Abstract
The Roseobacter clade represents one of the most abundant groups of marine bacteria and plays important biogeochemical roles in marine environments. Roseobacter genomes commonly contain a conserved gene transfer agent (GTA) gene cluster. A major capsid protein-encoding GTA (g5) has been used as a genetic marker to estimate the diversity of marine roseobacters. Here, the diversity of roseobacters in the coastal seawater of Arctic Kongsfjorden and Antarctic Maxwell Bay was investigated based on g5 gene clone library analysis. Four g5 gene clone libraries were constructed from microbial assemblages representing Arctic and Antarctic regions. The genus Phaeobacter was exclusively detected in Arctic seawater, whereas the genera Jannaschia, Litoreibacter and Pacificibacter were only observed in Antarctic seawater. More diverse genera within the Roseobacter clade were observed in Antarctic clones than in Arctic clones. The genera Sulfitobacter, Loktanella and Yoonia were dominant (higher than 10% of total clones) in both Arctic and Antarctic samples, implying their roles in polar marine environments. The results not only indicated a bipolar or even global distribution of roseobacters in marine environments but also showed their endemic distribution either in the Arctic or Antarctic. Endemic phylotypes were more frequently observed in polar regions than cosmopolitan phylotypes. In addition, endemic phylotypes were more abundant in Arctic samples (84.8% of Arctic sequences) than in Antarctic samples (54.3% of Antarctic sequences).
1. Introduction
The marine Roseobacter clade is the largest lineage within the family Rhodobacteraceae and is metabolically, phenotypically, and ecologically diverse [1,2,3]. Members of the roseobacters can comprise up to 25% of total marine bacterioplankton [1], making it one of the most abundant groups of marine bacteria [4]. In addition, the Roseobacter clade contains isolates capable of dimethylsulfoniopropionate (DMSP) degradation [5], carbon monoxide oxidation [6], anoxygenic photosynthesis [7], and quorum sensing [8], which play crucial ecological and environmental roles [9,10]. Moreover, some roseobacters have been found to be dominant in polar marine environments [11,12,13].
Gene transfer agents (GTAs) are DNA-containing phage-like particles encoded and produced by certain bacteria and archaea [14]. They can package fragments of the host genome and transfer the encapsidated genetic material to the recipient [15]. GTA-related gene transfer is regarded as a potential adaptive mechanism for microbes to maintain metabolic flexibility in changing environments. GTAs are common in genomes of the Roseobacter clade [5,16]. Among the GTA genes, a major capsid gene (g5) is highly conserved among those bacteria and has been widely used as a gene marker to estimate the diversity of marine roseobacters [17,18]. However, information on the diversity of GTA g5 genes in marine roseobacters at high latitudes is still limited.
Previous studies have revealed both bipolar and endemic distributions of marine Roseobacter clade members and their functional genes (e.g., pufM, dmdA and dddP) in polar regions [19,20]. It is hypothesized that a similar phenomenon can be found in the distribution of the g5 gene in roseobacters in polar marine environments. In this study, the GTA g5 gene was directly obtained from the marine environment to explore the diversity of roseobacters and the difference in the phylogenetic diversity of Roseobacter clade bacteria between Arctic and Antarctic coastal seawaters. The results will help improve our understanding of the distribution of Roseobacter clade bacteria and their adaptation to unique polar environments.
2. Materials and Methods
2.1. Sample Collection and DNA Extraction
Two Arctic surface seawater samples were collected from an outer station, St1, and inner station, St5, in Kongsfjorden in July 2011, whereas two Antarctic surface seawater samples were collected from Station A5 in Ardley Cove and Station G5 in Great Wall Cove of Maxwell Bay in December 2011 (Figure 1). The locations and sampling dates are summarized in Table 1 [12,21]. Microorganisms were collected by filtering water through a 0.2 μm-pore-size filter. DNA extraction was performed as previously described [22,23]. The purity and concentration of the extracted DNA were estimated using a Nanodrop 2000 spectrophotometer (Thermo Fischer Scientific, Hvidovre, Denmark).
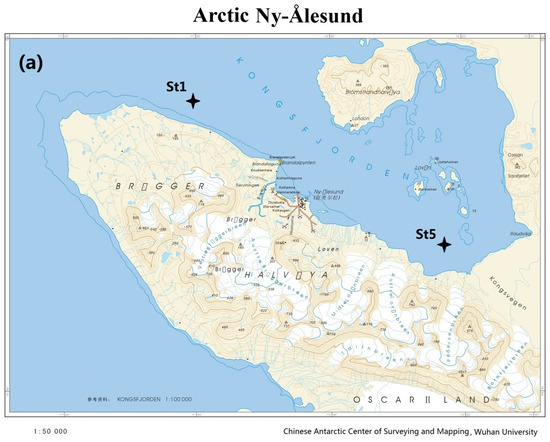
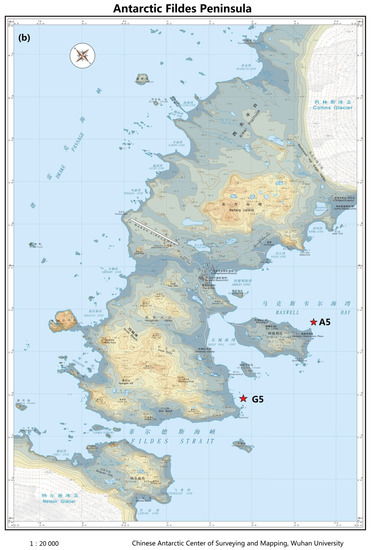
Figure 1.
Maps of sampling sites in Arctic Kongsfjorden (a) and Antarctic Maxwell Bay (b) in 2011.

Table 1.
Estimation of sequence diversity and genotype coverage of gene transfer agent g5 clone libraries.
