Abstract
Reduced trophic resources can pose relevant constraints to the development of freshwater animals with complex life cycles. For amphibians, food deprived environments, such as high-altitude ponds and springs and groundwaters are frequently used for breeding. The aim of this study is to outline trophic conditions leading to extreme cases of delayed larval development and increased size at metamorphosis of a European widespread amphibian, the fire salamander (Salamandra salamandra). We collected 150 fire salamander larvae, split them in two groups, one with high and one with low trophic resource availability. We then observed the effects of nutritional conditions on larval development recording time to metamorphosis and average day growth. Moreover, in the field, we surveyed larvae growth and size at metamorphosis in two artificial subterranean sites with low prey availability. Trophic conditions strongly affected larval development and under low food treatment time to metamorphosis reached up to 416 days. In the subterranean environments we observed a similar pattern, with larvae requiring more than one year to attain metamorphosis but reaching unexpected large sizes. Environmental trophic conditions experienced during early stages can induce strong delay in metamorphosis of the fire salamander; this plasticity makes fire salamander larvae optimal models for comparative studies and cross-environment experiments.
Keywords:
salamander; trophic; amphibians; tadpole; growth; stream; freshwater; method; carryover; Salamandra 1. Introduction
Complex life cycles represent adaptive mechanisms for decoupling the developmental processes and allow to respond independently to different selective forces [1]. In amphibians, the duration of larval development is a major life history trait that can show substantial variation across populations of the same species because of multifaceted interplay between development conditions and local adaptations. Larval development is determined by complex trade-offs in trait variation involving fitness consequences for subsequent life history stages; for instance, when predators occur a faster growth is advantageous, as it increases larval survival, but comes with the cost of reduced survival, size or performance during post-metamorphosis stages [2,3]. Furthermore, when in the aquatic environments trophic resources are limited, larval growth determines a significant physiological cost [4]. Therefore, low trophic resource availability often constraints growth, especially for predator species occupying upper positions of trophic webs [5,6,7]. Among amphibians, low prey availability has been observed to cause delayed larval growth in many newts’ and salamanders’ species [8,9,10,11], and under some circumstances this may even lead to particular developmental patterns such as paedomorphosis [12,13]. Nevertheless, both delayed larval development and paedomorphosis can be found in a wide range of habitats; they include both high altitude ponds with low level of trophic resources and temperate environments with well-established trophic webs, such as low-land semi-permanent marshes [14,15,16]. Moreover, ecological and environmental variability increase complexity as various strategies may occur depending on the different conditions encountered during growth and along species evolutionary history.
The study of fire salamander Salamadra salamandra (Linneus, 1758) is particularly interesting to understand the patterns that regulate the development of ectotherm populations facing contrasting conditions during larval phase. The fire salamander is a polytypic amphibian, widespread in the European continent with different subspecies, whose phylogenetic classification is still uncertain [17,18,19]. This species shows a high ecological plasticity, especially in the breeding site choice, with larvae able to exploit habitats with a wide variety of environmental conditions during development. Adults are generally found in wet deciduous mixed woodlands and typically breed in small streams and headwaters rich in oxygen content. [20,21]. However, some populations of this urodele also use different water bodies typologies [22,23], such as temporary and permanent ponds, artificial pools and cave habitats [24,25,26]. This species also shows intraspecific plasticity in developmental features, in morphological traits and in reproductive strategy under multiple environmental contexts [20,23,27,28,29]. Differing availability and quality of nutrients experienced under diverse ecological contexts impose contrasting constraints inducing variation in larval plasticity, including growth rate shifts behavioral changes [30], but the amplitude of these effects remains mostly unknown. In small underground pools the scarcity of food and high intraspecific competition has been reported to increase cannibalistic behavior with consequences on larval morphological and behavioral traits [31,32]. Even if other factors, besides trophic resource availability, can also affect the duration of salamander larval stage, the abundance of available prey is a key one and often interacts with the other ecological constraints. For instance, lower water temperatures experienced in colder environments reduce larva metabolic rate, slowing developmental changes [33,34]. The combined effects of prey availability can lead to striking differences in development patterns: in epigean breeding sites at lower altitudes where prey are abundant fire salamander larvae usually metamorphose after 3–4 months [35], while in cold, food deprived sites, such as caves, it is likely that they take more than one year [31,36,37].
The amplitude of the fire salamander larvae developmental plasticity induced by low trophic resources availability has never been explored to its extremes; with this contribution we aim to report cases of delayed larval development recorded both under experimental and field conditions, with individuals from food-deprived subterranean artificial pools attaining unexpected large sizes at metamorphosis.
