Abstract
Soil microorganisms that inhabit extreme environments have unique metabolic capacities and/or physical structures that allow them to survive in oligotrophic conditions. The bioprospecting of unknown bacteria in the context of current advances in genome mining is fundamental for the discovery of natural products with novel properties or applications. In this study, the plant growth-promoting and biocontrol traits of a Pseudomonas isolated from soil associated with plants from the Atacama Desert were characterized by whole-genome sequencing and in vitro assays. A high-quality genome draft of Pseudomonas sp. isolate C3 was obtained. An automated biosynthetic gene cluster analysis using antiSMASH 6.0 revealed the presence of a cluster of genes for the biosynthesis, regulation, and transport of the metabolite 2,4-diacetylphloroglucinol, which showed a high protein sequence identity (>89%) with a validated orthologous gene cluster from another Pseudomonas. In addition, via an in vitro assay, the biocontrol activity of Pseudomonas sp. isolate C3 against Botrytis cinerea, Monilinia fructicola, Phytium sp., Alternaria sp., Geotrichum candidum, and Fusarium oxysporum was corroborated. Finally, through KofamKOALA, the presence of genes involved in different metabolic pathways of plant growth-promoting traits was identified, which was corroborated by in vitro assays. This study provides information obtained from genomic analyses and culture tools on a bacterial isolate from the Atacama Desert characterized by plant growth-promoting capacities and biocontrol activity.
1. Introduction
Plant-associated bacteria play several ecological roles and are generally enhanced in the rhizosphere [1,2]. They can increase the availability of nutrients in the soil, known as a direct mechanism [3], and/or confer defense against phytopathogenic microorganisms, which can indirectly provide benefits for plant growth and health [4]. These bacteria are characterized as plant growth-promoting (PGP) bacteria, and, in a biotechnological context, their metabolites are of great interest for the agricultural industry regarding their potential applications for improving crop yields [5,6,7] through the replacement of chemical fertilizers and pesticides [8,9].
One of the metabolites produced by bacteria defined as PGP is indole acetic acid (IAA), a phytohormone of the auxin group that regulates vascular tissue differentiation and/or induces cell division and stem and root elongation [10]. Another compound produced by PGP is the enzyme ACC deaminase, which modulates ethylene synthesis by the plant, inactivating senescence processes in plant tissues in response to biotic or abiotic stress [11]. The ability of PGP to solubilize phosphate increases the availability of phosphate in the soil for metabolism by plants [12], similar to bacterium-mediated nitrogen fixation, which is also considered a PGP activity and depends on the presence of the enzyme nitrogenase, which fixes N2 into ammonium (NH3) [13]. Finally, siderophore production is also considered a PGP ability in bacteria because it increases the modification of iron hydroxide (Fe3+) into ferrous hydroxide (Fe2+), which is metabolically available to plants [14].
Antibiotic-producing PGP bacteria have been intensively studied, and special attention has been paid to bacteria of the Pseudomonas genus regarding the production of 2,4-diacetylphloroglucinol, a metabolite characterized by its ability to control a wide variety of diseases caused by plant pathogenic microorganisms, inducing systemic resistance mechanisms [15,16,17,18,19,20]. Bacteria with antimicrobial attributes have been widely studied [21,22,23]. Kumar et al. [24] demonstrated the low diversity and frequency of the reported antibiotic activity of bacteria of the genus Pseudomonas; however, they highlighted their potential as producers of 2,4-diacetylphloroglucinol, a potent broad-spectrum antibiotic, but the origins and evolutionary dynamics of the genes associated with this pathway remain undefined [25].
Two processes have been shown to be key for the application of these microorganisms: bacterial isolation and characterization as PGP [26]. However, if extreme environments are also used, there is the possibility of finding new, highly optimized molecules due to selection pressure, in accordance with the need for rapid evolution to survive [27].
The Atacama Desert is a hyper-arid region of Chile, which has been compared to Mars and termed “the dry edge of life” [28,29]. Specifically, the Talabre–Lejía transect (TLT) is in the western highlands of the Chilean Andes, between ~2500 and ~4500 m.a.s.l. (meters above sea level), bordering the active Lascar volcano and the saline Lejía Lake [30]. Despite vegetation in these areas being restricted by the temperature, pH, water availability, and radiation, among other factors [31,32], it is possible to observe vegetative patches along the entire location, from which bacteria with positive PGP capabilities have been isolated [33].
The aim of this study was to provide a broader view of PGP traits, not only through culture-based techniques, but also using different molecular tools to elucidate biosynthetic pathways associated with these beneficial attributes in plants. Thus, this study provides the first report of a bacterium of the genus Pseudomonas isolated from plant-associated soils in the Atacama Desert, characterized by genomic analysis and culture-based techniques as a bacterium with plant growth-promoting and biocontrol attributes, making it an excellent candidate for evaluation in in vivo or field assays.
2. Materials and Methods
2.1. Sampling Site Description
Pseudomonas sp. strain C3 is part of a microbial repository isolated in 2018 from the Talabre–Lejía transect, located in the Atacama Desert [34], where the most common plant species are Calamagrostis crispa and Nassella nardoides [31,32,35]. It has been deposited and is available in the microbial repository Colección Chilena de Recursos Genéticos Microbianos INIA (RGM, Chillán, Chile) under the internal code RGM2438.
2.2. Culture, DNA Isolation, and Whole-Genome Sequencing
Strain C3 was incubated in a stationary phase for 24 h at 30 °C in 2 mL of LB (Luria Bertani) culture medium, reaching an optical density (600 nm) of 1.3. DNA extraction was performed using the DNeasy Blood & Tissue (QIAGEN, Hilden, Germany) commercial kit following the manufacturer’s procedures. The isolated DNA was quantified by fluorometric analysis using the Broad-Range (BR) kit from Qubit (Invitrogen, Waltham, MA, USA) and sequenced using the NovaSeq system (Illumina, San Diego, CA, USA) by the Molecular Research DNA laboratory (Mr.DNA, Shallowater, TX, USA), with 2 × 250 bp paired-end sequencing and 4 million reads.
2.3. Genome Assembly and Annotation
De novo assembly was conducted using quality-filtered reads using the CLC Genomics Workbench (QIAGEN, Hilden, Germany) v12.0 with the default parameters [35]. The genome assembly was assessed by employing BUSCO v5.2.2 [36] in “genome” mode, “prodigal” v2.6.3 [37] was used for prediction and the genome representation was constructed using DNAPlotter [38]. The genome completeness was also evaluated with CheckM v1.1.2 [39]. Final gene prediction was performed using the NCBI Prokaryotic Genome Annotation Pipeline released in 2013 [40].
The 16S rDNA sequence used for the taxonomic classification of Pseudomonas sp. strain C3 has been deposited under GenBank code MT576541.1, and the genome sequence data have been deposited in DDBJ/ENA/GenBank under the accession number JAJNDW000000000 and linked to the National Center for Biotechnology Information (NCBI) under the BioProject accession number PRJNA783880.
2.4. Taxonomic Identification of the Complete Genome
The genome sequence data were uploaded and taxonomically analyzed by dDDH (digital DNA–DNA hybridization) using TYGS (Type Genome Server) [41]. The genome comparison was conducted using GBDP (Genome Blast Distance Phylogeny) [42]. The dDDH values and confidence intervals were calculated using the GGDC (Genome-to-Genome Distance Calculator) v3.0 [42,43]. The evolution tree was constructed with FASTME v2.1.6.1 [44] and supported with 100 pseudo-bootstrap replicates, rooted at the midpoint [45] and visualized with PhyD3 [46].
2.5. Identification of Metabolic Pathways and Specialized Metabolites
Genomic analyses were performed for the determination of metabolic pathways associated with PGP traits (nitrogen fixation, indole acetic acid (IAA) production, siderophore production, and iron and phosphate uptake) with KEGG (Kyoto Encyclopedia of Genes and Genomes) orthology [47] and complemented with KofamKOALA [48]. Additionally, the full genome of C3 was submitted to antiSMASH bacterial version v6.0.1 [49] for secondary metabolite biosynthetic gene cluster (BGC) detection with the strictness set to “relaxed”. The optional parameters clusterblast and knownclusterblast [50] were called in order to compare the genetic diversity of the 2,4-diacetylphloroglucinol-producing BGC components against the antiSMASH database and the MIBiG 2.0 repository, respectively.
