51. ISBN 978-0-3231-4865-8. [Google Scholar]
- Bekker, R.M.; Bakker, J.P.; Grandin, U.; Kalamees, R.; Milberg, P.; Poschlod, P.; Thompson, K.; Willems, J.H. Seed size, shape and vertical distribution in the soil: Indicators of seed longevity. Funct. Ecol. 1998, 12, 834–842. [Google Scholar] [CrossRef]
- Gioria, M.; Pyšek, P.; Moravcová, L. Soil seed banks in plant invasions: Promoting species invasiveness and long-term impact on plant community dynamics. Preslia 2012, 84, 327–350. [Google Scholar]
- Gioria, M.; Jarošík, V.; Pyšek, P. Impact of alien invasive plants on soil seed bank communities: Emerging patterns. Perspect. Plant Ecol. Evol. Syst. 2014, 16, 132–142. [Google Scholar] [CrossRef]
- Masaki, T.; Tanaka, H.; Shibata, M.; Nakashizuka, T. The seed bank dynamics of Cornus controversa and their role in regeneration. Seed Sci. Res. 1998, 8, 53–63. [Google Scholar] [CrossRef]
- Phillips, M.L.; Murray, B.R. Invasiveness in exotic plant species is linked to high seed survival in the soil. Evol. Ecol. Res. 2012, 14, 83–94. [Google Scholar]
- Wearne, L.J.; Morgan, J.W. Shrub invasion into subalpine vegetation: Implications for restoration of the native ecosystem. Plant Ecol. 2006, 183, 361–376. [Google Scholar] [CrossRef]
- Herrera, A.M.; Carruthers, R.I.; Mills, N.J. Introduced populations of Genista monspessulana (French broom) are more dense and produce a greater seed rain in California, USA, than native populations in the Mediterranean Basin of Europe. Biol. Invasions 2011, 13, 369–380. [Google Scholar] [CrossRef]
- Goets, S.A.; Kraaij, T.; Little, K.M. Seed bank and growth comparisons of native (Virgilia divaricata) and invasive alien (Acacia mearnsii and A. melanoxylon) plants: Implications for conservation. PeerJ 2018, 6, e5466. [Google Scholar] [CrossRef]
- Archibold, O.W.; Brooks, D.; Delanoy, L. An investigation of the invasive shrub European Buckthorn, Rhamnus cathartica L., near Saskatoon, Saskatchewan. Can. Field-Nat. 1997, 111, 617–621. [Google Scholar]
- Giantomasi, A.; Tecco, P.A.; Funes, G.; Gurvich, D.E.; Cabido, M. Canopy effects of the invasive shrub Pyracantha angustifolia on seed bank composition, richness and density in a montane shrubland (Córdoba, Argentina). Austral Ecol. 2008, 33, 68–77. [Google Scholar] [CrossRef]
- Gioria, M.; Pyšek, P.; Baskin, C.C.; Carta, A. Phylogenetic relatedness mediates persistence and density of soil seed banks. J. Ecol. 2020, 108, 2121–2131. [Google Scholar] [CrossRef]