2. Materials and Methods
From March 2017 to May 2017 we collected 150 new-born fire salamander larvae from 15 springs and streams of the Prealps of Northern Italy located at different altitudinal ranges (spanning between 250 and 1491 m a.s.l.; approx. 45.924 N, 9.967 E), i.e., 10 larvae for each site. We collected new-born larvae to minimize the potential influence of the exposure to environmental conditions occurring at the breeding sites. At the time of capture, salamander larvae had an average total body length of 33.66 mm (±0.26 mm). Experimental rearing lasted a total of 16 months: from March 2017 to July 2018. For each site we equally assigned experimental individuals to two rearing treatments: “low trophic resources availability” (hereafter LTA) and “high trophic resources availability” (hereafter HTA). During rearing, individuals were separately housed in perforated plastic cups (diameter 8 cm) that were divided into four plastic tanks (80 cm × 70 cm × 20 cm; two tanks for each treatment) with 5 cm of tap water. Rearing containers were placed in a thermostatic chamber where larvae were exposed to controlled temperature and natural photoperiod (night/day: 14/17 °C). Fire salamander larvae were fed with frozen commercial Chironomus sp. larvae. Overall, larvae in the rich food treatment received on average 3.09 (±0.17) chironomids per day. Addressing the low but sufficient (to allow development) amount of prey items to supply larvae in the LTA treatment was not an easy task [30]. Initially, we fed LTA larvae with 3 chironomids/week in the first month, raising this amount to 6 chironomids/week until July 2017. Subsequently, we slowly increased the food supply up to 25 chironomids a week; this quantity was maintained from March 2018 to June 2018. On the whole, larvae in the LTA treatment received on average 1.43 (±0.06) chironomids per day.
