Abstract
The pelagic ecosystem in the Ross Sea has one central component that is very important for energy exchanges between upper and lower trophic levels: the Middle Trophic Level. Krill species are the most important and abundant organisms within this level. Several acoustic surveys were conducted in the western Ross Sea over the past 25 years, revealing that Euphausia superba is by far the most abundant species of krill in the Ross Sea during austral summer, and that its core distribution is concentrated in the northern part, bordering the Southern Ocean. Euphausia crsytallorophias, the second most abundant krill species, is more concentrated in the central Ross Sea, generally near the coast. Data on krill biomass were collected in December and January from 1994 to 2016 and analyzed together with key environmental parameters by means of two-way ANOVA in order to explain species behavior and identify possible environmental drivers. Temperature and dissolved oxygen influenced the biomass of both species of krill, while other environmental parameters only affected one species. In conclusion, the biomass of both species has varied over the years, possibly due to a complex synergy of environmental drivers.
1. Introduction
Krill is a fundamental resource in the Ross Sea, sustaining the survival and wellness of many species of marine mammals and birds inhabiting this region [1,2]. Past studies have attempted to deepen our knowledge of the dynamics of the Ross Sea marine ecosystem at various trophic levels [3,4,5]. However, many aspects need further study. The recent establishment of a Marine Protected Area (MPA) in the Ross Sea and surrounding areas, which has been in effect since 1 December 2017 [6], has made specific studies on these very important animals even more interesting, allowing researchers to compare their population dynamics with those recorded in other areas around the Antarctic continent where specific fisheries are conducted.
Following a pilot survey in 1989–1990, eight acoustic surveys targeting Antarctic krill (Euphausia superba) and crystal krill (Euphausia crystallorophias) in the western Ross Sea were conducted between 1994 and 2016, yielding some intriguing information on the krill populations of the Ross Sea [7,8,9]. Taking into account ice coverage and krill spatial distribution, it was clearly evident that both E. superba and E. crystallorophias were concentrated around latitude 74° S in November, when ice coverage was quite dense and diffused. The situation differed in December-January when polynyas (ice-free areas) are typically much larger; both species traveled north, but to varying degrees, with E. superba reaching farther north, close to the shelf break than E. crystallorophias. These two species are probably in competition, having similar feeding appendages and a similar diet based primarily on phytoplankton [10,11], but with a non-negligible quota of zooplankton [12,13]. In the Ross Sea, however, E. superba appears to prefer water mixing at the shelf break and continental slope, while E. crystallorophias generally prefer shallow waters or areas close to ice [8,9], thus reducing overlap and competition for food.
There is evidence that environmental factors can influence krill populations around the Antarctic continent, resulting in changes in their abundance and spatial distribution [4,14]. For instance, phytoplankton blooms originating from ice melting through released algae appear to promote an increase in krill biomass [15,16]. Therefore, krill abundance may be locally favored by low salinity conditions and high chlorophyll a concentration, which means ice melting and consequent phytoplankton bloom [1]. Moreover, the ice edge may be a favorable environment for krill due to its high productivity conditions, also providing protection to early developmental stages from predators [17,18]. Other environmental factors, such as dissolved oxygen, especially if considered in conjunction with the general rise in temperature, which reduces the amount of oxygen in the water, with potential consequences for krill swarm formation and relative packing density, could also be important for krill dynamics [19]. This factor may also represent a direct threat to the survival of these species [20].
The Ross Sea has witnessed significant changes in recent years, including an increase in mean summer air temperature and a decrease in shelf water salinity from the 1950s to the present day [21]. On a decadal time scale (1995–2006), these factors affected the formation of Antarctic Bottom Water (AABW) in the western and central Ross Sea [22,23], as well as Ice Shelf Water (ISW) and High Salinity Shelf Water (HSSW). All these changes, and eventually others, may have influenced the behavior of krill.
In this paper, we analyzed Antarctic krill and crystal krill biomass data and satellite environmental data to determine whether recent environmental changes have altered the abundance and distribution of E. superba and E. crystallorophias in the western Ross Sea. We also investigated the potential ramifications for other levels of the local pelagic food web.
2. Materials and Methods
2.1. Acoustic and Biological Data
This study focuses on the western Ross Sea area (GSA 88.1, Figure 1). A stable presence of E. superba and E. crystallorophias populations was observed in this region following several acoustic surveys [8,9]. This region approximates a rectangular area delimited by latitudes 69°30′ and 78°6′ S (955 km) and longitudes 164°30′ E and 175°30′ W. This area has been surveyed acoustically eight times since 1989 under different ice coverage conditions. The six surveys selected from the total were all conducted between December and January during the austral summer.
Figure 1.
The Ross Sea, the reference study area.
Acoustic monitoring was carried out every 24 h from 15 to 200 m to obtain a more complete picture of krill abundance in the water column and avoid missing any krill aggregations due to nictemeral movements, which were not particularly significant in the study area [9]. In general, there was no discernible decline in schooling behavior. After noise testing, the vessel’s speed during the survey was generally set between 8 and 9.5 knots, depending mainly on water current and wind intensity conditions. Survey routes deviated from the original plan in the presence of ice.
The dB difference methodology [24,25] was used to identify krill swarms, taking into account specific dB intervals between pairs of available frequencies for the target species, verified with the help of pelagic hauls.
Acoustic density estimates per nautical mile were expressed as nautical area scattering coefficients (NASC; m2/nm2). NASC values at 120 kHz (generally the lead frequency for krill) of swarms identified as E. superba or E. crystallorophias were used for subsequent abundance calculation using the fluid sphere model [8,26,27,28]. A density contrast coefficient (g) of 1.0357 and a sound speed contrast coefficient (h) of 1.0279 were taken from the literature [29] and used for the two species; the average tilt angle was set to 15°. Acoustic data acquisition followed CCAMLR guidelines [30]. As regards data processing, the fluid sphere model [28] was applied in all the acoustic surveys carried out in the Ross Sea since 1989. This methodology [31,32,33,34] for estimating krill biomass has been validated and improved over time.
