Distribution and Environmental Impact Factors of Phytoplankton in the Bay of Bengal during Autumn
Abstract
:1. Introduction
2. Methods
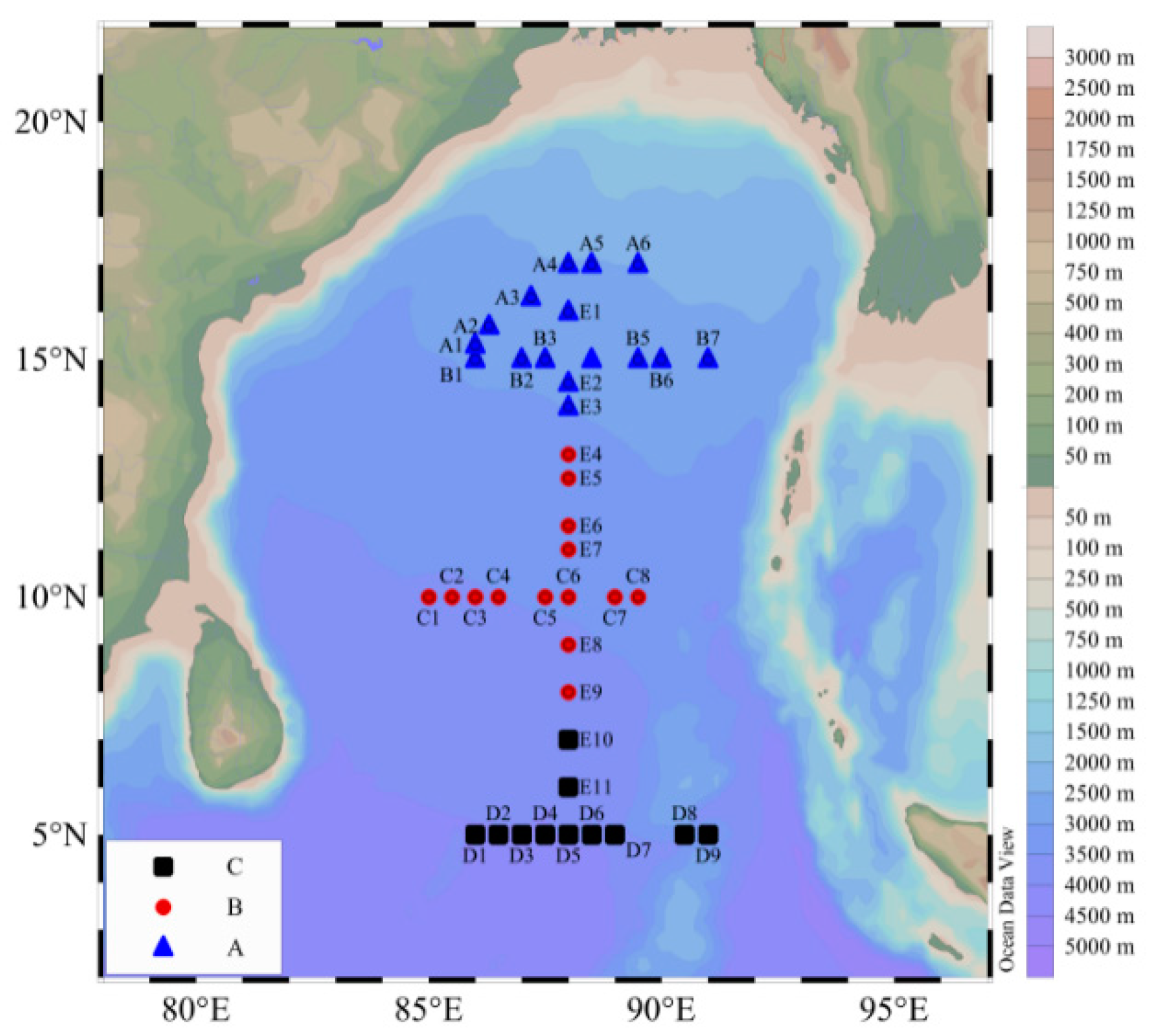
2.1. Sampling and Analysis
2.2. Data Analysis and Statistical Methods
3. Results
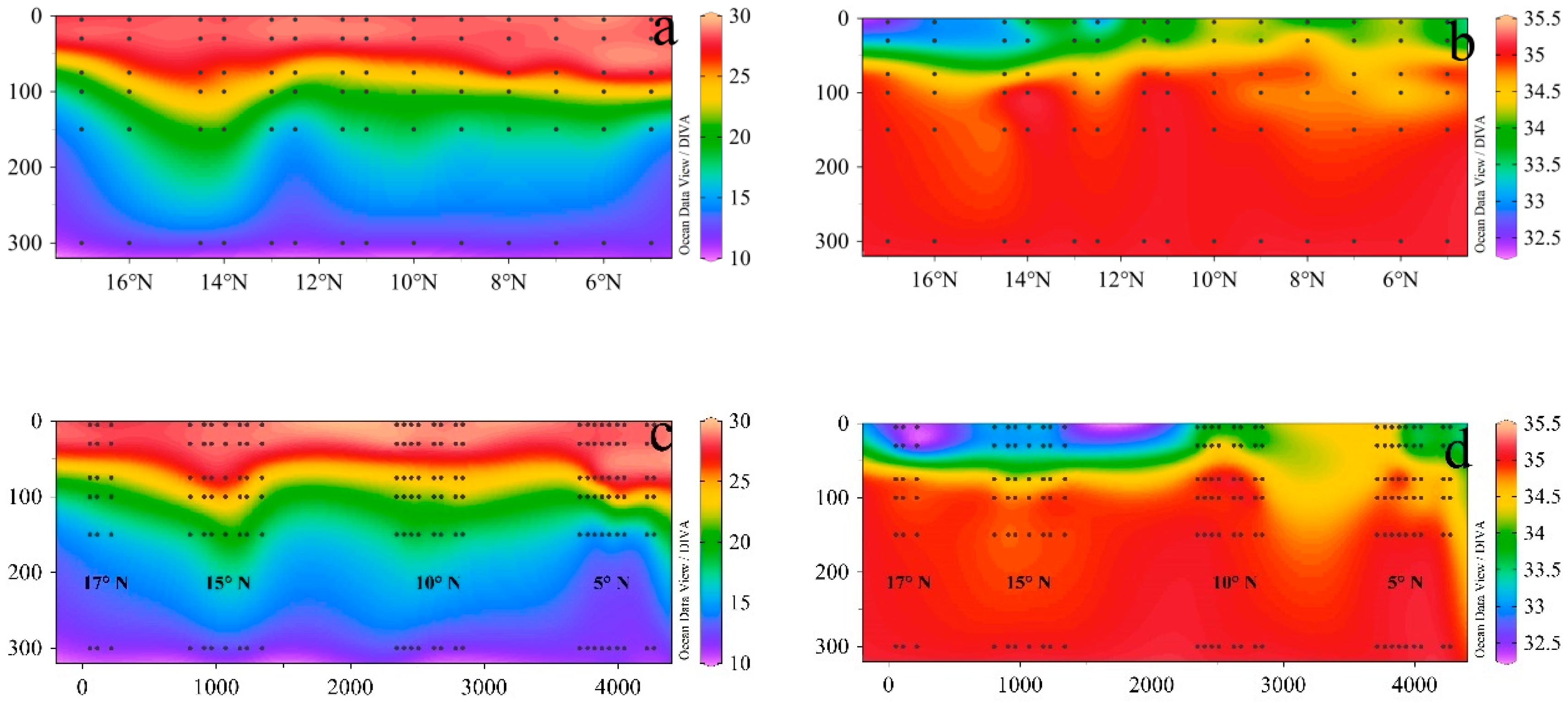
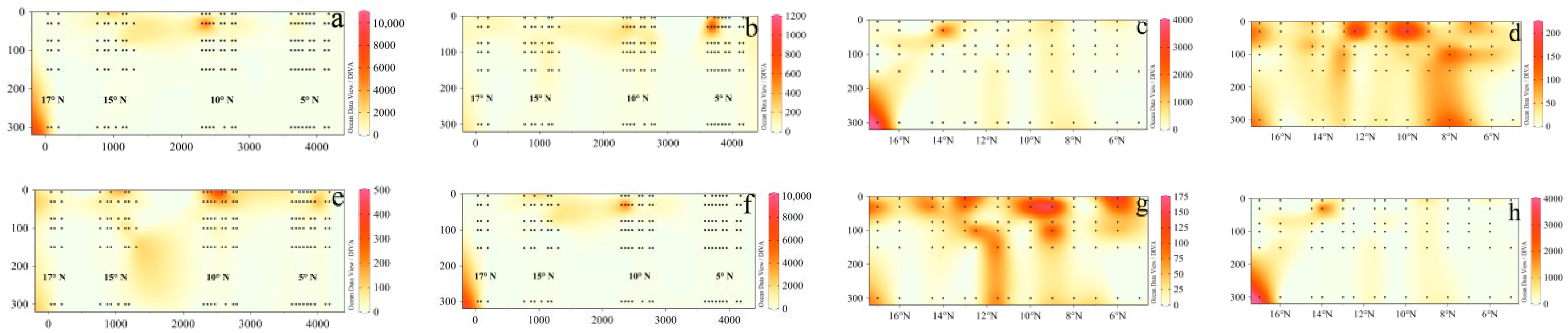
3.1. Environmental Parameters
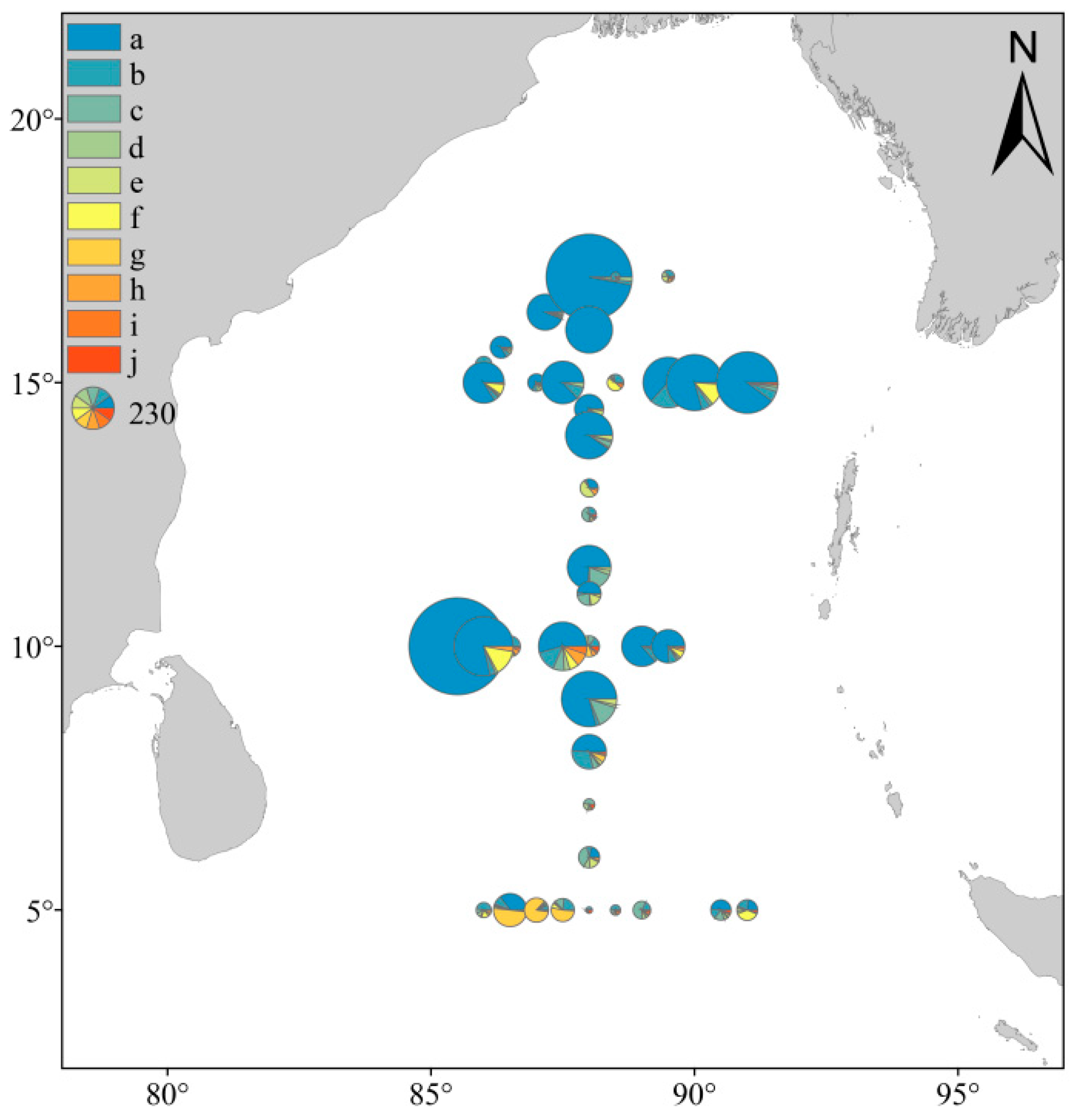
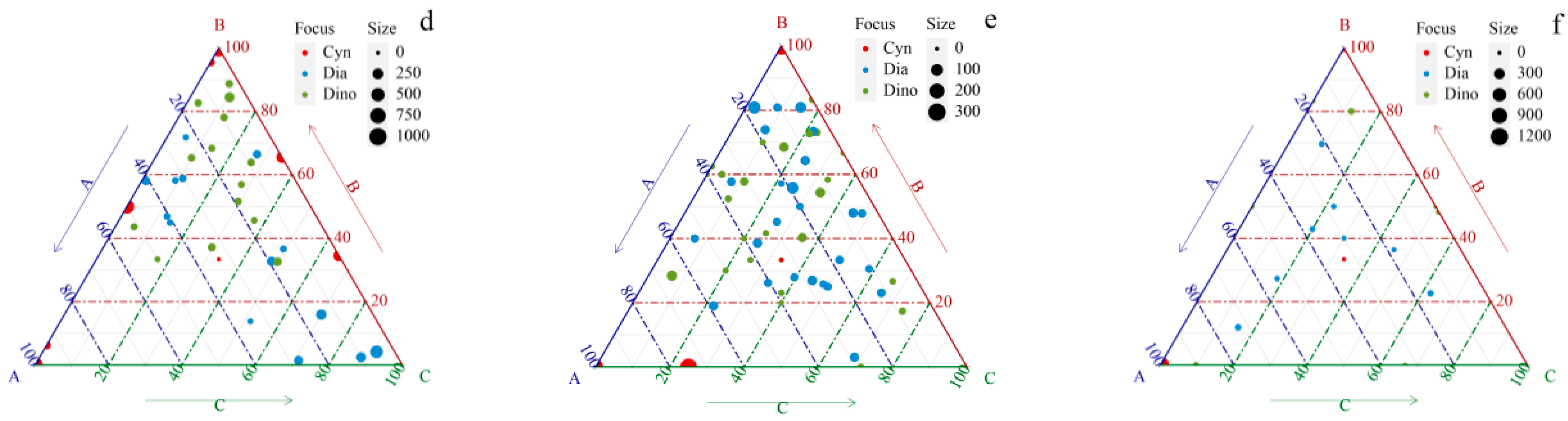
3.2. Species Composition and Dominant Species
3.3. Distribution of Phytoplankton Community Structure
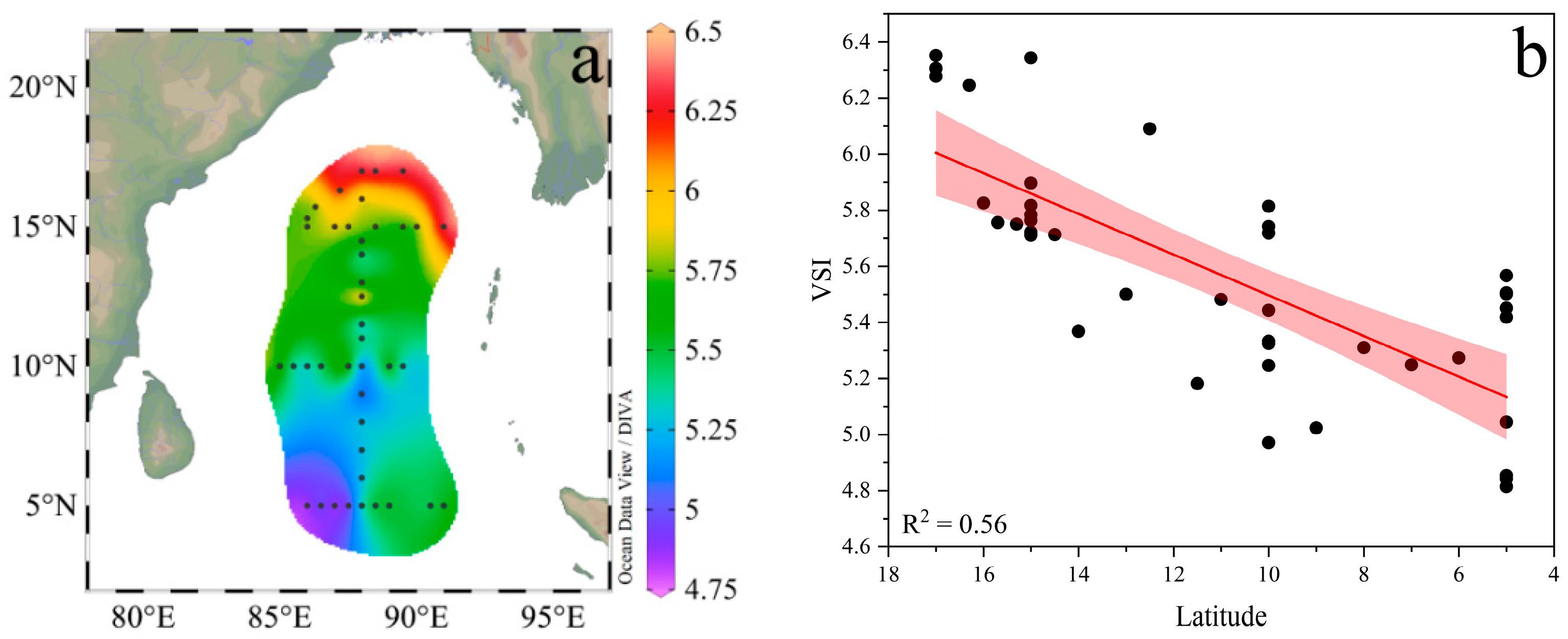
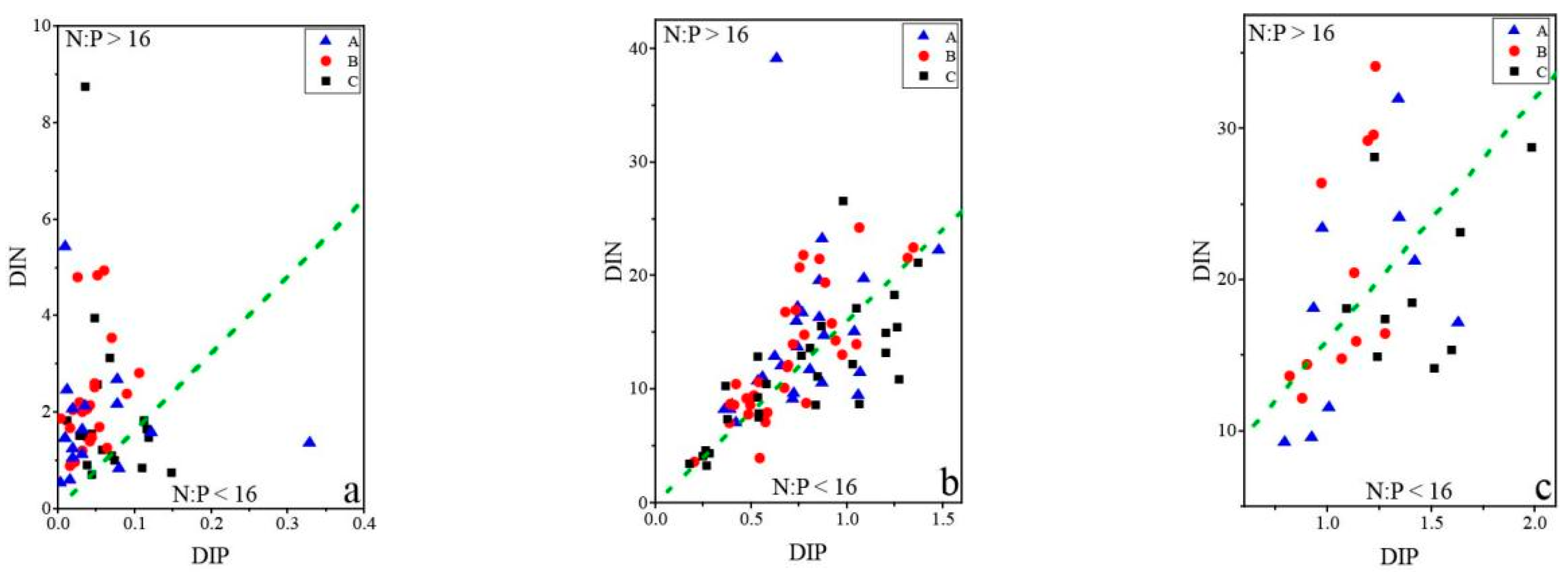
3.4. Relationships between Phytoplankton and Environmental Factors
4. Discussion
4.1. Hydrological Conditions and Corresponding Phytoplankton Community Structure
4.2. Vertical Stratification and Phytoplankton Community Structure
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Field, C.B.; Behrenfeld, M.J.; Randerson, J.T.; Falkowski, P. Primary production of the biosphere: Integrating terrestrial and oceanic components. Science 2016, 281, 237–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falkowski, P.G.; Barber, R.T.; Smetacek, V. Biogeochemical controls and feedbacks on ocean primary production. Science 1998, 281, 200–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schloss, I.R.; Abele, D.; Moreau, S.; Demers, S.; Bers, A.V.; González, O.; Ferreyra, G.A. Response of phytoplankton dynamics to 19-year (1991–2009) climate trends in Potter Cove (Antarctica). J. Mar. Syst. 2012, 92, 53–66. [Google Scholar] [CrossRef]
