The Impact of Drying Temperature on Basidiospore Size
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Shape and Size Range of Basidiospores
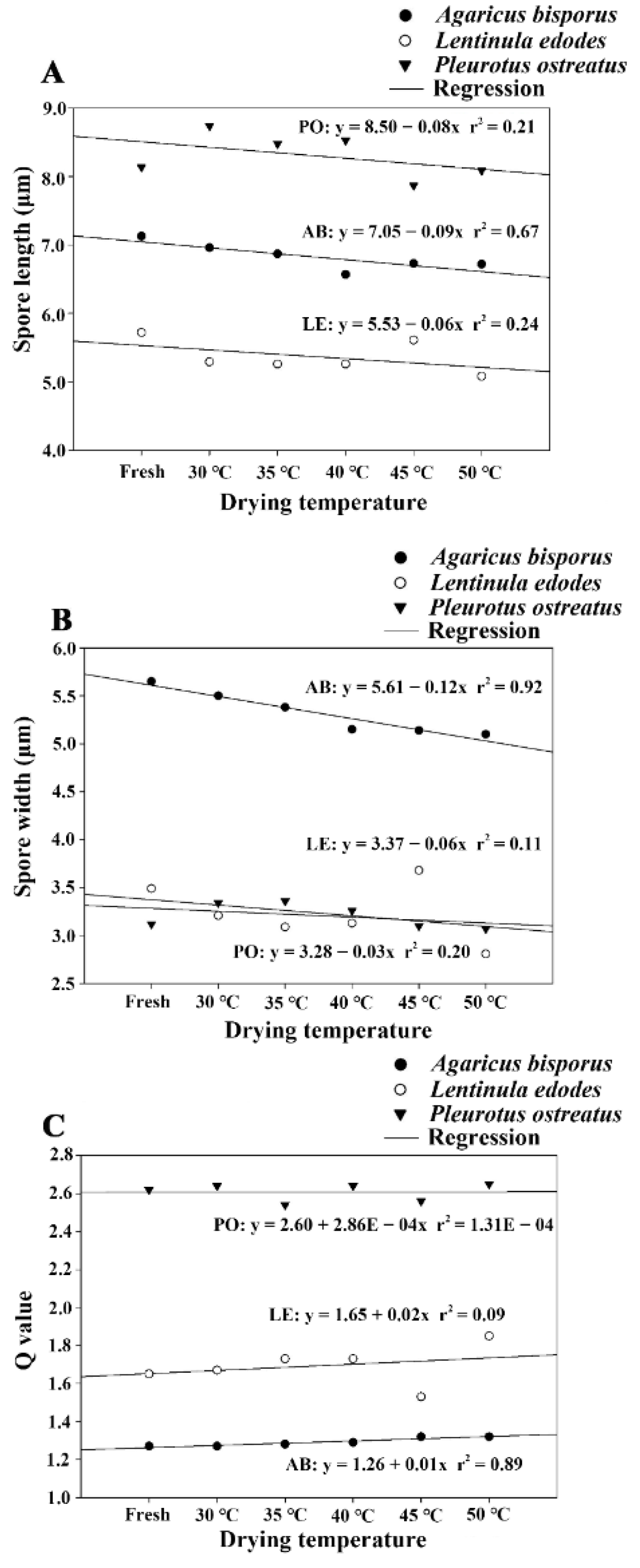
3.2. Correlations between Drying Temperatures and Basidiospore Size
3.3. Basidiospore Measurements Based on Previous Studies
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Halbwachs, H.; Bässler, C. Gone with the wind—A review on basidiospores of lamellate agarics. Mycosphere 2015, 6, 78–112. [Google Scholar] [CrossRef]
- Dramani, R.; Hegbe, A.D.M.T.; Tabe, A.; Badou, A.S.; Furneaux, B.; Ryberg, M.; Yorou, N.S. How are basidiospore size measurements affected by drying? Curr. Res. Environ. Appl. Mycol. 2020, 10, 63–70. [Google Scholar] [CrossRef]
- Decock, C.; Figueroa, S.H.; Robledo, G.; Castillo, G. Fomitiporia punctata (Basidiomycota, Hymenochaetales) and its presumed taxonomic synonyms in America: Taxonomy and phylogeny of some species from tropical/subtropical areas. Mycologia 2007, 99, 733–752. [Google Scholar] [CrossRef] [PubMed]
- Hoa, H.T.; Wang, C.L. The effects of temperature and nutritional conditions on mycelium growth of two Oyster mushrooms (Pleurotus ostreatus and Pleurotus cystidiosus). Mycobiology 2015, 43, 14–23. [Google Scholar] [CrossRef]
- Bruns, T.D.; Fogel, R.; Taylor, J.W. Amplification and sequencing of DNA from fungal herbarium specimens. Mycologia 1990, 82, 175–184. [Google Scholar] [CrossRef]
- Chin, S.K.; Law, C.L.; Supramaniam, C.V.; Cheng, P.G.; Mujumdar, A.S. Convective drying of Ganoderma tsugae Murrill and effect of temperature on basidiospores. Dry. Technol. 2008, 26, 1524–1533. [Google Scholar] [CrossRef]
- Yanaga, K.; Maekawa, N.; Shimomura, N.; Ishigaki, Y.; Nakamura, Y.; Takegami, T.; Tomosugi, N.; Miyazawa, S.; Kuwabata, S. Use of ionic liquid in fungal taxonomic study of ultrastructure of basidiospore ornamentation. Mycol. Prog. 2012, 11, 343–347. [Google Scholar] [CrossRef]