During acoustic surveys, pelagic hauls were also conducted to aid in the analysis of echograms, to differentiate between target krill swarms and non-target organisms, and to collect length-weight data of krill samples. Hauls along the acoustic survey routes were conducted using a 5 m2 Plankton Hamburg Net (HPN) in 1994, 1997, and 2000 [26], while HPRI-1000, a new plankton net designed at the CNR in Ancona (Italy), has been used since 2004 [27]. The net’s mesh size, which was 0.5 mm in 1994, changed to 1 mm from 1997 onwards. The number and position of the hauls were not fixed, depending on target visualization on the echosounder screen and ice coverage. Capture precision, that is, the capture of acoustically identified schools, was enhanced by connecting the echosounder to the SIMRAD ITI trawl positioning and monitoring system. Moreover, during the last two surveys [9], it was possible to plot the depth data flow on the sounder screen; this made it easier to check in real time whether the net was indeed catching the targeted schools, adjusting the depth stratum as necessary. During towing, net opening and trawl depth were displayed in real time on the screen, enabling accurate sampling in correspondence with echo traces. The total catch weight of each species was established after the catch was sorted. Total length, carapace length, weight, diameter of the compound eye, and sex were determined in 100 individuals per species. The length was measured using a gauge to the nearest millimeter, and the weight of each individual was measured with a high-precision scale (0.1 g) equipped with a motion compensation device (PL1020 Marel, Gardabaer, Iceland). In general, trawling was conducted horizontally in the stratum of interest between approximately 15 and 150 m; krill swarms were not very common below this depth. Haul duration was of one hour during the first expeditions and subsequently shortened to a duration of half an hour which, given the technological improvements in trawling operations, was considered to be sufficient to obtain a good sample for biometric measurements. The average vessel speed during pelagic trawling was 2.5 knots. The length-weight equation was computed using the best fit methodology from the specific graph.
Biomass estimates derived from acoustic surveys are expressed as the mean krill density in tons per square nautical mile per Elementary Statistical Sampling Rectangle (ESSR) [27]. The ESSR method was employed in all the above-cited surveys, given its suitability for the acoustic monitoring of large areas [28]. Consequently, the western Ross Sea was subdivided into rectangular grid cells that were spaced at intervals of 1° in longitude but varied in latitude following the Earth’s curvature variation at the poles, resulting in rectangles with a constant area of 600 nm2. Species abundance and biomass were calculated for each rectangle by considering the average NASC value of the portion of acoustic transects that lay within the rectangle. Biomass was estimated by referring to the depth stratum from 15 to 200 m. The first 15 m were excluded in order to account for vessel noise, noise owing to the presence of ice, and near field issues on the basis of the transducer draft.
Table 1 contains additional information on the acoustic surveys considered in this paper.

Table 1.
Specific details of the selected acoustic surveys. The EK echosounders are SIMRAD scientific equipment.
Acoustic data and associated pelagic haul data are stored in a database at the CNR IRBIM in Ancona (Italy) and can be obtained by submitting a request to the scientist responsible for the project.
2.2. Satellite Data
Satellite data were downloaded from the Copernicus Marine Service. Monthly means of temperature [35], salinity, and ice coverage variables at 1° of horizontal resolution for 75 vertical levels were selected and analyzed together with krill biomass data. For temperature and salinity, only superficial levels from the surface to around 200 m were selected for the analyses, since these are the strata monitored by the acoustic survey. An attempt was made to take into account the average values for the entire stratum; the values at 0.5, 97, and 199 m were then analyzed. Also selected were the monthly means of total chlorophyll a, total primary production, dissolved oxygen, and total phytoplankton at a horizontal resolution of 0.25° for 75 vertical levels, with the same depth levels as above. Temperature, salinity, water velocity, and ice coverage data were acquired from the new Mercator Ocean (Toulouse, FR) Global Ocean Ensemble Reanalysis [36]. These data consist of a numerical ocean model constrained with satellite data and in situ observations. The multi-model ensemble approach allows for the estimation of uncertainties in the ocean state. This reanalysis uses altimetry data observations that began in 1993. These data were obtained from the CMCC Global Ocean Physical Reanalysis System (C-GLORS), which simulates the state of the ocean over the past decades [37,38]. Ice coverage data were obtained from the same source as temperature and salinity and were expressed as a percentage of area coverage, i.e., sea ice area fraction (SIC). Total chlorophyll a, total primary production, dissolved oxygen, and total phytoplankton data use the PISCES biogeochemical model available on the NEMO [39] modeling platform. The forcings used were FREEGLORYS2V4 ocean physics generated at Mercator-Ocean and ERA-Interim [40] atmosphere produced at ECMWF on a daily basis. 3D mean fields were interpolated on a standard regular grid in NetCDF format.
2.3. Statistical Analyses
After preliminary data exploration with the statistical software R [41], biomass data were log-transformed (log10(x + 1), where x is the biomass) since they presented much higher absolute values than satellite environmental data. For each statistical rectangle used to estimate krill biomass, the corresponding environmental parameters were cut inside them by means of R software. This provided the most coherent information in each rectangle. If one of the rectangles lacked data and this was therefore deemed “not available” (=NA), data from that rectangle were discarded. This approach enabled us to compile the most complete dataset possible.
Two-way ANOVA (Analysis of Variance) was used to examine potential correlations between krill biomass and environmental data, based on four main case studies involving the selection of environmental data:
- Environmental data averaged from 0 to 200 m
- Environmental data at surface stratum
- Environmental data at a 97 m stratum
- Environmental data at a 199 m stratum
The selection of three case studies at fixed depths, in addition to the one addressing the entire water column from 0 to 200 m, was intended to determine whether the influence of certain environmental parameters on krill could be localized, preferably at specific depths. Separate ANOVA tests were conducted for each species, and the final reported results are those obtained by retaining only the statistically significant parameters from the first run.
The final models presented in this paper were chosen from a wide number of models. The best available models were identified by using the Q-Q plot technique, one of the well-known and widely used graphical techniques for testing conformity between empirical distribution and the given theoretical distribution [42]. Analyzing ANOVA model residuals to check normality for all groups at once is easier, and particularly useful when there are many groups, as in this case [43,44]. Levene’s test for homogeneity of variances was used to test the hypothesis of equality of group variances [45]. Finally, a post-hoc Tukey’s Honest Significant Difference test [46] was performed to highlight differences over the years.
3. Results
Figure 2 and Figure 3 depict Antarctic krill and crystal krill biomass, respectively, estimated acoustically for the ESSR covered by the six surveys held in the western Ross Sea. The bathymetry reference for these figures is [47], while the land is based on [48].