Fire salamander larvae were dorsally photographed at the first day of rearing and at metamorphosis. Larvae were photographed using an Olympus SP-81OUZ camera (14 megapixel, lens 36× wide optical zoom, ED 4.3–154.8 mm, 1:2.9–5.7) in a transparent container with 2 cm of tap water, placed on graph paper (accuracy ± 0.5 mm). Total body length (mm) was determined through ImageJ software (https://imagej.nih.gov/ij/ “5 September 2018”). Larval stage duration was calculated in days from capture up to metamorphosis. Once completed the study, we released juvenile salamanders to their respective sites of origin. The study design was approved and authorized by the ethical committee of the Lombardy Region Authority as complying with the regional law 10/2008, permission number: T1.2016.0052349. Statistical analyses were conducted in R environment, using linear mixed models (LMMs) with the function “lmer” of the package lmerTest. We considered larval stage duration (i.e., number of days spent as larvae) as dependent variable, while the food treatment was the fixed factor and the site of origin of the larvae was included as random factor. The same model structure was used to assess the effect of food treatments on larval growth, using individual length at metamorphosis as a dependent variable. We tested significance of food treatment in affecting time of larval development with a subsequent Wald F test.
Moreover, we combined this data with field observations, performing consecutive surveys to monitor fire salamander condition at two draining galleries within the same area (from October 2019 to May 2021) which are regularly used by this species to lay its larvae [38,39]. Both artificial sites are characterized by complete darkness, poor prey availability and constant water temperature (approximately 11 °C); the first site is occupied