Abstract
The copepod Nemesis santhadevii sp. nov. (Siphonostomatoida: Eudactylinidae), which is parasitizing the gill filaments of the Coral catshark Atelomycterus marmoratus (Anonymous (Bennett), 1830) off Kota Kinabalu waters, Malaysia, is described and illustrated in this article. The new species Nemesis santhadevii prominently differs from its congeners in the following features: (1) the cephalothorax sub-circular is 1.3 times as wide as long and overlapping the second pedigerous somite; (2) the fifth somite is 0.4 times the width of the fourth; (3) the genital double somite is slightly narrower than the fifth; (4) the lowest cephalothoracic shield’s body length (0.20:1) proportion; (5) the caudal rami is ovate, it has two large and three small setae; (6) and the second somite has antenna with a patch of 34–38 spinules. It is the first record of parasitic eudactilinid copepod from Sabah, East Malaysia. A checklist of global valid species of Nemesis Risso, 1826, is provided.
1. Introduction
The copepod family Eudactylinidae Wilson C.B., 1932, includes 61 valid species in 12 genera; among them, the genus Eudactylina van Beneden, 1853, is the most diverse with 38 species, followed by Nemesis Risso, 1826, with 12 valid species. Nine genera are monotypic and the remaining genus Eudactylinodes Wilson C.B., 1932, includes two species [1].
Nemesis was established as a monotypic genus for the type species N. lamna Risso, 1826 (=Nemesis lamna lamna Risso, 1826) [2]. Later, Wilson [3,4] described Nemesis versicolor Wilson C.B., 1913, from the smooth hammerhead shark, Sphyrna zygaena, and Nemesis atlantica Wilson C.B., 1922, from the Atlantic sharpnose shark, Rhizoprionodon (=Scoliodon) terraenovae. Two other species, Nemesis carchariaeglauci (Hesse, 1883) and Nemesis robusta (Beneden, 1851), were transferred to Nemesis [1]. The following species were, subsequently, described during the 20th century: Nemesis pallida Wilson C.B., 1932, Nemesis pilosus Pearse, 1951, Nemesis tiburo Pearse, 1952, Nemesis macrocephalus Shiino, 1957, Nemesis aggregatus Cressey, 1967, Nemesis spinulosus Cressey, 1970, and, the latest addition, Nemesis sphyrnae Rangnekar, 1984, from India [5,6,7,8,9,10,11,12]. The genus currently comprises 12 valid species [1].
The new species was found on the Coral catshark Atelomycterus marmoratus, off Kota Kinabalu waters, Malaysia. The host fish, A. marmoratus, is little known inshore, but is found on coral reefs, inhabiting crevices and holes on the reefs. It is caught occasionally by fisheries’ vessels over coral reefs and is utilized fresh and dried, and salted for food or processed for fishmeal and oil. Atelomycterus marmoratus is widely distributed in the Indo-West Pacific region from Pakistan and India to Malaysia, Singapore, Indonesia, New Guinea, Thailand, Viet Nam, the Philippines, and southern China, and north to Japan [13]. The present study describes a new species of Nemesis collected from A. marmoratus along with a checklist of global valid species of Nemesis.
2. Materials and Methods
Parasitic copepods were collected from the gill filaments of the host fish Atelomycterus marmoratus from Kota Kinabalu Fish Market (5.9825° N, 116.0717° E), Sabah, Malaysia, during a parasite survey of wild fish. The collected copepods were preserved in 70% ethanol and, subsequently, soaked in a drop of 80% lactic acid prior to examination using an Olympus BX51, Olympus, Tokyo, Japan differential phase contrast microscope. The copepods were examined by using the wooden slide method [14]. Drawings were prepared with the aid of a drawing tube mounted on a Nikon Eclipse 80i microscope, Tokyo, Japan. In the descriptions, body lengths were measured using a micrometer from the anterior margins of the cephalothorax to the posterior margins of the caudal rami, excluding the setae. All measurements are in micrometers, unless otherwise indicated. In the formula for the armature of legs 1–4 in the descriptions, Roman and Arabic numerals indicate spinules and setae of legs, respectively. Morphological terminology follows that used by Huys and Boxshall [15] and sources for the fish taxonomy and host nomenclature were confirmed using Fish Base [13] and Catalogue of Fishes [16]. The types were deposited in the Museum Collection Repository, Borneo Marine Research Institute, Universiti Malaysia Sabah, Kota Kinabalu.
