Invasive Alien Plant Species—Raising Awareness of a Threat to Biodiversity and Ecological Connectivity (EC) in the Adriatic-Ionian Region
Abstract
1. Introduction
2. Spread of the Selected Alien Plant Species in the Adriatic-Ionian Region
3. Species Description and Key Ecological Characteristics
3.1. Common Milkweed
3.2. Jerusalem Artichoke
3.3. Japanese Knotweed and Bohemian Knotweed
3.4. Giant Hogweed
3.5. Giant Goldenrod and Canadian Goldenrod
3.6. Bermuda Buttercup
4. Impact on Biodiversity—Evidence from the Broader European Territory
5. Available Management Practices
5.1. Preventative Measures
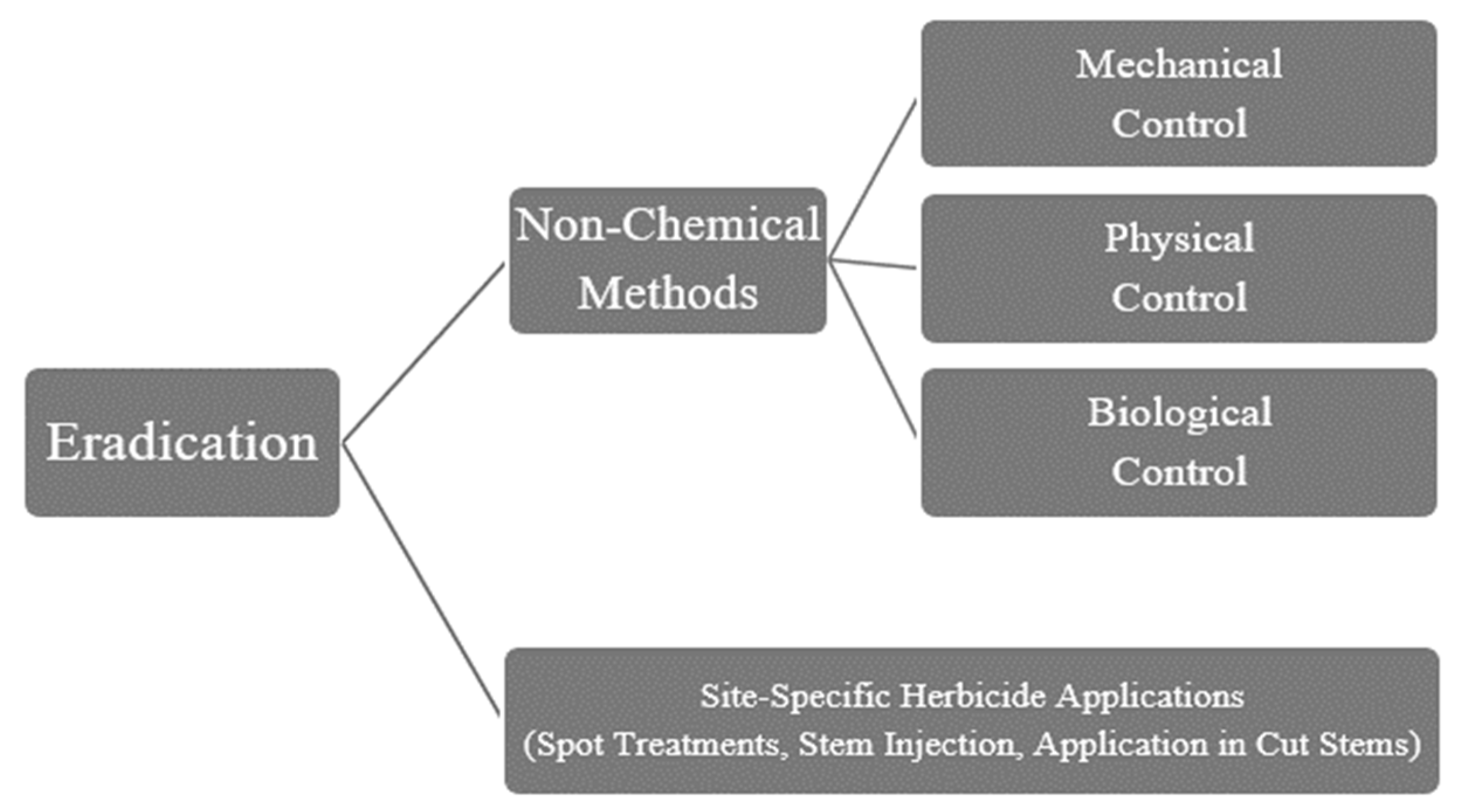
5.2. Eradication Methods
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haddad, N.M.; Brudvig, L.A.; Clobert, J.; Davies, K.F.; Gonzalez, A.; Holt, R.D.; Lovejoy, T.E.; Sexton, J.O.; Austin, M.P.; Collins, C.D.; et al. Habitat fragmentation and its lasting impact on earth’s ecosystems. Sci. Adv. 2015, 1, e1500052. [Google Scholar] [CrossRef] [PubMed]
- Perrin, M.; Bertrand, N.; Vanpeene, S. Ecological connectivity in spatial planning: From the EU framework to its territorial implementation in the French context. Environ. Sci. Policy 2022, 129, 118–125. [Google Scholar] [CrossRef]
- Transboundary Ecological Connectivity of Alps and Dinaric Mountains. Available online: https://dinalpconnect.adrioninterreg.eu/ (accessed on 13 April 2022).
- Hanski, I.; Ovaskainen, O. The metapopulation capacity of a fragmented landscape. Nature 2000, 404, 755–758. [Google Scholar] [CrossRef] [PubMed]
- Montgelard, C.; Zenboudji, S.; Ferchaud, A.-L.; Arnal, V.; van Vuuren, B.J. Landscape genetics in mammals. Mammalia 2014, 78, 139–157. [Google Scholar] [CrossRef]
- De la Fuente, B.; Beck, P.S. Invasive species may disrupt protected area networks: Insights from the pine wood nematode spread in Portugal. Forests 2018, 9, 282. [Google Scholar] [CrossRef]
- Gregory, A.; Spence, E.; Beier, P.; Garding, E. Toward best management practices for ecological corridors. Land 2021, 10, 140. [Google Scholar] [CrossRef]
- DINALPCONNECT, 2021: Report on Situation Analysis of Current Agricultural and Forestry Practices Affecting EC in Pilot Regions. EU Interreg Adrion; DINALPCONNECT Project. Available online: https://3.basecamp.com/3617449/buckets/15708478/documents/3948097125 (accessed on 13 April 2022).