2.6. In Vitro Growth Inhibition of Phytopathogenic Fungi
The antagonism assay was performed according to the method described by Sepúlveda-Chavera et al. [51] Briefly, potato dextrose agar (PDA) plates were inoculated with the plant pathogenic fungi Botrytis cinerea, Monilinia fructicola, Phytium sp., Alternaria sp., Geotrichum candidum, and Fusarium oxysporum in the center of each plate, and 20 µL of Pseudomonas sp. strain C3 culture in a stationary phase at 2.5 cm from the center of the Petri dish culture. Plates only inoculated with fungi in the center were used as a control.
The inhibition of the mycelial radial growth (IMRG) of the fungi was calculated using the following equation:
where C represents the growth radius (mm) of each fungus on the control plate, and T is the fungal growth radius (mm) from the center of the Petri dishes under the treatment. The plates were incubated at room temperature until the control plates were fully covered with the phytopathogenic fungi.
IMRG (%) = [(C − T)/C] × 100
2.7. In Vitro Identification of Plant Growth-Promoting Traits
The PGP assays were performed as described in Gaete et al. [33]. Briefly, specific culture media were used to determine four PGP attributes of Pseudomonas sp. strain C3. Siderophore production was assessed using CAS agar media [52]. A positive result for this colorimetric technique is a color change in the culture medium, from blue to an orange halo.
The phosphate solubilization and nitrogen-fixing assays were conducted according to the HIMEDIA Technical Data, using the PKV (Pikovskayas agar) [53] and NFM (Norris Glucose Nitrogen Free Medium) [54] culture media, respectively. Both methods are considered to show positive results when a transparent halo is observed in the plates. Finally, IAA production was measured using the Salkowski test following the suggestions described by Widawati [55], with a color change from yellow towards red being observed when IAA was synthesized.
2.8. Detection of In Vitro Hydrolytic Activity
The hydrolytic activity of Pseudomonas sp. strain C3 was evaluated using three specific culture media. For the chitinase activity assay, solid LB medium was supplemented with colloidal chitin [56]. A clear halo on a creamy background represents positivity for chitinase activity. The protease activity was evaluated using SMA (skim milk agar) media according to the protocol described by Bhowmik et al. [57]. The lipase activity was determined using a protocol described by Slifkin [58]. Again, for both assays, the appearance of a halo around the microorganism is indicative of a positive result.
3. Results
3.1. Genome Assembly and Annotation
The genome of Pseudomonas sp. strain C3 was assembled, obtaining completeness and contamination indexes of 99.38% and 0.1%, respectively, according to CheckM (Figure 1). This high-quality draft genome comprises 129 contigs with an N50 of 65,108 bp and a total length of 5,677,066 bp. In total, 5126 genes were predicted and analyzed with the BUSCO tool, obtaining 99.2% of the “bacterial” lineage markers in a single copy (Table 1).
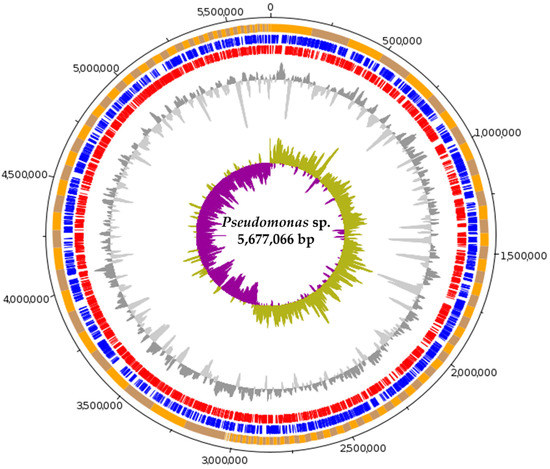
Figure 1.
Whole-genome representation of Pseudomonas sp. strain C3 using DNAPlotter tool. Orange and gray represent scaffolds ordered by GC bias and length. Blue and red represent CDS in the forward and reverse directions, respectively. Gray represents GC content with respect to the mean. The innermost circle represents the GC bias with respect to the mean.

Table 1.
Molecular features that determine the genome of Pseudomonas sp. strain C3.
Taxonomic identification based on the complete genome of Pseudomonas sp. strain C3 yielded 10 species grouped from other Pseudomonas genera, among which five (in addition to Pseudomonas sp. strain C3) formed a single paraphyletic clade (Figure 2). This phylogenetic tree was constructed based on the results obtained from the dDDH values of our strain (query strain) with each subject strain and its corresponding confidence interval (C.I., Table S1).
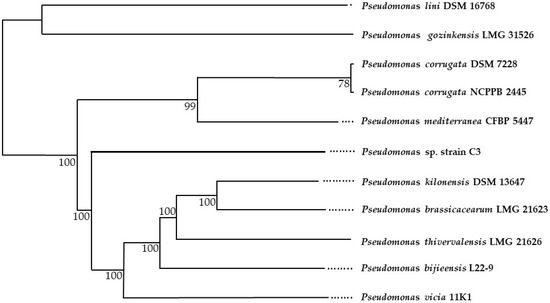
Figure 2.
Phylogenetic analysis with taxonomic inference based on the genomic sequence of Pseudomonas sp. strain C3 for microbial species delimitation by dDDH (digital DNA-DNA hybridization). The dots in the figure represent evolutionary events for each bacterium.
3.2. Molecular Analysis of Metabolites and Metabolic Pathways
The genomic exploration of secondary metabolites in Pseudomonas sp. strain C3 predicted 13 regions, all hosting single candidate clusters (Table 2). Seven of them had no matches for the ‘most similar known cluster’ output, corresponding to the types NAGGN (N-acetylglutaminylglutamine amide) (region 15.1), butyrolactone (region 17.1), RiPP-like (unspecified ribosomally synthesized and post-translationally modified peptide product) (region 31.1 and 46.1), NRPS-like (non-ribosomal peptide synthetase cluster) (region 59.1), lanthipeptide-class-ii (region 72.1), and siderophore (region 104.1) types. Five regions were partially matched with similarity scores ranging from 7% to 45% for the best hits: NRPS (non-ribosomal peptide synthetase cluster) (region 39.1) with crochelin A, betalactone (region 29.1) with fengycin, redox cofactor (region 30.1) with lankacidin C, NRPS-like (region 69.1) with fragin, and aryl polyene (region 12.1) with APE Vf (aryl polyene cluster), in that order. The T3PKS (type III polyketide synthase) was detected with 100% similarity to the BGC associated with 2,4-diacetylphloroglucinol production. After manual inspection, it was possible to confirm the presence of homologs for all the components of the phl gene cluster responsible for the biosynthesis, transport, and regulation of this metabolite (Figure 3).

Table 2.
Secondary metabolites identified in Pseudomonas sp. strain C3.

Figure 3.
Genes involved in the 2,4-diacetylphloroglucinol pathway reported in the genome of Pseudomonas sp. strain C3.
A genetic diversity analysis based on the protein sequence similarity of the eight genes involved in the 2,4-diacetylphloroglucinol pathway showed almost no variation in the protein sequence, compared to corresponding orthologues in other Pseudomonas isolates (Table S2). The gene with the highest identity corresponded to PhlF, involved in the regulatory function of this metabolite, followed by the genes involved in biosynthesis (phlB, phlC, phlD, phlG, and phlA, in that order), and then the phlE and phlH genes, involved in transport and regulation, respectively.
In order to perform a more extensive screening of the genomic sequence of Pseudomona sp. strain C3 for possible PGP attributes, a search for specific metabolic pathways was performed. As detailed in the methodology section, PGP-related metabolic pathways were retrieved using the KEEG orthology and KofamKOALA databases (Table 3). This analysis determined the presence of genes involved in the phosphate and iron uptake pathways, indole acetic acid (IAA) production, the nitrate reduction pathway, the siderophore production pathway (enterochelin), and the ACC deaminase gene (Figure 4).

Table 3.
Pseudomonas sp. strain C3 genes associated with plant growth-promoting and biocontrol traits discussed in this study.

Figure 4.
Different copies of genes involved in specific metabolic pathways associated with plant growth-promoting traits found in the genome of Pseudomonas sp. strain C3 through KEEG orthology and KofamKOALa databases.
