Abstract
Pinus contorta is considered one of the most invasive tree species worldwide, generating significant impacts on biodiversity and ecosystems. In several Patagonian ecosystems in southern Chile, it has escaped from plantations established mainly in the 1970s, and is now invading both forests and treeless environments. In this study, we evaluated the impact of the invasion of P. contorta on microenvironmental conditions in Araucaria araucana forest and Patagonian steppe ecosystems, and assessed how these changes related to the richness and abundance of native and non-native plant species. In each ecosystem, 24 plots of 100 m2 were established along a gradient of P. contorta biomass, where 18 environmental variables and the composition of native and non-native vegetation were measured at a local scale. Our results indicated that increased pine biomass was associated with differences in microclimatic conditions (soil and air temperature, photosynthetically active radiation (PAR), and soil moisture) and soil properties (potassium, nitrate, pH, and litter accumulation). These changes were ecosystem dependent, however, as well as associated with the level of invasion. Finally, the reduction in the richness and abundance of native plants was associated with the changes in soil properties (accumulation of leaf litter, pH, and organic matter) as well as in the microclimate (minimum air temperature, PAR) generated by the invasion of P. contorta. Overall, our results confirm that the invasion of P. contorta impacts microenvironmental conditions (i.e., canopy cover, litter accumulation, minimum air temperature, and maximum soil temperature) and reduces native plant diversity. For future restoration plans, more emphasis should be given to how environmental changes can influence the recovery of invaded ecosystems even after the removal of the living pine biomass (i.e., legacy of the invasion).
1. Introduction
The invasion of non-native trees generates a range of impacts on ecosystems, from loss of species [1] and of ecosystem functioning [2] to changes in environmental conditions [3], nutrient cycling, litter decomposition, and soil biota [4]. These impacts are context dependent and generally change in relation to the level of invasion [5,6,7]. Among non-native tree species, some of the most invasive species worldwide belong to the genus Pinus spp. [8]. These species have historically been introduced into different ecosystems worldwide because of the forestry industry, and have become especially problematic in South Africa, Australia, New Zealand, and more recently in South America [9]. Because of their wide invasive range and the multiple ecosystems they invade, they have been proposed as a model for understanding the impact of invasive species on ecosystems [10].
In Chile, Pinus spp. were introduced during the 16th century for ornamental use and later for erosion control and dune stabilization [11]. During the 20th century, use of Pinus species in forestry was promoted and many experimental monocultures were planted, generating a significant change in land use in native ecosystems, mainly in the central-southern zone of Chile [12]. These deliberate introductions, coupled with high anthropogenic pressure, have allowed some of these exotic species to disperse beyond their original introduction sites, invading a large part of central and southern Chile [11,13]. Among the different introduced Pinus species in Chile, Pinus contorta has been one of the most successful in invading sites beyond the original population, showing high invasibility, especially in cold ecosystems [14].
The high invasibility of P. contorta is mainly due to its early seed production, small seed size and short time intervals between massive seed production events, coupled with high growth rates under a broad range of environmental conditions [15,16]. This invasion success is further favored by natural and anthropogenic disturbances (e.g., fire), and the fact that tree-free ecosystems such as grasslands and shrublands facilitate the establishment of P. contorta [15,16]. A positive feedback loop between the level of invasion (establishment rate) and fire frequency has been shown to exist, but only in dense Pinus contorta stands (>1000 trees/ha) [16,17].
The study of the ecosystem impacts of P. contorta in Chile has been focused mainly on the impacts on the diversity of native plants, the influence on the fire cycle, and on the functional traits of native communities [6,18,19]. In Araucaria araucana forest and Patagonian steppe ecosystems, it has, for example, been shown that the presence of P. contorta decreases native species richness [6,19]. Furthermore, the increase in abundance of P. contorta has generated changes in the functional traits of the Patagonian steppe community, mainly favoring the presence of traits related to shade tolerance and the selection of conservative reproductive strategies. These changes are associated with the increase in tree cover generated by P. contorta and the subsequent decrease in photosynthetically active radiation (PAR) [19]. Finally, individuals of P. contorta have been shown to have a higher flammability compared with native species of the genus Nothofagus spp. in the Araucaria araucana forests, which increases the risk of fire in invaded ecosystems [18].
This increasing knowledge of the impacts of P. contorta invasion on plant diversity contrasts with what is known about the impact of its invasion on environmental variables, specifically on soil nutritional conditions (nutrients, pH, leaf litter, etc.) and microclimate (soil and air temperature, soil moisture). Quantifying these impacts can help to elucidate potential underlying mechanisms responsible for the decrease in native species richness and abundance associated with P. contorta invasion. For invasions by other tree species, significant changes in microclimatic conditions have already been observed, with important downstream effects on the richness and abundance patterns of native species. For example, the presence of the invasive species Robinia pseudoacacia in South African grasslands has been shown to decrease average temperatures by about 2 to 3 °C. It also changed light availability, which directly impacted the abundance of endemic arthropods (family Acrididae) due to habitat transformation [3]. Similarly, the invasion by Pinus mugo in alpine zones of central Europe influenced the microclimate (decreased water and light availability) and soil conditions (more fertile soils), generating changes in the storage of organic matter and increasing soil acidity. Here also, consequent modifications in the patterns of richness and abundance of native species in alpine ecosystems were observed [4].
Understanding these environmental changes generated by the invasion of P. contorta in Patagonian ecosystems can not only help to disentangle the mechanisms underlying the decline in the richness and abundance of native species but can also help to predict the long-term impact of the invasion on ecosystems, especially once the invasion has been controlled through conservation or restoration programs [20]. Therefore, considering the current patterns of P. contorta invasion in Araucaria araucana forest and Patagonian steppe in Chile, it is important to ask how the increased biomass of the P. contorta invasion impacts environmental conditions (i.e., microclimate, soil conditions and nutrients) of the invaded ecosystems and if these local changes in conditions can explain the decrease in native plant diversity generated by the invasion of P. contorta in these invaded ecosystems.
Knowing all this, we set out to evaluate the changes in environmental conditions across an invasion gradient of P. contorta in mountain forest (i.e., Araucaria araucana forest) and steppe (i.e., Patagonian steppe) ecosystems in southern Chile. We also analyzed how the resulting steep gradients in microenvironmental conditions were associated with trends in native plant diversity. We hypothesized that the increase in biomass of P. contorta modified the environmental conditions (microclimate, soil conditions and nutrients) of invaded ecosystems, generating conditions with less seasonal variation (i.e., cooler summers and warmer winters, drier summers) and lower nutrient availability. In addition, we hypothesized that the environmental changes generated by the increase in P. contorta biomass would coincide with a decrease in native plant richness in the invaded ecosystems.
2. Materials and Methods
2.1. Study Area
This study was carried out during the growing season of 2014–2015 in two sites in southern Chile, an open temperate mountain forest and a steppe ecosystem (Figure 1a). The Araucaria araucana forest was located on the southern slopes of the Lonquimay volcano, within the Malalcahuello National Reserve, in south-central Chile (38° S 25′20″–71° W 32′31″), at altitudes of ~1440 m.a.s.l. This ecosystem has a cool climate (mean annual temperatures of 8.5 °C) and a large amount of precipitation (~3083 mm), mainly in the form of winter snow. The vegetation of this ecosystem has been shaped by natural pressures resulting from glaciation processes and intense volcanic activity. Within this ecosystem, we can find Araucaria araucana forest near the treeline, often with an understory of Festuca scabriuscula, whereas the lower elevations are dominated by Nothofagus spp. forests (e.g., N. antarctica, N. obliqua, N. alpina) and a dense undergrowth of the native bamboo Chusquea culeou (Table S1) [11].