3. Results
3.1. Taxonomy
Order Siphonostomatoida Burmeister, 1835.
Family Eudactylinidae Wilson C.B., 1932.
Genus Nemesis Risso, 1826.
Nemesis Risso, 1826: Wilson, 1922, p. 32; Yamaguti, 1963, p. 166; and Pillai 1985, p. 657.
Type species: Nemesis lamna Risso, 1826.
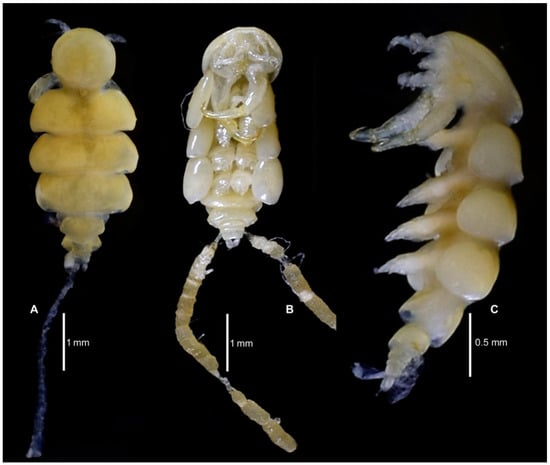
Figure 1.
Nemesis santhadevii sp. nov. from Atelomycterus marmoratus, habitus. (A) dorsal with one egg string, (B) ventral showing egg strings on both sides, and (C) lateral.
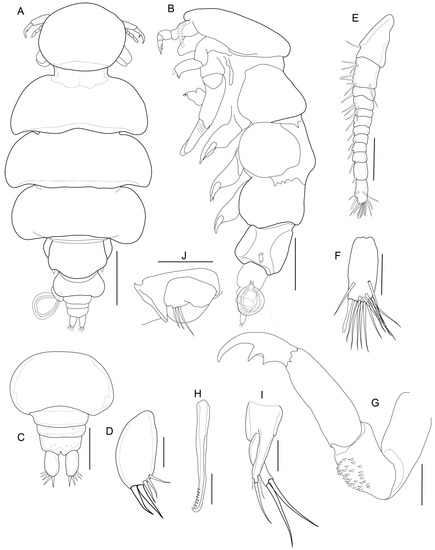
Figure 2.
Nemesis santhadevii sp. nov. from Atelomycterus marmoratus, paratype female. (A) habitus, dorsal view; (B) habitus, ventral view; (C) genito-abdomen; (D) caudal ramus; (E) antennule; (F) antennule apex; (G) antenna; (H) mandible; (I) maxillule; and (J) leg 5. Scale bar: (A,B) = 1 mm; (C) = 0.5 mm; and (D–J) = 0.01 mm.
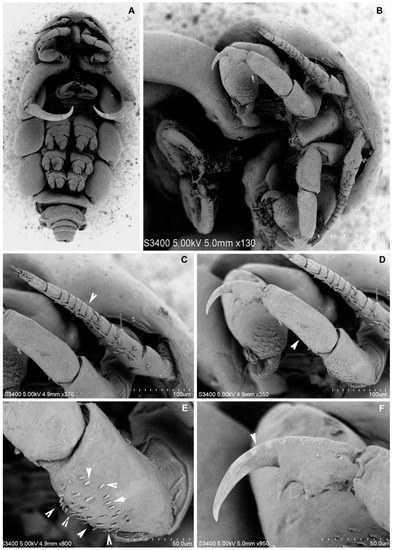
Figure 3.
Nemesis santhadevii sp. nov. from Atelomycterus marmoratus, paratype female. (A) habitus, ventral view; (B) ventral view showing cephalic appendages; (C) antennule, arrow showing segments; (D–F) antenna; (E) arrow showing spinules; and (F) arrow showing the bent claw of the distal segment.
