Application of DNA Barcoding for Monitoring Madagascar Fish Biodiversity in Coastal Areas
Abstract
:1. Introduction
2. Materials and Methods
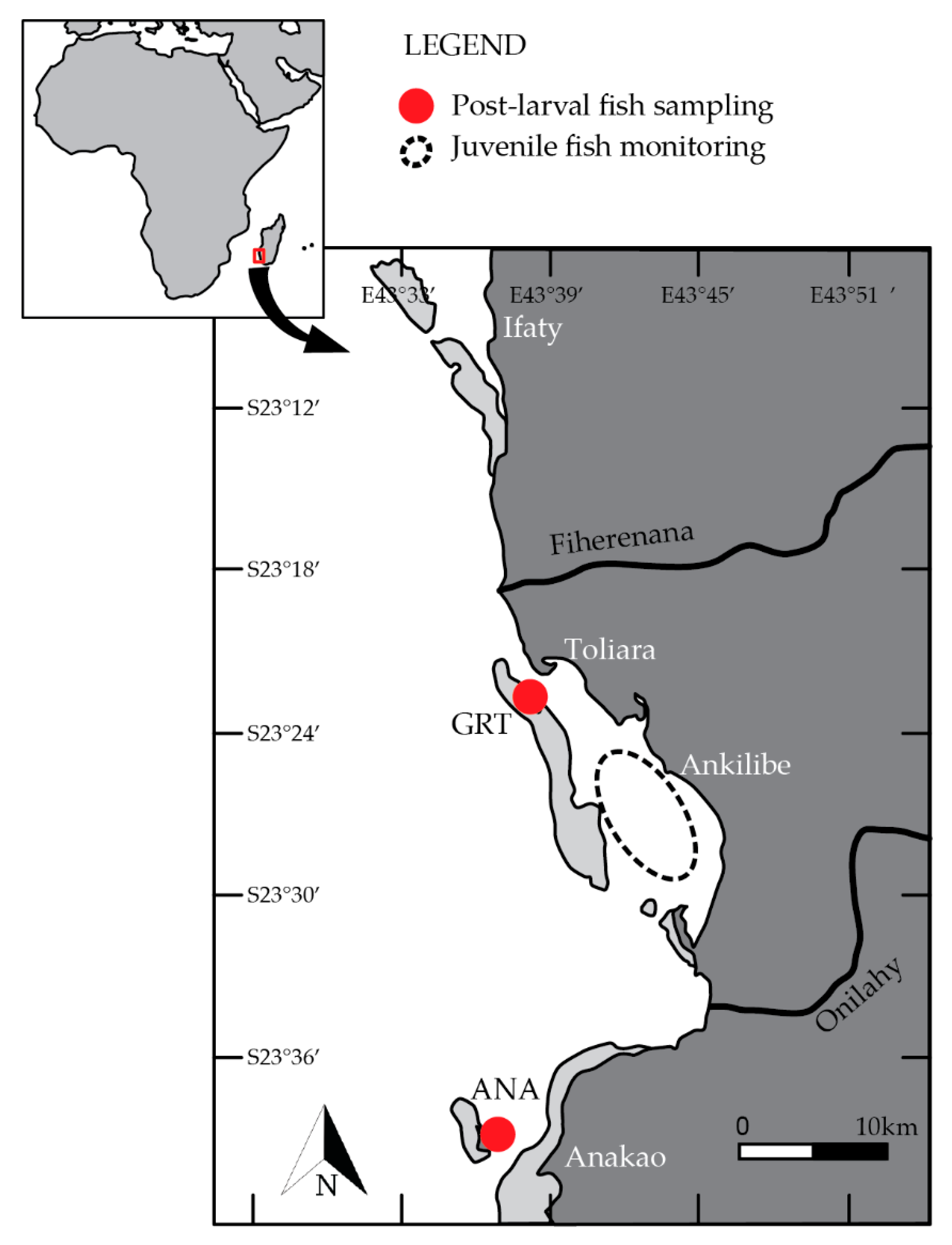
2.1. Study Sites and Sampling
2.2. Identification Process
2.3. Data Analyses
3. Results
3.1. Species Richness: Morphological vs. Molecular-Based Identification
3.2. Discordances in Species Assignment
3.3. Geographical Distribution of Ambiguous Species
3.4. DNA Barcoding Effort for Madagascar Fishes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- MEF. Fourth National Report on Biological Diversity Convention Madagascar; Ministry of Environment and Forests: Antananarivo, Madagascar, 2009; p. 144.
