Safe Passage or Hunting Ground? A Test of the Prey-Trap Hypothesis at Wildlife Crossing Structures on NH 44, Pench Tiger Reserve, Maharashtra, India
Abstract
:1. Introduction
- a.
- prey–predator latencies at crossing structures would be lower than those in control habitats, and lower than predator–prey latencies at crossing structures, indicating that predators scout for prey at crossing structures;
- b.
- predator–prey latencies at crossing structures would be lower than those in control habitats, and higher than prey–predator latencies at crossing structures, indicating an avoidance of crossing structures by prey; and
- c.
- prey–dog latencies at crossing structures would be similar to or lower than prey–predator latencies at crossing structures, indicating that free-ranging dogs scout for prey at crossing structures.
2. Materials and Methods
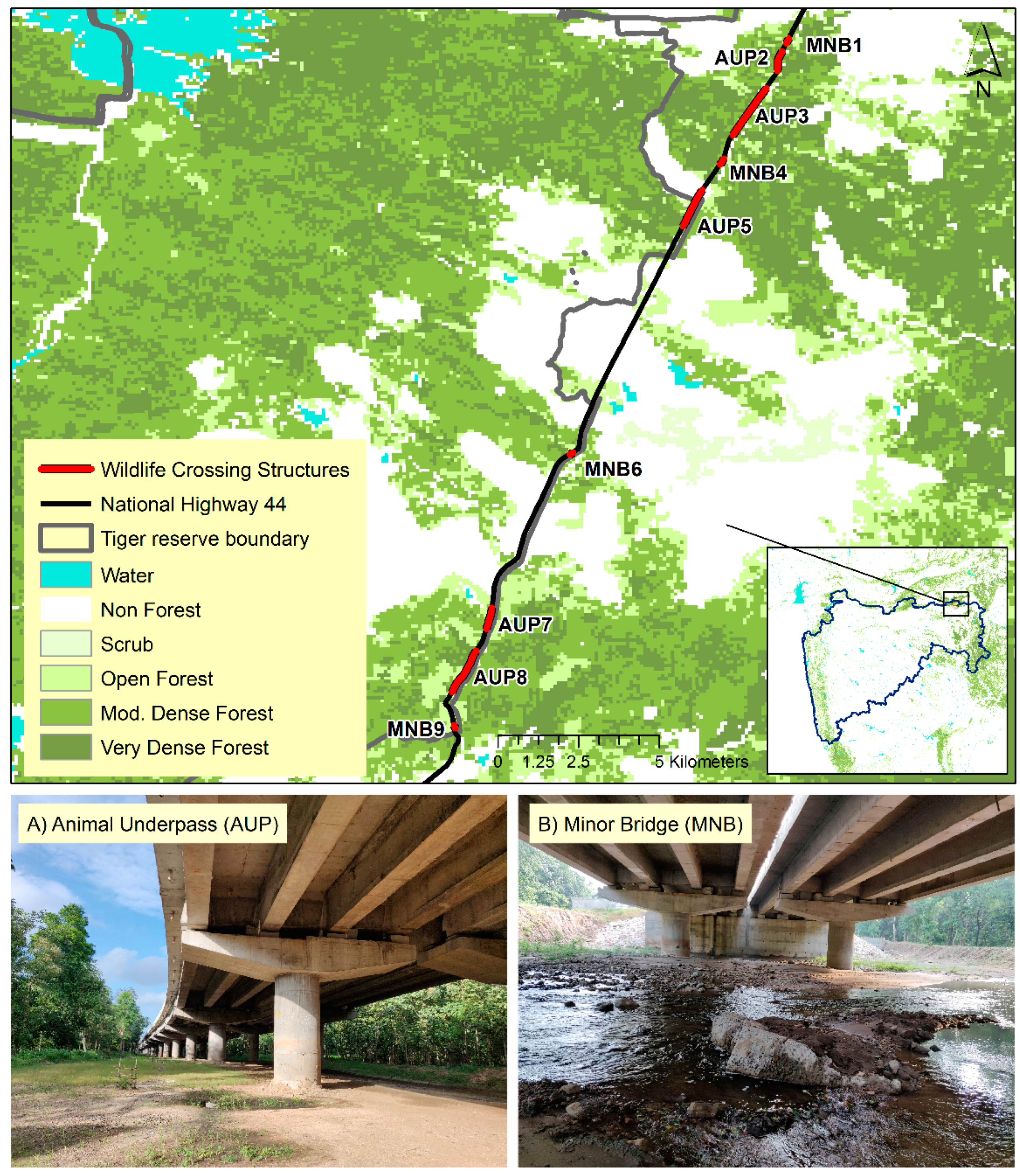
2.1. Study Area
2.2. Field Methods
2.3. Analytical Methods
2.3.1. Comparison of Geometric Mean Latencies between Group Pairs and Sites
2.3.2. Assessment of Factors Influencing Variation in Latencies
3. Results
3.1. Comparison of Geometric Mean Latencies between Group Pairs and Sites
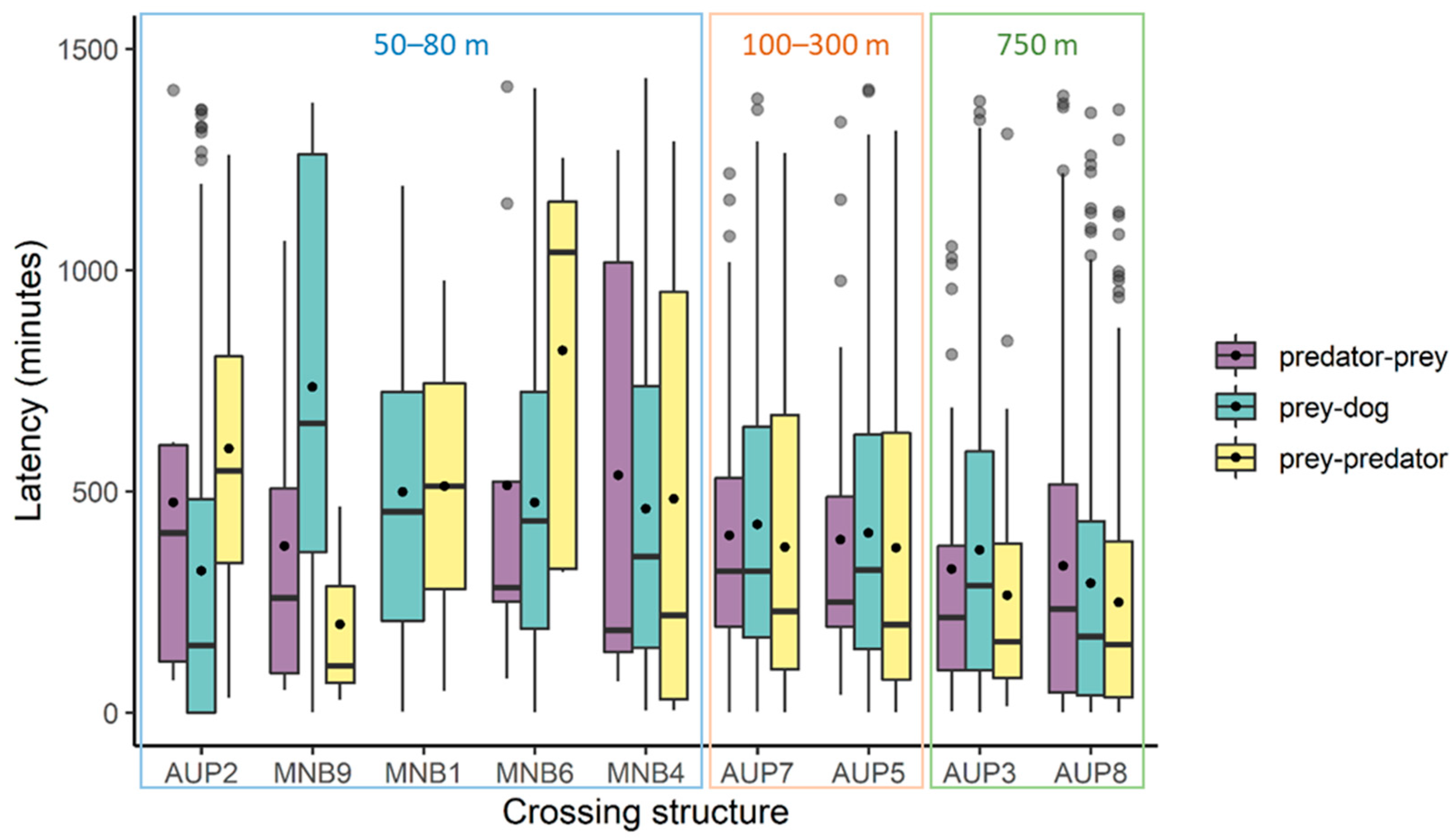
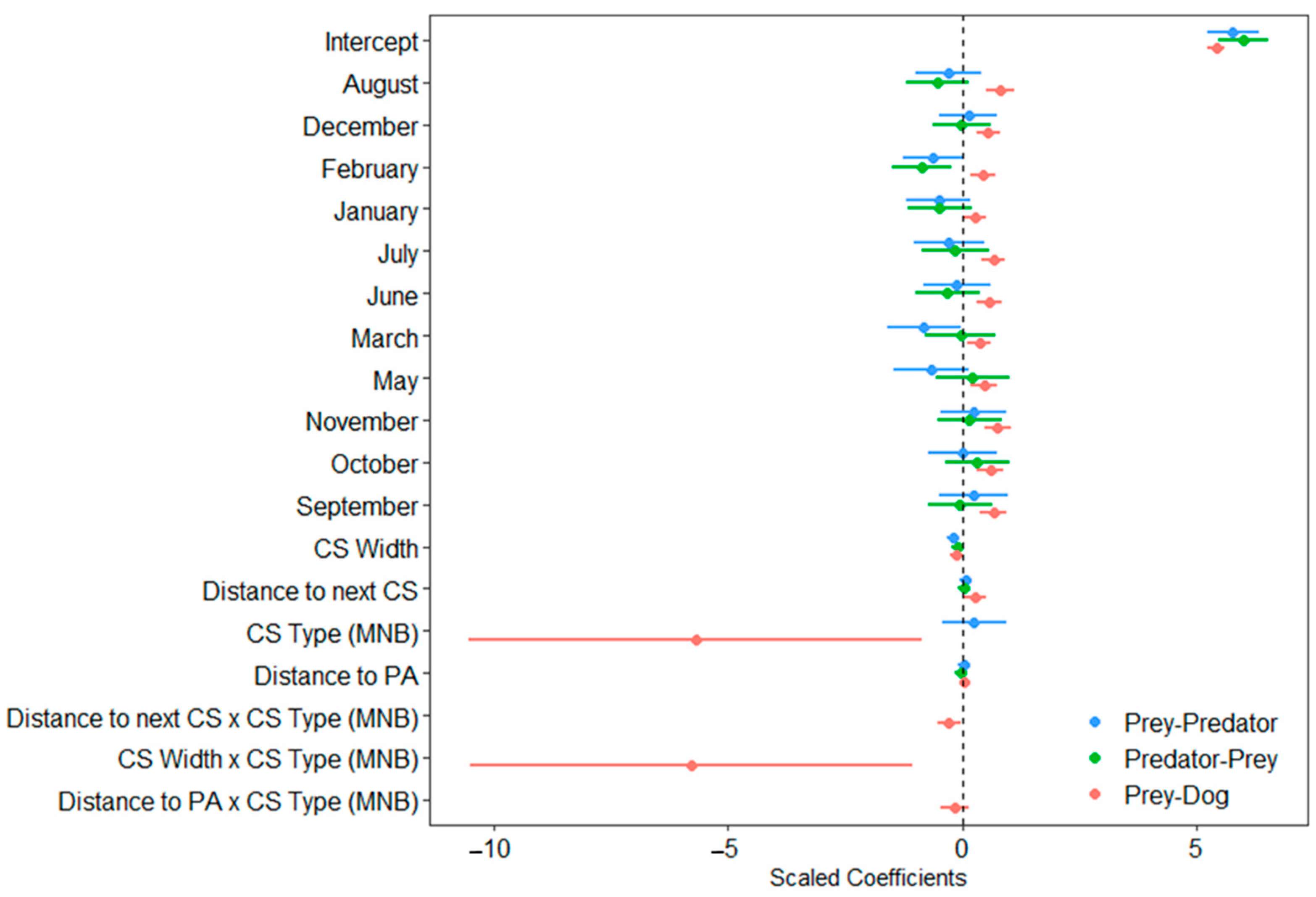
3.2. Assessment of Factors Influencing Variation in Latencies
4. Discussion
4.1. Predator Following Prey into Crossing Structures
4.2. Prey Following Predator into Crossing Structures
4.3. Free-Ranging Dogs Following Prey into Crossing Structures
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Der Ree, R.; Smith, D.J.; Grilo, C. The ecological effects of linear infrastructure and traffic: Challenges and opportunities of rapid global growth. In Handbook of Road Ecology; Van Der Ree, R., Smith, D.J., Grilo, C., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2015. [Google Scholar]
