Abstract
Landscape structure and configuration may affect bird body condition, with contrasting effects on resident and migratory species. There is little empirical evidence to support this hypothesis in tropical regions, where land-use change poses a major threat to biodiversity. We aimed to assess the effects of landscape structure and configuration on the body condition of neotropical migrant and resident bird species. We compiled body condition data (using the scaled mass index) of nine bird species (five resident and four migratory). We characterized landscape structure and configuration at 26 localities. We evaluated the effects of landscape metrics on bird body condition using Bayesian linear mixed models. The landscapes in our study largely varied in forest, crop, and grassland cover, as well as in landscape metrics. When we examined migrant birds, we found a positive effect of landscape connectivity and crop cover on body condition. Similarly, body condition of resident birds was positively affected by connectivity and crop cover, but also by forest patch area and capture day. Changes in landscape structure and configuration may indirectly alter the access to resources, causing additional energy expenditures, leading to a deteriorated body condition. Conversely, landscape heterogeneity may have a positive effect on bird body condition. Therefore, we recommend maintaining connectivity and complementary resources in the landscape.
1. Introduction
Tropical forests harbor more than half of all global biodiversity. Therefore, those forests are considered a priority for conserving threatened species, ecological interactions, and ecosystem services [1]. However, tropical forests are threatened by many biodiversity-loss drivers, with land-use change being one of the most important [2]. Approximately 70 million ha were deforested in the last decade, being converted to productive uses [3]. Such land-use changes have increased forest fragmentation in landscapes now dominated by large crop areas [3,4,5]. Total crop area in Latin America and the Caribbean is 160 million ha [6]. Additionally, native tropical forests in Latin America have undergone an attrition process, with 55 million forest fragments with a median size of 17 ha [7]. Therefore, habitat loss and fragmentation are considered key factors that explain changes in the vertebrate species richness and composition of this region [8,9,10].
While habitat fragmentation effects are commonly associated with species richness and abundance reductions due to area effects [11,12,13], it may have less obvious consequences (e.g., [14]). Among those less obvious effects, we may find an indirect consequence of forest fragmentation in bird survival probability due to alterations in body condition or energy reserves [15,16,17]. Thus, forest fragmentation may reduce access to food resources (e.g., insects or fruits) and increase energy expenditure when birds move among forest fragments while looking for resources [16,18,19]. Therefore, the remaining forest fragments and some landscape attributes (e.g., connectivity) are fundamental for migratory and resident bird populations in tropical regions [18]. Particularly, habitat quality is relevant for neotropical migratory birds during winter, and it can influence their reproductive success during spring [15,20,21].
The Colombian Tropical Andes ecoregion is among the highest-rated bird diversity places on Earth [22]. This ecoregion receives at least 139 migratory passerine species from North America [23,24]. It is also one of the regions with the greatest deforestation rates in the tropics (up to 67%; [25]). The drastic forest loss and fragmentation in the Andes are likely to be the cause of recent changes in species richness of migratory and resident birds, documented since the decade of 1990 [8,26,27]. However, we know very little about the effects of fragmentation on bird body condition. We aimed to assess the effects of landscape structure and configuration on nine bird species (four resident and five migratory species) in the tropical Andes. Given that body condition in birds is known to be affected by landscape attributes [17], and land-use changes have altered that landscape configuration in the Tropical Andes, we hypothesized that: (1) bird body condition will be negatively affected by crop cover and landscape connectivity, and (2) body conditions of migrant and resident bird species will have differential responses to landscape configuration.
2. Materials and Methods
2.1. Study Area
This study was conducted at 26 localities in the Department of Caldas, Colombia (6°5′24″ N, 75°37′8″ W). The 26 sampling localities were distributed across an elevational range between 179 and 2811 m (Figure 1, Table S1). Mean annual precipitation ranges from 2115 to 2800 mm, and the annual temperature varies between 14 and 23 °C. (Weather data obtained from http://www.ideam.gov.co (accessed on 1 September 2019)). The study landscapes are dominated by coffee (Coffea arabica L.), cacao (Theobroma cacao L.), avocado (Persea americana Mill.), plantain (Musa spp. L), and citrus (Citrus × limon L. and Citrus nobilis Lour) crops, along with forest plantations (Alnus acuminata Kunth, Pinus patula Schltdl. and Cham, and Fraxinus chinensis Roxb.), grasslands for cattle grazing, urban zones, and secondary forests. The territory is dominated by several smallholdings, of which, ~40% of the lands intended for agriculture have an area < 50 ha [28]. Several products are grown simultaneously on these smallholdings, including coffee, plantains, timber trees, citrus trees, and other fruit trees. Detailed information on the vegetation cover and climate conditions of the landscapes analyzed here is provided in Castaño-Villa et al. [29,30] and Martínez-Sánchez, et al. [31].
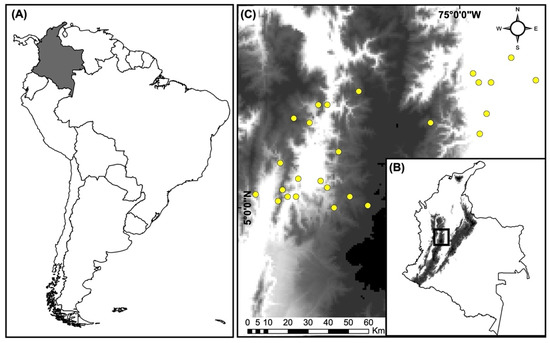
Figure 1.