- Abgrall, C.; Forey, E.; Mignot, L.; Chauvat, M. Invasion by Fallopia japonica alters soil food webs through secondary metabolites. Soil Biol. Biochem. 2018, 127, 100–109. [Google Scholar] [CrossRef]
- Jurová, J.; Renčo, M.; Gömöryová, E.; Čerevková, A. Effects of the invasive common milkweed (Asclepias syriaca) on nematode communities in natural grasslands. Nematology 2020, 22, 423–438. [Google Scholar] [CrossRef]
- Lazzaro, L.; Ferretti, G.; Bianchi, E.; Benesperi, R. Treatment by glyphosate—Based herbicide allowed recovering native species after Oxalis pes-caprae L. invasion: Indications from a Mediterranean island. Plant Biosyst. 2019, 153, 651–659. [Google Scholar] [CrossRef]
- Renčo, M.; Jurová, J.; Gömöryová, E.; Čerevková, A. Long-term giant hogweed invasion contributes to the structural changes of soil nematofauna. Plants 2021, 10, 2103. [Google Scholar] [CrossRef]
- Arianoutsou, M.; Bazos, I.; Delipetrou, P.; Kokkoris, Y. The alien flora of Greece: Taxonomy, life traits and habitat preferences. Biol. Invasions 2010, 12, 3525–3549. [Google Scholar] [CrossRef]
- Barina, Z.; Rakaj, M.; Somogyi, G.; Erős-Honti, Z.; Pifkó, D. The alien flora of Albania: History, current status and future trends. Weed Res. 2014, 54, 196–215. [Google Scholar] [CrossRef]
- Barudanović, S.; Zečić, E.; Macanović, A.; Duraković, B.; Mašić, E. Invasive alien plant species in global perspectives with special references to Bosnia and Herzegovina. In Invasive Alien Species: Observations and Issues from around the World, 1st ed.; Pullaiah, T., Ielmini, M.R., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2021; pp. 215–252. [Google Scholar]
- Boršić, I.; Ješovnik, A.; Mihinjač, T.; Kutleša, P.; Slivar, S.; Cigrovski Mustafić, M.; Desnica, S. Invasive alien species of Union concern (Regulation 1143/2014) in Croatia. Nat. Croat. 2018, 27, 357–398. [Google Scholar] [CrossRef]
- Galasso, G.; Conti, F.; Peruzzi, L.; Ardenghi, N.M.G.; Banfi, E.; Celesti–Grapow, L.; Albano, A.; Alessandrini, A.; Bacchetta, G.; Ballelli, S.; et al. An updated checklist of the vascular flora alien to Italy. Plant Biosyst. 2018, 152, 556–592. [Google Scholar] [CrossRef]
- Küzmič, F.; Šilc, U. Alien species in different habitat types of Slovenia: Analysis of vegetation database. Period. Biol. 2017, 119, 199–208. [Google Scholar] [CrossRef]
- Stešević, D.; Petrović, D. Preliminary list of plant invaders in Montenegro. Biol. Nyssana 2010, 1, 35–42. [Google Scholar]
- Barney, J.N.; Tharayil, N.; DiTommaso, A.; Bhowmik, P.C. The biology of invasive alien plants in Canada. 5. Polygonum cuspidatum Sieb. & Zucc.[= Fallopia japonica (Houtt.) Ronse Decr.]. Can. J. Plant Sci. 2006, 86, 887–906. [Google Scholar]
- Follak, S.; Bakacsy, L.; Essl, F.; Hochfellner, L.; Lapin, K.; Schwarz, M.; Tokarska-Guzik, B.; Wołkowycki, D. Monograph of invasive plants in Europe N°6: Asclepias syriaca L. Bot. Lett. 2021, 168, 422–451. [Google Scholar] [CrossRef]
- Paspatis, E.A. Chemical, cultural and biological control of Oxalis pes-caprae in vineyards in Greece. In Weed Control on Vine and Soft Fruits, 1st ed.; Cavalloro, R., Robinson, D.W., Eds.; CRC Press: Boca Raton, FL, USA, 1987; pp. 27–29. [Google Scholar]
- Weber, E.; Jakobs, G. Biological flora of central Europe: Solidago gigantea Aiton. Flora Morphol. Distrib. Funct. Ecol. Plants 2005, 200, 109–118. [Google Scholar] [CrossRef]
- Werner, P.A.; Gross, R.S.; Bradbury, I.K. The biology of Canadian weeds.: 45. Solidago canadensis L. Can. J. Plant Sci. 1980, 60, 1393–1409. [Google Scholar] [CrossRef]
- Bailey, J.P.; Bímová, K.; Mandák, B. Asexual spread versus sexual reproduction and evolution in Japanese Knotweed s.l. sets the stage for the “Battle of the Clones”. Biol. Invasions 2009, 11, 1189–1203. [Google Scholar] [CrossRef]
- Ministrstvo za Okolje in Proctor. Strokovne Podlage za Program Ukrepov za Obvladovanje Invazivnih Tujerodnih vrst za vrsto Sirska Svilnica (Asclepias syriaca L.) [Expert Basis for the Program of Measures for the Control of Invasive Alien Species for the Species Common Milkweed (Asclepias syriaca L.)]. Ljubljana (Slovenia): Zavod Republike Slovenije za Varstvo Narave. 2018. Available online: https://zrsvn-varstvonarave.si/wp-content/uploads/2020/08/Strokovne-podlage-za-sirsko-svilnico.pdf (accessed on 11 April 2022).
- Maslo, S. Preliminary list of invasive alien plant species (IAS) in Bosnia and Herzegovina. Herbologia 2016, 16, 1–14. [Google Scholar] [CrossRef]
- Tesio, F.; Weston, L.A.; Vidotto, F.; Ferrero, A. Potential allelopathic effects of Jerusalem artichoke (Helianthus tuberosus) leaf tissues. Weed Technol. 2010, 24, 378–385. [Google Scholar] [CrossRef]
- Mori, E.; Mazza, G.; Galimberti, A.; Angiolini, C.; Bonari, G. The porcupine as “Little Thumbling”: The role of Hystrix cristata in the spread of Helianthus tuberosus. Biologia 2017, 72, 1211–1216. [Google Scholar] [CrossRef]
- Zelnik, I. The presence of invasive alien plant species in different habitats: Case study from Slovenia. Acta Biol. Slov. 2012, 55, 25–38. [Google Scholar]
- Follak, S.; Eberius, M.; Essl, F.; Fürdös, A.; Sedlacek, N.; Trognitz, F. Invasive alien plants along roadsides in Europe. EPPO Bull. 2018, 48, 256–265. [Google Scholar] [CrossRef]
- Radisek, S.; Jakse, J.; Zhao, T.T.; Cho, S.E.; Shin, H.D. First report of powdery mildew of Helianthus tuberosus caused by Golovinomyces ambrosiae in Slovenia. J. Plant Pathol. 2018, 100, 331. [Google Scholar] [CrossRef]
- Hulina, N. “Planta hortifuga” in flora of the continental part of Croatia. Agric. Conspec. Sci. 2010, 75, 57–65. [Google Scholar]
- Vuković, N.; Bernardić, A.; Nikolić, T.; Hršak, V.; Plazibat, M.; Jelaska, S.D. Analysis and distributional patterns of the invasive flora in a protected mountain area—A case study of Medvednica Nature Park (Croatia). Acta Soc. Bot. Pol. 2010, 79, 285–294. [Google Scholar] [CrossRef]
- Mincheva, T.; Barni, E.; Siniscalco, C. From plant traits to invasion success: Impacts of the alien Fallopia japonica (Houtt.) Ronse Decraene on two native grassland species. Plant Biosyst. 2016, 150, 1348–1357. [Google Scholar] [CrossRef]
- Siniscalco, C.; Barni, E. Are non-native plant species a threat to the Alps? Insights and perspectives. In Climate Gradients and Biodiversity in Mountains of Italy, 1st ed.; Pedrotti, F., Ed.; Springer: Cham, Switzerland, 2018; pp. 91–107. [Google Scholar]
- Dorigo, W.; Lucieer, A.; Podobnikar, T.; Čarni, A. Mapping invasive Fallopia japonica by combined spectral, spatial, and temporal analysis of digital orthophotos. Int. J. Appl. Earth Obs. Geoinf. 2012, 19, 185–195. [Google Scholar] [CrossRef]
- Vuković, N.; Šegota, V.; Alegro, A.; Koletić, N.; Rimac, A.; Dekanić, S. “Flying under the radar”—How misleading distributional data led to wrong appreciation of knotweeds invasion (Reynoutria spp.) in Croatia. Bioinvasions Rec. 2019, 8, 175–189. [Google Scholar] [CrossRef]