- Ptacnik, R.; Solimini, A.G.; Andersen, T.; Tamminen, T.; Brettum, P.; Lepistö, L.; Rekolainen, S. Diversity predicts stability and resource use efficiency in natural phytoplankton communities. Proc. Natl. Acad. Sci. USA 2008, 105, 5134–5138. [Google Scholar] [CrossRef] [Green Version]
- Deppeler, S.L.; Davidson, A.T. Southern Ocean phytoplankton in a changing climate. Front. Mar. Sci. 2017, 4, 40. [Google Scholar] [CrossRef] [Green Version]
- Loeb, V.; Hofmann, E.E.; Klinck, J.M.; Holm-Hansen, O. Hydrographic control of the marine ecosystem in the South Shetland-Elephant Island and Bransfield Strait region. Deep Sea Res. Part II Top. Stud. Oceanogr. 2010, 57, 519–542. [Google Scholar] [CrossRef]
- Forcada, J.; Trathan, P.N.; Boveng, P.L.; Boyd, I.L.; Burns, J.M.; Costa, D.P.; Southwell, C.J. Responses of Antarctic pack-ice seals to environmental change and increasing krill fishing. Biol. Conserv. 2012, 149, 40–50. [Google Scholar] [CrossRef] [Green Version]
- Kerr, R.; Goyet, C.; da Cunha, L.C.; Orselli, I.B.; Lencina-Avila, J.M.; Mendes, C.R.B.; Tavano, V.M. Carbonate system properties in the Gerlache Strait, Northern Antarctic Peninsula (February 2015): II. Anthropogenic CO2 and seawater acidification. Deep Sea Res. Part II Top. Stud. Oceanogr. 2018, 149, 182–192. [Google Scholar] [CrossRef]
