Herpetofaunal Diversity in a Dahomey Gap Savannah of Togo (West Africa): Effects of Seasons on the Populations of Amphibians and Reptiles
Abstract
1. Introduction
- (a)
- The various diversity metrics should be very different between amphibians and reptiles, given their divergent tolerance to the climatic conditions (especially rainfall) between dry and wet seasons, and because of their different role within the savannah trophic chains. That is, the mean number of amphibian individuals per species should be higher than the mean number of reptile individuals per species, given that these latter are high-rank predators in the savannah trophic chains (for instance, some snakes, see [14]).
- (b)
- Amphibians should be more impacted than reptiles by the dry season months, as they are more linked to water bodies and humidity threshold than reptiles. That is, the number of amphibian species and individuals should be significantly lower by dry season, with consequent increase in the dominance and decrease in the evenness of the assemblage, whereas the same inter-seasonal difference should be less evident in reptiles.
- (c)
- In reptiles, dominance and evenness should be relatively independent on season, although excessive heat and drought may also depress the reptilian assemblages.
2. Materials and Methods
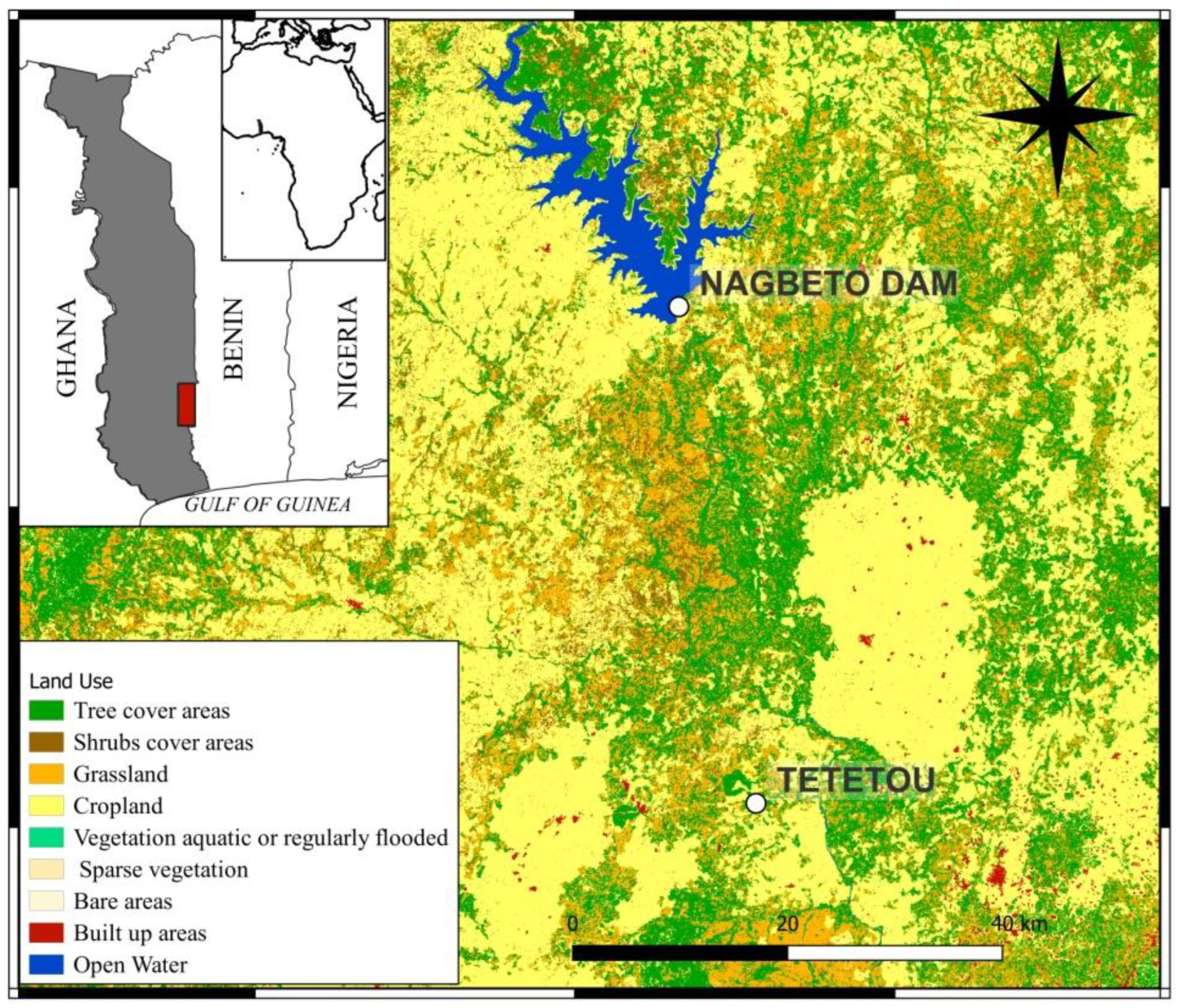
2.1. Study Area
2.2. Protocol
2.3. Statistical Analyses
- (a)
- Species richness, the total number of taxa recorded into each plantation type at each study area and during the wet or the dry season;
- (b)
- Dominance,and ranges from 0 (all taxa are equally present) to 1 (one taxon dominates the community completely);D = 1 − impson index
- (c)
- Shannon H’ index, varying from 0 for communities with only a single taxon to high values for communities with many taxa, each with few individuals [22]. Shannon’s index (H’) iswhere S is the total number of amphibian or reptile species recorded, fr = n/N, n is the number of individuals of each species in each season and N is the total number of individuals of each taxon (amphibians or reptiles) in the study area.H’ = − S(fr) × [ln(fr)],
- (d)
- Pielou’s Evenness index, calculated aswheree = H’/Hmax,with H’ representing Shannon’s index, and S the total number of amphibian or reptile species recorded. Hmax corresponds to the maximum value of diversity (i.e., when all species are equally represented in our samples [21]).Hmax = lnS,
- (e)
- Chao-1 index that calculated the predicted number of species at a given site given the observed sample size and the observed number of species. Chao-1 index iswhere F1 is the number of singleton species and F2 the number of doubleton species. A singleton is a species occurring only once in the total sample, and doubleton is a species occurring just twice in the total sample.Chao-1 = S + F1(F1 − 1)/(2 (F2 + 1),
3. Results
3.1. Amphibians
3.2. Reptiles
3.3. Comparisons between Amphibians and Reptiles
- (i)
- Dominance index was significantly higher in amphibians during the dry season than in all other pairwise comparisons, i.e., wet season amphibians and both seasons’ reptiles (ANOSIM: mean rank within seasons types = 102.4; mean rank between taxa = 1361.4; R = 0.311, P = 0.0012);
- (ii)
- Shannon’s index was significantly lower in dry season amphibians and significantly higher in wet season reptiles (ANOSIM: mean rank within seasons types = 116.3; mean rank between taxa = 118.3; R = 0.246, P = 0.0023);
- (iii)
- Evenness index was significantly lower in reptiles than in amphibians (ANOSIM: mean rank between taxa = 99.4; R = 0.221, P = 0.023).
4. Discussion
4.1. General Considerations
4.2. Diversity Metrics
4.3. Conservation Considerations
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Demenou, B.B.; Doucet, J.L.; Hardy, O.J. History of the fragmentation of the African rain forest in the Dahomey Gap: Insight from the demographic history of Terminalia ystem. Heredity 2018, 120, 547–561. [Google Scholar] [CrossRef] [PubMed]
- Luiselli, L.; Akani, G.C.; Ajong, S.N.; George, A.; Di Vittorio, M.; Eniang, E.A.; Dendi, D.; Hema, E.M.; Petrozzi, F.; Fa, J.E. Predicting the structure of turtle assemblages along a megatransect in West Africa. Biol. J. Linn. Soc. 2020, 130, 296–309. [Google Scholar] [CrossRef]
- Gbewaa, S.B.; Oppong, S.K.; Horne, B.D.; Tehoda, P.; Petrozzi, F.; Dendi, D.; Akani, G.C.; Vittorio, M.D.; Ajong, S.N.; Pacini, N.; et al. Community characteristics of sympatric freshwater turtles from savannah waterbodies in Ghana. Wetlands 2021, 41, 61. [Google Scholar] [CrossRef]
- Luiselli, L.; Dendi, D.; Petrozzi, F.; Segniagbeto, G.H. Lizard community structure and spatial resource use along a forest-savannah-urban habitat gradient in the Dahomey Gap (West Africa). Urban Ecosyst. 2022, 25, 1015–1026. [Google Scholar] [CrossRef]
- Segniagbeto, H. Herpétofaune du Togo: Taxinomie, Biogéographie; Thèse de Doctorat, University of Lomé: Lomé, Togo; MNHN: Paris, France, 2009; p. 456. [Google Scholar]
- Segniagbeto, G.H.; Trape, J.-F.; Afiademanyo, K.; Roedel, M.-O.; Ohler, A.; Dubois, A.; David, P.; Meirte, D.; Glitho, A.; Petrozzi, F.; et al. Checklist of the lizards of Togo, (West Africa), with comments on systematics, distribution, ecology, and conservation. Zoosystema 2015, 37, 381–402. [Google Scholar] [CrossRef]
- Segniagbeto, G.H.; Bour, R.; Ohler, A.; Dubois, A.; Roedel, M.-O.; Trape, J.-F.; Fretey, J.; Petrozzi, F.; Aïdam, A.; Luiselli, L. Turtles and tortoises of Togo: Historical data, distribution, ecology and conservation. Chel. Cons. Biol. 2014, 13, 152–165. [Google Scholar] [CrossRef]
- Segniagbeto, G.H.; Trape, J.-F.; David, P.; Ohler, A.-M.; Dubois, A.; Glitho, I.A. The snake fauna of Togo: Systematics, distribution, and biogeography, with remarks on selected taxonomic problems. Zoosystema 2011, 33, 325–360. [Google Scholar] [CrossRef]
- Segniagbeto, G.H.; Bowessidjaou, J.E.; Dubois, A.; Ohler, A. Les Amphibiens du Togo: État actuel des connaissances. Alytes 2007, 24, 72–90. [Google Scholar]
- IUCN. The Red List of Threatened Species. 2022. Available online: www.iucnredlist.org (accessed on 29 September 2022).
- Nelson, D.M.; Verschuren, D.; Urban, M.A.; Hu, F.S. Long-term variability and rainfall control of savanna fire regimes in equatorial East Africa. Glob. Chang. Biol. 2012, 18, 3160–3170. [Google Scholar] [CrossRef]
- Ogutu, J.O.; Piepho, H.P.; Dublin, H.T.; Bhola, N.; Reid, R.S. Rainfall influences on ungulate population abundance in the Mara-Serengeti ecosystem. J. Anim. Ecol. 2008, 77, 814–829. [Google Scholar] [CrossRef]
- Owusu, K. Rainfall changes in the savannah zone of northern Ghana 1961–2010. Weather 2018, 73, 46–50. [Google Scholar] [CrossRef]
- Luiselli, L.; Angelici, F.M.; Akani, G.C. Food habits of Python sebae in suburban and natural habitats. Afr. J. Ecol. 2001, 39, 116–118. [Google Scholar]
- Crump, M.L.; Scott, N.J. Visual encounter survey. In Measuring and Monitoring Biological Diversity, Standard Methods for Amphibians; Heyer, W.R., Donnelly, M.A., McDiarmid, R.W., Donnelly, M., Heyek, L.C., Foster, M.S., Eds.; Smithsonian Institution Press: Washington, DC, USA, 1994; pp. 84–91. [Google Scholar]
- Chippaux, J.-P. Les Serpents D’afrique Occidentale et Centrale; Collection Faune et Flore Tropicales; IRD Éditions: Paris, France, 2006; p. 311. [Google Scholar]
- Trape, J.-F.; Mané, Y. Guide Des Serpents D’afrique Occidentale (Savane et Désert); IRD Éditions: Paris, France, 2006; p. 226. [Google Scholar]
- Trape, J.-F.; Trape, S.; Chirio, L. Lézards, Crocodiles et Tortues D’afrique Occidentale et Du Sahara; IRD Editions: Marseille, France, 2012; p. 503. [Google Scholar]
- Channing, A.; Rödel, M.O. Field Guide to the Frogs and Other Amphibians of Africa; Struik Nature: Cape Town, South Africa, 2019; p. 408. [Google Scholar]
- Frost, D. Amphibian Species of the World: An Online Reference, Version 6.1; American Museum of Natural History: New York, NY, USA, 2021; Available online: https://amphibiansoftheworld.amnh.org/index.php (accessed on 1 October 2022). [CrossRef]
- Magurran, A.E. Ecological Diversity and Its Measurement; Princeton University Press: Princeton, NJ, USA, 1988. [Google Scholar]
- Hammer, O. PAST—Paleontological Statistics, version 2.17; Reference Manual 1999–2012; PAST: Oslo, Norway, 2012. [Google Scholar]
- Krebs, C.J. Ecological Methodology; Harper & Row: New York, NY, USA, 1989. [Google Scholar]
- Tóthmérész, B. Comparison of different methods for diversity ordering. J. Veg. Sci. 1995, 6, 283–290. [Google Scholar] [CrossRef]
- Oksanen, J.; Guillaume Blanchet, F.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; Wagner, H. Vegan: Community Ecology Package. 2010. Available online: http://vegan.r-forge.r-project.org/ (accessed on 13 August 2022).
- Rödel, M.-O. Herpetofauna of West Africa. In Amphibians of the West African Savana; Chimaira: Frankfurt am Main, France, 2000; Volume 1, p. 335. [Google Scholar]
- Tornier, G. Die Crocodile, Schildkröten und Eidechsen in Togo. Arch. Naturgeschichte 1901, 1901, 65–88. [Google Scholar]
- Luiselli, L. Resource partitioning and interspecific competition in snakes: The search for general geographical and guild patterns. Oikos 2006, 114, 193–211. [Google Scholar] [CrossRef]
- Luiselli, L. Do lizard communities partition the trophic niche? A worldwide meta-analysis using null models. Oikos 2008, 117, 321–330. [Google Scholar] [CrossRef]
- Akani, G.C.; Luiselli, L. Ecological studies on a population of the water snake Grayia smythii in a rainforest swamp of the Niger Delta, Nigeria. Contrib. Zool. 2001, 70, 139–146. [Google Scholar] [CrossRef]
- Luiselli, L.; Angelici, F.M. Ecological relationships in two Afrotropical cobra species (Naja melanoleuca and Naja nigricollis). Can. J. Zool. 2000, 78, 191–198. [Google Scholar] [CrossRef]
- Luiselli, L.; Angelici, F.M.; Akani, G.C. Comparative feeding strategies and dietary plasticity of the sympatric cobras Naja melanoleuca and Naja nigricollis in three diverging Afrotropical habitats. Can. J. Zool. 2002, 80, 55–63. [Google Scholar] [CrossRef]
- Maritz, B.; Alexander, G.J.; Maritz, R.A. The underappreciated extent of cannibalism and ophiophagy in African cobras. Ecology 2019, 100, 1–4. [Google Scholar] [CrossRef]
- Luiselli, L.; Akani, G.C.; Capizzi, D. Food resource partitioning of a community of snakes in a swamp rainforest of south-eastern Nigeria. J. Zool. Lond. 1998, 246, 125–133. [Google Scholar] [CrossRef]





| Family | Species | Type of Record | Observed Number in Dry Season | Observed Number in Wet Season | IUCN (2022) |
|---|---|---|---|---|---|
| Amphibia | |||||
| Arthroleptidae | Arthroleptis poecilonotus | O | 0 | 21 | LC |
| Arthroleptidae | Leptopelis spiritusnoctis | O | 8 | 35 | LC |
| Arthroleptidae | Leptopelis viridis | O | 0 | 3 | LC |
| Bufonidae | Sclerophrys maculatus | O | 0 | 51 | LC |
| Bufonidae | Sclerophrys regularis | O | 25 | 110 | LC |
| Hyperoliidae | Afrixalus dorsalis | O | 0 | 30 | LC |
| Hyperoliidae | Afrixalus vittiger | O | 0 | 23 | LC |
| Hyperoliidae | Afrixalus weidholzi | O | 0 | 3 | LC |
| Hemissotidae | Hemisus marmoratus | O | 3 | 1 | LC |
| Hyperoliidae | Hyperolius concolor | O | 0 | 50 | LC |
| Hyperoliidae | Hyperolius igbettensis | O | 0 | 40 | LC |
| Hyperoliidae | Hyperolius nitidulus | O | 0 | 18 | LC |
| Hyperoliidae | Kassina fusca | B | LC | ||
| Hyperoliidae | Kassina cassinoides | O | 0 | 2 | LC |
| Hyperoliidae | Kassina senegalensis | O | 0 | 17 | LC |
| Phrynobatrachidae | Phrynobatrachus latifrons | O | 20 | 17 | LC |
| Phrynobatrachidae | Phrynobatrachus natalensis | O | 5 | 0 | LC |
| Pipidae | Xenopus fischbergi | O | 0 | 36 | LC |
| Pipidae | Xenopus tropicalis | B | LC | ||
| Ranidae | Amniana galamensis | O | 3 | 16 | LC |
| Dicroglossidae | Hoplobratrachus occipitalis | O | 0 | 32 | LC |
| Ptychadenidae | Ptychadena bibroni | O | 16 | 1 | LC |
| Ptychadenidae | Ptychadena oxyrhynchus | O | 30 | 56 | LC |
| Ptychadenidae | Ptychadena pumilio | O | 6 | 43 | LC |
| Ptychadenidae | Ptychadena sp. | O | 24 | 46 | LC |
| Ptychadenidae | Ptychadena tellinii | O | 5 | 7 | LC |
| Microhylidae | Phrynomantis microps | B | LC | ||
| TOTAL | 145 | 658 | |||
| Reptilia | |||||
| Pelomedusidae | Pelusios castaneus | O | 32 | 18 | LC |
| Pelomedusidae | Pelomedusa subrufa olivacea | O | 23 | 16 | LC |
| Testidinidae | Kinixys nogueyi | O | 9 | 11 | VU |
| Tryonichidae | Cyclanorbis senegalensis | O | 2 | 48 | VU |
| Tryonichidae | Trionyx triunguis | O | 21 | 0 | VU |
| Crocodylidae | Crocodylus suchus | O | 6 | 2 | VU |
| Crocodylidae | Osteolaemus tetraspis | E | VU | ||
| Leptotyphlopidae | Leptotyphlops bicolor | B | LC | ||
| Typhlopidae | Typhlops punctatus | B | LC | ||
| Pythonidae | Python regius | O | 3 | 0 | LC |
| Pythonidae | Python sebae | O | 2 | 0 | LC |
| Atractaspididae | Atractaspis aterima | B | LC | ||
| Atractaspididae | Atractaspis dahomeyensis | B | LC | ||
| Atractaspididae | Atractaspis irregularis | O | 2 | 0 | LC |
| Colubridae | Afronatrix anoscopus | B | LC | ||
| Colubridae | Amblyodipsas unicolor | B | LC | ||
| Colubridae | Chamaelycus fasciatus | B | LC | ||
| Colubridae | Crotaphopeltis hotamboeia | O | 3 | 2 | LC |
| Colubridae | Dasypeltis fasciata | O | 0 | 1 | LC |
| Colubridae | Dasypeltis gansi | O | 2 | 0 | LC |
| Colubridae | Grayia smithii | O | 1 | 0 | LC |
| Colubridae | Lamprophis fuliginosus | O | 3 | 0 | LC |
| Colubridae | Lamprophis lineatus | O | 6 | 0 | LC |
| Colubridae | Lycophidion irroratum | O | 0 | 1 | LC |
| Colubridae | Lycophidion semicinctum | B | LC | ||
| Colubridae | Mehelya crossii | B | LC | ||
| Colubridae | Mehelya poensis | B | LC | ||
| Colubridae | Meizodon coronatus | B | LC | ||
| Colubridae | Meizodon regularis | B | LC | ||
| Colubridae | Philothamnus irregularis | O | 2 | 0 | LC |
| Colubridae | Philothamnus semivariegatus | O | 2 | 0 | LC |
| Colubridae | Prosymna meleagris | B | LC | ||
| Colubridae | Psammophis elegans | O | 2 | 1 | LC |
| Colubridae | Psammophis phillipsi | B | LC | ||
| Colubridae | Psammophis sibilans | B | LC | ||
| Colubridae | Rhamnophis aethiopissa | B | LC | ||
| Colubridae | Rhamphiophis oxyrhynchus | O | 3 | 0 | LC |
| Colubridae | Scaphiophis albopunctatus | B | LC | ||
| Colubridae | Telescopus variegatus | B | LC | ||
| Colubridae | Toxicodryas blandingii | B | LC | ||
| Colubridae | Toxicodryas pulverulenta | B | LC | ||
| Elapidae | Dendroaspis viridis | O | 2 | 1 | LC |
| Elapidae | Elapsoidea semiannulata | B | LC | ||
| Elapidae | Naja melanoleuca | O | 2 | 3 | LC |
| Elapidae | Naja nigricollis | O | 0 | 1 | LC |
| Viperidae | Bitis arietans | O | 2 | 3 | LC |
| Viperidae | Causus maculatus | O | 5 | 2 | LC |
| Viperidae | Echis ocellatus | O | 6 | 2 | LC |
| Agamidae | Agama agama | O | 44 | 36 | LC |
| Agamidae | Agama parafricana | B | LC | ||
| Agamidae | Agama sankaranica | O | 3 | 0 | LC |
| Chamaeleonidae | Chamaeleo gracilis | B | LC | ||
| Chamaeleonidae | Chamaeleo senegalensis | O | 2 | 2 | LC |
| Gekkonidae | Cnemaspis spinicollis | B | LC | ||
| Gekkonidae | Hemidactylus albituberculatus | O | 1 | 0 | LC |
| Gekkonidae | Hemidactylus angulatus | O | 10 | 7 | LC |
| Gekkonidae | Hemidactylus mabouia | O | 9 | 10 | LC |
| Gekkonidae | Hemidactylus matschiei | O | 4 | 1 | LC |
| Gekkonidae | Hemitheconyx caudicinctus | O | 3 | 1 | LC |
| Gekkonidae | Lygodactylus conraui | O | 2 | 0 | LC |
| Lacertidae | Heliobolus nitidus | O | 3 | 0 | LC |
| Scincidae | Mochlus guineensis | O | 12 | 0 | LC |
| Scincidae | Panaspis togoensis | O | 5 | 3 | LC |
| Scincidae | Trachylepis affinis | O | 18 | 8 | LC |
| Scincidae | Trachylepis maculilabris | O | 7 | 4 | LC |
| Scincidae | Trachylepis perrotetii | O | 8 | 15 | LC |
| Scincidae | Trachylepis quinquetaeniata | O | 20 | 14 | LC |
| Varanidae | Varanus exanthematicus | O | 2 | 1 | LC |
| Varanidae | Varanus niloticus | O | 6 | 3 | LC |
| Varanidae | Varanus ornatus | B | LC | ||
| TOTAL | 300 | 217 |
| Dry | Wet | Dry | Wet | |
|---|---|---|---|---|
| Amphibia | Reptilia | |||
| Species richness | 10 | 23 | 41 | 28 |
| Number of individuals | 145 | 658 | 323 | 194 |
| Dominance | 0.1339 | 0.08656 | 0.05867 | 0.08864 |
| Shannon | 2.184 | 2.75 | 3.199 | 2.749 |
| Evenness | 0.7398 | 0.6256 | 0.5978 | 0.5578 |
| Chao-1 | 12 | 26 | 41 | 33 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Segniagbeto, G.H.; Dekawole, J.K.; Ketoh, G.K.; Dendi, D.; Luiselli, L. Herpetofaunal Diversity in a Dahomey Gap Savannah of Togo (West Africa): Effects of Seasons on the Populations of Amphibians and Reptiles. Diversity 2022, 14, 964. https://doi.org/10.3390/d14110964
Segniagbeto GH, Dekawole JK, Ketoh GK, Dendi D, Luiselli L. Herpetofaunal Diversity in a Dahomey Gap Savannah of Togo (West Africa): Effects of Seasons on the Populations of Amphibians and Reptiles. Diversity. 2022; 14(11):964. https://doi.org/10.3390/d14110964
Chicago/Turabian StyleSegniagbeto, Gabriel Hoinsoudé, Jeanne Kafui Dekawole, Guillaume Koffivi Ketoh, Daniele Dendi, and Luca Luiselli. 2022. "Herpetofaunal Diversity in a Dahomey Gap Savannah of Togo (West Africa): Effects of Seasons on the Populations of Amphibians and Reptiles" Diversity 14, no. 11: 964. https://doi.org/10.3390/d14110964
APA StyleSegniagbeto, G. H., Dekawole, J. K., Ketoh, G. K., Dendi, D., & Luiselli, L. (2022). Herpetofaunal Diversity in a Dahomey Gap Savannah of Togo (West Africa): Effects of Seasons on the Populations of Amphibians and Reptiles. Diversity, 14(11), 964. https://doi.org/10.3390/d14110964