Specifically, in the case of phosphate uptake, appA was the only gene that was not found in the genomic context of isolate C3, while the rest of the genes were present in a single copy (phoA, pstA, phoU, and phoB), two copies (pstC and pstB), three copies (pstS), and five copies (phoR). Genes associated with iron uptake were also examined, and was detected two copies of fhuC, three of feoA and fur, six of fhuD, and seven of fhub; however, six genes were not present (fhuA, fecR, tonB, exbB, exbD, and irr). Two genes involved in an alternative metabolic pathway for IAA (tryptamine; TAM) were found, with only one copy of each (MAO and aldB). Regarding the nitrate reduction pathway, all the genes involved were present: napB in a single copy, nirB in three copies, and napA and nirD in six and seven copies, respectively. Four genes associated with enterochelin siderophore synthesis were detected, including one with one copy (entD) and three others with notably higher frequencies of 22, 11, and 23: entE, entB, and entF, respectively. Finally, the gene encoding ACC deaminase appeared to be present, with seven copies, in the genome of Pseudomonas sp. strain C3.
3.3. In Vitro Antifungal Activity of Pseudomonas sp. Strain C3
An in vitro assay involving six phytopathogenic fungi was performed to complement the molecular analysis that suggested a possible antifungal biocontrol activity attributed to the metabolite 2,4-diacetylphloroglucinol coded for in region 46.2. The exposure of Pseudomonas sp. strain C3 to these fungi evidenced its ability to control all of them, with different percentages of mycelial radial growth inhibition (%IMRG) (Figure 5). Alternaria sp. and Geotrichum candidum presented the highest inhibition rate (61.5%), followed, in descending order, by Botrytis cinerea (60%), Monilinia fruticola and Fusarium oxysporum (59.3%), and Phytium sp. (58.5%).
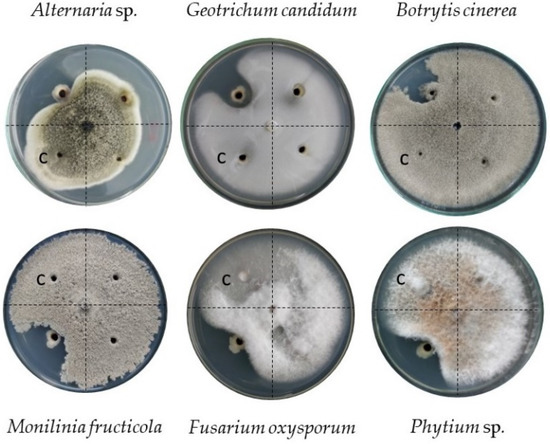
Figure 5.
In vitro determination of biocontrol of Pseudomonas sp. strain C3 against six phytopathogenic fungi. The letter “C” in the Petri dish indicates the quadrant that corresponds to the control without bacteria.
Through in vitro assays (Figure 6), four positive PGP activities were confirmed in Pseudomonas sp. strain C3, such as siderophore production (using CAS agar medium), nitrogen fixation (NFM culture medium), phosphate solubilization (PVK culture medium), and IAA production (Salkowski test). Complementary to this, hydrolase activity was also detected, specifically for proteases through the SMA medium culture.
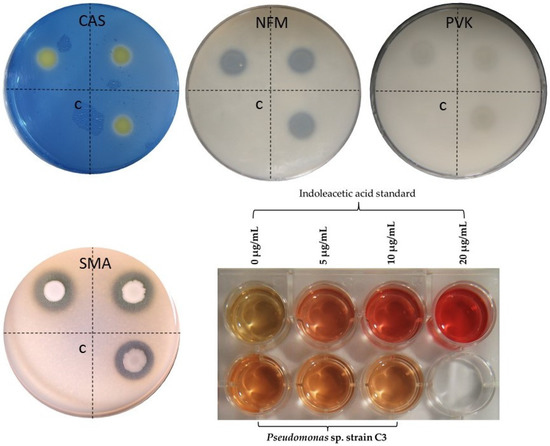
Figure 6.
In vitro assays showing PGP traits and proteolytic activity of Pseudomonas sp. strain C3. Siderophore production (CAS). Nitrogen fixing (NFM). Phosphate solubilization (PVK). Protease activity (SMA) and IAA quantification assay (Salkowski test). The letter “C” on the Petri dishes indicates the negative control corresponding to the culture medium without bacteria.
4. Discussion
Considering the high level of completeness (99.38%) and a contamination percentage close to zero (0.1%), was reported here, a high-quality genome draft [59] of Pseudomonas sp. strain C3, which contains 5,677,066 bp, with a GC content of 61.37% (Figure 1). Similar results have been obtained by other researchers who sequenced Pseudomonas wadenswilerensis isolated from forest soils in 2014, achieving a high-quality draft of 5,966,942 bp with a GC content of 63.39% [60], while Pseudomonas aeruginosa isolated from a polluted industrial metalworking environment has also been drafted in high quality, yielding a genome 6,985,358 bp in length and with a GC content of 66.08% [61].
Some studies have indicated that secondary metabolism plays a significant ecological role in inter- and intra-specific communication among soil microorganisms, where its metabolites exhibit a broad range of biological activities (e.g., antibiotic and antifungal activities and siderophores) that could be relevant for plants, as they affect plant growth and defense responses [62]. The mechanisms of the action of biocontrol microorganisms include antibiosis, parasitism, or competition with the pathogen for nutrients and space. They may also induce disease resistance in the host plant, acting in different steps of the infection process [63]. Biocontrol activity is assisted by the production of different types of compounds, including siderophores, antibiotics, volatile organic compounds, and lytic enzymes [64].
In general, the results obtained using molecular tools (Figure 4, Table 2 and Table 3) and culture-based techniques (Figure 5 and Figure 6) were consistent. Using PVK culture medium was determined the phosphate-solubilizing activity of Pseudomonas sp. strain C3, and the genomic analyses showed that eight genes involved in phosphate uptake were present. These genes are involved in the solubilization of inorganic phosphate by modulating uptake and transport into the bacterial cell. The passive diffusion of the compound is facilitated by the hydrolysis of inositol-polyphosphate structures by appA [65]; however, in Pseudomonas sp. strain C3, no ortholog of this gene was detected. Nevertheless, diffusion can also occur by hydrolysis by the phoA gene, which was present along with the ABC phosphate-transporter-associated pstSCAB gene, which allows the selective internalization of inorganic phosphate [66]. An analysis performed by Blus-Kadosh et al. [67] in Pseudomonas aeruginosa explicitly based on the pstS gene responsible for phosphate uptake and the phoB gene responsible for phosphate-deficient regulation showed that both genes were essential in the phosphate-uptake pathway. Furthermore, relevant to this context, an assay with mutants of Pseudomonas putida revealed that the pstSCAB genes responsible for the transport system negatively regulated the pho regulon [68]. Therefore, phosphate uptake can be expected if the pst and pho cassettes are present.
Through the CAS agar culture medium assay, was observed siderophore production by Pseudomonas sp. strain C3, supported by the antiSMASH analysis’ output. A siderophore-producing BGC was found in region 104.1 with no matches for the most similar known cluster against the MIBiG repository. In parallel, through genomic information using the KofamKOALA database for annotation analysis, it was determined that strain C3 contained the enterochelin synthesis gene cluster (entDEBF). Regarding the genes related to iron uptake, only five out of eleven genes were found. None of the genes classified by Clarke et al. [69] as essential for iron uptake, such as the transport-related fhuA gene or the TonB–ExbD system [70,71], were present. However, the absence of fhuA here does not limit iron uptake since the fhuBCD genes reported to be involved in the uptake of ferrichrome and ferrioxamine in other Pseudomonas aeruginosa strains were found to be present in the genome. An alternative way for bacteria to obtain iron from the environment is through the feo transporter [72]. Although there are not many studies based on this transporter, it has been proven that, if this gene is not present, iron absorption is reduced by 60%; thus, it has been described as essential for iron transport in bacteria [73]. The Fur gene is related to homeostasis control mechanisms in bacteria via iron storage and nutrient-dependent uptake [74]. A recent study of fur-deficient mutant lines demonstrated the effects of the Fur gene in the downregulation of more than a hundred genes, including the TonB-dependent and ABC-type transporters [75]. Both feo and fur were confirmed to be present in the genome of isolate C3.
