Reproductive Biology of Fritillaria aurea Schott (Liliaceae), a Rare Species Endemic to Turkey
Abstract
1. Introduction
2. Materials and Methods
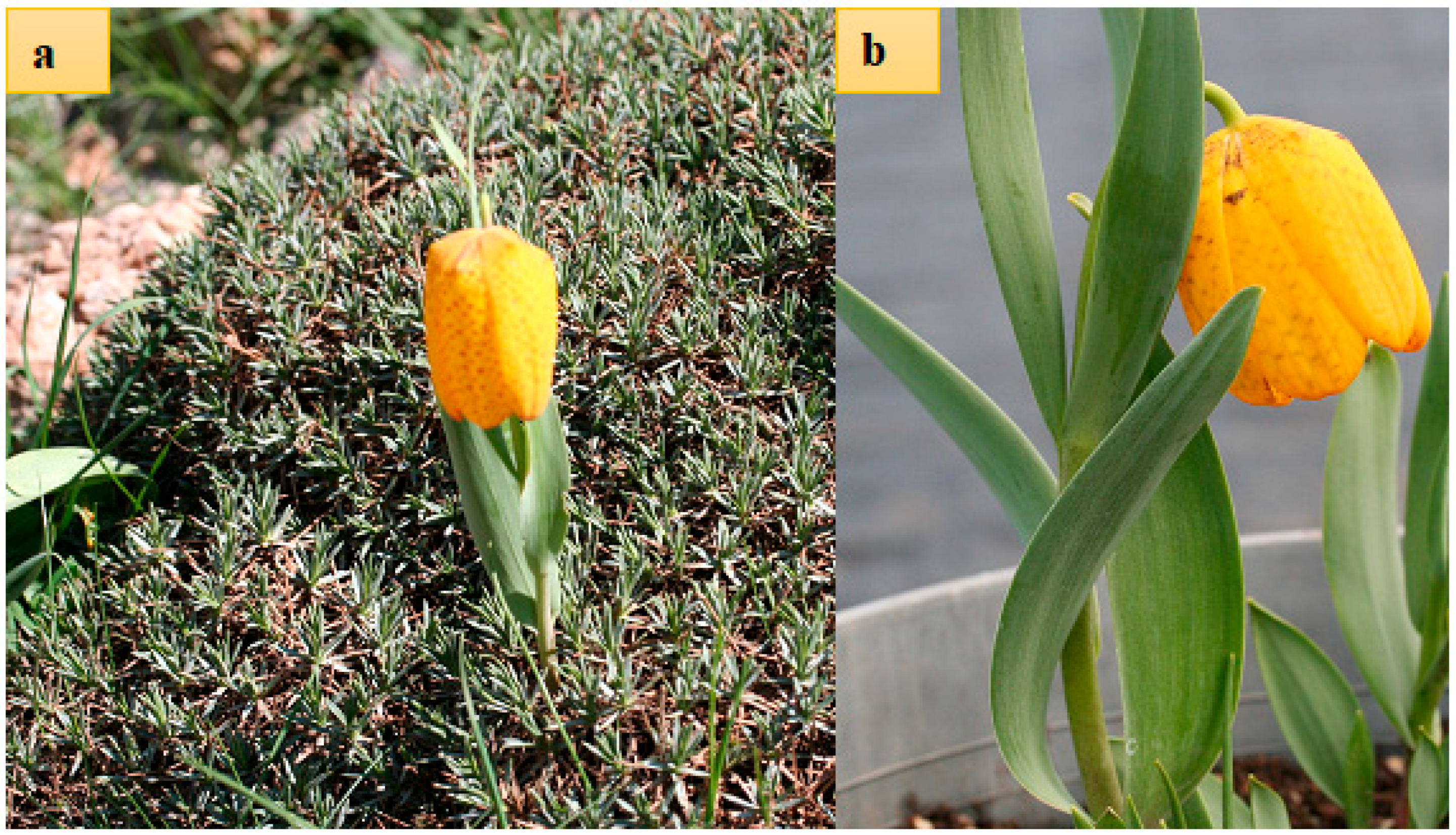
2.1. Study Site and Material
2.2. Pollination Experiments
2.3. Flowering Dynamics
2.4. Stigma Receptivity and Pollen Viability
2.5. Estimation of Pollen/Ovule Ratio (P/O) and Self-Incompatibility Index (SII)
2.6. Seed Viability and Germination
2.7. Pollinator Behavior Observation
2.8. Statistical Analysis
2.9. Collection Permits of Plant Materials
3. Results
3.1. Pollination Experiments
3.2. Flowering Dynamics
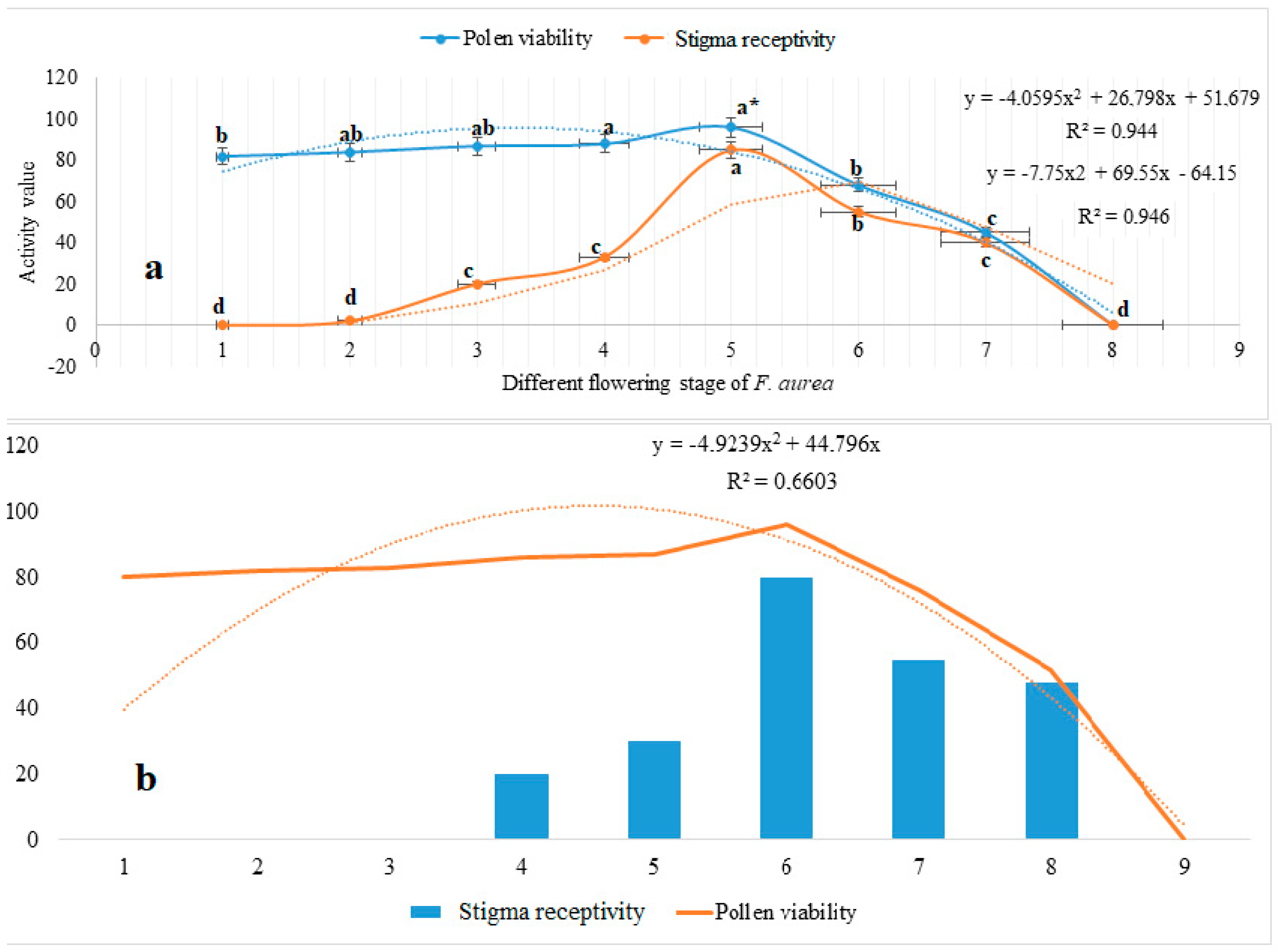
3.3. Stigma Receptivity and Pollen Viability
3.4. Estimation of Pollen/Ovule Ratio (P/O) and Self-Incompatibility Index (SII)
3.5. Seed Viability and Germination
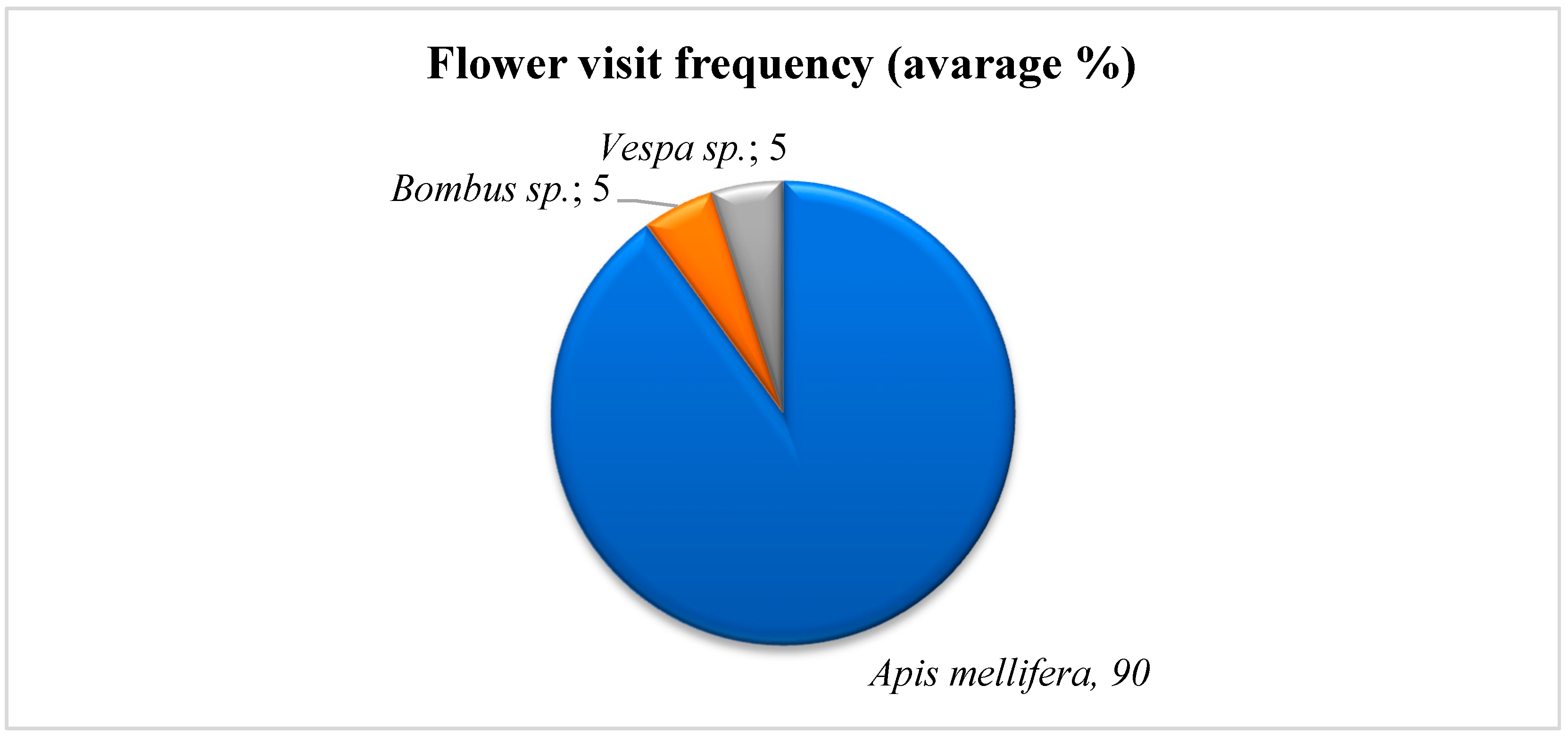
3.6. Pollinator Behavior Observation
4. Discussion
4.1. Pollination Experiments
4.2. Floral Traits, Stigma Receptivity, and Pollen Viability
4.3. Estimation of Pollen/Ovule Ratio (P/O) and Self-Incompatibility Index (SII)
4.4. Seed Viability and Germination
4.5. Pollinator Behavior Observation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giejsztowt, J.; Classen, A.T.; Deslippe, J.R. Climate change and invasion may synergistically affect native plant reproduction. Ecology 2020, 101, e02913. [Google Scholar] [CrossRef] [PubMed]
- Kaya, O.; Kose, C.; Gecim, T. An exothermic process involved in the late spring frost injury to flower buds of some apricot cultivars (Prunus armenica L.). Sci. Hortic. 2018, 241, 322–328. [Google Scholar] [CrossRef]
- Kaya, O.; Kose, C.; Donderalp, V.; Gecim, T.; Taskın, S. Last updates on cell death point, bud death time and exothermic characteristics of flower buds for deciduous fruit species by using differential thermal analysis. Sci. Hortic. 2020, 270, 109403. [Google Scholar] [CrossRef]