- Ibisch, P.L.; Hoffmann, M.T.; Kreft, S.; Pe’Er, G.; Kati, V.; Biber-Freudenberger, L.; DellaSala, D.A.; Vale, M.M.; Hobson, P.R.; Selva, N. A global map of roadless areas and their conservation status. Science 2016, 354, 1423–1427. [Google Scholar] [CrossRef] [PubMed]
- Pinto, F.A.S.; Bager, A.; Clevenger, A.P.; Grilo, C. Giant anteater (Myrmecophaga tridactyla) conservation in Brazil: Analysing the relative effects of fragmentation and mortality due to roads. Biol. Conserv. 2018, 228, 148–157. [Google Scholar] [CrossRef]
- Nayak, R.; Karanth, K.K.; Dutta, T.; Defries, R.; Ullas Karanth, K.; Vaidyanathan, S. Bits and pieces: Forest fragmentation by linear intrusions in India. Land Use Policy 2020, 99, 104619. [Google Scholar] [CrossRef]
- Colchero, F.; Conde, D.A.; Manterola, C.; Chávez, C.; Rivera, A.; Ceballos, G. Jaguars on the move: Modeling movement to mitigate fragmentation from road expansion in the Mayan Forest. Anim. Conserv. 2011, 14, 158–166. [Google Scholar] [CrossRef]
- Chen, H.L.; Koprowski, J.L. Barrier effects of roads on an endangered forest obligate: Influences of traffic, road edges, and gaps. Biol. Conserv. 2016, 199, 33–40. [Google Scholar] [CrossRef] [Green Version]
- Jacobson, S.L.; Bliss-Ketchum, L.L.; De Rivera, C.E.; Smith, W.P. A behavior-based framework for assessing barrier effects to wildlife from vehicle traffic volume. Ecosphere 2016, 7, 1–15. [Google Scholar] [CrossRef]
- Schwab, A.C.; Zandbergen, P.A. Vehicle-related mortality and road crossing behavior of the Florida panther. Appl. Geogr. 2011, 31, 859–870. [Google Scholar] [CrossRef]