- Tréguer, P.; Bowler, C.; Moriceau, B.; Dutkiewicz, S.; Gehlen, M.; Aumont, O.; Pondaven, P. Influence of diatom diversity on the ocean biological carbon pump. Nat. Geosci. 2018, 11, 27–37. [Google Scholar] [CrossRef]
- Platt, T.; Rao, D.V.; Irwin, B. Photosynthesis of picoplankton in the oligotrophic ocean. Nature 1983, 301, 702–704. [Google Scholar] [CrossRef]
- Vinayachandran, P.N.; Mathew, S. Phytoplankton bloom in the Bay of Bengal during the northeast monsoon and its intensification by cyclones. Geophys. Res. Lett. 2003, 30, 1572. [Google Scholar] [CrossRef] [Green Version]
- Vinayachandran, P.N.; McCreary, J.P., Jr.; Hood, R.R.; Kohler, K.E. A numerical investigation of the phytoplankton bloom in the Bay of Bengal during Northeast Monsoon. J. Geophys. Res. Ocean. 2005, 110, C12001. [Google Scholar] [CrossRef]
- Prasanna Kumar, S.; Nuncio, M.; Narvekar, J.; Kumar, A.; Sardesai, D.S.; De Souza, S.N.; Madhupratap, M. Are eddies nature’s trigger to enhance biological productivity in the Bay of Bengal? Geophys. Res. Lett. 2004, 31, L07309. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Ke, Z.; Lin, Q.; Ni, G.; Shen, P.; Liu, H.; Tan, Y. Longitudinal patterns of spring-intermonsoon phytoplankton biomass, species compositions and size structure in the Bay of Bengal. Acta Oceanol. Sin. 2012, 31, 121–128. [Google Scholar] [CrossRef]