- Razanajatovo, M.; Fischer, L.; van Kleunen, M. Do floral traits and the selfing capacity of Mimulus guttatus plastically respond to experimental temperature changes? Oecologia 2020, 192, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Tsiftsis, S.; Djordjević, V. Modelling sexually deceptive orchid species distributions under future climates: The importance of plant pollinator interactions. Sci. Rep. 2020, 10, 10623. [Google Scholar] [CrossRef]
- Cooper, E.J.; Dullinger, S.; Semenchuk, P. Late snowmelt delays plant development and results in lower reproductive success in the High Arctic. Plant Sci. 2011, 180, 157–167. [Google Scholar] [CrossRef]
- Humphreys, A.M.; Govaerts, R.; Ficinski, S.Z.; Nic Lughadha, E.; Vorontsova, M.S. Global dataset shows geography and life form predict modern plant extinction and rediscovery. Nat. Ecol. Evol. 2019, 3, 1043–1047. [Google Scholar] [CrossRef]
- Primack, R.B.; Miller-Rushing, A.J. Uncovering, collecting, and analyzing records to investigate the ecological impacts of climate change: A template from Thoreau’s Concord. BioScience 2012, 62, 170–181. [Google Scholar] [CrossRef]
- Holz, H.; Segar, J.; Valdez, J.; Staude, I.R. Assessing extinction risk across the geographic ranges of plant species in Europe. Plants People Planet 2022, 4, 303–311. [Google Scholar] [CrossRef]
- Zych, M.; Stpiczyńska, M. Neither protogynous nor obligatory out crossed: Pollination biology and breeding system of the European Red List Fritillaria meleagris L. (Liliaceae). Plant Biol. 2012, 14, 285–294. [Google Scholar] [CrossRef]
- Molina-Venegas, R.; Rodríguez, M.Á.; Pardo-de-Santayana, M.; Ronquillo, C.; Mabberley, D.J. Maximum levels of global phylogenetic diversity efficiently capture plant services for humankind. Nat. Ecol. Evol. 2021, 5, 583–588. [Google Scholar] [CrossRef]