- Jackson, N.D.; Fahrig, L. Relative effects of road mortality and decreased connectivity on population genetic diversity. Biol. Conserv. 2011, 144, 3143–3148. [Google Scholar] [CrossRef]
- Riley, S.P.D.; Pollinger, J.P.; Sauvajot, R.M.; York, E.C.; Bromley, C.; Fuller, T.K.; Wayne, R.K. A southern California freeway is a physical and social barrier to gene flow in carnivores. Mol. Ecol. 2006, 15, 1733–1741. [Google Scholar] [CrossRef]
- Ceia-Hasse, A.; Navarro, L.M.; Borda-de-Água, L.; Pereira, H.M. Population persistence in landscapes fragmented by roads: Disentangling isolation, mortality, and the effect of dispersal. Ecol. Modell. 2018, 375, 45–53. [Google Scholar] [CrossRef]
- Smith, D.J.; van der Ree, R.; Rossel, C. Wildlife crossing structures: An effective strategy to restore or maintain wildlife connectivity across roads. In Handbook of Road Ecology; van der Ree, R., Smith, D.J., Grilo, C., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2015. [Google Scholar]
- Gagnon, J.W.; Dodd, N.L.; Ogren, K.S.; Schweinsburg, R.E. Factors associated with use of wildlife underpasses and importance of long-term monitoring. J. Wildl. Manag. 2011, 75, 1477–1487. [Google Scholar] [CrossRef]
- Wang, Y.; Guan, L.; Piao, Z.; Wang, Z.; Kong, Y. Monitoring wildlife crossing structures along highways in Changbai Mountain, China. Transp. Res. Part D Transp. Environ. 2017, 50, 119–128. [Google Scholar] [CrossRef]