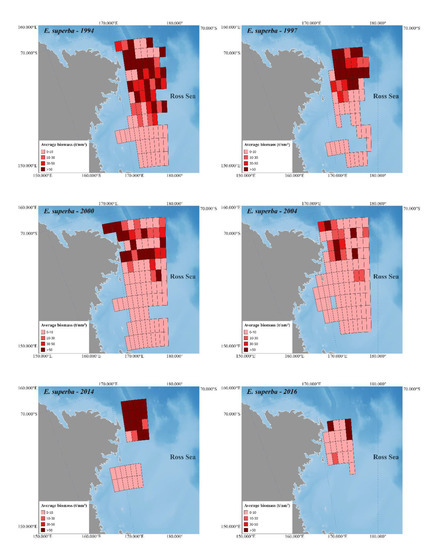
Figure 2.
The estimated biomass density of Antarctic krill in the western Ross Sea based on six selected acoustic surveys.
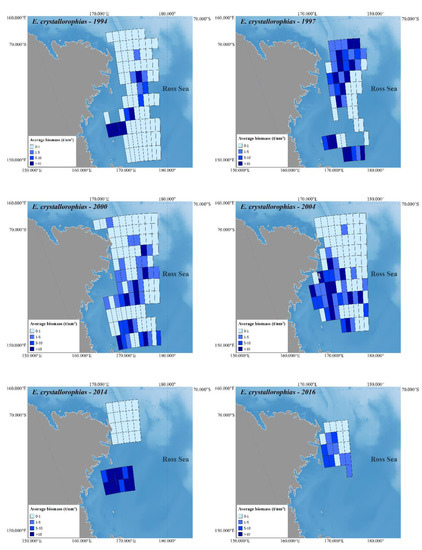
Figure 3.
The estimated biomass density of crystal krill in the western Ross Sea based on six selected acoustic surveys.
3.1. Mean Environmental Data in the Water Column Relevant for Krill Biomass Estimation (0–200 m)
The ANOVA results for E. crystallorophias, leaving the significant environmental parameters, are reported in Table 2, while the residuals’ histograms and QQ plots are shown in Figure 4.

Table 2.
Two-way ANOVA for E. crystallorophias and averaged environmental parameters.
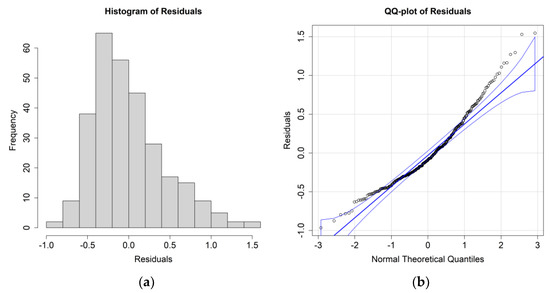
Figure 4.
(a) Histogram of the residuals for the E. crystallorophias biomass case study and environmental values averaged between 0 and 200 m; (b) QQ plot of the residuals.
The ANOVA results for E. superba, including only the significant environmental parameters, are reported in Table 3; residuals’ histograms and QQ plots are shown in Figure 5.

Table 3.
Two-way ANOVA for E. superba and averaged environmental parameters.
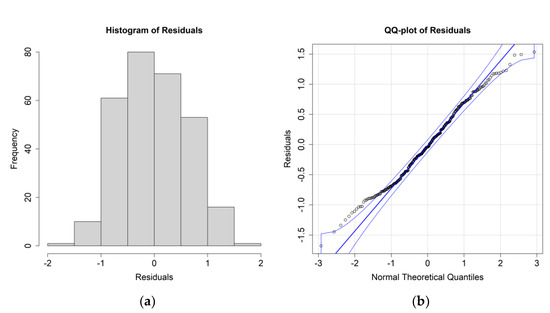
Figure 5.
(a) Histogram of the residuals for the E. superba biomass case study and environmental values averaged between 0 and 200 m; (b) QQ plot of the residuals.
To summarize the most important results, crystal krill biomass presented highly statistically significant correlations with the survey year, ice coverage, and dissolved oxygen, whereas significant correlations were found with salinity. Antarctic krill showed highly significant correlations with the survey month, survey year, chlorophyll a concentration, and dissolved oxygen, whereas significant correlations were found with salinity and water velocity. This indicates that both species are sensitive to dissolved oxygen levels and that their biomass has altered over the years. As regards crystal krill, ice coverage also seems to be important, while the E. superba biomass has a significant association with chlorophyll a concentration and the survey month.
3.2. Environmental Data at the Surface Layer (0.5 m)
The E. crystallorophias ANOVA results for this case study are reported in Table 4, while the residuals’ histograms and QQ plot are reported in Figure 6.

Table 4.
Two-way ANOVA for E. crystallorophias and environmental parameters at the surface layer (0.5 m).
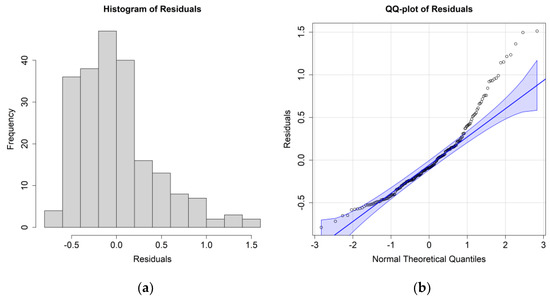
Figure 6.
(a) Histogram of the residuals for the E. crystallorophias biomass case study and environmental values at 0.5 m; (b) QQ plot of the residuals.
The E. superba ANOVA results analyzed together with environmental parameters at the surface layer are reported in Table 5; residuals’ histograms and QQ plots are shown in Figure 7.

Table 5.
Two-way ANOVA for E. superba and environmental parameters at the surface layer (0.5 m).
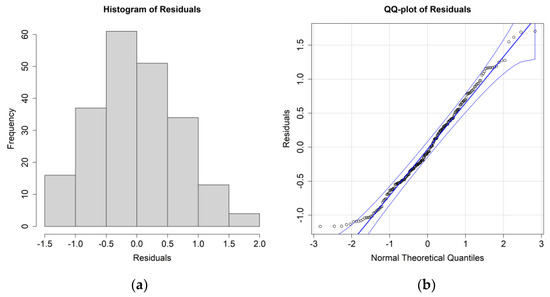
Figure 7.
(a) Histogram of the residuals for the E. superba biomass case study and environmental values at 0.5 m; (b) QQ plot of the residuals.