Moreover, when IAA production was analyzed by means of the Salkowski test, a low presence of this hormone in the culture medium was observed. A manual genomic search revealed the presence of the MAO and aldB genes, both involved in this metabolite’s synthesis. Multiple bacterial IAA synthesis pathways have recently been described by Duca et al., most of which use tryptophan as a precursor. An alternative pathway that uses tryptamine as a precursor is described in [76]. This tryptamine-involving pathway recruits an amine oxidase enzyme (MAO) to convert the primary substrate into indole acetaldehyde, and to subsequently obtain IAA through the effect of an aldehyde dehydrogenase (aldB). The function of aldB was characterized by mutagenesis assays in Pseudomonas syringae; the authors state that aldB is an alternative to aldA for IAA biosynthesis, and that aldB is directly related to a low production of IAA in the studied strain [77]. Additionally, previous work using in vitro assays demonstrated IAA production in two strains of the genus Pseudomonas together with plant-growth-promoting capabilities related to the germination percentage, shoot length, and root length, and increases in the vigor index in lentil (Lens culinaris) and barley (Hordeum vulgare) plants [78].
When in vitro atmospheric nitrogen fixation was evaluated using the PVK culture medium, Pseudomonas sp. strain C3 showed the expected halo around the inoculum indicative of a positive result. In addition to the manual gene search using the KofamKOALA tool, genes involved in the nitrate-reduction process were found. To this extent, Marzocchi [79] et al. and Huang et al. [80] recently highlighted the importance of the napA and napB genes in Gram-negative Candidatus electronema and of nirB and nirD in Pseudomonas putida, respectively, reported to be involved in the main reaction of nitrate reduction to ammonium and its assimilation/dissimilation rates, all of which were detected in the present study using the molecular tools detailed above. Moreover, previous work by Yan et al. [81] further elucidated the roles of nirB and nirD in nitrogen metabolism in unknown Pseudomonas sp. strain XS-18(). Finally, our genomic analysis for functional predictions indicated that isolate C3 encoded a specific BGC classified as T3PKS. Which exhibited a 100% similarity to the phl gene cluster (Table 2, Figure 3). Other studies that also included molecular analysis using antiSMASH in their workflows have detected 2,4-diacetylphloroglucinol-producing gene clusters in different strains of the genus Pseudomonas, including P. brassicacearum [82], P. protegens [83], and P. fluorescens [84], and in bacteria belonging to other genera such as Pseudogulbenkiania ferrooxidans [85]. Interestingly, a recent molecular analysis of P. putida has shown that several strains could control phytopathogenic fungi without the 2,4-diacetylphloroglucinol gene cluster in their genome [86], suggesting that this biocontrol activity is linked to the production of the siderophore pyoverdine.
The diversity analysis of all the genes involved in the 2,4-diactetylphloroglucinol pathway detected by antiSMASH in isolate C3 (Table S2) revealed high identity and coverage with respect to ten other Pseudomonas strains that were obtained from agricultural soils, for example, potatoes [82,87], rice [88], soybeans [89,90], and wheat crops [91], as well as to two Arabidopsis thaliana strains obtained from rhizospheres [92] and groundwater [93] and two others from unreported origins. Interestingly, our strain was isolated from the western slopes of the Andes mountains, in the Atacama region, where a plant community can be found [29,31,32], suggesting that this potential metabolic capacity could be relevant in the interaction of Pseudomonas sp. strain C3 with plants.
Despite 2,4-diactetylphloroglucinol having been mainly associated with members of the Pseudomonas genus, a recent study reported the presence of six genes out of the eight participants in its biosynthetic pathway in three Betaproteobacteria species (Pseudogulbenkiania ferrooxidans, Chromobacterium vaccini, and Chromobacterium piscinae) [17]. In the three of them, the phlG (biosynthesis) and phlH (regulation) genes were absent. In Pseudomonas sp. strain C3, both genes had the lowest identity and coverage percentage with respect to other Pseudomonas strains. Overall, a low frequency of metabolites associated with antimicrobial molecules has been detected in Pseudomonas, including 2,4-diactetylphloroglucinol [24]. This could be related to the synthesis of 2,4-diacetylphloroglucinol, which can act as an elicitor of induced systemic resistance [94], an undesirable trait for a plant pathogen, which has been proposed as a counter-selection mechanism in pseudomonads [25].
The compound 2,4-diacetylphloroglucinol was initially a subject of interest to researchers due to its antibiotic properties until it was later described as a potent broad-spectrum antifungal, leading to an application that gave it practical relevance in the agricultural industry [95]. Here, was determined that Pseudomonas sp. strain C3 affected the growth in the culture plates of six phytopathogenic fungi—Alternaria sp., Geotrichum candidum, Botrytis cinerea, Monilinia fruticola, Fusarium oxysporum, and Phytium sp—which are of agronomic interest (Figure 5). Other studies have exposed some of these pathogens to 2,4-diacetylphloroglucinol, obtaining similar results, e.g., Botrytis cinerea and Monilinia fructicola [83], Fusarium culmorum and Phytium sp. [96], and Alternaria sp. and Fusarium sp. [97]. To the best of our knowledge, there are no previous reports of biological biocontrol for Geotrichum candidum using this metabolite. It should be noted that other pathogens have been controlled and were not included in this study, such as Verticillium sp. [98], Ralstonia solanacearum [99], Magnaporthe oryzae and Rhizoctonia solani [100], and Pseudomonas syringae [101]. However, it cannot rule out the biocontrol ability of C3 being linked with other C3 metabolic capacities, including hydrolase activity and nutrient competition [64]. In fact, proteolytic activity was detected in vitro for Pseudomonas sp. strain C3 using the MSA medium, a result that was supported by the search for genes related to this activity in our strain of interest. Thus, the gacS and gacA genes have been reported in Pseudomonas fluorescens as regulators of extracellular proteases [102] and suggested to be crucial for its biocontrol activity as revealed through assays of mutant bacteria with mutations in these genes [103,104].
Thus, Pseudomonas sp. strain C3 is an excellent candidate isolated from an extreme environment for bioprospecting about the urgent global interest in antimicrobial discovery and bacteria with PGP attributes. Future work should address the elucidation of how the metabolite 2,4-diacetylphloroglucinol participates in the biocontrol action of phytopathogenic fungi, including in vivo assays to determine if all the attributes reported here in Pseudomonas the sp. strain C3 generate a significant beneficial effect on plants.
5. Conclusions
To the best our knowledge, this is the first study on a plant-associated bacterium isolated from soils of the Atacama Desert characterized through molecular tools and culture-based techniques. The bacterium exhibited biocontrol capacities on six phytopathogenic fungi associated with agriculturally important crops, which could be attributed to the production of 2,4-diactetylphloroglucinol by Pseudomonas sp. strain C3. The genes involved in this metabolic pathway showed low gene diversity based on the protein sequences, with percentages close to 100% identity with respect to other Pseudomonas isolates. In addition, using genomic information, the presence of genes necessary for iron and phosphate uptake, nitrogen fixation, and indole acetic acid and siderophore production, which could contribute to soil biogeochemical processes and improve crop yields, was determined. Thus, Pseudomonas sp. strain C3 is an excellent candidate for the evaluation of its contribution as a biocontrol and plant growth-promoting agent in field assays.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/d14050388/s1. Table S1: Pairwise comparison of genome sequences with 10 public genomes against Pseudomonas sp. strain C3; Table S2: Genetic diversity of 2,4-diacetylphloroglucinol in different Pseudomonas strains.
Author Contributions
A.G. conceived the study, designed the experiments, and wrote the first draft. J.E.M. performed the genome assembly. C.A.-G. analyzed the specific metabolites and participated in writing the draft. P.A.M.-T. evaluated the in vitro biocontrol assays. M.G. and G.F.S.-C. mainly funded the study. All the authors reviewed and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by ANID/FONDAP/15200002 and Fondecyt grants 1201278 to M.G. P.A.M.-T. was supported by Fondecyt grants 11200093, FIC-CORFO Project 13CEI2-21852 and the execution of the Project Co-execution Agreement between the Universidad de Tarapacá and the University of California Davis Chile (“Decreto Exento” Nº00.451/2018). J.E.M. was supported by ANID/FONDECYT grant N° 3190194. A.G. was supported by the National Agency for Research and Development (ANID)/Ph.D. Fellowship Nº 21210808 and “Dr. Abraham Stekel” Scholarship granted by INTA-Nestlé.