- González-Gallina, A.; Hidalgo-Mihart, M.G.; Castelazo-Calva, V. Conservation implications for jaguars and other neotropical mammals using highway underpasses. PLoS ONE 2018, 13, e0206614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andis, A.Z.; Huijser, M.P.; Broberg, L. Performance of arch-style road crossing structures from relative movement rates of large mammals. Front. Ecol. Evol. 2017, 5, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Little, S.J.; Harcourt, R.G.; Clevenger, A.P. Do wildlife passages act as prey-traps? Biol. Conserv. 2002, 107, 135–145. [Google Scholar] [CrossRef]
- Hunt, A.; Dickens, H.J.; Whelan, R.J. Movement of mammals through tunnels under railway lines. Aust. Zool. 1987, 24, 89–93. [Google Scholar] [CrossRef] [Green Version]
- Foster, M.L.; Humphrey, S.R. Use of highway underpasses by Florida panthers and other wildlife. Wildl. Soc. Bull. 1995, 23, 95–100. [Google Scholar]
- Soanes, K.; Mitchell, B.; van der Ree, R. Quantifying predation attempts on arboreal marsupials using wildlife crossing structures above a major road. Aust. Mammal. 2016, 39, 254–257. [Google Scholar] [CrossRef]
- Harris, I.M.; Mills, H.R.; Bencini, R. Multiple individual southern brown bandicoots (Isoodon obesulus fusciventer) and foxes (Vulpes vulpes) use underpasses installed at a new highway in Perth, Western Australia. Wildl. Res. 2010, 37, 127–133. [Google Scholar] [CrossRef]
- Mata, C.; Herranz, J.; Malo, J.E. Attraction and Avoidance between Predators and Prey at Wildlife Crossings on Roads. Diversity 2020, 12, 166. [Google Scholar] [CrossRef]
- Dickson, B.G.; Jenness, J.S.; Beier, P. Influence of vegetation, topography, and roads on cougar movement in southern California. J. Wildl. Dis. 2005, 69, 264–276. [Google Scholar] [CrossRef]