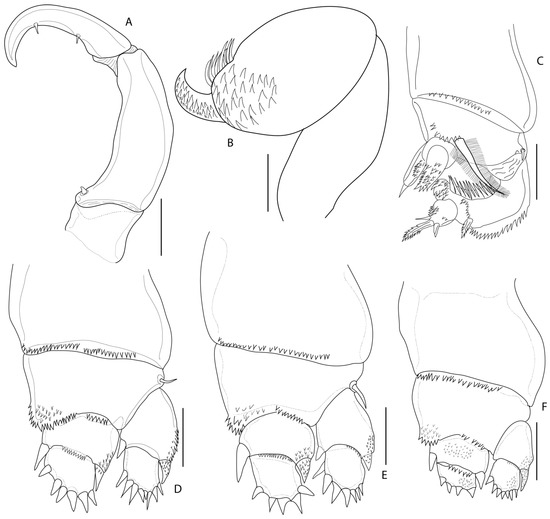
Figure 4.
Nemesis santhadevii sp. nov. from Atelomycterus marmoratus, paratype female. (A) maxilliped; (B) maxilla; (C) leg 1; (D) leg 2; (E) leg 3; and (F) leg 4. Scale bar: (A,B) = 0.02 mm; and (C–F) = 0.5 mm.
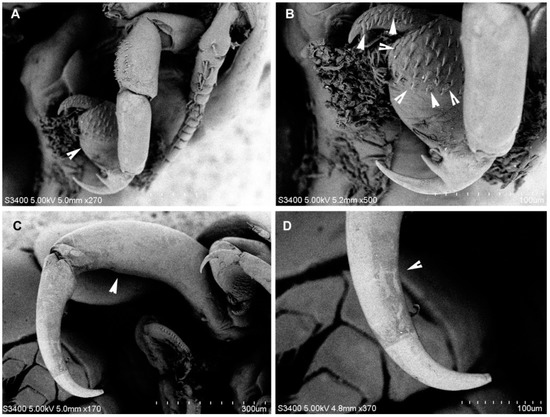
Figure 5.
Nemesis santhadevii sp. nov. from Atelomycterus marmoratus, female. (A) maxilla (arrow); (B) maxilla (arrow showing spinules); (C) maxilliped (arrow); and (D) maxilliped terminal segment (arrow).
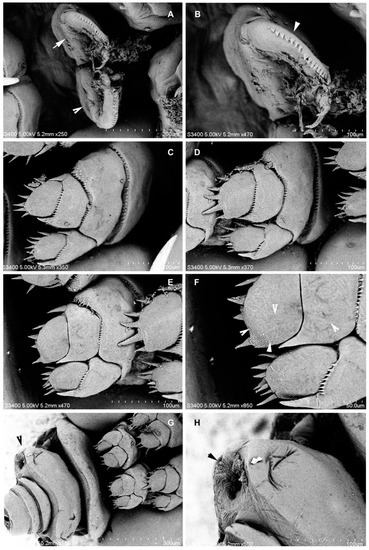
Figure 6.
Nemesis santhadevii sp. nov. from Atelomycterus marmoratus, ovigerous female. (A) leg 1, right and left (arrows); (B) leg 1, high magnification (arrow showing denticles); (C) leg 2; (D) leg 3; (E) leg 4; (F) leg 4, high magnification (arrow showing spinules); and (G,H) genital double somite (arrow).
https://zoobank.org/NomenclaturalActs/3b464950-ce0b-4bc6-84d6-82a538b9ce68 (accessed on 2 September 2022).
3.1.1. Type—Host
Coral catshark Atelomycterus marmoratus (Anonymous (Bennett), 1830) (Carcharhiniformes: Scyliorhinidae).
3.1.2. Type—Locality
Kota Kinabalu, East Malaysia (approximately 5.9825° N, 116.0717° E).
3.1.3. Type—Material
Holotype ♀ (3.2 mm) from the gill filaments of Atelomycterus marmoratus collected by BA Venmathi Maran 01 July 2022 (IPMB-Cr 01.00005). Paratypes: same data as holotype with the following measurements—10 ♀♀ (2.8 to 3.2 mm) (IPMB-Cr 01.00006 to IPMB-Cr 01.00015).
3.1.4. Site on Host
Gill filaments.
3.1.5. Etymology
The species is named in honour of Mrs. Santhadevi Alagarrajan, the late mother of the senior author (BAVM), as a tribute and in memory of her.
