Abstract
The Wnt gene family is of ancient origin and is involved in various biological processes. In this study, Wnt4 and Wnt16 were cloned from Daphnia pulex, named DpWnt4 and DpWnt16, respectively. In DpWnt4 cDNA, full-length 1684 bp, the open reading frame was 1122 bp and it encodes a 373 amino acid polypeptide. In DpWnt16 cDNA, full-length 1941 bp, the open reading frame was 1293 bp and it encodes a 430 amino acid polypeptide. The sequence analysis result showed that both DpWnt4 and DpWnt16 sequences contain a Wnt1 domain. Multiple sequence alignment and phylogenetic analysis revealed that DpWnt4 and DpWnt16 were most closely related to arthropods. The expression of DpWnt4 decreased at 0.5 mg/L group and was induced at 2 mg/L, while DpWnt16 was only induced at 2 mg/L nanoplastics group. These results help us understand more about the character of Wnt4 and Wnt16 in crustaceans and how Wnt genes respond to pollutants, especially nanoplastics.
1. Introduction
The Wnt gene family is of ancient origin and they are distributed widely from fruit flies to humans [1,2]. These genes encode for the synthesis of glycoproteins, usually containing a Wnt1 domain, several N-glycosylation sites, and 22–27 conserved cysteine residues [3,4]. The Wnt genes take part in a wide variety of cellular processes, such as cell proliferation, differentiation, apoptosis, and immunity against microbial infection [5,6]. In arthropods, there are 12 Wnt subfamilies, but the number varies depending on the species [2]. In Drosophila, the function of the Wnt genes has been well studied [7,8]. However, few studies have been conducted in other arthropods, particularly crustaceans, myriapods, and chelicerates. In decapods Litopenaeus vannamei and M. nipponense, the Wnt gene family was found to be involved in host defense against pathogens [4,9]. In Portunus trituberculatus, the Wnt4 and Wnt signalling pathways were found to play important roles in limb regeneration [10]. In previous studies from our laboratory, the expression of both Wnt4 and Wnt16 changed after three 21-day generations of a typical environmental nanoplastic concentration (1 mg/L) [11]. However, we cannot be sure if the expression changes of Wnt4 and Wnt16 are specific to NPs pollution or indirectly affected by other genes or pathways.
Daphnia pulex, a freshwater zooplankton, is crucial for the aquatic food chain. They possess many good characteristics, such as small-sized individuals and amenability to culture and they are easy to grow in the laboratory and have genetic homogeneity, etc. D. pulex has been used extensively to evaluate the toxic effects of chemicals in aquatic systems [12,13]. In addition, the genome of D. pulex is available, and the mode of action of poisons can be studied at the molecular and biochemical levels [14]. Furthermore, D. pulex has filter-feeding properties, and easily ingests the NPs on the surface of freshwater, so D. pulex is a suitable species for studying the effects of NPs [15]. Currently, global plastics production exceeds 320 million tons (Mt) per year [15,16]. Due to the mass production of plastics and their disposal without effective treatment, plastic fragments in the environment are rapidly increasing [17]. Nevertheless, the annual production of plastic has shown an increasing trend. Pollution due to plastics is one of the most serious issues facing all environmental media worldwide, including soils, sediments, and aquatic ecosystems [18,19,20,21]. The small size of micro- and nanoplastics means a higher specific surface area. The larger the specific surface area, the stronger the ability to adsorb other pollutants in aquatic ecosystems. Of all the plastic polymers, polystyrene (PS) is one of the most commonly used; therefore, we chose PS NPs as the research material in this study.
Nowadays, there is an increasing number of reports on the negative effects of MPs in marine and freshwater biota [22,23,24,25]. Previous reports found that these ingested MPs and NPs can accumulate in the gastrointestinal tract and even enter the circulatory system of aquatic animals, causing oxidative stress in these organisms [25,26]. Both MPs and NPs can be accumulated in different trophic levels and have demonstrated biomagnification, which may pose a threat to human health [27]. Moreover, NPs have an enormous surface area, which has the potential to bind even larger amounts of toxic compounds such as bisphenol A (BPA), and co-exposure to NPs and BPA led to a significant increase in BPA uptake in the head and viscera of zebrafish [28]. However, the impact mechanism of MPs and NPs on aquatic ecosystems is not yet fully understood, especially at the molecular level.