- Dupuis-Desormeaux, M.; Davidson, Z.; Mwololo, M.; Kisio, E.; Taylor, S.; MacDonald, S.E. Testing the Prey-Trap Hypothesis at Two Wildlife Conservancies in Kenya. PLoS ONE 2015, 10, e0139537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ford, A.T.; Clevenger, A.P. Validity of the Prey-Trap Hypothesis for Carnivore-Ungulate Interactions at Wildlife-Crossing Structures. Conserv. Biol. 2010, 24, 1679–1685. [Google Scholar] [CrossRef] [PubMed]
- Mysłajek, R.W.; Olkowska, E.; Wronka-Tomulewicz, M.; Nowak, S. Mammal use of wildlife crossing structures along a new motorway in an area recently recolonized by wolves. Eur. J. Wildl. Res. 2020, 66, 79. [Google Scholar] [CrossRef]
- Wildlife Institute of India. Eco-Friendly Measures to Mitigate the Impacts of Linear Infrastructure on Wildlife; Wildlife Institute of India: Dehradun, India, 2016. [Google Scholar]
- Cuddeback. Green Bay, Wisconsin, N.D. Available online: https://www.cuddeback.com/ (accessed on 17 November 2021).
- Karanth, K.U.; Sunquist, M.E. Prey Selection by Tiger, Leopard and Dhole in Tropical Forests. J. Anim. Ecol. 1995, 64, 439. [Google Scholar] [CrossRef]
- Majumder, A. Prey Selection, Food Habits and Population Structure of Sympatric Carnivores: Tiger panthera tigris tigris (L.), Leopard Panthera pardus (L.) and Dhole Cuon alpinus (PALLAS) in Pench Tiger Reserve, Madhya Pradesh (India). Ph.D. Thesis, Saurashtra University, Rajkot, India, 2011. [Google Scholar]
- Martinig, A.R.; Riaz, M.; St. Clair, C.C. Temporal clustering of prey in wildlife passages provides no evidence of a prey-trap. Sci. Rep. 2020, 10, 11489. [Google Scholar] [CrossRef]
- Barton, K. MuMIn: Multi-Model Inference. R Package. 2020. Available online: https://cran.r-project.org/package=MuMIn (accessed on 23 November 2021).