Crystal krill biomass presented highly significant connections with temperature and significant correlations with the survey year and chlorophyll a concentration. Antarctic krill exhibited highly significant connections with the survey month, survey year, and temperature, while a significant correlation was found with salinity. At the surface layer, both species were shown to be influenced by temperature, with Antarctic krill biomass being the only one to also vary in accordance with the survey year and month, as was the case for averaged environmental parameters throughout the water column.
3.3. Environmental Data at the 97 m Layer
The ANOVA results for the E. crystallorophias case study are reported in Table 6, while the histograms and QQ plots depicting residuals are reported in Figure 8.

Table 6.
Two-way ANOVA for E. crystallorophias and environmental parameters at the 97 m layer.
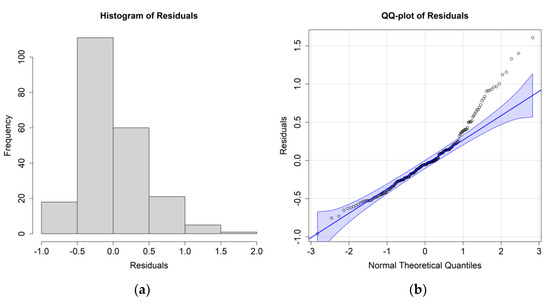
Figure 8.
(a) Histogram of the residuals for the E. crystallorophias biomass case study and environmental values at 97 m; (b) QQ plot of the residuals.
The E. superba ANOVA results analyzed together with environmental parameters at the 97 m layer are reported in Table 7; residuals’ histograms and QQ plots are shown in Figure 9.

Table 7.
Two-way ANOVA for E. superba and environmental parameters at the 97 m layer.
Figure 9.
(a) Histogram of the residuals for the E. superba biomass case study and environmental values at 97 m; (b) QQ plot of the residuals.
Temperature and dissolved oxygen were found to be highly significant for the E. crystallorophias biomass. E. superba exhibited highly significant correlations with the survey month and year, temperature, chlorophyll a concentration, and dissolved oxygen. At around 100 m depth, the factors that seem to exert the biggest influence are temperature and dissolved oxygen for both species. E. superba presented a highly significant connection with chlorophyll a concentration, survey month, and survey year. Other significant correlations for E. crystallorophias were found to be the survey year, water velocity, and chlorophyll concentration.
3.4. Environmental Data at the 199 m Layer
The ANOVA results for the E. crystallorophias case study are reported in Table 8, while the residuals’ histograms and QQ plot are depicted in Figure 10.

Table 8.
Two-way ANOVA for E. crystallorophias and environmental parameters at the 199 m layer.
Figure 10.
(a) Histogram of the residuals for the E. crystallorophias biomass case study and environmental values at 199 m; (b) QQ plot of the residuals.
The ANOVA results for E. superba analyzed together with environmental parameters at the 199 m layer are reported in Table 9; residuals’ histograms and QQ plots are shown in Figure 11.

Table 9.
Two-way ANOVA for E. superba and environmental parameters at the 199 m layer.
Figure 11.
(a) Histogram of the residuals for the E. superba biomass case study and environmental values at 199 m; (b) QQ plot of the residuals.
Temperature and phytoplankton correlations with the E. crystallorophias biomass were found to be highly significant. E. superba showed highly significant associations with the survey month, survey year, and temperature. At a depth of 200 m, temperature appears to influence both species. Phytoplankton concentration seems to be important for crystal krill, while the survey month and year influence Antarctic krill. Other significant connections were found with the survey year and chlorophyll a concentration for E. crystallorophias, as well as chlorophyll concentration in the case of E. superba.
All the above results, limited to highly significant correlations, are summarized in Table 10.

Table 10.
Summary of highly significant correlations between the two krill species and environmental parameters for each case study.
The results of Levene’s test and the Tukey test for each of the above case studies can be found in the Supplementary Materials.
4. Discussion
Crystal krill yielded very different results when environmental average values from 0 to 200 m were compared to values at fixed depth strata. In the former case, the most significant correlations involve the survey year, ice coverage, and dissolved oxygen. However, when fixed depth strata are considered, the variable most strongly correlated with krill biomass is temperature, followed by dissolved oxygen at a depth of 100 m and total phytoplankton concentration at a depth of 200 m. The correlation between the survey year and crystal krill biomass in the western Ross Sea suggests that the biomass has varied significantly across the years in which the surveys were conducted. Previous studies have revealed a correlation between E. crystallorophias biomass and ice coverage [9,49], as this species prefers to inhabit coastal and ice-covered areas and should be sensitive to ice dynamics. The relationship between crystal krill and sea ice can be explained by the availability of food and the desire to protect eggs and early life stages [50,51,52].
As regards the synergistic relationship between temperature and dissolved oxygen, we have already mentioned a general rise in water temperature in the Ross Sea, which would result in a decrease in the water’s oxygen concentration. This may have a deleterious impact on krill swarm formation and relative density [19], posing a potential threat to these species’ survival rate [20]. Based on our results, this synergistic effect on crystal krill should be stronger at a depth of around 100 m. In all fixed strata analyses, the temperature is by far the most prominent environmental parameter with significant connections in the case of crystal krill. This factor may be related to the importance of temperature in the regulation of krill metabolism [53]. Some experiments [54] demonstrated that food and oxygen demands tend to increase as temperatures rise, with a possible subtraction of energy from other activities, such as reproduction, in a global warming scenario such as the one we are helping to create. Temperature is also important for the stabilization of ice coverage, given that ice is important as a reserve of food and shelter for the protection of krill larvae [55,56,57,58]. Moreover, ice-free areas often demonstrate the predominance of krill rivals such as Salpa thompsoni [57]. Optimal summer temperatures seem to differ for these two krill species, with E. superba preferring higher temperatures at the shelf break in the northern part of the Ross Sea and E. crystallorophias preferring the shallower depths and colder temperatures of the south-western neritic part [8,9,50]. At least during the austral summer, this factor contributes to the spatial separation of these species and significantly reduces competition for food. The link between crystal krill abundance and phytoplankton has already been the subject of prior research [1,9]; however, the significance of its availability at greater depths (200 m), as demonstrated in this study, may not be as well documented. As also reported in other Antarctic areas [58], our data revealed that this species is quite abundant even at 100 m or more. For this reason, crystal krill at depths between 100–200 m could be quite influenced by the availability of phytoplankton in these or neighboring strata above.