by a laboratory for studies on subterranean biology (the “Enrico Pezzoli laboratory of Subterranean Biology”). The laboratory is naturally exploited by fire salamander females to lay their larvae. Here we followed the survival and growth of 35 larvae laid 15 October 2019, by a single female from the laying to metamorphosis, while in the second site (named G14) we recorded dorsal pictures of larvae at stages III a or III b [40]. For both subterranean sites, we performed multiple surveys (10 in the subterranean lab, 4 in G14). We subsequently assessed maximum total length of the larvae using ImageJ software.
3. Results
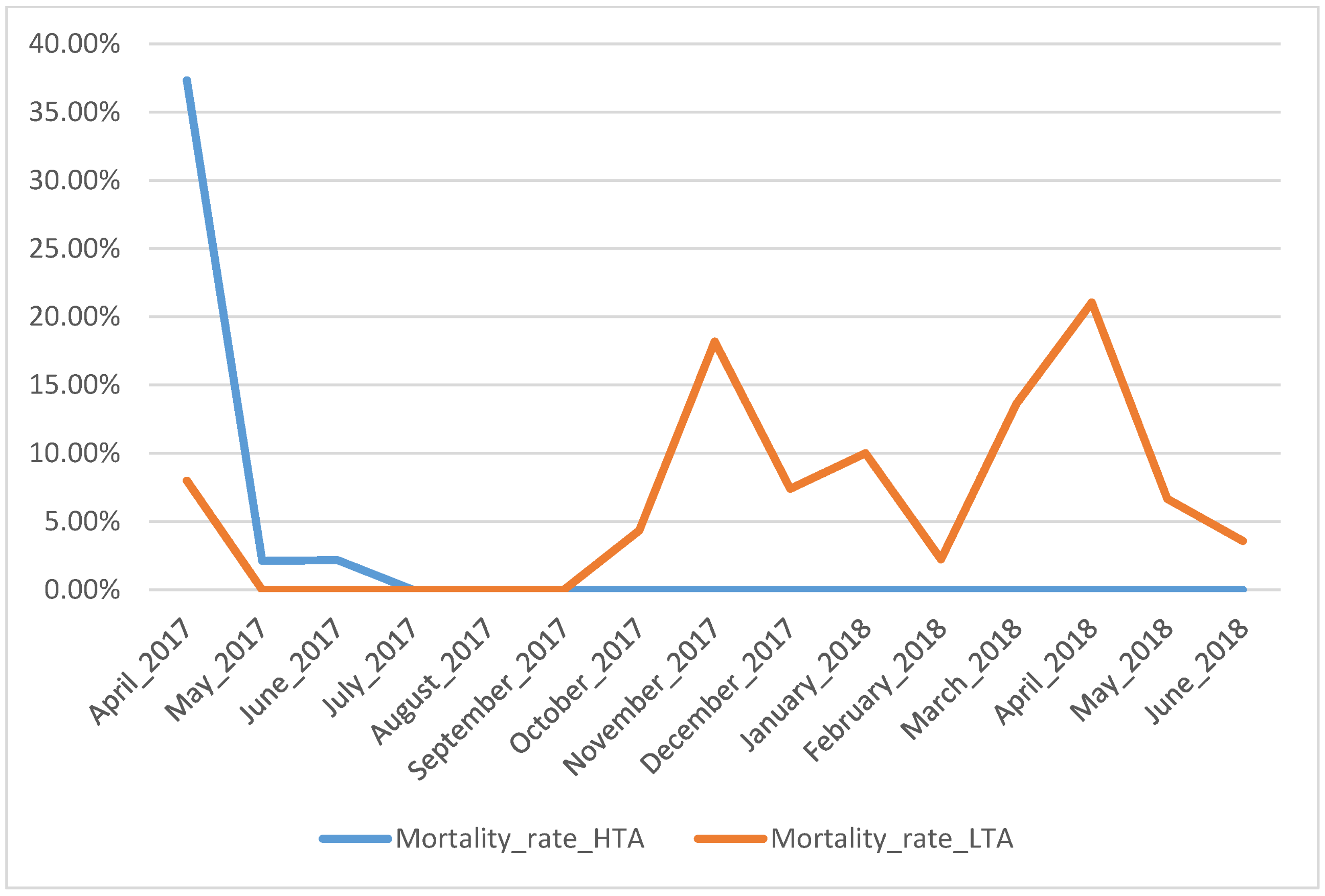
During the rearing experiment, 78 larvae (52%) did not survive until metamorphosis (30 for HTA treatment and 48 for LTA treatment; Figure 1). From October 2017 to February 2018, larvae reared under low trophic availability were attacked by an undetermined fungal infection, which spread in both LTA treatment tanks. We treated contaminated larvae with the medicine Sera Mycopur 50 mL (1 mL per 20 L of water for three days) and mortality strongly decreased. Mortality rate was significantly higher in the LTA treatment (χ21= 5.96, p = 0.01).
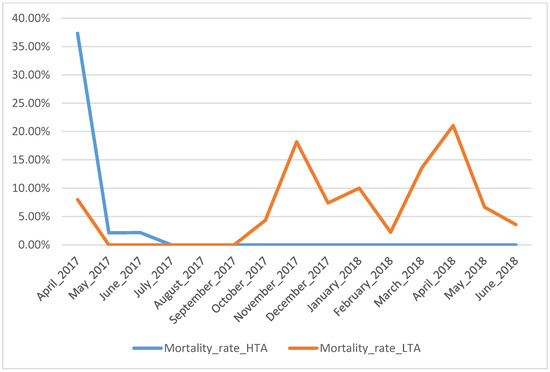
Figure 1.
Monthly mortality rate of fire salamander larvae according to rearing treatment. Blue line identifies larvae reared at high trophic resources abundance (HTA); orange line identifies larvae reared at low trophic resources availability (LTA).
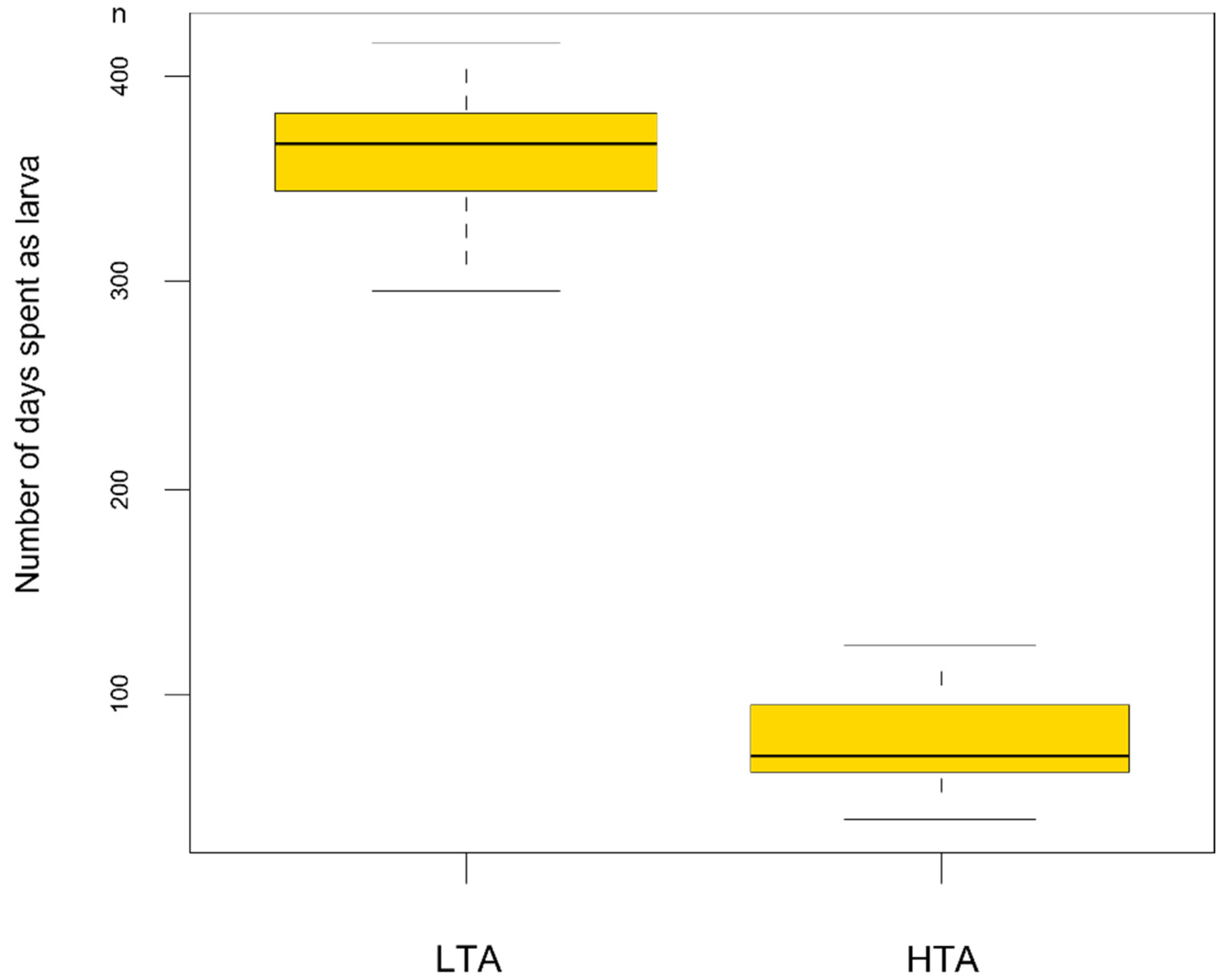
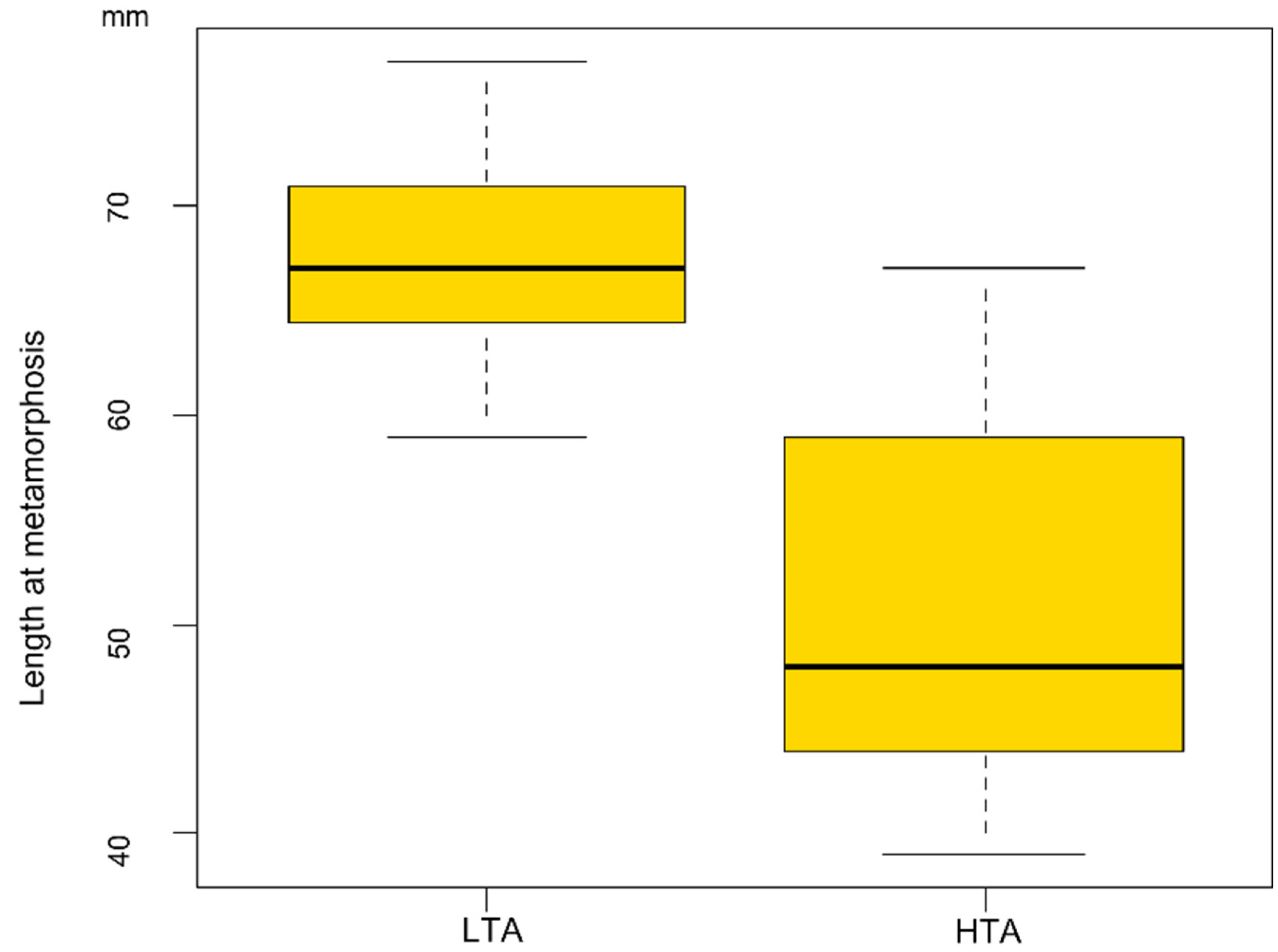