- RStudio Team. RStudio: Integrated Development Environment for R. 2020. Available online: https://www.rstudio.com/ (accessed on 15 July 2017).
- Mata, C.; Bencini, R.; Chambers, B.K.; Malo, J.E. Predator-prey interactions at wildlife crossing structures: Between myth and reality. In Handbook of Road Ecology; van der Ree, R., Smith, D.J., Grilo, C., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2015; pp. 190–197. [Google Scholar]
- Home, C.; Bhatnagar, Y.V.; Vanak, A.T. Canine Conundrum: Domestic dogs as an invasive species and their impacts on wildlife in India. Anim. Conserv. 2018, 21, 275–282. [Google Scholar] [CrossRef]
- Acosta-Jamett, G.; Chalmers, W.S.K.; Cunningham, A.A.; Cleaveland, S.; Handel, I.G.; Bronsvoort, B.M. Urban domestic dog populations as a source of canine distemper virus for wild carnivores in the Coquimbo region of Chile. Vet. Microbiol. 2011, 152, 247–257. [Google Scholar] [CrossRef] [Green Version]
- Furtado, M.M.; Hayashi, E.M.K.; Allendorf, S.D.; Coelho, C.J.; de Almeida Jácomo, A.T.; Megid, J.; Ramos Filho, J.D.; Silveira, L.; Tôrres, N.M.; Ferreira Neto, J.S. Exposure of Free-Ranging Wild Carnivores and Domestic Dogs to Canine Distemper Virus and Parvovirus in the Cerrado of Central Brazil. EcoHealth 2016, 13, 549–557. [Google Scholar] [CrossRef] [Green Version]