- Bailey, J. The Japanese knotweed invasion of Europe: Τhe potential for further evolution in non-native regions. In Biological Invasion of Ecosystems by Pests and Beneficial Organisms, 1st ed.; Yano, E., Matsuo, K., Shiyomi, M., Andow, D., Eds.; NIAES Series 3; NIAES: Tsukuba, Japan, 1999; pp. 27–37. [Google Scholar]
- Jovanović, S.; Hlavati-Širka, V.; Lakušić, D.; Jogan, N.; Nikolić, T.; Anastasiu, P.; Vladimirov, V.; Šinžar-Sekulić, J. Reynoutria niche modelling and protected area prioritization for restoration and protection from invasion: A Southeastern Europe case study. J. Nat. Conserv. 2018, 41, 1–15. [Google Scholar] [CrossRef]
- Giuliani, C.; Lastrucci, L.; Cresti, L.; Santini, G.; Fogg, B.; Lippi, M.M. The morphology and activity of the extrafloral nectaries in Reynoutria x bohemica (Polygonaceae). Plant Biol. 2019, 21, 975–985. [Google Scholar] [CrossRef] [PubMed]
- Lazzaro, L.; Bolpagni, R.; Barni, E.; Brundu, G.; Blasi, C.; Siniscalco, C.; Celesti-Grapow, L. Towards alien plant prioritization in Italy: Methodological issues and first results. Plant Biosyst. 2019, 153, 740–746. [Google Scholar] [CrossRef]
- Boršić, I.; Borovečki-Voska, L.; Kutleša, P.; Šemnički, P. New localities of Heracleum mantegazzianum Sommier et Levier (Apiaceae) in Croatia and control measures taken. Period. Biol. 2015, 117, 449–452. [Google Scholar] [CrossRef]
- Maslo, S. Giant hogweed Heracleum mantegazzianum Sommier &Levier—A new non-indigenous species in the flora of Bosnia and Herzegovina. Herbologia 2010, 11, 17–24. [Google Scholar]
- Jahodová, Š.; Fröberg, L.; Pyšek, P.; Geltman, D.; Trybush, S.; Karp, A. Taxonomy, identification, genetic relationships and distribution of large Heracleum species in Europe. In Ecology and Management of Giant Hogweed (Heracleum mantegazzianum); Pyšek, P., Cock, M.J.W., Nentwig, W., Ravn, H.P., Eds.; CABI: Cambridge, MA, USA, 2007; pp. 1–19. [Google Scholar]
- Marinšek, A.; Kutnar, L. Occurrence of invasive alien plant species in the floodplain forests along the Mura River in Slovenia. Period. Βiol. 2017, 119, 251–260. [Google Scholar] [CrossRef]
- Šajna, N. Alien plant species invading rare and protected habitats in Slovenia. In Weed and Pest Control—Molecular Biology, Practices and Environmental Impact, 1st ed.; Travlos, I.S., Bilalis, D., Chachalis, D., Eds.; Nova Publishers: New York, NY, USA, 2016; pp. 35–54. [Google Scholar]
- Boršić, I.; Milović, M.; Dujmović, I.; Bogdanović, S.; Cigić, P.; Rešetnik, I.; Nikolić, T.; Mitić, B. Preliminary check-list of invasive alien plant species (IAS) in Croatia. Nat. Croat. 2008, 17, 55–71. [Google Scholar]
- Novak, N.; Novak, M. The differences in the invasiveness of some alien plant species between continental and coastal part of Croatia. Poljoprivreda 2018, 24, 63–69. [Google Scholar] [CrossRef]
- Stešević, D.; Caković, D. Contribution to the alien flora of Montenegro and Supplementum to the Preliminary list of plant invaders. Biol. Nyssana 2013, 4, 1–7. [Google Scholar]
- Stesevic, D.; Bubanja, N. Five new alien species in the flora of Montenegro: Coreopsis tinctoria Nutt., Ipomoea indica (Burm.) Merr., Lupinus × regalis Bergmans, Physalis angulata L., and Solidago canadensis L. and new possible threats to the biodiversity. Acta Bot. Croat. 2017, 76, 98–102. [Google Scholar] [CrossRef]
- Lazzaro, L.; Bolpagni, R.; Buffa, G.; Gentili, R.; Lonati, M.; Stinca, A.; Acosta, A.T.R.; Adorni, M.; Aleffi, M.; Allegrezza, M.; et al. Impact of invasive alien plants on native plant communities and Natura 2000 habitats: State of the art, gap analysis and perspectives in Italy. J. Environ. Manag. 2020, 274, 111140. [Google Scholar] [CrossRef] [PubMed]
- Straub, S.C.; Parks, M.; Weitemier, K.; Fishbein, M.; Cronn, R.C.; Liston, A. Navigating the tip of the genomic iceberg: Next-generation sequencing for plant systematics. Am. J. Bot. 2012, 99, 349–364. [Google Scholar] [CrossRef] [PubMed]
- United States Department of Agriculture (USDA). PLANTS Database. Common Milkweed—Asclepias syriaca L. Available online: https://plants.usda.gov/home/plantProfile?symbol=assy (accessed on 9 February 2022).
- Bhowmik, P.C.; Bandeen, J.D. The biology of Canadian weeds: 19. Asclepias syriaca L. Can. J. Plant Sci. 1976, 56, 579–589. [Google Scholar] [CrossRef]
- United States Department of Agriculture (USDA). Plant Guide. Common Milkweed—Asclepias syriaca L. Available online: https://plants.usda.gov/DocumentLibrary/plantguide/pdf/pg_assy.pdf (accessed on 9 February 2022).
- Jeffery, L.S.; Robison, L.R. Growth characteristics of common milkweed. Weed Sci. 1971, 19, 193–196. [Google Scholar] [CrossRef]
- Gudžinskas, Z.; Petrulaitis, L.; Žalneravičius, E. Asclepias speciosa (Apocynaceae, Asclepiadoideae): A rare or unrecognized alien species in Europe? PhytoKeys 2019, 121, 29–41. [Google Scholar] [CrossRef]
- Wyatt, R.; Broyles, S.B. Ecology and evolution of reproduction in milkweeds. Annu. Rev. Ecol. Syst. 1994, 25, 423–441. [Google Scholar] [CrossRef]
- Anderson, W.P. Perennial Weeds. In Characteristics and Identification of Selected Herbaceous Species, 1st ed.; Iowa State University Press: Ames, IA, USA, 1999; p. 228. [Google Scholar]
- Csontos, P.; Bozsing, E.; Cseresnyes, I.; Penksza, K. Reproductive potential of the alien species Asclepias syriaca (Asclepiadaceae) in the rural landscape. Pol. J. Ecol. 2009, 57, 383–388. [Google Scholar]
- Oegama, T.; Fleitcher, R.A. Factors that influence dormancy in Milkweed seeds. Can. J. Bot. 1972, 50, 713–718. [Google Scholar] [CrossRef]
- Bagi, I. Common milkweed (Asclepias syriaca L.). In The Most Important Invasive Plants in Hungary, 1st ed.; Botta-Dukát, Z., Balogh, L., Eds.; HAS Institute of Ecology and Botany: Vácrátót, Hungary, 2008; pp. 151–158. [Google Scholar]
- Platt, W.J.; Weis, I.M. Resource partitioning and competition within a guild of fugitive Prairie plants. Am. Nat. 1977, 111, 479–513. [Google Scholar] [CrossRef]
- Moravcová, L.; Pyšek, P.; Jarošík, V.; Havlíčková, V.; Zákravský, P. Reproductive characteristics of neophytes in the Czech Republic: Traits of invasive and non-invasive species. Preslia 2010, 82, 365–390. [Google Scholar]
- Novák, R.; Dancza, I.; Szentey, L.; Karamán, J.; Béres, I.; Kazinczi, G.; Gólya, G. Arable Weeds of Hungary. In Fifth National Weed Survey (2007–2008); Ministry of Agriculture and Rural Development: Budapest, Hungary, 2009; p. 95. [Google Scholar]
- Szilassi, P.; Szatmári, G.; Pásztor, L.; Árvai, M.; Szatmári, J.; Szitár, K.; Papp, L. Understanding the environmental background of an invasive plant species (Asclepias syriaca) for the future: An application of LUCAS field photographs and machine learning algorithm methods. Plants 2019, 8, 593. [Google Scholar] [CrossRef]
- Tokarska-Guzik, B.; Pisarczyk, Ε. Risk Assessment of Asclepias syriaca. 2015. Available online: https://www.codeplantesenvahissantes.fr/fileadmin/PEE_Ressources/TELECHARGEMENT/Asclepias_syriaca_RA.pdf (accessed on 30 June 2020).