In the present work, we obtained the cDNA sequence of both Wnt4 and Wnt16 in D. pulex and investigated their expression levels in response to NPs. The results will provide further insight into the roles of Wnt4 and Wnt16 in regulating NPs pollution in crustaceans, and determine whether Wnts can be considered immune biomarkers for NPs.
2. Materials and Methods
2.1. Daphnia Culture
Daphnia pulex was kindly provided by the Zooplankton Adaptation and Evolution Laboratory of East China Normal University. 4 L glass beaker containing 3 L medium was used for Daphnia culture. Chlorella pyrenoidosa (Shanghai Coslight Biotechnology Co., Ltd., Shanghai, China) was used to feed/ as food for D. pulex. The experimental conditions were light:dark cycle of 16:8h and temperature of 20 °C, the concentration of dissolved oxygen (DO) was enough for survival (>5 mg/L). The third filial generation newborns (<24 h) were taken for the formal experiment to avoid the potential maternal effect.
2.2. Polystyrene Nanoplastics
Polystyrene (PS) NPs (BaseLine Chromtech Research Centre, Tianjin, China) with an average size of 75 nm in nominal diameter was selected for supply. Monodisperse PS microspheres were dissolved in 10 mL distilled water, and the final concentration of the stock solution was 25 mg/mL (1.06 × 1013 particles/mL). The composition of the virgin PS beads was confirmed by Fourier-transform infrared spectroscopy (FTIR) and the aggregation of NPs in water was confirmed by dynamic light scattering as described in the previous study [14].
2.3. Experimental Design
According to the 48-h LC50 of NPs for D. pulex and the results of previous studies [12,14], the following four concentration groups were prepared: 0.1, 0.5, 1, and 2 mg/L. A control group (0 mg/L) was also included. In each concentration group, 20 juveniles were placed in a 1 L beaker (500 mL culture medium). A parallel design was used in each group. During the experiment, the solution was changed every 48 h, and 400 μg C/L of Chlorella was supplied as feed. After 21 days, the samples were removed and stored in the refrigerator at −80 °C for subsequent measurement.
2.4. Extraction of Total RNA and Cloning the Full-Length Wnt4 and Wnt16 cDNA
Daphnia pulex from the control group were collected for RNA extraction, and the RNA concentration was evaluated by a NanoDrop 1000 spectrophotometer (Hach, Loveland, CO, USA). After checking the quality of the total RNA, the PrimeScript RT reagent Kit (Takara, Japan) was used to synthesise cDNA for qPCR. Two Wnt partial sequences were obtained from the database of our laboratory. NCBI BLASTx analysis revealed that they had high similarity to the Wnt family. cDNA and RACE cDNA template synthesis, full-length cDNA clone and sequence analysis, as well as expression of Wnt4 and Wnt16, were analysed as previously described (Wu et al., 2019). Primers used for RACE-PCR are listed in Table 1.

Table 1.
Primers used in cloning and characterising the gene of DpWnt4 and DpWnt16.
2.5. Quantitative Real-Time PCR (qRT-PCR)
The mRNA expression pattern of DpWnt4 and DpWnt16 was measured by qRT-PCR on a C1000 Bio-Rad CFX 96 Real-Time Thermal Cycler (Bio-Rad, Hercules, CA, USA). 18 s (GenBank accession number: AF014011) was used as reference gene. Total RNA from each treatment was extracted, followed by reverse transcription with same quantities of total RNA (500 ng). Double distilled water was used for cDNA dilution (1:5), then all the cDNA was used as template for qRT-PCR.
Each reaction was performed in a total reaction volume of 20 μL, which contained 10 μL TransStart Top Green qPCR SuperMix (TransGen Biotech Company, Beijing, China), 0.4 μL upstream and downstream primers, 1 μL template cDNA and 8.2 μL ddH2O. The PCR conditions used were as follows: 95 °C for 30 s; 40 cycles at 95 °C for 5 s, 57 °C for 20 s; and final extension at 72 °C for 10 min. Gene expression levels were calculated by 2−ΔΔCt method [29].
2.6. Data Analysis
The expression of DpWnt4 and DpWnt16 in different nanoplastics groups was analysed by one-way ANOVA, followed by Tukey’s test to determine whether the data were statistically significant (p < 0.05). All statistical analyses were performed using SPSS Statistics 19.0, and the graphs were created with GraphPad 7.0 (San Diego, CA, USA).