Larval stage duration analysis revealed that larvae from the rich food treatment showed a faster development (F1/76 = 2553.6 p < 0.001) than those reared with LTA availability (HTA treatment: development time = 78.7 ± 2.7 days; LTA treatment: 362.3 ± 5.4 days). Larvae reared in LTA treatments took a minimum of 296 days to metamorphose and a maximum of 416 days, with 17 larvae employing more than 365 days to attain metamorphosis (Figure 2). However, LMMs also showed food treatments significantly affected size at metamorphosis (F1/7.04 = 186.96 p < 0.001; Figure 3), with larvae reared under LTA treatments reaching larger size at metamorphosis than those from the HTA treatment (LTA treatment length: 67.81 ± 0.86 mm; HTA treatment length: 50.75 ± 0.86 mm).
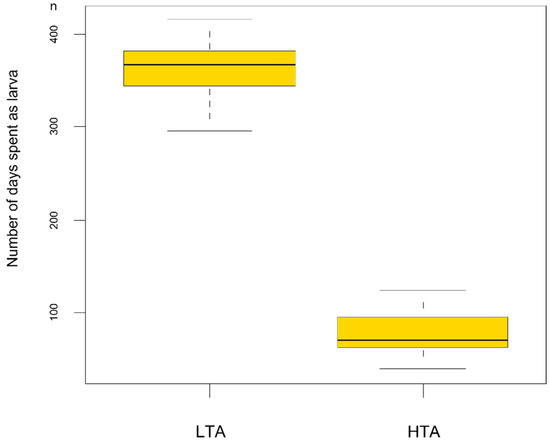
Figure 2.
Boxplot showing the relationship between larval stage duration and the food treatment experienced. LTA = low trophic resources availability; HTA = high trophic resources availability.
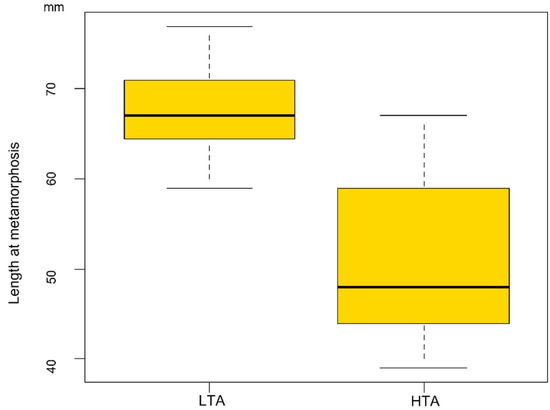
Figure 3.
Boxplot showing the relationship between the size at metamorphosis reached by larvae and the food treatment experienced. LTA = low trophic resources availability; HTA = high trophic resources availability.
In the subterranean laboratory, only one larva reached metamorphosis after more than one year from birth (23 October 2020), while all the other 34 individuals did not survive. In the last stage before metamorphosis the larva (measured the 16 October 2020) was 63 mm long. In the second subterranean site we recorded six larvae at pre-metamorphosis stage over a total of 85 larvae counted (Figure 4). The size of these six larvae was on average (±SE) 71.9 ± 0.35 mm; particularly one larva was 79.7 mm long and another one was 82.5 mm long.

Figure 4.
Larvae at late developmental stage collected in the draining gallery named G14 in the municipality of Castello di Brianza (Lombardy, Lecco district) at the last sampling performed in May 2021. The six larvae in the left and in the bottom were at stage III a or III b. The seventh larva on the upper right was still in later stage II.