In the ANOVA results, Antarctic krill presented a main difference with respect to crystal krill: the survey month and survey year had significant correlations with biomass in all case studies. This factor highlights the considerable variability of Antarctic krill biomass over the years, as well as a major difference between the situation in December, when ice is denser, and January, when most of the area is typically ice-free. The different levels of abundance and spatial distribution of Antarctic krill between December and January, as discussed in this paper, were also reported in previous studies [8,9]. In fact, a comparison of the beginning of austral summer with full summer reveals quite a diverse picture for these creatures, with the bulk of Antarctic krill distribution shifting northwards throughout the summer period. Even in the case of Antarctic krill, temperature appears to be a highly significant parameter for similar reasons as those reported for crystal krill; analogous considerations may also be made in this case, but taking into account these two species’ different preferences. Another, less-studied phenomenon connected to the rise in temperature and consequent increase in ice melting, is the stranded Antarctic krill individuals found in certain regions of Antarctica, such as Potter Cove [59], where the creatures’ guts were found to be full of particles suspended in the floods originating from melted ice. The latter is another example of the importance of a balance between temperature, ice presence, and marine organisms in Antarctic ecosystems. A significant association between E. superba biomass and dissolved oxygen was observed in the average values of the first 200 m and the 100 m depth strata. This factor reinforces the notion that both these krill species are sensitive to the same environmental parameters, despite their different preference ranges, as observed for temperature. Similarly, chlorophyll a concentration had an impact on Antarctic krill biomass when environmental parameters were averaged from 0 to 200 m and 100 m depth strata. This element highlights the trophic factor’s importance for Antarctic krill, particularly the availability of phytoplankton [60,61], in addition to boosting krill recruitment [62]. The ANOVA results suggest that the 100 m depth stratum has the highest phytoplankton concentration. Looking at the CTD profiles reported in [63], which were performed at the positions of pelagic hauls during the 2004 acoustic survey in the western Ross Sea, a peak in fluorescence can be seen at a 40–50 m depth, and in one case, even deeper. This suggests that chlorophyll concentration in these depth strata could exert a relevant influence on Antarctic krill. In any case, the analyses of fluorescence data from CTD sampling should be improved in order to support this hypothesis. Some authors also discovered an increase in Antarctic krill lipid reserves in conjunction with increasing levels of chlorophyll a concentration, estimated from remote sensing data collection and a decrease in sea surface temperature [11]. The present results showed significant associations between Antarctic krill biomass, chlorophyll a concentration, and temperature at a depth of 100 m, suggesting that something similar to the reported instance could also have happened in our study.
This paper demonstrates that acoustic estimations of krill biomass combined with satellite-derived environmental data can provide intriguing insights into the environmental variables that exert an influence on krill and, consequently, the Ross Sea’s local pelagic ecosystem. These preliminary results may provide an avenue for expanding our understanding of the spatial and temporal variations of krill biomass in the Ross Sea. The inclusion of krill spatial distribution and data on krill recruitment and krill predators should improve the precision of these conclusions.
5. Conclusions
The analyses in this paper have highlighted the importance of certain environmental parameters for Antarctic krill and crystal krill biomass and demonstrated that the levels of these stocks vary significantly from one summer to another. Temperature and dissolved oxygen are some of the parameters that are common to both species, and although chlorophyll a concentration was shown to be important for E. superba, ice coverage is a critical factor for E. crystallorophias. These preliminary analyses should be explored further in order to understand the complex intricate links between krill and the environment in the Ross Sea.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d14060433/s1, Table S1: Levene’s Test of Homogeneity of Variance (center = mean) for all E. crystal-lorophias case studies; Table S2: Levene’s Test of Homogeneity of Variance (center = mean) for all E. superba case studies; Table S3: Tukey multiple comparisons of means with a 95% family-wise confidence level for E. crystallorophias and averaged environmental parameters; Table S4: Tukey multiple comparisons of means with a 95% family-wise confidence level for E. crystallorophias and environmental parameters at 0.5 m; Table S5: Tukey multiple comparisons of means with a 95% family-wise confidence level for E. crystallorophias and environmental parameters at 97 m; Table S6: Tukey multiple comparisons of means with a 95% family-wise confidence level for E. crystallorophias and environmental parameters at 199 m; Table S7: Tukey multiple comparisons of means with a 95% family-wise confidence level for E. superba and averaged environmental parameters; Table S8: Tukey multiple comparisons of means with a 95% family-wise confidence level for E. superba and environmental parameters at 0.5 m; Table S9: Tukey multiple comparisons of means with a 95% family-wise confidence level for E. superba and environmental parameters at 97 m; Table S10: Tukey multiple comparisons of means with a 95% family-wise confidence level for E. superba and environmental parameters at 199 m.
Author Contributions
Conceptualization, A.D.F. and I.L.; methodology, A.D.F. and I.L.; software, G.C., I.B. and I.C.; validation, A.D.F., I.B., G.C., I.C., S.M., G.G. and I.L.; formal analysis, A.D.F., I.C. and I.L.; investigation, A.D.F., I.B., G.C., I.C., S.M., G.G. and I.L.; resources, I.L.; data curation, A.D.F., I.L., I.B. and G.C.; writing—original draft preparation, A.D.F.; writing—review and editing, A.D.F., I.B., G.C., I.C., S.M., G.G. and I.L.; visualization, A.D.F., I.L., I.B. and G.C.; supervision, A.D.F. and I.L.; project administration, I.L.; funding acquisition, I.L. All authors have read and agreed to the published version of the manuscript.
Funding
Authors are grateful to the Italian National Antarctic Scientific Commission (CSNA) and the Italian National Antarctic program (PNRA) for the endorsement of the Special Issue initiative and to the Italian National Antarctic Museum (MNA) for the financial support. Italian National Antarctic Research Program (PNRA): 2018-B1N1.01 (ROSSKRILL); Italian National Antarctic Research Program (PNRA): PNRA18_00276-B1 (ROSSKRILL).
Institutional Review Board Statement
The animal study protocol was approved by the Antarctic Technical Unit-Unit of Project Research, Technological Innovation, and Environmental Protection (UTA-RIA) of the National Agency for new Technologies, Energy, and Sustainable Economic Development (protocol code ENEA/2021/62121/UTA, 13/10/2021; antecedent analogous permits without protocol code were issued on 12 November 2013 and 23 January 2016).
