Abstract
Santa Catalina Island, located off the southern California coast, is home to the Blue Cavern Onshore State Marine Conservation Area (SMCA), which is recognized as a marine protected area. Here, we provide an updated species inventory of nearshore macroalgae, seagrasses, bony and cartilaginous fishes and invertebrates documented inside the Blue Cavern Onshore SMCA. Species richness data were compiled using scuba-based visual surveys conducted in the field, references from the primary and gray literature, museum records, unpublished species lists and online resources. The current checklist consists of 1091 marine species from 18 different taxonomic groups, which represents an ~43% increase in species diversity compared to the value reported previously. These data are indicative of the high biodiversity known from the Southern California Bight (SCB) region. The total number of intertidal and subtidal taxa reported represent approximately 85% and 45% of the documented macroalgae and plants, 41% and 24% invertebrates, and 62% and 20% of fishes from Catalina Island and the SCB, respectively. Among the marine taxa documented, 39 species either have undergone a geographic range shift or were introduced as the result of human activities, while another 4 species are listed as threatened, endangered or critically endangered. Research findings presented here offer an important baseline of species richness in the California Channel Islands and will help improve the efforts by resource managers and policy makers to conserve and manage similar habitats in the coastal waters off southern California.
1. Introduction
Ecosystem function is integral for effective management in marine systems [1], thus local and global species losses could threaten the stability of the ecosystem services on which humans depend [2]. An ongoing challenge to the documentation and management of biodiversity is that the number of taxa (species richness) in a given area is not typically known. Likewise, the patterns of species richness play an important role in studies of biogeography and conservation biology [3]. For example, while ~300,000 taxa have been described from the global ocean, the total number of marine organisms is estimated to include as many as 10 million species [4]. Not surprisingly, the taxonomic status of many of these organisms has yet to be evaluated [5], even among well-studied species, such as coastal fishes and invertebrates [6].
The California Channel Islands are located in the Southern California Bight (SCB), which stretches along ~700 km of coastline from Point Conception off California to Ensenada, just south of the US–Mexico border, and is known as a marine biodiversity hotspot [7]. The SCB is a dynamic region in which the subtropical, Southern California countercurrent flows nearshore along a northward trajectory, and the subarctic, California current moves offshore in a southerly direction. As these different water masses converge, this unique oceanographic circulation pattern acts as a biological transition zone, making the SCB one of the most productive and economically valuable coastal regions in the United States [8]. This area hosts nearly 500 species of macroalgae [9] and fishes [10], and more than 5000 species of invertebrates [11]. Concomitantly, the Bight coastal zone is also home to more than 22 million people, the busiest and largest container ports (Los Angeles and Long Beach) in the Western Hemisphere, as well as the second largest naval facility (San Diego) in the US [12].
Santa Catalina, the largest of the Southern Channel Islands (area = 194 km2), is the only island in the archipelago with a permanent civilian population. Surrounded by nearly ~87 km of rocky cliffs and sheltered bays, Catalina’s coastal zone is dominated by rocky reef and kelp forest habitat [13]. Located ~35 km south-southwest of Los Angeles, the island is easily accessible from multiple ports and marinas on the southern California mainland and is a popular tourist destination. Moreover, the diversity of its nearshore habitats and relatively inaccessible coastline makes the island an important resource to a range of stakeholders, including fishers, recreational groups, local residents and scientists. However, with more than one million visitors annually, Catalina’s marine biodiversity and ecosystems are under increasing pressure from anthropogenic stressors, such as nutrient pollution, habitat modification and climate change [14].
Recognized as an ecosystem unique for its species diversity, the State of California designated the Blue Cavern Onshore State Marine Conservation Area (SMCA), located on the leeward side of the island, as a marine protected area (MPA) in 2012. This site encompasses ~6.8 km2 of ocean habitat and is an expansion of the Catalina Marine Life Refuge, which was established by the state as a protected area in 1974. Situated adjacent to the University of Southern California (USC) Philip K. Wrigley Marine Science Center (WMSC), Blue Cavern Onshore SMCA is part of a network of nine MPAs established around the island in which the removal of living resources is either limited or prohibited altogether as outlined by California’s Marine Life Protection Act.
Here, we provide an updated species inventory of macroalgae, seagrasses, bony and cartilaginous fishes and invertebrates documented inside Blue Cavern Onshore SMCA. These findings are based on a recently published checklist of marine taxa from this area [15] and include updated records from two additional reef sites along with new data from previous data reports and articles published since 2021. It is worth noting that while the habitats and topographic complexities in and around Catalina are well known, species richness data on the intertidal and subtidal biota are far less complete [16]. Taxonomic data on species from this location are documented in an assortment of scholarly articles, technical reports, unpublished data and marine species databases, and likely represent a fraction of the biodiversity present, much of which remains unknown.
We anticipate that these new findings will provide an important baseline of species richness from Catalina Island, as well as prove useful to resource managers and policy makers for determining the mitigation costs associated with a loss in natural services. In particular, targeted conservation efforts that represent biodiversity in different regions and taxonomic groups require comprehensive inventories of the number of species present in a given area [17]. Data presented here will further improve coastal zone management by characterizing the marine biodiversity in Blue Cavern Onshore SMCA relative to other nearshore habitats in the California Channel Islands, as well as within the larger context of the SCB.
2. Materials and Methods
2.1. Study Sites
Species inventory data were recorded at 7 reef sites on the leeside of Santa Catalina Island in Blue Cavern Onshore SMCA: Big Fisherman’s Cove (33°26’43.34″ N, 118°29′11.40″ W), Bird Rock (33°27′01.7″ N, 118°29′11.7″ W), Blue Caverns (33°26′47.70″ N, 118°29′35.62″ W), Habitat Reef (33°26′41.50″ N, 118°29′17.00″ W), Intake Pipes (33°26′48.95″ N, 118°29′05.75″ W), Isthmus Reef (33°26′56.2″ N, 118°29′19.4″ W) and Pumpernickel Cove (33°26′54.08″ N, 118°29′47.90″ W) (Figure 1). These sites were selected because they support a variety of intertidal and subtidal species [13,15,18] and are the most convenient to access and use for research studies based at WMSC. The subtidal habitat structure of Blue Cavern Onshore SMCA is characterized as a major reef complex [19], which includes sandy areas with rock cobble and bedrock escarpments covered with fleshy macroalgae and kelp that provide a forest habitat for invertebrates and fishes [20,21]. Details on the nearshore reef habitat in and around Blue Cavern Onshore SMCA is reported elsewhere [15].