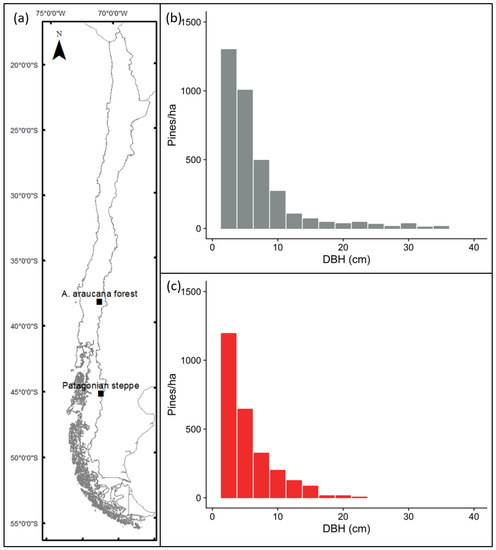
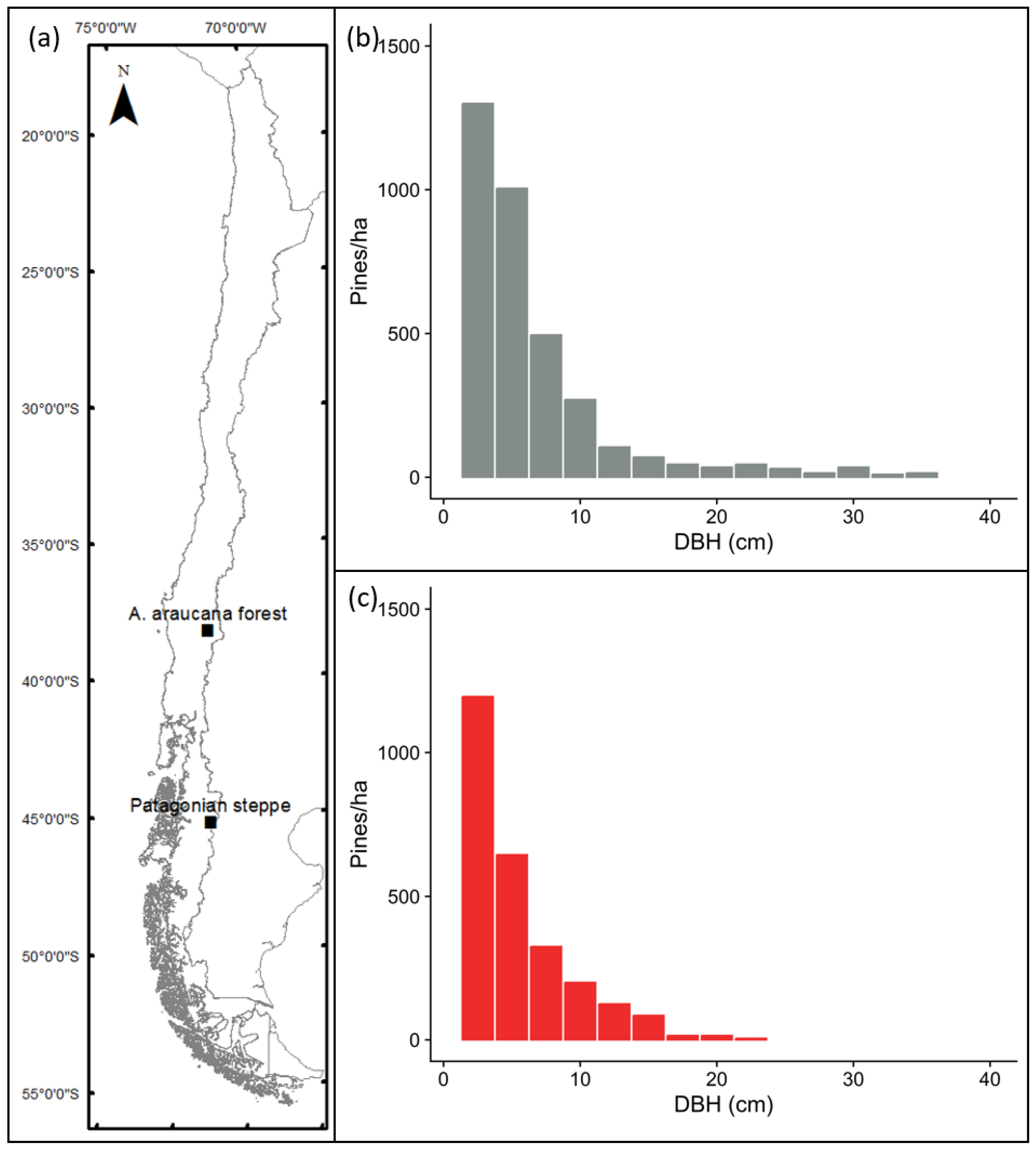
Figure 1.
Location of the study sites in the Malalcahuello National Reserve (A. araucana forest) and Coyhaique Alto (Patagonian steppe) (a). Average diametric distribution (pines > 1 m) along the invasion gradient for A. araucana forest (b) and Patagonian steppe (c) ecosystems.
The second studied ecosystem was a Patagonian steppe near the city of Coyhaique (45° S 30′05″–72° W 42′10″) at altitudes close to 730 m.a.s.l. The climate in this area is considered dry and cold, characterized by high precipitation, again mainly in the form of snow, with a precipitation of less than 80 mm during the growing season. Mean annual temperatures are around 6–9 °C. The vegetation is dominated by herbaceous species such as Festuca pallescens, and some cushion plants: Baccharis magellanica, Acaena integerrina, and Mulinum spinosum (Table S1).
In both ecosystems, P. contorta was introduced during the 1970s〒1980s, and since the original planting both areas have seen an increase in the distribution of P. contorta outside the original plantations [10,11]. In the A. araucana forest in Malalcahuello, there is now an invaded area greater than 200 ha, with about 5500 pines (>1 m) per ha in the core area, which is the product of an invasion process which began in the 1990s (Figure 1b). In the Patagonian steppe, the invasion is still at an earlier stage, with about 3100 pines per hectare. The currently invaded area of close to 100 ha results from only 8 years of invasion outside the original plantation (Figure 1c). The spread of pines has caused an invasion gradient, with higher pine biomass and older, bigger trees near the edge of the plantation and very sparse small trees and low biomass further from the edge [6,14].
2.2. Experimental Design
Across the P. contorta invasion gradient in both regions, 24 plots of 10 × 10 m were randomly selected, using stratified sampling to ensure that all pine biomass levels were adequately represented (Figure 2a,b). Within each 100 m2 plot, the dendrometric characteristics (i.e., DBH and height) of all tree individuals were measured and the total aerial biomass of P. contorta was estimated based on the mathematical functions previously developed for this species (Figure 1b,c) [18]. In the case of the A. araucana forest, pine biomass ranged from 0 to 156 t/ha−1 of P. contorta, whereas in the Patagonian steppe it ranged from 0 to 50.26 t/ha−1.
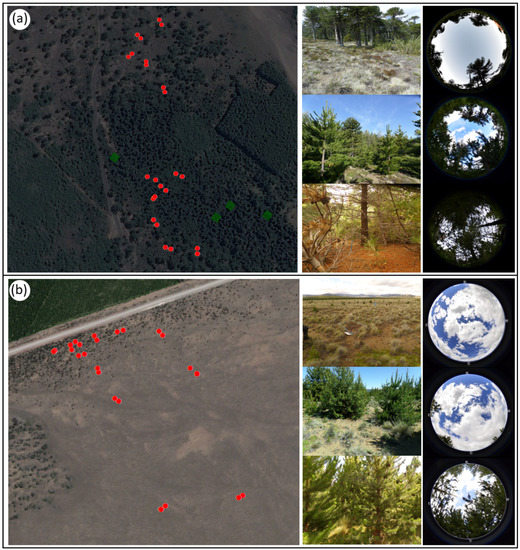
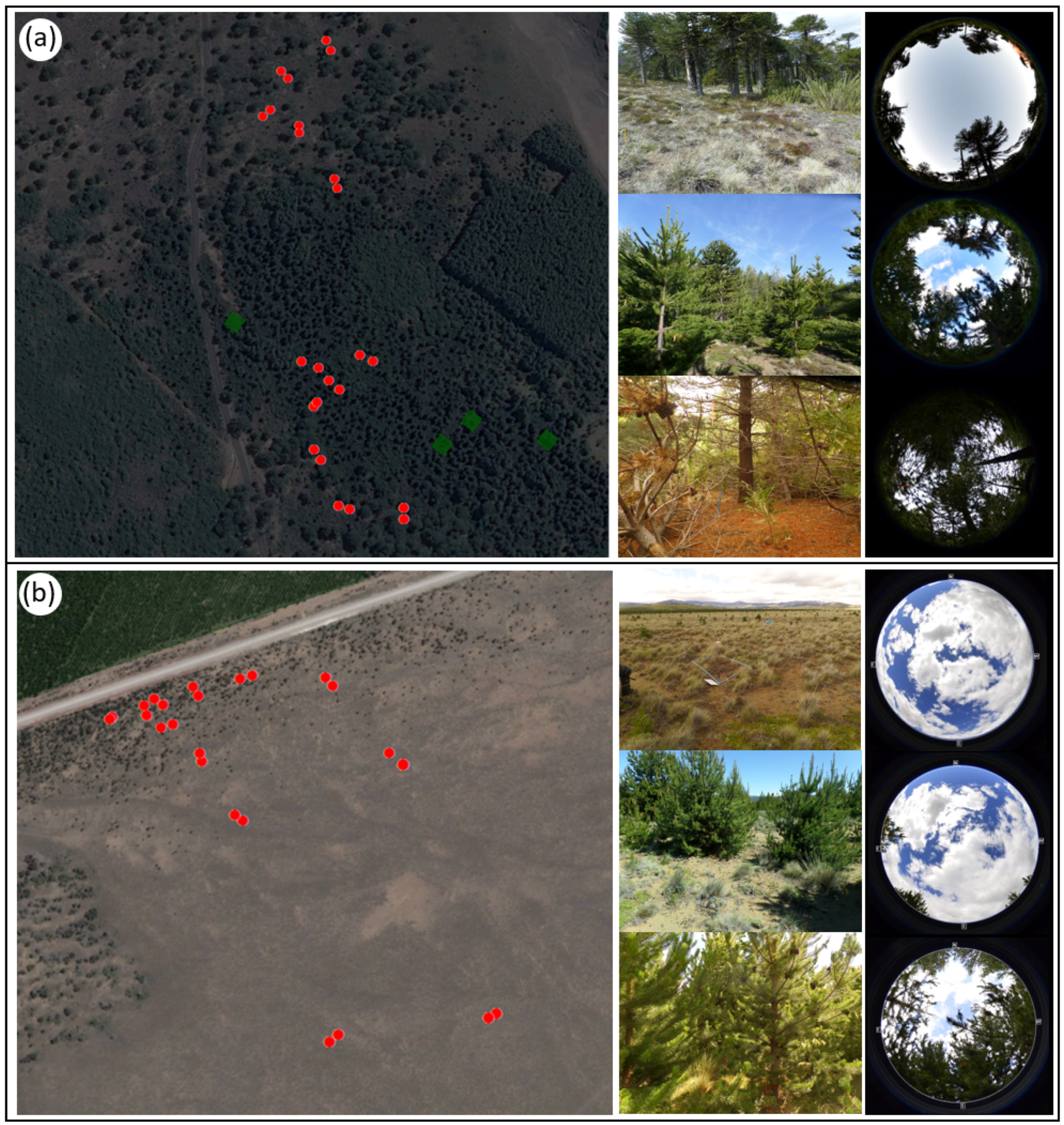
Figure 2.
Aerial photograph of the study sites with 10 × 10 m plots along an invasion gradient (red dots) and general characterization associated with hemispheric photos of the level of invasion (low, medium, and high) of P. contorta for A. araucana forests (a) and the Patagonian steppe (b). Dark green polygons show initial establishment of P. contorta plantations.