- Kalloo, G. Jerusalem artichoke: Helianthus tuberosus L. In Genetic Improvement of Vegetable Crops, 1st ed.; Kalloo, G., Bergh, B.O., Eds.; Pergamon Press: Oxford, UK, 1993; pp. 747–750. [Google Scholar]
- Kocurek, M.; Pilarski, J. Activity of C4 enzymes in C3-type herbaceous plants. Photosynthetica 2011, 49, 473–477. [Google Scholar] [CrossRef]
- Pacanoski, Z.; Mehmeti, A. The first report of the invasive alien weed Jerusalem artichoke (Helianthus tuberosus L.) in the Republic of North Macedonia. Agric. For. 2020, 66, 115–127. [Google Scholar] [CrossRef]
- Swanton, C.J.; Clements, D.R.; Moore, M.J.; Cavers, P.B. The biology of Canadian weeds. 101. Helianthus tuberosus L. Can. J. Plant Sci. 1992, 72, 1367–1382. [Google Scholar] [CrossRef]
- Bussmann, R.W.; Batsatsashvili, K.; Kikvidze, Z.; Paniagua-Zambrana, N.Y.; Khutsishvili, M.; Maisaia, I.; Sikharulidze, S.; Tchelidze, D. Helianthus annuus L. Helianthus tuberosus L. Asteraceae. In Ethnobotany of the Mountain Regions of Far Eastern Europe: Ural, Northern Caucasus, Turkey, and Iran, 1st ed.; Batsatsashvili, K., Kikvidze, Z., Bussmann, R.W., Eds.; Springer: Cham, Switzerland, 2020; pp. 453–458. [Google Scholar]
- Denisow, B.; Tymoszuk, K.; Dmitruk, M. Nectar and pollen production of Helianthus tuberosus L.—An exotic plant with invasiveness potential. Acta Bot. Croat. 2019, 78, 135–141. [Google Scholar] [CrossRef]
- Kanatas, P.; Gazoulis, I.; Zannopoulos, S.; Tataridas, A.; Tsekoura, A.; Antonopoulos, N.; Travlos, I. Shattercane (Sorghum bicolor (L.) Moench Subsp. Drummondii) and weedy sunflower (Helianthus annuus L.)—Crop Wild Relatives (CWRs) as weeds in agriculture. Diversity 2021, 13, 463. [Google Scholar] [CrossRef]
- Kays, S.J.; Nottingham, S.F. Biology and Chemistry of Jerusalem Artichoke: Helianthus tuberosus L.; Taylor & Francis Group: London, UK, 2008. [Google Scholar]
- Kosaric, N.; Cosentino, G.P.; Wieczorek, A.; Duvnjak, Z. The Jerusalem artichoke as an agricultural crop. Biomass 1984, 5, 1–36. [Google Scholar] [CrossRef]
- Kliszcz, A. Phenological Growth Stages and BBCH—Identification Keys of Jerusalem artichoke (Helianthus Tuberosus L.). Ann. Univ. Paedagog. Crac. Stud. Nat. 2021, 6, 203–225. [Google Scholar] [CrossRef]
- Podlaski, S.; Pietkiewicz, S.; Choluj, D.; Horaczek, T.; Wisniewski, G.; Gozdowski, D.; Kalaji, H.M. The relationship between the soil water storage and water-use efficiency of seven energy crops. Photosynthetica 2017, 55, 210–218. [Google Scholar] [CrossRef]
- Terzić, S.; Altagić, J.; Maksimović, I.; Zeremski, T.; Petrović, S.; Dedić, B. Influence of photoperiod on vegetation phases and tuber development in topinambour (Helianthus tuberosus L.). Arch. Biol. Sci. 2012, 64, 175–182. [Google Scholar] [CrossRef]
- Žgančíková, I.; Vereš, T.; Čurná, V. Monitoring of the Helianthus tuberosus (L.)—As an invasive weed of natural ecosystems. Res. J. Agric. Sci. 2012, 44, 127–130. [Google Scholar]
- Kompała-Bąba, A.; Błońska, A. Plant communities with Helianthus tuberosus L. in the towns of the Upper Silesian Industrial Region (southern Poland). Biodivers. Res. Conserv. 2008, 11, 57–64. [Google Scholar]
- Liava, V.; Karkanis, A.; Danalatos, N.; Tsiropoulos, N. Cultivation practices, adaptability and phytochemical composition of Jerusalem artichoke (Helianthus tuberosus L.): A weed with economic value. Agronomy 2021, 11, 914. [Google Scholar] [CrossRef]
- Rossini, F.; Provenzano, M.E.; Kuzmanović, L.; Ruggeri, R. Jerusalem artichoke (Helianthus tuberosus L.): A versatile and sustainable crop for renewable energy production in Europe. Agronomy 2019, 9, 528. [Google Scholar] [CrossRef]
- Filep, R.; Balogh, L.; Balázs, V.L.; Farkas, Á.; Pal, R.W.; Czigle, S.; Czégényi, D.; Papp, N. Helianthus tuberosus L. agg. in the Carpathian Basin: A blessing or a curse? Genet. Resour. Crop Evol. 2018, 65, 865–879. [Google Scholar] [CrossRef]
- Mandák, B.; Pyšek, P.; Lysák, M.; Suda, J.; Krahulcová, A.; Bímová, K. Variation in DNA—Ploidy levels of Reynoutria taxa in the Czech Republic. Ann. Bot. 2003, 92, 265–272. [Google Scholar] [CrossRef]
- Shaw, D. Fallopia japonica (Japanese knotweed). In Invasive Species Compendium; CABI: Wallingford, UK, 2013. [Google Scholar]
- Beerling, D.J.; Bailey, J.P.; Conolly, A.P. Fallopia japonica (Houtt.) Ronse Decraene. J. Ecol. 1994, 82, 959–979. [Google Scholar] [CrossRef]
- Bram, M.R.; McNair, J.N. Seed germinability and its seasonal onset of Japanese knotweed (Polygonum cuspidatum). Weed Sci. 2004, 52, 759–767. [Google Scholar] [CrossRef]
- Tiébré, M.S.; Bizoux, J.P.; Hardy, O.J.; Bailey, J.P.; Mahy, G. Hybridization and morphogenetic variation in the invasive alien Fallopia (Polygonaceae) complex in Belgium. Am. J. Bot. 2007, 94, 1900–1910. [Google Scholar] [CrossRef] [PubMed]
- Forman, J.; Kesseli, R.V. Sexual reproduction in the invasive species Fallopia japonica (Polygonaceae). Am. J. Bot. 2003, 90, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Engler, J.; Abt, K.; Buhk, C. Seed characteristics and germination limitations in the highly invasive Fallopia japonica (Polygonaceae). Ecol. Res. 2011, 26, 555–562. [Google Scholar] [CrossRef]
- Bímová, K.; Mandák, B.; Pyšek, P. Experimental study of vegetative regeneration in four invasive Reynoutria taxa (Polygonaceae). Plant Ecol. 2003, 166, 1–11. [Google Scholar] [CrossRef]
- Hollingsworth, M.L.; Bailey, J.P. Evidence for massive clonal growth in the invasive weed Fallopia japonica (Japanese Knotweed). Bot. J. Linn. Soc. 2000, 133, 463–472. [Google Scholar] [CrossRef]
- De Waal, L.C. A viability study of Fallopia japonica stem tissue. Weed Res. 2001, 41, 447–460. [Google Scholar] [CrossRef]
- Bashtanova, U.B.; Beckett, K.P.; Flowers, T.J. Physiological approaches to the improvement of chemical control of Japanese knotweed (Fallopia japonica). Weed Sci. 2009, 57, 584–592. [Google Scholar] [CrossRef]
- Adachi, N.; Terashima, Ι.; Takahashi, M. Central die–back of monoclonal stands of Reynoutria japonica in an early stage of primary succession on Mount Fuji. Ann. Bot. 1996, 77, 477–486. [Google Scholar] [CrossRef][Green Version]
- Price, E.; Gamble, R.; Williams, G.; Marshall, C. Seasonal patterns of partitioning and remobilization of 14C in the invasive rhizomatous perennial Japanese knotweed. Evol. Ecol. 2002, 15, 347–362. [Google Scholar] [CrossRef]
- Funkenberg, T.I.M.; Roderus, D.; Buhk, C. Effects of climatic factors on Fallopia japonica seedling establishment: Evidence from laboratory experiments. Plant Species Biol. 2012, 27, 218–225. [Google Scholar] [CrossRef]
- Weston, L.A.; Barney, J.N.; DiTommaso, A. A review of the biology and ecology three invasive perennials in New York State: Japanese knotweed (Polygonum cuspidatum), mugwort (Artemisia vulgaris) and pale swallow—Wort (Vincetoxicum rossicum). Plant Soil 2005, 277, 53–69. [Google Scholar] [CrossRef]
- Drazan, D.; Smith, A.G.; Anderson, N.O.; Becker, R.; Clark, M. History of knotweed (Fallopia spp.) invasiveness. Weed Sci. 2021, 69, 617–623. [Google Scholar] [CrossRef]
