A Review of the Giant Triton (Charonia tritonis), from Exploitation to Coral Reef Protector?
Abstract
1. Introduction
2. Taxonomy and Distinctive Characteristics
3. Distribution, Habitat and Abundance
4. Movement Ecology
5. Reproduction
6. Juvenile Growth, Development and Morphological Relationships
7. Management of Charonia tritonis
7.1. Threats to Population Recovery
7.2. Aquaculture and Stock Enhancement Potential
8. Predator-Prey Dynamics—Charonia tritonis and CoTS
8.1. Direct Interactions
8.2. Hunting
8.3. Prey Preference
8.4. Indirect Interactions
8.5. Attributes of a CoTS Biocontrol Agent
9. Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CoTS | Crown-of-Thorns starfish |
| dpf | days post-fertilization |
| eDNA | environmental Deoxyribonucleic acid |
| GBR | Great Barrier Reef |
| IPM | integrated pest management |
References
- Morton, B. Triton’s legacy. Mar. Pollut. Bull. 2012, 64, 891–892. [Google Scholar] [CrossRef] [PubMed]
- Nijman, V.; Spaan, D.; Nekaris, A.-I.K. Largescale trade in legally protected marine mollusc shells from Java and Bali, Indonesia. PLoS ONE 2015, 10, e0140593. [Google Scholar] [CrossRef]
- Haszprunar, G.; Vogler, C.; Wörheide, G. Persistent gaps of knowledge for naming and distinguishing multiple species of Crown-of-Thorns-Seastar in the Acanthaster planci species complex. Diversity 2017, 9, 22. [Google Scholar] [CrossRef]
- Cowan, Z.-L.; Pratchett, M.; Messmer, V.; Ling, S. Known predators of Crown-of-Thorns starfish (Acanthaster spp.) and their role in mitigating, if not preventing, population outbreaks. Diversity 2017, 9, 7. [Google Scholar] [CrossRef]
- Estes, J.A.; Terborgh, J.; Brashares, J.S.; Power, M.E.; Berger, J.; Bond, W.J.; Carpenter, S.R.; Essington, T.E.; Holt, R.D.; Jackson, J.B.C.; et al. Trophic downgrading of planet Earth. Science 2011, 333, 301–306. [Google Scholar] [CrossRef]
- Endean, R. Report on Investigations Made into Aspects of the Current Acanthaster planci (Crown-of-Thorns Starfish) Infestations on Certain Reefs of the Great Barrier Reef; Fisheries Branch Queensland, Department of Primary Industries: Brisbane, Australia, 1969; pp. 1–35. [Google Scholar]
- Endean, R.; Stablum, W. A study of some aspects of the Crown-of-thorns starfish (Acanthaster planci) infestations on reefs of Australia’s Great Barrier Reef. Atoll Res. Bull. 1973, 167, 1–76. [Google Scholar] [CrossRef]
- James, P. Requiem for the Reef; Foundation Press: Brisbane, Australia, 1976. [Google Scholar]
- Wells, S.M.; Pyle, R.M.; Collins, N.M. The International Union for Conservation of Nature Invertebrate Red Data Book; International Union for Conservation of Nature: Gland, Switzerland, 1983. [Google Scholar]
- Pratchett, M.S.; Caballes, C.F.; Rivera-Posada, J.A.; Sweatman, H.P.A. Limits to understanding and managing outbreaks of crown-of-thorns starfish (Acanthaster spp.). In Oceanography and Marine Biology: An Annual Review; Hughes, R., Hughes, D., Smith, I., Eds.; CRC Press: Boca Raton, FL, USA, 2014; pp. 133–200. [Google Scholar]
- Babcock, R.C.; Plagányi, É.E.; Condie, S.A.; Westcott, D.A.; Fletcher, C.S.; Bonin, M.C.; Cameron, D. Suppressing the next crown-of-thorns outbreak on the Great Barrier Reef. Coral Reefs 2020, 39, 1233–1244. [Google Scholar] [CrossRef]
- Babcock, R.C.; Dambacher, J.M.; Morello, E.B.; Plaganyi, E.E.; Hayes, K.R.; Sweatman, H.P.A.; Pratchett, M.S. Assessing different causes of Crown-of-Thorns starfish outbreaks and appropriate responses for management on the Great Barrier Reef. PLoS ONE 2016, 11, e0169048. [Google Scholar] [CrossRef] [PubMed]
- Caballes, C.F.; Pratchett, M.S. Reproductive biology and early life history of the crown-of-thorns starfish. In Echinoderms: Ecology, Habitats and Reproductive Biology; Whitmore, E., Ed.; Nova Science Publishers: New York, NY, USA, 2014. [Google Scholar]
- Uthicke, S.; Logan, M.; Liddy, M.; Francis, D.; Hardy, N.; Lamare, M. Climate change as an unexpected co-factor promoting coral eating seastar (Acanthaster planci) outbreaks. Sci. Rep. 2015, 5, 8402. [Google Scholar] [CrossRef]