3. Results
3.1. Molecular Characterisation of DpWnt4 and DpWnt16 Sequences
The newly cloned DpWnt4 cDNA was 1684 bp (GenBank accession number: MZ356166), including 1122 bp open reading frame (ORF) encoded a 373 amino acids polypeptide, with a 470 bp 5′ non-coding region (5′-UTR) and a 92 bp 3′-UTR (Figure 1A). The DpWnt4 protein had a molecular weight (MW) of 41.466 kDa and an isoelectric point (pI) of 8.87. Amino acid sequence analysis revealed that DpWnt4 contained a signal peptide (amino acids 1–25), a conserved cysteine-rich (24 cysteine residues) Wnt1 domain (amino acid 51 to 373), and three N-glycosylation sites (amino acids 94, 190, and 319). SMART analysis demonstrated that the DpWnt4 protein contained a transmembrane region (amino acids 7–29) at the N-terminal (Figure 1B).
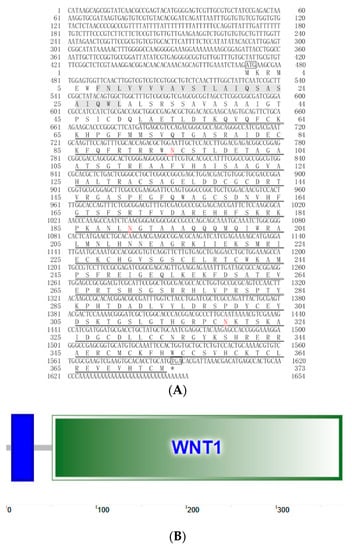
Figure 1.
Nucleotide and deduced amino acid sequences of DpWnt4 (A,B). Start (ATG) and stop (TGA/TAA) codons are marked with boxes. The transmembrane region is shaded blue rectangle in (B). The Wnt1 domain is indicated by double underlines. The N-glycosylation sites are highlighted in red.
The newly cloned DpWnt16 cDNA was 1941 bp (GenBank accession number: MN295983.1.), including the 1293 bp ORF encoded a protein of 430 amino acids without signal peptide, with a 524 bp 5′ non-coding region (5′-UTR) and a 648 bp 3′-UTR (Figure 2A). The DpWnt16 protein had a molecular mass of 47.43 kDa, with an estimated pI of 9.32. Amino acid sequence analysis revealed that DpWnt16 also contained a conserved cysteine-rich (24 cysteine residues) Wnt1 domain (amino acid 105–430), and two N-glycosylation sites (amino acid 148, and 192). SMART analysis demonstrated that the DpWnt16 protein contained three low-complexity regions (amino acids 18–29, 39–52, and 78–93, Figure 2B).

Figure 2.
Nucleotide and deduced amino acid sequences of DpWnt16 (A,B). Start (ATG) and stop (TGA/TAA) codons are marked with boxes. The Wnt1 domain is indicated by double underlines. The N-glycosylation sites are highlighted in red. The three pink rectangles represent the low-complexity regions (B).
3.2. Multiple Sequence Alignment
BLAST analysis showed that the DpWnt4 protein shared high identity with Daphnia magna (98.60%), Thamnocephalus platyurus (77.24%), and Euperipatoides kanangrensis (62.91%), but the similarity with other species was low (<60%, Figure 3).

Figure 3.
Amino acid sequence alignment of Wnt4 with homologs from other species. All the sequences used in this analysis had the GenBank accession numbers as follows: Daphnia magna (XP_032781217.1), Thamnocephalus platyurus (ALL53302.1), Euperipatoides kanangrensis (CDI40100.1), Branchiostoma floridae (XP_035697471.1), and Branchiostoma belcheri (XP_019642937.1).
BLAST analysis showed that the DpWnt16 protein shared high identity with Daphnia magna (94.97%), Penaeus monodon (65.68%), Penaeus vannamei (61.96%), Thamnocephalus platyurus (61.89%) (Figure 4). The result of amino acid sequences multiple alignment analysis revealed that the conservative domain from different species was highly conserved, including the Cys residues, Wnt1 domain, and signal peptide.

Figure 4.
