Composition and Structural Characteristics of Rhizosphere Microorganisms of Polygonum sibiricum (Laxm.) Tzvelev in the Yellow River Delta
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Sample Collection
2.3. Soil Index Determination
2.4. High-Throughput Sequencing of Soil Microbe
2.5. Statistical Analysis
3. Results
3.1. Soil Physicochemical Parameters
3.2. Microbial Community Structure Analysis
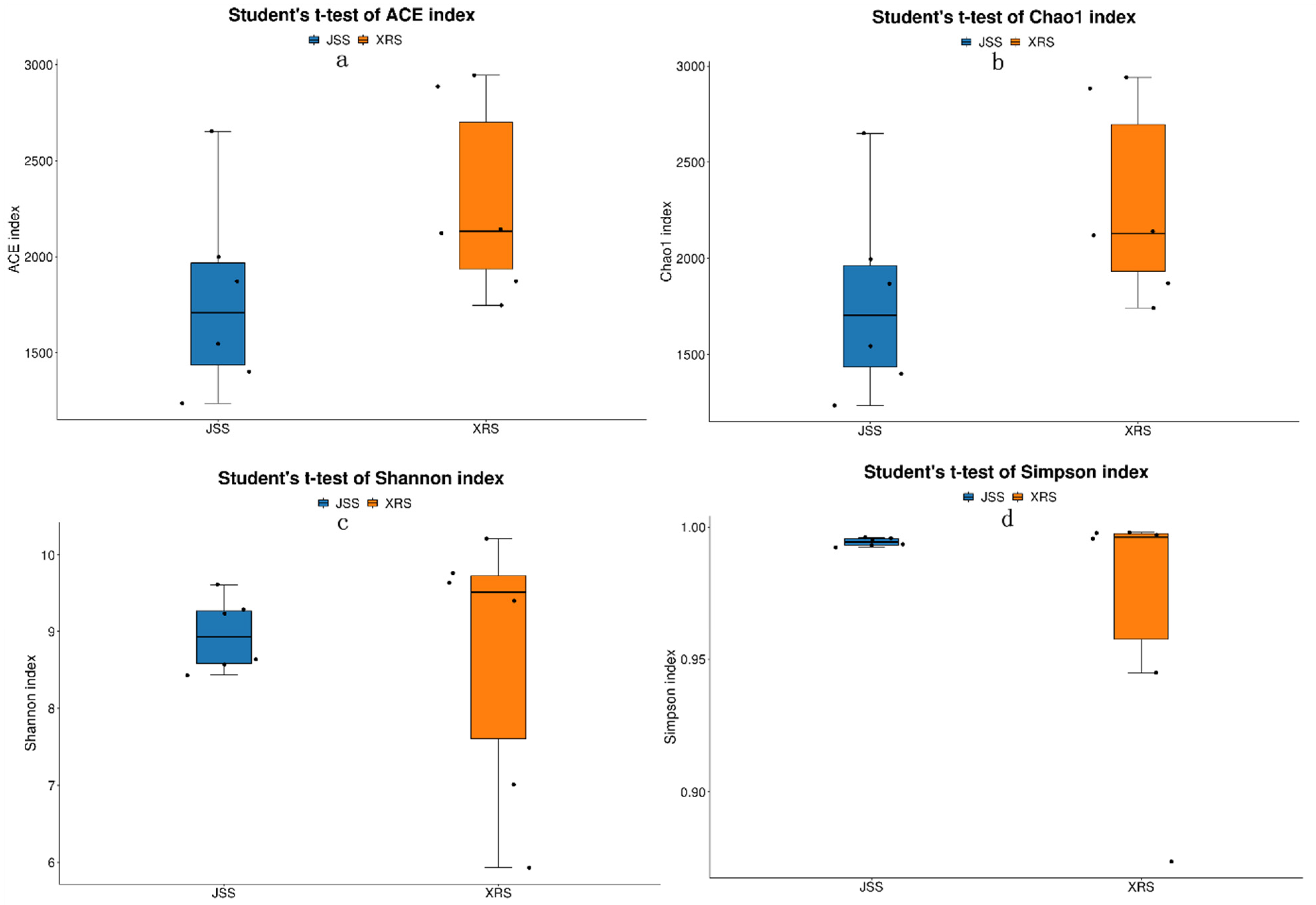
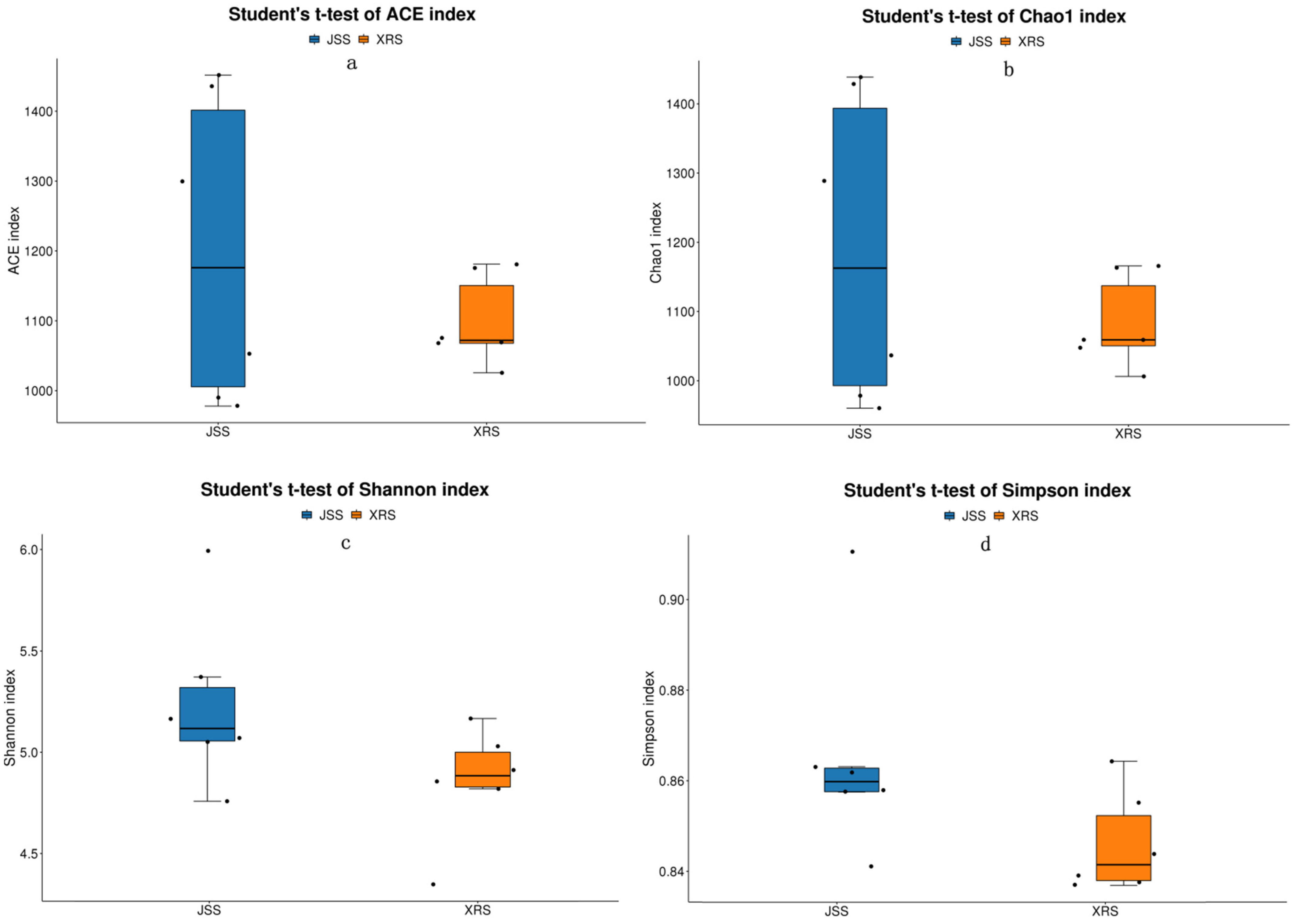
3.3. Alpha Diversity Index Difference Analysis among Different Groups
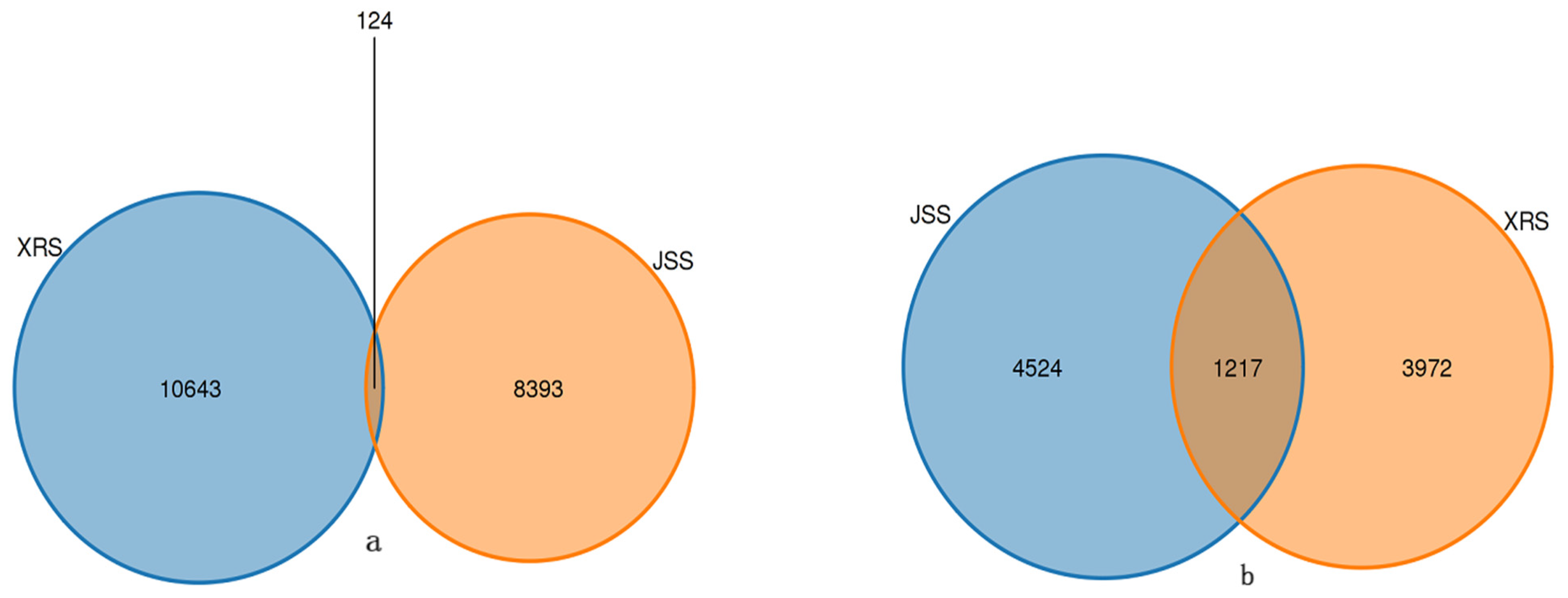
3.4. ASVs Abundance Analysisp
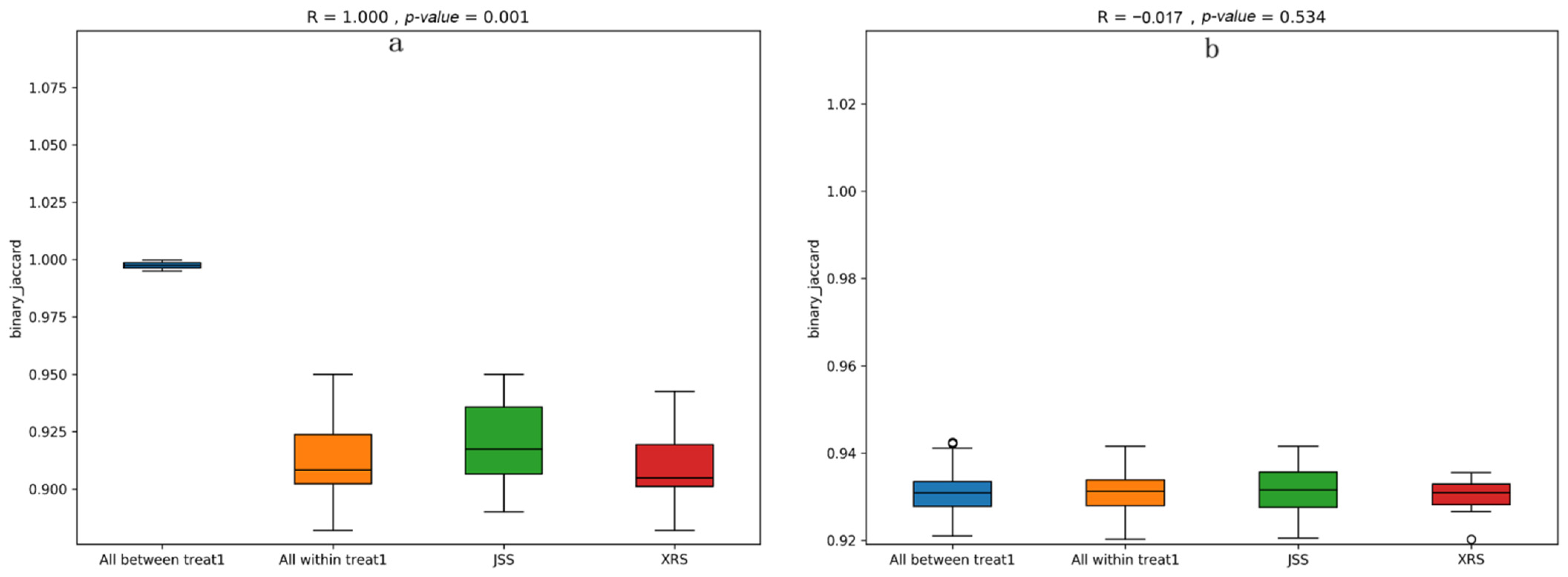
3.5. PCoA Analysis
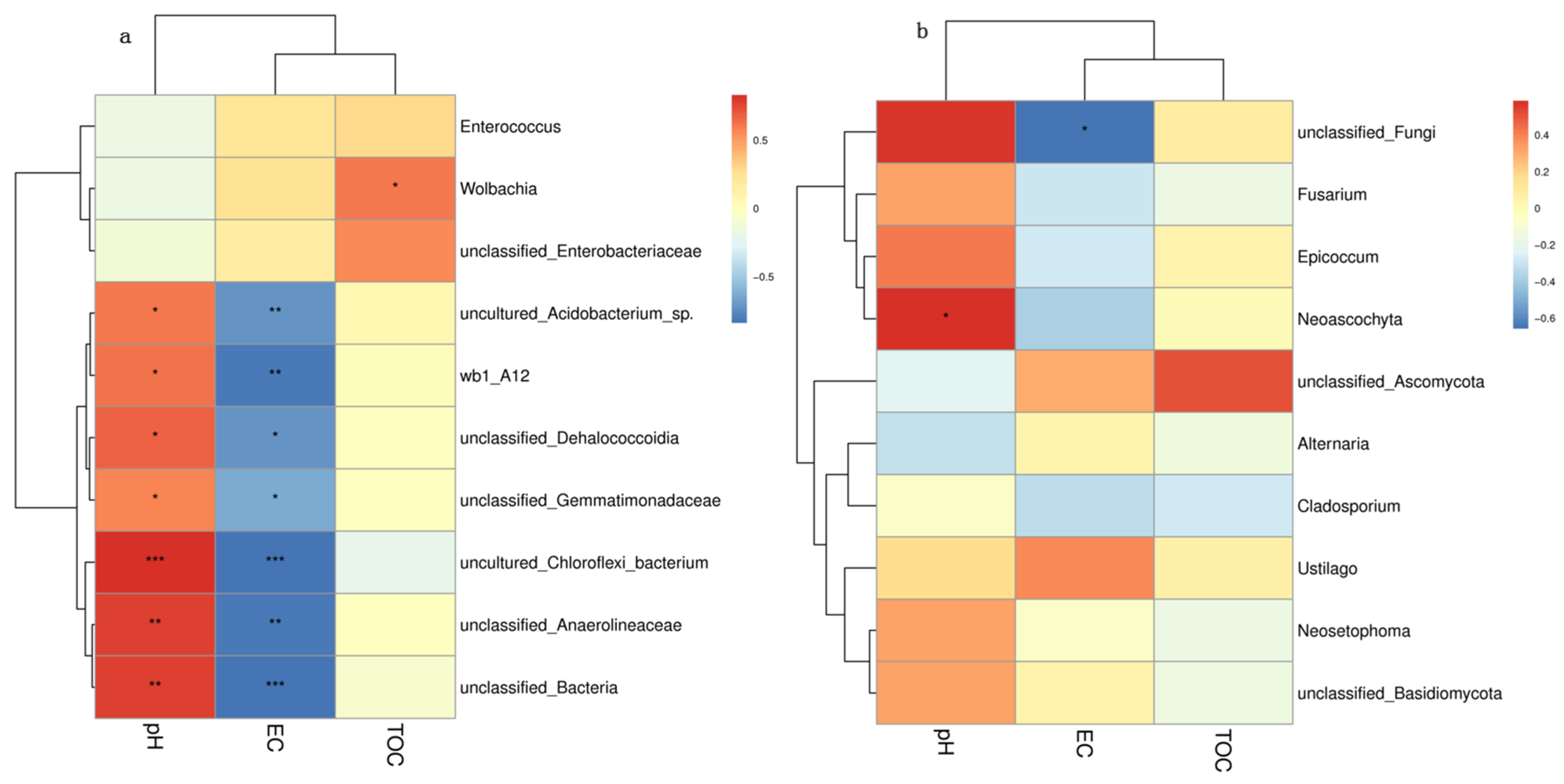
3.6. Correlation between Soil Microbiological Compositions and Soil Physicochemical Properties
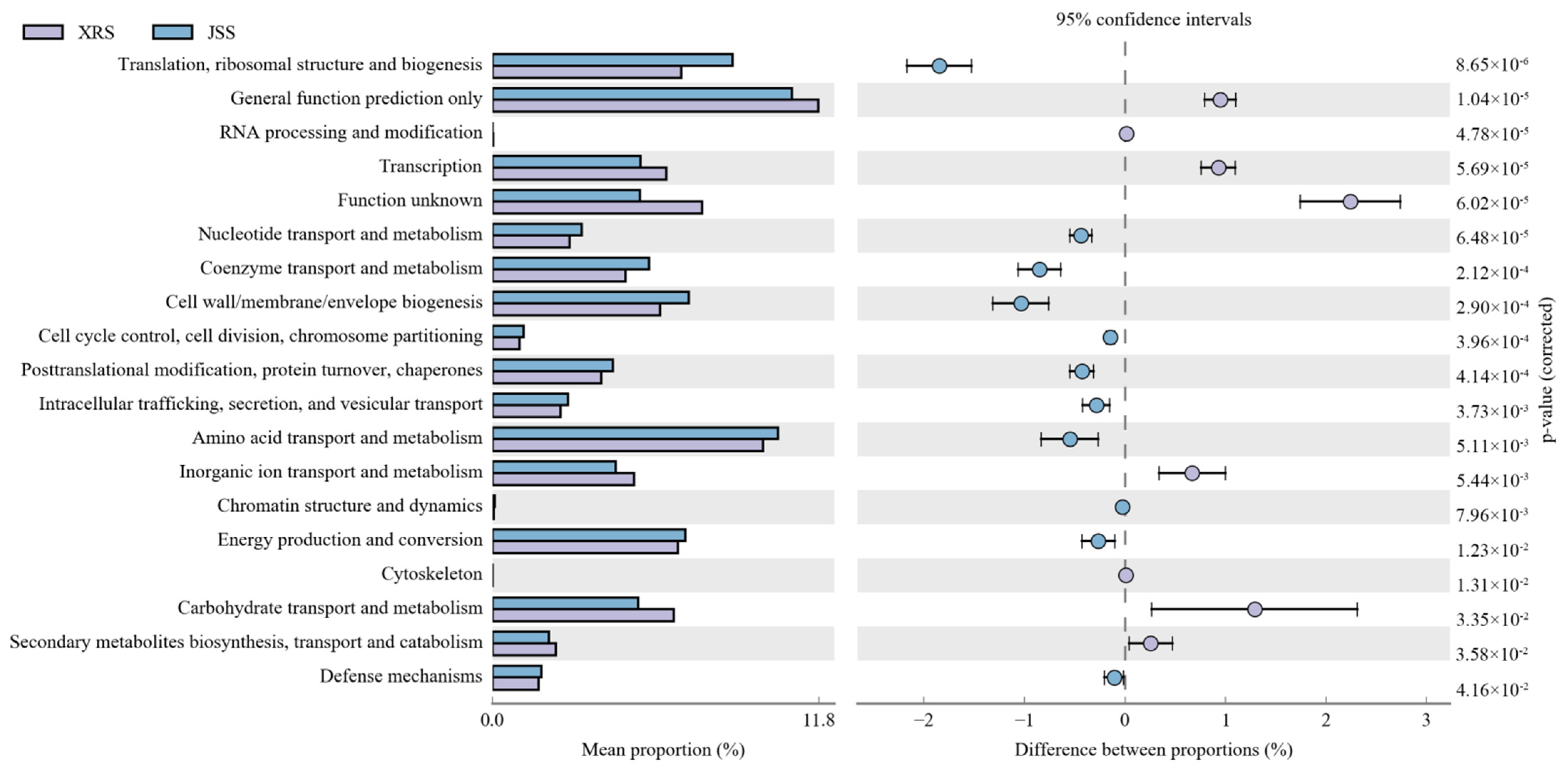