In the 100 m2 plots, canopy cover was measured in the center of the plot using a hemispheric camera at 1 m above the ground. In each plot, soil moisture and temperature were recorded at 5 cm depth with a Hobo Micro station H21-002 datalogger. Near-surface air temperature was recorded at 20 cm above the soil in each plot with a HOBO U23-001 Pro v2 Temp/HR sensor. Soil and air temperature and soil moisture data were recorded every hour over the summer.
In addition, each 10 × 10 m plot had four 1 m2 interior subplots which were used to measure local variables of species richness listing total native and non-native species, total abundance (measured as the added percentage cover of each species), light availability, soil nutrients, and litter content.
PAR (photosynthetically active radiation, in µmolm−2s−1) was measured in summer at midday (12–14 pm) with approximately 3 monthly measurements using a luxmeter. Soil samples were taken (25 g per subplot) until 100 g were obtained from the first 10 cm of soil (O-horizons). Each sample was analyzed for phosphorus (P) and potassium (K) content (mg/kg), pH in water, nitrogen measured as nitrate (NO3−) in mg/kg, and organic matter content by calcination. Analyses were carried out at the Soil, Water, and Forestry Laboratory of the Universidad de Concepción. Finally, within each 1 m2 subplot, total litter content and litter depth were measured. The total litter content was measured by collecting all litter present in 400 cm2 of each subplot, which was dried in an oven at 70 °C until it reached a constant weight and then weighed.
All variables were measured during the growing season (December–February). Microclimatic variables (soil temperature, air temperature, and humidity) were averaged over that same period.
2.3. Data Analysis
All analyses were run in R version 4.1.1 [21].
To evaluate the relationship between the P. contorta biomass and plant diversity (i.e., richness and abundance of native and non-native plant species), soil properties (i.e., litter content and soil nutrients), and microclimatic conditions (i.e., soil and air temperature, soil moisture, and light availability) in both the A. araucana forest and the Patagonian steppe, we used linear regression models, using the “lm” function of the base package. The biomass of P. contorta was used as the independent variable in all analyses, with the 18 environmental variables measured in this study as dependent variables.
Similarly, to determine whether changes in P. contorta biomass and soil and microclimate conditions correlated with changes in native plants’ richness, generalized linear models (GLM) were made, using native species richness as the dependent variable and soil and microclimate variables as the independent variables (only variables that were modified by the presence of P. contorta were included in this analysis). The “glm” function was used to perform the GLM, and the models were adjusted to a Poisson distribution error with a log link function to correct for log-normality in the species richness and total abundance data. All graphs were made with the ggplot2 package [22].
3. Results
3.1. Impact of P. contorta Invasion on Environmental Conditions
Increased density of P. contorta invasion was associated with significant changes in microclimatic conditions and soil properties. These changes were, however, different in the two regions (i.e., context dependent). For variables such as canopy cover, PAR light, total litter, nitrate content, and richness and abundance of native species, the effect of P. contorta was more intense in the Patagonian steppe than in the A. araucana forest (Table 1).

Table 1.
Estimated parameters, standard errors (SE), t-values, p-values, and Cohen’s coefficients for effect size (f2) for the models of both sites for all variables measured as a function of P. contorta biomass. Significant p-values are marked in bold (p-value < 0.05).
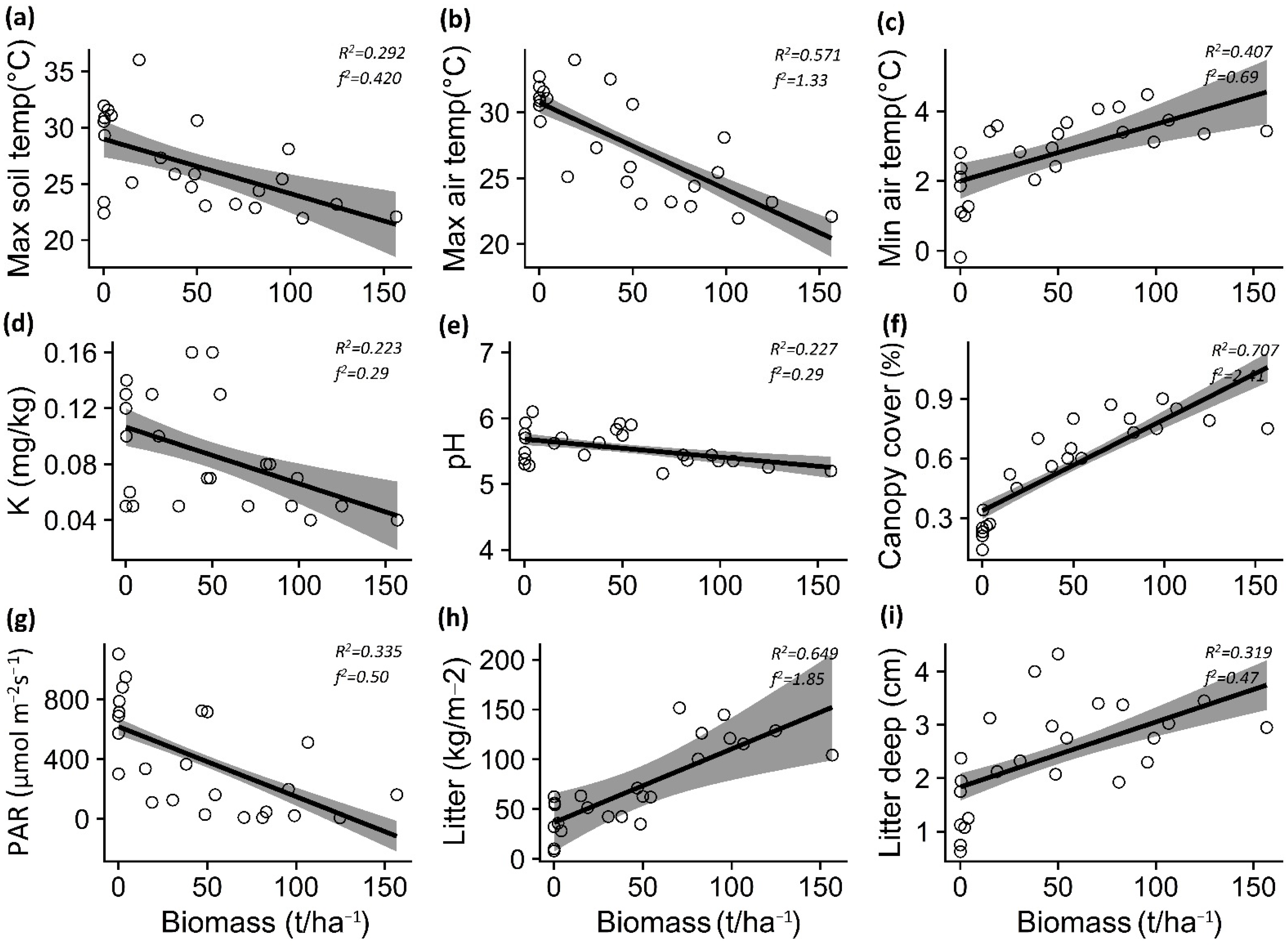
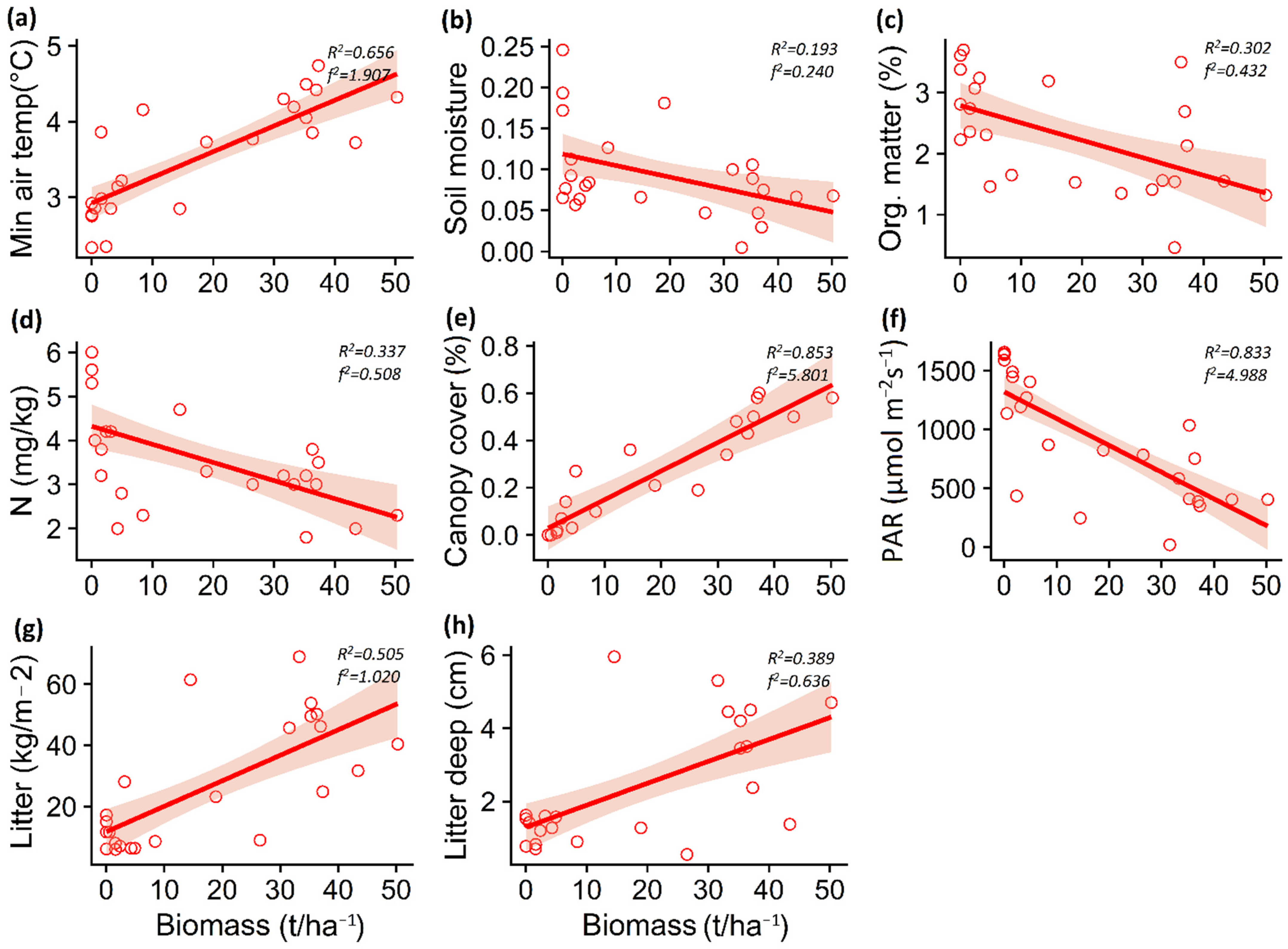
The increase in P. contorta biomass in the A. araucana forest decreased the maximum soil temperature (p = 0.003) and air temperature (p < 0.001), but increased the minimum air temperature (p = 0.004) (Table 1; Figure 3a–c). In the Patagonian steppe, the increase in P. contorta biomass generated an increase in minimum air temperature (p < 0.001) and a decrease in soil moisture (p = 0.031) (Table 1; Figure 4a,b).