- Gaskin, J.; Schwarzländer, M.; Grevstad, F.; Haverhals, M.; Bourchier, R.; Miller, T. Extreme differences in population structure and genetic diversity for three invasive congeners: Knotweeds in western North America. Biol. Invasions 2014, 16, 2127–2136. [Google Scholar] [CrossRef]
- Gillies, S.; Clements, D.R.; Grenz, J. Knotweed (Fallopia spp.) invasion of North America utilizes hybridization, epigenetics, seed dispersal (unexpectedly), and an arsenal of physiological tactics. Invasive Plant Sci. Manag. 2016, 9, 71–80. [Google Scholar] [CrossRef]
- Rouifed, S.; Puijalon, S.; Viricel, M.-R.; Piola, F. Achene buoyancy and germinability of the terrestrial invasive Fallopia × bohemica in aquatic environment: A new vector of dispersion? Écoscience 2011, 18, 79–84. [Google Scholar] [CrossRef]
- Mandák, B.; Pyšek, P.; Bímová, K. History of the invasion and distribution of Reynoutria taxa in the Czech Republic: A hybrid spreading faster than its parents. Preslia 2004, 76, 15–64. [Google Scholar]
- Tiley, G.E.D.; Dodd, F.S.; Wade, P.M. Heracleum mantegazzianum Sommier & Levier. J. Ecol. 1996, 84, 297–319. [Google Scholar]
- Page, N.A.; Wall, R.E.; Darbyshire, S.J.; Mulligan, G.A. The biology of invasive alien plants in Canada. 4. Heracleum mantegazzianum Sommier & Levier. Can. J. Plant Sci. 2006, 86, 569–589. [Google Scholar]
- Moravcová, L.; Perglová, I.; Pyšek, P.; Jarošík, V.; Pergl, J. Effects of fruit position on fruit mass and seed germination in the alien species Heracleum mantegazzianum (Apiaceae) and the implications for its invasion. Acta Oecol. 2005, 28, 1–10. [Google Scholar] [CrossRef]
- Perglová, I.; Pergl, J.; Pyšek, P. Flowering phenology and reproductive effort of the invasive alien plant Heracleum mantegazzianum. Preslia 2006, 78, 265–285. [Google Scholar]
- Tiley, G.E.D.; Philp, B. Effects of cutting flowering stems of Giant Hogweed Heracleum mantegazzianum on reproductive performance. Asp. Appl. Biol. 2000, 58, 77–80. [Google Scholar]
- Moravcovà, L.; Pyšek, P.; Krinke, L.; Pergl, J.; Perglová, I.; Thompson, K. Seed germination, dispersal and seed bank in Heracleum mantegazzianum. In Ecology and Management of Giant Hogweed (Heracleum mantegazzianum); CABI: Wallingford, UK, 2007; pp. 74–91. [Google Scholar]
- Otte, A.; Franke, R. The ecology of the Caucasian herbaceous perennial Heracleum mantegazzianum Somm. et Lev. (Giant Hogweed) in cultural ecosystems of Central Europe. Phytocoenologia 1998, 28, 205–232. [Google Scholar] [CrossRef]
- Neiland, M.R.M.; Proctor, J.; Sexton, R. Giant hogweed (H. mantegazzianum Somm. & Levier) by the River Allan and part of the River Forth. Forth Nat. Hist. 1987, 9, 51–56. [Google Scholar]
- Wadsworth, R.A.; Collingham, Y.C.; Willis, S.G.; Huntley, B.; Hulme, P.E. Simulating the spread and management of alien riparian weeds: Are they out of control? J. Appl. Ecol. 2000, 37, 28–38. [Google Scholar] [CrossRef]
- Invasive Alien Species of Union Concern. Available online: https://ec.europa.eu/environment/nature/pdf/IAS_brochure_species.pdf (accessed on 8 April 2022).
- Hull-Sanders, H.M.; Johnson, R.H.; Owen, H.A.; Meyer, G.A. Effects of polyploidy on secondary chemistry, physiology, and performance of native and invasive genotypes of Solidago gigantea (Asteraceae). Am. J. Bot. 2009, 96, 762–770. [Google Scholar] [CrossRef]
- Smart, A.J.; Larson, G.E.; Bauman, P.J. Grass and Canada goldenrod (Solidago canadensis) competition and implications for management in the northern tallgrass Prairie. Prairie Nat. 2012, 45, 4–12. [Google Scholar]
- Schlaepfer, D.R.; Edwards, P.J.; Semple, J.C.; Billeter, R. Cytogeography of Solidago gigantea (Asteraceae) and its invasive ploidy level. J. Biogeogr. 2008, 35, 2119–2127. [Google Scholar] [CrossRef]
- Malecka, J. Studies on the genus Solidago L. IV. Cytoembryology of Solidago canadensis L. var. “scabra”. Acta Biol. Cracoviensa Ser. Bot. 1989, 31, 85–95. [Google Scholar]
- Musial, K. Studies on the genus Solidago L. III. Embryology of Solidago canadensis L. var. canadensis. Acta Biol. Cracoviensa Ser. Bot. 1989, 31, 73–84. [Google Scholar]
- Šutovská, M.; Capek, P.; Kocmálová, M.; Fraňová, S.; Pawlaczyk, I.; Gancarz, R. Characterization and biological activity of Solidago canadensis complex. Int. J. Biol. Macromol. 2013, 52, 192–197. [Google Scholar] [CrossRef]
- Musiał, K.; Pagitz, K.; Gudžinskas, Z.; Łazarski, G.; Pliszko, A. Chromosome numbers in hybrids between invasive and native Solidago (Asteraceae) species in Europe. Phytotaxa 2020, 471, 267–275. [Google Scholar] [CrossRef]
- Huang, H.; Guo, S.; Chen, G. Reproductive biology in an invasive plant Solidago canadensis. Front. Biol. China 2007, 2, 196–204. [Google Scholar] [CrossRef]
- Weber, E. Strong regeneration ability from rhizome fragments in two invasive clonal plants (Solidago canadensis and S. gigantea). Biol. Invasions 2011, 13, 2947–2955. [Google Scholar] [CrossRef]
- Jakobs, G.; Weber, E.; Edwards, P.J. Introduced plants of the invasive Solidago gigantea (Asteraceae) are larger and grow denser than conspecifics in the native range. Divers. Distrib. 2004, 10, 11–19. [Google Scholar] [CrossRef]
- Cheng, J.; Yang, X.; Xue, L.; Yao, B.; Lu, H.; Tian, Z.; Li, J.; Zhou, X.; Zhang, Y.; Zia-ul-Haq, M.; et al. Polyploidization contributes to evolution of competitive ability: A long term common garden study on the invasive Solidago canadensis in China. Oikos 2020, 129, 700–713. [Google Scholar] [CrossRef]
- Botta-Dukat, Z.; Dancza, I. Morphological plasticity in the rhizome system of Solidago gigantea: Comparison of wet and dry habitats. In Proceedings of the Sixth International Conference on the Ecology and Management of Invasive Plants, Emapi, Loughborough, UK, 12–15 September 2001. [Google Scholar]
- Dudek, K.; Michlewicz, M.; Dudek, M.; Tryjanowski, P. Invasive Canadian goldenrod (Solidago canadensis L.) as a preferred foraging habitat for spiders. Arthropod Plant Interact. 2016, 10, 377–381. [Google Scholar] [CrossRef]
- Benelli, G.; Pavela, R.; Cianfaglione, K.; Nagy, D.U.; Canale, A.; Maggi, F. Evaluation of two invasive plant invaders in Europe (Solidago canadensis and Solidago gigantea) as possible sources of botanical insecticides. J. Pest Sci. 2019, 92, 805–821. [Google Scholar] [CrossRef]
- Schmid, B.; Puttick, G.M.; Burgess, K.H.; Bazzaz, F.A. Correlations between genet architecture and some life history features in three species of Solidago. Oecologia 1988, 75, 459–464. [Google Scholar] [CrossRef]
- Castro, S.; Ferrero, V.; Costa, J.; Sousa, A.J.; Navarro, L.; Loureiro, J. Reproductive strategy of the invasive Oxalis pes-caprae: Distribution patterns of flower morphs, ploidy levels and sexual reproduction. Biol. Invasions 2013, 15, 1863–1875. [Google Scholar] [CrossRef]
- Castro, S.; Castro, M.; Ferrero, V.; Costa, J.; Tavares, D.; Navarro, L.; Loureiro, J. Invasion fosters change: Independent evolutionary shifts in reproductive traits after Oxalis pes-caprae L. introduction. Front. Plant Sci. 2016, 7, 874. [Google Scholar] [CrossRef] [PubMed]
- Peirce, J.R. The biology of Australian weeds. 31. Oxalis pes-caprae L. Plant Prot. Q. 1997, 12, 110–119. [Google Scholar]