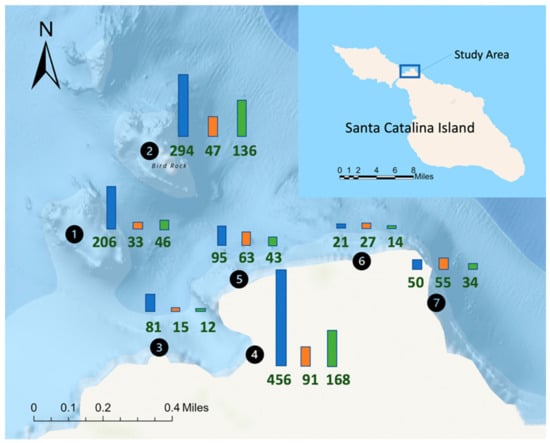
Figure 1.
Map indicating location of Blue Cavern Onshore State Marine Conservation Area reef sites off Santa Catalina Island, California. Numbers circled in black correspond to each study site: 1 = Isthmus Reef; 2 = Bird Rock; 3 = Habitat Reef; 4 = Big Fisherman’s Cove; 5 = Intake Pipes; 6 = Pumpernickel Cove; 7 = Blue Caverns. Values below each bar represent species count of marine invertebrates (blue bar), macroalgae and seagrasses (orange bar), and bony and cartilaginous fishes (green bar) recorded at each study location (n = 1091 species total).
2.2. Field Surveys
Timed, roving visual surveys were conducted by scuba divers between May 2015 and September 2016 and from May to September 2021 to identify individual species of subtidal marine macroalgae (excluding most crustose coralline algae), seagrasses, bony and cartilaginous fishes and invertebrates. This survey method is applicable for assessing the species richness of a variety of temperate [22] and tropical [23] marine taxa. Individual species were observed and recorded at each reef site based on methods reported previously [15,24]. Visual surveys were conducted while swimming ~1 m above the bottom contour (2–30 m depth). Total bottom time (35–60 min dive−1) varied among reef sites and was dependent on the amount of breathing gas available to a diver at depth, ambient water temperatures, survey area size and visibility beneath the surface. Priority was given to conspicuous (>1 cm) subtidal macrofauna and flora that could be identified to at least the genus level of classification. Survey data on deep-water (>30 m) species, marine parasites and most planktonic organisms (<1 cm) were not recorded in this study.
2.3. Species Records
An updated species inventory from Blue Cavern Onshore SMCA was compiled from the primary and gray literature, museum records and unpublished species lists described elsewhere [15]. The earliest collections of marine taxa from Catalina date back more than 100 years. Unfortunately, these published reports are often not readily available, and in some cases, the historic names used to describe the geographic locations where samples were collected have changed. We chose to focus the current checklist on research studies and collections completed over the last 57 years, beginning with the construction of WMSC at Big Fisherman’s Cove in 1965 up to the present year, 2022.
All marine taxa data and documents were either obtained via digital bibliographic resources or as a hard copy from the USC Libraries. Many intertidal and subtidal specimen records were sourced from electronic databases [25,26,27,28,29,30,31,32,33,34]. Only species that were explicitly reported as either observed or collected in water <30 m in depth at one of the study sites designated inside Blue Cavern Onshore SMCA were considered in this study. To eliminate synonyms and create a comprehensive list of valid species names for as many taxa reviewed as possible, the scientific nomenclature was confirmed for invertebrates and fishes [35], and for macroalgae and plants [29]. The open nomenclature abbreviations sp. and spp. were used to indicate that an individual or group of species within a genus were either unidentified or have yet to be described.
2.4. Data Analysis
Species richness of marine taxa from Blue Cavern Onshore SMCA was used to create an incidence matrix relating the presence of each species to a specific source citation. Individual references and field survey data used to construct the current checklist were recorded as discrete sampling units following methods established previously [15]. The numbers of bony and cartilaginous fishes, macroalgae, seagrasses and invertebrates were then used to estimate the expected richness using the mean value of 4 non-parametric incidence-based estimators (Chao2, Jack1, Jack2, Bootstrap) of species biodiversity [36,37,38]. These non-parametric statistical procedures are tractable for the analysis of binary species data [39] and have been demonstrated to be accurate estimators of species richness in other marine biodiversity studies [40,41,42]. Species richness was also evaluated by measuring the cumulative number of marine taxa documented in this study as a function of the first year in which they were reported by a specific reference. All data were analyzed using R Statistical Software [43] with the vegan [44] package.
3. Results
3.1. Species Biodiversity
A total of 1091 species of valid and unidentified marine taxa from 18 major phylogenetic groups were documented from 7 different reef sites in Blue Cavern Onshore SMCA (see Species checklist, Supplementary Table S1). A comprehensive inventory of 763 species of invertebrates, 225 species of macroalgae, 2 species of seagrass and 101 species of bony and cartilaginous fishes was compiled from a list of 158 discrete citations and observations. These findings include species reports from two phyla (Nemertea and Platyhelminthes) not reported in the previous inventory [15]. Despite a nearly 10.5-fold increase in the inventory of taxa reported in Blue Cavern Onshore SMCA since the construction of WMSC (from 104 in 1965 to a cumulative total of 1091 in 2022), none of the species-accumulation curves produced in this study approached an asymptote (Figure 2). Overall, a total of 33.8 h was spent underwater conducting roving visual surveys (n = 68 total dives) from which 105 species were documented. The majority of scuba-based surveys (47.1%) were performed in Big Fisherman’s Cove, with the remainder spread out among the other 6 reef sites (Bird Rock 6.9%, Blue Caverns 4.4%, Habitat Reef 10.3%, Intake Pipes 22.2%, Isthmus Reef 5.9%, Pumpernickel Cove 4.4%).
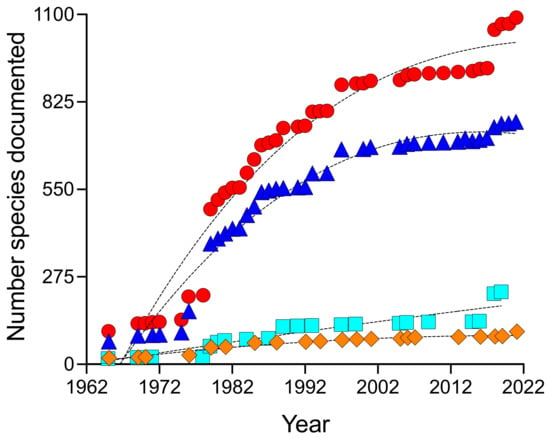
Figure 2.