3.1.6. Description
Adult Female (Figure 1, Figure 2, Figure 3, Figure 4, Figure 5 and Figure 6): Cephalothorax (Figure 1A–C and Figure 2A) sub-circular, 1.3 times as wide as long, narrowing posteriorly, producing distinct neck, overlapping second pedigerous somite. Pedigerous somites two to four separated by lateral constrictions, lateral borders of segments convex, segmentation dorsally distinct, somites subequal in size. Third somite slightly wider than second, fourth slightly narrower than third. Fifth somite 0.4 times width of fourth, with small lobes carrying fifth leg. Genital double somite slightly narrower than fifth (Figure 2A,C, Figure 3A and Figure 6G,H). Abdomen (Figure 2C) small, with three somites. Caudal rami (Figure 2C,D) ovate, with two large and three small setae.
Egg strings (Figure 1A,B) cylindrical; eggs uniseriate. Number of eggs per string ranged from 15 to 48, dependent on length of string.
Antennule (Figure 2E and Figure 3B–D) 12-segmented, elongated; segments 1 and 2 longer than others, segments 4–10 subequal in length, segments 12 longer than 11. All segments with setae, segments 3, 5–11 each with one seta on disto-lateral margin; segment 2 with eight setae, segment 4 with three setae, terminal segments with cluster of 12 setae and 1 aesthetasc. Antenna (Figure 2G and Figure 3D–F) 4-segmented, segment 1 unarmed, 2 stouter than third, with patches of 34–38 denticles, segment 3 unarmed, 1.5 times longer than 2, distal segment claw like, with two strong spinules (Figure 3F). Mandible (Figure 2H) unsegmented, elongated; inner distal margin with eight short denticles. Maxillule (Figure 2I), inner lobe (endite) with two long spinules, outer lobe (palp) with two short and one long spinules. Maxilla (Figure 4B and Figure 5A,B) 3-segmented, basal segment elongated unarmed, segment 2, 1.6 times as long as wide, 1.4 times as wide as segment 1, distally with patch of 25–29 denticles; distal segment claw like, armed with patches of 32–38 denticles. Maxilliped (Figure 4A and Figure 5C,D) 3-segmented, basal segment unarmed, shorter than others, segment 2 longest, 3 times as long as basal segment, inner proximal margin with one short spine, distal segment, claw like, with short spine on inner proximal and distal margins.
Legs 1–4 (Figure 4C–F) biramous, covered by spinules; rami 2-segmented. Leg 1 (Figure 4C and Figure 6A,B), coxae 1.2 times as wide as long, distal margin with 14–15 short spinules; basis with one short seta at outer margin, one long plumose seta on median margin; exopod with prominent incision on outer border, row of denticles followed by pectinate scales, distal lower lobe with three spinules, apical protrusion covered with denticles; endopod somites spinulose, distal segment with 1 apical spine. Leg 2 (Figure 4D and Figure 6C), coxae 1.9 times as wide as long, distal margin with 33–35 short spinules; basis with one short seta at outer margin, inner endopod basis with 30–35 marginal spinules and patches of 14–16 spinules; exopod distal segment with 8 spinules, unequal; proximal segment with 2 spinules; endopod with 6 and 2 spinules on distally and proximally, respectively. Leg 3 (Figure 4E and Figure 5D), coxae 1.6 times as wide as long, distal margin with 27–30 short spinules; basis with one seta at outer margin, inner endopod basis with 18–22 marginal spinules and patches of 7–10 spinules; exopod distal segment with 7 spinules, 3 smaller; 2 spinules proximally; endopod with 5 robust spinules and 2 spinules proximally; Leg 4 (Figure 4F and Figure 5E,F), coxae distal margin with 22–25 short spinules; basis inner margin with 11–14 marginal spinules and patches of 8–11 spinules; exopod and endopod with 7 and 4 spinules, respectively on distal segment (Table 1). Fifth leg, small lobe bearing 3 setae (Figure 2J).

Table 1.
The setal formula of rami of legs of the female Nemesis santhadevii sp. nov. (Roman numerals indicate the spine and the Arabic numerals indicate the setae).
3.1.7. Distribution
It is known only from the type locality, North Borneo of the South China Sea, Malaysia.