- Liu, H.; Ke, Z.; Song, X.; Tan, Y.; Huang, L.; Lin, Q. Primary production in the Bay of Bengal during spring intermonsoon period. Shengtai Xuebao/Acta Ecol. Sin. 2011, 31, 7007–7012. [Google Scholar]
- Shetye, S.R.; Shenoi, S.S.C.; Gouveia, A.D.; Michael, G.S.; Sundar, D.; Nampoothiri, G. Wind-driven coastal upwelling along the western boundary of the Bay of Bengal during the southwest monsoon. Cont. Shelf Res. 1991, 11, 1397–1408. [Google Scholar] [CrossRef]
- Sengupta, D.; Bharath Raj, G.N.; Shenoi, S.S.C. Surface freshwater from Bay of Bengal runoff and Indonesian throughflow in the tropical Indian Ocean. Geophys. Res. Lett. 2006, 33, L22609. [Google Scholar] [CrossRef] [Green Version]
- Yamaji, I. Illustrations of the Marine Plankton of Japan; Hoikusha: Tokyo, Japan, 1996. [Google Scholar]
- Jin, D. Chinese Marine Planktonic Diatoms; Shanghai Scientific and Technical Publishers: Shanghai, China, 1965. [Google Scholar]
- Sun, J.; Liu, D.Y. The preliminary notion on nomenclature of common phytoplankton in China Seas waters. Oceanol. Limnol. Sin. 2002, 33, 271–286. [Google Scholar]
- Pai, S.C.; Tsau, Y.J.; Yang, T.I. pH and buffering capacity problems involved in the determination of ammonia in saline water using the indophenol blue spectrophotometric method. Anal. Chim. Acta 2001, 434, 209–216. [Google Scholar] [CrossRef]