Institutional Review Board Statement
No applicable.
Data Availability Statement
All the data obtained through this study are shown in this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mendes, R.; Garbeva, P.; Raaijmakers, J.M. The rhizosphere microbiome: Significance of plant beneficial, plant pathogenic, and human pathogenic microorganisms. FEMS Microbiol. Rev. 2013, 37, 634–663. [Google Scholar] [CrossRef] [PubMed]
- Mendes, L.W.; Raaijmakers, J.M.; de Hollander, M.; Mendes, R.; Tsai, S.M. Influence of resistance breeding in common bean on rhizosphere microbiome composition and function. ISME J. 2018, 12, 212–224. [Google Scholar] [CrossRef] [PubMed]
- du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Goswami, D.; Dhandhukia, P.; Patel, P.; Thakker, J.N. Screening of PGPR from saline desert of Kutch: Growth promotion in Arachis hypogea by Bacillus licheniformis A2. Microbiol. Res. 2014, 169, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Jorquera, M.A.; Inostroza, N.G.; Lagos, L.M.; Barra, P.J.; Marileo, L.G.; Rilling, J.I.; Campos, D.C.; Crowley, D.E.; Richardson, A.E.; Mora, M.L. Bacterial community structure and detection of putative plant growth-promoting rhizobacteria associated with plants grown in Chilean agro-ecosystems and undisturbed ecosystems. Biol. Fertil. Soils 2014, 50, 1141–1153. [Google Scholar] [CrossRef]
- Ibort, P.; Molina, S.; Ruiz-Lozano, J.M.; Aroca, R. Molecular Insights into the Involvement of a Never Ripe Receptor in the Interaction Between Two Beneficial Soil Bacteria and Tomato Plants Under Well-Watered and Drought Conditions. Mol. Plant-Microbe Interact. 2018, 31, 633–650. [Google Scholar] [CrossRef]
- Subramanian, P.; Kim, K.; Krishnamoorthy, R.; Mageswari, A.; Selvakumar, G.; Sa, T. Cold Stress Tolerance in Psychrotolerant Soil Bacteria and Their Conferred Chilling Resistance in Tomato (Solanum lycopersicum Mill.) under Low Temperatures. PLoS ONE 2016, 11, e0161592. [Google Scholar] [CrossRef]
- Ashrafuzzaman, M.; Hossen, F.A.; Razi Ismail, M.; Hoque, A.; Islam, M.Z.; Shahidullah, S.M.; Meon, S. Efficiency of plant growth-promoting rhizobacteria (PGPR) for the enhancement of rice growth. Afr. J. Biotechnol. 2010, 8, 1247–1252. [Google Scholar] [CrossRef]
- Lehman, R.M.; Cambardella, C.A.; Stott, D.E.; Acosta-Martinez, V.; Manter, D.K.; Buyer, J.S.; Maul, J.E.; Smith, J.L.; Collins, H.P.; Halvorson, J.J.; et al. Understanding and Enhancing Soil Biological Health: The Solution for Reversing Soil Degradation. Sustainability 2015, 7, 988–1027. [Google Scholar] [CrossRef]
- Teale, W.D.; Paponov, I.A.; Palme, K. Auxin in action: Signalling, transport and the control of plant growth and development. Nat. Rev. Mol. Cell Biol. 2006, 7, 847–859. [Google Scholar] [CrossRef]
- Glick, B.R.; Todorovic, B.; Czarny, J.; Cheng, Z.; Duan, J.; McConkey, B. Promotion of Plant Growth by Bacterial ACC Deaminase. Crit. Rev. Plant Sci. 2007, 26, 227–242. [Google Scholar] [CrossRef]
- Krey, T.; Vassilev, N.; Baum, C.; Eichler-Löbermann, B. Effects of long-term phosphorus application and plant-growth promoting rhizobacteria on maize phosphorus nutrition under field conditions. Eur. J. Soil Biol. 2013, 55, 124–130. [Google Scholar] [CrossRef]
- Bhattacharjee, R.B.; Singh, A.; Mukhopadhyay, S.N. Use of nitrogen-fixing bacteria as biofertiliser for non-legumes: Prospects and challenges. Appl. Microbiol. Biotechnol. 2008, 80, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Ansari, R.A.; Mahmood, I.; Rizvi, R.; Sumbul, A. Safiuddin Siderophores: Augmentation of soil health and crop productivity. In Probiotics in Agroecosystem; Springer: Singapore, 2017; pp. 291–312. [Google Scholar] [CrossRef]
- Mavrodi, O.V.; McSpadden Gardener, B.B.; Mavrodi, D.V.; Bonsall, R.F.; Weller, D.M.; Thomashow, L.S. Genetic diversity of phlD from 2,4-diacetylphloroglucinol-producing fluorescent Pseudomonas spp. Phytopathology 2001, 91, 35–42. [Google Scholar] [CrossRef]
- Weller, D.M.; Landa, B.B.; Mavrodi, O.V.; Schroeder, K.L.; De La Fuente, L.; Blouin Bankhead, S.; Allende Molar, R.; Bonsall, R.F.; Mavrodi, D.V.; Thomashow, L.S. Role of 2,4-diacetylphloroglucinol-producing fluorescent Pseudomonas spp. in the defense of plant roots. Plant Biol. 2007, 9, 4–20. [Google Scholar] [CrossRef]
- Almario, J.; Bruto, M.; Vacheron, J.; Prigent-Combaret, C.; Moënne-Loccoz, Y.; Muller, D. Distribution of 2,4-diacetylphloroglucinol biosynthetic genes among the Pseudomonas spp. Reveals unexpected Polyphyletism. Front. Microbiol. 2017, 8, 1218. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, Y.P.; Zhang, L.Q. In silico and genetic analyses of cyclic lipopeptide synthetic gene clusters in Pseudomonas sp. 11K1. Front. Microbiol. 2019, 10, 544. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, W.; Jiang, Q.; Fei, Z.; Xiao, M. Genome analysis of plant growth-promoting rhizobacterium Pseudomonas chlororaphis subsp. aurantiaca JD37 and insights from comparasion of genomics with three Pseudomonas strains. Microbiol. Res. 2020, 237, 126483. [Google Scholar] [CrossRef]
- Liang, J.; Wang, S.; Yiming, A.; Fu, L.; Nie, W.; Chen, G.; Zhu, B. Genome resource for Pseudomonas sp. strain L22-9: A potential novel species with antifungal activity. Phytopathology 2021, 111, 425–428. [Google Scholar] [CrossRef]
- Yang, Y.; Babich, O.O.; Sukhikh, S.A.; Zimina, M.I.; Milentyeva, I.S. Antibiotic activity and resistance of lactic acid bacteria and other antagonistic bacteriocin-producing microorganisms. Foods Raw Mater. 2020, 8, 377–384. [Google Scholar] [CrossRef]
- Marcoleta, A.E.; Arros, P.; Varas, M.A.; Costa, J.; Rojas-Salgado, J.; Berríos-Pastén, C.; Tapia-Fuentes, S.; Silva, D.; Fierro, J.; Canales, N.; et al. The highly diverse Antarctic Peninsula soil microbiota as a source of novel resistance genes. Sci. Total Environ. 2022, 810, 152003. [Google Scholar] [CrossRef] [PubMed]
- Aguila-Torres, P.; Maldonado, J.; Gaete, A.; Figueroa, J.; González, A.; Miranda, R.; González-Stegmaier, R.; Martin, C.; González, M. Biochemical and Genomic Characterization of the Cypermethrin-Degrading and Biosurfactant-Producing Bacterial Strains Isolated from Marine Sediments of the Chilean Northern Patagonia. Mar. Drugs 2020, 18, 252. [Google Scholar] [CrossRef] [PubMed]