- Pratchett, M.S.; Caballes, C.F.; Wilmes, J.C.; Matthews, S.; Mellin, C.; Sweatman, H.P.A.; Nadler, L.E.; Brodie, J.; Thompson, C.A.; Hoey, J.; et al. Thirty years of research on Crown-of-Thorns Starfish (1986–2016): Scientific advances and emerging opportunities. Diversity 2017, 9, 41. [Google Scholar] [CrossRef]
- Hall, M.R.; Kocot, K.M.; Baughman, K.W.; Fernandez-Valverde, S.L.; Gauthier, M.E.A.; Hatleberg, W.L.; Krishnan, A.; McDougall, C.; Motti, C.A.; Shoguchi, E.; et al. The crown-of-thorns starfish genome as a guide for biocontrol of this coral reef pest. Nature 2017, 544, 231–234. [Google Scholar] [CrossRef] [PubMed]
- Høj, L.; Byrne, M.; Kroon, F.; Westcott, D. A Review of Biologically Based Control Technologies for Crown-of-Thorns Starfish: Options for Enhancing the Integrated Pest Management Approach. In Report to the National Environmental Science Program; Reef and Rainforest Research Centre Limited: Cairns, Australia, 2020; p. 133. [Google Scholar]
- Collier, T.; van Steenwyk, R. A critical evaluation of augmentative biological control. Biol. Control 2004, 31, 245–256. [Google Scholar] [CrossRef]
- Singh, S.P. Some Success Stories in Classical Biological Control in India; Asia-Pacific Association of Agricultural Research Institutions: Bangkok, Thailand, 2004; p. 73. [Google Scholar]
- Stiling, P.; Cornelissen, T. What makes a successful biocontrol agent? A meta-analysis of biological control agent performance. Biol. Control. 2005, 34, 236–246. [Google Scholar] [CrossRef]
- Van Driesche, R.G.; Carruthers, R.I.; Center, T.; Hoddle, M.S.; Hough-Goldstein, J.; Morin, L.; Smith, L.; Wagner, D.L.; Blossey, B.; Brancatini, V.; et al. Classical biological control for the protection of natural ecosystems. Biol. Control. 2010, 54, S2–S33. [Google Scholar] [CrossRef]
- Nicot, P.C. Classical and Augmentative Biological Control against Diseases and Pests: Critical Status Analysis and Review of Factors Influencing Their Success; International Organization for Biological and Integrated Control of Noxious Animals and Plants, West Palaearctic Regional Section (IOBC/WPRS): Zurich, Switzerland, 2011; p. 184. [Google Scholar]
- van Lenteren, J.C. The state of commercial augmentative biological control: Plenty of natural enemies, but a frustrating lack of uptake. BioControl 2011, 57, 1–20. [Google Scholar] [CrossRef]
- Begg, G.S.; Cook, S.M.; Dye, R.; Ferrante, M.; Franck, P.; Lavigne, C.; Lövei, G.L.; Mansion-Vaquie, A.; Pell, J.K.; Petit, S.; et al. A functional overview of conservation biological control. Crop Prot. 2017, 97, 145–158. [Google Scholar] [CrossRef]
- Motti, C.; Cummins, S.; Francis, D.; Hall, M.; Hillberg, A.; Klein, A.; Menéndez, P.; Rudd, D.; Thomas-Hall, P. Charonia tritonis: A natural biocontrol agent for crown-of-thorns starfish. In Final Report Prepared for Reef2050 Grant Id: 3600000775; Australian Institute of Marine Science: Townsville, Australia, 2019; p. 172. [Google Scholar]
- Pratchett, M.S.; Caballes, C.F.; Cvitanovic, C.; Raymundo, M.L.; Babcock, R.C.; Bonin, M.C.; Bozec, Y.-M.; Burn, D.; Byrne, M.; Castro-Sanguino, C.; et al. Knowledge gaps in the biology, ecology, and management of the pacific crown-of-thorns sea star, Acanthaster sp., on Australia’s Great Barrier Reef. Biol. Bull. 2021, 241, 330–346. [Google Scholar] [CrossRef]
- Hall, M.R.; Motti, C.A.; Kroon, F.J. The potential role of the giant triton snail, Charonia tritonis (Gastropoda: Ranellidae) in mitigating population outbreaks of the crown-of-thorns starfish. In NESP Project 2.1.1: Integrated Pest Management of Crown-of-Thorns Starfish; Australian Institute of Marine Science: Townsville, Australia, 2018; pp. 1–52. [Google Scholar]
- Ponder, W.F.; Colgan, D.J.; Healy, J.; Nützel, A.; Simone, L.R.L.; Strong, E.E. Caenogastropoda. In Phylogeny and Evolution of the Mollusca; Ponder, W., Lindberg, D.L., Eds.; University of California Press: Berkeley, CA, USA, 2008; pp. 331–383. [Google Scholar]
- Ponder, W.F.; Lindberg, D.R.; Ponder, J.M. Biology and Evolution of the Mollusca; CRC Press: Boca Raton, FL, USA, 2019; Volume 1. [Google Scholar]
- Bouchet, P.; Gofas, S. Charonia. World Register of Marine Species. Available online: http://www.marinespecies.org/aphia.php?p=taxdetails,id=138425 (accessed on 10 September 2022).