Amino acid sequence alignment of Wnt16 with homologs from other species. All the sequences used in this analysis had the GenBank accession numbers as follows: Daphnia magna (XP_032781217.1), Thamnocephalus platyurus (ALL53298.1), Penaeus monodon (XP_037793890.1), Penaeus vannamei (ALO81632.1), Zootermopsis nevadensis (XP_021925369.1), Cryptotermes secundus (XP_023712832.1), Macrobrachium nipponense (QIV66986.1), Dinothrombium tinctorium (RWS07889.1) and Centruroides sculpturatus (XP_023224171.1).
3.3. Phylogenetic Analysis
In order to further explore the evolution of the two proteins, a phylogenetic tree was constructed by Mega 5.0 software for Wnt4 and Wnt16 proteins from both vertebrates and invertebrates. In the tree of the Wnt4 group, DpWnt4 was clustered with sequences of Daphnia magna first, then clustered with Thamnocephalus platyurus (Figure 5). In the tree of the Wnt16 group, the relation between DpWnt16 and Wnt16 from D. magna was closest and then clustered with other Wnt16 proteins from invertebrates (Figure 6).
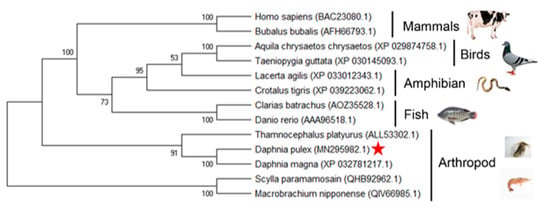
Figure 5.
Phylogenetic tree of the Wnt4 clans from multiple species. Phylogenetic trees were constructed by the neighbour-joining (NJ) method using MEGA 5.0 software [30]. Red pentagram: it is Daphnia pluex (MN295982.1).
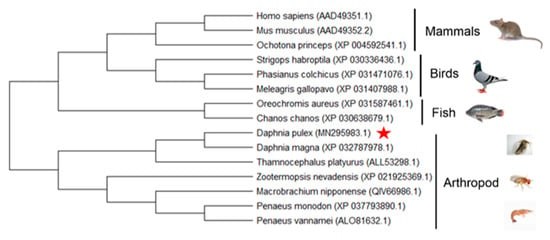
Figure 6.
Phylogenetic tree of the Wnt16 clans from multiple species. Phylogenetic trees were constructed by the neighbour-joining (NJ) method using MEGA 5.0 software [30]. Red pentagram: it is Daphnia pluex (MN295982.1).
3.4. mRNA Expression of DpWnt4 and DpWNT16 after Nanoplastics Treatment
DpWnt4 and DpWnt16 genes expression profiles in response to different concentrations of nanoplastics were evaluated by qRT-PCR. The expression pattern of DpWnt4 (Figure 7A) was inhibited at the 0.1 mg/L NPs concentration level and significantly increased at the highest NPs concentration (2 mg/L). There were no significant changes in the 0.5 and 1 mg/L exposure groups when compared with the control group (p > 0.05). The expression of DpWnt16 increased slightly in the 1 mg/L NPs treatment group (p < 0.05; Figure 7B) and then showed a large increase in the 2 mg/L group (p < 0.05).
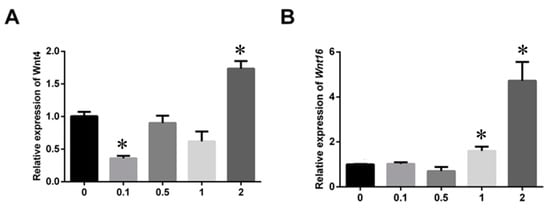
Figure 7.
Effects of nanoplastics on mRNA levels of DpWnt4 (A) and DpWnt16 (B) genes after 21 days. Values are represented as means ± SE, asterisks indicate significant differences (p < 0.05).
4. Discussion
Wnt ligand proteins are a family of conserved signalling molecules. The Wnt genes encode secreted glycoprotein ligands, and these glycoprotein ligands can bind to various transmembrane receptors and then trigger a plethora of biological processes [3], such as tissue development, homeostasis, cell apoptosis, diseases, and immunity [16,31]. In human and mouse, there are 19 Wnt genes which are divided into 12 subfamilies. In arthropods, the number of Wnt genes vary from 7 (Drosophila) to 12 (D. pulex). In this study, the full-length sequences of Wnt4 and Wnt16 cDNA were obtained from D. pulex. The DpWnt4 and DpWnt16 encoded 373 and 430 amino acid residues, respectively. Protein sequence analysis revealed that both proteins contain multiple structures similar to other Wnt family members, including a Wnt1 domain, 2 or 3 N-glycosylation sites, and 24 Cys residues, indicating that the structure of WNT family genes is highly conserved. The N-glycosylation sites were thought to be associated with the solubility of Wnt. The Cys residues were described as a site for palmitoylation and interaction with Frizzled Wnt receptors, and play important roles in the proper folding of Wnt proteins [32].