- Fricke, R.; Mahafina, J.; Behivoke, F.; Jaonalison, H.; Léopold, M.; Ponton, D. Annotated Checklist of the Fishes of Madagascar, Southwestern Indian Ocean, with 158 New Records. FishTaxa 2018, 3, 432. [Google Scholar]
- Collet, A.; Durand, J.-D.; Desmarais, E.; Cerqueira, F.; Cantinelli, T.; Valade, P.; Ponton, D. DNA Barcoding Post-Larvae Can Improve the Knowledge about Fish Biodiversity: An Example from La Reunion, SW Indian Ocean. Mitochondrial DNA Part A 2018, 29, 905–918. [Google Scholar] [CrossRef] [PubMed]
- Leis, J.M.; Carson-Ewart, B.M. The Larvae of Indo-Pacific Coastal Fishes: An Identification Guide to Marine Fish Larvae, 2nd ed.; Leiden; Brill: Boston, FL, USA, 2004; ISBN 978-90-04-11577-4. [Google Scholar]
- Maamaatuaiahutapu, M.; Remoissenet, G.; Galzin, R. Guide d’identification Des Larves de Poissons Récifauxde Polynésie Française; Coral Reef Initiative for the South Pacific; Téthys: Nouméa, New Calédonie, 2006; 108p. [Google Scholar]
- Juncker, M. Young Coral Reef Fish of Wallis and the Central Pacific: Identification Guide; Wallis and Futuna-Environment: Noumea, New Caledonia, 2007; 170p. [Google Scholar]
- Frantine-Silva, W.; Sofia, S.H.; Orsi, M.L.; Almeida, F.S. DNA Barcoding of Freshwater Ichthyoplankton in the Neotropics as a Tool for Ecological Monitoring. Mol. Ecol. Resour. 2015, 15, 1226–1237. [Google Scholar] [CrossRef] [PubMed]
- Brodeur, R.; Peterson, W.; Auth, T.; Soulen, H.; Parnel, M.; Emerson, A. Abundance and Diversity of Coastal Fish Larvae as Indicators of Recent Changes in Ocean and Climate Conditions in the Oregon Upwelling Zone. Mar. Ecol. Prog. Ser. 2008, 366, 187–202. [Google Scholar] [CrossRef]
- Carassou, L.; Ponton, D.; Mellin, C.; Galzin, R. Predicting the Structure of Larval Fish Assemblages by a Hierarchical Classification of Meteorological and Water Column Forcing Factors. Coral Reefs 2008, 27, 867–880. [Google Scholar] [CrossRef]
- Mahafina, J. Perception et Comportement Des Pêcheurs Pour Une Gestion Durable de La Biodiversité et de La Pêcherie Récifale Du Sud-Ouest de Madagascar. Ph.D. Thesis, Université de La Réunion et Université de Toliara, Toliara, Madagascar, 2011. [Google Scholar]
- Jaonalison, H.; Mahafina, J.; Ponton, D. Fish Post-Larvae Assemblages at Two Contrasted Coral Reef Habitats in Southwest Madagascar. Reg. Stud. Mar. Sci. 2016, 6, 62–74. [Google Scholar] [CrossRef]
- Chen, L.-C.; Lan, K.-W.; Chang, Y.; Chen, W.-Y. Summer Assemblages and Biodiversity of Larval Fish Associated with Hydrography in the Northern South China Sea. Mar. Coast. Fish. 2018, 10, 467–480. [Google Scholar] [CrossRef] [Green Version]
- Grande, H.; Rezende, S.d.M.; Simon, T.E.; Félix-Hackradt, F.C.; García-Charton, J.A.; Maida, M.; Gaspar, A.L.B.; Francini-Filho, R.B.; Fredou, T.; Ferreira, B.P. Diversity of Settlement-stage Reef Fishes Captured by Light-trap in a Tropical South-west Atlantic Ocean Coastal Reef System. J. Fish Biol. 2018, 94, 210–222. [Google Scholar] [CrossRef] [Green Version]
- Pegg, G.G.; Sinclair, B.; Briskey, L.; Aspden, W.J. MtDNA Barcode Identification of Fish Larvae in the Southern Great Barrier Reef—Australia. Sci. Mar. 2006, 70, 7–12. [Google Scholar] [CrossRef]