Location of the 26 landscapes included in the analyses: (A) location of Colombia in South America; (B) location of the study area in Colombia; and (C) location of the 26 landscapes in the Colombian Andes.
2.2. Information Compilation on Landscapes and Birds
We used the information published by Cardona-Salazar et al. [32] in a recent data paper. We also used unpublished data collected by the GIET and GEBIOME research groups during the periods of 2005–2007, 2011, 2015, and 2021–2022 (Projects: SanFranciscoBird, PalynologicalBird, TickBirds1, and HemoparasitesBirds) and by ISAGEN from 2014 to 2021 (Project: Monitoreo de la fauna vertebrada silvestre en zonas de influencia de los centros productivos de ISAGEN en el oriente de Caldas). From those databases, we extracted morphological information (wing length in mm and body mass in g) for 1235 individual birds, recorded in 26 localities in the West and Central Andes and in the inter-Andean valleys of the Cauca and Magdalena Rivers from Colombia (Table S2). All individual birds selected for analysis were captured at the understory level using mist nets installed up to 3 m above ground level. As many bird species use a large portion of the vertical profile, considering only those captures made at the understory level provides a common criterion to pool different species, with different habits, for data analysis purposes.
Those 1235 records correspond to individuals captured in the field between 2005 and 2022. Our sampling localities (denoted by Ln hereafter) were separated at least 20 km from each other (except L39 and L40, which were separated by 3 km; see Table S1 and Figure 1). We have chosen these 26 localities as they represent different landscape conditions (within a disturbance gradient), representing the current situation in the Andes (i.e., a heterogeneous habitat mosaic with native forest remnants immersed in a productive matrix with different land uses). Individual birds included in our analyses corresponded to four resident species and five neotropical migrant species (i.e., birds that breed in North America and winter in the neotropics, to which we refer as ‘migratory birds’ hereafter). The resident species included were the Golden-Faced Tyrannulet (Zimmerius chrysops, Tyrannidae, n = 59), the Ochre-bellied Flycatcher (Mionectes oleagineus, Tyrannidae, n = 637), and the Common Tody-Flycatcher (Todirostrum cinereum, Tyrannidae, n = 23), and the Thick-Billed Euphonia (Euphonia laniirostris, Fringillidae, n = 92). Those four resident species are naturally distributed in Colombia at elevations ranging from 0 to 2200 m. Those species were also characterized as actively foraging at forest edges and across the landscape, using scattered trees and some crops, feeding on insects and fruits [33].
The migratory species included were the Gray-Cheeked Thrush (Catharus minimus, Turdidae, n = 54) the Swainson’s Thrush (Catharus ustulatus, Turdidae, n = 292), the Northern Waterthrush (Parkesia noveboracensis, Parulidae, n = 41), the Connecticut Warbler (Oporornis agilis, Parulidae, n = 12), and the Canada Warbler (Cardellina canadensis, Parulidae, n = 25). These five species migrate to Colombia during the fall (by mid-September) and return to North America in March during the spring [34]. These species have been recorded in Colombia at 0 to 3000 m of elevation [35]. Bird taxonomy followed Remsen, et al. [36].
We assessed landscape configuration using Google Earth Pro 7.0 satellite imagery. For each locality, we plotted a circumference using a 1-km radius (threshold value determined according to the daily movement ranges of passerines [37,38]) over the images. Then, we identified three major land-use types in the landscape (i.e., forest, crops, and grasslands) within the 1-km radius buffer. We used ArcGIS v10.6 (ESRI, Redlands, CA, USA) to conduct the landscape characterization.
2.3. Data Analysis
Body condition in each individual was estimated using the Scaled Mass Index (SMI) proposed by Peig and Green [39,40]. This index scales the mass of each individual to the mass it should have for a given body length. This process can eliminate the variation in body mass that would be dependent on sex, age, and species. This standardization allows for intra- and interspecies comparisons in the body condition among individuals. SMI has been used in previous studies on temperate and tropical birds [18,41].
To characterize our 26 study landscapes, we used 4 configuration metrics (following the FRAGSTATS standards): the connectivity index, Euclidean nearest-neighbor distance, largest patch index, and patch area according to Forman and Godron [42], Turner [43], McGarigal, et al. [44], and Gergel, et al. [45] (Table 1). We used a cell size of 15 m × 15 m to calculate those landscape metrics, using FRAGSTATS software v4.2 [44].

Table 1.
Description of the different landscape indices used to characterize the study area. FRAGSTATS acronyms are provided in parentheses. MN = average area used.
We included landscape metrics as dependent variables; we also included capture day (CAPTURE DAY: the dates were converted to Julian days, and then to radians; 1 January was Day 1 of sampling for each year) as a covariate, and we used body condition (SMI) as the response variable. We included bird species as a random effect to account for interspecies variability. We have z-transformed (centered and standardized) the fixed effects to make the regression coefficients across models comparable [46].