- Oberlander, K.C.; Emshwiller, E.; Bellstedt, D.U.; Dreyer, L.L. A model of bulb evolution in the eudicot genus Oxalis L. Mol. Phylogenet. Evol. 2009, 51, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Pütz, N. Vegetative spreading of Oxalis pes-caprae (Oxalidaceae). Plant Syst. Evol. 1994, 191, 57–67. [Google Scholar] [CrossRef]
- Brandes, D. Sociology and ecology of Oxalis pes-caprae L. in the Mediterranean region with special attention to Malta. Phytocoenologia 1991, 19, 285–306. [Google Scholar] [CrossRef]
- Damanakis, M.; Markaki, Μ. Studies on the biology of Oxalis pes-caprae L. under field conditions in Crete Greece. Zizaniology 1990, 2, 145–154. [Google Scholar]
- Roets, F.; Oberlander, K.C.; Dreyer, L.L. New relatives of Oxalis pes-caprae (Oxalidaceae) from South Africa. Blumea 2014, 59, 131–138. [Google Scholar] [CrossRef]
- Ornduff, R. Reproductive systems and chromossome races of Oxalis pes-caprae L. and their bearing on the genesis of a noxious weed. Ann. Mo. Bot. Gard. 1987, 74, 79–84. [Google Scholar] [CrossRef]
- Castro, S.; Loureiro, J.; Santos, C.; Ater, M.; Ayensa, G.; Navarro, L. Distribution of flower morphs, ploidy level and sexual reproduction of the invasive weed Oxalis pes-caprae in the Western area of the Mediterranean region. Ann. Bot. 2007, 99, 507–517. [Google Scholar] [CrossRef]
- Vilà, M.; Bartomeus, I.; Gimeno, I.; Traveset, A.; Moragues, E.V.A. Demography of the invasive geophyte Oxalis pes-caprae across a Mediterranean island. Ann. Bot. 2006, 97, 1055–1062. [Google Scholar] [CrossRef]
- Verdaguer, D.; Sala, A.; Vila, M. Effect of environmental factors and bulb mass on the invasive geophyte Oxalis pes-caprae development. Acta Oecol. 2010, 36, 92–99. [Google Scholar] [CrossRef]
- Petsikos, C.; Dalias, P.; Troumbis, A.Y. Effects of Oxalis pes-caprae L. invasion in olive groves. Agric. Ecosyst. Environ. 2007, 120, 325–329. [Google Scholar] [CrossRef]
- Bacieczko, W.; Borcz, A. Structure of Asclepias syriaca L. population against phytocenotic and habitat conditions in Widuchowa (West Pomerania). Biodivers. Res. Conserv. 2015, 40, 69–75. [Google Scholar] [CrossRef][Green Version]
- Evetts, L.L.; Burnside, O.C. Germination and seedling development of common milkweed and other species. Weed Sci. 1972, 20, 371–378. [Google Scholar] [CrossRef]
- Kelemen, A.; Valkó, O.; Kröel-Dulay, G.; Deák, B.; Török, P.; Tóth, K.; Miglécz, T.; Tóthmérész, B. The invasion of common milkweed (Asclepias syriaca) in sandy old-fields—Is it a threat to the native flora? Appl. Veg. Sci. 2016, 19, 218–224. [Google Scholar] [CrossRef]
- Gallé, R.; Erdélyi, N.; Szpisjak, N.; Tölgyesi, C.; Maák, I. The effect of the invasive Asclepias syriaca on the ground—Dwelling arthropod fauna. Biologia 2015, 70, 104–112. [Google Scholar] [CrossRef]
- Filep, R.; Pal, R.W.; Balázs, V.L.; Mayer, M.; Nagy, D.U.; Cook, B.J.; Farkas, Á. Can seasonal dynamics of allelochemicals play a role in plant invasions? A case study with Helianthus tuberosus L. Plant Ecol. 2016, 217, 1489–1501. [Google Scholar] [CrossRef]
- Dassonville, N.; Vanderhoeven, S.; Vanparys, V.; Hayez, M.; Gruber, W.; Meerts, P. Impacts of alien invasive plants on soil nutrients are correlated with initial site conditions in NW Europe. Oecologia 2008, 157, 131–140. [Google Scholar] [CrossRef]
- Lavoie, C. The impact of invasive knotweed species (Reynoutria spp.) on the environment: Review and research perspectives. Biol. Ιnvasions 2017, 19, 2319–2337. [Google Scholar] [CrossRef]
- Gerber, E.; Krebs, C.; Murrell, C.; Moretti, M.; Rocklin, R.; Schaffner, U. Exotic invasive knotweeds (Fallopia spp.) negatively affect native plant and invertebrate assemblages in European riparian habitats. Biol. Conserv. 2008, 141, 646–654. [Google Scholar] [CrossRef]
- Kato-Noguchi, H. Allelopathy of knotweeds as invasive plants. Plants 2021, 11, 3. [Google Scholar] [CrossRef]
- Neupert, M.; Margerie, P.; Forey, E.; Chauvat, M.; Bureau, F.; Aubert, M.; Prével, S.; Langlois, E.; Vincenot, L. The best of both worlds? Hybridization potentiates exotic Bohemian Knotweed’s (Reynoutria × bohemica) impacts on native plant and faunal communities. Biol. Life Sci. Forum 2021, 2, 20. [Google Scholar]
- Dassonville, N.; Guillaumaud, N.; Piola, F.; Meerts, P.; Poly, F. Niche construction by the invasive Asian knotweeds (species complex Fallopia): Impact on activity, abundance and community structure of denitrifiers and nitrifiers. Biol. Invasions 2011, 13, 1115–1133. [Google Scholar] [CrossRef]
- Dostál, P.; Müllerová, J.; Pyšek, P.; Pergl, J.; Klinerová, T. The impact of an invasive plant changes over time. Ecol. Lett. 2013, 16, 1277–1284. [Google Scholar] [CrossRef] [PubMed]
- Gioria, M.; Osborne, B. Assessing the impact of plant invasions on soil seed bank communities: Use of univariate and multivariate statistical approaches. J. Veg. Sci. 2009, 20, 547–556. [Google Scholar] [CrossRef]
- Thiele, J.; Otte, A. Impact of Heracleum mantegazzianum on invaded vegetation and human activities. In Ecology and Management of Giant Hogweed (Heracleum mantegazzianum); Pyšek, P., Cock, M.J.W., Nentwig, W., Ravn, H.P., Eds.; CABI: Cambridge, MA, USA, 2007; pp. 144–156. [Google Scholar]
- Jandová, K.; Dostál, P.; Cajthaml, T. Searching for Heracleum mantegazzianum allelopathy in vitro and in a garden experiment. Biol. Invasions 2015, 17, 987–1003. [Google Scholar] [CrossRef]
- Koutika, L.S.; Vanderhoeven, S.; Chapuis-Lardy, L.; Dassonville, N.; Meerts, P. Assessment of changes in soil organic matter after invasion by exotic plant species. Biol. Fertil. Soils 2007, 44, 331–341. [Google Scholar] [CrossRef]
- Pal, R.W.; Chen, S.; Nagy, D.U.; Callaway, R.M. Impacts of Solidago gigantea on other species at home and away. Biol. Invasions 2015, 17, 3317–3325. [Google Scholar] [CrossRef]
- Quist, C.W.; Vervoort, M.T.; Van Megen, H.; Gort, G.; Bakker, J.; Van der Putten, W.H.; Helder, J. Selective alteration of soil food web components by invasive giant goldenrod Solidago gigantea in two distinct habitat types. Oikos 2014, 123, 837–845. [Google Scholar] [CrossRef]
- Fenesi, A.; Vágási, C.I.; Beldean, M.; Földesi, R.; Kolcsár, L.-P.; Shapiro, J.T.; Török, E.; Kovács-Hostyánszki, A. Solidago canadensis impacts on native plant and pollinator communities in different—Aged old fields. Basic Appl. Ecol. 2015, 16, 335–346. [Google Scholar] [CrossRef]
- Fenesi, A.; Geréd, J.; Meiners, S.J.; Tóthmérész, B.; Török, P.; Ruprecht, E. Does disturbance enhance the competitive effect of the invasive Solidago canadensis on the performance of two native grasses? Biol. Invasions 2015, 17, 3303–3315. [Google Scholar] [CrossRef]
- De Groot, M.; Kleijn, D.; Jogan, N. Species groups occupying different trophic levels respond differently to the invasion of semi-natural vegetation by Solidago canadensis. Biol. Conserv. 2007, 136, 612–617. [Google Scholar] [CrossRef]
- Bielecka, A.; Borkowska, L.; Królak, E. Environmental changes caused by the clonal invasive plant Solidago canadensis. Ann. Bot. Fenn. 2020, 57, 33–48. [Google Scholar] [CrossRef]