- Kumar, U.; Panneerselvam, P.; Banik, A.; Annapurna, K. Lower Frequency and Diversity of Antibiotic-Producing Fluorescent Pseudomonads in Rhizosphere of Indian Rapeseed–Mustard (Brassica juncea L. Czern.). Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2018, 88, 579–586. [Google Scholar] [CrossRef]
- Biessy, A.; Filion, M. Phloroglucinol Derivatives in Plant-Beneficial Pseudomonas spp.: Biosynthesis, Regulation, and Functions. Metabolites 2021, 11, 182. [Google Scholar] [CrossRef]
- Ambrosini, A.; Passaglia, L.M.P. Plant Growth—Promoting Bacteria (PGPB): Isolation and Screening of PGP Activities. Curr. Protoc. Plant Biol. 2017, 2, 190–209. [Google Scholar] [CrossRef]
- Li, S.J.; Hua, Z.S.; Huang, L.N.; Li, J.; Shi, S.H.; Chen, L.X.; Kuang, J.L.; Liu, J.; Hu, M.; Shu, W.S. Microbial communities evolve faster in extreme environments. Sci. Rep. 2014, 4, 6205. [Google Scholar] [CrossRef]
- Azua-Bustos, A.; González-Silva, C.; Arenas-Fajardo, C.; Vicuña, R. Extreme environments as potential drivers of convergent evolution by exaptation: The Atacama Desert Coastal Range case. Front. Microbiol. 2012, 3, 426. [Google Scholar] [CrossRef]
- Crits-Christoph, A.; Robinson, C.K.; Barnum, T.; Fricke, W.F.; Davila, A.F.; Jedynak, B.; McKay, C.P.; DiRuggiero, J. Colonization patterns of soil microbial communities in the Atacama Desert. Microbiome 2013, 1, 28. [Google Scholar] [CrossRef]
- Mandakovic, D.; Maldonado, J.; Pulgar, R.; Cabrera, P.; Gaete, A.; Urtuvia, V.; Seeger, M.; Cambiazo, V.; González, M. Microbiome analysis and bacterial isolation from Lejía Lake soil in Atacama Desert. Extremophiles 2018, 22, 665–673. [Google Scholar] [CrossRef]
- Díaz, F.P.; Frugone, M.; Gutiérrez, R.A.; Latorre, C. Nitrogen cycling in an extreme hyperarid environment inferred from δ15N analyses of plants, soils and herbivore diet. Sci. Rep. 2016, 6, 22226. [Google Scholar] [CrossRef] [PubMed]
- Eshel, G.; Araus, V.; Undurraga, S.; Soto, D.C.; Moraga, C.; Montecinos, A.; Moyano, T.; Maldonado, J.; Díaz, F.P.; Varala, K.; et al. Plant ecological genomics at the limits of life in the Atacama Desert. Proc. Natl. Acad. Sci. USA 2021, 118, e2101177118. [Google Scholar] [CrossRef] [PubMed]
- Gaete, A.; Mandakovic, D.; González, M. Isolation and Identification of Soil Bacteria from Extreme Environments of Chile and Their Plant Beneficial Characteristics. Microorganisms 2020, 8, 1213. [Google Scholar] [CrossRef] [PubMed]
- Maza, F.; Maldonado, J.; Vásquez-Dean, J.; Mandakovic, D.; Gaete, A.; Cambiazo, V.; González, M. Soil bacterial communities from the Chilean Andean highlands: Taxonomic composition and culturability. Front. Bioeng. Biotechnol. 2019, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Mandakovic, D.; Cintolesi, Á.; Maldonado, J.; Mendoza, S.N.; Aïte, M.; Gaete, A.; Saitua, F.; Allende, M.; Cambiazo, V.; Siegel, A.; et al. Genome-scale metabolic models of Microbacterium species isolated from a high altitude desert environment. Sci. Rep. 2020, 10, 5560. [Google Scholar] [CrossRef]
- Manni, M.; Berkeley, M.R.; Seppey, M.; Simão, F.A.; Zdobnov, E.M. BUSCO update: Novel and streamlined workflows along with broader and deeper phylogenetic coverage for scoring of eukaryotic, prokaryotic, and viral genomes. Mol. Biol. Evol. 2021, 38, 4647–4654. [Google Scholar] [CrossRef]
- Hyatt, D.; Chen, G.L.; LoCascio, P.F.; Land, M.L.; Larimer, F.W.; Hauser, L.J. Prodigal: Prokaryotic gene recognition and translation initiation site identification. BMC Bioinform. 2010, 11, 119. [Google Scholar] [CrossRef]
- Carver, T.; Thomson, N.; Bleasby, A.; Berriman, M.; Parkhill, J. DNAPlotter: Circular and linear interactive genome visualization. Bioinformatics 2009, 25, 119–120. [Google Scholar] [CrossRef]
- Parks, D.H.; Imelfort, M.; Skennerton, C.T.; Hugenholtz, P.; Tyson, G.W. CheckM: Assessing the quality of microbial genomes recovered from isolates, single cells, and metagenomes. Genome Res. 2015, 25, 1043–1055. [Google Scholar] [CrossRef]
- Tatusova, T.; Dicuccio, M.; Badretdin, A.; Chetvernin, V.; Nawrocki, E.P.; Zaslavsky, L.; Lomsadze, A.; Pruitt, K.D.; Borodovsky, M.; Ostell, J. NCBI prokaryotic genome annotation pipeline. Nucleic Acids Res. 2016, 44, 6614–6624. [Google Scholar] [CrossRef]
- Meier-Kolthoff, J.P.; Göker, M. TYGS is an automated high-throughput platform for state-of-the-art genome-based taxonomy. Nat. Commun. 2019, 10, 2182. [Google Scholar] [CrossRef] [PubMed]
- Meier-Kolthoff, J.P.; Auch, A.F.; Klenk, H.P.; Göker, M. Genome sequence-based species delimitation with confidence intervals and improved distance functions. BMC Bioinform. 2013, 14, 60. [Google Scholar] [CrossRef] [PubMed]
- Meier-Kolthoff, J.P.; Carbasse, J.S.; Peinado-Olarte, R.L.; Göker, M. YGS and LPSN: A database tandem for fast and reliable genome-based classification and nomenclature of prokaryotes. Nucleic Acids Res. 2022, 50, D801–D807. [Google Scholar] [CrossRef] [PubMed]
- Lefort, V.; Desper, R.; Gascuel, O. FastME 2.0: A Comprehensive, Accurate, and Fast Distance-Based Phylogeny Inference Program. Mol. Biol. Evol. 2015, 32, 2798–2800. [Google Scholar] [CrossRef] [PubMed]
- Farris, J.S. Estimating Phylogenetic Trees from Distance Matrices. Am. Nat. 2015, 106, 645–668. [Google Scholar] [CrossRef]
- Kreft, L.; Botzki, A.; Coppens, F.; Vandepoele, K.; Van Bel, M. PhyD3: A phylogenetic tree viewer with extended phyloXML support for functional genomics data visualization. Bioinformatics 2017, 33, 2946–2947. [Google Scholar] [CrossRef]
- Aoki-Kinoshita, K.F.; Kanehisa, M. Gene annotation and pathway mapping in KEGG. Methods Mol. Biol. 2007, 396, 71–91. [Google Scholar] [CrossRef]
- Aramaki, T.; Blanc-Mathieu, R.; Endo, H.; Ohkubo, K.; Kanehisa, M.; Goto, S.; Ogata, H. KofamKOALA: KEGG Ortholog assignment based on profile HMM and adaptive score threshold. Bioinformatics 2020, 36, 2251–2252. [Google Scholar] [CrossRef]
- Blin, K.; Shaw, S.; Kloosterman, A.M.; Charlop-Powers, Z.; Van Wezel, G.P.; Medema, M.H.; Weber, T.H. General rights antiSMASH 6.0: Improving cluster detection and comparison capabilities antiSMASH 6.0: Improving cluster detection and comparison capabilities. Nucleic Acids Res. 2021, 49, W29–W35. [Google Scholar] [CrossRef]
- Buchfink, B.; Xie, C.; Huson, D.H. Fast and sensitive protein alignment using DIAMOND. Nat. Agron. 2014, 12, 59–60. [Google Scholar] [CrossRef]