4. Discussion
The genus Nemesis is distinguished from other eudactylinids by the following combinations of characteristics: (1) the rounded cephalothorax is narrower than the second pedigerous somite; (2) the pedigerous somites from two to four are free, and laterally folded downwards, and dorsal projections are absent; (3) the tergites of the free pedigerous somites are well defined and ornamented; (4) the fifth somite is generally much narrower than the fourth, having postero-lateral lobes carrying the vestigial legs; (5) antennule 13–14 are segmented; and (6) the first leg is highly modified, and coxal setae are absent in legs 2–4 [4,17,18].
The species of Nemesis can be determined based on: the proportion of the dorsal cephalothoracic shield compared to body length; the comparative sizes of the second to the fifth pedigerous somites; the size of the fifth somite; and variation in the appendages [3,6,8,9,11,18].
The most combinations of diagnostic characteristics of Nemesis santhadevii sp. nov. include: (1) the sub-circular cephalothorax is 1.3 times as wide as it is long, and it overlaps the second pedigerous somite; (2) the fifth somite is 0.4 times the width of fourth; (3) the genital double somite is slightly narrower than the fifth somite; (4) the caudal rami has two large and three small spinules; (5) segment 2 of the antenna has a patch of 34 to 38 short spinules; and (6) the structure and the number of setae or spines on legs 1–4 (Table 1). N. santhadevii sp. nov. appears to be the 13th species of the genus Nemesis and the first from Malaysia and the South China Sea. Species of Nemesis are distinguished from each other mainly by one or two minor differences [18].
By observing the following characteristics, N. santhadevii sp. nov. can be distinguished from most of its congeners (see Table 2) in: the lowest cephalothoracic shield’s proportion to body length (0.20:1) in the female of N. santhadevii sp. nov. (compared with more than 0.25:1 in all other species); the size of the fifth somite is 0.4 times the width of the fourth (compared with being slightly narrower than the fourth in most congeners and equal to the fourth in N. lamna); the comparative sizes of the second to the fifth pedigerous somites—in N. santhadevii sp. nov., somites two and three are equal, and somite four is slightly narrower than three (compared with somites two to five being equal or subequal or decreasing in size from somites two to five in other species). In the structure of antennae, N. tiburo and N. santhadevii sp. nov. have only one patch of spinules on the second somite, but both species can be distinguished from each other by the third somite of the antenna—without spinules in N. santhadevii sp. nov. and with spinules in N. tiburo.

Table 2.
Inter-specific characteristics between the species of Nemesis Risso, 1826.
Most species of Nemesis have been reported from more than one host (see Table 3). The type species N. lamna has been reported from seven species of elasmobranch fishes. Both N. atlantica, and N. pallida, have been reported from six different host fishes. Four species, such as N. santhadevii sp. nov., N. tiburo, and N. sphyrnae, have so far been reported only from their respective type hosts [3,6,8,9,11,12,18].

Table 3.
List of valid species of Nemesis Risso, 1826, with their fish hosts and distribution record.
Author Contributions
B.A.V.M.: conceptualization, collection, identification, writing the original draft, editing, funding, and correspondence; and P.T.A. and S.Y.M.: assistance in reviewing the illustrations and editing the draft. All authors have read and agreed to the published version of the manuscript.
Funding
We thank Universiti Malaysia Sabah Project, SDN0073-2019 and the MABIK Project LPA2007.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The types were deposited in the Museum Collection Repository, Borneo Marine Research Institute, Universiti Malaysia Sabah, Kota Kinabalu.
Acknowledgments
We thank Danny Tang, USA, Susumu Ohtsuka and Kunihiko Izawa, Japan, for providing valuable literature. Thanks to the reviewers for their constructive comments which were very helpful in improving the manuscript. SYM thank the National Institute of Fisheries Science, Ministry of Oceans and Fisheries, Republic of Korea (R2022037).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Walter, T.C.; Boxshall, G.A. World of Copepods Database. 2022. Available online: http://www.marinespecies.org/copepoda (accessed on 5 September 2022).