- Kintsch, J.; Jacobson, S.L.; Cramer, P. The wildlife crossing guild decision framework: A behavior-based approach to designing effective wildlife crossing structures. In Proceedings of the 2015 International Conference on Ecology and Transportation (ICOET 2015) Session, Raleigh, NC, USA, 20–24 September 2015. [Google Scholar]
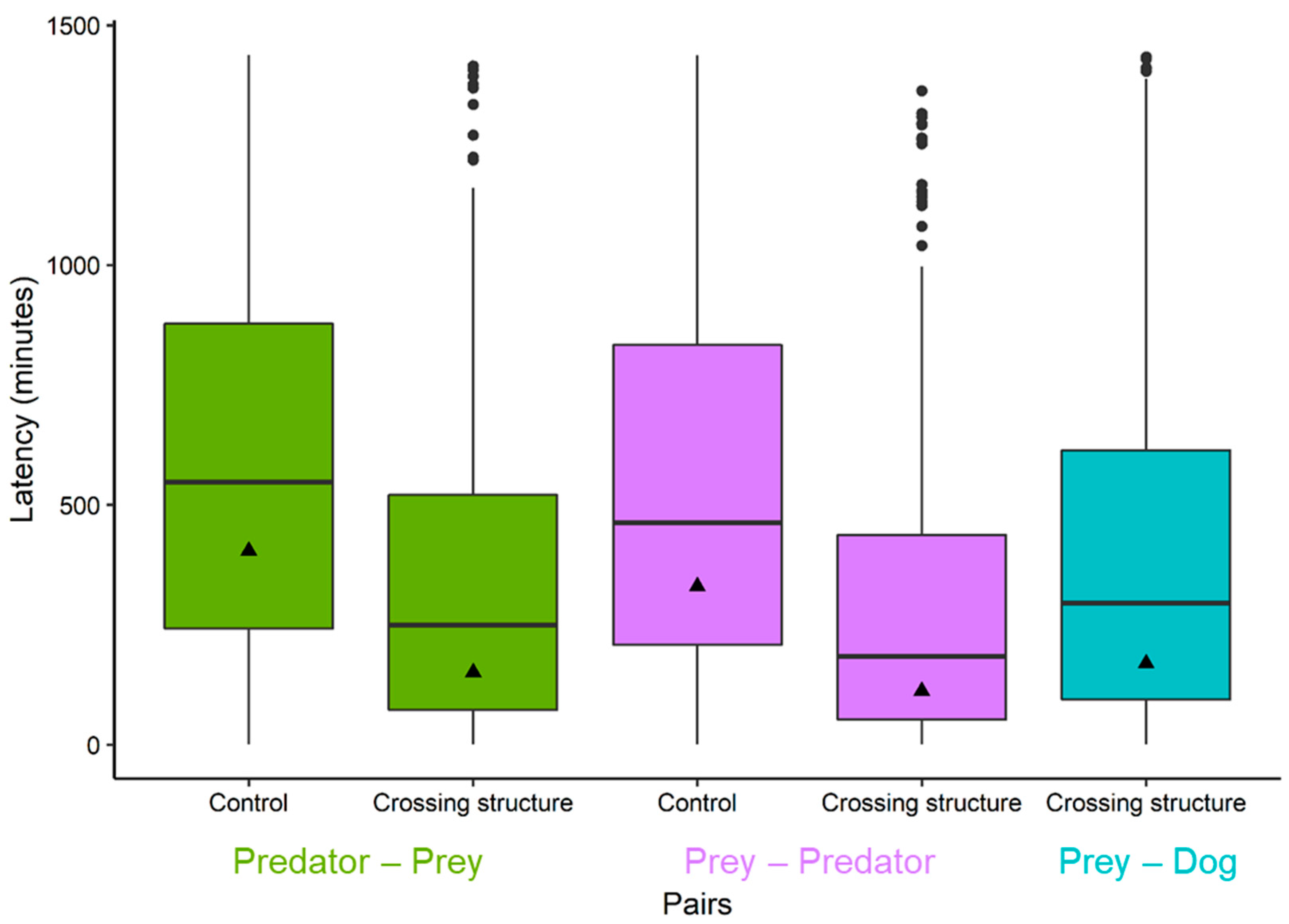
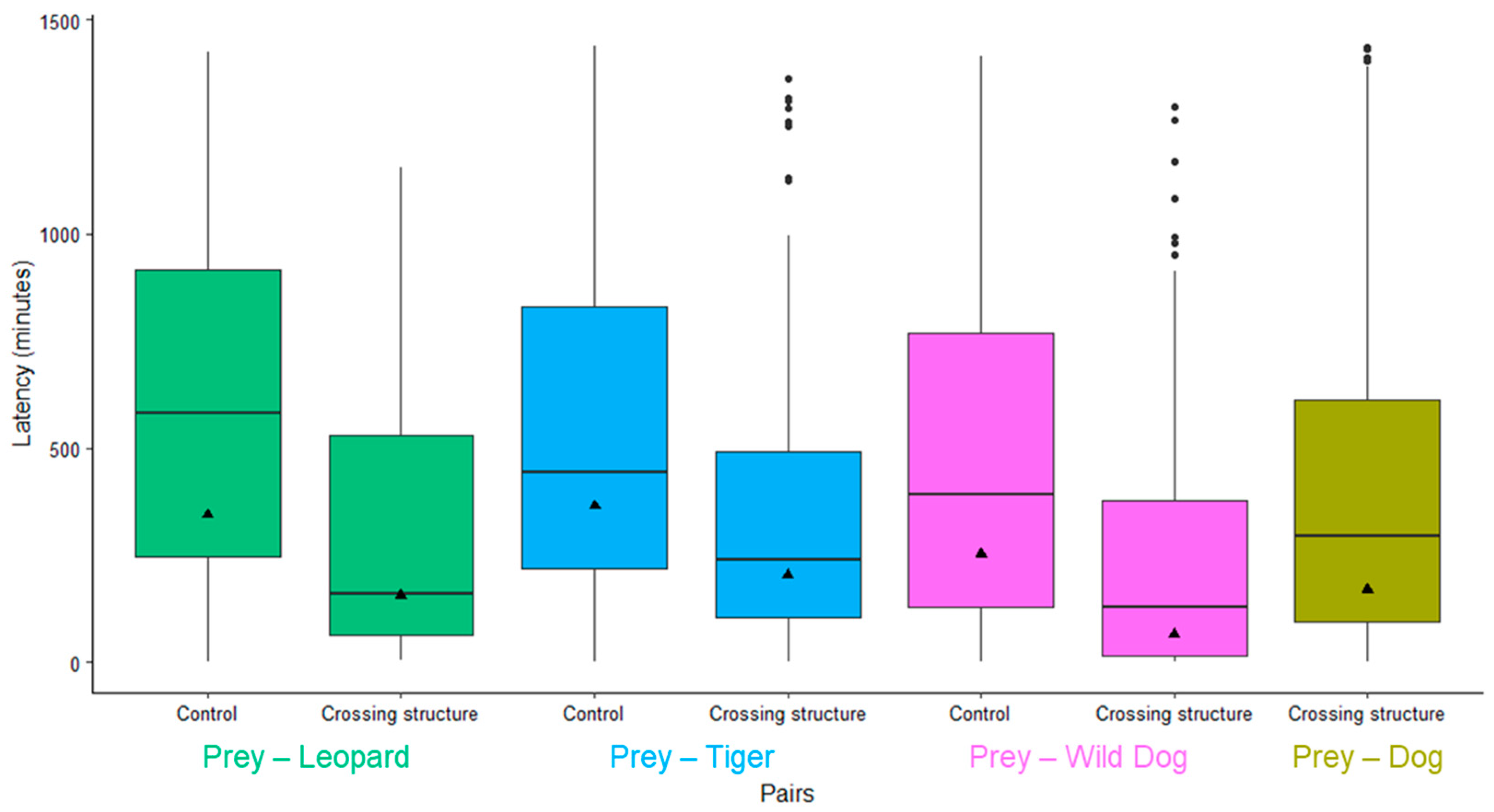
| Pair | Site | n | Geometric Mean (Minutes) | Mean | Standard Deviation | Standard Error | 95% Confidence Interval | |
|---|---|---|---|---|---|---|---|---|
| 1 | Predator–prey | Control | 475 | 405 | 589 | 134–1220 | 385–426 | 366–447 |
| Crossing structure | 374 | 151 | 355 | 24.1–948 | 137–166 | 125–182 | ||
| 2 | Prey–dog | Crossing structure | 1602 | 170 | 385 | 27.3–1060 | 162–178 | 155–186 |
| 3 | Prey–predator | Control | 492 | 331 | 540 | 88–1240 | 311–351 | 294–372 |
| Crossing structure | 387 | 112 | 295 | 17.4–726 | 102–123 | 93.2–135 |
| Prey–Predator Species | Site | n | Geometric Mean (Minutes) | SD | SE | CI | |
|---|---|---|---|---|---|---|---|
| 1 | Prey–dog | Crossing structure | 1602 | 170 | 27.3–1060 | 162–178 | 155–186 |
| 2 | Prey–Leopard | Control | 129 | 344 | 79.5–1490 | 302–391 | 266–444 |
| Crossing structure | 72 | 156 | 39.1–625 | 133–184 | 113–216 | ||