- Dai, M.; Wang, L.; Guo, X.; Zhai, W.; Li, Q.; He, B.; Kao, S.J. Nitrification and inorganic nitrogen distribution in a large perturbed river/estuarine system: The Pearl River Estuary, China. Biogeosciences 2008, 5, 1227–1244. [Google Scholar] [CrossRef] [Green Version]
- Guo, S.; Feng, Y.; Wang, L.; Dai, M.; Liu, Z.; Bai, Y.; Sun, J. Seasonal variation in the phytoplankton community of a continental-shelf sea: The East China Sea. Mar. Ecol. Prog. Ser. 2014, 516, 103–126. [Google Scholar] [CrossRef] [Green Version]
- Sun, R. The Principle of Animal Ecology; Beijing Normal University Press: Beijing, China, 1987. [Google Scholar]
- Tan, J.Y.; Huang, L.M.; Tan, Y.H.; Lian, X.P.; Hu, Z.F. The influences of water-mass on phytoplankton community structure in the Luzon strait. Acta Oceanol. Sin. 2013, 35, 178–189. [Google Scholar]
- Mena, C.; Reglero, P.; Hidalgo, M.; Sintes, E.; Santiago, R.; Martín, M.; Moyà, G.; Balbín, R. Phytoplankton community structure is driven by stratification in the oligotrophic mediterranean sea. Front. Microbiol. 2019, 10, 1698. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Song, S.Q.; Le, F.F.; Wang, D.; Dai, M.H.; Ning, X.R. Phytoplankton in northern South China Sea in the winter of 2004. Acta Oceanol. Sin. 2007, 29, 132–145. [Google Scholar]