- Trivedi, S.; Aloufi, A.A.; Ansari, A.A.; Ghosh, S.K. Role of DNA Barcoding in Marine Biodiversity Assessment and Conservation: An Update. Saudi J. Biol. Sci. 2016, 23, 161–171. [Google Scholar] [CrossRef] [Green Version]
- Ko, H.-L.; Wang, Y.-T.; Chiu, T.-S.; Lee, M.-A.; Leu, M.-Y.; Chang, K.-Z.; Chen, W.-Y.; Shao, K.-T. Evaluating the Accuracy of Morphological Identification of Larval Fishes by Applying DNA Barcoding. PLoS ONE 2013, 8, e53451. [Google Scholar] [CrossRef] [PubMed]
- Meredith, C.; Hoffman, J.; Trebitz, A.; Peterson, G.; Lietz, J.; Hatzenbuhler, C.; Pilgrim, E.; Okum, S.; Martinson, J. Development of DNA-Based Identification Methods to Track the Species Composition of Fish Larvae within Nearshore Areas of the Great Lakes; Minnesota Chapter of the American Fisheries Society: St. Cloud, MN, USA, 2017. [Google Scholar]
- Leis, J.M. Taxonomy and Systematics of Larval Indo-Pacific Fishes: A Review of Progress since 1981. Ichthyol. Res. 2015, 62, 9–28. [Google Scholar] [CrossRef] [Green Version]
- Bruggemann, J.H.; Rodier, M.; Guillaume, M.M.M.; Andréfouët, S.; Arfi, R.; Cinner, J.E.; Pichon, M.; Ramahatratra, F.; Rasoamanendrika, F.; Zinke, J.; et al. Wicked Social-Ecological Problems Forcing Unprecedented Change on the Latitudinal Margins of Coral Reefs: The Case of Southwest Madagascar. Ecol. Soc. 2012, 17, 47. [Google Scholar] [CrossRef]
- Ranaivomanana, L.N.J. Identification Des Conditions d’appropriation de La Gestion Durable Des Ressources Naturelles et Des Écosystèmes: Cas Du Grand Récif de Toliara. Ph.D. Thesis, Institut Halieutique et des Sciences Marines (IH.SM) de l’Université de Toliara, Toliara, Madagascar, 2006. [Google Scholar]
- Dufour, V. Colonisation Des Recifs Coralliens Par Les Larves de Poissons. Ph.D. Thesis, Université Pierre & Marie Curie, Paris, France, 1992. [Google Scholar]
- Marchetti, M.P.; Esteban, E.; Limm, M.; Kurth, R. Evaluating Aspects of Larval Light Trap Bias and Specificity in the Northern Sacramento River System: Do Size and Color Matter? Am. Fish. Soc. Symp. 2004, 39, 269–279. [Google Scholar]
- Lindquist, D.C.; Shaw, R.F. Effects of Current Speed and Turbidity on Stationary Light-Trap Catches of Larval and Juvenile FIshes. Fish. Bull. 2005, 103, 438–444. [Google Scholar]
- Wilson, D. Patterns of Replenishment of Coral-Reef Fishes in the Nearshore Waters of the San Blas Archipelago, Caribbean Panama. Mar. Biol. 2001, 139, 735–753. [Google Scholar] [CrossRef]
- Anderson, T.W.; Bartels, C.T.; Hixon, M.A.; Bartels, E.; Carr, M.H.; Shenker, J.M. Current Velocity and Catch Efficiency in Sampling Settlement-Stage Larvae of Coral-Reef Fishes. Fish. Bull. 2002, 100, 404–413. [Google Scholar]
- Laroche, J.; Ramananarivo, N. A Preliminary Survey of the Artisanal Fishery on Coral Reefs of the Tulear Region (Southwest Madagascar). Coral Reefs 1995, 14, 193–200. [Google Scholar] [CrossRef]
- Raharinaivo, L.R.; Jaonalison, H.; Mahafina, J.; Ponton, D. How to Efficiently Determine the Size at Maturity of Small-sized Tropical Fishes: A Case Study Based on 144 Species Identified via DNA Barcoding from Southwestern Madagascar. J. Appl. Ichthyol. 2020, 36, 402–413. [Google Scholar] [CrossRef]
- Bauchot, M.L.; Bianchi, G. Fiches FAO d’identification Des Especes Pour Les Besoins de La Peche. Guide Des Poissons Commerciaux de Madagascar (Especes Marines et d’eaux Saumatres), Avec Le Support Du Programme Des Nations Unies Pour Le Developpement (Projet RAF/79/065); FAO: Rome, Italy, 1984; 135p. [Google Scholar]
- Smith, M.M.; Heemstra, P.C. (Eds.) Smiths’ Sea Fishes; Springer: Berlin/Heidelberg, Germany, 1986; ISBN 978-3-642-82860-7. [Google Scholar]
- Collet, A.; Bosc, P.; Pothin, K.; Chabanet, P. Guide d’identification Des Post-Larves de Poissons Des Récifs Coralliens; ARDA et OCEA Consult’: La Réunion, France, 2013; 176p. [Google Scholar]
- Werner, F.; Fröndt, R. Automated Purification of Genomic DNA from Tissue with the Macherey-Nagel NucleoMag® Tissue Kit on the EpMotion® 5075. Appl. Note 2015, 365, 1–7. [Google Scholar]
- Ward, R.D.; Zemlak, T.S.; Innes, B.H.; Last, P.R.; Hebert, P.D.N. DNA Barcoding Australia’s Fish Species. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2005, 360, 1847–1857. [Google Scholar] [CrossRef] [PubMed]
- Ratnasingham, S.; Hebert, P.D.N. A DNA-Based Registry for All Animal Species: The Barcode Index Number (BIN) System. PLoS ONE 2013, 8, e66213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. 2019. Available online: https://www.scirp.org/(S(351jmbntvnsjt1aadkposzje))/reference/ReferencesPapers.aspx?ReferenceID=1778707 (accessed on 12 October 2021).
- Poulin, R. Comparison of Three Estimators of Species Richness in Parasite Component Communities. J. Parasitol. 1998, 84, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Meredith, M. Quick and Dirty Estimates for Wildlife Populations. 2019. Available online: http://www.r-project.org/ (accessed on 12 October 2021).
- Smith, E.P.; Van Belle, G. Nonparametric Estimation of Species Richness. Biometrics 1984, 40, 119–129. [Google Scholar] [CrossRef] [Green Version]
- Pinheiro, H.T.; Rocha, L.A.; Macieira, R.M.; Carvalho-Filho, A.; Anderson, A.B.; Bender, M.G.; Di Dario, F.; Ferreira, C.E.L.; Figueiredo-Filho, J.; Francini-Filho, R.; et al. South-Western Atlantic Reef Fishes: Zoogeographical Patterns and Ecological Drivers Reveal a Secondary Biodiversity Centre in the Atlantic Ocean. Divers. Distrib. 2018, 24, 951–965. [Google Scholar] [CrossRef] [Green Version]
- Steinke, D.; Connell, A.D.; Hebert, P.D.N. Linking Adults and Immatures of South African Marine Fishes. Genome 2016, 59, 959–967. [Google Scholar] [CrossRef] [Green Version]
- Reid, K.; Crochelet, E.; Bloomer, P.; Hoareau, T.B. Investigating the Origin of Vagrant Dusky Groupers, Epinephelus Marginatus (Lowe, 1834), in Coastal Waters of Réunion Island. Mol. Phylogenet. Evol. 2016, 103, 98–103. [Google Scholar] [CrossRef] [Green Version]
- Weigt, L.A.; Baldwin, C.C.; Driskell, A.; Smith, D.G.; Ormos, A.; Reyier, E.A. Using DNA Barcoding to Assess Caribbean Reef Fish Biodiversity: Expanding Taxonomic and Geographic Coverage. PLoS ONE 2012, 7, e41059. [Google Scholar] [CrossRef] [Green Version]
- Healey, A.J.E.; McKeown, N.J.; Taylor, A.L.; Provan, J.; Sauer, W.; Gouws, G.; Shaw, P.W. Cryptic Species and Parallel Genetic Structuring in Lethrinid Fish: Implications for Conservation and Management in the Southwest Indian Ocean. Ecol. Evol. 2018, 8, 2182–2195. [Google Scholar] [CrossRef] [Green Version]
- Ponton, D.; Carassou, L.; Raillard, S.; Borsa, P. Geometric Morphometrics as a Tool for Identifying Emperor Fish (Lethrinidae) Larvae and Juveniles. J. Fish Biol. 2013, 83, 14–27. [Google Scholar] [CrossRef]
- Wibowo, A.; Wahlberg, N.; Vasemägi, A. DNA Barcoding of Fish Larvae Reveals Uncharacterised Biodiversity in Tropical Peat Swamps of New Guinea, Indonesia. Mar. Freshw. Res. 2017, 68, 1079. [Google Scholar] [CrossRef]
- Durand, J.-D.; Pham, M.H.; Tran, T.T.V.; Hoang, D.H.; Van Vo, Q. Sorting the Wheat from the Chaff: A Review of BINs Associated with Groupers of Vietnam and the Implications for Species Identification from DNA Barcoding. Mar. Biodivers. 2020, 50, 38. [Google Scholar] [CrossRef]
- Collins, R.A.; Cruickshank, R.H. The Seven Deadly Sins of DNA Barcoding. Mol. Ecol. Resour. 2013, 13, 969–975. [Google Scholar] [CrossRef]
- Semmens, B.; Buhle, E.; Salomon, A.; Pattengill-Semmens, C. A Hotspot of Non-Native Marine Fishes: Evidence for the Aquarium Trade as an Invasion Pathway. Mar. Ecol. Prog. Ser. 2004, 266, 239–244. [Google Scholar] [CrossRef] [Green Version]
- Blaxter, M. Imagining Sisyphus Happy: DNA Barcoding and the Unnamed Majority. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2016, 371, 20150329. [Google Scholar] [CrossRef] [Green Version]


| Species Name in BOLD | Current Work | Other Projects in BOLD Database | ||
|---|---|---|---|---|
| BINs | Distribution in BOLD | Other BINs | Distribution in BOLD | |
| Amblygobius phalaena | BOLD: AAB8727 | West IO & PO | BOLD: AAB8728 | Center PO |
| Antennarius striatus | BOLD: AAO6018 | East AO & IO, South PO | BOLD: AAF2509 | West AO, North PO |
| Apogon semiornatus | BOLD: AAD2206 | West IO | BOLD: AAD2207 | West PO |
| Asterropteryx semipunctata | BOLD: AAC0108 | West IO | BOLD: AAC0105/BOLD: AAC0106/ BOLD: AAC0107/BOLD: AAC0109 | South & North PO, North AO |
| Cheilinus chlorourus | BOLD: AAB4186 | West IO | BOLD: AAB4185/BOLD: ACE8465 | PO |
| Corythoichthys flavofasciatus | BOLD: AAI8860 | West IO | BOLD: AAE9785/BOLD: ACR0421 | North PO |
| Dendrochirus brachypterus | BOLD: AAC9564 | West IO | BOLD: AAC9563 | East IO, West PO |
| Diagramma pictum | BOLD: AAD4477 | West IO | BOLD: AAB9940 | North & East IO, West PO |
| Epinephelus rivulatus | BOLD: ACZ9919 | West IO | BOLD: AAF0433 | East IO, West PO |
| Fowleria variegata | BOLD: AAD8726 | West IO | BOLD: AAD8727/BOLD: ACC5212 | West PO |
| Gazza minuta | BOLD: AAB7096 | West IO | BOLD: AAA9823 | North IO & Northwest PO |
| Gerres longirostris | BOLD: AAE6359 | West IO | BOLD: AAE6360 | Souther PO |
| Halichoeres nebulosus | BOLD: AAC7896 | West IO | BOLD: AAC7897/BOLD: AAD5811 | East IO, North PO |
| Halichoeres zeylonicus | BOLD: AAF7654 | West IO | BOLD: AAF7655 | North & East IO |
| Hippichthys spicifer | BOLD: AAE5342 | West IO | BOLD: ADA9516 | North PO |
| Hippocampus histrix | BOLD: AAE5356 | Southwest & North IO | BOLD: AAE5355 | North PO |
| Lethrinus harak | BOLD: AAC1521 | West IO | BOLD: AAC1520 | East IO, West PO |
| Lethrinus lentjan | BOLD: AAB0511 | West IO | BOLD: AAB0508 | West PO |
| Lethrinus miniatus | BOLD: AAC8078 | southwest & north IO | BOLD: AAC8077 | East IO |
| Lethrinus nebulosus | BOLD: ABY6363 | West IO | BOLD: ACE7416/BOLD: AAE3737/ BOLD: AAB2308 | Northeast IO, West PO |
| Lutjanus lutjanus | BOLD: AAA8168 | West, North & east IO | BOLD:AAA8169 | North PO |
| Nectamia fusca | BOLD: AAL9262 | West IO | BOLD: AAF8240/BOLD: ABZ1276 | West PO |
| Nectamia savayensis | BOLD: AAD9453 | West IO | BOLD:AAD6832/BOLD: AAD9454/ BOLD: AAF1913/BOLD: ABY4865 | North & East IO, West PO |
| Neoniphon sammara | BOLD: AAC8278 | West IO | BOLD: AAC6632 | East IO, West PO |
| Ostorhinchus aureus | BOLD: ACE9301 | West IO | BOLD: AAD5599 | East IO, West PO |
| Ostorhinchus cookii | BOLD: AAC2084 | West & North IO | BOLD: AAC2085/BOLD: AAC2086 BOLD: AAC2086 | West PO |
| Ostorhinchus taeniophorusa | BOLD: AAD8453 BOLD: AAD8454 | West & North IO | BOLD: ACP7798 | West & Center PO |
| Oxycheilinus bimaculatus | BOLD: AAC3195 | West IO | BOLD: AAC7530/BOLD: AAC7531 | East IO, West PO |
| Petroscirtes mitratus | BOLD: AAE6131 | West IO | BOLD: AAE3283/BOLD: ADK6555 | North IO, West PO |
| Plectroglyphidodon lacrymatus | BOLD: AAB6988 | West IO | BOLD: AAB6989 | West PO |
| Plesiops coeruleolineatus | BOLD: AAE4785 | West IO | BOLD: AAE4786/BOLD: AAE4787 | South PO |
| Pomacentrus caeruleus | BOLD: AAB9539 | West & East IO | BOLD: ADW3804/BOLD: ADW3805 | North IO |
| Pristiapogon fraenatus | BOLD: AAJ1264 | West IO | BOLD: AAD6833/BOLD: ADC1718 | East IO, West PO |
| Pristiapogon kallopterus | BOLD: AAB4082 | West IO & PO | BOLD: ABZ7996 | Center PO |
| Pseudalutarius nasicornis | BOLD: AAI4531 | West IO | BOLD: AAE8257 | South PO |
| Sargocentron diadema | BOLD: AAB3424 | West, North & East IO | BOLD: AAB3425 | PO |
| Scarus ghobban | BOLD: ABY4451 | West IO | BOLD: AAB3544/BOLD: AAB3545 | East IO, West & East PO |
| Sphyraena flavicauda | BOLD: AAF8900 | West IO | BOLD: AAG2679 | East IO |
| Stolephorus indicus | BOLD: AAG4825 | West IO | BOLD: AAB7978/BOLD: AAB7978 | North IO & North PO |
| Synanceia verrucosa | BOLD: AAE2821 | West IO, south & center PO | BOLD: AAE2820 | North PO |
| Tylosurus crocodilus | BOLD: AAC4148 | IO, North & center PO | BOLD:AAB9821/BOLD: ABY6772 | West AO, East PO |
| Valenciennea puellaris | BOLD: AAC4124 | West & North IO | BOLD: AAC4125 | West PO |
| Vanderhorstia ornatissima | BOLD: AAF0288 | West & North IO | BOLD: ACC5236 | Southern PO |
| Species Name in BOLD Database | Current Work | Other Projects in BOLD Database | ||
|---|---|---|---|---|
| BINs | Distribution in BOLD | BINs | Distribution in BOLD | |
| Species Assigned to More Than Two BINs | ||||
| Trachinocephalus myopsa | BOLD: AAA9578 BOLD: ABX6347 | IO, PO | BOLD: AAA9580/BOLD: AAA9581 BOLD: AAA9582/BOLD: ACF3856 BOLD: ACF3856 | AO, IO, North PO |
| Selar crumenophthalmus | BOLD: AAB0871 | West IO, West PO | BOLD: AAB0870 | West AO, West IO, West PO |
| Parupeneus heptacanthus | BOLD: AAB2590 | West & East IO | BOLD: AAB2589/BOLD: AAI4268 | East IO, West PO |
| Caesio caerulaurea | BOLD: AAB4822 | West IO | BOLD: AAB4823/BOLD: ABZ2197 | West IO, East IO, West PO |
| Synodus variegatus | BOLD: AAB5069 | West & East IO, West PO | BOLD: AAB5072 | East IO |