- Tekşen, M. Fritillaria L. In Resimli Türkiye Florası (Illustrated Flora of Turkey), 2nd ed.; Güner, A., Kandemir, A., Menemen, Y., Yıldırım, H., Aslan, S., Ekşi, G., Güner, I., Çimen, A.Ö., Eds.; ANG Vakfı Nezahat Gökyiğit Botanik Bahçesi Yayınları: İstanbul, Turkey, 2018; Volume 2, pp. 800–876. [Google Scholar]
- Huang, J.; Yu, Y.; Liu, Y.M.; Xie, D.F.; He, X.J.; Zhou, S.D. Comparative chloroplast genomics of Fritillaria (Liliaceae), inferences for phylogenetic relationships between Fritillaria and Lilium and plastome evolution. Plants 2020, 9, 133. [Google Scholar] [CrossRef]
- Wang, D.; Li, Z.; Zhang, L.; Atanasov, A.G.; Wang, S. Characterization of the isosteroidal alkaloid chuanbeinone from bulbus of Fritillaria pallidiflora as novel antitumor agent in vitro and in vivo. Planta Med. 2016, 82, 195–204. [Google Scholar] [CrossRef]
- Wang, Y.; Aamer, M.; Aslay, M.; Sener, B.; Khan, F.; Wahab, A.; Rahman, A.; Choudhary, M. A new steroidal alkaloid from Fritillaria michailovskyi Fomin. Nat. Prod. Res. 2020, 36, 361–366. [Google Scholar] [CrossRef]
- Shi, Z.J.; Li, J.H. Research progress on key cultivation techniques and planting patterns of Fritillaria. Mod. Agric. Sci. Technol. 2017, 20, 59–61. [Google Scholar]
- Zych, M.; Stpiczyńska, M.; Roguz, K. Pollination biology and breeding system of European Fritillaria meleagris L. (Liliaceae). In Reproductive Biology of Plants; CRC Press: Boca Raton, FL, USA, 2014; pp. 147–163. [Google Scholar]
- Zhang, X.J.; Cui, D.L.; Zong, X.C.; Ren, R.Y.; Wei, J.C.; SITU, L.L.; Zhang, Y.L. Pollination biology and breeding system of Fritillaria ussuriensis Maxim. Acta Bot. Sin. 2010, 30, 1404–1408. [Google Scholar]
- Ma, S.M.; Wang, Y.F.; Ye, X.L.; Zhao, N.X.; Liang, C.Y. Progress in study of apomixis in monocotyledonous plants. Chin. Bull. Bot. 2002, 19, 530–537. [Google Scholar]
- Aslay, M.K.; Çukadar, H.M.; Ünlü, Z.; Kadıoğlu, M.; Tekşen, E.; Kaya, Ö. Determination of Some Productivity Situations of the Soils of Terslale (Fritillaria L.) Species of Eastern Anatolia Flora. V. In Proceedings of the Uluslararası Katılımlı Toprak ve Su Kaynakları Kongresi, Kırklareli, Turkey, 12–15 September 2017. [Google Scholar]
- Türktaş, M.; Aslay, M.; Kaya, E.; Ertuğrul, F. Molecular characterization of phylogeneticrelationships in Fritillaria species inferred from chloroplast trnL-trnF sequences. Turk. J. Biol. 2012, 36, 552–560. [Google Scholar] [CrossRef]
- Rix, E.M. Fritillaria L. In Flora of Turkey and the East Aegean Islands, 1st ed.; Davis, P.H., Ed.; Edinburgh University Press: Edinburgh, UK, 1975; Volume 8, pp. 284–302. [Google Scholar]
- Dafni, A.; Maues, J. A rapid and simple procedure to determine stigma receptivity. Sex. Plant Reprod. 1998, 11, 177–180. [Google Scholar] [CrossRef]
- Shivanna, K.R.; Tandon, R. Reproductive Ecology of Flowering Plants: A Manual; Springer: New Delhi, India, 2014; pp. 107–123. [Google Scholar]
- Cruden, R.W. Pollen-ovule ratios: A conservative indicator of breeding systems in flowering plants. Evolution 1977, 31, 32–46. [Google Scholar] [CrossRef]
- Zapata, T.R.; Arroyo, M.T.K. Plant reproductive ecology of a secondary deciduous tropical forest in Venezuela. Biotropica 1978, 10, 221–230. [Google Scholar] [CrossRef]
- Dafni, A. Pollination Ecology: A Practical Approach; University Press: New York, NY, USA, 1992. [Google Scholar]
- Aslay, M.; Çukadar, K.; Ünlü, H.M.; Kadıoğlu, Z.; Tekşen, M. Ekim Öncesi Uygulamalar ve Farklı Çimlenme Ortamlarının Tchihatchewia isatidea Boiss. (Brassicaceae) Tohumlarının Çimlenmesi Üzerine Etkisi. IV. In Proceedings of the Süs Bitkileri Kongresi, Mersin, Turkey, 20–22 October 2010. [Google Scholar]
- Bernardello, G.; Anderson, G.J.; Lopez, S.P.; Cleland, M.A.; Stuessy, T.F.; Crawford, D.J. Reproductive biology of Lactoris fernandeziana (Lactoridaceae). Am. J. Bot. 1999, 86, 829–840. [Google Scholar] [CrossRef] [PubMed]
- Anderson, S. Identifying Important Plant Areas; Plantlife International: London, UK, 2002. [Google Scholar]
- Nyman, Y. Pollination mechanisms in six Campanula species (Campanulaceae). Plant Syst. Evol. 1992, 181, 97–108. [Google Scholar] [CrossRef]
- Barrett, S.C.H.; Cruzan, M.B. Incompatibility in heterostylous plants. In Genetic Control of Self-Incompatibility and Reproductive Development in Flowering Plants; Williams, E.G., Clarke, A.E., Knox, R.B., Eds.; Kluwer: Dordrecht, The Netherland, 1994; pp. 189–219. [Google Scholar]
- Brys, R.; Jacquemyn, H.; Hermy, M. Impact of mate availability, population size, and spatial aggregation of morphs on sexual reproduction in a distylous, aquatic plant. Am. J. Bot. 2007, 94, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Lei, F.W.; Wu, Y.M.; Shen, X.L.; Xia, X.F.; Zhang, D.H.; Xian, Y.M.; Zhang, Z.X. Multiple reproductive strategies of a spring ephemeral plant, Fritillaria maximowiczii, enable its adaptation to harsh environments. Plant Species Biol. 2022, 37, 38–51. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, C.; Song, B.; Du, F. Corolla retention after pollination facilitates the development of fertilized ovules in Fritillaria delavayi (Liliaceae). Sci. Rep. 2019, 9, 729. [Google Scholar] [CrossRef]
- Yashima, T.; Kinoshita, E.; Shimizu, T. Flowering phenology and self-incompatibility in Fritillaria camtschatcensis (L.) Ker-Gawl. J. Phytogeogr. Taxon. 1997, 45, 129–133. [Google Scholar]
- de Jong, T.; Klinkhamer, P. Evolutionary Ecology of Plant Reproductive Strategies; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Aslay, M.; Çukadar, K.; Ünlü, H.M.; Kadıoğlu, Z.; Tekşen, M.; Kaya, E. Doğu Anadolu Fritillaria’larının Kültür Şartlarına Adaptasyonu. V. In Proceedings of the Süs Bitkileri Kongresi, Yalova, Turkey, 6–9 May 2013. [Google Scholar]
- Aslay, M.; Tekşen, M.; Alp, Ş.; Ellialtıoğlu, Ş. 13. Terslale. In Süs Bitkileri Islahı (Türler) Kitabı; Kazaz, S., Yalçın Mendi, N.Y., Eds.; Gece Kitaplığı: Ankara, Turkey, 2021. [Google Scholar]
- Aslay, M. Tescil Edilmiş Ters Lale Süs Bitkisi Çeşidi ”Doğu Güneşi”. VII. In Proceedings of the Ulusal Bahçe Bitkileri Kongresi, Çanakkale, Turkey, 25–29 August 2015. [Google Scholar]
- Karakaş Metin, Ö.; Türktaş, M.; Aslay, M.; Kaya, E. Evaluation of the Genetic Relationship Between Fritillaria Species From Turkey’s Flora Using Fluorescent-based AFLP. Turk. J. Biol. 2013, 37, 273–279. [Google Scholar]
- Yucel, G.; Mengüç, A. Production of Fritillaria imperialis L. Bulbs Using Different Methods and Monitoring the Development of Bulb Groups Under Export Size. Egypt. J. Hortic. 2021, 48, 257–266. [Google Scholar] [CrossRef]
- Newstrom, L.E.; Frankie, G.W.; Baker, H.G.; Colwell, R.K. Diversity of Long-Term Flowering Patterns. La Selva: Ecology and Natural History of A Neotropical Rain Forest; University of Chicago Press: Chicago, IL, USA, 1994; Volume 1, pp. 142–160. [Google Scholar]
- Kumari, P.; Khajuria, A.; Wani, I.A.; Khan, S.; Verma, S. Effect of floral size reduction on Pollination and reproductive efficiency of female flowers of Valeriana wallichii, a threatened medicinal plant. Nat. Acad. Sci. Lett. 2021, 44, 75–79. [Google Scholar] [CrossRef]
- Castro, S.; Silveira, P.; Navarro, L. Effect of pollination on floral longevity and costs of delaying fertilization in the out-crossing Polygala vayredae Costa (Polygalaceae). Ann. Bot. 2008, 102, 1043–1048. [Google Scholar] [CrossRef]
- Hermanutz, L.; Innes, D.; Denham, A.; Whelan, R. Very low fruit:flower ratios in Grevillea (Proteaceae) are independent of breeding system. Aust. J. Bot. 1998, 46, 465–478. [Google Scholar] [CrossRef]
- Rocha, O.J.; Aguilar, G. Reproductive biology of the dry forest tree Enterolobium cyclocarpum (Guanacaste) in Costa Rica: A comparison between trees left in pastures and in continuous forest. Am. J. Bot. 2001, 88, 1607–1614. [Google Scholar] [CrossRef]
- Byers, D.L. Pollen quantity and quality as explanations for low seed set in small populations exemplified by Eupatorium (Asteraceae). Am. J. Bot. 2004, 82, 1000–1006. [Google Scholar] [CrossRef]
- Dorken, M.E.; Eckert, C.G. Severely reduced sexual reproduction in northern populations of a clonal plant, Decodon verticillatus (Lythraceae). J. Ecol. 2001, 89, 339–350. [Google Scholar] [CrossRef]
- Colling, G.; Reckinger, C.; Matthies, D. Effects of pollen quantity and quality on reproduction and offspring vigour in the rare plant Scorzonera humilis (Asteraceae). Am. J. Bot. 2004, 91, 1774–1782. [Google Scholar] [CrossRef]
- Gopalakrishnan, K.K.; Thomas, T.D. Reproductive biology of Pittosporum dasycaulon Miq., (Family Pittosporaceae) a rare medicinal tree endemic to Western Ghats. Bot. Stud. 2014, 55, 15. [Google Scholar] [CrossRef][Green Version]
- Tamura, M.N. Liliaceae. In The Families and Genera of Vascular Plants. III. Flowering Plants. Monocotyledons, Lilianae (Except Orchidaceae); Kubitzki, K., Ed.; Springer: Berlin, Germany, 1998; pp. 343–353. [Google Scholar]
- Kawano, S.; Masuda, J.; Hayashi, K. Life history monographs of Japanese plants. 10: Fritillaria koidzumiana Ohwi (Liliaceae). Plant Species Biol. 2008, 23, 51–57. [Google Scholar] [CrossRef]
- Mancuso, E.; Peruzzi, L. Male individuals in cultivated Fritillaria persica L. (Liliaceae): Real androdioecy or gender disphasy? Turk. J. Bot. 2010, 34, 435–440. [Google Scholar] [CrossRef]
- Kulloli, S.K.; Sreekala, A.K.; Pandurangan, A.G. Floral biology of Impatiens trichocarpa Hook. f., (Balsaminaceae) an endemic Balsam of Western Ghats. Indian J. Sci. Technol. 2009, 2, 30–34. [Google Scholar] [CrossRef]
- Ramsey, M.; Vaughton, G. Effect of environment on the magnitude of inbreeding depression in seed germination in a partially self-fertile perennial herb (Blandfordia grandiflora, Liliaceae). Int. J. Plant Sci. 1998, 159, 98–104. [Google Scholar] [CrossRef]
- Bewley, J.D.; Black, M. Mobilization of stored seed reserves. In Seeds; Springer: Boston, MA, USA, 1994; pp. 293–343. [Google Scholar]
- Goulson, D. Bumblebees: Their Behaviour and Ecology; Oxford University Press: Oxford, UK, 2003. [Google Scholar]
- Schoonhoven, L.M.; van Loon, J.J.A.; Dicke, M. Insect-Plant Biology, 2nd ed.; Oxford University Press: Oxford, UK, 2007. [Google Scholar]
- Nic Lughadha, E.; Bachman, S.P.; Leão, T.C.; Forest, F.; Halley, J.M.; Moat, J.; Carmen, A.; Karen, L.B.; Ryan, F.A.B.; Gildas, G.; et al. Extinction risk and threats to plants and fungi. Plants People Planet 2020, 2, 389–408. [Google Scholar] [CrossRef]



| Treatment | Number of Flowers | Number to Recycle * | Fruit Setting Rate % | Seed Viability (%) | Germination (%) |
|---|---|---|---|---|---|
| Open-pollination | 50 | 48 | 68.0 ± 8.3 ns | 67.0 ± 6.3 ns | 45.0 ± 5.3 ns |
| Spontaneous self-pollination | 50 | 50 | - | - | - |
| Spontaneous cross-pollination | 40 | 39 | 63.0 ± 9.4 | 71.0 ± 9.2 | 40.0 ± 4.9 |
| Apomixis | 30 | 30 | - | - | - |
| Hand self-pollination | 50 | 49 | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yıldız, F.; Aslay, M.; Kandemir, A.; Kaya, O. Reproductive Biology of Fritillaria aurea Schott (Liliaceae), a Rare Species Endemic to Turkey. Diversity 2022, 14, 1052. https://doi.org/10.3390/d14121052
Yıldız F, Aslay M, Kandemir A, Kaya O. Reproductive Biology of Fritillaria aurea Schott (Liliaceae), a Rare Species Endemic to Turkey. Diversity. 2022; 14(12):1052. https://doi.org/10.3390/d14121052
Chicago/Turabian StyleYıldız, Faruk, Meral Aslay, Ali Kandemir, and Ozkan Kaya. 2022. "Reproductive Biology of Fritillaria aurea Schott (Liliaceae), a Rare Species Endemic to Turkey" Diversity 14, no. 12: 1052. https://doi.org/10.3390/d14121052
APA StyleYıldız, F., Aslay, M., Kandemir, A., & Kaya, O. (2022). Reproductive Biology of Fritillaria aurea Schott (Liliaceae), a Rare Species Endemic to Turkey. Diversity, 14(12), 1052. https://doi.org/10.3390/d14121052