2.2. Clone Library Construction and Sequencing of the GTA g5 Gene
Four g5 gene clone libraries were constructed using nucleic acids obtained from surface water samples. Degenerate PCR primers MCP-109F (5′-GGC TAY CTG GTS GAT CCS CAR AC-3′) and MCP-368R (5′-TAG AAC AGS ACR TGS GGY TTK GC-3′) were used to amplify GTA g5 genes in the present study [18]. Amplification was conducted using a total volume of 25 μL containing 200 ng of template DNA, 12.5 μL of DreamTaq Green PCR Master Mix (2×; Thermo Scientific, Waltham, USA), 2 μg of bovine serum albumin (BSA), and 0.4 μmol/L of each primer. The thermocycling conditions were listed as follows: 30 s at 98 °C; 35 cycles at 98 °C for 10 s, 60 °C for 30 s, and 72 °C for 30 s; and a final extension step at 72 °C for 7 min. The success of PCR was determined through electrophoresis of 4 μL of the reaction mixture in 0.8% (w/v) agarose gels. Genomic DNA from one reference strain Ruegeria pomeroyi DSS-3, which possesses a g5 gene (CP000031), was used as a template for the positive control. Both distilled water and genomic DNA from Escherichia coli DH5α were used as a template for negative controls. The g5 gene PCR products were ligated to the vector pMD18-T (Takara, Dalian, China) and used to transform E. coli DH5α competent host cells. All clones were screened for inserts through colony PCR with the M13 primer sequences flanking the pMD18-T cloning site as described by Zeng et al. [24]. After verification through gel electrophoresis, all positive clones were sent to Majorbio Biopharm Technology Co., Ltd. (Shanghai, China) for Sanger sequencing.
2.3. Data Analysis
The obtained g5 gene sequences were checked for chimeras using the Bellerophon program [25]. A 98% identity in the putative g5 sequence was employed to group sequences into the same operational taxonomic unit (OTU) or genotype in this study [18]. From each OTU, a single sequence was selected as a representative. Coverage, Shannon’s diversity and Simpson’s diversity indices of the clone library were estimated using the SpadeR (https://chao.shinyapps.io/SpadeR; accessed on 16 January 2022). The DNA sequences were translated into amino acid sequences and then aligned and compared with reference sequences from bacterial taxa in the GenBank database. Amino acid sequences were grouped at the 90% similarity level [18]. Neighbor-joining phylogenetic trees were constructed using the MEGA 5.1 software. The evolutionary distances were calculated under the Jones–Taylor–Thornton model. Neighbor-joining bootstrap tests of phylogeny were performed using 1000 replicates.
2.4. Nucleotide Sequence Accession Numbers
The g5 nucleotide sequences of representative from each OTU determined in the present study have been deposited in GenBank under accession numbers KC906230 to KC906242, KC951540 to KC951570, KF018557 to KF018562, KF537694 to KF537765, and KF686584 to KF686735.
3. Results and Discussion
3.1. Statistical Analysis of the g5 Gene Library
A total of 715 clones were sequenced, including 404 and 311 clones from Arctic and Antarctic samples, respectively. The coverage of each clone library ranged from 68% to 90% (Table 1). Arctic samples showed higher coverage than Antarctic samples. However, Antarctic samples exhibited higher g5 gene diversity than Arctic samples: the cloned sequences fell into 274 OTUs, including 122 and 152 OTUs from Arctic and Antarctic regions, respectively. In addition, both Shannon–Wiener and Simpson indices suggested that the Antarctic samples had more diverse g5 gene genotypes than the Arctic samples (Table 1). The results implied that more diverse roseobacters existed in Antarctic coastal waters than in Arctic coastal waters, consistent with the findings of previous studies [19,20].
3.2. Diversity and Distribution of Arctic g5 Genes
All g5 gene sequences were subsequently translated into amino acid sequences. In congruence with the diversity of DNA sequences, Arctic g5 gene product amino acid sequences fell into eight phylogenetic groups, including the genera Ascidiaceihabitans, Loktanella, Octadecabacter, Phaeobacter, Sulfitobacter, Tateyamaria and Yoonia (Figure 2). Sulfitobacter was the most abundant genus (49.0%) in the Arctic g5 clones, followed by Loktanella (29.5%) and Yoonia (11.1%). Phylogenetic analysis of the bacterial g5 gene clones (Figure 2) showed that there were seven cloned sequences in total (1.7% of Arctic clones) exhibiting a close relationship (>90% amino acid identity) to both Loktanella sp. 1ANDIMAR09 (KQB98268) and Yoonia rosea (WP076658629); thus, the seven clones were placed in the Loktanella/Yoonia phylogenetic group. In fact, Loktanella sp. 1ANDIMAR09 exhibited identical g5 gene product amino acid sequence to Yoonia rosea (Figure 2). A similar phenomenon was observed in another 26 clones (6.4%), which showed a close relationship (>83% amino acid identity) to Ascidiaceihabitans donghaensis (WP108828037) and Tateyamaria omphalii (WP 076630136). Both Ascidiaceihabitans donghaensis and Tateyamaria omphalii were first reported from marine organisms in eastern Asia [26,27].
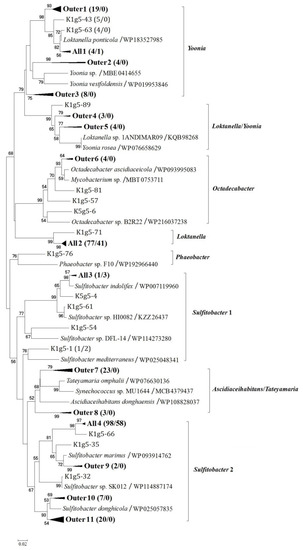
Figure 2.