| Dactyloptena orientalis | BOLD: AAB5966 | West & East IO, West PO | BOLD: AAB5965 | East IO, West PO |
| Parupeneus barberinusa | BOLD: AAB5978 BOLD: AAB5980 | IO, West PO | BOLD: AAB5979 | IO, West PO |
| Gerres filamentosus | BOLD: AAC0377 | West & North IO | BOLD: AAC0379/BOLD: AAC0380/ BOLD: AAC0382/BOLD: AAC0383/ BOLD: AAL5737/BOLD: AAY1477/ BOLD: ADC2070 | North & East IO, West PO |
| Hemiramphus far | BOLD: AAC0565 | West IO | BOLD: AAC0563/BOLD: AAX1441 | West, North & East IO, West PO |
| Gerres oyena | BOLD: AAC1291 | West IO | BOLD: AAC1288/BOLD: AAC1289/ BOLD: AAC1290/BOLD: AAC1290 | West, North & East IO, West PO |
| Sebastapistes strongia | BOLD: AAC4542 BOLD: AAC4543 | South West IO, West PO | BOLD: AAC4545/BOLD: ACD1673 | West PO |
| Lagocephalus sceleratus | BOLD: AAC5565 | IO, Mediterranean Sea, West PO | BOLD: AAC5566/BOLD: AAC5567 | East IO, West PO |
| Epinephelus flavocaeruleus | BOLD: AAD1767 | West & East IO, West PO | BOLD: ACE3678/BOLD: ACE8264 | West IO |
| Carangoides coeruleopinnatus | BOLD: AAD2297 | West & East IO, West PO | BOLD: AAB3475 BOLD: AAD2298 | West & East IO, West PO |
| Stethojulis albovittata | BOLD: AAD4824 | West IO | BOLD: ACF2947 | West IO |
| Lactoria fornasini | BOLD: AAF2668 | West IO, West PO | BOLD: AAF2667 | West IO |
| Zebrasoma desjardinii | BOLD: AAF6311 | West IO | BOLD: ACV8450 | West IO |
| Apogon coccineus | BOLD: AAU1535 | West IO | BOLD: AAJ8751 | West IO |
| Chromis opercularis | BOLD: ACF0042 | West IO | BOLD: AAJ0279 | West IO |
| Upeneus vittatus | BOLD: ACV4665 | West & East IO | BOLD: ABZ7416 | West & East IO |
| Terapon jarbua | BOLD: ACV8977 | West IO | BOLD: AAA9351/BOLD: AAA9352/ BOLD: AAA9354/BOLD: ADR6412 | West, North & East IO, West PO |
| Species Assigned to a BIN | ||||
| Myripristis hexagona & Myripristis murdjan | BOLD: AAA9764 | West IO, PO | West IO, PO | |
| Pempheris adusta & Pempheris nesogallica | BOLD: AAD1777 | IO, Mediterranean Sea | IO, Mediterranean Sea | |
| Apogon erythrinus & Ostorhinchus aureus | BOLD: AAD5600 | West & East IO, North PO | North PO | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaonalison, H.; Durand, J.-D.; Mahafina, J.; Valade, P.; Collet, A.; Cerqueira, F.; Ponton, D. Application of DNA Barcoding for Monitoring Madagascar Fish Biodiversity in Coastal Areas. Diversity 2022, 14, 377. https://doi.org/10.3390/d14050377
Jaonalison H, Durand J-D, Mahafina J, Valade P, Collet A, Cerqueira F, Ponton D. Application of DNA Barcoding for Monitoring Madagascar Fish Biodiversity in Coastal Areas. Diversity. 2022; 14(5):377. https://doi.org/10.3390/d14050377
Chicago/Turabian StyleJaonalison, Henitsoa, Jean-Dominique Durand, Jamal Mahafina, Pierre Valade, Adeline Collet, Frédérique Cerqueira, and Dominique Ponton. 2022. "Application of DNA Barcoding for Monitoring Madagascar Fish Biodiversity in Coastal Areas" Diversity 14, no. 5: 377. https://doi.org/10.3390/d14050377
APA StyleJaonalison, H., Durand, J.-D., Mahafina, J., Valade, P., Collet, A., Cerqueira, F., & Ponton, D. (2022). Application of DNA Barcoding for Monitoring Madagascar Fish Biodiversity in Coastal Areas. Diversity, 14(5), 377. https://doi.org/10.3390/d14050377