We evaluated the effects of the landscape metric estimated with FRAGSTATS in bird body condition using Bayesian linear mixed models (BLMM) with a Gaussian error distribution. We fitted separate models for (a) all birds, (b) migratory birds only, and (c) resident birds only. First, we fitted models, including all fixed effects, and estimated each variable’s variance inflation factor (VIF). To avoid multicollinearity, we excluded those variables with VIF values > 2 and ran the models again [47]. (As a result of this, we excluded forest and grassland cover from the final model.) All analyses were performed on R version 3.6.1 [48] using the ‘usdm’ package [49] and the Bayesian methods implemented in the ‘MCMCglmm’ package [50]. We used the default priors in ‘MCMCglmm’ for fixed and random effects. To obtain a minimum of 1000 posterior distributions, we ran 13,000 iterations for each model, with a burn-in period of 1000 iterations and a thinning interval of 3000 iterations. We considered an instance as significant if the confidence interval did not overlap with zero. Additionally, we developed the summary plot of the Markov Chain for each model for the intercept of the predictors for the Bayesian linear mixed model. (See details in the Supplementary Material Figures S1–S3).
3. Results
3.1. Bird Occurrence across Landscapes
We recorded migrant bird species in 92% of the landscapes assessed at elevations between 179 and 2811 m. For instance, C. ustulatus was recorded in 80.7% of the 26 landscapes assessed, while we found O. agilis and C. canadensis in only seven and eight landscapes, respectively. All migrant birds were captured between the end of September and April (Figure S4). Conversely, resident birds were captured throughout the year and occurred at 88.3% of the landscapes, at elevations between 179 and 2811 m (Figure S5).
3.2. Landscape Characterization
Overall, the landscapes studied showed different anthropogenic disturbance levels as a result of land-use changes to establish coffee crops and grasslands for cattle grazing (Table 2). The average forest cover across the 26 landscapes examined was 28.4% (according to the largest patch index), followed by crop fields with an average cover of 19.6%. Grasslands had an average cover of 16.4%, showing the largest variation range (Table 2) as this land-use type is dominant in livestock farming areas. Regarding landscape connectivity, the mean connectance was 28.0%, according to our connectance index (expressed as the percentage of functional joinings among forest patches over a 200 m threshold distance). Landscape connectivity varied from 9 to 66% across landscapes, reaching the largest values in those landscapes with creeks/woody vegetation. When we examined connectivity using the Euclidean nearest-neighbor distance (EEN) index, we found an average distance of 73.7 m, with a large variation (30 to 344 m). We found an average patch area of 66.8 ha, with a large variation across landscapes (6.5 to 247.1 ha). Detailed results on landscape metrics for each sampling locality are available in Table S3.

Table 2.
Summary of the landscape indices (described in Table 1; acronyms correspond to standard FRAGSTATS metrics) obtained for the 26 landscapes analyzed. We present mean, minimum, and maximum values. MN = average area used.
3.3. Effects of Landscape Metrics on Body Condition
When we examined all birds (i.e., resident and migrant species together), body condition was positively affected by landscape connectivity (CONNECT, pMCMC = 0.004), crop cover in the landscape (LPI_crops, pMCMC = 0.006), and capture day (CAPTURE DAY, pMCMC = 0.032), showing similar effect sizes (Figure 2, Table S4). Then, examining migrant and resident birds separately, we found that migrant bird body condition followed the same trend, being positively affected by landscape connectivity and crop cover (CONNECT, pMCMC = 0.036; LPI_crops, pMCMC = 0.004; Figure 3A, Table S5). On the other hand, resident birds’ body condition was positively affected by landscape connectivity (CONNECT, pMCMC = 0.024) and crop cover (LPI_crops, pMCMC = 0.006), but in this case, we also found a positive effect of forest patch area (PATCH_AREA_MN, pMCMC = 0.036) and capture day (CAPTURE DAY, pMCMC = 0.040) (Figure 3B, Table S6), which had no significant effects on migratory species. Finally, body condition was not significantly affected by the Euclidian distance to the nearest neighbor (ENN_MN) in any case (Tables S3–S5).
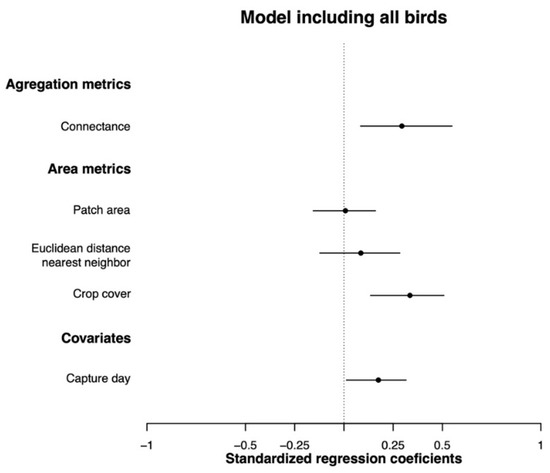
Figure 2.
Standard regression coefficients obtained from the BLMM model, including all bird species. The dot represents the posterior mean, and the lines represent confidence intervals of 95%. We consider a given variable to have a statistically significant effect on body condition when the confidence intervals do not overlap with zero.
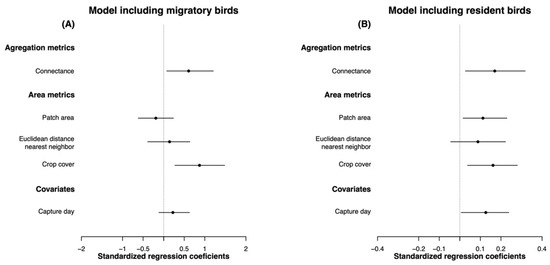
Figure 3.
Standard regression coefficients obtained from the BLMM models for: (A) migrant species and (B) resident species. The dot represents the posterior mean, and the lines represent confidence intervals of 95%. We consider a given variable to have a statistically significant effect on body condition when the confidence intervals do not overlap with zero.