- Buyck, B.; Kauff, F.; Cruaud, C.; Hofstetter, V. Molecular evidence for novel Cantharellus (Cantharellales, Basidiomycota) from tropical African miombo woodland and a key to all tropical African chanterelles. Fungal Divers. 2013, 58, 281–298. [Google Scholar] [CrossRef]
- Cho, H.J.; Lee, H.; Park, M.S.; Kim, C.; Wisitrassameewong, K.; Lupala, A.; Park, K.H.; Kim, M.J.; Fong, J.J.; Lim, Y.W. Macrolepiota in Korea: New records and a new species. Mycobiology 2019, 47, 368–377. [Google Scholar] [CrossRef]
- Li, Y.; Liang, M.; Shu, X.; Liu, C.; Shu, J. Differentiation of basidiospores by MALDI-TOF lipid profiling. Int. J. Mass Spectrom. 2013, 352, 44–50. [Google Scholar] [CrossRef]
- Mata, G.; Medel, R.; Callac, P.; Billette, C.; Garibay-Orijel, R. First report of wild Agaricus bisporus (Basidiomycota, Agaricaceae) from Tlaxcala and Veracruz, Mexico. Rev. Mex. Biodivers. 2016, 87, 10–17. [Google Scholar] [CrossRef]
- Sarma, T.C.; Sarma, I.; Patiri, B.N. Wild edible mushrooms used by some ethnic tribes of Western Assam. The Bioscan 2010, 3, 613–625. [Google Scholar] [CrossRef]
- Callac, P.; Billette, C.; Imbernon, M.; Kerrigan, R.W. Morphological, genetic, and interfertility analyses reveal a novel, tetrasporic variety of Agaricus bisporus from the Sonoran Desert of California. Mycologia 1993, 85, 835–851. [Google Scholar] [CrossRef]
- Mitchell, A.D.; Walter, M. Species of Agaricus occurring in New Zealand. N. Z. J. Bot. 1999, 37, 715–725. [Google Scholar] [CrossRef]
- First Nature. Available online: https://www.first-nature.com/fungi/agaricus-bisporus.php (accessed on 5 December 2021).
- Mushroomexpert.com. Available online: http://www.mushroomexpert.com/agaricus_bisporus.html (accessed on 5 December 2021).