- Gioria, M.; Le Roux, J.J.; Hirsch, H.; Moravcová, L.; Pyšek, P. Characteristics of the soil seed bank of invasive and non-invasive plants in their native and alien distribution range. Biol. Invasions 2019, 21, 2313–2332. [Google Scholar] [CrossRef]
- Chambers, J.C.; MacMahon, J.A. A day in the life of a seed: Movements and fates of seeds and their implications for natural and managed systems. Annu. Rev. Ecol. Syst. 1994, 25, 263–292. [Google Scholar] [CrossRef]
- Chee-Sanford, J.C.; Williams, M.M.; Davis, A.S.; Sims, G.K. Do microorganisms influence seed-bank dynamics? Weed Sci. 2006, 54, 575–587. [Google Scholar] [CrossRef]
- Moravcová, L.; Gudžinskas, Z.; Pyšek, P.; Pergl, J.; Perglová, I. Seed ecology of Heracleum mantegazzianum and H. sosnowskyi, two invasive species with different distributions in Europe. In Ecology and Management of Giant Hogweed (Heracleum mantegazzianum); Pyšek, P., Cock, M.J.W., Nentwig, W., Ravn, H.P., Eds.; CABI: Wallingford, UK; Cambridge, MA, USA, 2007; pp. 157–169. [Google Scholar]
- Taura, L.; Kamaitytė-Bukelskienė, L.; Sinkevičienė, Z.; Gudžinskas, Z. Study on the Rare Semiaquatic Plant Elatine hydropiper (Elatinaceae) in Lithuania: Population Density, Seed Bank and Conservation Challenges. Front. Biosci. Landmark 2022, 27, 162. [Google Scholar] [CrossRef] [PubMed]
- Van Clef, M.; Stiles, E.W. Stiles. Seed longevity in three pairs of native and non-native congeners: Assessing invasive potential. Northeast. Nat. 2001, 8, 301–310. [Google Scholar] [CrossRef]
- Kollman, J.; Grubb, P.J. Biological flora of Central Europe: Cornus sanguinea L. Flora 2001, 196, 161–179. [Google Scholar] [CrossRef]
- Wawrzyniak, M.; Michalak, M.; Chmielarz, P. Effect of different conditions of storage on seed viability and seedling growth of six European wild fruit woody plants. Ann. For. Sci. 2020, 77, 58. [Google Scholar] [CrossRef]
- Bomanowska, A.; Adamowski, W.; Kirpluk, I.; Otręba, A.; Rewicz, A. Invasive alien plants in Polish national parks—Threats to species diversity. PeerJ 2019, 7, e8034. [Google Scholar] [CrossRef]
- Gudžinskas, Z.; Petrulaitis, L. New alien taxa of the genus Cornus (Cornaceae) recorded in Lithuania and Latvia. Botanica 2021, 27, 160–169. [Google Scholar] [CrossRef]
- Takos, I.; Efthimiou, G. Germination results on dormant seeds of fifteen tree species autumn sown in a northern Greek nursery. Silvae Genet. 2003, 52, 67–70. [Google Scholar]
- Pipinis, E.; Milios, E.; Mavrokordopoulou, O.; Smiris, P. Effect of sowing date on seedling emergence of species with seeds enclosed in a stony endocarp. J. Sustain. For. 2018, 37, 375–388. [Google Scholar] [CrossRef]