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Qu, C.P.; Xu, Z.R.; Liu, G.J.; Liu, C.; Li, Y.; Wei, Z.G.; Liu, G.F. Differential Expression of Copper-Zinc Superoxide Dismutase Gene of Polygonum sibiricum Leaves, Stems and Underground Stems, Subjected to High-Salt Stress. Int. J. Mol. Sci. 2010, 11, 5234–5245. [Google Scholar] [CrossRef] [PubMed]
- De Cesare, F.; Di Mattia, E.; Macagnano, A. Nanorhizosphere: A new approach to study the interactions between plant and soil microorganisms—The effect of pollutants. In Proceedings of the Egu General Assembly Conference, Vienna, Austria, 23–28 April 2017. [Google Scholar]
- Volpiano, C.G.; Lisboa, B.B.; Jose, J.; Beneduzi, A.; Granada, C.E.; Vargas, L.K. Soil-plant-microbiota interactions to enhance plant growth. Rev. Bras. De Cienc. Do Solo 2022, 46, e0210098. [Google Scholar] [CrossRef]
- Zhang, B.H.; Hong, J.P.; Zhang, Q.; Jin, D.S.; Gao, C.H. Contrast in soil microbial metabolic functional diversity to fertilization and crop rotation under rhizosphere and non-rhizosphere in the coal gangue landfill reclamation area of Loess Hills. PLoS ONE 2020, 15, e0229341. [Google Scholar] [CrossRef]