- Abhilasha, D.; Quintana, N.; Vivanco, J.; Joshi, J. Do allelopathic compounds in invasive Solidago canadensis restrain the native European flora? J. Ecol. 2008, 96, 993–1001. [Google Scholar] [CrossRef]
- Novak, N.; Novak, M.; Barić, K.; Šćepanović, M.; Ivić, D. Allelopathic potential of segetal and ruderal invasive alien plants. J. Cent. Eur. Agric. 2018, 19, 408–422. [Google Scholar] [CrossRef]
- Jakobsson, A.; Padrón, B.; Traveset, A. Competition for pollinators between invasive and native plants: Effects of spatial scale of investigation (note). Écoscience 2009, 16, 138–141. [Google Scholar] [CrossRef]
- Glen, A.S.; Pech, R.P.; Byrom, A.E. Connectivity and invasive species management: Towards an integrated landscape approach. Biol. Ιnvasions 2013, 15, 2127–2138. [Google Scholar] [CrossRef]
- Vicente, J.R.; Fernandes, R.F.; Randin, C.F.; Broennimann, O.; Gonçalves, J.; Marcos, B.; Pôças, I.; Alves, P.; Guisan, A.; Honrado, J.P. Will climate change drive alien invasive plants into areas of high protection value? An improved model-based regional assessment to prioritise the management of invasions. J. Environ. Manag. 2013, 131, 185–195. [Google Scholar] [CrossRef]
- Christopoulou, A.; Christopoulou, A.; Fyllas, N.M.; Dimitrakopoulos, P.G.; Arianoutsou, M. How effective are the protected areas of the Natura 2000 network in halting biological invasions? A case study in Greece. Plants 2021, 10, 2113. [Google Scholar] [CrossRef]
- Szilassi, P.; Soóky, A.; Bátori, Z.; Hábenczyus, A.A.; Frei, K.; Tölgyesi, C.; van Leeuwen, B.; Tobak, Z.; Csikós, N. Natura 2000 areas, road, railway, water, and ecological networks may provide pathways for biological invasion: A country scale analysis. Plants 2021, 10, 2670. [Google Scholar] [CrossRef]
- Wilkerson, M.L. Invasive plants in conservation linkages: A conceptual model that addresses an underappreciated conservation issue. Ecography 2013, 36, 1319–1330. [Google Scholar] [CrossRef]
- Vilà, M.; Ibáñez, I. Plant invasions in the landscape. Landsc. Ecol. 2011, 26, 461–472. [Google Scholar] [CrossRef]
- Kanatas, P.; Travlos, I.S.; Gazoulis, I.; Tataridas, A.; Tsekoura, A.; Antonopoulos, N. Benefits and limitations of decision support systems (DSS) with a special emphasis on weeds. Agronomy 2020, 10, 548. [Google Scholar] [CrossRef]
- Novoa, A.; Dehnen-Schmutz, K.; Fried, J.; Vimercati, G. Does public awareness increase support for invasive species management? Promising evidence across taxa and landscape types. Biol. Ιnvasions 2017, 19, 3691–3705. [Google Scholar] [CrossRef]
- Gazoulis, I.; Kanatas, P.; Papastylianou, P.; Tataridas, A.; Alexopoulou, E.; Travlos, I. Weed management practices to improve establishment of selected lignocellulosic crops. Energies 2021, 14, 2478. [Google Scholar] [CrossRef]
- Cordeiro, B.; Marchante, H.; Castro, P.; Marchante, E. Does public awareness about invasive plants pays off? An analysis of knowledge and perceptions of environmentally aware citizens in Portugal. Biol. Invasions 2020, 22, 2267–2281. [Google Scholar] [CrossRef]
- Schuh, B.; Dax, T.; Andronic, C.; Derszniak-Noirjean, M.; Gaupp-Berghausen, M.; Hsiung, C.-H.; Münch, A.; Machold, I.; Schroll, K.; Brkanovic, S. The Challenge of Land Abandonment after 2020 and Options for Mitigating Measures. In Research for AGRI-Committee, 2020; European Parliament, Policy Department for Structural and Cohesion Policies, Directorate—General for Internal Policies: Brussels, Belgium, 2020; Available online: https://bit.ly/39ElcFJ (accessed on 17 April 2022).
- Barr, S.; Jonas, J.L.; Paschke, M.W. Optimizing seed mixture diversity and seeding rates for grassland restoration. Restor. Ecol. 2017, 25, 396–404. [Google Scholar] [CrossRef]
- Firn, J.; Price, J.N.; Whalley, R.D. Using strategically applied grazing to manage invasive alien plants in novel grasslands. Ecol. Process. 2013, 2, 26. [Google Scholar] [CrossRef]
- Pattison, Z.; Vallejo-Marín, M.; Willby, N. Riverbanks as battlegrounds: Why does the abundance of native and invasive plants vary? Ecosystems 2019, 22, 578–586. [Google Scholar] [CrossRef]
- Úbeda, X.; Sarricolea, P. Wildfires in Chile: A review. Glob. Planet. Chang. 2016, 146, 152–161. [Google Scholar] [CrossRef]
- Fogliatto, S.; Milan, M.; Vidotto, F. Control of Ailanthus altissima using cut stump and basal bark herbicide applications in an eighteenth-century fortress. Weed Res. 2020, 60, 425–434. [Google Scholar] [CrossRef]
- Bakacsy, L.; Bagi, I. Survival and regeneration ability of clonal common milkweed (Asclepias syriaca L.) after a single herbicide treatment in natural open sand grasslands. Sci. Rep. 2020, 10, 14222. [Google Scholar] [CrossRef] [PubMed]
- Delbart, E.; Mahy, G.; Weickmans, B.; Henriet, F.; Crémer, S.; Pieret, N.; Vanderhoeven, S.; Monty, A. Can land managers control Japanese knotweed? Lessons from control tests in Belgium. Environ. Manag. 2012, 50, 1089–1097. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.L.; Jiang, H.W.; Fang, F.; Chen, G.Q. Influences of herbicides, uprooting and use as cut flowers on sexual reproduction of Solidago canadensis. Weed Res. 2009, 49, 291–299. [Google Scholar] [CrossRef]
- Labant-Hoffmann, É.; Kazinczi, G. Chemical and mechanical methods for suppression of Jerusalem artichoke (Helianthus tuberosus L.). Herbologia 2014, 14, 63–71. [Google Scholar] [CrossRef]
- Nielsen, C.; Vanaga, I.; Treikale, O.; Priekule, I. Mechanical and chemical control of Heracleum mantegazzianum and H. sosnowskyi. In Ecology and Management of Giant Hogweed (Heracleum mantegazzianum); Pyšek, P., Cock, M.J.W., Nentwig, W., Ravn, H.P., Eds.; CABI: Cambridge, MA, USA, 2007; pp. 226–239. [Google Scholar]
- Duke, S.O.; Powles, S.B. Glyphosate: A once-in-a-century herbicide. Pest Manag. Sci. 2008, 64, 319–325. [Google Scholar] [CrossRef]
- Fogliatto, S.; Ferrero, A.; Vidotto, F. Current and future scenarios of glyphosate use in Europe: Are there alternatives? In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Cambridge, MA, USA, 2020; Volume 163, pp. 219–278. [Google Scholar]
- Kanatas, P.; Antonopoulos, N.; Gazoulis, I.; Travlos, I.S. Screening glyphosate—Alternative weed control options in important perennial crops. Weed Sci. 2021, 69, 704–718. [Google Scholar] [CrossRef]
- Travlos, I.; Kanatas, P.; Tsekoura, A.; Gazoulis, I.; Papastylianou, P.; Kakabouki, I.; Antonopoulos, N. Efficacy of different herbicides on Echinochloa colona (L.) Link control and the first case of its glyphosate resistance in Greece. Agronomy 2020, 10, 1056. [Google Scholar] [CrossRef]
- Janikova, A.; Svehlakova, H.; Turcova, B.; Stalmachova, B. Influence of management on vegetative reproduction of invasive species of Helianthus tuberosus in Poodri PLA. IOP Conf. Ser. Earth Environ. Sci. 2020, 444, 012025. [Google Scholar] [CrossRef]
- Svehlakova, H.; Janikova, A.; Kupka, J.; Sotkova, N.; Rajdus, T. Possibilities of the management of Helianthus tuberosus species in Poodri PLA (Czech Republic). IOP Conf. Ser. Earth Environ. Sci. 2017, 92, 012066. [Google Scholar] [CrossRef]
- Brühl, C.A.; Zaller, J.G. Indirect herbicide effects on biodiversity, ecosystem functions, and interactions with global changes. In Herbicides, 1st ed.; Mesnage, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 231–272. [Google Scholar]