- Risso, A. Histoire Naturelle des Principales Productions de 1 ‘Europe Meridionale et Particulierement de Celles des Environs de Nice et des Alpes Maritimes; Levrault: Paris, France, 1826; Volume 5, pp. 1–402. [Google Scholar]
- Wilson, C.B. Crustacean parasites of West Indian fishes and land crabs with descriptions of new genera and species. Proc. U. S. Nat. Mus. 1913, 44, 189–277. [Google Scholar] [CrossRef]
- Wilson, C.B. North American Parasitic Copepods Belonging to the Family Dichelesthiidae; US Government Printing Office: Washington, DC, USA, 1922; Volume 60, pp. 1–100.
- Scott, A. The copepod parasites of Irish Sea fishes. Rep. Lancashire Sea Fish Lab. 1929, 81–118. [Google Scholar]
- Wilson, C.B. The Copepoda of the Woods Hole region, Massachusetts. Bull. U. S. Natl. Mus. 1932, 158, 1–635. [Google Scholar]
- Pearse, A.S. Parasitic Crustacea from Bimini Bahamas. Proc. U. S. Natl. Mus. 1951, 101, 341–372. [Google Scholar] [CrossRef]
- Pearse, A.S. Parasitic Crustacea from the Texas coast. Pubis. Inst. Mar. Sci. Univ. Tex. 1952, 2, 5–42. [Google Scholar]
- Shiino, S.M. Copepods parasitic on Japanese fishes. 15. Eudactylinidae and Dichelesthiidae. Rep. Faculty Fish. Prefect. Univ. Mie. 1957, 2, 392–410. [Google Scholar]
- Cressey, R.F. Caligoid copepods parasitic on sharks of the Indian Ocean. Proc. U. S. Natl. Mus. 1967, 12, 1–21. [Google Scholar] [CrossRef]
- Cressey, R.F. Copepods parasitic on sharks from the east coast of Florida. Smith. Contr. Zool. 1970, 38, 1–30. [Google Scholar] [CrossRef]
- Rangnekar, M.P. Parasitic Copepods from Marine Fishes of Bombay; Crustaceana, Supplement, No. 7, Studies on Copepoda II; Brill: Leiden, The Netherlands, 1984; pp. 344–351. [Google Scholar]
- Froese, R.; Pauly, D. (Eds.) FishBase. World Wide Web Electronic Publication, Version (09/2009). 2022. Available online: www.fishbase.org (accessed on 20 August 2022).
- Humes, A.G.; Gooding, R.U. A method for studying the external anatomy of copepods. Crustaceana 1964, 6, 238–240. [Google Scholar] [CrossRef]
- Huys, R.; Boxshall, G.A. Copepod Evolution; The Ray Society: London, UK, 1991; p. 468. [Google Scholar]
- Fricke, R.; Eschmeyer, W.N.; van der Laan, R. (Eds.) Catalog of Fishes: Genera, Species, References. 2020. Available online: http://research.calacademy.org/research/ichthyology/catalog/fishcatmain.asp (accessed on 10 March 2022).
- Yamaguti, S. Parasitic Copepoda and Branchiura of Fishes; Interscience Publishers, John Wiley: New York, NY, USA, 1963; p. 723. [Google Scholar]
- Pillai, N.K. The Fauna of India: Copepod parasites of marine fishes. In The Fauna of India. Zoological Society of India; Calcutta; Government of India: New Delhi, India, 1985; p. 900. [Google Scholar]
- Hesse, C.E. Crustaces. Raresounoveaux des cotes de France. Ann. Sci. Natl. 1883, 15, 1–48. [Google Scholar]
- Raux, P. Crustaces de la Mediterraneeet de son Littoral; Levrault: Paris, France, 1828; p. 176. [Google Scholar]
- Heller, C. Crustaceen. In Zoologischer Teil in Reise der Oesterreichischen Fregatte Novara; Steindachner: Berlin, Germany, 1868; Volume 2, p. 280. [Google Scholar]
- Heegaard, P.E. Parasitic Copepoda from Australian waters. Rec. Aust. Mus. 1962, 25, 149–234. [Google Scholar] [CrossRef]
- Hewitt, G.C. Some New Zealand Parasitic Copepoda of the Family Eudactylinidae; Victoria University of Wellington: Wellington, New Zealand, 1969; Volume 49, pp. 1–31. [Google Scholar]
- Beneden, P.J.V. Note surun nouveau genre de Crustace parasite Eudactylina (E. acuta). Bull. Acad. R. Belg. 1853, 20, 235–239. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).