- Pegler, D.N. The genus Lentinula (Tricholomataceae tribe Collybieae). Sydowia 1983, 36, 227–239. [Google Scholar]
- Stamets, P. Growing Gourmet and Medicinal Mushroom, 3rd ed.; Ten Speed Press: Olympia, WA, USA; Berkeley, CA, USA, 2000; pp. 259–262. [Google Scholar]
- Asef, M.R. Intersterility groups of Pleurotus ostreatus complex in Iran. Mycology 2012, 3, 147–152. [Google Scholar] [CrossRef]
- Singh, R.K.; Pandey, S.K.; Singh, D.; Masurkar, P. First report of edible mushroom Pleurotus ostreatus from India with potential to kill plant parasitic nematodes. Indian Phytopathol. 2019, 72, 173–176. [Google Scholar] [CrossRef]
- Venturella, G.; Gargano, M.L.; Compagno, R. The genus Pleurotus in Italy. Flora Mediterr. 2015, 25, 143–155. [Google Scholar] [CrossRef]
- Junior, N.M.; Asai, T.; Capelari, M.; Paccola-Meirelles, L.D. Morphological and molecular identification of four Brazilian commercial isolates of Pleurotus spp. and cultivation on corncob. Braz. Arch. Biol. Technol. 2010, 53, 397–408. [Google Scholar] [CrossRef]
- Lechner, B.E.; Wright, J.E.; Albertó, E. The genus Pleurotus in Argentina. Mycologia 2004, 96, 845–858. [Google Scholar] [CrossRef]
- Nath, R.K.; Sarma, T.C. Edible macrofungi of Kaliabar sub-division of Nagaon district, Assam, India. Ann. Plant Sci. 2018, 7, 2161–2165. [Google Scholar] [CrossRef][Green Version]
- Mushroomexpert.com. Available online: http://www.mushroomexpert.com/pleurotus_ostreatus.html (accessed on 5 December 2021).
- Hosaka, K.; Uno, K. Assessment of the DNA quality in mushroom specimens: Effect of drying temperature. Bull. Natl. Mus. Nat. Sci. Ser. B 2011, 37, 101–111. [Google Scholar]

| Macrofungal Species | Drying Temperature | Basidiospore Size Range (μm) | Q Value Range | Length (μm) | Width (μm) | Q Value |
|---|---|---|---|---|---|---|
| Agaricus bisporus | Fresh | 6.31–8.01 × 5.02–6.44 | 1.12–1.56 | 7.13 ± 0.41 a | 5.65 ± 0.29 a | 1.27 ± 0.09 a |
| 30 °C | 4.49–7.94 × 4.40–6.65 | 0.81–1.61 | 6.96 ± 0.67 ab | 5.50 ± 0.38 b | 1.27 ± 0.15 a | |
| 35 °C | 4.38–8.48 × 4.44–6.40 | 0.86–1.70 | 6.87 ± 0.58 b | 5.38 ± 0.37 b | 1.28 ± 0.14 a | |
| 40 °C | 4.69–8.22 × 3.27–6.46 | 0.86–2.03 | 6.57 ± 0.61 c | 5.15 ± 0.55 c | 1.29 ± 0.21 a | |
| 45 °C | 4.20–8.41 × 3.46–6.09 | 0.83–1.92 | 6.73 ± 0.66 bc | 5.14 ± 0.38 c | 1.32 ± 0.17 a | |
| 50 °C | 3.54–8.42 × 4.42–5.93 | 0.66–1.66 | 6.72 ± 0.75 bc | 5.10 ± 0.30 c | 1.32 ± 0.17 a | |
| Lentinula edodes | Fresh | 3.60–8.52 × 2.68–4.00 | 1.03–2.52 | 5.72 ± 0.76 a | 3.49 ± 0.24 b | 1.65 ± 0.25 b |
| 30 °C | 2.71–7.47 × 2.00–4.55 | 1.00–2.64 | 5.29 ± 0.72 b | 3.21 ± 0.40 c | 1.67 ± 0.30 b | |
| 35 °C | 3.93–6.70 × 2.16–3.75 | 1.28–2.69 | 5.26 ± 0.57 b | 3.09 ± 0.35 c | 1.73 ± 0.27 b | |