- Mendes, K.F. Pesticides in Agriculture and Environment, 1st ed.; B P International: West Bengal, India, 2022; p. 1. [Google Scholar]
- Schiffleithner, V.; Essl, F. Is it worth the effort? Spread and management success of invasive alien plant species in a Central European National Park. NeoBiota 2016, 31, 43–61. [Google Scholar]
- Miller, T.W. Integrated strategies for management of perennial weeds. Invasive Plant Sci. Manag. 2016, 9, 148–158. [Google Scholar] [CrossRef]
- Nagy, D.U.; Rauschert, E.S.; Henn, T.; Cianfaglione, K.; Stranczinger, S.; Pal, R.W. The more we do, the less we gain? Balancing effort and efficacy in managing the Solidago gigantea invasion. Weed Res. 2020, 60, 232–240. [Google Scholar] [CrossRef]
- Rajdus, T.; Svehlakova, H.; Plohak, P.; Stalmachova, B. Management of invasive species Solidago canadensis in Ostrava region (Czech Republic). IOP Conf. Ser. Earth Environ. Sci. 2020, 444, 012046. [Google Scholar] [CrossRef]
- Švehláková, H.; Turčová, B.; Rajdus, T.; Plohák, P.; Nováková, J. Effective combination of management methods suppresses invasive Jerusalem artichoke. IOP Conf. Ser. Earth Environ. Sci. 2021, 900, 012045. [Google Scholar] [CrossRef]
- Andersen, U.V.; Calov, B. Long-term effects of sheep grazing on giant hogweed (Heracleum mantegazzianum). Hydrobiologia 1996, 340, 277–284. [Google Scholar] [CrossRef]
- Ducs, A.; Kazi, A.; Bilko, A.; Altbaecker, V. Milkweed control by food imprinted rabbits. Behav. Processes 2016, 130, 75–80. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, X.; Zhu, Y.; Li, L.; Zhang, Y.; Li, J.; Qiang, S. Biological control of Solidago canadensis using a bioherbicide isolate of Sclerotium rolfsii SC64 increased the biodiversity in invaded habitats. Biol. Control 2019, 139, 104093. [Google Scholar] [CrossRef]
- Hagner, M.; Lindqvist, B.; Vepsäläinen, J.; Samorì, C.; Keskinen, R.; Rasa, K.; Hyvönen, T. Potential of pyrolysis liquids to control the environmental weed Heracleum mantegazzianum. Environ. Technol. Innov. 2020, 20, 101154. [Google Scholar] [CrossRef]
- Seier, M.K.; Wittenberg, R.; Ellison, C.A.; Djeddour, D.H.; Evans, H.C. Surveys for natural enemies of giant hogweed (Heracleum mantegazzianum) in the Caucasus Region and assessment for their classical biological control potential in Europe. In Proceedings of the XI International Symposium on Biological Control of Weeds, Canberra, Australia, 27 April–2 May 2003; SCIRO Entomology: Canberra, Australia, 2004; p. 149. [Google Scholar]
- Shaw, R.H.; Tanner, R.; Djeddour, D.; Cortat, G. Classical biological control of Fallopia japonica in the United Kingdom—Lessons for Europe. Weed Res. 2011, 51, 552–558. [Google Scholar] [CrossRef]
- Tóth, T.; Szilágyi, A.; Kövics, G. Preliminary estimation of the efficacy of Fusarium sporotrichioides Sherb. as biological control agent against common milkweed (Asclepias syriaca L.). Acta Agrar. Debr. 2018, 74, 201–204. [Google Scholar] [CrossRef]
- Podroužková, Š.; Janovský, Z.; Horáčková, J.; Juřičková, L. Do snails eat exotic plant species invading river floodplains? J. Molluscan Stud. 2015, 81, 139–146. [Google Scholar] [CrossRef]

| Binomial Name | Botanical Family | Common Name | Origin | Infested Countries 1 |
|---|---|---|---|---|
| Asclepias syriaca | Apocynaceae | Common milkweed | North America | IT, SI, HR, BA, ME |
| Helianthus tuberosus | Asteraceae | Jerusalem artichoke | North America | IT, SI, HR, BA, ME, AL, GR |
| Reynoutria japonica | Polygonaceae | Japanese knotweed | East Asia | IT, SI, HR, BA |
| Reynoutria × bohemica | Polygonaceae | Japanese knotweed | East Asia | IT, SI, HR, BA |
| Heracleum mantegazzianum | Apiaceae | Giant hogweed | Eastern Europe | IT, SI, HR, BA |
| Solidago gigantea | Asteraceae | Giant goldenrod | North America | IT, SI, HR, BA, ME |
| Solidago canadensis | Asteraceae | Canadian goldenrod | North America | IT, SI, HR, BA, ME |
| Oxalis pes-caprae L. | Oxalidaceae | Bermuda buttercup | South Africa | IT, HR, AL, GR |
| Species | Invaded Habitats | Countries | Affected Organisms/Parameters | Case Studies |
|---|---|---|---|---|
| Common milkweed | Grasslands, abandoned agricultural fields | Slovakia | Native plant communities | [10] |
| Poplar forest | Hungary | Soil arthropods | [147] | |
| Jerusalem artichoke | Riparian areas | Hungary, Romania, Ukraine | Native plant communities | [85] |
| Japanese knotweed | Forest edges, abandoned agricultural fields, field edges, grasslands | Belgium | Native Plant communities, soil properties | [149] |
| Riparian areas | France | Soil properties, arthropods | [9] | |
| Riparian areas | Switzerland, Germany, France | Native plant communities, invertebrates | [151] | |
| Bohemian knotweed | Grasslands | France | Native plant communities, invertebrates | [153] |
| Willow forest, grasslands, wastelands | Belgium, France | Soil properties | [154] | |
| Riparian areas | Switzerland, Germany, France | Native plant communities, invertebrates | [151] | |
| Giant hogweed | Grasslands | Czech Republic | Native plant communities | [155] |
| Riparian areas | Slovakia | Soil properties, soil nematodes | [12] | |
| Abandoned agricultural fields, grasslands | Belgium | Soil properties | [159] | |
| Giant goldenrod | Riparian areas | Hungary | Native plant communities | [160] |
| Grasslands | Netherlands | Native plant communities, Soil properties, Soil fungi, Soil nematodes | [161] | |
| Canadian goldenrod | Ruderal areas | Slovenia | Native plant communities, insect-pollinators | [164] |
| Grasslands | Poland | Native plant communities, soil properties | [165] | |
| Bermuda buttercup | Orchards | Greece | Native plant communities, soil properties | [143] |
| Abandoned agricultural fields | Italy | Native plant communities | [11] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gazoulis, I.; Antonopoulos, N.; Kanatas, P.; Karavas, N.; Bertoncelj, I.; Travlos, I. Invasive Alien Plant Species—Raising Awareness of a Threat to Biodiversity and Ecological Connectivity (EC) in the Adriatic-Ionian Region. Diversity 2022, 14, 387. https://doi.org/10.3390/d14050387
Gazoulis I, Antonopoulos N, Kanatas P, Karavas N, Bertoncelj I, Travlos I. Invasive Alien Plant Species—Raising Awareness of a Threat to Biodiversity and Ecological Connectivity (EC) in the Adriatic-Ionian Region. Diversity. 2022; 14(5):387. https://doi.org/10.3390/d14050387
Chicago/Turabian StyleGazoulis, Ioannis, Nikolaos Antonopoulos, Panagiotis Kanatas, Nikolas Karavas, Irena Bertoncelj, and Ilias Travlos. 2022. "Invasive Alien Plant Species—Raising Awareness of a Threat to Biodiversity and Ecological Connectivity (EC) in the Adriatic-Ionian Region" Diversity 14, no. 5: 387. https://doi.org/10.3390/d14050387
APA StyleGazoulis, I., Antonopoulos, N., Kanatas, P., Karavas, N., Bertoncelj, I., & Travlos, I. (2022). Invasive Alien Plant Species—Raising Awareness of a Threat to Biodiversity and Ecological Connectivity (EC) in the Adriatic-Ionian Region. Diversity, 14(5), 387. https://doi.org/10.3390/d14050387