Neighbor-joining phylogenetic tree based on partial g5 gene product amino acid sequences (ca. 260 aa) showing the phylogenetic diversity of g5 from Arctic Kongsfjorden bacterial communities. Groups from Outer1 to Outer11 represent closely related clones exclusively found at the outer station St1, whereas groups from All1 to All4 represent closely related clones shared by the outer station St1 and inner station St5. The numbers in parentheses following clone names or group names indicate the number of sequences found at stations St1 and St5, respectively. Bootstrap numbers are shown as percentages based on 1000 replicates, and values of less than 50 were omitted. The scale bar indicates evolutionary distance.
Although showing lower coverage for the clone library, the outer station sample St1 exhibited higher g5 gene diversity than the inner station sample St5 (Table 1). Members within the phylogenetic groups Ascidiaceihabitans/Tateyamaria and Phaeobacter were exclusively detected at outer station St1. In addition, represented by groups from Outer1 to Outer11 (Figure 2), genotypes exclusively found at outer station St1 comprised 28.7% of the total Arctic clones. In contrast, genotypes exclusively detected at inner station St5 accounted for only 0.5% of the total clones. Represented by groups from All1 to All4, genotypes shared by the outer and inner stations comprised most of the Arctic clones. Kongsfjorden is a typical glacier fjord in the European Arctic and is influenced by freshwater inputs mainly from glacier meltwater in summer. Consistent with previous studies [13,28], the results of this study indicate an influence of freshwater input on the diversity and distribution of marine roseobacters in Kongsfjorden during Arctic summers.
Genotype K1g5-64 (KF537731) within the genus Sulfitobacter accounted for 32.3% and 51.1% at outer station St1 and inner station St5, respectively. Gene product of this genotype showed 100% amino acid identity to strain Sulfitobacter sp. BSw21498 (WP138923583), which was isolated from Kongsfjorden and possessed the dimethylsulfoniopropionate (DMSP) lyase gene dddL [29]. Members of the genus Sulfitobacter can exhibit algicidal effects against microalgae [30] and degrade algae-derived DMSP [31]. This result is not only consistent with those of previous studies [20,32], indicating a role of Sulfitobacter species in sulfur metabolism, but also, implies that GTA-related gene transfer can be helpful for the environmental adaptation and ecological function of Sulfitobacter species in the Arctic coastal region.
Genotypes K1g5-34 (KF537711) and K1g5-39 (KF537714) within the genus Loktanella together comprised 19.2% and 30.6% of the St1 and St5 clones, respectively. They show higher than 98% g5 gene product amino acid similarity to strain Loktanella salsilacus (WP090187762). Members of Loktanella can participate in DMSP degradation and aerobic anoxygenic photosynthesis in polar marine environments [19,20]. Except for group All1 (Figure 2) containing genotypes shared by the outer and inner stations, sequences within the Loktanella/Yoonia and Yoonia phylogenetic groups were exclusively detected at outer station St1, together accounting for 15.8% of the St1 clones. The genus Yoonia contains many species (e.g., Loktanella litorea, Loktanella vestfoldensis and Roseobacter sp. CCS2) formerly classified within the genera Loktanella and Roseobacter [33]. These Yoonia species have also been found to possess the aerobic anoxygenic phototrophy gene pufM and DMSP degradation genes [19,20,32]. Most species of Yoonia require NaCl to grow, indicating that Yoonia-related sequences detected in this study originated from marine environments.
3.3. Diversity and Distribution of Antarctic g5 Genes
In congruence with the diversity of DNA sequences, Antarctic g5 gene product amino acid sequences fell into ten phylogenetic groups, including the genera Ascidiaceihabitans, Jannaschia, Litoreibacter, Loktanella, Octadecabacter, Pacificibacter, Sulfitobacter, Tateyamaria and Yoonia (Figure 3). Among them, Sulfitobacter was the most abundant genus (34.0%) in the Antarctic g5 clones. However, different from Arctic samples, Ascidiaceihabitans/Tateyamaria-related sequences were unexpectedly abundant, accounting for 19.2% of the Antarctic clones. The genera Loktanella and Yoonia were also dominant in Antarctic g5 sequences, comprising 15.1% and 12.8% of the Antarctic clones, respectively. In addition, sequences within the Loktanella/Yoonia phylogenetic group comprised 10.6% of the Antarctic clones.
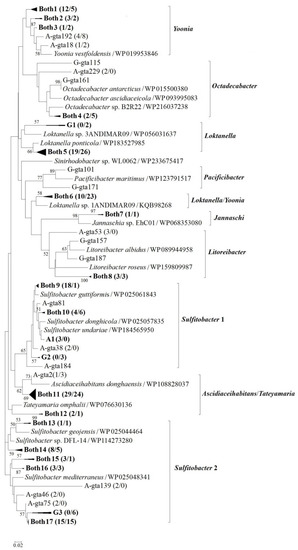
Figure 3.
Neighbor-joining phylogenetic tree based on partial g5 gene product amino acid sequences (ca. 260 aa) showing the phylogenetic diversity of g5 from Antarctic Maxwell Bay bacterial communities. Group A1 represents closely related clones exclusively found in Ardley Cove, whereas groups from G1 to G3 represent closely related clones exclusively found in Great Wall Cove. Groups from Both1 to Both17 are closely related clones shared by the two coves. The numbers in parentheses following clone names or group names indicate the number of sequences found at stations A5 (Ardley Cove) and G5 (Great Wall Cove), respectively. Bootstrap numbers are shown as percentages based on 1000 replicates, and values of less than 50 were omitted. The scale bar indicates evolutionary distance.
Similar values of library coverage and diversity indices were observed between Ardley Cove sample A5 and Great Wall Cove sample G5 (Table 1). Represented by groups from Both1 to Both17 (Figure 3) containing closely related clones shared by the two coves, genotypes found in both Antarctic libraries comprised 89.0% of the total Antarctic clones. Mixing exists between the bacterioplankton communities in the two coves due to seawater exchange when high tide occurs, resulting in genotypes shared by the two adjacent coves can comprise most of the Antarctic clones. Represented by group A1, genotypes exclusively found in Ardley Cove accounted for only 5.5% of the total Antarctic clones. The same value (5.5%) was observed for the genotypes (e.g., groups from G1 to G3) exclusively found in Great Wall Cove.
Represented by genotype A-gta103 (KF686586), group Both11 accounted for 18.4% and 15.8% of the A5 and G5 clones, respectively, and showed higher than 88% g5 gene product amino acid identity to Tateyamaria omphalii (WP076630136) and Ascidiaceihabitans donghaensis (WP108828037). Members of Tateyamaria species show antimicrobial activity and are abundant in healthy marine organisms [34,35]. Moreover, Ascidiaceihabitans species have been connected to the health of fish and shrimp in marine environments [36,37]. However, the reason for the relatively high abundance of Ascidiaceihabitans/Tateyamaria-related sequences detected in Antarctic seawater remains uncertain.
Represented by genotypes A-gta153 (KF686627), group Both17 comprised 9.5% and 9.7% of the A5 and G5 clones, respectively, showing higher than 82% g5 gene product amino acid identity to Sulfitobacter mediterraneus (WP203198950). Cells of the strain type S. mediterraneus can undergo a morphological change during adsorption on polymeric surfaces [38], which may aid Sulfitobacter cells in exhibiting algicidal effects. Represented by genotype A-gta131 (KF686603), group Both5 accounted for 12.1% and 16.8% of the A5 and G5 clones, respectively. Sequences of the clones showed higher than 91% g5 gene product amino acid identity to Loktanella ponticola (WP183527985). In addition, represented by genotype A-gta174 (KF686599), group Both6 comprised 6.3% and 14.9% of the A5 and G5 clones, respectively, showing higher than 90% g5 gene product amino acid similarity to strain Loktanella sp. 1ANDIMAR09 (KQB98268) and Yoonia rosea (WP076658629). Combined with the Arctic clone libraries described above, the results revealed that Sulfitobacter, Loktanella and Yoonia were dominant groups of marine roseobacters in both Arctic and Antarctic coastal regions, playing similar ecological roles in bipolar marine environments.
3.4. Relationships between Arctic and Antarctic g5 Genes
Phylogenetic analysis based on g5 gene product amino acid sequences was conducted to investigate the similarity or difference between Arctic and Antarctic g5 genotypes. Compared with Phaeobacter (represented by genotype K1g5-76), which was exclusively detected in Arctic seawater, the genera Jannaschia (represented by A-gta48), Litoreibacter (represented by G-gta157) and Pacificibacter (represented by G-gta101) were only observed in Antarctic seawater in this study. In addition, phylogenetic groups Ant1 within the genus Octadecabacter, Ant2 within the genus Yoonia, Ant5 and Ant6 within the genus Loktanella, Ant7 and Ant8 within Ascidiaceihabitans/Tateyamaria, and Ant9, Ant10, Ant11, Ant12, Ant13 and Ant14 within the genus Sulfitobacter were exclusively detected in Antarctic samples (Figure 4). In contrast, phylogenetic groups from Arc1 to Arc7 were exclusively observed in Arctic seawater. Much more abundant Ascidiaceihabitans/Tateyamaria-related sequences were detected in Antarctic seawater (19.2% of Antarctic clones) than in Arctic seawater (6.4% of Arctic clones). Differences in environmental parameters (e.g., water temperature, salinity and nutrients) and bacterial abundance [12,21] can be observed between the two investigated Arctic and Antarctic regions, suggesting niche adaptation of specific roseobacters to unique environments.
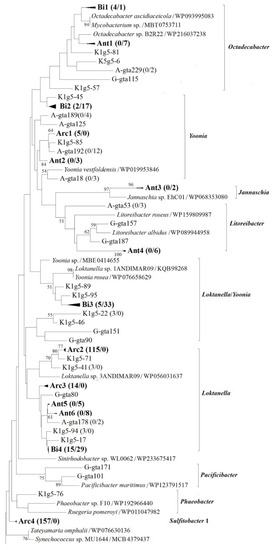
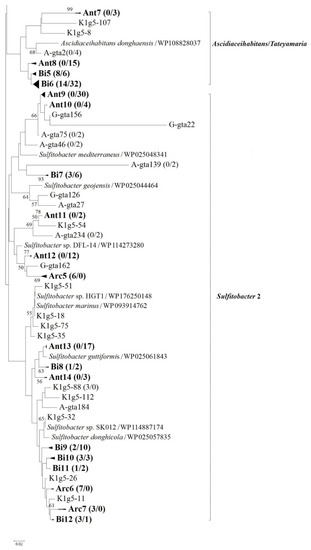
Figure 4.
Neighbor-joining phylogenetic tree based on partial g5 gene product amino acid sequences (ca. 260 aa) showing the phylogenetic diversity of g5 genes from Arctic and Antarctic bacterial communities. Groups from Arc1 to Arc7 represent closely related clones exclusively found in Arctic region, whereas groups from Ant1 to Ant14 represent closely related clones exclusively found in Antarctic region. Groups from Bi1 to Bi12 are closely related clones detected in bipolar regions. The numbers in parentheses following clone names or group names indicate the number of sequences found in Arctic and Antarctic samples, respectively. Bootstrap numbers are shown as percentages based on 1000 replicates, and values of less than 50 were omitted. The scale bar indicates evolutionary distance.
Although the genera Sulfitobacter, Loktanella and Yoonia were dominant in both Arctic and Antarctic coastal seawater, an endemic distribution of the genotypes was observed in this study. For example, represented by genotype K1g5-64 (KF537731) showing identical to Sulfitobacter sp. BSw21498, the phylogenetic group Arc4 (Figure 4) was dominant (39.0% of Arctic clones) and exclusively detected in Arctic clone libraries. On the other hand, represented by genotype A-gta40 (KF686607) showing higher than 96% sequence similarity to strain Sulfitobacter guttiformis (WP025061843), the phylogenetic group Ant13 was abundant (5.4% of Antarctic clones) and exclusively detected in Antarctic seawater. Similar to Sulfitobacter mediterraneus, S. guttiformis (formerly classified as Staleya guttiformis) can also attach to polymeric surfaces and produce extracellular polymeric substances [39]. The strain type S. guttiformis was first isolated from an Antarctic hypersaline lake [40]. Within the genus Yoonia, genotypes K1g5-45 (KF537719; 0.2% of Arctic clones) and A-gta18 (KF686595; 1.0% of Antarctic clones) were exclusively detected in Arctic and Antarctic seawater, respectively. The two genotypes showed 88.1% g5 gene product amino acid sequence similarity between each other, both exhibiting higher than 87% similarity to Yoonia vestfoldensis (WP019953846). The strain type Yoonia vestfoldensis was isolated from an Antarctic salt lake [41]. Endemic distributions of PufM, DmdA, and DddP genotypes have been observed in the Arctic or Antarctic region [19,20,32]. Sulfitobacter-related dddL gene sequences are detected in Kongsfjorden but absent in Maxwell Bay, whereas Loktanellar-related dddP gene sequences are abundant in Maxwell Bay but absent in Kongsfjorden [20,32].
Represented by phylogenetic groups from Arc1 to Arc7 (Figure 4), genotypes limited in Arctic samples accounted for 84.8% of total Arctic clones, whereas the proportion of genotypes (represented by groups from Ant1 to Ant14) exclusively found in Antarctic samples comprised 54.3% of Antarctic sequences. The results suggested that the proportion of endemic phylotypes of roseobacters distributed in the Arctic was higher than that in the Antarctic. The endemic phylotypes accounted for a larger share (71.5% on average) of all g5 clones, suggesting that marine roseobacters in polar regions comprise mainly endemic phylotypes, which are distributed either in the Arctic or Antarctica. Though Louca [42] reports that most prokaryotic species and even closely related strains are globally distributed, geographic endemism at the species or strain level is observed in thermophilic microorganisms. Global-scale microbial distribution patterns are likely the result of recent or current environmental filtering rather than geographic endemism [42]. Polar region is colonized principally by psychrophilic and psychrotrophic microorganisms. Dispersal between poles is problematic for psychrophilic bacteria because of the long distances and the difficulty of transporting across the equator [43]. Whether the high proportion of endemic phylotypes in roseobacters observed in this study is connected to phenotypical characteristics of the bacteria requires further study.
Represented by genotype K1g5-34 (KF537711) showing identical to Loktanella salsilacus (WP 090187762), which was first isolated from an Antarctic salt lake [41], phylogenetic group Arc2 comprised 29.6% of Arctic clones but was not detected in Antarctic samples. In addition, represented by genotypes A-gta166 (KF686615) and A-gta221 (KF686666) showing higher than 93% similarity to Loktanella ponticola (WP183527985), groups Ant5 and Ant6 were exclusively observed in Antarctic samples and accounted for 4.1% of the total Antarctic clones. However, the type strain of Loktanella ponticola was isolated from seawater in the Korean South Sea [44]. Therefore, Loktanella-related genotypes within the phylogenetic groups Arc2, Ant5 and Ant6 can actually be considered to have a bipolar distribution.
The bipolar distribution of roseobacter phylotypes has been reported in Arctic and Antarctic water samples [12,43]. In the present study, phylogenetic groups from Bi1 to Bi12 accounted for 15.2% and 45.7% of the Arctic and Antarctic g5 gene clones, respectively. Groups from Bi1 to Bi12 (Figure 4) represented closely related clones detected in bipolar regions. Represented by OTU A-gta222 (KF686644), which showed 93.4% similarity to Loktanella ponticola (WP183527985), bipolar group Bi4 comprised 3.7% and 9.3% of the Arctic and Antarctic clones, respectively. Moreover, including groups from Bi7 to Bi12, Sulfitobacter-related phylotypes accounted for 3.2% and 7.7% of the Arctic and Antarctic clones, respectively. Represented by OTU A-gta103 (KF686586), which showed 89.2% similarity to Tateyamaria omphalii (WP076630136), bipolar group Bi6 comprised 3.4% and 10.2% of the Arctic and Antarctic clones, respectively. The results support the bipolar or even global distribution of roseobacters within the Loktanella, Sulfitobacter and Ascidiaceihabitans/Tateyamaria groups. Different from previous studies, in this study Ascidiaceihabitans/Tateyamaria group was abundant in both Arctic and Antarctic coastal seawaters. Neither Ascidiaceihabitans nor Tateyamaria are reported in previous studies based on analysis of functional genes [19,20,32]. The genera Sulfitobacter and Loktanella are the dominant members of the Alphaproteobacteria in bacterioplankton community in both Arctic Kongsfjorden [21] and Antarctic Maxwell Bay [12]. Sulfitobacter-related sequences are also abundant in Arctic and Antarctic coastal seawaters based on analysis of aerobic anoxygenic phototrophy gene pufM [19] and DMSP demethylase gene dmdA [20]. In addition, Loktanella-related sequences are detected in Kongsfjorden and Maxwell Bay based on pufM and dmdA genes [19,20]. The bipolar distribution of g5 genotypes suggests that GTA transduction may contribute to the adaptation of some roseobacters (e.g., Loktanella and Sulfitobacter) to bipolar marine environments.
The GTA g5 gene has served as a genetic marker to investigate the diversity of roseobacters in marine environments due to the congruence between g5 and 16S rRNA gene phylogenies [17,18,45]. However, in the present study, high g5 gene product amino acid sequence similarities were observed between members of different genera (e.g., Loktanella/Yoonia, and Ascidiaceihabitans/Tateyamaria) within the Roseobacter clade. It could result in a difficulty in clarifying the taxonomic status of closely related members of the Roseobacter clade. Therefore, aside from the g5 gene focusing on roseobacters harboring GTA, the 16S rRNA gene should be used simultaneously to estimate the diversity of the Roseobacter clade in natural environments. It could be helpful for us to obtain more insight into the role of GTA transduction in adaptation and evolution of the Roseobacter clade in polar marine environments.
4. Conclusions
Based on analysis of the major capsid protein-encoding GTA g5 gene, the present study supports previous findings of both bipolar and endemic distributions of roseobacters and their functional genes in polar marine environments [19,20]. Endemic phylotypes were more frequently observed in polar regions than cosmopolitan phylotypes. The genera Sulfitobacter, Loktanella and Yoonia were dominant in the Roseobacter clade detected in Arctic and Antarctic coastal seawaters. This may be attributed to GTAs that are maintained in bacterial genomes due to the advantages associated with gene exchange in stressful conditions [14]. The results also indicate that, similar to temperate oceans [46,47,48], GTAs are common in the Roseobacter clade in polar marine environments. However, identical g5 gene product amino acid sequences were observed between Loktanella sp. 1ANDIMAR09 (KQB98268) and Yoonia rosea (WP076658629) in this study, suggesting a potential problem for clarifying the taxonomic status of closely related members of the Roseobacter clade based on the g5 gene. Further research on the characterization of bacterial isolates of the Roseobacter clade should be performed to improve our understanding of the distribution, environmental adaptation and ecological role of marine roseobacters in polar regions.
Author Contributions
Conceptualization, Y.-X.Z.; methodology, Y.-X.Z. and H.-R.L.; formal analysis, Y.-X.Z.; writing—original draft preparation, Y.-X.Z.; supervision, Y.-X.Z.; project administration, Y.-X.Z.; funding acquisition, Y.-X.Z. and W.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Key Research and Development Program of China (Grant No. 2018YFC1406903) and the National Natural Science Foundation of China (Grant Nos 91851201 and 41476171).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The g5 nucleotide sequences determined in the present study have been deposited in GenBank under accession numbers KC906230 to KC906242, KC951540 to KC951570, KF018557 to KF018562, KF537694 to KF537765, and KF686584 to KF686735.
Acknowledgments
We appreciate the assistance of Chinese Antarctic Center of Surveying and Mapping, Wuhan University, for providing maps. We also are grateful to the anonymous reviewers for their helpful comments.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Buchan, A.; Gonzaìlez, J.M.; Moran, M.A. Overview of the marine roseobacter lineage. Appl. Environ. Microbiol. 2005, 71, 5665–5677. [Google Scholar] [CrossRef]
- Garrity, G.M.; Bell, J.A.; Lilburn, T.; Family, I. Rhodobacteraceae fam. nov. In Bergey’s Manual of Systematic Bacteriology, 2nd ed.; Volume 2 (The Proteobacteria), Part C (The Alpha-, Beta-, Delta-, and Epsilonproteobacteria); Brenner, D.J., Krieg, N.R., Staley, J.T., Garrity, G.M., Eds.; Springer: New York, NY, USA, 2005; p. 161. [Google Scholar]
- Liang, K.Y.H.; Orata, F.D.; Boucher, Y.F.; Case, R.J. Roseobacters in a sea of poly- and paraphyly: Whole genome-based taxonomy of the family Rhodobacteraceae and the proposal for the split of the“Roseobacter clade” into a novel family, Roseobacteraceae fam. nov. Front. Microbiol. 2021, 12, 683109. [Google Scholar] [CrossRef]
- Moran, M.A.; Belas, R.; Schell, M.A.; Gonzaìlez, J.M.; Sun, F.; Sun, S.; Binder, B.J.; Edmonds, J.; Ye, W.; Orcutt, B.; et al. Ecological genomics of marine roseobacters. Appl. Environ. Microbiol. 2007, 73, 4559–4569. [Google Scholar] [CrossRef]
- Luo, H.; Moran, M.A. Evolutionary ecology of the marine Roseobacter clade. Microbiol. Mol. Biol. Rev. 2014, 78, 573–587. [Google Scholar] [CrossRef]
- Tolli, J.D.; Sievert, S.M.; Taylor, C.D. Unexpected diversity of bacteria capable of carbon monoxide oxidation in a coastal marine environment, and contribution of the Roseobacter-associated clade to total CO oxidation. Appl. Environ. Microbiol. 2006, 72, 1966–1973. [Google Scholar] [CrossRef]
- Imhoff, J.F.; Rahn, T.; Künzel, S.; Neulinger, S.C. Phylogeny of anoxygenic photosynthesis based on sequences of photosynthetic reaction center proteins and a key enzyme in bacteriochlorophyll biosynthesis, the chlorophyllide reductase. Microorganisms 2019, 7, 576. [Google Scholar] [CrossRef]
- Cude, W.N.; Buchan, A. Acyl-homoserine lactone-based quorum sensing in the roseobacter clade: Complex cell-to-cell communication controls multiple physiologies. Front. Microbiol. 2013, 4, 336. [Google Scholar] [CrossRef]
- Moran, M.A.; González, J.M.; Kiene, R.P. Linking a bacterial taxon to sulfur cycling in the sea: Studies of the marine roseobacter group. Geomicrobiol. J. 2003, 20, 375–388. [Google Scholar] [CrossRef]
- Reisch, C.R.; Moran, M.A.; Whitman, W.B. Bacterial catabolism of dimethylsulfoniopropionate (DMSP). Front. Microbiol. 2011, 2, 172. [Google Scholar] [CrossRef]
- Brinkmeyer, R.; Knittel, K.; Jürgens, J.; Weyland, H.; Amann, R.; Helmke, E. Diversity and structure of bacterial communities in Arctic versus Antarctic pack ice. Appl. Environ. Microbiol. 2003, 69, 6610–6619. [Google Scholar] [CrossRef]
- Zeng, Y.X.; Yu, Y.; Qiao, Z.Y.; Jin, H.Y.; Li, H.R. Diversity of bacterioplankton in coastal seawaters of Fildes Peninsula, King George Island, Antarctica. Arch. Microbiol. 2014, 196, 137–147. [Google Scholar] [CrossRef]
- Zeng, Y.X.; Luo, W.; Li, H.R.; Yu, Y. High diversity of planktonic prokaryotes in Arctic Kongsfjorden seawaters in summer 2015. Polar Biol. 2021, 44, 195–208. [Google Scholar] [CrossRef]
- Esterman, E.S.; Wolf, Y.I.; Kogay, R.; Koonin, E.V.; Zhaxybayeva, O. Evolution DNA packaging in gene transfer agents. Virus Evol. 2021, 7, veab015. [Google Scholar] [CrossRef]
- Hynes, A.P.; Mercer, R.G.; Watton, D.E.; Buckley, C.B.; Lang, A.S. DNA packaging bias and differential expression of gene transfer agent genes within a population during production and release of the Rhodobacter capsulatus gene transfer agent, RcGTA. Mol. Microbiol. 2012, 85, 314–325. [Google Scholar] [CrossRef]
- Brinkhoff, T.; Giebel, H.A.; Simon, M. Diversity, ecology, and genomics of the Roseobacter clade: A short overview. Arch. Microbiol. 2008, 189, 531–539. [Google Scholar] [CrossRef]
- Lang, A.S.; Beatty, J.T. Importance of widespread gene transfer agent genes in alpha-proteobacteria. Trends Microbiol. 2007, 15, 54–62. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, K.; Budinoff, C.; Buchan, A.; Lang, A.; Jiao, N.; Chen, F. Gene transfer agent (GTA) genes reveal diverse and dynamic Roseobacter and Rhodobacter populations in the Chesapeake Bay. ISME J. 2009, 3, 364–373. [Google Scholar] [CrossRef]
- Zeng, Y.X.; Dong, P.Y.; Qiao, Z.Y.; Zheng, T.L. Diversity of the aerobic anoxygenic phototrophy gene pufM in Arctic and Antarctic coastal seawaters. Acta Oceanol. Sin. 2016, 35, 68–77. [Google Scholar] [CrossRef]
- Zeng, Y.X.; Qiao, Z.Y. Diversity of dimethylsulfoniopropionate degradation genes reveals the significance of marine Roseobacter clade in sulfur metabolism in coastal areas of Antarctic Maxwell Bay. Curr. Microbiol. 2019, 76, 967–974. [Google Scholar] [CrossRef]
- Zeng, Y.X.; Zhang, F.; He, J.F.; Lee, S.H.; Qiao, Z.Y.; Yu, Y.; Li, H.R. Bacterioplankton community structure in the Arctic waters as revealed by pyrosequencing of 16S rRNA genes. Antonie Leeuwenhoek 2013, 103, 1309–1319. [Google Scholar] [CrossRef]
- Bosshard, P.P.; Santini, Y.; Grüter, D.G.; Stettler, R.; Bachofen, R. Bacterial diversity and community composition in the chemocline of the meromictic alpine Lake Cadagno as revealed by 16S rRNA gene analysis. FEMS Microbiol. Ecol. 2000, 31, 173–182. [Google Scholar] [CrossRef]
- Bano, N.; Hollibaugh, J.T. Diversity and distribution of DNA sequences with affinity to ammonia-oxidizing bacteria of the β subdivision of the class Proteobacteria in the Arctic Ocean. Appl. Environ. Microbiol. 2000, 66, 1960–1969. [Google Scholar] [CrossRef]
- Zeng, Y.; Zheng, T.; Li, H. Community composition of the marine bacterioplankton in Kongsfjorden (Spitsbergen) as revealed by 16S rRNA gene analysis. Polar Biol. 2009, 32, 1447–1460. [Google Scholar] [CrossRef][Green Version]
- Huber, T.; Faulkner, G.; Hugenholtz, P. Bellerophon: A program to detect chimeric sequences in multiple sequence alignments. Bioinformatics 2004, 20, 2317–2319. [Google Scholar] [CrossRef]
- Kurahashi, M.; Yokota, A. Tateyamaria omphalii gen. nov., sp. nov., an α-Proteobacterium isolated from a top shell Omphalius pfeifferi pfeifferi. Syst. Appl. Microbiol. 2007, 30, 371–375. [Google Scholar] [CrossRef]
- Kim, Y.O.; Park, S.; Nam, B.H.; Lee, C.; Park, J.M.; Kim, D.G.; Yoon, J.H. Ascidiaceihabitans donghaensis gen. nov., sp. nov., isolated from the golden sea squirt Halocynthia aurantium. Int. J. Syst. Evol. Microbiol. 2014, 64, 3970–3975. [Google Scholar] [CrossRef]
- Sinha, R.K.; Krishnan, K.P.; Kerkar, S.; Thresyamma, D.D. Influence of glacial melt and Atlantic water on bacterioplankton community of Kongsfjorden, an Arctic fjord. Ecol. Indic. 2017, 82, 143–151. [Google Scholar] [CrossRef]
- Zeng, Y.X.; Zhang, Y.H.; Li, H.R.; Luo, W. Complete genome of Sulfitobacter sp. BSw21498 isolated from seawater of Arctic Kongsfjorden. Mar. Genom. 2020, 53, 100769. [Google Scholar] [CrossRef]
- Barak-Gavish, N.; Frada, M.J.; Ku, C.; Lee, P.A.; DiTullio, G.R.; Malitsky, S.; Aharoni, A.; Green, S.J.; Rotkopf, R.; Kartvelishvily, E.; et al. Bacterial virulence against an oceanic bloom-forming phytoplankter is mediated by algal DMSP. Sci. Adv. 2018, 4, eaau5716. [Google Scholar] [CrossRef]
- Amin, S.A.; Hmelo, L.R.; van Tol, H.M.; Durham, B.P.; Carlson, L.T.; Heal, K.R.; Morales, R.L.; Berthiaume, C.T.; Parker, M.S.; Djunaedi, B.; et al. Interaction and signalling between a cosmopolitan phytoplankton and associated bacteria. Nature 2015, 522, 98–101. [Google Scholar] [CrossRef]
- Zeng, Y.X.; Qiao, Z.Y.; Yu, Y.; Li, H.R.; Luo, W. Diversity of bacterial dimethylsulfoniopropionate degradation genes in surface seawater of Arctic Kongsfjorden. Sci. Rep. 2016, 6, 33031. [Google Scholar] [CrossRef] [PubMed]
- Wirth, J.S.; Whitman, W.B. Phylogenomic analyses of a clade within the roseobacter group suggest taxonomic reassignments of species of the genera Aestuariivita, Citreicella, Loktanella, Nautella, Pelagibaca, Ruegeria, Thalassobius, Thiobacimonas and Tropicibacter, and the proposal of six novel genera. Int. J. Syst. Evol. Microbiol. 2018, 68, 2393–2411. [Google Scholar]
- Chen, Y.H.; Kuo, J.; Sung, P.J.; Chang, Y.C.; Lu, M.C.; Wong, T.Y.; Liu, J.K.; Weng, C.F.; Twan, W.H.; Kuo, F.W. Isolation of marine bacteria with antimicrobial activities from cultured and field-collected soft corals. World J. Microbiol. Biotechnol. 2012, 28, 3269–3279. [Google Scholar] [CrossRef] [PubMed]
- Villasante, A.; Catalán, N.; Rojas, R.; Lohrmann, K.B.; Romero, J. Microbiota of the digestive gland of red abalone (Haliotis rufescens) is affected by withering syndrome. Microorganisms 2020, 8, 1411. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, M.; Wang, B.; Jiang, K.; Wang, M.; Wang, L. Response of the Litopenaeus vananmei intestinal bacteria and antioxidant system to rearing density and exposure to Vibrio paraheamolyticus E1. J. Invertebr. Pathol. 2020, 170, 107326. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Zhou, S.; Lukwambe, B.; Nicholaus, R.; Yang, W.; Zheng, Z. Bacterioplankton community assembly in migratory fish habitat: A case study of the southern East China Sea. Environ. Sci. Pollut. Res. Int. 2022, 29, 33725–33736. [Google Scholar] [CrossRef]
- Ivanova, E.P.; Pham, D.K.; Wright, J.P.; Nicolau, D.V. Detection of coccoid forms of Sulfitobacter mediterraneus using atomic force microscopy. FEMS Microbiol Lett. 2002, 214, 177–181. [Google Scholar] [CrossRef][Green Version]
- Ivanova, E.P.; Mitik-Dineva, N.; Wang, J.; Pham, D.K.; Wright, J.P.; Nicolau, D.V.; Mocanasu, R.C.; Crawford, R.J. Staleya guttiformis attachment on poly(tert-butylmethacrylate) polymeric surfaces. Micron 2008, 39, 1197–1204. [Google Scholar] [CrossRef]
- Labrenz, M.; Tindall, B.J.; Lawson, P.A.; Collins, M.D.; Schumann, P.; Hirsch, P. Staleya guttiformis gen. nov., sp. nov. and Sulfitobacter brevis sp. nov., alpha-3-Proteobacteria from hypersaline, heliothermal and meromictic antarctic Ekho Lake. Int. J. Syst. Evol. Microbiol. 2000, 50, 303–313. [Google Scholar] [CrossRef]
- Van Trappen, S.; Mergaert, J.; Swings, J. Loktanella salsilacus gen. nov., sp. nov., Loktanella fryxellensis sp. nov. and Loktanella vestfoldensis sp. nov., new members of the Rhodobacter group, isolated from microbial mats in Antarctic lakes. Int. J. Syst. Evol. Microbiol. 2004, 54, 1263–1269. [Google Scholar] [CrossRef]
- Louca, S. The rates of global bacterial and archaeal dispersal. ISME J. 2022, 16, 159–167. [Google Scholar] [CrossRef]
- Staley, J.T.; Gosink, J.J. Poles apart: Biodiversity and biogeography of sea ice bacteria. Ann. Rev. Microbiol. 1999, 53, 198–215. [Google Scholar] [CrossRef]
- Jung, Y.T.; Park, S.; Park, J.M.; Yoon, J.H. Loktanella ponticola sp. nov., isolated from seawater. Int. J. Syst. Evol. Microbiol. 2014, 64, 3717–3723. [Google Scholar] [CrossRef]
- Sun, F.L.; Wang, Y.S.; Wu, M.L.; Jiang, Z.Y.; Sun, C.C.; Cheng, H. Genetic diversity of bacterial communities and gene transfer agents in Northern South China Sea. PLoS ONE 2014, 9, e111892. [Google Scholar] [CrossRef]
- Dang, H.; Li, T.; Chen, M.; Huang, G. Cross-ocean distribution of Rhodobacterales bacteria as primary surface colonizers in temperate coastal marine waters. Appl. Environ. Microbiol. 2008, 74, 52–60. [Google Scholar] [CrossRef]
- Roux, S.; Brum, J.R.; Dutilh, B.E.; Sunagawa, S.; Duhaime, M.B.; Loy, A.; Poulos, B.T.; Solonenko, N.; Lara, E.; Poulain, J.; et al. Ecogenomics and potential biogeochemical impacts of globally abundant ocean viruses. Nature 2016, 537, 689–693. [Google Scholar] [CrossRef]
- Bárdy, P.; Füzik, T.; Hrebík, D.; Pantůček, R.; Thomas Beatty, J.; Plevka, P. Structure and mechanism of DNA delivery of a gene transfer agent. Nat. Commun. 2020, 11, 3034. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).