- Pantigoso, H.A.; Newberger, D.; Vivanco, J.M. The rhizosphere microbiome: Plant-microbial interactions for resource acquisition. J. Appl. Microbiol. 2022, 133, 2864–2876. [Google Scholar] [CrossRef]
- Cúcio, C.; Engelen, A.H.; Costa, R.; Muyzer, G. Rhizosphere Microbiomes of European Seagrasses Are Selected by the Plant, but Are Not Species Specific. Front. Microbiol. 2016, 7, 440. [Google Scholar] [CrossRef] [PubMed]
- Kong, H.G.; Song, G.C.; Ryu, C.M. Inheritance of seed and rhizosphere microbial communities through plant-soil feedback and soil memory. Environ. Microbiol. Rep. 2019, 11, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhuang, Z.; Han, D.; Qi, X. The sedimentary characteristics and formation mechanism of shell ridges along the southwest coast of Bohai Bay. J. Ocean. Univ. China 2005, 4, 124–130. [Google Scholar] [CrossRef]
- Podile, A.R.; Kishore, G.K. Plant growth-promoting rhizobacteria. In Plant-Associated Bacteria; Gnanamanickam, S.S., Ed.; Springer: Dordrecht, The Netherlands, 2006; pp. 195–230. [Google Scholar]
- Xu, J.; Wang, W.Y.; Sun, J.H.; Zhang, Y.; Ge, Q.; Du, L.G.; Yin, H.X.; Liu, X.J. Involvement of auxin and nitric oxide in plant Cd-stress responses. Plant Soil 2011, 346, 107–119. [Google Scholar] [CrossRef]
- Jia, X.; Li, X.D.; Zhao, Y.H.; Wang, L.; Zhang, C.Y. Soil microbial community structure in the rhizosphere of Robinia pseudoacacia L. seedlings exposed to elevated air temperature and cadmium-contaminated soils for 4 years. Sci. Total Environ. 2019, 650, 2355–2363. [Google Scholar] [CrossRef]
- Garbeva, P.; van Elsas, J.D.; van Veen, J.A. Rhizosphere microbial community and its response to plant species and soil history. Plant Soil 2008, 302, 19–32. [Google Scholar] [CrossRef]
- Logue, J.B.; Stedmon, C.A.; Kellerman, A.M.; Nielsen, N.J.; Andersson, A.F.; Laudon, H.; Lindstrom, E.S.; Kritzberg, E.S. Experimental insights into the importance of aquatic bacterial community composition to the degradation of dissolved organic matter. ISME J. 2016, 10, 533–545. [Google Scholar] [CrossRef] [PubMed]