Species-accumulation curve for bony and cartilaginous fishes (diamonds), macroalgae and seagrasses (squares), invertebrates (triangles), as well as all marine taxa (circles) documented from seven different reef sites in Blue Cavern Onshore SMCA from 1965 to 2022.
3.2. Species Richness
The expected species richness of bony and cartilaginous fishes (123.2 ± 0.93), macroalgae and seagrasses (369.4 ± 5.2), invertebrates (1345.7 ± 11.4), as well as all taxa combined (1828.7 ± 11.7) recorded in Blue Cavern State Onshore SMCA were 1.2- to 1.8-times greater than the total number of organisms cataloged from all external sources and scuba-based surveys (Table 1). A heat map analysis was also performed on the number of species recorded within a given taxonomic group at each reef site assessed (Figure 3). Squares highlighted in red indicate taxa with high species richness, while squares colored in yellow represent taxonomic groups with the least number of species present. Marine taxa documented with the highest biodiversity (>40 species per reef site) include the Rhodophyta macroalgae, Vertebrata bony and cartilaginous fishes and the Mollusca and Arthropoda invertebrates, whereas those with the lowest richness (<2 species per site) include the Tracheophyta seagrasses, as well as the Phoronida, Sipuncula, Chaetognatha, Nemertea and Platyhelminthes invertebrates. Finally, the current species inventory for Blue Cavern State Onshore SMCA contains a total of 21 nonindigenous and invasive species, 18 species that have undergone a geographic range shift and 4 taxa listed as either a species of concern, endangered or critically endangered (Supplementary Table S1).

Table 1.
Species richness of marine biota documented from Blue Cavern Onshore SMCA. Expected species richness was estimated using the mean value (±1 SE) of four non-parametric incidence-based estimators (Chao and Chiu 2016) of the number of bony and cartilaginous fishes, macroalgae and plants, invertebrates, as well as all marine taxa recorded in this study.
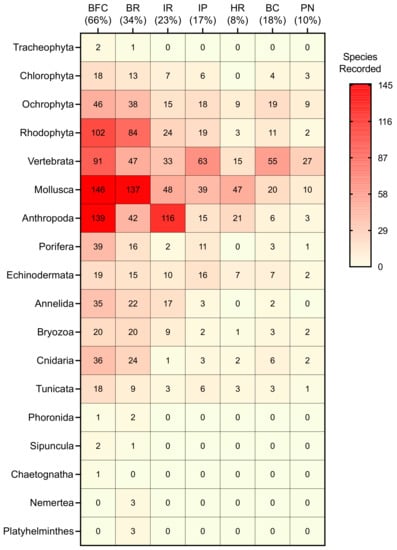
Figure 3.
Heat map showing species richness of seagrasses, macroalgae, bony and cartilaginous fishes and invertebrates documented at seven reef sites in Blue Cavern Onshore SMCA. Each square indicates number of species recorded within a taxonomic group. Values in parentheses indicate percentage of studies (n = 158 total; does not include scuba-based surveys from this study) conducted at each reef site (BFC = Big Fisherman’s Cove, BR = Bird Rock, IR = Isthmus Reef, IP = Intake Pipes, HR = Habitat Reef, BC = Blue Caverns, PN = Pumpernickel Cove). Color gradient (ranging from yellow to red, as shown in key) signifies changes in the number of species present (rows) among marine taxa recorded at different study sites (columns).
4. Discussion
4.1. Nearshore Biodiversity
The species inventory of macroalgae, seagrasses, bony and cartilaginous fishes and invertebrates presented here provides an important baseline of the nearshore biodiversity in the California Channel Islands and will help improve the efforts to conserve and manage similar habitats in the region. The current checklist consists of 1091 individual marine species from 18 different taxonomic groups, which represents an ~43% increase in species diversity compared to the value reported previously [15]. This increase is due in part to advances in environmental DNA (eDNA) techniques (i.e., genetic material obtained from seawater or soil samples rather than directly from an individual organism), which has increasingly been used to measure the biodiversity of marine ecosystems [45,46]. Specifically, eDNA methods were recently used [47] to survey more than 50 different species of fishes in Blue Cavern SMCA. Nonetheless, the total number of marine taxa documented here continues to represent only 60% of the estimated species richness from this area (Table 1). As previously suggested [15], this value might indicate that as much as 40% of the macrofauna and flora from this area have yet to be reported.
These findings corroborate our species-accumulation curves (Figure 2), which are regarded as well-established predictive tools for estimating species richness and sampling effort [38]. Species-accumulation data infer that the current species inventory represents only a fraction of a more highly diverse ecosystem. This underrepresentation of true species richness is likely due in part to the under-sampling of some habitats compared to others [6]. Among the many reef sites within Blue Cavern Onshore SMCA, those closest and with the most convenient access for conducting research studies at WMSC have received the most attention. Based on 158 discrete references and collections used to evaluate biodiversity data in this study, Big Fisherman’s Cove, Bird Rock and Isthmus Reef were selected as survey sites 2- to 8-times more often than other locations and were host to the greatest numbers of marine taxa documented (Figure 3). Future efforts to investigate patterns of species richness in this area might close the apparent gap in the estimated number of taxa (compared to the number observed) by focusing on reef sites that are less frequented, such as Intake Pipes, Habitat Reef, Blue Caverns, Pumpernickel Cove, as well as other areas in Blue Cavern Onshore SMCA, which covers nearly 7 km2 of nearshore habitat.
Still, the current inventory of marine taxa is reflective of the high biodiversity recognized in the coastal waters off southern California [11,18]. Overall, these data are beneficial for improving marine biodiversity conservation actions in the California Channel Islands, as well as within the larger context of the SCB [48]. The total number of species reported from Blue Cavern Onshore SMCA represents approximately 85% and 45% of the documented macroalgae and plants, 41% and 24% invertebrates and 62% and 20% of fishes known from Catalina Island [49] and the SCB [9,10], respectively. Although more than 5000 species of marine invertebrates are known from the SCB [11], the taxonomic status of many of these organisms is not yet known. Thus, a more conservative assessment of 3250 valid benthic taxa [32] was used here to estimate the number of invertebrate species documented off Catalina relative to the SCB.
4.2. Colonizers and Nonindigenous Species
Species checklists provide a means to track and monitor marine communities over time and are beneficial for detecting changes in the presence and condition of select organisms [50]. Findings from this study highlight the temporal occurrence and spatial distribution of a variety of marine taxa recorded inside Blue Cavern Onshore SMCA. In particular, the eelgrass Zostera marina (one of two species of seagrasses known from this area) was not documented in Big Fisherman’s Cove until 1996 and is likely the result of natural colonization from populations in the Northern Channel Islands [51]. Eelgrass beds are an important refuge for marine invertebrates and fishes and provide ecosystem services whose economic value, in terms of their overall abundance and density of eelgrass habitats as a whole, outweighs their ecological function [52]. Along the Pacific coast of the United States, state and federal resource agencies recognize eelgrass as habitat areas of particular concern that provide ecologically important habitat for species to survive and reproduce and are high priorities for conservation. Once established, the presence of Z. marina likely attracted a variety of conspicuous species associated with eelgrass habitats to the Big Fisherman’s Cove reef site, such as the California sea cucumber Apostichopus californicus, orangethroat pikeblenny Chaenopsis alepidota, Pacific angelshark Squatina californica, as well as the rays Myliobatis californica and Urolophus halleri.
Additionally, a total of 39 species from Blue Cavern Onshore SMCA were either introduced or have undergone a geographic range shift, while another 4 species are listed as threatened, endangered or critically endangered (Supplementary Table S1). For example, the invasive brown seaweed Sargassum horneri has raised concerns about its impact on native ecosystems in southern California, particularly off the Channel Islands [29,53]. Populations of S. horneri can cause a decrease in the abundance of fleshy macroalgae and kelp that provide a refuge for invertebrates and fishes [54], as well as significant economic losses to a variety of commercial industries ranging from fisheries and boating to tourism [55].
4.3. Survivors, Visitors and Missing in Action
Among the 864 species of invertebrates and fishes documented in Blue Cavern Onshore SMCA, 18 taxa have experienced either a geographic range expansion or contraction. For example, the range of the California dorid nudibranch Felimare californiensis was once widespread throughout the SCB; however, by the mid-1980s, this species was extirpated from the region [56]. After disappearing for nearly 20 years, the first sightings of F. californiensis were reported in 2003. Currently, only a handful of populations are known to exist off the southern California mainland and Channel Islands, which include the Big Fisherman’s Cove reef site on Catalina Island. The marked decline of F. californiensis populations is likely due to a variety of factors, including significant increases in coastal eutrophication, loss of essential habitat and historical overharvesting by the aquarium trade [56].
Over the past 200 years, 133 local- to global-scale marine extinctions are known to have taken place [57]. Therefore, our findings are in agreement with previous studies, which suggest that that species loss and ecosystem change have become more widespread over shorter ecological timescales [58,59,60]. For instance, a northward shift in the species ranges of several subtropical fishes from the Pacific coast of Mexico has resulted in the frequent occurrence of both the finescale triggerfish Balistes polylepis and largemouth blenny Labrisomus xanti in Big Fisherman’s Cove [15,61]. Other species of fish whose northern ranges have expanded into Blue Cavern Onshore SMCA include the Rainbow scorpionfish Scorpaenodes xyris [62], as well as the cardinalfishes Apogon guadalupensis and A. pacificus [63]. While the presence of these species in the SCB was once considered a relatively rare occurrence, such sightings have become more common and are likely attributed to the increasing frequency and spatial extent of marine heatwaves [64,65] and El Niño events [66,67].
Furthermore, several widespread, cryptogenic invertebrate species reported from Blue Cavern Onshore SMCA, such as the bryozoans Bugula neretina, Watersipora subatra, W. subtorquata, the colonial ascidian Diplosoma listerianum and the sea anemone Bunodeopsis sp., are cause for concern, given their ability to quickly settle and encrust hard substrates [26,68,69]. These species can alter the diversity of benthic ecosystems by competing with native biota for space, facilitate the spread of other nonindigenous taxa and cause significant damage to marine ecosystem services [70,71]. Other marine invertebrates, however, once commonly found in Blue Cavern Onshore SMCA, are now extremely rare. For example, two different species of echinoderms, the sea stars Patiria miniata and Pisaster giganteus, were documented in at least 15 different studies performed between 1965 and 1988 at the reef sites evaluated in this study. The sudden disappearance of these species from subtidal habitats (stretching from Alaska to Mexico) is linked to an infectious pathogen known as sea star-associated densovirus (SSaDV) [72], which caused a mass die-off of both species from Catalina and other locations in the Channel Islands [73]. Although SSaDV is not fully understood, mortality events appear to be most prevalent when sea surface temperatures are anomalously warmer than usual in the East Pacific Ocean [74]. Since 1988, there have been no reports of P. miniata in Blue Cavern Onshore SMCA, while P. giganteus has been documented twice (in 1997 and 2004).
4.4. Vulnerable and Endangered Species
Currently, 13 species of marine invertebrates and fishes living in California’s nearshore waters are in danger of extinction, as outlined by the Endangered Species Act (ESA). Among the animals listed, three species of gastropod mollusks are known from Blue Cavern Onshore SMCA (Supplementary Table S1). One of these, the endangered black abalone Haliotis cracherodii is a large herbivorous sea snail that inhabits both intertidal and subtidal habitat. It is worth noting that H. cracherodii was last documented in Big Fisherman’s Cove more than 40 years ago [75]. Two additional species, the pink (H. corrugata) and green abalones (H. fulgens), are recognized as species of concern. Interestingly, H. corrugata and H. fulgens are frequently observed in Blue Cavern Onshore SMCA, serving as a reminder that MPAs are important refuges for populations facing multiple threats, such as overfishing, habitat degradation and climate change [76]. Nevertheless, despite the closure of the regional fishery in 1997, as well as the implementation of numerous restoration programs over the past three decades, abalone populations throughout the SCB are still recovering from a combination of natural and human-induced stock collapses [77] and disease events [78].
Other vulnerable species recorded in Blue Cavern Onshore SMCA include the giant sea bass Stereolepis gigas, the largest bony fish known from California’s kelp forest habitat and classified as critically endangered by the IUCN Red List. Truly a behemoth fish, giant sea bass are members of the wreckfish family and can grow to more than 2 m in total length and 255 kg in weight [79]. One of the largest individuals documented was observed off Catalina Island at Goat Harbor (~5 km southeast of Blue Cavern Onshore SMCA) and measured 2.75 m in total length with an estimated weight of 380 kg [80]. Although never listed as endangered under the ESA [81], S. gigas stocks along the California coast were so severely impacted by overfishing that a moratorium was declared in 1982 [82]. Remarkably though, the number of giant sea bass documented in Blue Cavern Onshore SMCA has become more common in recent years, in which encounters with these fish are known from four of the reef sites (Blue Caverns, Big Fisherman’s Cove, Intake Pipes and Isthmus Reef) assessed in this study. Finally, East Pacific green turtles (Chelonia mydas), listed as threatened under the ESA, regularly visit the highly productive coastal waters off southern California from their nesting beaches in Mexico. Although not one of the species included in this study, one of the authors (Ginsburg) recently observed an adult green turtle in Big Fisherman’s Cove, which is the first report to our knowledge to document their presence at this location.
5. Conclusions
Further investigations of species richness from Blue Cavern Onshore SMCA and other nearby protected areas on Catalina Island (and the California Channel Islands archipelago altogether) are required to provide new insights into the mechanisms that contribute to both the spatial and temporal connectivity among populations in the region. Such studies will help communicate the significance of conserving marine biodiversity for future generations with both the general public and stakeholder groups.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d14050366/s1, Supplementary Table S1. Species checklist of (I) Macroalgae (excluding most crustose coralline algae), (II) Seagrasses, (III) Invertebrates and (IV) Bony and Cartilaginous Fishes documented from Blue Cavern Onshore SMCA (n = 1091 species total). The abbreviations sp. and spp. indicate that an individual or group of species within a genus were either unidentified or have yet to be described. Taxa organized alphabetically by phylum and class (columns organized top to bottom, left to right). Superscripted letters indicate the following: a Nonindigenous species; b Species range shift; c Species of concern; d Endangered species; e Critically endangered species; f Identification based on morphology; g New taxon added to checklist.
Author Contributions
Conceptualization, D.W.G. and A.H.H.; Data curation, D.W.G. and A.H.H.; Formal analysis, D.W.G. and A.H.H.; Methodology, D.W.G.; Validation, D.W.G. and A.H.H.; Writing—original draft, D.W.G.; Writing—review and editing, D.W.G. and A.H.H. All authors have read and agreed to the published version of the manuscript.
Funding
Funding and resources for this study provided by NSF-OCE (#1559941), USC Sea Grant, USC Dornsife College, the Zinsmeyer Family Endowed Undergraduate Research Fund and the Wrigley Institute for Environmental Studies.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available from authors upon reasonable request.
Acknowledgments
We would like to thank the USC Wrigley Institute for Environmental Studies and Wrigley Marine Science Center staff for their assistance with boat and dive operations for this project. Special thanks to A. Looby for getting this project started and to J. Engle, M. Fourriére, G. Hendler and K.A. Miller for their suggestions regarding the analysis of species inventory data. The authors would also like to acknowledge C. Dreja, J. McCarty, J. Beck, K. Relf, and P. Samwell-Smith for inspiring the title of this article. This study utilized data collected by the Multi-Agency Rocky Intertidal Network (MARINe): a long-term ecological consortium primarily supported by BOEM, NPS, The David & Lucile Packard Foundation and USN, D. Pondella and J. Williams from the Vantuna Research Group at Occidental College and J. Freiwald from Reef Check California. This is contribution no. 258 from the USC Wrigley Marine Science Center on Santa Catalina Island.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Caldow, C.; Monaco, M.E.; Pittman, S.J.; Kendall, M.S.; Goedeke, T.L.; Menza, C.; Kinlan, B.P.; Costa, B.M. Biogeographic assessments: A framework for information synthesis in marine spatial planning. Mar. Policy 2015, 51, 423–432. [Google Scholar] [CrossRef]
- McCann, K.S. The diversity-stability debate. Nature 2000, 405, 228–233. [Google Scholar] [CrossRef]
- Gotelli, N.J.; Colwell, R.K. Quantifying biodiversity: Procedures and pitfalls in the measurement and comparison of species richness. Ecol. Lett. 2001, 4, 379–391. [Google Scholar] [CrossRef]
- Briggs, J.C. Species diversity: Land and sea compared. Syst. Biol. 1994, 43, 130–135. [Google Scholar] [CrossRef]
- Sala, E.; Knowlton, N. Global marine biodiversity trends. Annu. Rev. Environ. Resour. 2006, 31, 93–122. [Google Scholar] [CrossRef]
- Bearham, D.; Strzelecki, J.; Hara, A.; Hosie, A.; Kirkendale, L.; Richards, Z.; Huisman, J.M.; Liu, D.; McLaughlin, J.; Naughton, K.M.; et al. Habitats and benthic biodiversity across a tropical estuarine–marine gradient in the eastern Kimberley region of Australia. Reg. Stud. Mar. 2022, 49, 102039. [Google Scholar] [CrossRef]
- Spalding, M.D.; Fox, H.E.; Allen, G.R.; Davidson, N.; Ferdaña, Z.A.; Finlayson, M.A.X.; Halpern, B.S.; Jorge, M.A.; Lombana, A.L.; Lourie, S.A.; et al. Marine ecoregions of the world, a bioregionalization of coastal and shelf areas. BioScience 2007, 57, 573–583. [Google Scholar] [CrossRef]
- Fautin, D.; Dalton, P.; Incze, L.S.; Leong, J.A.C.; Pautzke, C.; Rosenberg, A.; Sandifer, P.; Sedberry, G.; Tunnell, J.W., Jr.; Abbott, I.; et al. An overview of marine biodiversity in United States waters. PLoS ONE 2010, 5, e11914. [Google Scholar] [CrossRef]
- Murray, S.N.; Bray, R.N. Benthic macrophytes. In Ecology of the Southern California Bight, a Synthesis and Interpretation; Dailey, M.D., Reish, D.J., Anderson, J.W., Eds.; University of California Press: Berkeley, CA, USA, 1993; pp. 304–368. [Google Scholar] [CrossRef]
- Cross, J.N.; Allen, L.G. Fishes. In Ecology of the Southern California Bight, a Synthesis and Interpretation; Dailey, M.D., Reish, D.J., Anderson, J.W., Eds.; University of California Press: Berkeley, CA, USA, 1993; pp. 459–540. [Google Scholar] [CrossRef]
- Dailey, M.D.; Anderson, J.W.; Reish, D.J.; Gorsline, D.S. The Southern California Bight: Background and Setting. In Ecology of the Southern California Bight, a Synthesis and Interpretation; Dailey, M.D., Reish, D.J., Anderson, J.W., Eds.; University of California Press: Berkeley, CA, USA, 1993; pp. 1–18. [Google Scholar] [CrossRef]
- Schiff, K.; Greenstein, D.; Dodder, N.; Gillett, D.J. Southern California bight regional monitoring. Reg. Stud. Mar. Sci. 2016, 4, 34–46. [Google Scholar] [CrossRef][Green Version]
- Zahn, L.A.; Claisse, J.T.; Williams, J.P.; Williams, C.M.; Pondella, D.J. The biogeography and community structure of kelp forest macroinvertebrates. Mar. Ecol. 2016, 37, 770–785. [Google Scholar] [CrossRef]
- Harley, C.D.; Rogers-Bennett, L. The potential synergistic effects of climate change and fishing pressure on exploited invertebrates on rocky intertidal shores. Calif. Coop. Ocean. Fish. Investig. Rep. 2004, 45, 98. [Google Scholar]
- Looby, A.; Ginsburg, D.W. Nearshore species biodiversity of a marine protected area off Santa Catalina Island, California. West. N. Am. Nat. 2021, 81, 113–130. [Google Scholar] [CrossRef]
- Engle, J.M. Distribution patterns of rocky subtidal fishes around the California Islands. In Proceedings of the Third California Islands Symposium; Hochberg, F.G., Ed.; Santa Barbara Museum of Natural History: Santa Barbara, CA, USA, 1993; pp. 475–484. [Google Scholar]
- Luypaert, T.; Hagan, J.G.; McCarthy, M.L.; Poti, M. Status of Marine Biodiversity in the Anthropocene. In YOUMARES 9—The Oceans: Our Research, Our Future; Jungblut, S., Liebich, V., Bode-Dalby, M., Eds.; Springer: Cham, Switzerland, 2020; pp. 57–82. [Google Scholar] [CrossRef]
- Claisse, J.T.; Blanchette, C.A.; Dugan, J.E.; Williams, J.P.; Freiwald, J.; Pondella, D.J.; Schooler, N.K.; Hubbard, D.M.; Davis, K.; Zahn, L.A. Biogeographic patterns of communities across diverse marine ecosystems in southern California. Mar. Ecol. 2018, 39, e12453. [Google Scholar] [CrossRef]
- Pondella, D.J.; Williams, J.; Claisse, J.; Schaffner, B.; Ritter, K.; Schiff, K. The physical characteristics of nearshore rocky reefs in the Southern California Bight. Bull. South. Calif. Acad. Sci. 2015, 114, 105–122. [Google Scholar] [CrossRef]
- Abbott, I.A.; Hollenberg, G.J. Marine Algae of California; Stanford University Press: Stanford, CA, USA, 1992; p. 844. [Google Scholar] [CrossRef]
- Parnell, P.E.; Miller, E.F.; Lennert-Cody, C.E.; Dayton, P.K.; Carter, M.L.; Stebbins, T.D. The response of giant kelp (Macrocystis pyrifera) in southern California to low-frequency climate forcing. Limnol. Oceanogr. 2010, 55, 2686–2702. [Google Scholar] [CrossRef]
- Davis, G.E.; Kushner, D.J.; Mondragon, J.M.; Mondragon, J.E.; Lerma, D.; Richards, D.V. Sampling protocol. In Kelp Forest Monitoring Handbook; Channel Islands National Park: Ventura, CA, USA, 1997; Volume 1, pp. 8–33. [Google Scholar]
- Holt, B.G.; Rioja-Nieto, R.; MacNeil, M.A.; Lupton, J.; Rahbek, C. Comparing diversity data collected using a protocol designed for volunteers with results from a professional alternative. Methods Ecol. Evol. 2013, 4, 383–392. [Google Scholar] [CrossRef]
- Schmitt, E.F.; Sluka, R.D.; Sullivan-Sealey, K.M. Evaluating the use of roving diver and transect surveys to assess the coral reef fish assemblage off southeastern Hispaniola. Coral Reefs. 2002, 21, 216–223. [Google Scholar] [CrossRef]
- Catania, D. CAS Ichthyology (ICH). v150.315. California Academy of Sciences. Available online: http://ipt.calacademy.org:8080/resource?r=ich&v=150.315 (accessed on 30 April 2022).
- Fofonoff, P.W.; Ruiz, G.M.; Steves, B.; Simkanin, C.; Carlton, J.T. National Exotic Marine and Estuarine Species Information System. Available online: http://invasions.si.edu/nemesis (accessed on 4 April 2022).
- marine.ucsc.edu. Long-Term Monitoring Program. Available online: https://marine.ucsc.edu/sitepages/bigfisherman-bio.html (accessed on 30 April 2022).
- National Museum of Natural History, Smithsonian Institution Invertebrate Zoology Collection Database. Available online: https://collections.nmnh.si.edu/search/iz/ (accessed on 30 April 2022).
- Miller, K.A. California Seaweeds eFlora. Available online: http://ucjeps.berkeley.edu/seaweedflora/ (accessed on 30 April 2022).
- Natural History Museum of Los Angeles County Vertebrate Collection. Available online: http://ipt.vertnet.org:8080/ipt/resource.do?r=lacm_verts (accessed on 30 April 2022).
- Reef Check California, Global Reef Tracker. Available online: http://data.reefcheck.org/ (accessed on 30 April 2022).
- Southern California Association of Marine Invertebrate Taxonomists: Taxonomic Listing of Macro- and Megainvertebrates from Infaunal and Epibenthic Programs in the Southern California Bight, 12th Edition. Available online: https://www.scamit.org/publications/SCAMIT%20Ed%2012-2018.pdf (accessed on 30 April 2022).
- Scripps Institution of Oceanography Marine Vertebrate Collection. Available online: http://ipt.vertnet.org:8080/ipt/resource.do?r=sio_marine_vertebrates (accessed on 30 April 2022).
- Pondella, D.J.; Caselle, J.E.; Claisse, J.T.; Williams, J.P.; Davis, K.; Williams, C.M.; Zahn, L.A. South Coast Baseline Program Final Report: Kelp and Shallow Rock Ecosystems. Available online: https://caseagrant.ucsd.edu/sites/default/files/SCMPA-27-Final-Report_0.pdf (accessed on 30 April 2022).
- World Register of Marine Species. Available online: https://www.marinespecies.org (accessed on 30 April 2022).
- Burnham, K.P.; Overton, W.S. Estimation of the size of a closed population when capture probabilities vary among animals. Biometrika 1978, 65, 625–633. [Google Scholar] [CrossRef]
- Chao, A. Estimating the population size for capture-recapture data with unequal catchability. Biometrics 1987, 43, 783–791. [Google Scholar] [CrossRef]
- Colwell, R.K.; Coddington, J.A. Estimating terrestrial biodiversity through extrapolation. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1994, 345, 101–118. [Google Scholar] [CrossRef]
- Chao, A.; Chiu, C.-H. Nonparametric Estimation and Comparison of Species Richness; John Wiley and Sons, Ltd.: Hoboken, NJ, USA, 2016; pp. 1–11. [Google Scholar] [CrossRef]
- Foggo, A.; Attrill, M.J.; Frost, M.T.; Rowden, A.A. Estimating marine species richness: An evaluation of six extrapolative techniques. Mar. Ecol. Prog. Ser. 2003, 248, 15–26. [Google Scholar] [CrossRef]
- Drew, J.A.; Buxman, C.L.; Holmes, D.D.; Mandecki, J.L.; Mungkaje, A.J.; Richardson, A.C.; Westneat, M.W. Biodiversity inventories and conservation of the marine fishes of Bootless Bay, Papua New Guinea. BMC Ecol. 2012, 12, 15. [Google Scholar] [CrossRef] [PubMed]
- Fourriére, M.; Reyes-Bonilla, H.; Rodríguez-Zaragoza, F.A.; Nicole, N. Fishes of Clipperton Atoll, Eastern Pacific: Checklist, endemism, analysis of completeness of the inventory. Pac. Sci. 2014, 68, 375–395. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing, Vienna, Austria. Available online: https://www.R-project.org/ (accessed on 10 February 2022).
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. R Package Version 2.5-6. Available online: https://CRAN.R-project.org/package=vegan (accessed on 10 February 2022).
- Thomsen, P.F.; Kielgast, J.; Iversen, L.L.; Møller, P.R.; Rasmussen, M.; Willerslev, E. Detection of a Diverse Marine Fish Fauna Using Environmental DNA from Seawater Samples. PLoS ONE 2012, 7, e41732. [Google Scholar] [CrossRef] [PubMed]
- Bohmann, K.; Evans, A.; Gilbert, M.T.P.; Carvalho, G.R.; Creer, S.; Knapp, M.; Yu, D.W.; Bruyn, M. Environmental DNA for wildlife biology and biodiversity monitoring. Trends Ecol. Evol. 2014, 29, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Ely, T.; Barber, P.H.; Man, L.; Gold, Z. Short-lived detection of an introduced vertebrate eDNA signal in a nearshore rocky reef environment. PLoS ONE 2021, 16, e0245314. [Google Scholar] [CrossRef]
- Costello, M.J.; Ballantine, B. Biodiversity conservation should focus on no-take marine reserves: 94% of marine protected areas allow fishing. Trends Ecol. Evol. 2015, 30, 507–509. [Google Scholar] [CrossRef]
- Engle, J.M. Provisional Checklist of the Marine Species of Santa Catalina Island; Unpublished Report; University of Southern California, Wrigley Marine Center: Santa Catalina Island, CA, USA, 1978; p. 259. [Google Scholar]
- Hammond, P. Species inventory. In Global Biodiversity: Status of the Earth’s Living Resources; Groombridge, B., Ed.; Chapman and Hall: London, UK, 1992; pp. 17–39. [Google Scholar] [CrossRef]
- Engle, J.M.; Miller, K.A. Distribution and Morphology of Eelgrass (Zostera marina L.) at the California Channel Islands; Garcelon, D.K., Schween, C.A., Eds.; Institute for Wildlife Studies: Arcata, CA, USA, 2005; pp. 405–414. [Google Scholar]
- Barbier, E.B.; Hacker, S.D.; Kennedy, C.; Koch, E.W.; Stier, A.C.; Silliman, B.R. The value of estuarine and coastal ecosystem services. Ecol. Monogr. 2011, 81, 169–193. [Google Scholar] [CrossRef]
- Marks, L.; Reed, D.; Obaza, A. Assessment of control methods for the invasive seaweed Sargassum horneri in California, USA. Manag. Biol. Invasions 2017, 8, 205–213. [Google Scholar] [CrossRef]
- Ginther, S.C.; Steele, M.A. Limited recruitment of an ecologically and economically important fish Paralabrax clathratus to an invasive alga. Mar. Ecol. Prog. Ser. 2018, 602, 213–224. [Google Scholar] [CrossRef]
- Williams, S.L.; Smith, J.E. A global review of the distribution, taxonomy, and impacts of introduced seaweeds. Annu. Rev. Ecol. Evol. Syst. 2007, 38, 327–359. [Google Scholar] [CrossRef]
- Goddard, J.H.R.; Schaefer, M.C.; Hoover, C.; Valdés, Á. Regional extinction of a conspicuous dorid nudibranch (Mollusca: Gastropoda) in California. Mar. Biol. 2013, 160, 1497–1510. [Google Scholar] [CrossRef]
- Dulvy, N.K.; Sadovy, Y.; Reynolds, J.D. Extinction vulnerability in marine populations. Fish Fish. 2003, 4, 25–64. [Google Scholar] [CrossRef]
- Harnik, P.G.; Lotze, H.K.; Anderson, S.C.; Finkel, Z.V.; Finnegan, S.; Lindberg, D.R.; Liow, L.H.; Lockwood, R.; McClain, C.R.; McGuire, J.L.; et al. Extinctions in ancient and modern seas. Trends Ecol. Evol. 2012, 27, 608–617. [Google Scholar] [CrossRef] [PubMed]
- McCauley, D.J.; Pinsky, M.L.; Palumbi, S.R.; Estes, J.A.; Joyce, F.H.; Warner, R.R. Marine defaunation: Animal loss in the global ocean. Science 2015, 347, 1255641. [Google Scholar] [CrossRef]
- Johnson, C.N.; Balmford, A.; Brook, B.W.; Buettel, J.C.; Galetti, M.; Guangchun, L.; Wilmshurst, J.M. Biodiversity losses and conservation responses in the Anthropocene. Science 2017, 356, 270–275. [Google Scholar] [CrossRef]
- Love, M.S.; Passarelli, J.K.; Cantrell, B.; Hastings, P.A. The largemouth blenny, Labrisomus xanti, new to the California marine fauna with a list of and key to the species of Labrisomidae, Clinidae, Chaenopsidae found in California waters. Bull. South. Calif. Acad. Sci. 2016, 115, 191–197. [Google Scholar] [CrossRef]
- Lea, R.N.; Rosenblatt, R.H. Observations on fishes associated with the 1997-98 El Niño off California. Calif. Coop. Ocean. Fish. Investig. Rep. 2000, 41, 117–129. [Google Scholar]
- Lea, R.N.; Fraser, T.H.; Baldwin, C.C.; Craig, M.T. Five Valid Species of Cardinalfishes of the Genus Apogon (Apogonidae) in the Eastern Pacific Ocean, with a Redescription of A. atricaudus and Notes on the Distribution of A. atricaudus and A. atradorsatus. Ichthyol. Herpetol. 2022, 110, 106–114. [Google Scholar] [CrossRef]
- Hobday, A.J.; Alexander, L.V.; Perkins, S.E.; Smale, D.A.; Straub, S.C.; Oliver, E.C.; Benthuysen, J.A.; Burrows, M.T.; Donat, M.G.; Feng, M.; et al. A hierarchical approach to defining marine heatwaves. Prog. Oceanogr. 2016, 141, 227–238. [Google Scholar] [CrossRef]
- Sanford, E.; Sones, J.L.; García-Reyes, M.; Goddard, J.H.; Largier, J.L. Widespread shifts in the coastal biota of northern California during the 2014–2016 marine heatwaves. Sci. Rep. 2019, 9, 4216. [Google Scholar] [CrossRef] [PubMed]
- Rosales-Casián, J.A. Finescale triggerfish (Balistes polylepis) and roosterfish (Nematistius pectoralis) presence in temperate waters off Baja California, México, evidence of El Niño conditions. Calif. Cooperative Ocean. Fish. Investig. Rep. 2013, 54, 81–84. [Google Scholar]
- Walker, H.J.; Hastings, P.A.; Hyde, J.R.; Lea, R.N.; Snodgrass, O.E.; Bellquist, L.F. Unusual occurrences of fishes in the southern California current system during the warm water period of 2014–2018. Estuar. Coast. Shelf Sci. 2020, 236, 106634. [Google Scholar] [CrossRef]
- Obaza, A.K.; Williams, J.P. Spatial and temporal dynamics of the overwater structure fouling community in southern California. Mar. Freshw. Res. 2018, 69, 1771–1783. [Google Scholar] [CrossRef]
- Susick, K.; Scianni, C.; Mackie, J.A. Artificial structure density predicts fouling community diversity on settlement panels. Biol. Invasions. 2019, 22, 271–292. [Google Scholar] [CrossRef]
- Molnar, J.L.; Gamboa, R.L.; Revenga, C.; Spalding, M.D. Assessing the global threat of invasive species to marine biodiversity. Front. Ecol. Environ. 2008, 6, 485–492. [Google Scholar] [CrossRef]
- Mackie, J.A.; Darling, J.A.; Geller, J.B. Ecology of cryptic invasions: Latitudinal segregation among Watersipora (Bryozoa) species. Sci. Rep. 2012, 2, 871. [Google Scholar] [CrossRef] [PubMed]
- Hewson, I.; Button, J.B.; Gudenkauf, B.M.; Miner, B.; Newton, A.L.; Gaydos, J.K.; Wynne, J.; Groves, C.L.; Hendler, G.; Murray, S.; et al. Densovirus associated with sea-star wasting disease and mass mortality. Proc. Natl. Acad. Sci. USA 2014, 111, 17278–17283. [Google Scholar] [CrossRef]
- Eckert, G.L.; Engle, J.M.; Kushner, D.J. Sea star disease and population declines at the Channel Islands. In Proceedings of the Fifth California Islands Symposium; Browne, D.R., Mitchell, K.L., Chaney, H.W., Eds.; U.S. Department of the Interior, Minerals Management Service: Camarillo, CA, USA, 2000; pp. 390–393. [Google Scholar]
- Harvell, C.D.; Montecino-Latorre, D.; Caldwell, J.M.; Burt, J.M.; Bosley, K.; Keller, A.; Heron, S.F.; Salomon, A.K.; Lee, L.; Pontier, O.; et al. Disease epidemic and a marine heat wave are associated with the continental-scale collapse of a pivotal predator (Pycnopodia helianthoides). Sci. Adv. 2019, 5, eaau7042. [Google Scholar] [CrossRef]
- Given, R.R.; Robertson, D. Biological Studies on the Rock Mole-Pier Complex; Unpublished Report; University of Southern California, Wrigley Marine Center: Santa Catalina Island, CA, USA, 1981; p. 134. [Google Scholar]
- Nickols, K.J.; White, J.W.; Malone, D.; Carr, M.H.; Starr, R.M.; Baskett, M.L.; Hastings, A.; Botsford, L.W. Setting ecological expectations for adaptive management of marine protected areas. J. Appl. Ecol. 2019, 56, 2376–2385. [Google Scholar] [CrossRef]
- Karpov, K.A.; Haaker, P.L.; Taniguchi, I.K.; Rogers-Bennett, L. Serial depletion and the collapse of the California abalone fishery (Haliotis spp.) fishery. Can. Spec. Publ. Fish. Aquat. Sci. 2000, 130, 11–24. [Google Scholar]
- Harvell, C.D.; Lamb, J.B. Disease outbreaks can threaten marine biodiversity. In Marine Disease Ecology; Oxford University Press: Oxford, UK, 2020; pp. 141–158. [Google Scholar] [CrossRef]
- Allen, L.G. GIANTS! Or… The Return of the Kelp Forest King. Copeia 2017, 105, 10–13. [Google Scholar] [CrossRef]
- House, P.H.; Clark, B.L.; Allen, L.G. The return of the king of the kelp forest: Distribution, abundance, biomass of giant sea bass (Stereolepis gigas) off Santa Catalina Island, California, 2014–2015. Bull. South. Calif. Acad. Sci. 2016, 115, 1–14. [Google Scholar] [CrossRef]
- Ramírez-Valdez, A.; Rowell, T.J.; Dale, K.E.; Craig, M.T.; Allen, L.G.; Villaseñor-Derbez, J.C.; Cisneros-Montemayor, A.M.; Hernández-Velasco, A.; Torre, J.; Hofmeister, J.; et al. Asymmetry across international borders: Research, fishery and management trends and economic value of the giant sea bass (Stereolepis gigas). Fish Fish. 2021, 22, 1392–1411. [Google Scholar] [CrossRef]
- Hawk, H.A.; Allen, L.G. Age and growth of the giant sea bass, Stereolepis gigas. Coop. Ocean. Fish. Investig. Rep. 2014, 55, 128–134. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).