Early Development of the Endemic Delminichthys krbavensis (Leuciscidae, Cypriniformes) from a Karstic Field in Croatia
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ćaleta, M.; Marčić, Z.; Buj, I.; Zanella, D.; Mustafić, P.; Duplić, A.; Horvatić, S. A review of extant Croatian freshwater fish and lampreys. Annotated list and distribution. Croat. J. Fisheries 2019, 77, 136–232. [Google Scholar] [CrossRef] [Green Version]
- Ćaleta, M.; Buj, I.; Mrakovčić, M.; Mustafić, P.; Zanella, D.; Marčić, Z.; Duplić, A.; Mihinjač, T.; Katavić, I. Endemic Fishes of Croatia; Croatian Environmental Agency: Zagreb, Croatia, 2015. [Google Scholar]
- Martinić, I. (Ed.) An Overview of the State of Biological and Landscape Diversity of Croatia with the Protection Strategy and Action Plans; Ministry of Environmental Protection and Physical Planning: Zagreb, Croatia, 2000; p. 158. [Google Scholar]
- Bognar, A. Gorski Kotar i Ogulinsko-Plašćanska Udolina. U: PAVIĆ, R. (ur.) Geografija SR Hrvatske, Knjiga 4, Gorska Hrvatska; Školska Knjiga: Zagreb, Croatia, 1975. [Google Scholar]
- Vukelić, M. Laudonov gaj. Šumarski List 2001, 7/8, 425–436. [Google Scholar]
- Bioportal. HR2000632 Krbavsko Polje. Department of Nature Conservation of the Ministry of Economy and Sustainable Development. 2022. Available online: http://www.bioportal.hr (accessed on 1 March 2022).
- Zupančič, P.; Bogutskaya, N.G. Description of two new species, Phoxinellus krbavensis and P. jadovensis, re-description of P. fontinalis Karaman, 1972, and discussion of the distribution of Phoxinellus species (Teleostei: Cyprinidae) in Croatia and in Bosnia and Herzegovina. Nat. Croat. 2002, 11, 411–437. [Google Scholar]
- Kottelat, M.; Freyhof, J. Handbook of European Freshwater Fishes; Kottelat: Cornol, Switzerland; Freyhof: Berlin, Germany, 2007. [Google Scholar]
- Mihinjač, T.; Marčić, Z.; Buj, I.; Zanella, D.; Mustafić, P.; Mrakovčić, M.; Ćaleta, M. Threatened fishes of the world: Delminichthys krbavensis (Zupančič & Bogutskaya, 2002) (Cyprinidae). Croat. J. Fish. 2015, 73, 33–34. [Google Scholar] [CrossRef] [Green Version]
- Crivelli, A.J. Delminichthys krbavensis. IUCN Red List. Threat. Species 2006, 2006, eT60446A12367313. [Google Scholar] [CrossRef]
- Mrakovčić, M.; Brigić, A.; Buj, I.; Ćaleta, M.; Mustafić, P.; Zanella, D. Red Book of Freshwater Fish of Croatia; Ministry of Culture, State Institute for Nature Protection: Zagreb, Croatia, 2006; pp. 61–62. [Google Scholar]
- Mustafić, P.; Buj, I.; Opašić, M.; Zanella, D.; Marčić, Z.; Ćaleta, M.; Šanda, R.; Horvatić, S.; Mrakovčić, M. Morphological comparison of Delminichthys ghetaldii (Steindachner, 1882), D. adspersus (Heckel, 1843), D. jadovensis (Zupančič and Bogutskaya, 2002) and D. krbavensis (Zupančič & Bogutskaya, 2002), endemic species of the Dinaric karst, Croatia. J. Appl. Ichthyol. 2017, 33, 256–262. [Google Scholar] [CrossRef]
- Official Gazette of the Republic of Croatia. Ordinance on Strictly Protected Taxa (OG 144/13, 73/16). Available online: https://narodne-novine.nn.hr/clanci/sluzbeni/2013_12_144_3086.html (accessed on 16 February 2022.).
- Zupančič, P. Rare and Threatened Freshwater Fish of the Adriatic Basin of Croatia, Slovenia and Bosnia and Herzegovina; Narodna in Univerzitetna Knjižnica: Ljubljana, Slovenia, 2008. (In Slovenian) [Google Scholar]
- Hutchings, J.A. Handbook of fish biology and fisheries. In Fish Biology; Hart, P.J.B., Reynolds, J.D., Eds.; Blackwell Science: Oxford, UK, 2002; Volume 12002, pp. 149–174. [Google Scholar]
- García, V.B.; Lucifora, L.O.; Myers, R.A. The importance of habitat and life history to extinction risk in sharks, skates, rays, and chimaeras. Proc. R. Soc. B 2008, 275, 83–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peňáz, M. A general framework of fish ontogeny: A review of the ongoing debate. Folia Zool. 2001, 50, 241–256. [Google Scholar]
- Kamler, E. Parent-egg-progeny relationships in teleost fishes: An energetics perspective. Rev. Fish Biol. Fish. 2005, 15, 399–421. [Google Scholar] [CrossRef]
- Marčić, Z.; Milković, A.; Ćaleta, M.; Buj, I.; Zanella, D.; Mustafić, P. Reproductive biology of the endemic dace Telestes karsticus Marčić and Mrakovčić 2011 (Cyprinidae, Leuciscinae) endemic to Croatia. J. Appl. Ichthyol. 2017, 33, 203–208. [Google Scholar] [CrossRef]
- Barbieri, R.; Stoumboudi, M.; Kalogianni, E.; Leonardos, I. First report on the spawning migration and early life development of a cyprinid species of the genus Telestes. J. Appl. Ichthyol. 2020, 36, 817–824. [Google Scholar] [CrossRef]
- Marčić, Z.; Abramović, A.; Ćaleta, M.; Buj, I.; Zanella, D.; Horvatić, S.; Mustafić, P. Early development of the endemic dace Telestes karsticus (Leuciscidae, Cypriniformes) in a Dinaric karst stream in Croatia. J. Appl. Ichthyol. 2021, 37, 99–105. [Google Scholar] [CrossRef]
- Korzelecka-Orkisz, A.; Bonisławska, M.; Taǹski, A.; Smaruj, I.; Szulc, J.; Formicki, K. Embryonic development of Aspius aspius L. (Actinopterygii: Cypriniformes: Cyprinidae). Electron. J. Pol. Agric. Univ. 2013, 16, #09. Available online: http://www.ejpau.media.pl/volume16/issue3/art-09.html (accessed on 15 March 2022).
- Kupren, K.; Rams, I.; Żarski, D.; Kucharczyk, D. Early development and allometric growth patterns of rheophilic cyprinid common dace Leuciscus leuciscus (Cyprinidae: Leuciscinae). Ichthyol. Res. 2016, 63, 382–390. [Google Scholar] [CrossRef] [Green Version]
- Kupren, K.; Żarski, D.; Kucharczyk, D. Early development and allometric growth patterns in ide Leuciscus idus (Linnaeus 1758). J. Appl. Ichthyol. 2015, 31, 509–517. [Google Scholar] [CrossRef]
- Peňáz, M.; Prokeš, M.; Kouřil, J.; Hamačkowa, J. Early development of the carp, Cyprinus carpio. Acta Sci. Nat. Acad. Sci. Bohem. Brno 1983, 17, 1–39. [Google Scholar]
- Korzelecka-Orkisz, A.; Bonisławska, M.; Pawlos, D.; Szulc, J.; Winnicki, A.; Formicki, K. Morphophysiological aspects of the embryonic development of tench (Tinca tinca L.). Electron. J. Pol. Agric. Univ. 2009, 12, #21. Available online: http://www.ejpau.media.pl/volume12/issue4/art-21.html (accessed on 15 March 2022).
- Korwin-Kossakowski, M. The influence of temperature during the embryonic period on larval growth and development in carp, Cyprinus carpio L.; and grass carp, Ctenopharyngodon idella (Val.): Theoretical and practical aspects. Arch. Pol. Fish. 2008, 16, 231–314. [Google Scholar] [CrossRef]
- Biondić, R.; Rubinić, J.; Biondić, B.; Meaški, H.; Radišić, M. Defining of Trends and Assessment of Groundwater Status in the Karst Area in Croatia; Technical Report; Faculty of Geotechnical Engineering and Rijeka, University of Zagreb: Varaždin, Croatia; Faculty of Civil Engineering, University of Rijeka: Varaždin, Croatia, 2016. (In Croatian) [Google Scholar]
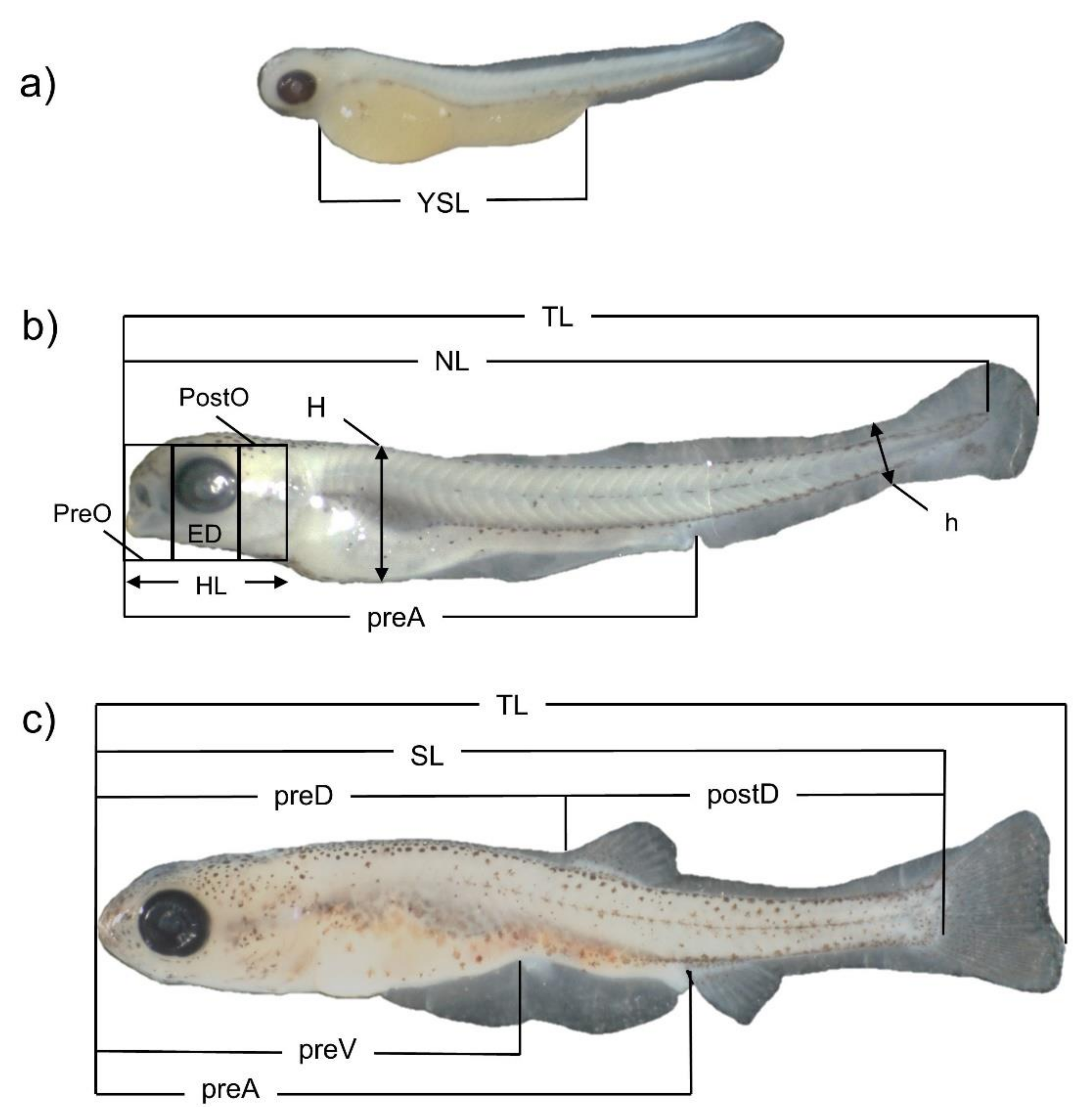
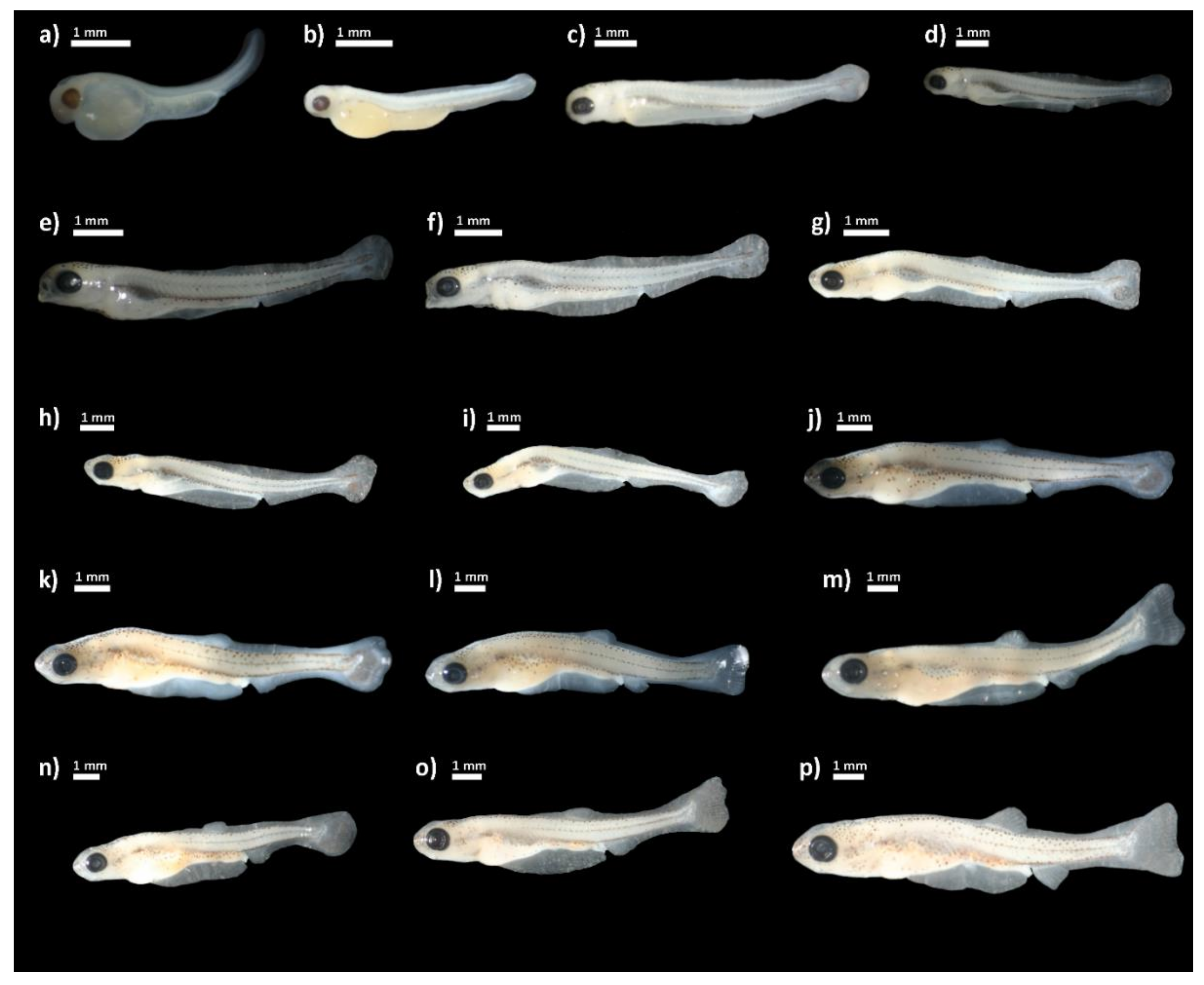
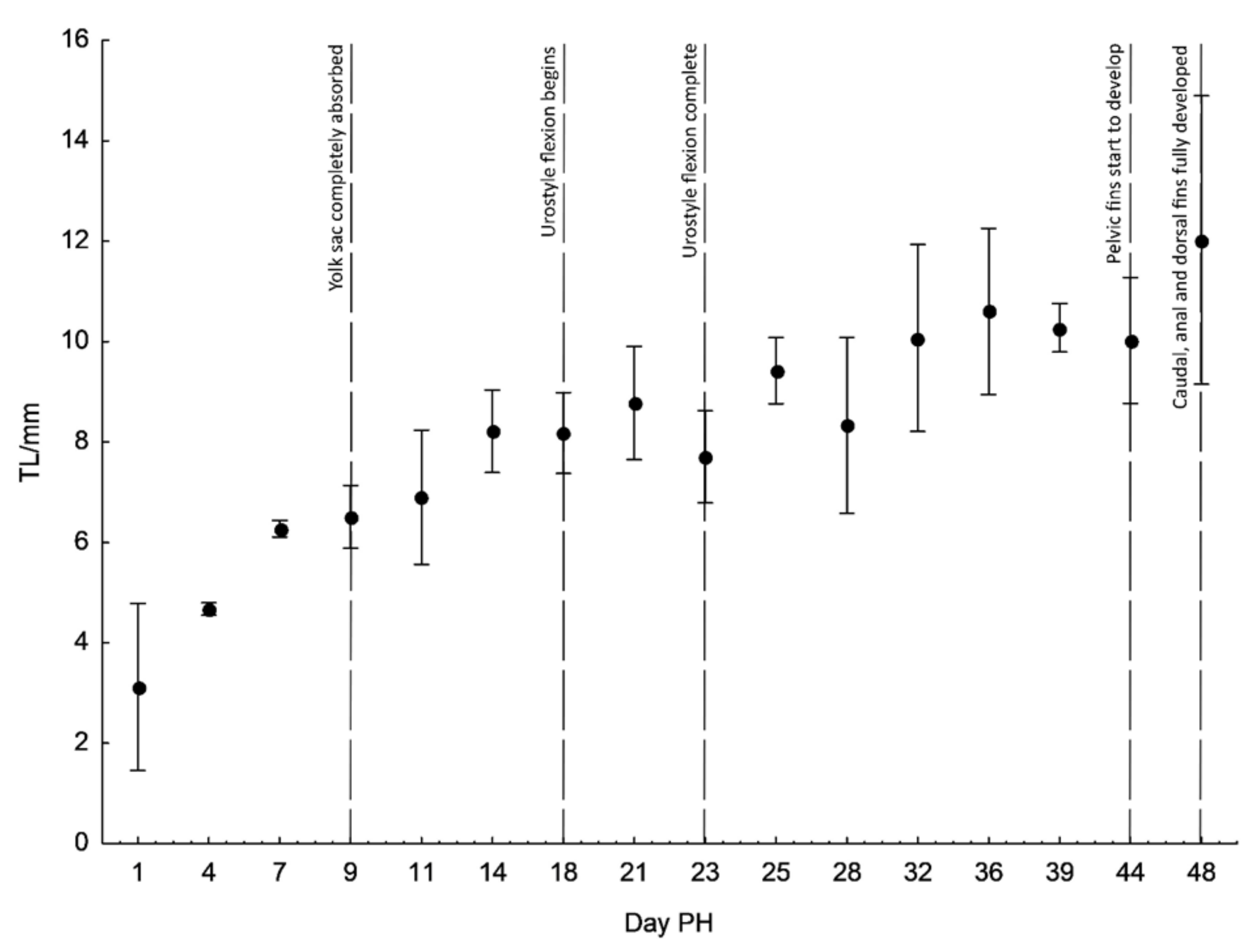
| Days PH | TL ± S.D. | NL ± S.D. | SL ± S.D. | HL ± S.D. | PreO ± S.D. | ED ± S.D. | PostO ± S.D. | H ± S.D. | ||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 3.12 ± 0.67 | 0.97 | 0.29 | |||||||
| 4 | 4.67 ± 0.10 | 0.98 | 0.21 | |||||||
| 7 | 6.27 ± 0.13 | 0.97 | 0.19 | 0.12 | 0.37 | 0.5 | 0.14 | |||
| 9 | 6.51 ± 0.39 | 0.96 | 0.19 | 0.14 | 0.37 | 0.48 | 0.13 | |||
| 11 | 6.90 ± 1.08 | 0.94 | 0.21 | 0.21 | 0.34 | 0.45 | 0.15 | |||
| 14 | 8.21 ± 0.66 | 0.84 | 0.17 | 0.17 | 0.36 | 0.46 | 0.13 | |||
| 18 | 8.18 ± 0.65 | 0.85 | 0.95 | 0.17 | 0.21 | 0.36 | 0.47 | 0.11 | ||
| 21 | 8.78 ± 0.91 | 0.88 | 0.18 | 0.20 | 0.34 | 0.44 | 0.13 | |||
| 23 | 7.71 ± 0.74 | 0.83 | 0.20 | 0.26 | 0.29 | 0.44 | 0.14 | |||
| 25 | 9.42 ± 0.53 | 0.90 | 0.22 | 0.25 | 0.31 | 0.48 | 0.16 | |||
| 28 | 8.33 ± 1.41 | 0.85 | 0.21 | 0.18 | 0.33 | 0.48 | 0.15 | |||
| 32 | 10.08 ± 1.50 | 0.80 | 0.22 | 0.19 | 0.32 | 0.47 | 0.17 | |||
| 36 | 10.60 ± 1.33 | 0.84 | 0.19 | 0.22 | 0.34 | 0.43 | 0.15 | |||
| 39 | 10.28 ± 0.39 | 0.82 | 0.22 | 0.21 | 0.33 | 0.45 | 0.15 | |||
| 44 | 10.03 ± 1.01 | 0.72 | 0.29 | 0.15 | 0.23 | 0.34 | 0.14 | |||
| 48 | 12.03 ± 2.31 | 0.83 | 0.21 | 0.21 | 0.32 | 0.46 | 0.15 | |||
| Days PH | h ± S.D. | PreA ± S.D. | PreD ± S.D. | PostD ± S.D. | PreV ± S.D. | YSL ± S.D. | Days PH | h ± S.D. | ||
| 1 | 0.70 | |||||||||
| 4 | 0.05 | 0.49 | ||||||||
| 7 | 0.07 | 0.61 | 0.44 | |||||||
| 9 | 0.06 | 0.58 | 0.48 | |||||||
| 11 | 0.03 | 0.64 | ||||||||
| 14 | 0.02 | 0.48 | ||||||||
| 18 | 0.02 | 0.53 | ||||||||
| 21 | 0.03 | 0.56 | ||||||||
| 23 | 0.03 | 0.59 | ||||||||
| 25 | 0.04 | 0.64 | ||||||||
| 28 | 0.04 | 0.53 | 0.55 | 0.43 | ||||||
| 32 | 0.04 | 0.62 | 0.48 | 0.36 | ||||||
| 36 | 0.04 | 0.62 | 0.49 | 0.36 | ||||||
| 39 | 0.03 | 0.60 | 0.48 | 0.35 | ||||||
| 44 | 0.04 | 0.60 | 0.46 | 0.34 | ||||||
| 48 | 0.05 | 0.59 | 0.49 | 0.31 | 0.43 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marčić, Z.; Jerković, A.; Ćaleta, M.; Buj, I.; Zanella, D.; Horvatić, S.; Mustafić, P. Early Development of the Endemic Delminichthys krbavensis (Leuciscidae, Cypriniformes) from a Karstic Field in Croatia. Diversity 2022, 14, 393. https://doi.org/10.3390/d14050393
Marčić Z, Jerković A, Ćaleta M, Buj I, Zanella D, Horvatić S, Mustafić P. Early Development of the Endemic Delminichthys krbavensis (Leuciscidae, Cypriniformes) from a Karstic Field in Croatia. Diversity. 2022; 14(5):393. https://doi.org/10.3390/d14050393
Chicago/Turabian StyleMarčić, Zoran, Ana Jerković, Marko Ćaleta, Ivana Buj, Davor Zanella, Sven Horvatić, and Perica Mustafić. 2022. "Early Development of the Endemic Delminichthys krbavensis (Leuciscidae, Cypriniformes) from a Karstic Field in Croatia" Diversity 14, no. 5: 393. https://doi.org/10.3390/d14050393
APA StyleMarčić, Z., Jerković, A., Ćaleta, M., Buj, I., Zanella, D., Horvatić, S., & Mustafić, P. (2022). Early Development of the Endemic Delminichthys krbavensis (Leuciscidae, Cypriniformes) from a Karstic Field in Croatia. Diversity, 14(5), 393. https://doi.org/10.3390/d14050393