- Shang, S.; Hu, S.X.; Liu, X.X.; Zang, Y.; Chen, J.; Gao, N.; Li, L.Y.; Wang, J.; Liu, L.X.; Xu, J.K.; et al. Effects of Spartina alterniflora invasion on the community structure and diversity of wetland soil bacteria in the Yellow River Delta. Ecol. Evol. 2022, 12, e8905. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Telatin, A. Qiime Artifact eXtractor (qax): A Fast and Versatile Tool to Interact with Qiime2 Archives. Biotech 2021, 10, 5. [Google Scholar] [CrossRef]
- McMurdie, P.J.; Holmes, S. phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, X.Y. D-MANOVA: Fast distance-based multivariate analysis of variance for large-scale microbiome association studies. Bioinformatics 2022, 38, 286–288. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.M.; Motesharezadeh, B.; Hosseini, H.M.; Alikhani, H.; Zolfaghari, A.A. Root-induced changes of Zn and Pb dynamics in the rhizosphere of sunflower with different plant growth promoting treatments in a heavily contaminated soil. Ecotoxicol. Environ. Saf. 2018, 147, 206–216. [Google Scholar] [CrossRef]
- Li, Z.G.; Zu, C.; Wang, C.; Yang, J.F.; Yu, H.; Wu, H.S. Different responses of rhizosphere and non-rhizosphere soil microbial communities to consecutive Piper nigrum L. monoculture. Sci. Rep. 2016, 6, 35825. [Google Scholar] [CrossRef]
- Patkowska, E. Effect of Bio-Products on Bean Yield and Bacterial and Fungal Communities in the Rhizosphere and Non-Rhizosphere. Pol. J. Environ. Stud. 2009, 18, 255–263. [Google Scholar]
- Ugarelli, K.; Laas, P.; Stingl, U. The Microbial Communities of Leaves and Roots Associated with Turtle Grass (Thalassia testudinum) and Manatee Grass (Syringodium filliforme) are Distinct from Seawater and Sediment Communities, but Are Similar between Species and Sampling Sites. Microorganisms 2019, 7, 4. [Google Scholar] [CrossRef]