4. Discussion
Landscape plays a major role in many ecological processes, but assessing those effects in the real world is challenging [51]. Our results show that some aspects of landscape structure and configuration influence body condition in migrants and resident birds. We found a large variation in connectivity and land-use patterns across the landscapes assessed in the Colombian Andes. In particular, crop cover and landscape connectivity were positively related to body condition. In this regard, we observed the lowest SMI values in those landscapes with connectivity values under 13%, which suggests that shorter distances between the forest fragments lead to lower energy expenditures of the birds in the landscape, as shown by Camacho, et al. [52] in southwestern Spain. Contrarily, we observed the largest SMI values in those landscapes dominated by crop fields (covering 40–60%) and with connectivity values over 40%. Those landscapes present a heterogeneous mosaic composed of crops (e.g., coffee, plantain, and timber trees), patches, and forest remnants. Therefore, our results are consistent with the eighth landscape-complexity hypothesis proposed by Tscharntke et al. [51], which states that intermediate complexity habitats perform better (for conservation and management purposes) than those with extremely simplified or complex landscapes. In the tropical Andes, the extremely simplified situation is represented by grasslands; the forests represent an extremely complex situation. In contrast, the intermediate situation is represented by those heterogeneous crop fields (mainly coffee and cacao) intertwined with forest remnants. It is important to notice that the bird species included in this study belong to different taxonomic groups and, more importantly, present different habits. Thus, one source of variation that may have confounding effects on our analyses is whether species are ground-dwellers (e.g., Catharus spp.) or prefer moving higher in vegetation (e.g., Cardellina canadensis). Thus, field studies at the species level are needed to assess whether there are idiosyncratic responses to landscape configuration.
4.1. The Role of Landscape Connectivity
Landscape connectivity was one of the relevant metrics influencing body condition in our study area. We used two connectivity proxies: the number of functional joinings (CONNECT) and the Euclidean distance to the nearest neighbor (ENN), but only the first affected body condition. This result suggests that the number of functional connections is more relevant than the distance to the nearest patch in high-mobility species. Reduced connectivity across forest fragments at the landscape scale may increase the movement distances that birds must travel when searching for food, increasing the energy costs of those movements [53]. This is more relevant for those bird species that avoid crossing open areas [54]. Also, landscape connectivity would be more important to migrant species. This outcome is consistent with a study conducted in Australia that found little evidence of landscape effects on the body condition and physiological parameters of 13 forest-dependent resident birds [55], which probably respond to patch-level variations instead.
Bird movement among scattered forest fragments can increase foraging energy expenditure and, consequently, will have a negative effect on body condition [16,17]. Particularly, some small frugivorous bird species analyzed here (i.e., C. ustulatus, C. minimus, M. oleagineus, and Z. chrysops) might be conditioned to engaging in constant movement among forest fragments to find spatially-clumped food resources (i.e., fruits in plants scattered along the landscape) [56,57]. Patch size can also influence movement behavior (although it had a non-significant effect on migratory species), as large patches can sustain larger populations and provide more resources [53]. Therefore, birds may be avoiding small patches (with limited resource availability) and increasing energy costs by moving longer distances when searching for larger patches. We will need telemetry-based data to formally test this hypothesis in our study area.
4.2. The Role of Landscape Heterogeneity and Forest Patch Area
Contrary to our initial expectations, crop cover (LPI_crops) significantly affected bird body condition. Therefore, the fact that crop cover positively affected body condition may be associated with complementary food resources provided by some agroecosystems (e.g., citrus crops) or agroforestry systems (e.g., coffee and cacao planted within shade trees), increasing resource diversity and abundance for many bird species. Some species can positively respond to habitat fragmentation and landscape heterogeneity depending on their life-history traits. High-mobility species can exploit complementary resources across the landscape [58]. Such positive effects of landscape heterogeneity and the increase in resource availability to sustain animal populations in degraded and productive lands have been reported for other systems as well [59,60,61,62,63].
While native forest remnants are important breeding sites, there is an important effect on the surrounding matrix [64]. Birds in forest remnants surrounded by crop fields (providing complementary resources) showed better reproductive outputs than those inhabiting forest remnants surrounded by other land uses, providing no complementary resources [64]. In the Andean region, migratory and resident insectivorous birds also inhabit different agroforestry systems (e.g., shaded coffee crops) to prey upon many arthropods [31,65,66]. Such abundant food resources in productive lands may favor a better body condition in some migratory insectivorous bird species (e.g., P. noveboracensis, O. agilis, and C. canadensis), which are common during winter in crop fields in this region [33]. Coffee and cacao crops in the Central Andes are often grown within shade vegetation, harboring relatively diverse systems with an intermediate complexity. Such agroecosystems have fewer negative effects than do others, such as those of the oil palm, which are also common in the tropics [67]. Further, the changes in the body condition of the resident birds (mostly with a frugivorous diet, such as E. laniirostris, M. oleaginous, and Z. chrysops) during the year could be associated with the availability of appropriate fruit resources [68]. The body condition of frugivorous resident birds could possibly be affected by the offer of ornithochorous fruits that exhibit a drastic difference in abundance between the rainy and dry seasons in the tropical Andean region [57]. Patch area had a positive effect on body condition only for resident species. This may be explained by an area and edge effects over available resources (i.e., larger forest patches have more resources). While migratory birds depend on these resources for some months out of the year, resident species rely on those resources completely.
5. Conclusions
The effects of forest fragmentation and land-use changes have not only altered bird diversity in the tropics [9,67], but they can also lead to changes in body condition, which can have negative effects on the survival and reproductive success of birds [69,70]. Changes in landscape structure and configuration could indirectly alter resource availability and access to those resources, causing additional energy expenditures that deteriorate body condition. Moreover, crop heterogeneity found in smallholder shade-coffee systems may positively affect body condition. Therefore, landscape management can be optimized, taking the responses of migrant and resident bird species into consideration, prioritizing forest connectivity restoration, and promoting agricultural development based on biodiversity-friendly agroecosystems (e.g., shade coffee and mixed crops).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d14060432/s1: Table S1: Coordinates and elevation of the study sites; Table S2: Bird species included in the analysis; Table S3: Configuration and landscape composition; Table S4: Results from BLMM model for all birds; Table S5: Results from BLMM model for migrant birds; Table S6: Results from BLMM model for resident birds; Figure S1: Summary of Markov Chain of the BLMM model for all birds; Figure S2: Summary of Markov Chain of the BLMM model for migrant birds; Figure S3: Summary of Markov Chain of the BLMM model for resident birds; Figure S4: Number of migrant birds registered on a monthly basis; Figure S5: Number of resident birds registered on a monthly basis.
Author Contributions
Conceptualization, D.A.M.-M., J.C.R.-R., F.E.F., F.A.R.-P. and G.J.C.-V.; methodology, D.A.M.-M., J.C.R.-R., F.E.F., C.E.L. and G.J.C.-V.; formal analysis, D.A.M.-M., J.C.R.-R., F.E.F., C.E.L. and G.J.C.-V.; investigation, D.A.M.-M., J.C.R.-R., F.E.F. and G.J.C.-V.; data curation, D.A.M.-M. and J.C.R.-R.; writing—original draft preparation, D.A.M.-M., J.C.R.-R., F.E.F., C.E.L. and G.J.C.-V.; writing—review and editing, D.A.M.-M., F.E.F., C.E.L. and G.J.C.-V.; visualization, D.A.M.-M. and C.E.L.; supervision, G.J.C.-V.; project administration, G.J.C.-V. and F.A.R.-P.; funding acquisition, G.J.C.-V. and F.A.R.-P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Vicerrectoría de Investigación y Postgrados of the Universidad de Caldas—VIPUCa (grant 0311321).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Original data and R code used are freely available from the figshare digital repository: https://doi.org/10.6084/m9.figshare.13169516.
Acknowledgments
We are grateful to B. Toro (Project: Monitoreo de la fauna vertebrada silvestre en zonas de influencia de los centros productivos de ISAGEN en el oriente de Caldas—Código 0481419), for their collaboration and the database supplied. We are also grateful to three anonymous reviewers for their suggestions to improve the manuscript. FEF acknowledges the support of project ANID/PIA/ACT192027 and the Fundación San Ignacio del Huinay. The publication of this article was supported by the Federal University of Pará (UFPA) (PROPESP-PAPQ 02/2022—Qualified Publication Support Program).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Groombridge, B.; Jenkins, M. World Atlas of Biodiversity: Earth’s Living Resources in the 21st Century; University of California Press: Berkeley, CA, USA, 2002; p. 340. [Google Scholar]
- Murphy, G.E.P.; Romanuk, T.N. Data gaps in anthropogenically driven local-scale species richness change studies across the Earth’s terrestrial biomes. Ecol. Evol. 2016, 6, 2938–2947. [Google Scholar] [CrossRef] [PubMed]
- FAO; UNEP. The State of the World’s Forests 2020: Forests, Biodiversity and People; FAO and UNEP: Rome, Italy, 2020; p. 214. [Google Scholar]
- Lewis, S.L.; Edwards, D.P.; Galbraith, D. Increasing human dominance of tropical forests. Science 2015, 349, 827–832. [Google Scholar] [CrossRef] [PubMed]
- FAO. FAOSTAT Statistics Database; Food and Agriculture Organization of the United Nations: Rome, Italy, 2019. [Google Scholar]
- Dixon, J.; Gibbon, D.P.; Gulliver, A.; Hall, M. Farming Systems and Poverty: Improving Farmers’ Livelihoods in a Changing World; FAO—World Bank: Rome, Italy; Washington, DC, USA, 2001; p. 412. [Google Scholar]
- Taubert, F.; Fischer, R.; Groeneveld, J.; Lehmann, S.; Müller, M.S.; Rödig, E.; Wiegand, T.; Huth, A. Global patterns of tropical forest fragmentation. Nature 2018, 554, 519–522. [Google Scholar] [CrossRef] [PubMed]
- Kattan, G.H.; Alvarez-Lopez, H.; Giraldo, M. Forest Fragmentation and Bird Extinctions: San Antonio Eighty Years Later. Conserv. Biol. 1994, 8, 138–146. [Google Scholar] [CrossRef]
- Laurance, W.F.; Lovejoy, T.E.; Vasconcelos, H.L.; Bruna, E.M.; Didham, R.K.; Stouffer, P.C.; Gascon, C.; Bierregaard, R.O.; Laurance, S.G.; Sampaio, E. Ecosystem Decay of Amazonian Forest Fragments: A 22-Year Investigation. Conserv. Biol. 2002, 16, 605–618. [Google Scholar] [CrossRef] [Green Version]
- Prugh, L.R.; Hodges, K.E.; Sinclair, A.R.E.; Brashares, J.S. Effect of habitat area and isolation on fragmented animal populations. Proc. Natl. Acad. Sci. USA 2008, 105, 20770–20775. [Google Scholar] [CrossRef] [Green Version]
- Fischer, J.; Lindenmayer, D.B. Landscape modification and habitat fragmentation: A synthesis. Glob. Ecol. Biogeogr. 2007, 16, 265–280. [Google Scholar] [CrossRef]
- Haddad, N.M.; Brudvig, L.A.; Clobert, J.; Davies, K.F.; Gonzalez, A.; Holt, R.D.; Lovejoy, T.E.; Sexton, J.O.; Austin, M.P.; Collins, C.D.; et al. Habitat fragmentation and its lasting impact on Earth’s ecosystems. Sci. Adv. 2015, 1, e1500052. [Google Scholar] [CrossRef] [Green Version]
- Henle, K.; Davies, K.F.; Kleyer, M.; Margules, C.; Settele, J. Predictors of Species Sensitivity to Fragmentation. Biodivers. Conserv. 2004, 13, 207–251. [Google Scholar] [CrossRef]
- Rodríguez-Cabal, M.A.; Aizen, M.A.; Novaro, A.J. Habitat fragmentation disrupts a plant-disperser mutualism in the temperate forest of South America. Biol. Conserv. 2007, 139, 195–202. [Google Scholar] [CrossRef]
- Sherry, T.W.; Holmes, R.T. Winter Habitat Quality, Population Limitation, and Conservation of Neotropical-Nearctic Migrant Birds. Ecology 1996, 77, 36–48. [Google Scholar] [CrossRef]
- Hinsley, S.A. The costs of multiple patch use by birds. Landsc. Ecol. 2000, 15, 765–775. [Google Scholar] [CrossRef]
- Keinath, D.A.; Doak, D.F.; Hodges, K.E.; Prugh, L.R.; Fagan, W.; Şekercioğlu, H.; Buchart, S.H.M.; Kauffman, M. A global analysis of traits predicting species sensitivity to habitat fragmentation. Glob. Ecol. Biogeogr. 2017, 26, 115–127. [Google Scholar] [CrossRef]
- Deikumah, J.P.; McAlpine, C.; Maron, M. Matrix Intensification Affects Body and Physiological Condition of Tropical Forest-Dependent Passerines. PLoS ONE 2015, 10, e0128521. [Google Scholar] [CrossRef]
- Pavlacky, D.C.; Possingham, H.P.; Goldizen, A.W. Integrating life history traits and forest structure to evaluate the vulnerability of rainforest birds along gradients of deforestation and fragmentation in eastern Australia. Biol. Conserv. 2015, 188, 89–99. [Google Scholar] [CrossRef]
- Norris, D.R.; Marra, P.; Kyser, T.K.; Sherry, T.W.; Ratcliffe, L.M. Tropical winter habitat limits reproductive success on the temperate breeding grounds in a migratory bird. Proc. R. Soc. B Biol. Sci. 2004, 271, 59–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bearhop, S.; Hilton, G.M.; Votier, S.C.; Waldron, S. Stable isotope ratios indicate that body condition in migrating passerines is influenced by winter habitat. Proc. R. Soc. B Biol. Sci. 2004, 271, S215–S218. [Google Scholar] [CrossRef] [Green Version]
- Kattan, G.H.; Franco, P. Bird diversity along elevational gradients in the Andes of Colombia: Area and mass effects. Glob. Ecol. Biogeogr. 2004, 13, 451–458. [Google Scholar] [CrossRef]
- Fierro, K. Aves migratorias en Colombia. In Plan Nacional de Las Especies Migratorias Diagnóstico e Identificación de Acciones Para la Conservación y el Manejo Sostenible de Las Especies Migratorias de La Biodiversidad en Colombia; Naranjo, L.G., Amaya, J.D., Eds.; Ministro de Ambiente, Vivienda y Desarrollo Territorial: Bogotá, Colombia, 2009; pp. 63–75. [Google Scholar]
- Avendaño, J.E.; Bohórquez, C.I.; Rosselli, L.; Arzuza-Buelvas, D.; Cuervo, A.M.; Stiles, F.G.; Renjifo, L.M. Lista de chequeo de las aves de Colombia: Una síntesis del estado del conocimiento desde Hilty & Brown (1986). Ornitol. Colomb. 2017, 16, eA01. [Google Scholar]
- Armenteras, D.; Rodríguez, N.; Retana, J.; Morales, M. Understanding deforestation in montane and lowland forests of the Colombian Andes. Reg. Environ. Chang. 2011, 11, 693–705. [Google Scholar] [CrossRef]
- Renjifo, L.M. Composition Changes in a Subandean Avifauna after Long-Term Forest Fragmentation. Conserv. Biol. 1999, 13, 1124–1139. [Google Scholar] [CrossRef]
- Castaño-Villa, G.J.; Patiño-Zabala, Y.J.C. Extinciones locales de aves en fragmentos de bosque en la región de Santa Elena, Andes Centrales. Colombia. Hornero 2008, 23, 23–34. [Google Scholar]
- Velásquez-Chaverra, E.d.J.; Arias, C.H. Anuario Estadístico de Frutas y Hortalizas 2004–2008 y Sus Calendarios de Siembras y Cosechas; Ministerio de Agricultura y Desarrollo Rural: Bogotá, Colombia, 2009.
- Castaño-Villa, G.J.; Estevez, J.V.; Fontúrbel, F.E. The role of native forest plantations in the conservation of Neotropical birds: The case of the Andean alder. J. Nat. Conserv. 2014, 22, 547–551. [Google Scholar] [CrossRef]
- Castaño-Villa, G.J.; Ramos-Valencia, S.A.; Fontúrbel, F.E. Fine-scale habitat structure complexity determines insectivorous bird diversity in a tropical forest. Acta Oecologica 2014, 61, 19–23. [Google Scholar] [CrossRef]
- Martínez-Sánchez, E.T.; Romero, M.C.; Páez, F.A.R.; Cárdenas, J.E.P.; Castaño-Villa, G.J. Contribution of agroecosystems to the conservation of bird diversity in the department of Caldas. Rev. Fac. Nac. Agron. 2018, 71, 8445–8457. [Google Scholar] [CrossRef] [Green Version]
- Cardona-Salazar, L.J.; Benavides-Ossa, Y.A.; Vargas-Daza, M.; Betancurt-Grisales, J.F.; Bohada-Murillo, M.; Martínez-Sánchez, E.T.; Cardona-Romero, M.; Busi, A.; Tobón-Escobar, W.D.; Ortíz-Giraldo, M.; et al. A morphological, reproductive, and molt phenology database for 379 bird species from the Colombian Tropical Andes. Ecology 2020, 101, e03016. [Google Scholar] [CrossRef] [Green Version]
- Hilty, S.L.; Brown, W.L. A Guide to the Birds of Colombia; Princeton University Press: Princeton, NJ, USA, 1986; p. 836. [Google Scholar]
- Marra, P.P.; Francis, C.M.; Mulvihill, R.S.; Moore, F.R. The influence of climate on the timing and rate of spring bird migration. Oecologia 2005, 142, 307–315. [Google Scholar] [CrossRef]
- Ayerbe-Quiñones, F. Guía Ilustrada de La Avifauna Colombiana, 1st ed.; Wildlife Conservation Society: Bogotá, Colombia, 2018. [Google Scholar]
- Remsen, J.V.; Areta, J.I.; Bonaccorso, S.; Claramunt, S.; Jaramillo, A.; Pacheco, J.F.; Ribas, C.; Robbins, M.B.; Stiles, F.G.; Stotz, D.F.; et al. A classification of the bird species of South America. Am. Ornithol. Soc. 2019. Available online: http://www.museum.lsu.edu/~Remsen/SACCBaseline.html (accessed on 5 September 2019).
- Aborn, D.A.; Moore, F.R. Pattern of Movement by Summer Tanagers (Piranga Rubra) During Migratory Stopover: A Telemetry Study. Behaviour 1997, 134, 1077–1100. [Google Scholar] [CrossRef]
- Boscolo, D.; Metzger, J.P. Is bird incidence in Atlantic forest fragments influenced by landscape patterns at multiple scales? Landsc. Ecol. 2009, 24, 907–918. [Google Scholar] [CrossRef]
- Peig, J.; Green, A.J. New perspectives for estimating body condition from mass/length data: The scaled mass index as an alternative method. Oikos 2009, 118, 1883–1891. [Google Scholar] [CrossRef]
- Peig, J.; Green, A.J. The paradigm of body condition: A critical reappraisal of current methods based on mass and length. Funct. Ecol. 2010, 24, 1323–1332. [Google Scholar] [CrossRef]
- Bell, S.C.; Owens, I.P.; Lord, A.M. Quality of breeding territory mediates the influence of paternal quality on sex ratio bias in a free-living bird population. Behav. Ecol. 2014, 25, 352–358. [Google Scholar] [CrossRef] [Green Version]
- Forman, R.T.T.; Godron, M. Patches and Structural Components for a Landscape Ecology. BioScience 1981, 31, 733–740. [Google Scholar] [CrossRef]
- Turner, M.G. Landscape Ecology: The Effect of Pattern on Process. Annu. Rev. Ecol. Syst. 1989, 20, 171–197. [Google Scholar] [CrossRef]
- McGarigal, K.; Cushman, S.; Ene, E. FRAGSTATS 4.2: Spatial Pattern Analysis Program for Categorical Maps; University of Massachusetts: Amherst, MA, USA, 2012. [Google Scholar]
- Gergel, S.E.; Turner, M.G.; Miller, J.R.; Melack, J.M.; Stanley, E.H. Landscape indicators of human impacts to riverine systems. Aquat. Sci. 2002, 64, 118–128. [Google Scholar] [CrossRef]
- Schielzeth, H. Simple means to improve the interpretability of regression coefficients. Methods Ecol. Evol. 2010, 1, 103–113. [Google Scholar] [CrossRef]
- Zuur, A.F.; Ieno, E.N.; Elphick, C.S. A protocol for data exploration to avoid common statistical problems. Methods Ecol. Evol. 2010, 1, 3–14. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing, Reference Index Version 3.6.1; Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Naimi, B. Usdm: Uncertainty Analysis for Species Distribution Models, R package version 1.1–15; R Core Team: Vienna, Austria, 2010. [Google Scholar]
- Hadfield, J.D. MCMC Methods for Multi-Response Generalized Linear Mixed Models: TheMCMCglmmRPackage. J. Stat. Softw. 2010, 33, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Tscharntke, T.; Tylianakis, J.; Rand, T.A.; Didham, R.K.; Fahrig, L.; Batary, P.; Bengtsson, J.; Clough, Y.; Crist, T.O.; Dormann, C.; et al. Landscape moderation of biodiversity patterns and processes—Eight hypotheses. Biol. Rev. 2012, 87, 661–685. [Google Scholar] [CrossRef]
- Camacho, C.; Palacios, S.; Sáez, P.; Sánchez, S.; Potti, J. Human-Induced Changes in Landscape Configuration Influence Individual Movement Routines: Lessons from a Versatile, Highly Mobile Species. PLoS ONE 2014, 9, e104974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matthews, S.N.; Rodewald, P.G. Movement behaviour of a forest songbird in an urbanized landscape: The relative importance of patch-level effects and body condition during migratory stopover. Landsc. Ecol. 2010, 25, 955–965. [Google Scholar] [CrossRef]
- Richard, Y.; Armstrong, D.P. Cost distance modelling of landscape connectivity and gap-crossing ability using radio-tracking data. J. Appl. Ecol. 2010, 47, 603–610. [Google Scholar] [CrossRef]
- Amos, J.N.; Balasubramaniam, S.; Grootendorst, L.; Harrisson, K.A.; Lill, A.; Mac Nally, R.; Pavlova, A.; Radford, J.Q.; Takeuchi, N.; Thomson, J.R.; et al. Little evidence that condition, stress indicators, sex ratio, or homozygosity are related to landscape or habitat attributes in declining woodland birds. J. Avian Biol. 2013, 44, 45–54. [Google Scholar] [CrossRef]
- Loiselle, B.; Blake, J.G. Temporal Variation in Birds and Fruits Along an Elevational Gradient in Costa Rica. Ecology 1991, 72, 180–193. [Google Scholar] [CrossRef]
- Morales-Betancourt, J.A.; Castaño-Villa, G.J.; Fontúrbel, F.E. Resource abundance and frugivory in two manakin species (Aves: Pipridae) inhabiting a reforested area in Colombia. J. Trop. Ecol. 2012, 28, 511–514. [Google Scholar] [CrossRef]
- Ewers, R.M.; Didham, R.K. Confounding factors in the detection of species responses to habitat fragmentation. Biol. Rev. 2006, 81, 117–142. [Google Scholar] [CrossRef]
- Hewison, A.M.; Morellet, N.; Verheyden, H.; Daufresne, T.; Angibault, J.-M.; Cargnelutti, B.; Merlet, J.; Picot, D.; Rames, J.-L.; Joachim, J.; et al. Landscape fragmentation influences winter body mass of roe deer. Ecography 2009, 32, 1062–1070. [Google Scholar] [CrossRef]
- Öberg, S. Influence of landscape structure and farming practice on body condition and fecundity of wolf spiders. Basic Appl. Ecol. 2009, 10, 614–621. [Google Scholar] [CrossRef]
- Salazar, D.A.; Fontúrbel, F.E. Beyond habitat structure: Landscape heterogeneity explains the monito del monte (Dromiciops gliroides) occurrence and behavior at habitats dominated by exotic trees. Integr. Zool. 2016, 11, 413–421. [Google Scholar] [CrossRef]
- Fontúrbel, F.E.; Salazar, D.A.; Medel, R. Increased resource availability prevents the disruption of key ecological interactions in disturbed habitats. Ecosphere 2017, 8, e01768. [Google Scholar] [CrossRef]
- Hansen, N.A.; Scheele, B.C.; Driscoll, D.A.; Lindenmayer, D.B. Amphibians in agricultural landscapes: The habitat value of crop areas, linear plantings and remnant woodland patches. Anim. Conserv. 2019, 22, 72–82. [Google Scholar] [CrossRef]
- Okada, S.; Lindenmayer, D.B.; Wood, J.T.; Crane, M.J.; Pierson, J. How does a transforming landscape influence bird breeding success? Landsc. Ecol. 2017, 32, 1039–1048. [Google Scholar] [CrossRef]
- Greenberg, R.; Bichier, P.; Angon, A.C.; Reitsma, R. Bird Populations in Shade and Sun Coffee Plantations in Central Guatemala. Conserv. Biol. 1997, 11, 448–459. [Google Scholar] [CrossRef]
- Boesing, A.L.; Nichols, E.; Metzger, J.P. Effects of landscape structure on avian-mediated insect pest control services: A review. Landsc. Ecol. 2017, 32, 931–944. [Google Scholar] [CrossRef]
- Murillo, M.B.; Castaño-Villa, G.J.; Fontúrbel, F.E. The effects of forestry and agroforestry plantations on bird diversity: A global synthesis. Land Degrad. Dev. 2019, 31, 646–654. [Google Scholar] [CrossRef]
- Campo-Celada, M.; Jordano, P.; Benítez-López, A.; Gutiérrez-Expósito, C.; Rabadán-González, J.; Mendoza, I. Assessing short and long-term variations in diversity, timing and body condition of frugivorous birds. Oikos 2022, 2022, e08387. [Google Scholar] [CrossRef]
- Marra, P.P.; Holmes, R.T. Consequences of Dominance-Mediated Habitat Segregation in American Redstarts During the Nonbreeding Season. Ornithology 2001, 118, 92–104. [Google Scholar] [CrossRef]
- Johnson, M.D.; Sherry, T.W.; Holmes, R.T.; Marra, P.P. Assessing Habitat Quality for a Migratory Songbird Wintering in Natural and Agricultural Habitats. Conserv. Biol. 2006, 20, 1433–1444. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).