4. Discussion
One of the most relevant aspects emerging from the reported data is the extremely high developmental plasticity of fire salamander larvae triggered by different environmental conditions, which in our experiment were represented by the different levels of trophic resources availability. All larvae under HTA treatment completed metamorphosis within periods consistent to those reported in other studies, i.e., 2–3 months after birth [30,41], whereas the larval growth pattern with LTA availability described in this study extends previously published data and reaches an unprecedented extreme of 416 days. Larvae under LTA treatment required one year to metamorphose (i.e., 296–416 days of larval development). Krause, Steinfartz and Caspers [30] showed that the duration of larval phase differed by 20 days between fire salamander larvae fed twice a week and larvae fed six times a week. On the contrary, in our experiment, with a lower dose of nutrients, we observed a temporal variation closer to natural larval overwintering [35,42], but with an impressive delay of larval stage duration. Larvae under HTA treatment had a lower length at metamorphosis. Despite the larger body size, larvae under LTA treatment had a higher mortality rate that limited the total number of larvae metamorphosed under this condition. However, it is worth noting only this treatment group has been infected by a fungal disease. Unfortunately, it has not been possible to identify which fungal infection was involved, but the fact that it occurred only in LTA treatment suggests low resource availability might have facilitated an increase in larvae susceptibility to pathogens. This possibility is also supported in the light of previous studies on this species that did not find a significant correlation between survival and nutritional condition [30]. Remarkably, despite of it, the increase in size at metamorphosis we observed in LTA larvae suggests suboptimal conditions may have promoted shifts in the growth/development patterns of fire salamander larvae. Thus, consequences of low food availability can unexpectedly affect larval development in urodeles. Another limit of the study is linked to the fact that in LTA treatment the amount of prey supplied was not constant. Larvae minimum energy requirements changed according to the increase in size; we had to adjust the number of preys along time to allow survival and development. On average the total number of preys consumed by LTA larvae was half of the prey consumed by HTA larvae. In general, we can state that the equivalent of a dose below 10 chironomids/week is not enough for larvae at later stages to complete metamorphosis. Two picks of mortality occurred in LTA treatment (Figure 1), one corresponding with fungus detection and the other with pre-metamorphosis stages. This suggests that under low trophic resources availability larvae can resist less to stressful conditions, such as pathogen infections and metabolic costs of metamorphosis. Further studies, specifically designed, could investigate and test these outcomes. HTA larvae experienced mortality only at the beginning of the experiment; no apparent explanations can be retrieved for this pattern.
Although data obtained from field observations are mainly anecdotical, they add evidence on the role that oligotrophic conditions can play in delaying metamorphosis in plastic urodele species. Field surveys revealed that time to reach metamorphosis was similar to the time took in the LTA treatment, even if the overall survival was considerably lower. Particularly noticeable is the big size observed in some of the larvae from the second subterranean site. This field observation fits with the longer sizes recorded in larvae reared with low trophic resources. While it is reported that in Italian epigean sites fire salamander larvae can grow at maximum till 75 mm [43,44], the maximum total length of fire salamander larvae previously observed in a subterranean breeding site was of 78 mm [37]. Notably, one of the larvae observed during our surveys significantly encompassed these records. The large abundance of conspecifics observed in the site suggests that biggest larvae are likely cannibalistic, as fire salamander intraspecific aggressiveness is enhanced in sites with high density of larvae and low trophic availability [32].
The observations described in this study suggest that environmental trophic conditions experienced during early stages can induce strong delay in metamorphosis of the fire salamander and provide useful insights and comparisons for further studies; the amount of food effectively gathered during experimental rearing of salamanders’ larvae, if not taken into consideration, can bring to unexpected and counterintuitive results. Despite the limitations outlined above about larvae mortality, the research can be seen as an interesting starting point and methodological framework to study differences in amphibian larval development among distinct environmental conditions.
Author Contributions
Conceptualization, R.M. and G.F.F.; methodology, P.C., B.B., R.M. and A.M.; formal analysis, R.M.; larvae rearing and data collection, P.C.; field investigations, R.M.; P.C., R.M. and A.M., wrote the first draft of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Review Board and Ethics Committee of LOMBARDY REGION AUTHORITY (T1.2016.0052349. of 10 December 2016).
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to sensitiveness of the sites.