- Ball, P.W. Cornus L. Flora Europaea: Rosaceae to Umbelliferae; Tutin, T.G., Heywood, V.H., Burges, N.H., Moore, D.M., Valentine, D.H., Walters, S.M., Webb, D.A., Eds.; Cambridge University Press: Cambridge, UK, 1968; Volume 2, pp. 313–314. ISBN 978-0-5210-6662-4. [Google Scholar]
- Gudžinskas, Z.; Petrulaitis, L.; Žalneravičius, E. New woody alien plant species recorded in Lithuania. Bot. Lith. 2017, 23, 153–168. [Google Scholar] [CrossRef][Green Version]
- Wangerin, W. Cornaceae. In Das Pflanzenreich. Series IV, Family 229 (Heft 41); Engler, H.G.A., Ed.; W. Engelmann: Leipzig, Germany, 1910; pp. 1–101. [Google Scholar]
- Zieliński, J.; Tomaszewski, D.; Gawlak, M.; Orlova, L. Kłopotliwe derenie–Cornus alba L. i C. sericea L. (Cornaceae). Dwa gatunki czy jeden? Roc. Pol. Tow. Dendrol. 2014, 62, 9–23. [Google Scholar]
- Murell, Z.E.; Poindexter, D.B. Cornaceae Bechtold & J. Presl. In Flora of North America North of Mexico, Magnoliophyta: Vitaceae to Garryaceae; Flora of North America Editorial Committee, Ed.; Oxford University Press: New York, NY, USA, 2016; Volume 12, pp. 443–457. ISBN 978-0-1906-4372-0. [Google Scholar]
- Lindelof, K.; Lindo, J.A.; Zhou, W.; Ji, X.; Xiang, Q.Y. Phylogenomics, biogeography, and evolution of the blue-or white-fruited dogwoods (Cornus)—Insights into morphological and ecological niche divergence following intercontinental geographic isolation. J. Syst. Evol. 2020, 58, 604–645. [Google Scholar] [CrossRef]
- Woźnicka, A.; Melosik, I.; Morozowska, M. Quantitative and qualitative differences in morphological traits of endocarps revealed between Cornus L. species. Plant Syst. Evol. 2015, 301, 291–308. [Google Scholar] [CrossRef][Green Version]
- Riebl, R.; Meve, U.; Aas, G. Morphologische Variabilität und taxonomische Differenzierung von Cornus sanguinea: Nordbayerische Naturstandorte und Strassenbegleitgrün im Vergleich. Ber. Bayer. Bot. Ges. Erforsch. Heim. Flora 2017, 87, 39–54. [Google Scholar]
- Kelly, D.L. Cornus sericea L. in Ireland: An incipient weed of wetlands. Watsonia 1990, 18, 33–36. [Google Scholar]
- Chytrý, M.; Tichý, L.; Hennekens, S.M.; Knollová, I.; Janssen, J.A.M.; Rodwell, J.S.; Peterka, T.; Marcenò, C.; Landucci, F.; Danihelka, J.; et al. EUNIS Habitat Classification: Expert system, characteristic species combinations and distribution maps of European habitats. Appl. Veg. Sci. 2020, 23, 648–675. [Google Scholar] [CrossRef]
- Csontos, P. Seed banks: Ecological definitions and sampling considerations. Commun. Ecol. 2007, 8, 75–85. [Google Scholar] [CrossRef]
- Starfinger, U.; Karrer, G. A standard protocol for testing viability with the Triphenyl Tetrazolium Chloride (TTC) Test. Jul. Kuehn-Arch. 2016, 255, 65–66. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Fondation for Statistical Computing: Vienna, Austria; Available online: https://www.R-project.org/ (accessed on 19 April 2022).
- Fournier, D.A.; Skaug, H.J.; Ancheta, J.; Ianelli, J.; Magnusson, A.; Maunder, M.N.; Nielsen, A.; Sibert, J. AD Model Builder: Using automatic differentiation for statistical inference of highly parameterized complex nonlinear models. Optim. Methods Softw. 2012, 27, 233–249. [Google Scholar] [CrossRef]
- Pérez-Fernández, A.M.; Gómez-Gutiérrez, J.M.; Martín-Berrocoso, A.; Reinier, M. Effect of seed shape and size on their distribution in the soil seed bank. J. Mediterr. Ecol. 2002, 3, 11–17. [Google Scholar]
- Martínez-Ghersa, M.A.; Ghersa, C.M. The relationship of propagule pressure to invasion potential in plants. Euphytica 2006, 148, 87–96. [Google Scholar] [CrossRef]
- Wall, S.B.V. How rodents smell buried seeds: A model based on the behavior of pesticides in soil. J. Mammal. 2003, 84, 1089–1099. [Google Scholar] [CrossRef]
- Bojňanský, V.; Fargašová, A. Atlas of Seeds and Fruits of Central and East-European Flora; Springer: Dordrecht, The Netherlands, 2007; pp. 439–441. ISBN 978-9-4017-7670-7. [Google Scholar]
- Thompson, J.N.; Pellmyr, O. Origins of variance in seed number and mass: Interaction of sex expression and herbivory in Lomatium salmoniflorum. Oecologia 1989, 79, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Konarska, A. The biology of flowering and structure of selected elements of Cornus alba L. flowers. Acta Agrobot. 2009, 62, 9–15. [Google Scholar] [CrossRef][Green Version]
- Warzecha, B.; Parker, V.T. Differential post-dispersal seed predation drives chaparral seed bank dynamics. Plant Ecol. 2014, 215, 1313–1322. [Google Scholar] [CrossRef]
- Smith, S.B.; DeSando, S.A.; Pagano, T. The value of native and invasive fruit-bearing shrubs for migrating songbirds. Northeast Nat. 2013, 20, 171–184. [Google Scholar] [CrossRef]
- Brown, D. Estimating the composition of a forest seed bank: A comparison of the seed extraction and seedling emergence methods. Can. J. Bot. 1992, 70, 1603–1612. [Google Scholar] [CrossRef]
- Lennon, J.T.; Den Hollander, F.; Wilke-Berenguer, M.; Blath, J. Principles of seed banks and the emergence of complexity from dormancy. Nat. Commun. 2021, 12, 4807. [Google Scholar] [CrossRef]
- Acharya, S.N.; Chu, C.B.; Hermesh, R.; Schaalje, G.B. Factors affecting red-osier dogwood seed germination. Can. J. Bot. 1992, 70, 1012–1016. [Google Scholar] [CrossRef]
- Kollmann, J. Hypotheses on the regeneration niche of fleshy-fruited species in natural forest gaps and edges in central Europe. Verh. Ges. Ökol. 1997, 27, 85–91. [Google Scholar]
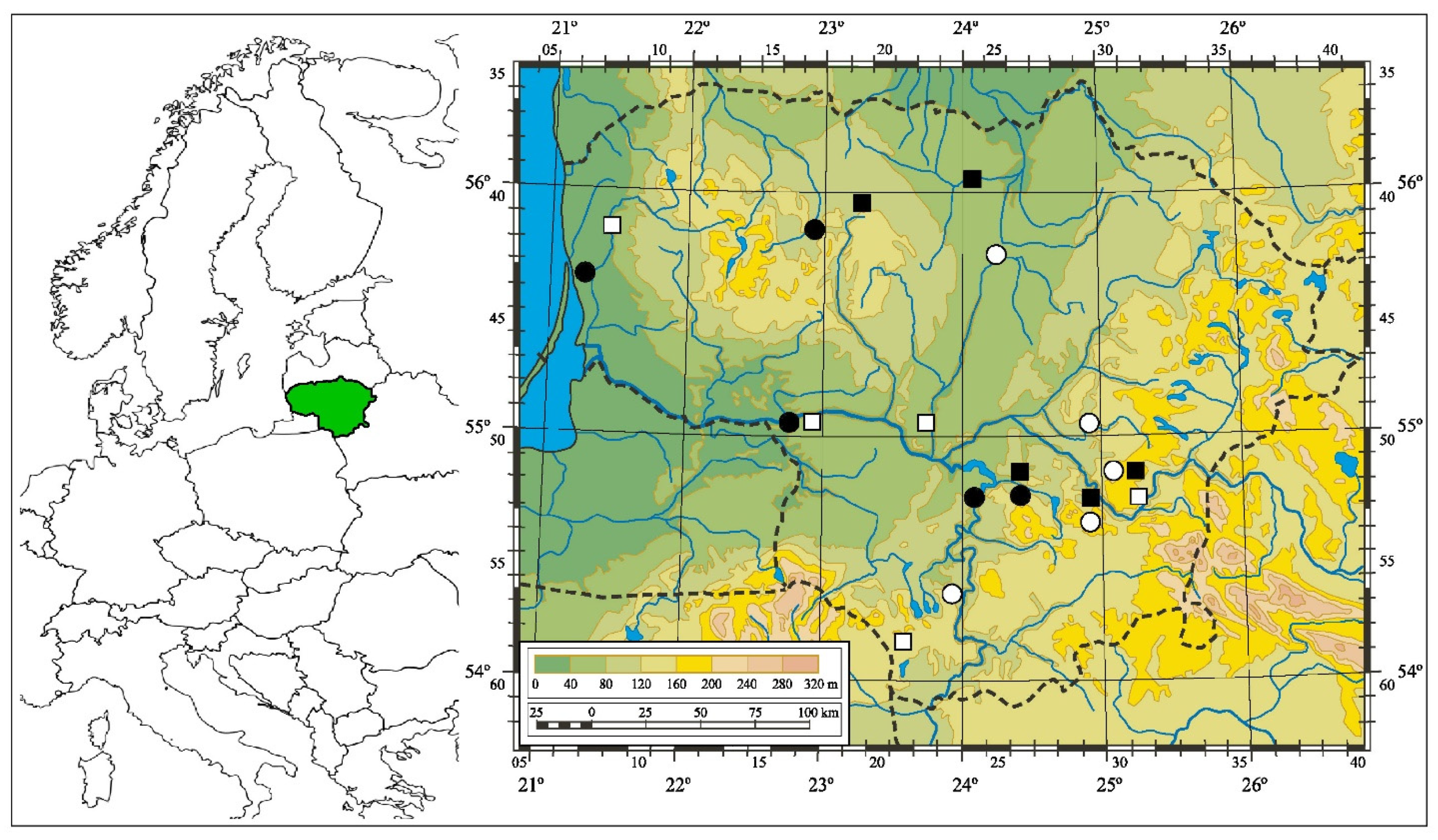
| Site Name | Administrative Unit | Longitude (°E) | Latitude (°N) | EUNIS Habitat Type |
|---|---|---|---|---|
| Cornus alba | ||||
| Panevėžys | Panevėžys city | 55.75270 | 24.31168 | S92 |
| Surgėliai | Širvintos distr. | 55.01496 | 24.90851 | S92 |
| Kiemeliai | Vilnius distr. | 54.85709 | 25.01377 | T1J |
| Trakai | Trakai distr. | 54.65630 | 24.90519 | V64 |
| Alytus | Alytus city | 54.38806 | 24.00010 | T1J |
| Cornus sericea | ||||
| Juodlė | Kelmė distr. | 55.81489 | 22.92023 | T1F |
| Klaipėda | Klaipėda city | 55.65867 | 21.23459 | T12 |
| Jurbarkas | Jurbarkas city | 55.08171 | 22.72513 | V64 |
| Žiežmariai | Kaišiadorys distr. | 54.81564 | 24.44084 | T1J |
| Darsūniškis | Kaišiadorys distr. | 54.74778 | 24.13509 | T12 |
| Cornus sanguinea subsp. sanguinea | ||||
| Raguviškiai | Kretinga distr. | 55.85995 | 21.39046 | T11 |
| Šilinė | Jurbarkas distr. | 55.08856 | 22.94780 | T1B |
| Piepaliai | Kaunas distr. | 55.07423 | 23.78337 | S35 |
| Vilnius | Vilnius city | 54.75331 | 25.29198 | T1E |
| Liūnelis | Lazdijai distr. | 54.12713 | 23.66146 | S35 |
| Cornus sanguinea subsp. australis | ||||
| Švobiškis | Pasvalys distr. | 56.05463 | 24.16271 | V64 |
| Šiauliai | Šiauliai city | 55.93154 | 23.28444 | S35 |
| Kaišiadorys | Kaišiadorys city | 54.87099 | 24.43677 | V64 |
| Maišiagala | Vilnius distr. | 54.86959 | 25.05138 | T1J |
| Vievis | Elektrėnai distr. | 54.77094 | 24.83838 | V64 |
| Taxa | C. alba | C. sericea | C. sanguinea subsp. sanguinea | C. sanguinea subsp. australis |
|---|---|---|---|---|
| Total number in both layers | 1183 | 433 | 712 | 1547 |
| Upper soil layer (0–5 cm) | ||||
| Total number of seeds | 1100 | 402 | 631 | 1347 |
| Percentage of the total number of seeds | 93.3 | 92.8 | 88.6 | 87.1 |
| Number of damaged seeds | 318 | 168 | 201 | 317 |
| Percentage of damaged seed in the layer | 28.9 | 41.8 | 31.9 | 23.5 |
| Number of intact seeds | 782 | 234 | 430 | 1030 |
| Percentage of intact seed in the layer | 71.1 | 58.2 | 68.1 | 76.5 |
| Number of viable seeds | 318 | 96 | 80 | 466 |
| Number of non-viable seeds | 464 | 138 | 350 | 564 |
| Lower soil layer (5–10 cm) | ||||
| Total number of seeds | 83 | 31 | 81 | 200 |
| Percentage of the total number of seeds | 6.8 | 7.2 | 11.4 | 12.9 |
| Number of intact seeds | 74 | 10 | 44 | 117 |
| Percentage of intact seed in the layer | 89.2 | 38.7 | 54.3 | 58.5 |
| Number of damaged seeds | 9 | 19 | 37 | 83 |
| Percentage of damaged seed in the layer | 10.8 | 61.3 | 45.7 | 41.5 |
| Number of viable seeds | 39 | 6 | 8 | 52 |
| Number of non-viable seeds | 35 | 4 | 36 | 65 |
| Site Name | Intact Seeds | Viable Seeds | Non-Viable Seeds | Viable Seeds (%) | Non-Viable Seeds (%) |
|---|---|---|---|---|---|
| Cornus alba | |||||
| Panevėžys | 1095 ± 480 a | 430 ± 267 a | 665 ± 283 a | 39.3 | 60.7 |
| Surgėliai | 555 ± 361 b | 250 ± 191 ab | 305 ± 199 b | 45.0 | 55.0 |
| Kiemeliai | 590 ± 346 b | 185 ± 224 b | 405 ± 246 b | 31.4 | 68.6 |
| Trakai | 415 ± 308 b | 185 ± 182 b | 230 ± 210 b | 44.6 | 55.4 |
| Alytus | 1255 ± 857 a | 540 ± 554 a | 715 ± 434 a | 43.0 | 57.0 |
| All sites pooled | 782 ± 601 A | 318 ± 348 A | 464 ± 348 A | 40.7 | 59.3 |
| Cornus sericea | |||||
| Juodlė | 240 ± 330 a | 65 ± 79 abc | 175 ± 268 a | 27.1 | 72.9 |
| Klaipėda | 285 ± 323 a | 180 ± 248 ab | 105 ± 124 a | 63.2 | 36.8 |
| Jurbarkas | 255 ± 161 a | 30 ± 56 ac | 225 ± 126 b | 11.8 | 88.2 |
| Žiežmariai | 150 ± 105 a | 80 ± 87 abc | 70 ± 78 a | 53.3 | 46.7 |
| Darsūniškis | 240 ± 266 a | 125 ± 122 b | 115 ± 174 a | 52.1 | 47.9 |
| All sites pooled | 234 ± 252 B | 96 ± 147 B | 138 ± 177 B | 41.0 | 59.0 |
| Cornus sanguinea subsp. sanguinea | |||||
| Raguviškiai | 825 ± 795 a | 215 ± 347 a | 610 ± 500 a | 26.1 | 73.9 |
| Šilinė | 120 ± 161 b | 15 ± 36 b | 105 ± 143 b | 12.5 | 87.5 |
| Piepaliai | 615 ± 453 a | 105 ± 156 ac | 510 ± 356 a | 17.1 | 82.9 |
| Vilnius | 70 ± 117 b | 20 ± 51 b | 50 ± 81 b | 28.6 | 71.4 |
| Liūnelis | 525 ± 265 a | 45 ± 59 bc | 480 ± 238 a | 8.6 | 91.4 |
| All sites pooled | 431 ± 517 C | 80 ± 197 B | 351 ± 386 C | 18.6 | 81.4 |
| Cornus sanguinea subsp. australis | |||||
| Švobiškis | 2280 ± 1248 a | 585 ± 408 a | 1695 ± 998 a | 25.7 | 74.3 |
| Šiauliai | 345 ± 268 bd | 170 ± 149 b | 175 ± 176 bd | 49.3 | 50.7 |
| Kaišiadorys | 605 ± 309 c | 1390 ± 1810 a | 345 ± 269 ac | 80.1 | 19.9 |
| Maišiagala | 185 ± 193 b | 130 ± 152 bc | 55 ± 74 d | 70.3 | 29.7 |
| Vievis | 1735 ± 1912 cd | 55 ± 112 c | 550 ± 291 e | 9.1 | 90.9 |
| All sites pooled | 1030 ± 1315 A | 466 ± 978 A | 564 ± 769 AC | 45.2 | 54.8 |
| Effects | Estimate | SE | z-Value | p-Value | Variance | SD |
|---|---|---|---|---|---|---|
| Upper layer | ||||||
| f C. alba | 1.966 | 0.298 | 6.60 | <0.001 | ||
| f C. sericea | 0.820 | 0.303 | 2.71 | <0.01 | ||
| f C. sanguinea subsp. sanguinea | 1.079 | 0.304 | 3.55 | <0.001 | ||
| f C. sanguinea subsp. australis | 1.839 | 0.299 | 6.15 | <0.001 | ||
| r Locality | 0.436 | 0.660 | ||||
| Lower layer | ||||||
| f C. alba | −0.440 | 0.458 | −0.96 | 0.336 | ||
| f C. sericea | −2.365 | 0.546 | −4.33 | <0.001 | ||
| f C. sanguinea subsp. sanguinea | −1.495 | 0.515 | −2.90 | <0.001 | ||
| f C. sanguinea subsp. australis | −0.414 | 0.468 | −0.89 | 0.376 | ||
| r Locality | 0.961 | 0.980 |
| Effects | Estimate | SE | z-Value | p-Value | Variance | SD |
|---|---|---|---|---|---|---|
| Upper layer | ||||||
| f C. alba | 1.939 | 0.241 | 8.04 | <0.001 | ||
| f C. sericea | 0.650 | 0.252 | 2.59 | <0.01 | ||
| f C. sanguinea subsp. sanguinea | 1.224 | 0.243 | 5.04 | <0.001 | ||
| f C. sanguinea subsp. australis | 1.988 | 0.242 | 8.22 | <0.001 | ||
| r Cornus cover | 0.279 | 0.528 | ||||
| Lower layer | ||||||
| f C. alba | −0.399 | 0.498 | −0.80 | 0.423 | ||
| f C. sericea | −1.167 | 0.573 | −2.82 | <0.01 | ||
| f C. sanguinea subsp. sanguinea | −1.313 | 0.519 | −2.53 | 0.011 | ||
| f C. sanguinea subsp. australis | 0.661 | 0.504 | 1.31 | 0.190 | ||
| r Cornus cover | 1.127 | 1.062 |
| Site Name | Intact Seeds | Viable Seeds | Non-Viable Seeds | Viable Seeds (%) | Non-Viable Seeds (%) |
|---|---|---|---|---|---|
| Cornus alba | |||||
| Panevėžys | 25 ± 64 a | 10 ± 31 a | 15 ± 49 a | 40.0 | 60.0 |
| Surgėliai | 50 ± 100 ab | 25 ± 64 a | 25 ± 44 ab | 50.0 | 50.0 |
| Kiemeliai | 90 ± 129 a | 40 ± 82 a | 50 ± 76 ab | 44.4 | 55.6 |
| Trakai | 110 ± 137 b | 60 ± 109 a | 50 ± 61 b | 54.5 | 45.5 |
| Alytus | 95 ± 123 b | 60 ± 109 a | 35 ± 49 ab | 63.2 | 36.8 |
| All sites pooled | 74 ± 116 A | 39 ± 84 A | 35 ± 56 A | 52.7 | 47.3 |
| Cornus sericea | |||||
| Juodlė | 10 ± 31 a | 10 ± 31 a | 0 a | 100 | 0 |
| Klaipėda | 0 a | 0 a | 0 a | 0 | 0 |
| Jurbarkas | 15 ± 67 a | 5 ± 22 a | 10 ± 44 a | 33.3 | 66.7 |
| Žiežmariai | 10 ± 30 a | 10 ± 30 a | 0 a | 100 | 0 |
| Darsūniškis | 15 ± 37 a | 5 ± 22 a | 10 ± 31 a | 33.3 | 66.7 |
| All sites pooled | 10 ± 43 B | 6 ± 24 B | 4 ± 24 B | 60.0 | 40.0 |
| Cornus sanguinea subsp. sanguinea | |||||
| Raguviškiai | 125 ± 141 a | 30 ± 57 a | 95 ± 94 a | 24.0 | 76.0 |
| Šilinė | 0b | 0 b | 0 b | 0 | 0 |
| Piepaliai | 15 ± 37 b | 5 ± 22 b | 10 ± 31 b | 33.3 | 66.7 |
| Vilnius | 5 ± 22 b | 0 b | 5 ± 22 b | 0 | 100 |
| Liūnelis | 75 ± 107 a | 5 ± 22 b | 70 ± 103 a | 6.7 | 93.3 |
| All sites pooled | 44 ± 94 C | 8 ± 31 B | 36 ± 75 A | 18.2 | 81.8 |
| Cornus sanguinea subsp. australis | |||||
| Švobiškis | 315 ± 287 a | 95 ± 94 a | 220 ± 238 a | 30.2 | 69.8 |
| Šiauliai | 35 ± 59 b | 35 ± 59 bc | 0 b | 100 | 0 |
| Kaišiadorys | 170 ± 210 c | 110 ± 174 ab | 60 ± 88 c | 64.7 | 35.3 |
| Maišiagala | 5 ± 22 bd | 5 ± 22 c | 0b | 100 | 0 |
| Vievis | 60 ± 114 a | 15 ± 37 c | 45 ± 115 bc | 25.0 | 75.0 |
| All sites pooled | 117 ± 201 A | 52 ± 102 A | 65 ± 147 A | 44.4 | 55.6 |
| Effects | Estimate | SE | z-Value | p-Value | Variance | SD |
|---|---|---|---|---|---|---|
| Upper layer | ||||||
| f C. alba | 2.014 | 0.146 | 13.81 | <0.001 | ||
| f C. sericea | 0.990 | 0.155 | 6.38 | <0.001 | ||
| f C. sanguinea subsp. sanguinea | 1.545 | 0.150 | 10.33 | <0.001 | ||
| f C. sanguinea subsp. australis | 1.971 | 0.146 | 13.53 | <0.001 | ||
| r Habitat type | 0.0579 | 0.2407 |
| Taxa | Scrub (S) | Forest (T) | Human-Made (V) |
|---|---|---|---|
| Upper layer | |||
| C. alba | 825 ± 500 a | 923 ± 728 a | 415 ± 308 b |
| C. sericea | – | 229 ± 271 a | 255 ± 161 a |
| C. sanguinea subsp. sanguinea | 570 ± 369 a | 337 ± 193 b | – |
| C. sanguinea subsp. australis | 345 ± 268 a | 185 ± 193 a | 1540 ± 1485 b |
| Lower layer | |||
| C. alba | 38 ± 84 a | 93 ± 125 b | 110 ± 137 b |
| C. sericea | – | 10 ± 34 a | 20 ± 70 a |
| C. sanguinea subsp. sanguinea | 45 ± 85 a | 43 ± 100 a | – |
| C. sanguinea subsp. australis | 35 ± 59 a | 5 ± 22 a | 182 ± 237 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrulaitis, L.; Rašomavičius, V.; Uogintas, D.; Gudžinskas, Z. Soil Seed Bank of Alien and Native Cornus (Cornaceae) Taxa in Lithuania: What Determines Seed Density and Vertical Distribution in Soil? Diversity 2022, 14, 488. https://doi.org/10.3390/d14060488
Petrulaitis L, Rašomavičius V, Uogintas D, Gudžinskas Z. Soil Seed Bank of Alien and Native Cornus (Cornaceae) Taxa in Lithuania: What Determines Seed Density and Vertical Distribution in Soil? Diversity. 2022; 14(6):488. https://doi.org/10.3390/d14060488
Chicago/Turabian StylePetrulaitis, Lukas, Valerijus Rašomavičius, Domas Uogintas, and Zigmantas Gudžinskas. 2022. "Soil Seed Bank of Alien and Native Cornus (Cornaceae) Taxa in Lithuania: What Determines Seed Density and Vertical Distribution in Soil?" Diversity 14, no. 6: 488. https://doi.org/10.3390/d14060488
APA StylePetrulaitis, L., Rašomavičius, V., Uogintas, D., & Gudžinskas, Z. (2022). Soil Seed Bank of Alien and Native Cornus (Cornaceae) Taxa in Lithuania: What Determines Seed Density and Vertical Distribution in Soil? Diversity, 14(6), 488. https://doi.org/10.3390/d14060488