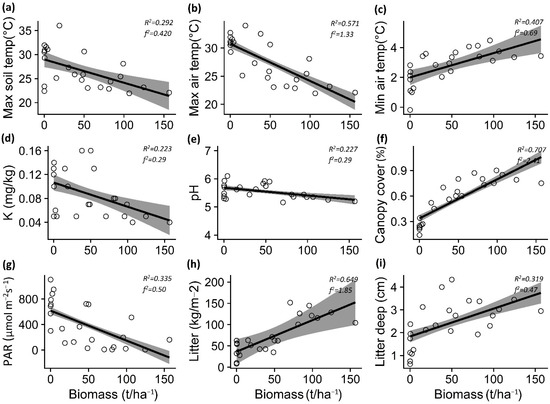
Figure 3.
Relationship between Pinus contorta biomass and maximum soil and air temperatures (a,b), minimum air temperature (c), potassium (d), pH (e), canopy cover (f), PAR light (g), total litter (h), and litter depth (i) for Araucaria araucana forest. The lines show the linear model fit and the 95% confidence interval. R2 is the adjusted R2.
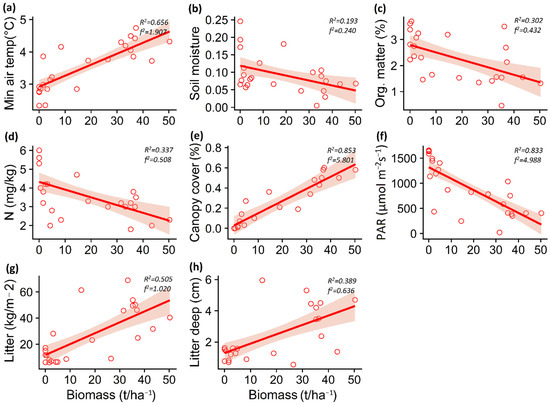
Figure 4.
Relationship between Pinus contorta biomass and minimum air temperature (a), soil moisture (b), organic matter (c), nitrate content (d), canopy cover (e), PAR light (f), total litter (g), and litter depth (h) for Patagonian steppe. The lines show the linear model fit and the 95% confidence interval. R2 is the adjusted R2.
For both ecosystems, PAR light decreased (p < 0.001) whereas canopy cover, total litter, and litter depth increased (p < 0.001) significantly with increasing P. contorta invasion (Table 1; Figure 3g–i and Figure 4f–h).
In the case of soil properties, the increase in P. contorta biomass in the A. araucana forest generated a decrease in potassium content (p = 0.019) and pH levels (p = 0.018) (Table 1; Figure 3d,e), whereas in the Patagonian steppe, the increase in biomass of P. contorta decreased both the percentage of organic matter (p = 0.005) and the nitrate content in the soil (p = 0.002) (Table 1, Figure 4c,d).
In summary, in the Patagonian steppe, P. contorta had the strongest effects on minimum air temperature, organic matter, nitrate content, canopy cover, PAR, and total litter, whereas in the A. araucana forest, it had the strongest effects on maximum soil and air temperature, minimum air temperature, canopy cover, PAR, total litter, and litter depth (Table 1).
3.2. Effect of Microenvironmental Change on Native Plant Diversity in Ecosystems Invaded by P. contorta
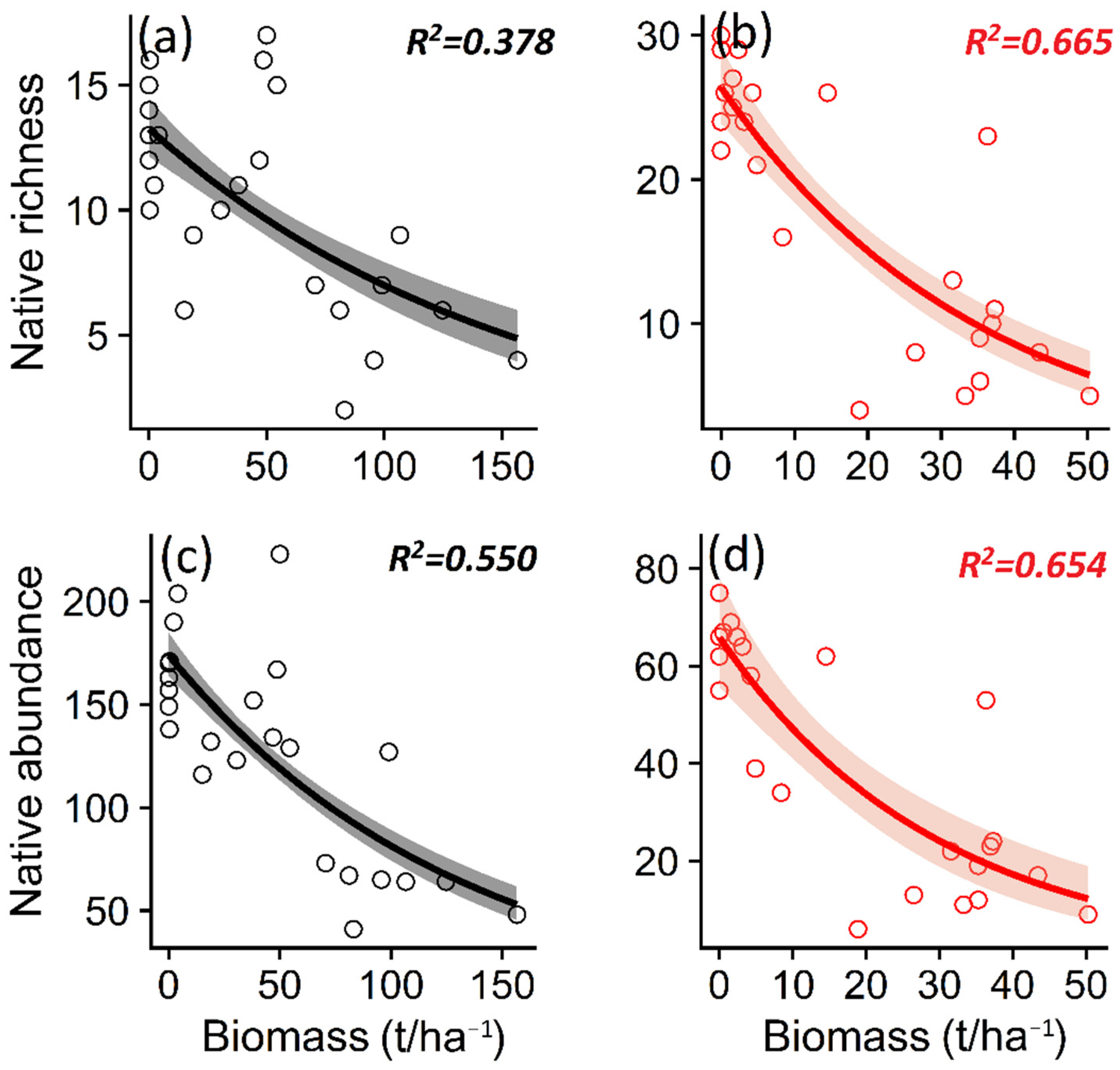
We observed that the invasion of P. contorta in both ecosystems was related to a lower native plant richness (p = 0.008/p < 0.001) and abundance (p < 0.001) (Table 1, Figure 5a–d), as well as non-native plant richness (p = 0.007/p = 0.005) and abundance (p < 0.001) (Table 1, Figure S1a–d).
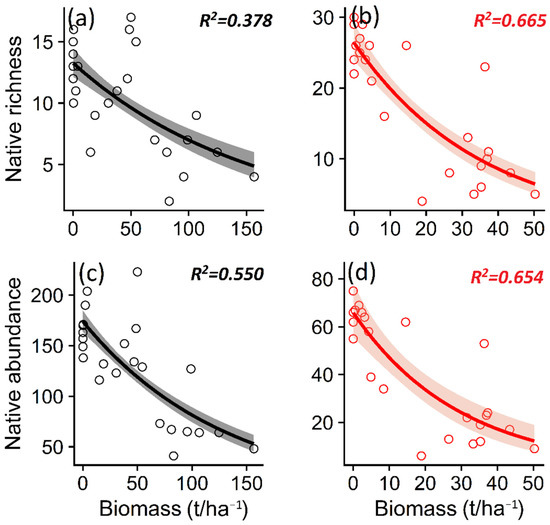
Figure 5.