| 3 | Prey–Tiger | Control | 253 | 365 | 120–1110 | 340–391 | 318–419 |
| Crossing structure | 129 | 203 | 54.3–758 | 181–228 | 161–255 | ||
| 4 | Prey–Wild dog | Control | 110 | 252 | 53.2–1190 | 217–292 | 188–337 |
| Crossing structure | 186 | 65.6 | 7.42–580 | 55.9–76.9 | 47.8–89.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saxena, A.; Habib, B. Safe Passage or Hunting Ground? A Test of the Prey-Trap Hypothesis at Wildlife Crossing Structures on NH 44, Pench Tiger Reserve, Maharashtra, India. Diversity 2022, 14, 312. https://doi.org/10.3390/d14050312
Saxena A, Habib B. Safe Passage or Hunting Ground? A Test of the Prey-Trap Hypothesis at Wildlife Crossing Structures on NH 44, Pench Tiger Reserve, Maharashtra, India. Diversity. 2022; 14(5):312. https://doi.org/10.3390/d14050312
Chicago/Turabian StyleSaxena, Akanksha, and Bilal Habib. 2022. "Safe Passage or Hunting Ground? A Test of the Prey-Trap Hypothesis at Wildlife Crossing Structures on NH 44, Pench Tiger Reserve, Maharashtra, India" Diversity 14, no. 5: 312. https://doi.org/10.3390/d14050312
APA StyleSaxena, A., & Habib, B. (2022). Safe Passage or Hunting Ground? A Test of the Prey-Trap Hypothesis at Wildlife Crossing Structures on NH 44, Pench Tiger Reserve, Maharashtra, India. Diversity, 14(5), 312. https://doi.org/10.3390/d14050312