- Busseni, G.; Rocha Jimenez Vieira, F.; Amato, A.; Pelletier, E.; Pierella Karlusich, J.J.; Ferrante, M.I.; Iudicone, D. Meta-omics reveals genetic flexibility of diatom nitrogen transporters in response to environmental changes. Mol. Biol. Evol. 2019, 36, 2522–2535. [Google Scholar] [CrossRef] [Green Version]
- Caputi, L.; Carradec, Q.; Eveillard, D.; Kirilovsky, A.; Pelletier, E.; Pierella Karlusich, J.J.; Tara Oceans Coordinators. Community-level responses to iron availability in open ocean plankton ecosystems. Glob. Biogeochem. Cycles 2019, 33, 391–419. [Google Scholar] [CrossRef]
- Estrada, M.; Berdalet, E. Phytoplankton in a turbulent world. Sci. Mar. 1997, 61, 125–140. [Google Scholar] [CrossRef]
- Thomas, W.H.; Gibson, C.H. Quantified small-scale turbulence inhibits a red tide dinoflagellate, Gonyaulax polyedra Stein. Deep Sea Res. Part A Oceanogr. Res. Pap. 1990, 37, 1583–1593. [Google Scholar] [CrossRef]
- Smayda, T.J. Harmful algal blooms: Their ecophysiology and general relevance to phytoplankton blooms in the sea. Limnol. Oceanogr. 1997, 42, 1137–1153. [Google Scholar] [CrossRef]
- Stal, L.J.; Severin, I.; Bolhuis, H. The ecology of nitrogen fixation in cyanobacterial mats. In Recent Advances in Phototrophic Prokaryotes; Springer: Cham, Switzerland, 2010; pp. 31–45. [Google Scholar] [CrossRef]
- Zehr, J.P.; Ward, B.B. Nitrogen cycling in the ocean: New perspectives on processes and paradigms. Appl. Environ. Microbiol. 2002, 68, 1015–1024. [Google Scholar] [CrossRef] [Green Version]