Data Availability Statement
Survey data are available on request. Satellite environmental data presented in this study are freely accessible via the search engine at https://resources.marine.copernicus.eu/products (access on 25 March 2022).
Acknowledgments
This paper is an Italian contribution to the Commission for the Conservation of Antarctic Marine Living Resources CONSERVATION MEASURE 91-05 (2016) for the Ross Sea region Marine Protected Area, specifically, addressing the priorities of Annex 91-05/C. The authors would like to thank the Italian National Research Program in Antarctica (PNRA) for its financial support of the acoustic surveys in the Ross Sea. We also wish to express our gratitude to the crew of R/V Italica and all the researchers involved for their assistance in conducting the surveys.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kang, M.; Fajaryanti, R.; Son, W.; Kim, J.-K.; La, H.S. Acoustic Detection of Krill Scattering Layer in the Terra Nova Bay Polynya, Antarctica. Front. Mar. Sci. 2020, 7, 584550. [Google Scholar] [CrossRef]
- Meyer, B.; Atkinson, A.; Bernard, K.S.; Brierley, A.S.; Driscoll, R.; Hill, S.L.; Marschoff, E.; Maschette, D.; Perry, F.A.; Reiss, C.S.; et al. Successful ecosystem-based management of Antarctic krill should address uncertainties in krill recruitment, behaviour and ecological adaptation. Commun. Earth Environ. 2020, 1, 28. [Google Scholar] [CrossRef]
- Bolinesi, F.; Saggiomo, M.; Ardini, F.; Castagno, P.; Cordone, A.; Fusco, G.; Rivaro, P.; Saggiomo, V.; Mangoni, O. Spatial-related community structure and dynamics in phytoplankton of the Ross Sea, Antarctica. Front. Mar. Sci. 2020, 7, 574963. [Google Scholar] [CrossRef]
- Smith, W.O.; Sedwick, P.N.; Arrigo, K.R.; Ainley, D.G.; Orsi, A.H. The Ross Sea in a sea of change. Oceanography 2012, 25, 90–103. [Google Scholar] [CrossRef] [Green Version]
- Smith, W.O.; Ainley, D.G.; Arrigo, K.R.; Dinniman, M.S. The Oceanography and Ecology of the Ross Sea. Annu. Rev. Mar. Sci. 2014, 6, 469–487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brooks, C.M.; Bloom, E.; Kavanagh, A.; Nocito, E.S.; Watters, G.M.; Weller, J. The Ross Sea, Antarctica: A highly protected MPA in international waters. Mar. Pol. 2021, 134, 104795. [Google Scholar] [CrossRef]
- Azzali, M.; Kalinowski, J. Spatial and temporal distribution of krill E. superba biomass in the Ross Sea (1989–1990 and 1994). In Ross Sea Ecology; Faranda, F.M., Guglielmo, L., Ionora, A., Eds.; Springer: Berlin, Germany, 1999; pp. 433–455. [Google Scholar]
- Azzali, M.; Leonori, I.; De Felice, A.; Russo, A. Spatial–temporal relationships between two euphausiid species in the Ross Sea. Chem. Ecol. 2006, 22, S219–S233. [Google Scholar] [CrossRef]
- Leonori, I.; De Felice, A.; Canduci, G.; Costantini, I.; Biagiotti, I.; Giuliani, G.; Budillon, G. Krill distribution in relation to environmental parameters in mesoscale structures in the Ross Sea. J. Mar. Sys. 2017, 166, 159–171. [Google Scholar] [CrossRef]
- Haberman, K.L.; Ross, R.M.; Quetin, L.B. Diet of the Antarctic krill (Euphausia superba Dana): II. Selective grazing in mixed phytoplankton assemblages. J. Exp. Mar. Biol. Ecol. 2003, 283, 97–113. [Google Scholar] [CrossRef]
- Hellessey, N.; Johnson, R.; Ericson, J.A.; Nichols, P.D.; Kawaguchi, S.; Nicol, S.; Hoem, N.; Virtue, P. Antarctic krill lipid and fatty acid content variability is associated to satellite derived chlorophyll a and sea surface temperatures. Sci. Rep. 2020, 10, 6060. [Google Scholar] [CrossRef]
- Schmidt, K.; Atkinson, A.; Pond, D.W.; Ireland, L.C. Feeding and overwintering of Antarctic krill across its major habitats: The role of sea ice cover, water depth, and phytoplankton abundance. Limn. Ocean. 2014, 59, 17–36. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, K.; Atkinson, A. Feeding and Food Processing in Antarctic Krill (Euphausia superba Dana). In Biology and Ecology of Antarctic Krill. Advances in Polar Ecology; Siegel, V., Ed.; Springer: Cham, Switzerland, 2016. [Google Scholar] [CrossRef]
- Flores, H.; Atkinson, A.; Kawaguchi, S.; Krafft, B.A.; Milinevsky, G.; Nicol, S.; Reiss, C.; Tarling, G.A.; Werner, R.; Bravo Rebolledo, E.; et al. Impact of climate change on Antarctic krill. Mar. Ecol. Prog. Ser. 2012, 458, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Biggs, T.E.G.; Alvarez-Fernandez, S.; Evans, C.; Mojica, K.D.A.; Rozema, P.D.; Venables, H.J.; Pond, D.W.; Brussaard, C.P.D. Antarctic phytoplankton community composition and size structure: Importance of ice type and temperature as regulatory factors. Pol. Biol. 2019, 42, 1997–2015. [Google Scholar] [CrossRef] [Green Version]
- Moreau, S.; Boyd, P.W.; Strutton, P.G. Remote assessment of the fate of phytoplankton in the Southern Ocean sea-ice zone. Nat. Commun. 2020, 11, 3108. [Google Scholar] [CrossRef]
- Brierley, A.S.; Fernandes, P.G.; Brandon, M.A.; Armstrong, F.; Millard, N.W.; McPhail, S.D.; Stevenson, P.; Pebody, M.; Perrett, J.; Squires, M.; et al. Antarctic krill under sea ice: Elevated abundance in a narrow band just south of ice edge. Science 2002, 295, 1890–1892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicol, S. Krill, currents, and sea ice: Euphausia superba and its changing environment. Bioscience 2006, 56, 111–120. [Google Scholar] [CrossRef] [Green Version]
- Brierley, A.S.; Cox, M.J. Shapes of krill swarms and fish schools emerge as aggregation members avoid predators and access oxygen. Curr. Biol. 2010, 20, 1758–1762. [Google Scholar] [CrossRef] [Green Version]
- Tremblay, N.; Abele, D. Response of three krill species to hypoxia and warming: An experimental approach to oxygen minimum zones expansion in coastal ecosystems. Mar. Ecol. 2016, 37, 179–199. [Google Scholar] [CrossRef] [Green Version]
- Fusco, G.; Budillon, G.; Spezie, G. Surface heat fluxes and thermohaline variability in the Ross Sea and in Terra Nova Bay polynya. Cont. Shelf Res. 2009, 29, 1887–1895. [Google Scholar] [CrossRef]
- Jacobs, S.S.; Giulivi, C.F.; Mele, P.A. Freshening of the Ross Sea during the late 20th century. Science 2002, 297, 386–389. [Google Scholar] [CrossRef] [Green Version]
- Budillon, G.; Castagno, P.; Aliani, S.; Spezie, G.; Padman, L. Thermohaline variability and Antarctic bottom water formation at the Ross Sea shelf break. Deep-Sea Res. I Oceanogr. Res. Pap. 2011, 58, 1002–1018. [Google Scholar] [CrossRef]
- Madureira, L.S.P.; Everson, I.; Murphy, E.J. Interpretation of acoustic data at two frequencies to discriminate between Antarctic krill (Euphausia superba Dana) and other scatterers. J. Plank. Res. 1993, 1, 787–802. [Google Scholar] [CrossRef]
- Watkins, J.L.; Brierley, A.S. Verification of the acoustic techniques used to identify Antarctic krill. ICES J. Mar. Sci. 2002, 59, 1326–1336. [Google Scholar] [CrossRef]
- Azzali, M.; Leonori, I.; Lanciani, G. A hybrid approach to acoustic classification and length estimation of krill. CCAMLR Sci. 2004, 11, 33–58. [Google Scholar]
- Azzali, M.; Russo, A.; Sala, A.; De Felice, A.; Catalano, B. Preliminary Results of A Survey on Krill, Environment and Predators in CCAMLR Division 88.1 Carried out in December 2003 and in January 2004 (Project 8.4); CCAMLR WG-EMM-04/71; CCAMLR: Hobart, Australia, 2004; 23p. [Google Scholar]
- Simmonds, E.J.; MacLennan, D.N. Fisheries Acoustics; Blackwell Science: Oxford, UK, 2005; p. 456. [Google Scholar]
- Foote, K.G.; Everson, I.; Watkins, J.L.; Bone, D.G. Target strengths of Antarctic krill (Euphausia superba) at 38 and 120 kHz. J. Acoust. Soc. Am. 1990, 87, 16–24. [Google Scholar] [CrossRef] [Green Version]
- Anonymous. Report of the Third Meeting of the Subgroup on Acoustic Survey and Analysis Methods (Cambridge, UK, 30 April to 2 May 2007); CCAMLR: Hobart, Australia, 2007. [Google Scholar]
- Anderson, V.C. Sound scattering from a fluid sphere. J. Acoust. Soc. Am. 1950, 22, 426–431. [Google Scholar] [CrossRef]
- Johnson, R.K. Sound scattering from a fluid sphere revisited. J. Acoust. Soc. Am. 1977, 61, 375–377. [Google Scholar] [CrossRef]
- Stanton, T.K.; Clay, C.S.; Chu, D. Ray representation of sound scattering by weakly scattering deformed fluid cylinders: Simple physics and application to zooplankton. J. Acoust. Soc. Am. 1993, 94, 3452–3454. [Google Scholar] [CrossRef]
- Macaulay, M.C. A generalized target strength model for euphausiids, with application to other zooplankton. J. Acoust. Soc. Am. 1994, 95, 2452–2466. [Google Scholar] [CrossRef]
- Copernicus Marine Service. Available online: https://resources.marine.copernicus.eu/products (accessed on 25 March 2022).
- Lellouche, J.-M.; Greiner, E.; Le Galloudec, O.; Garric, G.; Regnier, C.; Drevillon, M.; Benkiran, M.; Testut, C.-E.; Bourdalle-Badie, R.; Gasparin, F.; et al. Recent updates to the Copernicus Marine Service global ocean monitoring and forecasting real-time 1/12° high-resolution system. Ocean Sci. 2018, 14, 1093–1126. [Google Scholar] [CrossRef] [Green Version]
- Dobricic, S.; Pinardi, N. An oceanographic three-dimensional variational data assimilation scheme. Ocean Mod. 2008, 22, 89–105. [Google Scholar] [CrossRef]
- Storto, A.; Masina, S. C-GLORSv5: An improved multi-purpose global ocean eddy-permitting physical reanalysis. Earth Syst. Sci. Data Discuss. 2016, 8, 679–696. [Google Scholar] [CrossRef] [Green Version]
- Pelletier, C.; Fichefet, T.; Goosse, H.; Haubner, K.; Helsen, S.; Huot, P.V.; Kittel, C.; Klein, F.; van Lipzig, N.P.; Marchi, S.; et al. PARASO, a circum-Antarctic fully coupled ice-sheet-ocean-sea-ice-atmosphere-land model involving f.ETISh1.7, NEMO3.6, LIM3.6, COSMO5.0 and CLM4.5. Geosci. Mod. Dev. 2022, 15, 553–594. [Google Scholar] [CrossRef]
- Berrisford, P.; Dee, D.P.; Poli, P.; Brugge, R.; Fielding, M. The ERA-Interim archive Version 2.0. Ecmwf Era Rep. Ser. 2011, 13–23. Available online: https://www.ecmwf.int/en/elibrary/8174-era-interim-archive-version-20 (accessed on 15 April 2022).
- R Core Team. R: A language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. 2021. Available online: https://www.R-project.org/ (accessed on 15 April 2022).