According to the result of multiple alignment, Wnt4 and Wnt16 showed high similarities with their homologs in arthropods. The phylogenetic tree results also showed that Wnt4 and Wnt16 had the closest evolutionary relationship with Daphnia magna and Thamnocephalus platyurus, both belonging to arthropods. Previous studies found that both Daphnia and Platynereis contain 12 Wnt subfamilies [2], indicating that arthropods belong to the same ancestors and may have the same function. The combined results of sequence features, homologous analysis, and phylogenetic analysis demonstrated that the cloned genes were DpWnt4 and DpWnt16.
In mammals, when tissue is injured by some challenges (e.g., viruses, helminths, and fungus), the host will initiate tissue/organ repair by activating the canonical Wnt pathways, which are important targets of immunotherapy in many immunological diseases. The activation of canonical Wnt signals is triggered by the binding of a Wnt ligand to the FZD family receptor and low-density co-receptor LRP5 or LRP-6. Then the downstream signalings are triggered [33,34]. In Macrobrachium nipponense, Mn-Wnt4 and Mn-Wnt16 were found to activate the expression of Antimicrobial Peptides (AMPs) and then take part in the immune response to bacterial and viral invasion, indicating that the Wnt signals also play important roles in the immune response of invertebrates [9,35]. In the present study, both DpWnt4 and DpWnt16 were significantly upregulated at the highest NP concentration (2 mg/L). Thus, the upregulation of Wnt4 and Wnt16 can also be regarded as an immune response to NPs. Similar results were also found for Mytilus galloprovincialis and Eriocheir sinensis, where gene expression associated with cell stress response and innate immunity was significantly altered after exposure to NPs [19,26].
Nanoplastics have been reported to induce many adverse effects in both marine and freshwater organisms [21,36]. It was found that NP particles can affect the reproduction and immunity of D. magna, and were much more toxic than MP particles [18]. In addition, a large number of studies have found that nanomaterials can cause biological stress responses. In D. magna, the expression of Glutathione-S-transferase (GST), a biomarker for oxidative stress evaluation, was significantly induced by NPs [37]. In C. fluminea, the accumulation of polystyrene NPs can cause an imbalance in the antioxidation system and oxidative stress, then trigger an immune response in C. fluminea [38]. In D. pulex, studies on immune response caused by NPs have also been carried out where the accumulation of reactive oxygen species (ROS) and the induction of oxidative stress were observed, as well as immunosuppression [14]. Similar results were also found in Larimichthys crocea, Platymonas helgolandica, and Chlorella pyrenoidosa [4,39,40]. In the present study, two immune-related genes, DpWnt4 and DpWnt16 also upregulated after a high concentration of NPs exposure, indicating the Wnt signals may also be involved in the immune response to NPs pollution. However, the effects of NPs on the immune regulation mechanism have not been investigated thoroughly, especially at the molecular level, and the underlying mechanism still requires further examination. The abnormal expression of Wnt4 and Wnt16 genes may affect downstream pathways regulated by these genes. More research on other species is needed to determine whether Wnt genes are immune biomarkers of NPs pollution.
In conclusion, this study cloned the Wnt4 and Wnt16 genes in D. pulex and determined their expression profiles in response to five concentrations of NPs particles. The results suggested that Wnt4 and Wnt16 mRNA may play critical roles in the immunity regulation of the toxic response in D. pulex. However, further studies are required to examine the underlying molecular mechanisms and clarify the function of these genes in D. pulex.
Author Contributions
Conceptualization, C.M. and D.Z.; methodology, C.M.; software, C.M.; validation, C.M.; formal analysis, C.M.; investigation, C.M.; resources, Z.L.; data curation, C.M.; writing—original draft preparation, C.M. and D.Z.; writing—review and editing, C.M., D.Z. and Z.L.; visualization, C.M. and D.Z.; supervision, Z.L.; project administration, C.M.; funding acqui-sition, Z.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the Natural Science Foundation of Zhejiang Province of China (LQ22C030003), the National Natural Science Foundation of China (42207323), Shanghai Urban Construction Vocational College Talent Introduction and Faculty Construction Project (B001-22-002), the special fund of State Environmental Protection Key Laboratory of Environmental Health Impact Assessment of Emerging Contaminants (SEPKL-EHIAEC-202201), and the Scientific Research Foundation for Scholars of Hangzhou Normal University (2021QDL063).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
No conflict of interest, financial or otherwise, are declared by the authors.
References
- Garriock, R.J.; Warkman, A.S.; Meadows, S.M.; D’Agostino, S.; Krieg, P.A. Census of vertebrate Wnt genes: Isolation and developmental expression of Xenopus Wnt2, Wnt3, Wnt9a, Wnt9b, Wnt10a, and Wnt16. Dev. Dyn. 2007, 236, 1249–1258. [Google Scholar] [CrossRef] [PubMed]
- Murat, S.; Hopfen, C.; McGregor, A.P. The function and evolution of Wnt genes in arthropods. Arthropod. Struct. Dev. 2010, 39, 446–452. [Google Scholar] [CrossRef]
- Norollahi, S.E.; Hamidian, S.M.T.; Vahidi, S.; Babaei, K.; Samadani, A.A. Modifications of WNT signaling pathway genes including WNT1, KLF5 and WNT16 in colorectal cancer. Gene Rep. 2020, 20, 100733. [Google Scholar] [CrossRef]
- Wang, K.; Dai, X.; Zhang, C.; Cao, X.; Zhang, R.; Zhang, Z.; Huang, X.; Ren, Q. Two Wnt genes regulate the expression levels of antimicrobial peptides during Vibrio infection in Macrobrachium nipponense. Fish Shellfish. Immunol. 2020, 101, 225–233. [Google Scholar] [CrossRef]
- Chen, H.; Li, S.; Xiao, L.; Zhang, Y.; Li, G.; Liu, X.; Lin, H. Wnt4 in protogynous hermaphroditic orange-spotted grouper (Epinephelus coioides): Identification and expression. Comp. Biochem. Physiol., Part B: Biochem. Mol. Biol. 2015, 183, 67–74. [Google Scholar] [CrossRef]
- Chen, J.; Li, Y.; Zhang, J.; Liu, H.; Li, Y. Identification and expression analysis of two Wnt4 genes in the spotted scat (Scatophagus argus). Electron. J. Biotechnol. 2016, 20, 20–27. [Google Scholar] [CrossRef][Green Version]
- Packard, M.; Koo, E.S.; Gorczyca, M.; Sharpe, J.; Cumberledge, S.; Budnik, V.J.C. The Drosophila Wnt, wingless, provides an essential signal for pre-and postsynaptic differentiation. Cell 2002, 111, 319–330. [Google Scholar] [CrossRef]
- Swarup, S.; Verheyen, E.M. Wnt/wingless signaling in Drosophila. Cold Spring Harb. Perspect. Biol. 2012, 4, a007930. [Google Scholar] [CrossRef]
- Du, J.; Zhang, X.; Yuan, J.; Zhang, X.; Li, F.; Xiang, J. Wnt gene family members and their expression profiling in Litopenaeus vannamei. Fish Shellfish. Immunol. 2018, 77, 233–243. [Google Scholar] [CrossRef]
- Fu, Y.; Liu, L.; Wang, C.; Zhu, F.; Liu, X. Suppression of limb regeneration by RNA interference of WNT4 in the swimming crab Portunus trituberculatus. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2019, 234, 41–49. [Google Scholar] [CrossRef]
- Liu, Z.; Cai, M.; Wu, D.; Yu, P.; Jiao, Y.; Jiang, Q.; Zhao, Y. Effects of nanoplastics at predicted environmental concentration on Daphnia pulex after exposure through multiple generations. Environ. Pollut. 2020, 256, 113506. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yu, P.; Cai, M.; Wu, D.; Zhang, M.; Huang, Y.; Zhao, Y. Polystyrene nanoplastic exposure induces immobilization, reproduction, and stress defense in the freshwater cladoceran Daphnia pulex. Chemosphere 2019, 215, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Liu, Z.; Cai, M.; Jiao, Y.; Li, Y.; Chen, Q.; Zhao, Y. Molecular characterisation of cytochrome P450 enzymes in waterflea (Daphnia pulex) and their expression regulation by polystyrene nanoplastics. Aquat. Toxicol. 2019, 217, 105350. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Cai, M.; Yu, P.; Chen, M.; Wu, D.; Zhang, M.; Zhao, Y. Age-dependent survival, stress defense, and AMPK in Daphnia pulex after short-term exposure to a polystyrene nanoplastic. Aquat. Toxicol. 2018, 204, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, Z.; Tang, S.; Li, D.; Jiang, Q.; Zhang, T. Transcriptional response provides insights into the effect of chronic polystyrene nanoplastic exposure on Daphnia pulex. Chemosphere 2020, 238, 124563. [Google Scholar] [CrossRef] [PubMed]
- Greven, A.C.; Merk, T.; Karagoz, F.; Mohr, K.; Klapper, M.; Jovanovic, B.; Palic, D. Polycarbonate and polystyrene nanoplastic particles act as stressors to the innate immune system of fathead minnow (Pimephales promelas). Environ. Toxicol. Chem. 2016, 35, 3093–3100. [Google Scholar] [CrossRef]
- Filho, W.L.; Saari, U.; Fedoruk, M.; Iital, A.; Moora, H.; Klöga, M.; Voronova, V. An overview of the problems posed by plastic products and the role of extended producer responsibility in Europe. J. Clean. Prod. 2019, 214, 550–558. [Google Scholar] [CrossRef]
- Besseling, E.; Wang, B.; Lurling, M.; Koelmans, A.A. Nanoplastic affects growth of S. obliquus and reproduction of D. magna. Environ. Sci. Technol. 2014, 48, 12336–12343. [Google Scholar] [CrossRef]
- Canesi, L.; Ciacci, C.; Bergami, E.; Monopoli, M.P.; Dawson, K.A.; Papa, S.; Canonico, B.; Corsi, I. Evidence for immunomodulation and apoptotic processes induced by cationic polystyrene nanoparticles in the hemocytes of the marine bivalve Mytilus. Mar. Environ. Res. 2015, 111, 34–40. [Google Scholar] [CrossRef]
- Cole, M.; Lindeque, P.; Fileman, E.; Halsband, C.; Goodhead, R.; Moger, J.; Galloway, T.S. Microplastic ingestion by zooplankton. Environ. Sci. Technol. 2013, 47, 6646–6655. [Google Scholar] [CrossRef]
- Gaylarde, C.C.; Baptista Neto, J.A.; da Fonseca, E.M. Nanoplastics in aquatic systems—Are they more hazardous than microplastics? Environ. Pollut. 2021, 272, 115950. [Google Scholar] [CrossRef] [PubMed]
- Jeong, C.B.; Won, E.J.; Kang, H.M.; Lee, M.C.; Hwang, D.S.; Hwang, U.K.; Zhou, B.; Souissi, S.; Lee, S.J.; Lee, J.S. Microplastic size-dependent toxicity, oxidative stress induction, and p-JNK and p-p38 activation in the monogonont rotifer (Brachionus koreanus). Environ. Sci. Technol. 2016, 50, 8849–8857. [Google Scholar] [CrossRef]
- Mak, C.W.; Yeung, K.C.-F.; Chan, K.M. Acute toxic effects of polyethylene microplastic on adult zebrafish. Ecotoxicol. Environ. Saf. 2019, 182, 109442. [Google Scholar] [CrossRef] [PubMed]
- Mazurais, D.; Ernande, B.; Quazuguel, P.; Severe, A.; Huelvan, C.; Madec, L.; Mouchel, O.; Soudant, P.; Robbens, J.; Huvet, A.; et al. Evaluation of the impact of polyethylene microbeads ingestion in European sea bass (Dicentrarchus labrax) larvae. Mar. Environ. Res. 2015, 112, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, Z.; Yang, Y.; Jiang, Q.; Wu, D.; Huang, Y.; Jiao, Y.; Chen, Q.; Huang, Y.; Zhao, Y. Effects of nanoplastics on energy metabolism in the oriental river prawn (Macrobrachium nipponense). Environ. Pollut. 2020, 268, 115890. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Liu, Z.; Wu, D.; Chen, M.; Lv, W.; Zhao, Y. Accumulation of polystyrene microplastics in juvenile Eriocheir sinensis and oxidative stress effects in the liver. Aquat. Toxicol. 2018, 200, 28–36. [Google Scholar] [CrossRef]
- Banerjee, A.; Shelver, W.L. Micro- and nanoplastic induced cellular toxicity in mammals:A review. Sci. Total Environ. 2020, 755 Pt 2, 142518. [Google Scholar] [CrossRef]
- Chen, Q.; Yin, D.; Jia, Y.; Schiwy, S.; Legradi, J.; Yang, S.; Hollert, H. Enhanced uptake of BPA in the presence of nanoplastics can lead to neurotoxic effects in adult zebrafish. Sci. Total Environ. 2017, 609, 1312–1321. [Google Scholar] [CrossRef]
- Kenneth, J.; Livak, T.D.S. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef]
- Wang, S.; Liu, M.; Wang, J.; Huang, J.; Wang, J. Polystyrene nanoplastics cause growth inhibition, morphological damage and physiological disturbance in the marine microalga Platymonas helgolandica. Mar. Pollut. Bull. 2020, 158, 111403. [Google Scholar] [CrossRef]
- Liu, L.; Fu, Y.; Zhu, F.; Mu, C.; Li, R.; Song, W.; Shi, C.; Ye, Y.; Wang, C. Transcriptomic analysis of Portunus trituberculatus reveals a critical role for WNT4 and WNT signalling in limb regeneration. Gene 2018, 658, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Chae, W.J.; Bothwell, A.L.M. Canonical and Non-Canonical Wnt Signaling in Immune Cells. Trends Immunol. 2018, 39, 830–847. [Google Scholar] [CrossRef] [PubMed]
- Staal, F.J.; Luis, T.C.; Tiemessen, M.M. WNT signalling in the immune system: WNT is spreading its wings. Nat. Rev. Immunol. 2008, 8, 581–593. [Google Scholar] [CrossRef]
- Zhang, S.; Li, C.Z.; Yang, Q.H.; Dong, X.H.; Chi, S.Y.; Liu, H.Y.; Shi, L.L.; Tan, B.P. Molecular cloning, characterization and expression analysis of Wnt4, Wnt5, Wnt6, Wnt7, Wnt10 and Wnt16 from Litopenaeus vannamei. Fish Shellfish. Immunol. 2016, 54, 445–455. [Google Scholar] [CrossRef]
- Pedersen, A.F.; Meyer, D.N.; Petriv, A.V.; Soto, A.L.; Shields, J.N.; Akemann, C.; Baker, B.B.; Tsou, W.L.; Zhang, Y.; Baker, T.R. Nanoplastics impact the zebrafish (Danio rerio) transcriptome: Associated developmental and neurobehavioral consequences. Environ. Pollut. 2020, 266, 115090. [Google Scholar] [CrossRef] [PubMed]
- Fadare, O.O.; Wan, B.; Guo, L.-H.; Xin, Y.; Qin, W.; Yang, Y. Humic acid alleviates the toxicity of polystyrene nanoplastic particles to Daphnia magna. Environ. Sci. Nano 2019, 6, 1466–1477. [Google Scholar] [CrossRef]
- Li, Z.; Feng, C.; Wu, Y.; Guo, X. Impacts of nanoplastics on bivalve: Fluorescence tracing of organ accumulation, oxidative stress and damage. J. Hazard. Mater. 2020, 392, 122418. [Google Scholar] [CrossRef]
- Li, L.; Gu, H.; Chang, X.; Huang, W.; Sokolova, I.M.; Wei, S.; Sun, L.; Li, S.; Wang, X.; Hu, M.; et al. Oxidative stress induced by nanoplastics in the liver of juvenile large yellow croaker Larimichthys crocea. Mar. Pollut. Bull. 2021, 170, 112661. [Google Scholar] [CrossRef]
- Yang, W.; Gao, P.; Nie, Y.; Huang, J.; Wu, Y.; Wan, L.; Ding, H.; Zhang, W. Comparison of the effects of continuous and accumulative exposure to nanoplastics on microalga Chlorella pyrenoidosa during chronic toxicity. Sci. Total Environ. 2021, 788, 147934. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).