Acknowledgments
We are grateful to Roberto Vignarca for help with logistic.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Moran, N.A. Adaptation and constraint in the complex life cycles of animals. Annu. Rev. Ecol. Evol. Syst. 1994, 25, 573–600. [Google Scholar] [CrossRef]
- Ficetola, G.F.; De Bernardi, F. Trade-off between larval development rate and post-metamorphic traits in the frog Rana latastei. Evol. Ecol. 2006, 20, 143–158. [Google Scholar] [CrossRef]
- Melotto, A.; Manenti, R.; Ficetola, G.F. Rapid adaptation to invasive predators overwhelms natural gradients of intraspecific variation. Nat. Commun. 2020, 11, 3608. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.S.; Monaghan, P.; Metcalfe, N.B. The trade-off between growth rate and locomotor performance varies with perceived time until breeding. J. Exp. Biol. 2010, 213, 3289–3298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, T.L.; Rowland, F.E.; Semlitsch, R.D. Variation in phenology and density differentially affects predator-prey interactions between salamanders. Oecologia 2017, 185, 475–486. [Google Scholar] [CrossRef]
- Reinhardt, T.; Steinfartz, S.; Paetzold, A.; Weitere, M. Linking the evolution of habitat choice to ecosystem functioning: Direct and indirect effects of pond-reproducing fire salamanders on aquatic-terrestrial subsidies. Oecologia 2013, 173, 281–291. [Google Scholar] [CrossRef]
- Rowland, F.E.; Rawlings, M.B.; Semlitsch, R.D. Joint effects of resources and amphibians on pond ecosystems. Oecologia 2017, 183, 237–247. [Google Scholar] [CrossRef]
- Benard, M.F. Warmer winters reduce frog fecundity and shift breeding phenology, which consequently alters larval development and metamorphic timing. Glob. Change Biol. 2015, 21, 1058–1065. [Google Scholar] [CrossRef]
- Bohenek, J.R.; Leary, C.J.; Resetarits, W.J. Exposure to glucocorticoids alters life history strategies in a facultatively paedomorphic salamander. J. Exp. Zool. Part A—Ecol. Integr. Physiol. 2021, 335, 329–338. [Google Scholar] [CrossRef]
- Dahl, E.; Orizaola, G.; Nicieza, A.G.; Laurila, A. Time constraints and flexibility of growth strategies: Geographic variation in catch-up growth responses in amphibian larvae. J. Anim. Ecol. 2012, 81, 1233–1243. [Google Scholar] [CrossRef]
- Van Buskirk, J. Amphibian phenotypic variation along a gradient in canopy cover: Species differences and plasticity. Oikos 2011, 120, 906–914. [Google Scholar] [CrossRef]
- Denoel, M.; Hervant, F.; Schabetsberger, R.; Joly, P. Short- and long-term advantages of an alternative ontogenetic pathway. Biol. J. Linn. Soc. 2002, 77, 105–112. [Google Scholar] [CrossRef] [Green Version]
- Denoël, M.; Ficetola, G.F.; Cirovic, R.; Radovic, D.; Dzukic, G.; Kalezic, M.L.; Vukov, T.D. A multi-scale approach to facultative paedomorphosis of European newts (Salamandridae) in the Montenegrin karst: Distribution pattern, environmental variables, and conservation. Biol. Conserv. 2009, 142, 509–517. [Google Scholar] [CrossRef]
- Ringia, A.M.; Lips, K.R. Oviposition, early development and growth of the cave salamander, Eurycea lucifuga: Surface and subterranean influences on a troglophilic species. Herpetologica 2007, 63, 258–268. [Google Scholar] [CrossRef]
- Ficetola, G.F.; Scali, S.; Denoel, M.; Montinaro, G.; Vukov, T.D.; Zuffi, M.A.L.; Padoa-Schioppa, E. Ecogeographical variation of body size in the newt Triturus carnifex: Comparing the hypotheses using an information-theoretic approach. Glob. Ecol. Biogeogr. 2010, 19, 485–495. [Google Scholar] [CrossRef]
- Denoël, M.; Ficetola, G.F. Using kernels and ecological niche modeling to delineate conservation areas in an endangered patch-breeding phenotype. Ecol. Appl. 2015, 25, 1922–1931. [Google Scholar] [CrossRef] [Green Version]
- Hendrix, R.; Hauswaldt, J.S.; Veith, M.; Steinfartz, S. Strong correlation between cross-amplification success and genetic distance across all members of ’True Salamanders’ (Amphibia: Salamandridae) revealed by Salamandra salamandra-specific microsatellite loci. Mol. Ecol. Resour. 2010, 10, 1038–1047. [Google Scholar] [CrossRef]
- Najbar, A.; Konowalik, A.; Halupka, K.; Najbar, B.; Ogielska, M. Body size and life history traits of the fire salamander Salamandra salamandra from Poland. Amphib. -Reptil. 2020, 41, 63–74. [Google Scholar] [CrossRef]
- Steinfartz, S.; Veith, M.; Tautz, D. Mitochondrial sequence analysis of Salamandra taxa suggests old splits of major lineages and postglacial recolonizations of Central Europe from distinct source populations of Salamandra salamandra. Mol. Ecol. 2000, 9, 397–410. [Google Scholar] [CrossRef]
- Caspers, B.A.; Junge, C.; Weitere, M.; Steinfartz, S. Habitat adaptation rather than genetic distance correlates with female preference in fire salamanders (Salamandra salamandra). Front. Zool. 2009, 6, 13. [Google Scholar] [CrossRef] [Green Version]
- Manenti, R.; Conti, A.; Pennati, R. Fire salamander (Salamandra salamandra) males’ activity during breeding season: Effects of microhabitat features and body size. Acta Herpetol. 2017, 12, 29–36. [Google Scholar]