- Strong, E.E.; Puillandre, N.; Beu, A.G.; Castelin, M.; Bouchet, P. Frogs and tuns and tritons—A molecular phylogeny and revised family classification of the predatory gastropod superfamily Tonnoidea (Caenogastropoda). Mol. Phylogenet. Evol. 2019, 130, 18–34. [Google Scholar] [CrossRef]
- Toscano, A.; Bentivegna, F.; Cirino, P. Holothurians’ responses to attack by the tonnid gastropod Tonna galea. In Echinoderm Research; Scalera-Liaci, L., Ed.; CRC Press: London, UK, 1991; p. 204. [Google Scholar]
- Morton, B. Prey capture, preference and consumption by Linatella caudata (Gastropoda: Tonnoidea: Ranellidae) in Hong Kong. J. Molluscan Stud. 1990, 56, 477–486. [Google Scholar] [CrossRef]
- Cornman, I. Toxic properties of the saliva of Cassis. Nature 1963, 200, 88–89. [Google Scholar] [CrossRef]
- Houbrick, J.R.; Fretter, V. Some aspects of the functional anatomy and biology of Cymatium and Bursa. J. Molluscan Stud. 1969, 38, 415–429. [Google Scholar]
- Laxton, J.H. Feeding in some Australasian Cymatiidae (Gastropoda: Prosobranchia). Zool. J. Linn. Soc. 1971, 50, 1–9. [Google Scholar] [CrossRef]
- Bose, U.; Wang, T.; Zhao, M.; Motti, C.A.; Hall, M.R.; Cummins, S.F. Multiomics analysis of the giant triton snail salivary gland, a crown-of-thorns starfish predator. Sci. Rep. 2017, 7, 6000. [Google Scholar] [CrossRef]
- Morton, B. Foregut anatomy and predation by Charonia lampas (Gastropoda: Prosobranchia: Neotaenioglossa) attacking Ophidaster ophidianus (Asteroidea:Ophidiasteridae) in the Açores, with a review of triton feeding behaviour. J. Nat. Hist. 2012, 46, 2621–2637. [Google Scholar] [CrossRef]
- Beu, A. The Mollusca of the genus Charonia (Family Cymatiidae). Trans. R. Soc. N. Z. 1970, 11, 205–223. [Google Scholar]
- Dodge, H. A historical review of the mollusks of Linnaeus. Part 5 the genus Murex of the Class Gastropoda. Bull. Am. Mus. Nat. Hist. N. Y. 1957, 113, 73–224. [Google Scholar]
- Beu, A.G.; Kay, A. Taxonomy of gastropods of the families Ranellidae (=Cymatiidae) and Bursidae. Part IV the Cymatium pileare complex. J. R. Soc. N. Z. 1988, 18, 185–223. [Google Scholar] [CrossRef]
- Beu, A. Neogene tonnoidean gastropods of tropical and South America: Contributions to the Dominican Republic and Panama Paleontology projects and uplift of the Central American Isthmus. Bull. Am. Paleontol. 2010, 377–378, 1–550. [Google Scholar]
- Vermeij, G.J.; Cambridge, M.A. Biogeography and Adaptation: Patterns of Marine Life; Harvard University Press: Cambridge, UK, 1978. [Google Scholar]
- Wagner, P.J. Gastropod phylogenetics: Progress, problems and implications. J. Paleontol. 2001, 75, 1128–1140. [Google Scholar] [CrossRef]
- Strong, E.E. Refining molluscan characters: Morphology, character coding and a phyologeny of the Caenogastropoda. Zool. J. Linn. Soc. 2003, 137, 447–554. [Google Scholar] [CrossRef]
- Ponder, W.F.; Lindberg, D.R. Towards a phylogeny of gastropod molluscs—An analysis using morphological characters. J. Zool. Soc. 1997, 119, 83–265. [Google Scholar] [CrossRef]
- Bigatti, G.; Sacristán, H.; Rodríguez, M.; Stortz, C.; Penchaszadeh, P. Diet, prey narcotization and biochemical composition of salivary gland secretions of the volutid snail Odontocymbiola magellanica. J. Mar. Biol. Assoc. UK 2010, 90, 959–967. [Google Scholar] [CrossRef]
- Taylor, J.D.; Morris, N.J.; Taylor, C.N. Food specialization and the evolution of predatory prosobranch gastropods. Palaeontology 1980, 23, 375–409. [Google Scholar]
- Coelho, A.C.S.; Matthwes, H.R.; Leal, J.H. Superfamily Tonnacea do Brasil. VI Familia Cymatiidae (Mollusca, Gaqstropoda). Arq. Mus. Nac. RioJan. Braz. 1981, 56, 111–136. [Google Scholar]
- Beu, A. Indo-West Pacific Ranellidae, Bursidae and Personidae (Mollusca: Gastropoda). A monograph of the New Caledonian fauna and revisions of related taxa. Mem. Am. Mus. Nat. Hist. 1998, 178, 1–255. [Google Scholar]
- Fairweather, P.G. Correlations of predatory whelks with intertidal prey at several scales of space and time. Mar. Ecol. Prog. Ser. 1988, 45, 237–243. [Google Scholar] [CrossRef]
- Wilson, B.R. Australian Marine Shells. Il Prosobranch Gastropods. Part One; Odyssey Publishing: Perth, Australia, 1993. [Google Scholar]
- Lai, K.-Y. The family Cymatiidae of Taiwan. Bull. Malacol. Rep. China 1989, 14, 107–128. [Google Scholar]
- Clench, W.J.; Turner, R.D. The family Cymatiidae in the Western Atlantic. Johnsonia 1957, 3, 189–244. [Google Scholar]
- Percharde, P.L. Observations on the gastropod, Charonia variegata, in Trinidad and Tobago. Nautilus 1972, 85, 84–92. [Google Scholar]
- Atlas of Living Australia. Available online: http://biocache.ala.org.au/occurrences/search?q=charoniatritonis#tab_mapView (accessed on 15 June 2017).
- Nateewathana, A.; Aungtonya, C. The Indo-Pacific trumpet triton snail, Charonis tritonis L.: Morphometrics of a species on the verge of local extinction. Phuket Mar. Biol. Cent. Spec. Publ. 1994, 13, 137–140. [Google Scholar]
- Kay, E.A. Pacific Island Marine Mollusks: Systematics. In Marine Coastal Biodiversity in the Tropical Island Pacific Region; Maragos, J.E., Peterson, M.N.A., Eldredge, L.G., Bardach, J.E., Takeuchi, H.F., Eds.; EastWest Center: Honolulu, HI, USA, 1995; pp. 135–159. [Google Scholar]
- Kubota, S. Record of a rare species Charonia tritonis (Linnaeus, 1758) (Gastropoda: Renellidae) from Shirahama, Wakayama Prefecture, central Honshu, Japan. Molluscan Divers. 2012, 3, 95–96. [Google Scholar]
- Montoya, M. A 3000 mile range extension: Charonia tritonis in the Eastern Pacific. Hawaii. Shell News 1983, 31, 8. [Google Scholar]
- Emerson, W.K. On the occurrence of Charonia tritonis in the eastern Pacific (Ranellidae: Cymatiinae). Festivus 1989, 21, 13–15. [Google Scholar]
- Bouchet, F.; Héros, V.; Lozouet, F.P.; Maestrati, P. A quarter-century of deep-sea malacological exploration in the South and West Pacific: Where do we stand? How far to go ? In Tropical Deep-Sea Benthos 25; Héros, V., Cowie, R.H., Bouchet, P., Eds.; Mémoires du Muséum National d’Histoire Naturelle: Paris, France, 2008; Volume 196. [Google Scholar]
- Tröndlé, J.; Boutet, M. Inventory of marine molluscs of French Polynesia. Atoll Res. Bull. 2009, 570, 1–90. [Google Scholar] [CrossRef]
- Paterson, J.C. Preliminary survey of the giant triton (Charonia tritonis) on selected reefs in the Cairns Region during January. In Charonia Research Report to Great Barrier Reef Marine Park Authority COTSREC; 1990; Available online: https://charonia.wordpress.com/report-to-gbrmpa-1990/ (accessed on 13 March 2021).
- Cho, I.-Y.; Kim, K.-Y.; Yi, C.H.; Kim, I.H.; Jung, Y.-H.; Hwang, S.-J.; Bae, J.; Yoon, M.; Kim, M.-S. Full-length mitochondrial genome of the triton trumpet Charonia lampas (Littorinimorpha: Ranellidae). Mitochondrial DNA Part B Resour. 2017, 2, 759–760. [Google Scholar] [CrossRef][Green Version]
- Klein, A.H. Establishment of Multi-Omics Resources for the Giant Triton Snail and the Silver-Lipped Pearl Oyster. Ph.D. Thesis, University of the Sunshine Coast, Sippy Downs, Australia, 2021. [Google Scholar]
- Uthicke, S.; Lamare, M.; Doyle, J.R. eDNA detection of corallivorous seastar (Acanthaster cf. solaris) outbreaks on the Great Barrier Reef using digital droplet PCR. Coral Reefs 2018, 37, 1229–1239. [Google Scholar]
- Adams, C.I.M.; Knapp, M.; Gemmell, N.J.; Jeunen, G.-J.; Bunce, M.; Lamare, M.D.; Taylor, H.R. Beyond biodiversity: Can environmental DNA (eDNA) cut it as a population genetics tool? Genes Genomes Genet. 2019, 10, 192. [Google Scholar] [CrossRef]
- Allen, A.M.; Singh, N.J. Linking movement ecology with wildlife management and conservation. Front. Ecol. Evol. 2016, 3, 155. [Google Scholar] [CrossRef]
- Supp, S.R.; Bohrer, G.; Fieberg, J.; La Sorte, F.A. Estimating the movements of terrestrial animal populations using broad-scale occurrence data. Mov. Ecol. 2021, 9, 60. [Google Scholar] [CrossRef]
- Mueller, T.; Fagan, W.F. Search and navgation in dynamic environments—From individual behaviours to population distributions. Oikos 2008, 117, 654–664. [Google Scholar] [CrossRef]
- Schlaff, A.; Menéndez, P.; Hall, M.; Heupel, M.; Armstrong, T.; Motti, C. Acoustic tracking of a large predatory marine gastropod, Charonia tritonis, on the Great Barrier Reef. Mar. Ecol. Prog. Ser. 2020, 642, 147–161. [Google Scholar] [CrossRef]
- Pratchett, M.S.; Caballes, C.F.; Messmer, V.; Fletcher, C.S.; Westcott, D.A. Movement patterns of Pacific crown-of-thorns starfish (Acanthaster cf. solaris) linked to habitat structure and prey availability. In Report to the National Environmental Science Program; Reef and Rainforest Research Centre Limited: Cairns, Australia, 2020; p. 40. [Google Scholar]
- Bose, U.; Suwansa-ard, S.; Maikaeo, L.; Motti, C.A.; Hall, M.R.; Cummins, S.F. Neuropeptides encoded within a neural transcriptome of the giant triton snail Charonia tritonis, a Crown-of-Thorns Starfish predator. Peptides 2017, 98, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Moomjian, L.; Nystrom, S.; Rittschof, D. Behavioral responses of sexually active mud snails: Kariomones and pheromones. J. Chem. Ecol. 2003, 29, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.-P.; Xia, J.-J.; Peng, P.-F.; Li, H.-P.; Luo, P.; Hu, C.-Q. Characterization of embryogenesis and early larval development in the Pacific triton, Charonia tritonis (Gastropoda: Caenogastropod). Invertebr. Reprod. Dev. 2013, 57, 237–246. [Google Scholar] [CrossRef]
- Strathmann, M.F. Reproduction and Development of Marine Invertebrates of the Northern Pacific Coast, Data and Methods for the Study of Eggs, Embryos and Larvae; University of Washington Press: Seattle, WA, USA, 1987. [Google Scholar]
- Nugranad, J.; Promjinda, K.; Varaibal, T.; Chantara, S. Reproduction of the trumpet triton Charonia tritonis in captivity. Phuket Mar. Biol. Cent. Spec. Publ. 2001, 25, 153–160. [Google Scholar]
- Berg, C. Egg capsule and early veliger of Charonia tritonis (Linnaeus). Veliger 1971, 13, 298. [Google Scholar]
- Nugranad, J.; Chantrapornsilp, S.; Varapibal, T. Feeding and spawning behaviour of the trumpet triton, Charonia tritonis in captivity. Phuket Mar. Biol. Cent. Spec. Publ. 2000, 21, 51–56. [Google Scholar]
- Laxton, J.H. Reproduction in some New Zealand Cymatiida. Zool. J. Linn. Soc. 1969, 48, 237–253. [Google Scholar] [CrossRef]
- Thorson, G. Reproductive and larval ecology of marine bottom invertebrates. Biol. Rev. 1950, 25, 1–38. [Google Scholar] [CrossRef]
- Cañete, J.; Gallardo, G.; Céspedes, T.; Cárdenas, C.; Santana, M. Encapsulated development, spawning and early veliger of the ranellid snail Fusitritonis magellanicus (Röding, 1798) in the cold waters of the Magellan Strait, Chile. Lat. Am. J. Aquat. Res. 2012, 40, 914–928. [Google Scholar] [CrossRef]
- Webber, H.H. Gastropoda: Prosobranchia. In Reproduction in Marine Invertebrates; Giese, A., Pearse, J., Eds.; Academic Press: New York, NY, USA, 1977; p. 369. [Google Scholar]
- Kilburn, R.; Rippey, E. Seashells of Southern Africa; Macmillan: Johannesburg, South Africa, 1982. [Google Scholar]
- Cazaux, C. Ponte et larves du gasteropode prosobranche Tritonalia nodifer. Bull. Société Linnéenne Bordx. 1972, 11, 143–148. [Google Scholar]
- Motti, C.; (Australian Institute of Marine Science, Townsville, Australia). Personal Communications, Observed paternal care by adult charonia tritonis for egg capsules laid in captivity. Both males and females were observed in close proximity to the egg capsules. Egg capsules that were removed from the tank and maintained in separate aquaria showed greater amounts of biofouling. Overall rate of prey consumption dropped during mating, spawning and embryonic development. 2017.
- D’Asaro, C.N. Egg capsules of prosobranch mollusks from South Florida and the Bahamas and notes on spawning in the laboratory. Bull. Mar. Sci. 1970, 20, 414–440. [Google Scholar]
- Latigan, M.J. Some aspects of the breeding biology of Charonia lampas pustulata and Mayena australasia gemmifera under aquarium conditions. Ann. Cape Prov. Mus. Nat. Hist. 1976, 11, 47–53. [Google Scholar]
- Riedel, F. A re-evaluation of the ontogeny of Cabestana spengleri (Perry, 1811). Veliger 1992, 35, 117–121. [Google Scholar]
- Bouchet, P.; Lozouet, P.; Maestrati, P.; Heros, V. Assessing the magnitude of species richness in tropical marine environments: Exceptionally high numbers of molluscs at a New Caledonia site. Biol. J. Linn. Soc. Lond. 2002, 75, 421–436. [Google Scholar] [CrossRef]
- Shuto, T. Larval ecology of prosobranch gastropods and its bearing on biogeography and paleontology. Lethaia 1974, 7, 239–256. [Google Scholar] [CrossRef]
- Bode, M.; Bode, L.; Armsworth, P.R. Larval dispersal reveals regional sources and sinks in the Great Barrier Reef. Mar. Ecol. Prog. Ser. 2006, 308, 17–25. [Google Scholar] [CrossRef]
- Klein, A.H.; Motti, C.A.; Hillberg, A.K.; Ventura, T.; Thomas-Hall, P.; Armstrong, T.; Barker, T.; Whatmore, P.; Cummins, S.F. Development and Interrogation of a Transcriptomic Resource for the Giant Triton Snail (Charonia tritonis). Mar. Biotechnol. 2021, 23, 501–515. [Google Scholar] [CrossRef]
- Klein, A.H.; Ballard, K.R.; Storey, K.B.; Motti, C.A.; Zhao, M.; Cummins, S.F. Multi-omics investigations within the Phylum Mollusca, Class Gastropoda: From ecological application to breakthrough phylogenomic studies. Brief. