Effects of Bamboo Forest Type and Density on the Growth of Bletilla striata and Root Endophytic Fungi
Abstract
:1. Introduction
2. Materials and Methods
2.1. Overview of the Study Area
2.2. The Sample Set
2.3. Sample Collection and Processing
2.4. Environmental Factor Index Measurement
2.5. High-Throughput Sequencing
2.6. Data Processing and Analysis
3. Results
3.1. Effects of Bamboo Forest Type and Density on the Growth of B. striata
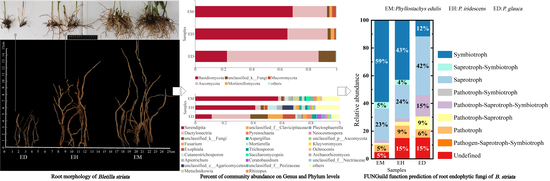
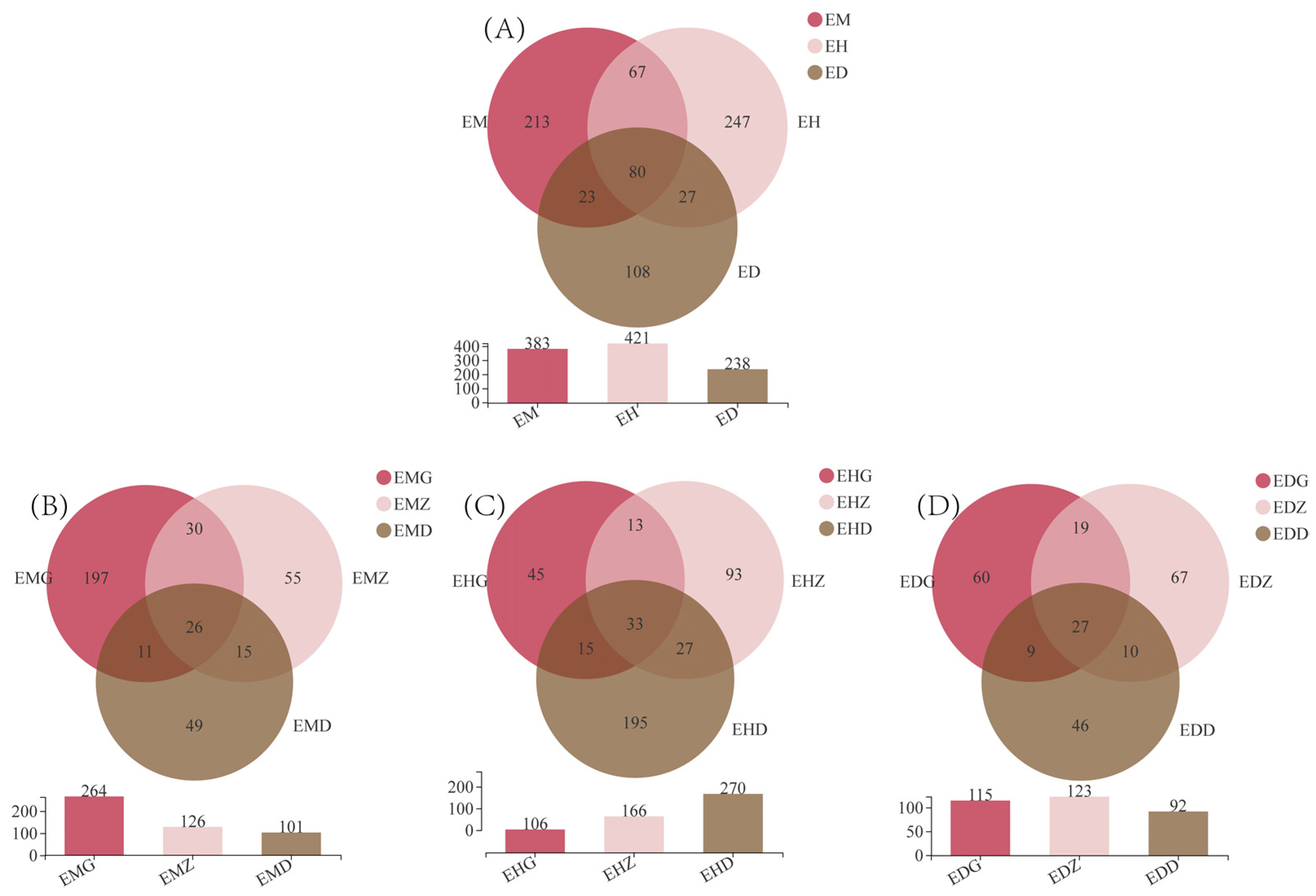
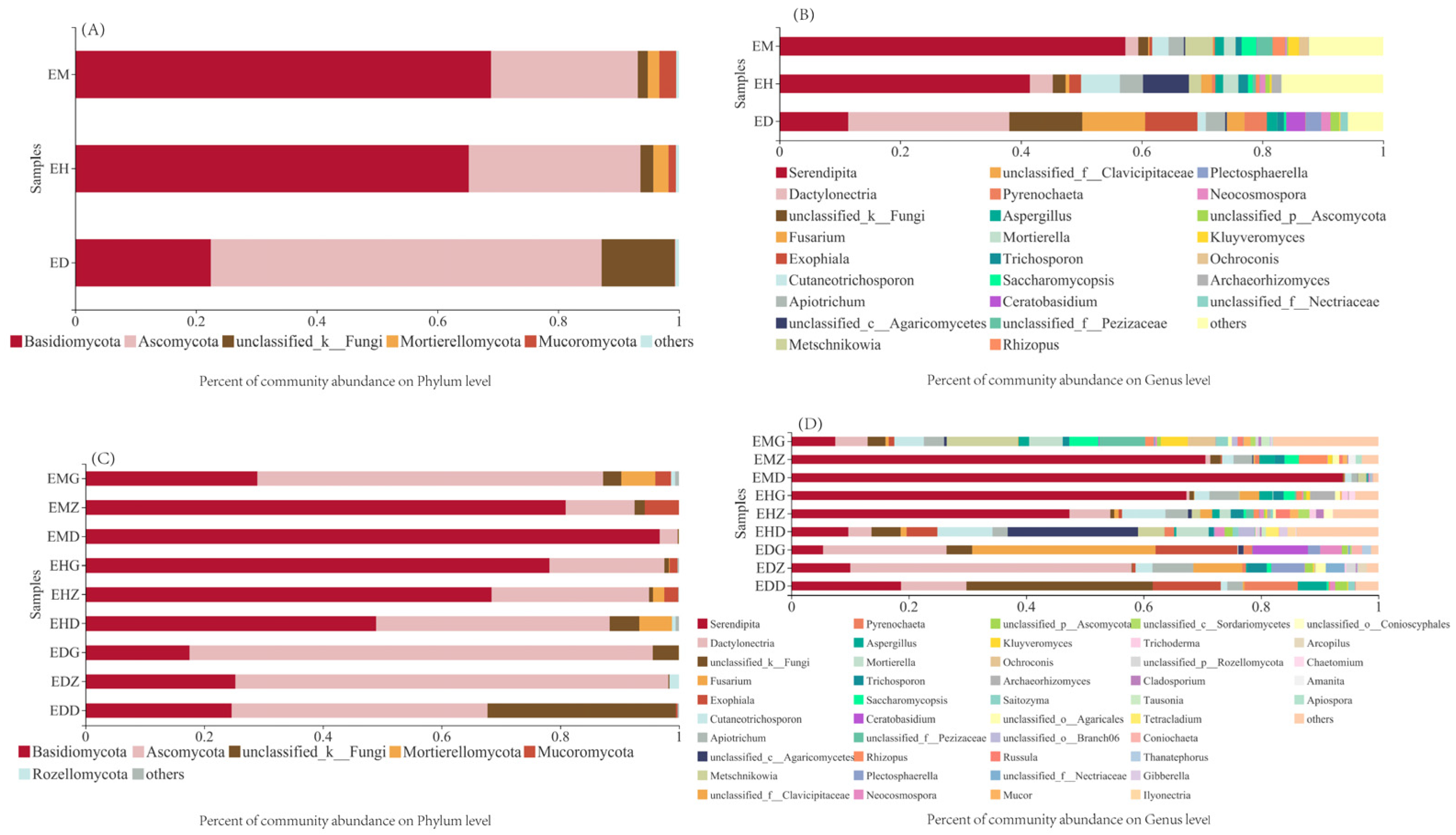
3.2. Composition of Endophytic Fungal OTUs in B. striata under Different Bamboo Forest Types and Densities
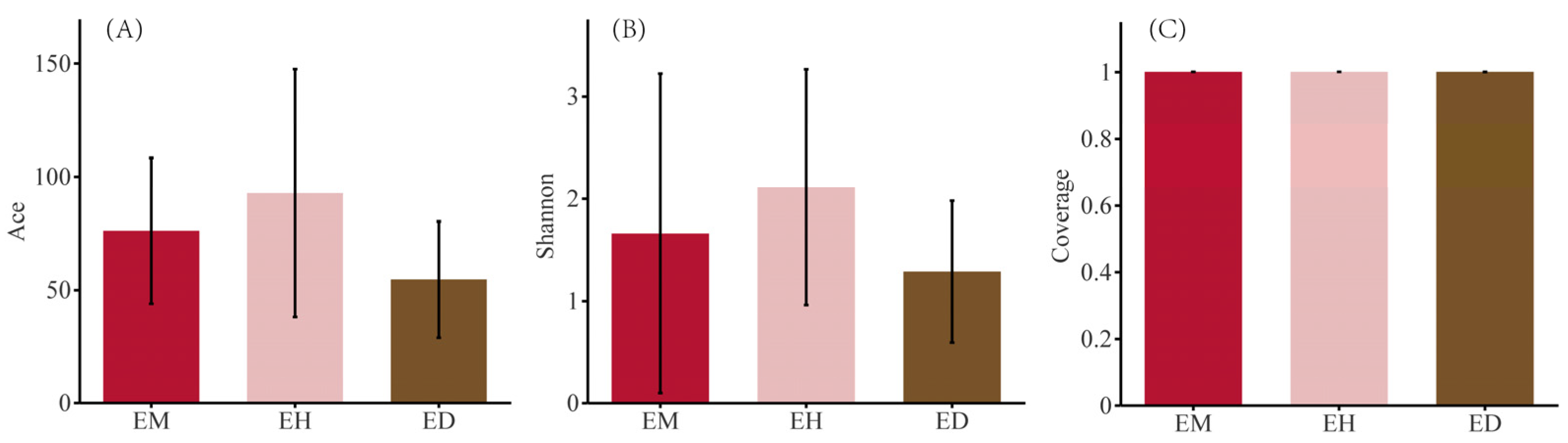
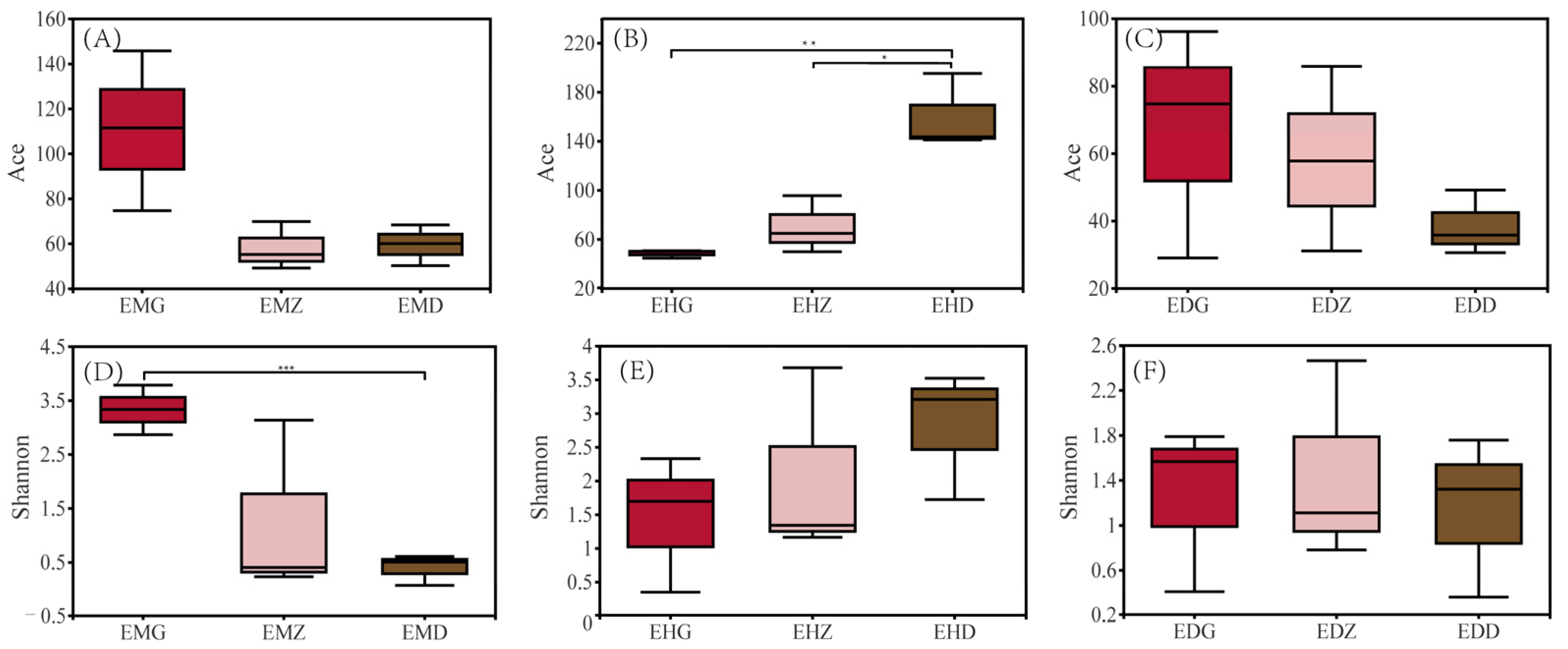
3.3. Diversity Analysis of the Endophytic Fungal Community in B. striata
3.4. Analysis of the Community Composition of Endophytic Fungi in B. striata Roots
3.5. LefSe Difference Analysis of Endophytic Fungi Community in the B. striata Roots
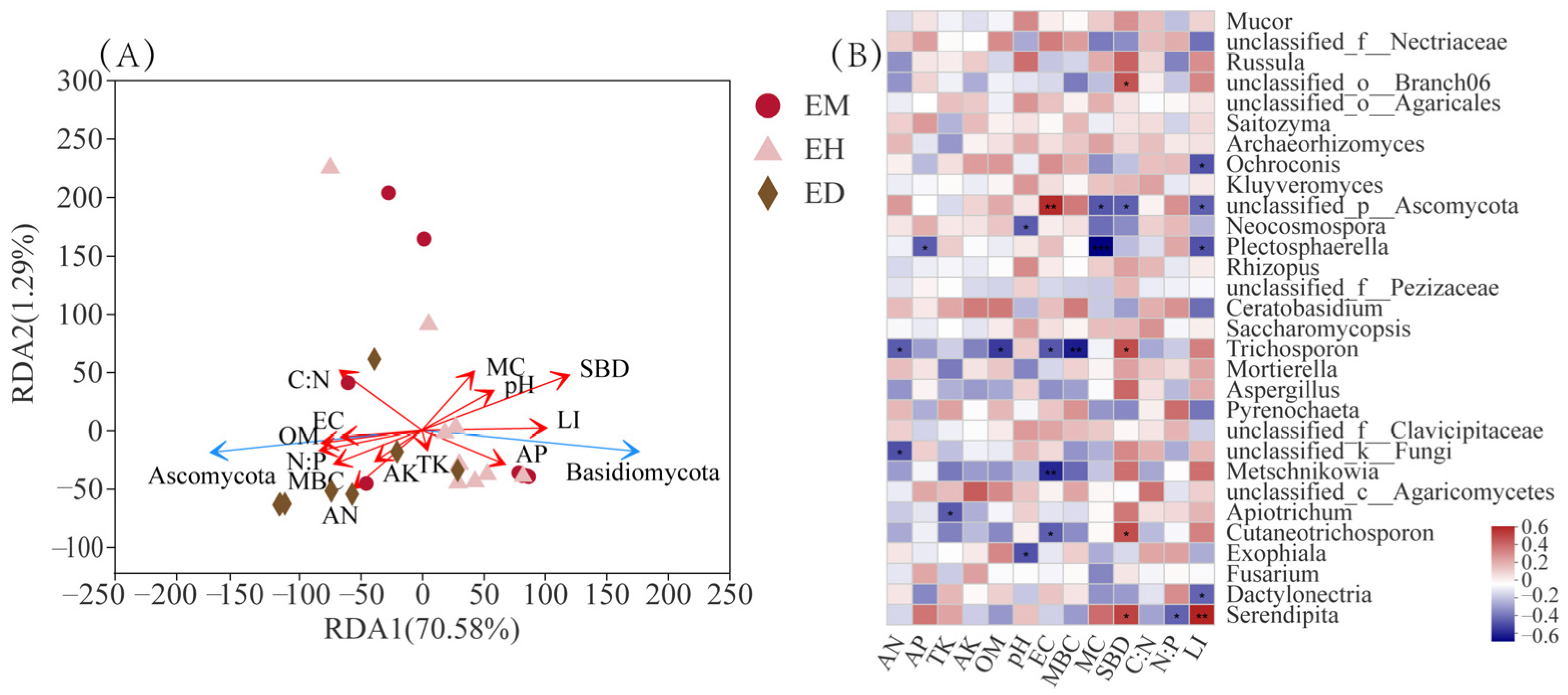
3.6. Correlation Analysis of Environmental Factors and the Endophytic Fungi Community in B. striata Roots
3.7. FUNGuild Function Prediction Analysis of the Endophytic Fungi in B. striata Roots
4. Discussion
4.1. Effects of Different Bamboo Forest Types and Densities on the Growth of B. striata
4.2. Effects of Different Bamboo Forest Types and Densities on the Endophytic Fungal Community in B. striata Roots
4.3. Potential Effects of Serendipita on the Growth of B. striata
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fan, W.; Meng, P.; Zhang, J.S. Chinese Agroforestry Research; China Forestry Press: Beijing, China, 2003. [Google Scholar]
- Mortimer, R.; Saj, S.; David, C. Supporting and regulating ecosystem services in cacao agroforestry systems. Agroforest. Syst. 2018, 92, 1639–1657. [Google Scholar] [CrossRef]
- Wolz, K.J.; Branham, B.E.; de Lucia, E.H. Reduced nitrogen losses after conversion of row crop agriculture to alley cropping with mixed fruit and nut trees. Agr. Ecosyst. Environ. 2018, 258, 172–181. [Google Scholar] [CrossRef]
- Li, W.H.; Lai, S.D. Agroforestry in China; Science Press: Beijing, China, 1994. [Google Scholar]
- Fan, L.J.; Guo, X.Y.; Ma, N.X. Comparative study on phylogenetics and sequences composition of bamboos and cereals. Forest Res. 2006, 2, 165–169. [Google Scholar]
- Yang, C.B. Characteristics Stabilization Mechanisms Soil Organic Carbon Moso Bamboo Plantations; Chinese Academy of Forestry: Beijing, China, 2019. [Google Scholar]
- Peng, L.Y.; Peng, C.; Ai, W.S.; Yang, M.; Meng, Y.; Tu, J.; Li, M.Q. Research advance and forecast on the tridimensional compound management of bamboo forest. Hunan Forest. Sci. Technol. 2019, 46, 72–76. [Google Scholar]
- Lobovikov, M.; Schoene, D.; Lou, Y. Bamboo in climate change and rural livelihoods. Mitig. Adapt. Strat. Gl. 2012, 17, 261–276. [Google Scholar] [CrossRef]
- Bhardwaj, G.D.R.; Kaushal, R.; Devi, M. Performance of ginger crop in response to integrated nutrient management under bamboo-based agroforestry system in mid-hill sub-humid conditions of Himachal Pradesh. Int. J. Farm Sci. 2016, 6, 24–29. [Google Scholar]
- Fan, Y.R.; Chen, S.L.; Yang, Q.P.; Li, Y.C.; Guo, Z.W.; Chen, S. Population growth and biomass allocation of Polygonatum cyrtonema within a Phyllostachys edulis forest utilizing bamboo density treatments. J. Zhejiang Agrofor. Univ. 2013, 30, 199–205. [Google Scholar]
- Cai, C.J.; Fan, S.H.; Liu, G.L.; Wang, S.M.; Feng, Y. Research and development advance of compound management of bamboo forests. World Bamboo Ratt. 2018, 16, 47–52. [Google Scholar]
- Chen, S.L.; Chen, C.Y.; Yang, Q.P.; Wang, W.H. A study on the optimized mode of forest structure of Dendrocalamus latiflorus for Bamboo shoot. Acta Agri. Univ. Jiangxiensis 2005, 17, 191–194. [Google Scholar]
- Zhou, X.H.; Xiao, Z.Y.; Zeng, P.S.; Yao, J.B.; Wu, X.Y.; Huang, Y.T. Effects of different forest habitats and growth years on the growth of Polygonatum cyrtonema and the content of medicinal active components. J. Southwest Forest. Univ. 2019, 39, 155–160. [Google Scholar]
- Huang, Y.P.; Wang, B.F.; Fan, F.R.; Shen, Q.T.; Chen, S.H.; Huang, S.M.; Su, S.J. Effect of forest types and canopy density on polysaccharide content of Polygonatum cyrtonema. Chin. Agr. Sci. Bull. 2016, 32, 102–105. [Google Scholar]
- Feng, J.Y. Effects of Bamboo Density and Drought Stress on Photosynthetic Characteristics of Five Medicinal Plants; Zhejiang A&F University: Hangzhou, China, 2021. [Google Scholar]
- Flora of China Editorial Committee. Flora of China; Science Press: Beijing, China, 1999. [Google Scholar]
- Qian, C.D.; Jiang, F.S.; Yu, H.S.; Fu, Y.H.; Cheng, D.Q.; Gan, L.S.; Ding, Z.S. Antibacterial biphenanthrenes from the fibrous roots of Bletilla striata. J. Nat. Prod. 2015, 78, 939–943. [Google Scholar] [CrossRef] [PubMed]
- Herrera, H.; Valadares, R.; Oliveira, G.; Fuentes, A.; Almonacis, L.; Bashan, Y.; Arriagada, C. Adaptation and tolerance mechanisms developed by mycorrhizal Bipinnula fimbriata plantlets (Orchidaceae) in a heavy metal-polluted ecosystem. Mycorrhiza 2018, 28, 651–663. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis, 3rd ed.; Academic Press: New York, NY, USA, 2008. [Google Scholar]
- Schiebold, J.M.I.; Bidartondo, M.I.; Karasch, P.; Gravendeel, B.; Gebauer, G. You are what you get from your fungi: Nitrogen stable isotope patterns in Epipactis species. Ann. Bot. 2017, 119, 1085–1095. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.S.; Chen, J.; Li, S.C.; Zeng, X.; Meng, Z.X.; Guo, S.X. Comparative transcriptome analysis of genes involved in GA-GID1-DELLA regulatory module in symbiotic and asymbiotic seed germination of Anoectochilus roxburghii (Wall.) Lindl. (Orchidaceae). Int. J. Mol. Sci. 2015, 16, 30190–30203. [Google Scholar] [CrossRef] [Green Version]
- Gonneau, C.; Jersakova, J.; de Tredern, E.; Till-Bottraud, I.; Saarinen, K.; Sauve, M.; Roy, M.; Hajek, T.; Selosse, M.A. Photosynthesis in perennial mixotrophic Epipactis spp. (Orchidaceae) contributes more to shoot and fruit biomass than to hypogeous survival. J. Ecol. 2014, 102, 1183–1194. [Google Scholar] [CrossRef]
- Liebel, H.T.; Bidartondo, M.I.; Gebauer, G. Are carbon and nitrogen exchange between fungi and the orchid Goodyera repens affected by irradiance? Ann. Bot. 2015, 115, 251–261. [Google Scholar] [CrossRef] [Green Version]
- Burke, R.M.; Cairney, J.W.G. Carbohydrate oxidases in ericoid and ectomycorrhizal fungi: A possible source of Fenton radicals during the degradation of lignocelluloses. New Photol. 1998, 139, 637–645. [Google Scholar] [CrossRef]
- Slezack, S.; Dumas-Gaudot, E.; Rosendahl, S.; Kjoller, R. Endoproteolytic activities in pea roots inoculated with the arbuscular mycorrhizal fungus Glomus mosseae and/or Aphanomyces euteiches in relation to bioprotection. New Photol. 1999, 142, 517–529. [Google Scholar] [CrossRef]
- McCormick, M.K.; Taylor, D.L.; Juhaszova, K.; Burnett, J.R.; Whigham, D.F.; Oneill, J.P. Limitations on orchid recruitment: Not a simple picture. Mol. Ecol. 2012, 21, 1511–1523. [Google Scholar] [CrossRef]
- Zeng, X.H.; Diao, H.X.; Ni, Z.Y.; Shao, L.; Jiang, K.; Hu, C.; Huang, Q.J.; Huang, W.C. Temporal variation in community composition of root associated endophytic fungi and carbon and nitrogen stable isotope abundance in two Bletilla species (Orchidaceae). Plants 2021, 10, 18. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.H.; Qi, Y.Q.; Ren, Q.F.; Liu, F.; Ou, M.Z.; Chen, Y.F. Effects of nitrogen on the growth and polysaccharides accumulation of Bletilla striata. Plant Physiol. J. 2022, 58, 331–338. [Google Scholar]
- Tao, J.X.; Lv, H.B.; Zhou, W.H.; Luo, Z.Y. Exploration of natural Rubber+ Bletilla striata (Thunb.) Reichb. f. planting mode. Mod. Agric. Sci. Technol. 2018, 17, 138–144. [Google Scholar]
- Huang, X.J.; Zhang, W.B.; Lai, G.W.; Zhang, F.G.; Jiang, Z.H.; Qiu, W.Q. Effects of different shading degrees on the growth of Bletilla striata and rhizoma huangjing under compound management. Mod. Agric. Sci. Technol. 2019, 4, 49–51. [Google Scholar]
- Liu, X.Q. The Botanical Garden in Shanghai Mountain Bamboo Landscaping Art of W Transform the Design Application; Shanghai Normal University: Shanghai, China, 2016. [Google Scholar]
- Lu, R.K. Methods of Soil Agrochemical Analysis; China Agricultural Science and Technology Press: Beijing, China, 2000; pp. 12–264. [Google Scholar]
- Xu, W.D. Effects of Light, Planting Density and Harvesting Time on Yield and Quality of Lettuce in Plant Factory; Nanjing Agricultural University: Nanjing, China, 2015. [Google Scholar]
- Wang, X.W.; Wang, Y.G.; Fu, Q.S.; Zhao, B.; Guo, Y.D. Effects of low light stress on morphological trait, physiological characters and leaf ultrastructure of tomato (Lycopersicon esculentum) seedlings. North China Agri. J. 2009, 24, 144–149. [Google Scholar]
- Bai, H.N.; Niu, X.; Wang, B.; Song, Q.F.; Tao, Y.Z. Effects of expansion of Phyllostachys edulis on community diversity of endophytic fungi in leaves of Rhododendron latoucheae. Chin. J. Ecol. 2021, 12, 1–13. [Google Scholar]
- Wang, W.S. Seedling and understory cultivation techniques of Bletilla striata. Anhui Forest. Sci. Technol. 2018, 44, 61–63. [Google Scholar]
- Zhou, Y.R. Effects of Different Light Intensities on Photosynthetic and Physiological Characteristics of Bletilla striata; Zhengzhou University: Zhengzhou, China, 2020. [Google Scholar]
- Tuominen, A. Defensive strategies in Geranium sylvaticum, Part 2: Roles of water-soluble tannins, flavonoids and phenolic acids against natural enemies. Phytochemistry 2013, 95, 408–420. [Google Scholar] [CrossRef]
- Xi, G.J.; Li, J.B.; Shi, J.; Han, Z.M. Diversity of endophytic fungi in Bletilla striata. Acta Agric. Zhejiangensis 2017, 29, 2077–2083. [Google Scholar]
- Den, W.X.; Zhao, M.L.; Wang, Z.L.; Li, Y.M. Analysis of species composition of endophytic fungi in roots of Bletilla striata based on high throughput sequencing. J. Southwest Forest. Univ. 2021, 41, 76–84. [Google Scholar]
- Reiter, N.; Phillips, R.D.; Swarts, N.D.; Wright, M.; Holmes, G.; Sussmilch, F.C.; Davis, B.J.; Whitehead, M.R.; Linde, C.C. Specific mycorrhizal associations involving the same fungal taxa in common and threatened Caladenia (Orchidaceae): Implications for conservation. Ann. Bot. 2020, 5, 1–51. [Google Scholar] [CrossRef] [PubMed]
- Oktalira, F.T.; May, T.W.; Dearnaley, J.D.W.; Linde, C.C. Seven new Serendipita species associated with Australian terrestrial orchids. Mycologia 2021, 113, 968–987. [Google Scholar] [CrossRef] [PubMed]
- Bokati, D.; Craven, K.D. The cryptic Sebacinales: An obscure but ubiquitous group of root symbionts comes to light. Fungal Ecol. 2016, 22, 115–119. [Google Scholar] [CrossRef]
- Qin, Y.; Druzhinina, I.S.; Pan, X.Y.; Yuan, Z.L. Microbially mediated plant salt tolerance and microbiome-based solutions for saline agriculture. Biotechnol. Adv. 2016, 34, 1245–1259. [Google Scholar] [CrossRef] [PubMed]
- Weiß, M.; Waller, F.; Zuccaro, A.; Selosse, M.A. Sebacinales-one thousand and one interactions with land plants. New Phytol. 2016, 211, 20–40. [Google Scholar] [CrossRef]
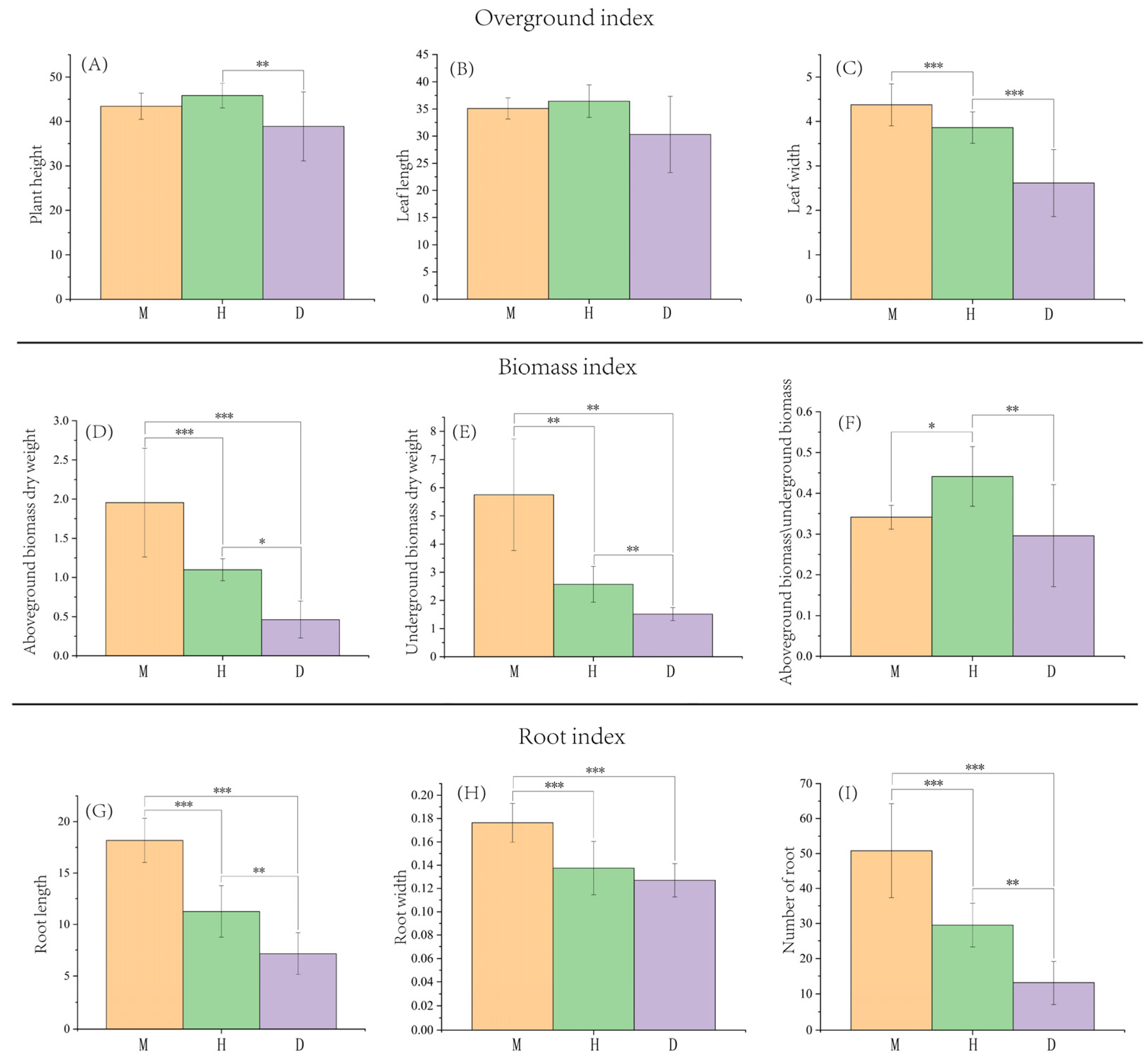


| Plant Height (cm) | Leaf Length (cm) | Leaf Width (cm) | Root Length (cm) | Root Width (cm) | Number of Roots (num.) | Aboveground Biomass (g) | Belowground Biomass (g) | Aboveground/Belowground Biomass | ||
|---|---|---|---|---|---|---|---|---|---|---|
| P. edulis | high | 44.24 ± 4.97 a | 35.51 ± 0.78 a | 4.01 ± 0.22 b | 17.60 ± 2.38 a | 0.16 ± 0.00 b | 41.3 ± 4.49 a | 1.32 ± 0.18 b | 3.6 ± 0.28 b | 0.36 ± 0.02 a |
| medium | 42.38 ± 0.08 a | 34.39 ± 1.19 a | 4.28 ± 0.48 a b | 18.44 ± 2.15 a | 0.17 ± 0.01 a b | 48.83 ± 14.37 a | 1.92 ± 0.57 a b | 5.9 ± 1.17 a | 0.32 ± 0.03 a | |
| low | 43.64 ± 2.70 a | 35.39 ± 3.49 a | 4.84 ± 0.28 a | 18.50 ± 2.70 a | 0.19 ± 0.02 a | 62.27 ± 12.56 a | 2.63 ± 0.52 a | 7.75 ± 1.14 a | 0.34 ± 0.02 a | |
| P. iridescens | high | 45.45 ± 2.93 a | 36.12 ± 3.29 a b | 3.97 ± 0.44 a | 10.14 ± 2.14 a | 0.14 ± 0.03 a | 22.77 ± 1.00 b | 0.97 ± 0.05 a | 1.89 ± 0.200 b | 0.52 ± 0.07 a |
| medium | 43.89 ± 1.93 a | 34.14 ± 1.82 b | 3.92 ± 0.48 a | 10.01 ± 2.80 a | 0.14 ± 0.02 a | 33.97 ± 6.69 a | 1.18 ± 0.19 a | 2.77 ± 0.62 a | 0.43 ± 0.03 a b | |
| low | 48.18 ± 2.10 a | 39.03 ± 1.88 a | 3.69 ± 0.16 a | 13.62 ± 0.38 a | 0.12 ± 0.02 a | 31.73 ± 2.29 a | 1.15 ± 0.05 a | 3.06 ± 0.26 a | 0.38 ± 0.02 b | |
| P. glauca | high | 40.53 ± 3.17 a | 31.39 ± 2.76 a | 2.64 ± 0.38 a | 7.08 ± 2.20 a | 0.12 ± 0.02 a | 13.05 ± 3.27 a | 0.5 ± 0.17 a | 1.65 ± 0.13 a | 0.30 ± 0.08 a |
| medium | 33.68 ± 6.35 a | 25.67 ± 5.82 a | 2.17 ± 0.60 a | 6.61 ± 1.79 a | 0.12 ± 0.01 a | 9.72 ± 3.28 a | 0.31 ± 0.20 a | 1.29 ± 0.27 b | 0.24 ± 0.12 a | |
| low | 42.44 ± 11.30 a | 33.85 ± 10.17 a | 3.03 ± 1.10 a | 7.83 ± 2.65 a | 0.13 ± 0.02 a | 16.55 ± 9.58 a | 0.57 ± 0.31 a | 1.61 ± 0.05 a | 0.35 ± 0.18 a |
| AN | AP | TK | AK | OM | Ph | EC | MBC | MC | SBD | C:N | N:P | LI | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Endophytic fungi | p | 0.13 | 0.18 | 0.96 | 0.48 | 0.07 | 0.17 | 0.19 | 0.08 | 0.25 | 0.00 | 0.12 | 0.05 | 0.02 |
| R2 | 0.17 | 0.14 | 0.00 | 0.06 | 0.22 | 0.14 | 0.14 | 0.19 | 0.12 | 0.57 | 0.17 | 0.24 | 0.33 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, H.; Song, Z.; Li, S.; Lan, S.; Zeng, X.; Huang, W. Effects of Bamboo Forest Type and Density on the Growth of Bletilla striata and Root Endophytic Fungi. Diversity 2022, 14, 391. https://doi.org/10.3390/d14050391
Fu H, Song Z, Li S, Lan S, Zeng X, Huang W. Effects of Bamboo Forest Type and Density on the Growth of Bletilla striata and Root Endophytic Fungi. Diversity. 2022; 14(5):391. https://doi.org/10.3390/d14050391
Chicago/Turabian StyleFu, Hao, Zhilin Song, Shanmin Li, Siren Lan, Xinhua Zeng, and Weichang Huang. 2022. "Effects of Bamboo Forest Type and Density on the Growth of Bletilla striata and Root Endophytic Fungi" Diversity 14, no. 5: 391. https://doi.org/10.3390/d14050391
APA StyleFu, H., Song, Z., Li, S., Lan, S., Zeng, X., & Huang, W. (2022). Effects of Bamboo Forest Type and Density on the Growth of Bletilla striata and Root Endophytic Fungi. Diversity, 14(5), 391. https://doi.org/10.3390/d14050391