| 40 °C | 3.41–8.36 × 2.19–5.44 | 0.94–2.85 | 5.26 ± 0.73 b | 3.13 ± 0.49 c | 1.73 ± 0.36 b | |
| 45 °C | 4.24–6.96 × 2.96–4.44 | 1.11–2.04 | 5.61 ± 0.52 a | 3.68 ± 0.33 a | 1.53 ± 0.20 c | |
| 50 °C | 3.51–7.57 × 1.98–3.86 | 1.02–3.04 | 5.08 ± 0.68 b | 2.81 ± 0.38 d | 1.85 ± 0.40 a | |
| Pleurotus ostreatus | Fresh | 4.92–10.85 × 2.47–3.78 | 1.68–3.83 | 8.14 ± 1.25 bc | 3.12 ± 0.27 b | 2.62 ± 0.43 a |
| 30 °C | 5.87–11.79 × 2.70–3.99 | 1.55–4.06 | 8.74 ± 1.27 a | 3.34 ± 0.32 a | 2.64 ± 0.46 a | |
| 35 °C | 6.74–10.59 × 2.87–4.11 | 1.90–3.16 | 8.48 ± 0.77 ab | 3.36 ± 0.26 a | 2.54 ± 0.27 a | |
| 40 °C | 5.61–10.93 × 2.43–3.87 | 1.79–3.78 | 8.53 ± 1.02 ab | 3.26 ± 0.29 a | 2.64 ± 0.43 a | |
| 45 °C | 3.94–10.85 × 2.44–4.07 | 1.37–3.89 | 7.87 ± 1.40 c | 3.10 ± 0.32 b | 2.56 ± 0.53 a | |
| 50 °C | 5.71–9.88 × 2.58–3.61 | 1.78–3.33 | 8.09 ± 0.81 bc | 3.07 ± 0.24 b | 2.65 ± 0.33 a |
| Macrofungal Species | Basidiospore Size (μm) | Status of Specimen | References |
|---|---|---|---|
| Agaricus bisporus | 6–8.5 × 5–6 | fresh | [10] |
| 6–9 × 5.5–9 | fresh | [11] | |
| 4.2–6.2 × 3.2–4.4 | dry | [12] | |
| 4.8–6.8 × 4.0–5.1 | dry | [13] | |
| 6.8–8.5 × 5.6–6.6 | dry | [14] | |
| 4–7.5 × 4–5.5 | ns | [15] | |
| 6–8 × 5–6 | ns | [16] | |
| 6.31–8.01 × 5.02–6.44 | fresh | This study | |
| Lentinula edodes | 5–7 × 3–3.7 | dry | [17] |
| 7.25–8.5 × 5.2–6.7 | dry | [12] | |
| 5–6.5 × 3–3.5 | ns | [18] | |
| 3.60–8.52 × 2.68–4.00 | fresh | This study | |
| Pleurotus ostreatus | 8–10.5 × 3–3.6 | fresh | [19] |
| 10–12 × 4–5 | fresh | [20] | |
| 6.2–8 × 4–6.5 | dry | [21] | |
| 8.7–11.2 × 3.7 | dry | [22] | |
| 8–13 × 3.1–3.6 | dry | [23] | |
| 5–7 × 3.5 | ns | [24] | |
| 7–11 × 2–4 | ns | [25] | |
| 4.92–10.85 × 2.47–3.78 | fresh | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Y.; Karunarathna, S.C.; Li, H.; Galappaththi, M.C.A.; Zhao, C.-L.; Kakumyan, P.; Mortimer, P.E. The Impact of Drying Temperature on Basidiospore Size. Diversity 2022, 14, 239. https://doi.org/10.3390/d14040239
Hu Y, Karunarathna SC, Li H, Galappaththi MCA, Zhao C-L, Kakumyan P, Mortimer PE. The Impact of Drying Temperature on Basidiospore Size. Diversity. 2022; 14(4):239. https://doi.org/10.3390/d14040239
Chicago/Turabian StyleHu, Yuwei, Samantha C. Karunarathna, Huili Li, Mahesh C. A. Galappaththi, Chang-Lin Zhao, Pattana Kakumyan, and Peter E. Mortimer. 2022. "The Impact of Drying Temperature on Basidiospore Size" Diversity 14, no. 4: 239. https://doi.org/10.3390/d14040239
APA StyleHu, Y., Karunarathna, S. C., Li, H., Galappaththi, M. C. A., Zhao, C.-L., Kakumyan, P., & Mortimer, P. E. (2022). The Impact of Drying Temperature on Basidiospore Size. Diversity, 14(4), 239. https://doi.org/10.3390/d14040239