- Kobayashi, T.; Ralph, T.J.; Sharma, P.; Mitrovic, S.M. Influence of historical inundation frequency on soil microbes (Cyanobacteria, Proteobacteria, Actinobacteria) in semi-arid floodplain wetlands. Mar. Freshw. Res. 2020, 71, 617–625. [Google Scholar] [CrossRef]
- Goldfarb, K.C.; Karaoz, U.; Hanson, C.A.; Santee, C.A.; Bradford, M.A.; Treseder, K.K.; Wallenstein, M.D.; Brodie, E.L. Differential growth responses of soil bacterial taxa to carbon substrates of varying chemical recalcitrance. Front. Microbiol. 2011, 2, 94. [Google Scholar] [CrossRef] [PubMed]
- Mujakic, I.; Piwosz, K.; Koblizek, M. Phylum Gemmatimonadota and Its Role in the Environment. Microorganisms 2022, 10, 151. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, Y.Z.; Liu, Y.H.; Chen, H.; Hu, Y.L. Response of Bacterial and Fungal Soil Communities to Chinese Fir (Cunninghamia lanceolate) Long-Term Monoculture Plantations. Front. Microbiol. 2020, 11, 181. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, L.; Shang, S.; Shi, D.; Xu, H.; Wang, J. Composition and Structural Characteristics of Rhizosphere Microorganisms of Polygonum sibiricum (Laxm.) Tzvelev in the Yellow River Delta. Diversity 2022, 14, 965. https://doi.org/10.3390/d14110965
Zhao L, Shang S, Shi D, Xu H, Wang J. Composition and Structural Characteristics of Rhizosphere Microorganisms of Polygonum sibiricum (Laxm.) Tzvelev in the Yellow River Delta. Diversity. 2022; 14(11):965. https://doi.org/10.3390/d14110965
Chicago/Turabian StyleZhao, Liping, Shuai Shang, Dongli Shi, Hui Xu, and Jun Wang. 2022. "Composition and Structural Characteristics of Rhizosphere Microorganisms of Polygonum sibiricum (Laxm.) Tzvelev in the Yellow River Delta" Diversity 14, no. 11: 965. https://doi.org/10.3390/d14110965
APA StyleZhao, L., Shang, S., Shi, D., Xu, H., & Wang, J. (2022). Composition and Structural Characteristics of Rhizosphere Microorganisms of Polygonum sibiricum (Laxm.) Tzvelev in the Yellow River Delta. Diversity, 14(11), 965. https://doi.org/10.3390/d14110965