- Chavera, G.F.S.; Macuer, M.A.; Torres, P.M. Endospore-Forming Bacteria Present in a Commercial Stabilized Poultry Manure Determines the Fusarium Biocontrol and the Tomato Growth Promotion. Agronomy 2020, 10, 1636. [Google Scholar] [CrossRef]
- Louden, B.C.; Haarmann, D.; Lynne, A.M. Use of Blue Agar CAS Assay for Siderophore Detection. J. Microbiol. Biol. Educ. 2011, 12, 51–53. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, R.N. Isolation of rhizobia from soybean cultivated in latur area & study of its phosphate solubilization activity. Biosci. Discov. 2013, 4, 100–103. [Google Scholar]
- Wafula, E.N.; Murunga, S.; Nalianya Wafula, E.; Murunga, S.I.; Wafula, E.N. Isolation and Identification of Phosphate Solubilizing and Nitrogen-Fixing Bacteria from Lake Ol’Bolossat Sediments, Kenya. Mod. Appl. Sci. 2020, 14. [Google Scholar] [CrossRef]
- Widawati, S. Isolation of indole acetic acid (IAA) producing Bacillus siamensis from peat and optimization of the culture conditions for maximum IAA production. IOP Conf. Ser. Earth Environ. Sci. 2020, 572, 012025. [Google Scholar] [CrossRef]
- Saima; Kuddus, M.; Roohi; Ahmad, I.Z. Isolation of novel chitinolytic bacteria and production optimization of extracellular chitinase. J. Genet. Eng. Biotechnol. 2013, 11, 39–46. [Google Scholar] [CrossRef]
- Bhowmik, S.; Islam, S.; Ahmed, M.M.; Belal Hossain, M.; Hossain, M.A. Protease producing bacteria and activity in gut of tiger shrimp (Penaeus monodon). J. Fish. Aquat. Sci. 2015, 10, 489–500. [Google Scholar] [CrossRef]
- Slifkin, M. Tween 80 opacity test responses of various Candida species. J. Clin. Microbiol. 2000, 38, 4626–4628. [Google Scholar] [CrossRef]
- Bowers, R.M.; Kyrpides, N.C.; Stepanauskas, R.; Harmon-Smith, M.; Doud, D.; Reddy, T.B.K.; Schulz, F.; Jarett, J.; Rivers, A.R.; Eloe-Fadrosh, E.A.; et al. Minimum information about a single amplified genome (MISAG) and a metagenome-assembled genome (MIMAG) of bacteria and archaea. Nat. Biotechnol. 2017, 35, 725–731. [Google Scholar] [CrossRef]
- Rutz, D.; Frasson, D.; Sievers, M.; Blom, J.; Rezzonico, F.; Pothier, J.F.; Smits, T.H.M. High-Quality Draft Genome Sequence of Pseudomonas wadenswilerensis CCOS 864 T. Microbiol. Resour. Announc. 2018, 7, e01059-18. [Google Scholar] [CrossRef]
- Izrael-Živković, L.; Beškoski, V.; Rikalović, M.; Kazazić, S.; Shapiro, N.; Woyke, T.; Gojgić-Cvijović, G.; Vrvić, M.M.; Maksimović, N.; Karadžić, I. High-quality draft genome sequence of Pseudomonas aeruginosa san ai, an environmental isolate resistant to heavy metals. Extremophiles 2019, 23, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Tyc, O.; Song, C.; Dickschat, J.S.; Vos, M.; Garbeva, P. The Ecological Role of Volatile and Soluble Secondary Metabolites Produced by Soil Bacteria. Trends Microbiol. 2017, 25, 280–292. [Google Scholar] [CrossRef] [PubMed]
- Vurukonda, S.S.K.P.; Giovanardi, D.; Stefani, E. Plant Growth Promoting and Biocontrol Activity of Streptomyces spp. as Endophytes. Int. J. Mol. Sci. 2018, 19, 952. [Google Scholar] [CrossRef]
- Syed Ab Rahman, S.F.; Singh, E.; Pieterse, C.M.J.; Schenk, P.M. Emerging microbial biocontrol strategies for plant pathogens. Plant Sci. 2018, 267, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Fei, B.; Xu, H.; Zhang, F.; Li, X.; Ma, S.; Cao, Y.; Xie, J.; Qiao, D.; Cao, Y. Relationship between Escherichia coli AppA phytase’s thermostability and salt bridges. J. Biosci. Bioeng. 2013, 115, 623–627. [Google Scholar] [CrossRef]
- Vuppada, R.K.; Hansen, C.R.; Strickland, K.A.P.; Kelly, K.M.; McCleary, W.R. Phosphate signaling through alternate conformations of the PstSCAB phosphate transporter. BMC Microbiol. 2018, 18, 8. [Google Scholar] [CrossRef] [PubMed]
- Blus-Kadosh, I.; Zilka, A.; Yerushalmi, G.; Banin, E. The Effect of pstS and phoB on Quorum Sensing and Swarming Motility in Pseudomonas aeruginosa. PLoS ONE 2013, 8, e74444. [Google Scholar] [CrossRef]
- Wu, H.; Kosaka, H.; Kato, J.; Kuroda, A.; Ikeda, T.; Takiguchi, N.; Ohtake, H. Cloning and characterization of Pseudomonas putida genes encoding the phosphate-specific transport system. J. Biosci. Bioeng. 1999, 87, 273–279. [Google Scholar] [CrossRef]
- Clarke, T.; Tari, L.; Vogel, H. Structural Biology of Bacterial Iron Uptake Systems. Curr. Top. Med. Chem. 2005, 1, 7–30. [Google Scholar] [CrossRef]
- Schalk, I.J.; Guillon, L. Fate of ferrisiderophores after import across bacterial outer membranes: Different iron release strategies are observed in the cytoplasm or periplasm depending on the siderophore pathways. Amino Acids 2013, 44, 1267–1277. [Google Scholar] [CrossRef]
- Noinaj, N.; Guillier, M.; Barnard, T.J.; Buchanan, S.K. TonB-Dependent Transporters: Regulation, Structure, and Function. Annu. Rev. Microbiol. 2010, 64, 43–60. [Google Scholar] [CrossRef] [PubMed]
- Cartron, M.L.; Maddocks, S.; Gillingham, P.; Craven, C.J.; Andrews, S.C. Feo—Transport of ferrous iron into bacteria. BioMetals 2006, 19, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Lau, C.K.Y.; Krewulak, K.D.; Vogel, H.J. Bacterial ferrous iron transport: The Feo system. FEMS Microbiol. Rev. 2016, 40, 273–298. [Google Scholar] [CrossRef]
- Pasqua, M.; Visaggio, D.; Sciuto, A.L.; Genah, S.; Banin, E.; Visca, P.; Imperi, F. Ferric Uptake Regulator Fur Is Conditionally Essential in Pseudomonas aeruginosa. J. Bacteriol. 2017, 199, 22. [Google Scholar] [CrossRef] [PubMed]
- Becerra, G.; Igeño, M.I.; Merchán, F.; Sánchez-Clemente, R.; Blasco, R. New evolving strategies revealed by transcriptomic analysis of a fur-mutant of the cyanotrophic bacterium Pseudomonas pseudoalcaligenes CECT 5344. Microb. Biotechnol. 2020, 13, 148–161. [Google Scholar] [CrossRef]
- Duca, D.R.; Glick, B.R. Indole-3-acetic acid biosynthesis and its regulation in plant-associated bacteria. Appl. Microbiol. Biotechnol. 2020, 104, 8607–8619. [Google Scholar] [CrossRef]
- McClerklin, S.A.; Lee, S.G.; Harper, C.P.; Nwumeh, R.; Jez, J.M.; Kunkel, B.N. Indole-3-acetaldehyde dehydrogenase-dependent auxin synthesis contributes to virulence of Pseudomonas syringae strain DC3000. PLoS Pathog. 2018, 14, e1006811. [Google Scholar] [CrossRef]
- Amina, M. Plant Growth-Promotion and IAA Secretion with Pseudomonas fluorescens and Pseudomonas putida Pseudomonas biofilm. J. Bot. Sci. 2017, 6. Available online: https://www.rroij.com/open-access/plant-growthpromotion-and-iaa-secretion-withpseudomonas-fluorescens-and-pseudomonas-putida-.pdf (accessed on 24 March 2022).