- Gao, K.; Beardall, J.; Häder, D.P.; Hall-Spencer, J.M.; Gao, G.; Hutchins, D.A. Effects of ocean acidification on marine photosynthetic organisms under the concurrent influences of warming, UV radiation, and deoxygenation. Front. Mar. Sci. 2019, 6, 322. [Google Scholar] [CrossRef]
- Huang, B.; Ou, L.; Hong, H.; Luo, H.; Wang, D. Bioavailability of dissolved organic phosphorus compounds to typical harmful dinoflagellate Prorocentrum donghaiense Lu. Mar. Pollut. Bull. 2005, 51, 838–844. [Google Scholar] [CrossRef] [PubMed]
- Stoecker, D.K. Mixotrophy among Dinoflagellates 1. J. Eukaryot. Microbiol. 1999, 46, 397–401. [Google Scholar] [CrossRef]
- Tilman, D. Green, blue-green and diatom algae: Taxonomic differences in competitive ability for phosphorus, silicon and nitrogen. Arch. Hydrobiol. 1986, 106, 473–485. [Google Scholar] [CrossRef]
- Pérez, V.; Fernández, E.; Marañón, E.; Morán, X.A.G.; Zubkov, M.V. Vertical distribution of phytoplankton biomass, production and growth in the Atlantic subtropical gyres. Deep Sea Res. Part I Oceanogr. Res. Pap. 2006, 53, 1616–1634. [Google Scholar] [CrossRef]




| Special | fi | pi | Y |
|---|---|---|---|
| Trichodesmium Ehrenberg ex Gomont | 0.2155 | 0.6415 | 0.1382 |
| Synedra C.G. Ehrenberg | 0.75 | 0.0459 | 0.0344 |
| Prorocentrum lenticulatum (Matzenauer) F.J.R.Taylor | 0.5216 | 0.0372 | 0.0194 |
| Coscinodiscus granii Gough | 0.6164 | 0.0169 | 0.0104 |
| Eunotogramma debile Grunow in Van Heurck | 0.5905 | 0.0118 | 0.007 |
| Dictyocha fibula Ehrenberg | 0.2198 | 0.0285 | 0.0063 |
| Thalassionema frauenfeldii (Grunow) Tempère and Peragallo | 0.1724 | 0.0349 | 0.006 |
| Tripos furca (Ehrenberg) F.Gómez | 0.2328 | 0.0135 | 0.0031 |
| Planktoniella formosa (Karsten) Qian and Wang | 0.3664 | 0.0067 | 0.0024 |
| Coscinodiscus subtilis Ehrenberg | 0.4009 | 0.006 | 0.0024 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Sun, J.; Yu, H. Distribution and Environmental Impact Factors of Phytoplankton in the Bay of Bengal during Autumn. Diversity 2022, 14, 361. https://doi.org/10.3390/d14050361
Wang X, Sun J, Yu H. Distribution and Environmental Impact Factors of Phytoplankton in the Bay of Bengal during Autumn. Diversity. 2022; 14(5):361. https://doi.org/10.3390/d14050361
Chicago/Turabian StyleWang, Xingzhou, Jun Sun, and Hao Yu. 2022. "Distribution and Environmental Impact Factors of Phytoplankton in the Bay of Bengal during Autumn" Diversity 14, no. 5: 361. https://doi.org/10.3390/d14050361
APA StyleWang, X., Sun, J., & Yu, H. (2022). Distribution and Environmental Impact Factors of Phytoplankton in the Bay of Bengal during Autumn. Diversity, 14(5), 361. https://doi.org/10.3390/d14050361