- Anonymous. Q-Q Plot (Quantile to Quantile Plot). In The Concise Encyclopedia of Statistics; Springer: New York, NY, USA, 2008. [Google Scholar] [CrossRef]
- Shapiro, S.S.; Wilk, M.B. An analysis of variance test for normality (complete samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Royston, P. An extension of Shapiro and Wilk’s W test for normality to large samples. Appl. Stat. 1982, 31, 115–124. [Google Scholar] [CrossRef]
- Brown, M.B.; Forsythe, A.B. Robust Tests for the Equality of Variances. J. Am. Stat. Ass. 1974, 69, 364–367. [Google Scholar] [CrossRef]
- Brillinger, D.R. The Collected Works of John, W. Tukey; Time Series, 1949–1964; CRC Press: Boca Raton, FL, USA, 1984; Volume 1. [Google Scholar]
- Arndt, J.E.; Schenke, H.W.; Jakobsson, M.; Nitsche, F.-O.; Buys, G.; Goleby, B.; Rebesco, M.; Bohoyo, F.; Hong, J.K.; Black, J.; et al. The International Bathymetric Chart of the Southern Ocean (IBCSO)—Digital bathymetric model. Pangaea https://doi.org/10.1594/PANGAEA.805734; In supplement to: Arndt, J.E.; Schenke, H.W.; Jakobsson, M.; Nitsche, F.-O.; Buys, G.; Goleby, B.; Rebesco, M.; Bohoyo, F.; Hong, J.K.; Black, J.; et al. The International Bathymetric Chart of the Southern Ocean Version 1.0—A new bathymetric compilation covering circum-Antarctic waters. Geoph. Res. Lett. 2013, 40, 3111–3117. [Google Scholar]
- Gerrish, L.; Fretwell, P.; Cooper, P. Medium Resolution Vector Polygons of the Antarctic Coastline (Version 7.3). UK Polar Data Centre; Natural Environment Research Council; UK Research & Innovation: Swindon, UK, 2020. [Google Scholar] [CrossRef]
- Pakhomov, E.A.; Perissinotto, R. Antarctic neritic krill Euphausia crystallorophias: Spatio-temporal distribution, growth and grazing rates. DeepSea Res. I 1996, 43, 59–87. [Google Scholar] [CrossRef]
- Davis, L.B.; Hofmann, E.E.; Klinck, J.M.; Piñones, A.; Dinniman, M.S. Distributions of krill and Antarctic silverfish and correlations with environmental variables in the western Ross Sea, Antarctica. Mar. Ecol. Prog. Ser. 2017, 584, 45–65. [Google Scholar] [CrossRef] [Green Version]
- Saenz, B.T.; Ainley, D.G.; Daly, K.L.; Ballard, G.; Conlisk, E.; Elrod, M.L.; Stacy, K.L. Drivers of concentrated predation in an Antarctic marginal-ice-zone food web. Sci. Rep. 2020, 10, 7282. [Google Scholar] [CrossRef] [PubMed]
- David, C.L.; Schaafsma, F.L.; van Franeker, J.A.; Pakhomov, E.A.; Hunt, B.P.V.; Lange, B.A.; Castellani, G.; Brandt, A.; Flores, H. Sea-ice habitat minimizes grazing impact and predation risk for larval Antarctic krill. Pol. Biol. 2021, 44, 1175–1193. [Google Scholar] [CrossRef]
- Tarling, G.A. Routine metabolism of Antarctic krill (Euphausia superba) in South Georgia waters: Absence of metabolic compensation at its range edge. Mar. Biol. 2020, 167, 108. [Google Scholar] [CrossRef]
- Michael, K.; Suberg, L.A.; Wessels, W.; Kawaguchi, S.; Meyer, B. Facing Southern Ocean warming: Temperature effects on whole animal performance of Antarctic krill (Euphausia superba). Zool. 2021, 146, 125910. [Google Scholar] [CrossRef] [PubMed]
- Stretch, J.J.; Hamner, P.P.; Hamner, W.M.; Michel, W.C.; Cook, J.; Sullivan, C.W. Foraging behavior of antarctic krill Euphausia superba on sea ice microalgae. Mar. Ecol. Prog. Ser. 1988, 44, 131–139. [Google Scholar] [CrossRef]
- Veytia, D.; Bestley, S.; Kawaguchi, S.; Meiners, K.M.; Murphy, E.J.; Fraser, A.D.; Kusahara, K.; Kimura, N.; Corney, S. Overwinter sea-ice characteristics important for Antarctic krill recruitment in the southwest Atlantic. Ecol. Ind. 2021, 129, 107934. [Google Scholar] [CrossRef]
- Loeb, V.; Siegel, V.; Holm-Hansen, O.; Hewitt, R.; Fraser, W.; Trivelpiece, W.; Trivelpiece, S. Effects of sea-ice extent and krill or salp dominance on the Antarctic food web. Nature 1997, 387, 897–900. [Google Scholar] [CrossRef]
- Nordhausen, W. Winter abundance and distribution of Euphausia superba, E. crystallorophias, and Thysanoessa macrura in Gerlache Strait and Crystal Sound, Antarctica. Mar. Ecol. Prog. Ser. 1994, 109, 131–142. [Google Scholar] [CrossRef]
- Fuentes, V.; Alurralde, G.; Meyer, B.; Aguirre, G.E.; Canepa, A.; Wölfl, A.-C.; Hass, H.C.; Williams, G.N.; Schloss, I.R. Glacial melting: An overlooked threat to Antarctic krill. Sci. Rep. 2016, 6, 27234. [Google Scholar] [CrossRef] [Green Version]
- Silk, J.R.D.; Thorpe, S.E.; Fielding, S.; Murphy, E.J.; Trathan, P.N.; Watkins, J.L.; Hill, S.L. Environmental correlates of Antarctic krill distribution in the Scotia Sea and southern Drake Passage. ICES J. Mar. Sci. 2016, 73, 2288–2301. [Google Scholar] [CrossRef] [Green Version]
- Hill, S.L.; Phillips, T.; Atkinson, A. Potential climate change effects on the habitat of Antarctic krill in the Weddell quadrant of the Southern Ocean. PLoS ONE 2013, 8, e72246. [Google Scholar] [CrossRef] [Green Version]
- Marrari, M.; Daly, K.L.; Hu, C. Spatial and temporal variability of SeaWiFS chlorophyll a distributions west of the Antarctic Peninsula: Implications for krill production. Deep Sea Res. II 2008, 55, 377–392. [Google Scholar] [CrossRef] [Green Version]
- De Felice, A. Stock e Bioenergetica di due Specie di Eufausiacei (E. superba ed E. crystallorophias) nel Mare di Ross. Ph.D. Thesis, Università Politecnica delle Marche, Ancona, Italy, 9 February 2010. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).