- Marzocchi, U.; Thorup, C.; Dam, A.S.; Schramm, A.; Risgaard-Petersen, N. Dissimilatory nitrate reduction by a freshwater cable bacterium. ISME J. 2021, 16, 50–57. [Google Scholar] [CrossRef]
- Huang, X.; Weisener, C.G.; Ni, J.; He, B.; Xie, D.; Li, Z. Nitrate assimilation, dissimilatory nitrate reduction to ammonium, and denitrification coexist in Pseudomonas putida Y-9 under aerobic conditions. Bioresour. Technol. 2020, 312, 123597. [Google Scholar] [CrossRef]
- Yan, L.; Wang, C.; Jiang, J.; Liu, S.; Zheng, Y.; Yang, M.; Zhang, Y. Nitrate removal by alkali-resistant Pseudomonas sp. XS-18 under aerobic conditions: Performance and mechanism. Bioresour. Technol. 2022, 344, 126175. [Google Scholar] [CrossRef] [PubMed]
- Nelkner, J.; Tejerizo, G.T.; Hassa, J.; Lin, T.W.; Witte, J.; Verwaaijen, B.; Winkler, A.; Bunk, B.; Spröer, C.; Overmann, J.; et al. Genetic Potential of the Biocontrol Agent Pseudomonas brassicacearum (Formerly P. trivialis) 3Re2-7 Unraveled by Genome Sequencing and Mining, Comparative Genomics and Transcriptomics. Genes 2019, 10, 601. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.X.; Kong, X.W.; Li, S.Y.; Chen, X.J.; Chen, X.J. Antibiotics of Pseudomonas protegens FD6 are essential for biocontrol activity. Australas. Plant Pathol. 2020, 49, 307–317. [Google Scholar] [CrossRef]
- Dutta, S.; Yu, S.-M.; Lee, Y.H. Assessment of the Contribution of Antagonistic Secondary Metabolites to the Antifungal and Biocontrol Activities of Pseudomonas fluorescens NBC275. Plant Pathol. J. 2020, 36, 491. [Google Scholar] [CrossRef]
- Talkal, R.; Tikariha, H.; Purohit, H. An Approach to In Silico Dissection of Bacterial Intelligence Through Selective Genomic Tools. Indian J. Microbiol. 2018, 58, 278–286. [Google Scholar] [CrossRef]
- Daura-Pich, O.; Hernández, I.; Pinyol-Escala, L.; Lara, J.M.; Martínez-Servat, S.; Fernández, C.; López-García, B. No antibiotic and toxic metabolites produced by the biocontrol agent Pseudomonas putida strain B2017. FEMS Microbiol. Lett. 2020, 367, fnaa075. [Google Scholar] [CrossRef]
- Krechel, A.; Faupel, A.; Hallmann, J.; Ulrich, A.; Berg, G. Potato-associated bacteria and their antagonistic potential towards plant-pathogenic fungi and the plant-parasitic nematode Meloidogyne incognita (Kofoid & White) Chitwood. Can. J. Microbiol. 2011, 48, 772–786. [Google Scholar] [CrossRef]
- Moronta-Barrios, F.; Gionechetti, F.; Pallavicini, A.; Marys, E.; Venturi, V. Bacterial Microbiota of Rice Roots: 16S-Based Taxonomic Profiling of Endophytic and Rhizospheric Diversity, Endophytes Isolation and Simplified Endophytic Community. Microorganisms 2018, 6, 14. [Google Scholar] [CrossRef]
- Nishu, S.D.; Hyun, H.R.; Lee, T.K. Complete genome sequence of drought tolerant plant growth-promoting rhizobacterium Glutamicibacter halophytocola DR408. Microbiol. Soc. Korea 2019, 55, 300–302. [Google Scholar] [CrossRef]
- Deng, P.; Wang, X.; Baird, S.M.; Lu, S.E. Complete genome of Pseudomonas chlororaphis strain UFB2, a soil bacterium with antibacterial activity against bacterial canker pathogen of tomato. Stand. Genom. Sci. 2015, 10, 117. [Google Scholar] [CrossRef]
- Loper, J.E.; Hassan, K.A.; Mavrodi, D.V.; Davis, E.W.; Lim, C.K.; Shaffer, B.T.; Elbourne, L.D.H.; Stockwell, V.O.; Hartney, S.L.; Breakwell, K.; et al. Comparative Genomics of Plant-Associated Pseudomonas spp.: Insights into Diversity and Inheritance of Traits Involved in Multitrophic Interactions. PLoS Genet. 2012, 8, e1002784. [Google Scholar] [CrossRef] [PubMed]
- Ortet, P.; Barakat, M.; Lalaouna, D.; Fochesato, S.; Barbe, V.; Vacherie, B.; Santaella, C.; Heulin, T.; Achouak, W. Complete Genome Sequence of a Beneficial Plant Root-Associated Bacterium, Pseudomonas brassicacearum. J. Bacteriol. 2011, 193, 3146. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Price, M.N.; Wetmore, K.M.; Waters, R.J.; Callaghan, M.; Ray, J.; Liu, H.; Kuehl, J.V.; Melnyk, R.A.; Lamson, J.S.; Suh, Y.; et al. Mutant phenotypes for thousands of bacterial genes of unknown function. Nature 2018, 557, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Chae, D.H.; Kim, D.R.; Cheong, M.S.; Lee, Y.B.; Kwak, Y.S. Investigating the Induced Systemic Resistance Mechanism of 2,4-Diacetylphloroglucinol (DAPG) using DAPG Hydrolase-Transgenic Arabidopsis. Plant Pathol. J. 2020, 36, 255. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Tan, H.; Chen, F.; Li, T.; Zhu, J.; Jian, Q.; Yuan, D.; Xu, L.; Hu, W.; Jiang, Y.; et al. Novel synthesized 2, 4-DAPG analogues: Antifungal activity, mechanism and toxicology. Sci. Rep. 2016, 6, 32266. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Verma, P.P.; Kaur, M. Identification of secondary metabolites produced byfluorescent Pseudomonads for controlling fungal pathogensof apple. Indian Phytopathol. 2017, 70, 452–456. [Google Scholar] [CrossRef]
- Müller, T.; Behrendt, U.; Ruppel, S.; Von Der Waydbrink, G.; Müller, M.E.H. Fluorescent Pseudomonads in the Phyllosphere of Wheat: Potential Antagonists against Fungal Phytopathogens. Curr. Microbiol. 2016, 72, 383–389. [Google Scholar] [CrossRef]
- Nesemann, K.; Braus-Stromeyer, S.A.; Harting, R.; Höfer, A.; Kusch, H.; Ambrosio, A.B.; Timpner, C.; Braus, G.H. Fluorescent pseudomonads pursue media-dependent strategies to inhibit growth of pathogenic Verticillium fungi. Appl. Microbiol. Biotechnol. 2017, 102, 817–831. [Google Scholar] [CrossRef]
- Suresh, P.; Varathraju, G.; Shanmugaiah, V.; Almaary, K.S.; Elbadawi, Y.B.; Mubarak, A. Partial purification and characterization of 2, 4-diacetylphloroglucinol producing Pseudomonas fluorescens VSMKU3054 against bacterial wilt disease of tomato. Saudi J. Biol. Sci. 2021, 28, 2155–2167. [Google Scholar] [CrossRef]
- Patel, J.K.; Archana, G. Engineered production of 2,4-diacetylphloroglucinol in the diazotrophic endophytic bacterium Pseudomonas sp. WS5 and its beneficial effect in multiple plant-pathogen systems. Appl. Soil Ecol. 2018, 124, 34–44. [Google Scholar] [CrossRef]
- Weller, D.M.; Mavrodi, D.V.; van Pelt, J.A.; Pieterse, C.M.J.; van Loon, L.C.; Bakker, P.A.H.M. Induced Systemic Resistance in Arabidopsis thaliana against Pseudomonas syringae pv. tomato by 2,4-Diacetylphloroglucinol-Producing Pseudomonas fluorescens. Phytopathology 2012, 102, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Sacherer, P.; Défago, G.; Haas, D. Extracellular protease and phospholipase C are controlled by the global regulatory gene gacA in the biocontrol strain Pseudomonas fluorescens CHA0. FEMS Microbiol. Lett. 1994, 116, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Duffy, B.K.; Defago, G. Controlling instability in gacS-gacA regulatory genes during inoculant production of Pseudomonas fluorescens biocontrol strains. Appl. Environ. Microbiol. 2000, 66, 3142–3150. [Google Scholar] [CrossRef] [PubMed]
- Van Den Broek, D.; Chin-A-Woeng, T.F.C.; Eijkemans, K.; Mulders, I.H.M.; Bloemberg, G.V.; Lugtenberg, B.J.J. Biocontrol Traits of Pseudomonas spp. Are Regulated by Phase Variation. Mol. Plant-Microbe Interact. 2007, 16, 1003–1012. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).