- Balogova, M.; Jelic, D.; Kyselova, M.; Uhrin, M. Subterranean systems provide a suitable overwintering habitat for Salamandra salamandra. Int. J. Speleol. 2017, 46, 321–329. [Google Scholar] [CrossRef]
- Manenti, R.; Ficetola, G.F. Salamanders breeding in subterranean habitats: Local adaptations or behavioural plasticity? J. Zool. 2013, 289, 182–188. [Google Scholar] [CrossRef]
- Manenti, R.; De Bernardi, F.; Ficetola, G.F. Pastures vs forests: Do traditional pastoral activities negatively affect biodiversity? The case of amphibians communities. North-West. J. Zool. 2013, 9, 284–292. [Google Scholar]
- Manenti, R.; Siesa, M.E.; Ficetola, G.F. Odonata occurence in caves: Active or accidentals? A new case study. J. Cave Karst Stud. 2013, 75, 205–209. [Google Scholar] [CrossRef]
- Weitere, M.; Tautz, D.; Neumann, D.; Steinfartz, S. Adaptive divergence vs. environmental plasticity: Tracing local genetic adaptation of metamorphosis traits in salamanders. Mol. Ecol. 2004, 13, 1665–1677. [Google Scholar] [CrossRef]
- Buckley, D.; Alcobendas, M.; Garcia-Paris, M.; Wake, M.H. Heterochrony, cannibalism, and the evolution of viviparity in Salamandra salamandra. Evol. Dev. 2007, 9, 105–115. [Google Scholar] [CrossRef] [Green Version]
- Caspers, B.A.; Steinfartz, S. Preference for the other sex: Olfactory sex recognition in terrestrial fire salamanders (Salamandra salamandra). Amphib. -Reptil. 2011, 32, 503–508. [Google Scholar] [CrossRef]
- Caspers, B.A.; Steinfartz, S.; Krause, E.T. Larval deposition behaviour and maternal investment of females reflect differential habitat adaptation in a genetically diverging salamander population. Behav. Ecol. Sociobiol. 2015, 69, 407–413. [Google Scholar] [CrossRef]
- Krause, E.T.; Steinfartz, S.; Caspers, B.A. Poor nutritional conditionsduring the early larval stage reduce risk-taking activities of fire salamander larvae (Salamandra salamandra). Ethology 2011, 117, 416–421. [Google Scholar] [CrossRef]
- Limongi, L.; Ficetola, G.F.; Romeo, G.; Manenti, R. Environmental factors determining growth of salamander larvae: A field study. Curr. Zool. 2015, 61, 421–427. [Google Scholar] [CrossRef] [Green Version]
- Melotto, A.; Ficetola, G.F.; Manenti, R. Safe as a cave? Intraspecific aggressiveness rises in predator-devoid and resource-depleted environments. Behav. Ecol. Sociobiol. 2019, 73, 68. [Google Scholar] [CrossRef]
- Berven, K.A.; Grudzen, T.A. Dispersal in the wood frog (Rana sylvatica): Implications for genetic population structure. Evolution 1990, 44, 2047–2056. [Google Scholar] [CrossRef] [PubMed]
- Ficetola, G.F.; Garner, T.W.J.; De Bernardi, F. Genetic diversity, but not hatching success, is jointly affected by postglacial colonization and isolation in the threatened frog. Mol. Ecol. 2007, 16, 3285. [Google Scholar] [CrossRef]
- Romeo, G.; Giovine, G.; Ficetola, G.F.; Manenti, R. Development of the fire salamander larvae at the altitudinal limit in Lombardy (north-western Italy): Effect of two cohorts occurrence on intraspecific aggression. North-West. J. Zool. 2015, 11, 234–240. [Google Scholar]
- Barzaghi, B.; Ficetola, G.F.; Pennati, R.; Manenti, R. Biphasic predators provide biomass subsidies in small freshwater habitats: A case study of spring and cave pools. Freshw. Biol. 2017, 62, 1637–1644. [Google Scholar] [CrossRef]
- Manenti, R.; Ficetola, G.F.; Marieni, A.; De Bernardi, F. Caves as breeding sites for Salamandra salamandra: Habitat selection, larval development and conservation issues. North-West. J. Zool. 2011, 7, 304–309. [Google Scholar]
- Manenti, R.; Ficetola, G.F.; Bianchi, B.; De Bernardi, F. Habitat features and distribution of Salamandra salamandra in underground springs. Acta Herpetol. 2009, 4, 143–151. [Google Scholar]
- Manenti, R.; Lunghi, E.; Ficetola, G.F. Cave exploitation by an usual epigean species: A review on the current knowledge on fire salamander breeding in cave. Biogeographia 2017, 32, 31–46. [Google Scholar] [CrossRef] [Green Version]
- Jusczcyk, W.; Zakrzewski, M. External morphology of larval stages of the spotted salamander Salamandra salamandra (L.). Acta Biol. Crac. 1981, 23, 127–135. [Google Scholar]
- Reilly, S.M. The ontogeny of aquatic feeding behavior in Salamandra salamandra: Stereotypy and isometry in feeding kinematics. J. Exp. Biol. 1995, 198, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Giovine, G. Analisi dello sviluppo e osservazioni sullo svernamento delle larve di Salamandra salamandra salamandra (L.) nei colli di Bergamo (lombardia). Nat. Brescia. 1996, 31, 263–269. [Google Scholar]
- Lanza, B.; Andreone, F.; Bologna, M.A.; Corti, C.; Razzetti, E.P.C. Fauna d’Italia, vol. XLII, Amphibia; Calderini: Bologna, Italy, 2007; Volume XLII. [Google Scholar]
- Lanza, B.; Nistri, A.; Vanni, S. Anfibi d’Italia; Ministero dell’Ambiente e della Tutela del Territorio e del Mare, I.S.P.R.A.; Grandi & Grandi Editori: Avignano sul Panaro, Italy, 2009.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).