Relationship between the biomass of Pinus contorta in Araucaria araucana forest and Patagonian steppe and native plant richness (a,b) and native plant total abundance (c,d). The lines show the linear model fit and the 95% confidence interval. R2 is the adjusted R2.
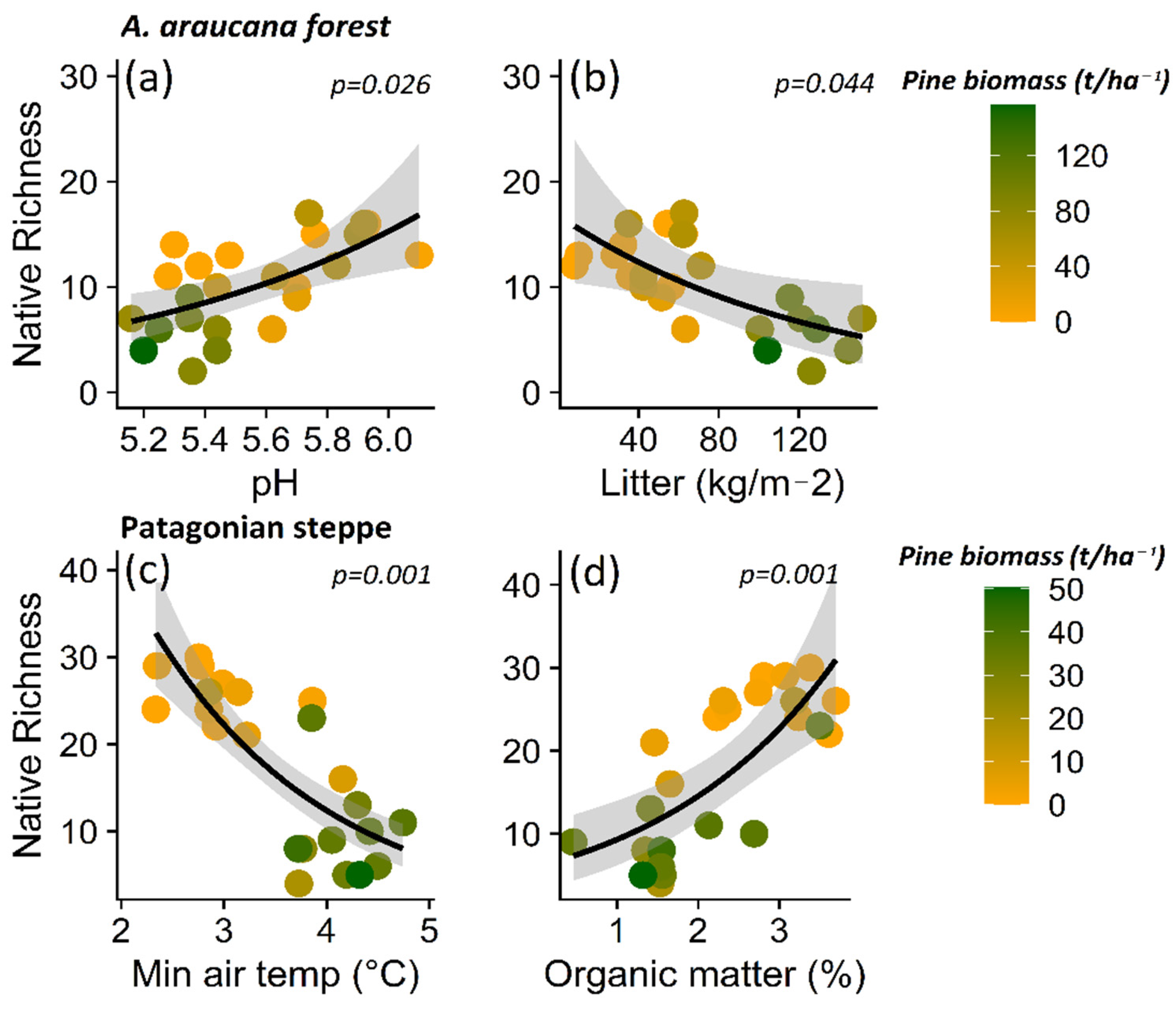
Based on these patterns, the decrease in native plant richness in the A. araucana forest was related most strongly to the increase in total litter content (p = 0.044) and decrease in pH levels (p = 0.034) (Table S2; Figure 6a,b). In the case of the Patagonian steppe, the decrease in native plant richness is explained by the increase in minimum air temperature (p = 0.001) and the decrease in organic matter (p = 0.001) (Table S3, Figure 6c,d).
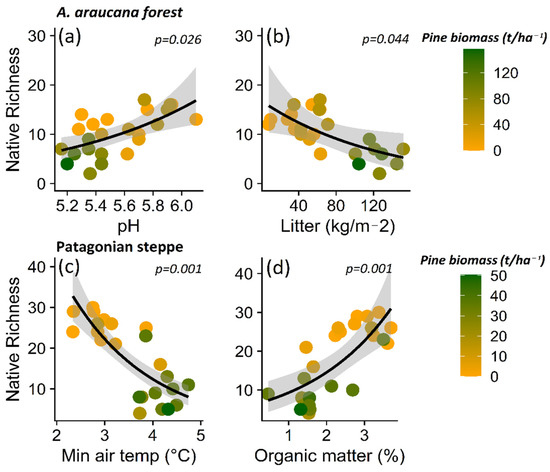
Figure 6.
Relationship between native plant species richness and pH (a) and litter content (b) for Araucaria araucana forest, and the relationship between native plants and minimum air temperature (c) and percentage organic matter (%) (d) for the Patagonian steppe. The lines show the linear model fit and the 95% confidence interval and the color gradient at the points corresponds to the biomass gradient of Pinus contorta.
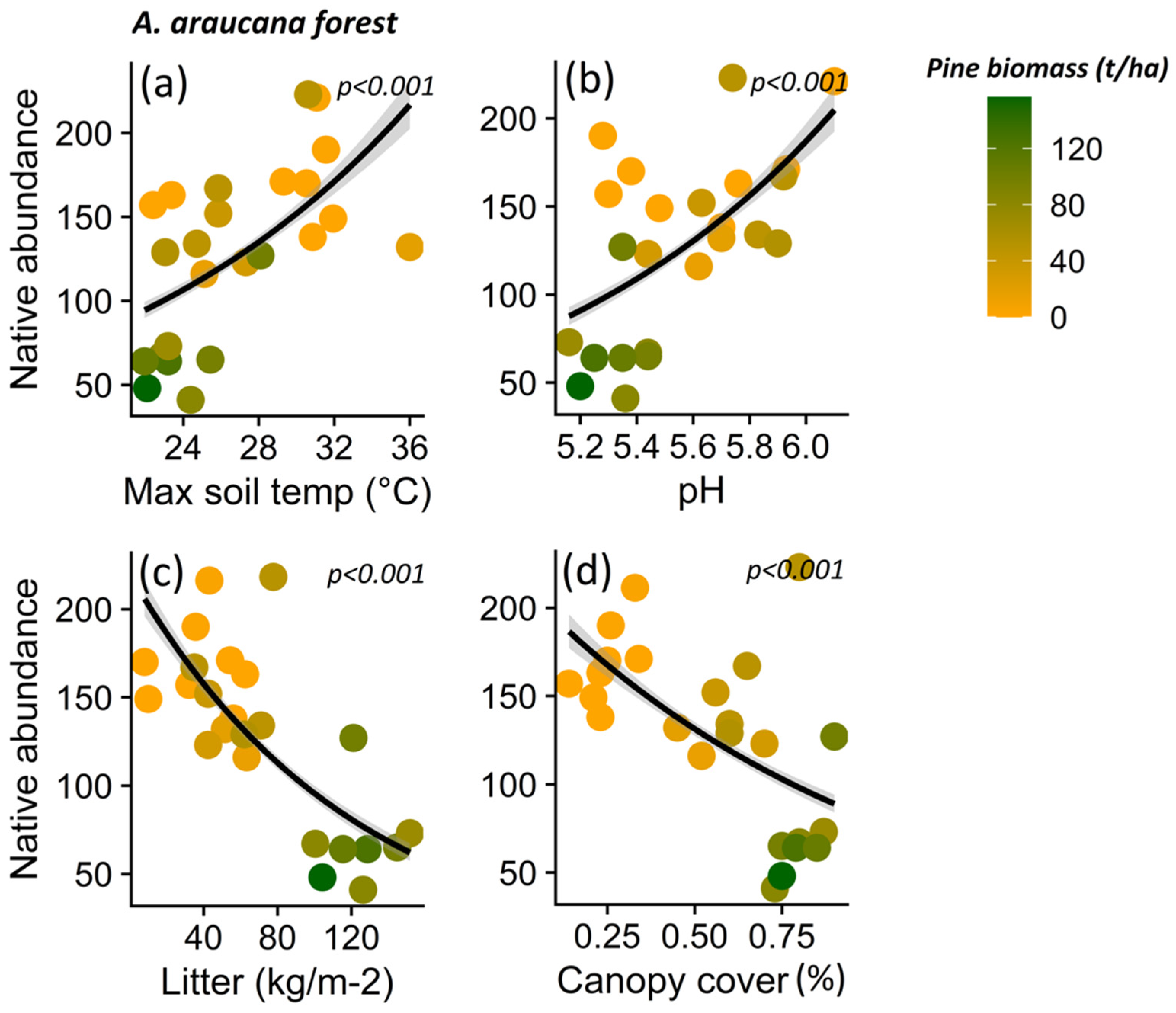
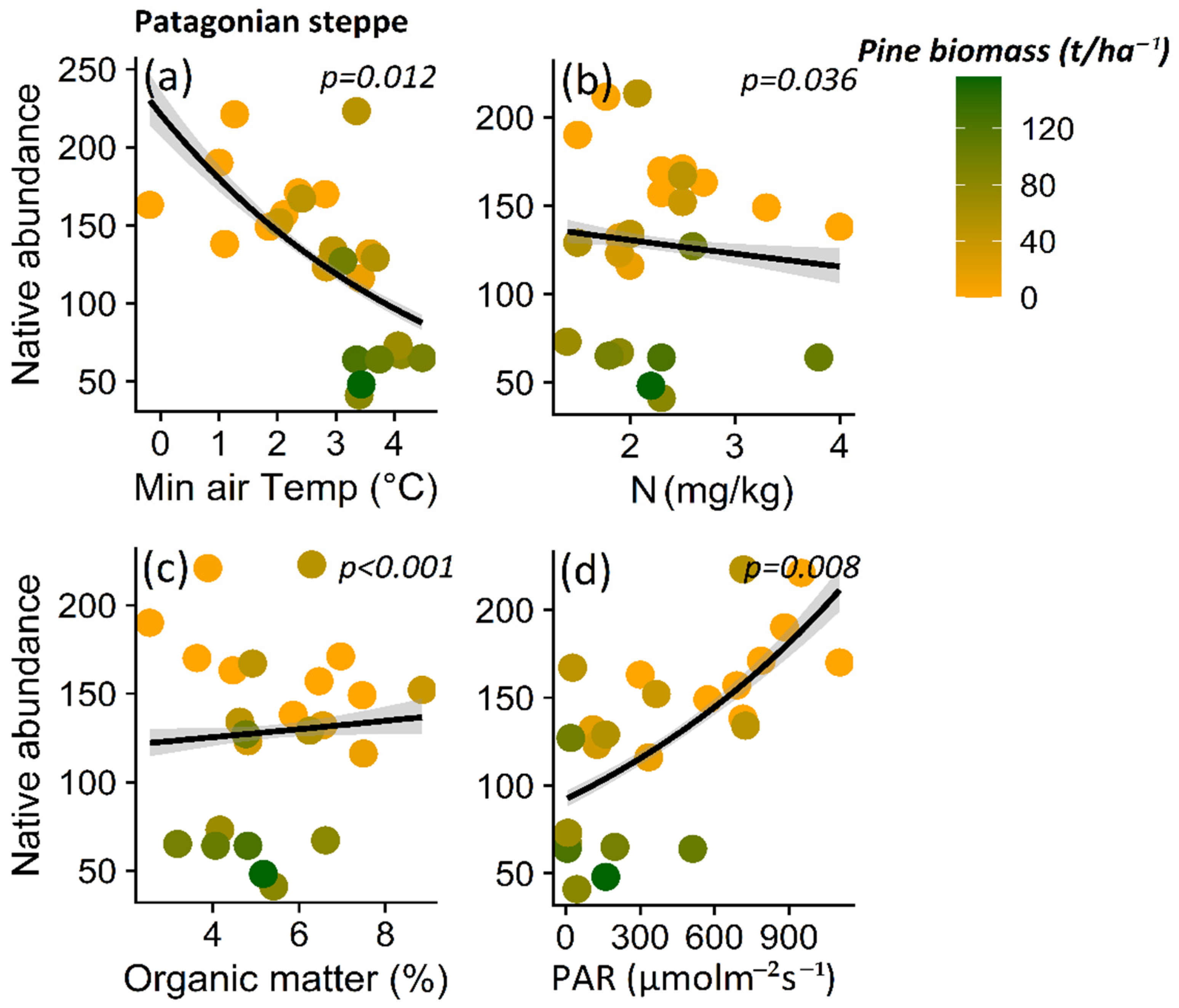
Native plant abundance for A. araucana forest decreased in parallel to decreasing maximum soil temperature (p < 0.001) (Figure 7a), pH (p < 0.001) (Figure 7b), litter content (p < 0.001) (Figure 7c), and canopy cover (p < 0.001) (Figure 7d), and other microclimatic and soil condition variables (i.e., maximum air temperature (p < 0.001) and potassium content (p = 0.037)) (Table S4). In the case of the Patagonian steppe, the abundance of native plants decreases with increasing minimum air temperature (p = 0.012) (Table S5; Figure 8a), nitrate content (p = 0.036) (Table S5; Figure 8b), and with decreasing organic matter content (p < 0.001) (Table S5; Figure 8c) and PAR light (p = 0.008) (Table S5; Figure 8d).
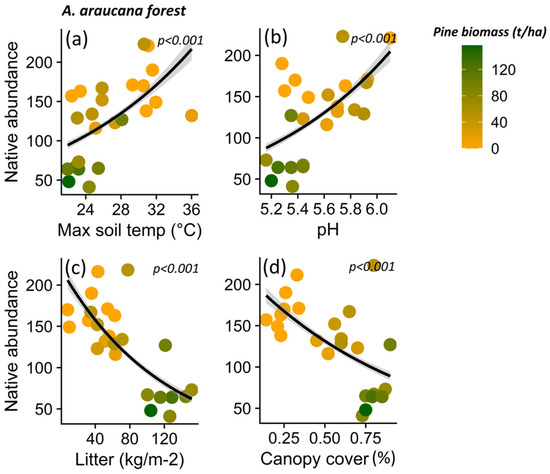
Figure 7.
Relationship between native plant species, total abundance and maximum soil temperature (a), pH (b), litter content (c), and canopy cover (d) for Araucaria araucana forest. The lines show the linear model fit and the 95% confidence interval, and the color gradient at the points corresponds to the biomass gradient of Pinus contorta for the Araucaria araucana forest.
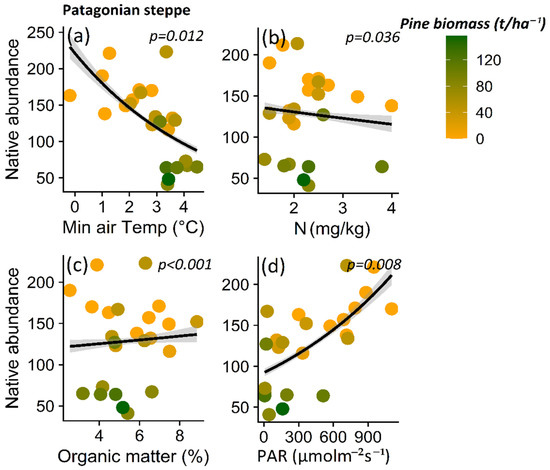
Figure 8.
Relationship between native plant total abundance and minimum air temperature (a), nitrate content (b), organic matter (c), and PAR light (d) for the Patagonian steppe. The lines show the linear model fit and the 95% confidence interval and the color gradient at the points corresponds to the biomass gradient of Pinus contorta for the Patagonian steppe.
4. Discussion
4.1. Impact of P. contorta Invasion on Microenvironmental Conditions
Our results showed a significant impact of P. contorta invasion on microclimatic conditions (i.e., maximum/minimum soil and air temperatures, light availability, and soil moisture), soil nutrients (i.e., nitrate, potassium) and soil properties (i.e., pH, organic matter accumulation, and litter depth). These environmental changes observed along the gradient of P. contorta invasion also correlated strongly with the reduction in the richness and abundance of native and even non-native plants.
Recent studies had already documented the impacts of P. contorta on litter content (accumulation) in Patagonian ecosystems in both Chile and Argentina [23]. To our knowledge, however, there have up until now been no studies evaluating the impacts of P. contorta on microclimatic conditions or other soil properties. Nevertheless, literature has shown comparable effects of other invasive tree species on these local conditions as found in our study. For example, the invasive tree Bellucia pentamera invading forest ecosystems in Indonesia caused an increase in mean air temperature and phosphorus and nitrogen content, while reducing water content [24]. The invasion of Pinus mugo in alpine ecosystems of central Europe has a similar effect, where ecosystems invaded by P. mugo generated drier, shadier, and more nutrient-rich ecosystems compared with non-invaded alpine grasslands [4]. Unlike these other case studies, however, the invasion of P. contorta in this study largely reduced temperatures. For example, it has also been reported that the presence of Robinia pseudoacacia species generated significant decreases in light intensity and average temperature (decrease of about 2 °C) in grassland ecosystems [3], whereas the same species generated less humid and warmer environments in the Carpathian-Pannonian region [25]. This reduction will have implications not only for plant communities, but also animal species, especially ectotherms [3].
Our study reinforces the idea that there is a tendency for treeless ecosystems such as the Patagonian steppe to be more susceptible to changes in conditions, compared with ecosystems that have historically presented a forested structure (tree cover and understory) such as the Araucaria araucana forest. This low presence of non-native plants may be correlated with the effect of P. contorta on nutrient availability (i.e., nitrogen content) and low PAR light availability. In ecosystems without trees invaded by P. contorta, the loss of species is greater compared with forested ecosystems such as the Araucaria araucana forest [6,19,26].
4.2. Variables Explaining the Reduction in Native Plant Richness in Invaded Ecosystems
The reduction in native plant richness is one of the most documented impacts of Pinus contorta invasion [6,23,27]. Similarly, in our study we found a negative correlation between pine biomass and species richness and total abundance of native plants (Figure 5). This effect on the composition of native plants was also mirrored in the reduction in richness and abundance of non-native plants in both studied ecosystems.
Reduced plant diversity of both native and non-native plants in ecosystems invaded by P. contorta has for years been attributed to the increased accumulation of P. contorta leaf litter, which inhibits plant recruitment [23,28]. In addition, especially in treeless ecosystems, this decrease is associated with a decrease in light availability resulting from the increase in canopy cover, which affects the growth of shade-intolerant species [19]. This effect is common in invasive trees but also in over-dominant shrubs and herbs, and has been documented in different ecosystems, for example, the invasion of Hakea drupaceae in South Africa [29], Acacia dealbata in Spain [30], Prosopis juliflora in the Arab Emirates [31], and Carpobrotus spp. in Mediterranean islands [32].
In our study, we found similar patterns in which litter accumulation is correlated with decreased native plant richness and abundance. On the other hand, canopy cover is negatively correlated with native plant abundance in both ecosystems, without being significantly related to species richness. However, we also determined that for the Araucaria araucana forest the increase in the biomass of P. contorta was also correlated with more acidic soils that could decrease the richness of native plants, whereas abundance of native plants was strongly correlated with a decrease in minimum soil temperature and changes in pH and potassium content. As for the Patagonian steppe ecosystems, the richness and abundance of native plants was related most strongly to the increase in minimum soil temperature and the decrease in organic matter. These results are consistent with studies showing that the invasion of Carpobrotus spp., Alianthus altissima, and Oxalis pes-caprae modified soil conditions (increasing K and decreasing pH) which contributed to a decrease in native plant richness on Mediterranean islands [32]. Similar effects were caused by the invasion of Acacia dealbata in Spain, which increased soil nutrients (N, P, K) and acidified the soil, allowing non-native plants to become established and leading to a decrease in the richness and abundance of native species [30].
As for changes in microclimatic conditions, mainly in cold ecosystems (e.g., mountains or Patagonia), changes in temperature and soil moisture contribute significantly to the loss of native plants [33]. Our results are consistent with experimental studies of temperature manipulation indicating that native plant richness locally decreases with increasing soil temperature, especially in cold ecosystems [34]. As such, the reduction in native plant richness could be reflecting a scarcity of species with high thermal optima [35] or a more complex response to drought and temperature interactions, especially considering that most of these ecosystems are composed of native or endemic plants that have evolved under particular environmental conditions during the last ice age [6,26,34]. Both the Araucaria araucana forest (high elevation) and the Patagonian steppe (high latitude) are cold ecosystems; therefore, the decrease in native species richness could be associated with an interaction between the increase in soil temperature and the decrease in PAR light availability.
In this context, it is also important to consider that both ecosystems (A. araucana forest and Patagonian steppe) have marked differences between extreme diurnal temperatures, so that the reduction in this temperature difference, towards more temperate conditions, could be generating phenological changes in the native plant community. Changes in the phenology of native plants may be generating a mismatch between biotic interactions with pollinators, which over time directly affects the richness and abundance of the invaded communities [36,37].
It is important to note that the richness and abundance of non-native plants also decreased with the invasion of P. contorta. These results are contradictory to the invasive collapse hypothesis, which suggests that the presence of non-native species in a territory facilitates the establishment of new non-native species [30,38]. This low presence of non-native plants may be associated with P. contorta’s impact on nutrients (i.e., low nitrogen content) and light (i.e., low PAR). Most of the other non-native species recorded in the area are herbaceous species that have been dispersed through cattle and horses. So far, these non-native species have clearly not adapted to the shady and drier conditions underneath the pine invasion.
Within this context, we are aware of the potential limitations of an observational study such as the one presented here [39]. However, the two study sites have been well studied over several years and our sampling design has addressed local variations [6,26]. Thus, we are confident that our results show a clear association between the reduction in native plants and the changes caused by P. contorta on environmental conditions and are not caused by other local variables prior to the invasion.
Finally, our results are important for planning and implementing ecological restoration of invaded ecosystems. The strong impacts on microenvironmental conditions (mainly in A. araucana forest) and plant diversity (mainly in Patagonian steppe) generated by the invasion of P. contorta have been shown to be maintained over time, even once the species is eradicated, in what has been called the legacy effect [28,40]. Generally, invasive tree management increases the legacy of its short-term impacts, mainly associated with large inputs of organic matter, disruption of plant–soil feedback and changes in soil conditions (changes in pH and availability of essential nutrients) [20,40]. Therefore, understanding the impacts of P. contorta on soil conditions (presence of Pinus leaf litter), soil nutritional conditions (acid soils), and even microclimatic conditions (soil and air temperature), is key information for managers. In addition, understanding the link between microenvironmental conditions and plant diversity in invaded sites allows us to determine the level of tolerance of native species to these microenvironmental changes and to determine the threshold of invasion that can be tolerated to avoid important losses of biodiversity. This would allow the refining of control methods in the early stages of an invasion to obtain optimal results, mainly on the recruitment of native and endemic plants and community reassembly of ecosystems invaded by P. contorta after its removal.
5. Conclusions
Our results confirm that the impact of P. contorta is not only present in the reported reduction in the richness and abundance of native and non-native plants, but that there is also an important effect on microclimatic and soil conditions, specifically on the temperature extremes of both air and soil, as well as on nutrient availability (i.e., nitrate content and phosphorus).
Several studies have already shown that the impact of P. contorta on litter accumulation and canopy cover leads to a decrease in the richness and abundance of native plants. Our study showed that the impact generated by P. contorta on other microenvironmental variables, such as changes in microclimatic conditions (soil and air temperature) and soil conditions (decreased pH and organic matter), could also explain the reduced richness and abundance of native plants. The mechanisms behind the loss of biodiversity of native species associated with plant invasion would thus not only depend on the competition exerted by P. contorta, but also on the modifications that this species exerts on the abiotic environment. Moreover, these microenvironmental changes can have significant effects on other functional groups (e.g., pollinators, decomposers) with important consequences for the whole trophic network of the invaded ecosystems. To further disentangle these drivers, we recommend field experiments to manipulate P. contorta biomass in invaded systems, to assess the resulting changes in microenvironmental conditions and plant species richness and abundance over time.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d15030320/s1, Figure S1: Relationship between the biomass of Pinus contorta and the richness and abundance of non-native plant species; Table S1: List of plant species and growth forms that make up each study ecosystem. P. contorta, the predominant invasive tree species in each ecosystem; Table S2: Estimates, standard error, z-value, and p-values of all measured environmental variables (variables that change with P. contorta biomass) that influence changes in native plant richness in the A. araucana forest; Table S3: Estimates, standard error, z-value, and p-values of all measured environmental variables (variables that change with P. contorta biomass) that influence changes in native plant richness in the Patagonian steppe; Table S4: Estimates, standard error, z-value, and p-values of all measured environmental variables (variables that change with P. contorta biomass) that influence changes in native plant abundance in the A. araucana forest; Table S5: Estimates, standard error, z-value, and p-values of all measured environmental variables (variables that change with P. contorta biomass) that influence changes in native plant abundance in the Patagonian steppe.
Author Contributions
R.A.G., E.F.-L. and A.P.: writing—original draft; R.A.G., M.N. and A.J.C.-C.: data collection; E.F.-L. and J.J.L.: data curation and formal analysis; R.A.G., E.F.-L., L.C., A.J.C.-C., K.T.D., M.N., M.A.N., B.D.M., J.J.L. and A.P.: review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work is part of the research carried out by the Laboratorio de Invasiones Biológicas (LIB), and much of the field work could not have been done without the support of the LIB team. Study funded by FONDECYT 1140485 and 1180205. R.A.G., E.F.-L., and A.P. are funded by ANID/Basal FB210006. J.J.L. is funded by the ASICS project (ANR-20-EBI5-0004, BiodivERsA, BiodivClim call 2019–2020).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Brundu, G.; Pauchard, A.; Pyšek, P.; Pergl, J.; Bindewald, A.M.; Brunori, A.; Canavan, S.; Campagnaro, T.; Celesti-Grapow, L.; de Sá Dechoum, M.; et al. Global Guidelines for the Sustainable Use of Non-Native Trees to Prevent Tree Invasions and Mitigate Their Negative Impacts. NeoBiota 2020, 61, 65–116. [Google Scholar] [CrossRef]
- Castro-Díez, P.; Alonso, Á.; Saldaña-López, A.; Granda, E. Effects of Widespread Non-Native Trees on Regulating Ecosystem Services. Sci. Total Environ. 2021, 778, 146141. [Google Scholar] [CrossRef] [PubMed]
- Chikowore, G.; Martin, G.D.; Chidawanyika, F. An Assessment of the Invasive Alien Tree, Robinia pseudoacacia Canopy Traits and Its Effect on Grassland Microclimates and Subsequent Arthropod Assemblages. J. Insect Conserv. 2021, 25, 429–439. [Google Scholar] [CrossRef]
- Zeidler, M.; Duchoslav, M.; Banaš, M.; Lešková, M. Impacts of Introduced Dwarf Pine (Pinus mugo) on the Diversity and Composition of Alpine Vegetation. Community Ecol. 2012, 13, 213–220. [Google Scholar] [CrossRef]
- Terwei, A.; Zerbe, S.; Mölder, I.; Annighöfer, P.; Kawaletz, H.; Ammer, C. Response of Floodplain Understorey Species to Environmental Gradients and Tree Invasion: A Functional Trait Perspective. Biol. Invasions 2016, 18, 2951–2973. [Google Scholar] [CrossRef]
- Franzese, J.; Urrutia, J.; García, R.A.; Taylor, K.; Pauchard, A. Pine Invasion Impacts on Plant Diversity in Patagonia: Invader Size and Invaded Habitat Matter. Biol. Invasions 2017, 19, 1015–1027. [Google Scholar] [CrossRef]
- Dyderski, M.K.; Jagodziński, A.M. How Do Invasive Trees Impact Shrub Layer Diversity and Productivity in Temperate Forests? Ann. For. Sci. 2021, 78, 20. [Google Scholar] [CrossRef]
- Richardson, D.M.; Rejmánek, M. Conifers as Invasive Aliens: A Global Survey and Predictive Framework. Divers. Distrib. 2004, 10, 321–331. [Google Scholar] [CrossRef]
- Simberloff, D.; Nuñez, M.A.; Ledgard, N.J.; Pauchard, A.; Richardson, D.M.; Sarasola, M.; Van Wilgen, B.W.; Zalba, S.M.; Zenni, R.D.; Bustamante, R.; et al. Spread and Impact of Introduced Conifers in South America: Lessons from Other Southern Hemisphere Regions. Austral Ecol. 2010, 35, 489–504. [Google Scholar] [CrossRef]
- Sapsford, S.J.; Brandt, A.J.; Davis, K.T.; Peralta, G.; Dickie, I.A.; Gibson, R.D.; Green, J.L.; Hulme, P.E.; Nuñez, M.A.; Orwin, K.H.; et al. Towards a Framework for Understanding the Context Dependence of Impacts of Non-Native Tree Species. Funct. Ecol. 2020, 34, 944–955. [Google Scholar] [CrossRef]
- Peña, E.; Hidalgo, M.; Langdon, B.; Pauchard, A. Patterns of Spread of Pinus contorta Dougl. Ex Loud. Invasion in a Natural Reserve in Southern South America. For. Ecol. Manag. 2008, 256, 1049–1054. [Google Scholar] [CrossRef]
- Echeverria, C.; Coomes, D.; Salas, J.; Rey-Benayas, J.M.; Lara, A.; Newton, A. Rapid Deforestation and Fragmentation of Chilean Temperate Forests. Biol. Conserv. 2006, 130, 481–494. [Google Scholar] [CrossRef]
- Pauchard, A.; Bustamante, R.O. Forest Ecology and Management Habitat Suitability of Five Commonly Planted Non-Native Trees in Chile: Implications for an Invasion Process. For. Ecol. Manag. 2023, 529, 120726. [Google Scholar] [CrossRef]
- Langdon, B.; Pauchard, A.; Aguayo, M. Pinus contorta Invasion in the Chilean Patagonia: Local Patterns in a Global Context. Biol. Invasions 2010, 12, 3961–3971. [Google Scholar] [CrossRef]
- Langdon, B.; Cavieres, L.A.; Pauchard, A. At a Microsite Scale, Native Vegetation Determines Spatial Patterns and Survival of Pinus contorta Invasion in Patagonia. Forests 2019, 10, 654. [Google Scholar] [CrossRef]
- Davis, K.T.; Maxwell, B.D.; Caplat, P.; Pauchard, A.; Nuñez, M.A. Simulation Model Suggests That Fire Promotes Lodgepole Pine (Pinus contorta) Invasion in Patagonia. Biol. Invasions 2019, 1, 2287–2300. [Google Scholar] [CrossRef]
- Taylor, K.T.; Maxwell, B.D.; McWethy, D.B.; Pauchard, A.; Nuñez, M.A.; Whitlock, C. Pinus contorta Invasions Increase Wildfire Fuel Loads and May Create a Positive Feedback with Fire. Ecology 2017, 98, 678–687. [Google Scholar] [CrossRef]
- Cóbar-Carranza, A.J.; García, R.A.; Pauchard, A.; Peña, E. Effect of Pinus contorta Invasion on Forest Fuel Properties and Its Potential Implications on the Fire Regime of Araucaria Araucana and Nothofagus Antarctica Forests. Biol. Invasions 2014, 16, 2273–2291. [Google Scholar] [CrossRef]
- Bravo-Monasterio, P.; Pauchard, A.; Fajardo, A. Pinus contorta Invasion into Treeless Steppe Reduces Species Richness and Alters Species Traits of the Local Community. Biol. Invasions 2016, 18, 1883–1894. [Google Scholar] [CrossRef]
- Dickie, I.A.; St John, M.G.; Yeates, G.W.; Morse, C.W.; Bonner, K.I.; Orwin, K.; Peltzer, D.A. Belowground Legacies of Pinus contorta Invasion and Removal Result in Multiple Mechanisms of Invasional Meltdown. AoB Plants 2014, 6, plu056. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2015. [Google Scholar]
- Wickham, H. Ggplot2: Elganta Graphics for Data Analysis; Springer: New York, NY, USA, 2008; ISBN 978-0-387-78170-9. [Google Scholar] [CrossRef]
- Taylor, K.T.; Maxwell, B.D.; Pauchard, A.; Nuñez, M.A.; Rew, L.J. Native versus Non-Native Invasions: Similarities and Differences in the Biodiversity Impacts of Pinus contorta in Introduced and Native Ranges. Divers. Distrib. 2016, 22, 578–588. [Google Scholar] [CrossRef]
- Solfiyeni, S.; Syamsuardi, S.; Chairul, C.; Mukhtar, E. Impacts of Invasive Tree Species Bellucia pentamera on Plant Diversity, Microclimate and Soil of Secondary Tropical Forest in West Sumatra, Indonesia. Biodiversitas 2022, 23, 3135–3146. [Google Scholar] [CrossRef]
- Slabejová, D.; Bacigál, T.; Hegedüšová, K.; Májeková, J.; Medvecká, J.; Mikulová, K.; Šibíková, M.; Škodová, I.; Zaliberová, M.; Jarolímek, I. Comparison of the Understory Vegetation of Native Forests and Adjacent Robinia pseudoacacia Plantations in the Carpathian-Pannonian Region. For. Ecol. Manag. 2019, 439, 28–40. [Google Scholar] [CrossRef]
- Urrutia, J.; Pauchard, A.; García, R.A. Diferencias En La Composición Vegetal de Un Bosque de Araucaria araucana (Molina) K.Koch y Nothofagus antarctica (G. Forst.) Oerst. Asociadas a Un Gradiente de Invasión de Pinus contorta Douglas Ex Loudon. Gayana-Bot. 2013, 70, 92–100. [Google Scholar] [CrossRef]
- Pauchard, A.; Escudero, A.; García, R.A.; de la Cruz, M.; Langdon, B.; Cavieres, L.A.; Esquivel, J. Pine Invasions in Treeless Environments: Dispersal Overruns Microsite Heterogeneity. Ecol. Evol. 2016, 6, 447–459. [Google Scholar] [CrossRef] [PubMed]
- Franzese, J.; Raffaele, E.; Chiuffo, M.C.; Blackhall, M. The Legacy of Pine Introduction Threatens the Fuel Traits of Patagonian Native Forests. Biol. Conserv. 2022, 267, 109472. [Google Scholar] [CrossRef]
- Erckie, L.; Adedoja, O.; Geerts, S.; van Wyk, E.; Boatwright, J.S. Impacts of an Invasive Alien Proteaceae on Native Plant Species Richness and Vegetation Structure. South Afr. J. Bot. 2022, 144, 332–338. [Google Scholar] [CrossRef]
- González-Muñoz, N.; Costa-Tenorio, M.; Espigares, T. Invasion of Alien Acacia Dealbata on Spanish Quercus Robur Forests: Impact on Soils and Vegetation. For. Ecol. Manag. 2012, 269, 214–221. [Google Scholar] [CrossRef]
- El-Keblawy, A.; Al-Rawai, A. Impacts of the Invasive Exotic Prosopis juliflora (Sw.) D.C. on the Native Flora and Soils of the UAE. Plant Ecol. 2007, 190, 23–35. [Google Scholar] [CrossRef]
- Vilà, M.; Tessier, M.; Suehs, C.M.; Brundu, G.; Carta, L.; Galanidis, A.; Lambdon, P.; Manca, M.; Médail, F.; Moragues, E.; et al. Local and Regional Assessments of the Impacts of Plant Invaders on Vegetation Structure and Soil Properties of Mediterranean Islands. J. Biogeogr. 2006, 33, 853–861. [Google Scholar] [CrossRef]
- Thuiller, W.; Lavorel, S.; Araújo, M.B.; Sykes, M.T.; Prentice, I.C. Climate Change Threats to Plant Diversity in Europe. Proc. Natl. Acad. Sci. USA 2005, 102, 8245–8250. [Google Scholar] [CrossRef]
- Robinson, S.I.; McLaughlin, Ó.B.; Marteinsdóttir, B.; O’Gorman, E.J. Soil Temperature Effects on the Structure and Diversity of Plant and Invertebrate Communities in a Natural Warming Experiment. J. Anim. Ecol. 2018, 87, 634–646. [Google Scholar] [CrossRef]
- De Sassi, C.; Lewis, O.T.; Tylianakis, J.M. Plant-Mediated and Nonadditive Effects of Two Global Change Drivers on an Insect Herbivore Community. Ecology 2012, 93, 1892–1901. [Google Scholar] [CrossRef] [PubMed]
- Forrest, J.R.K. Plant-Pollinator Interactions and Phenological Change: What Can We Learn about Climate Impacts from Experiments and Observations? Oikos 2015, 124, 4–13. [Google Scholar] [CrossRef]
- Giejsztowt, J.; Classen, A.T.; Deslippe, J.R. Climate Change and Invasion May Synergistically Affect Native Plant Reproduction. Ecology 2020, 101, e02913. [Google Scholar] [CrossRef] [PubMed]
- Simberloff, D.; Von Holle, B. Positive Interactions of Nonindigenous Species. Biol. Invasions 1999, 1, 21–32. [Google Scholar] [CrossRef]
- Sagarin, R.; Pauchard, A. Observation and Ecology: Broadening the Scope of Science to Understand a Complex World; Island Press: Washington, DC, USA, 2013; pp. 1–213. [Google Scholar]
- Dickie, I.A.; Bennett, B.M.; Burrows, L.E.; Nuñez, M.A.; Peltzer, D.A.; Porté, A.; Richardson, D.M.; Rejmánek, M.; Rundel, P.W.; van Wilgen, B.W. Conflicting Values: Ecosystem Services and Invasive Tree Management. Biol. Invasions 2014, 16, 705–719. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).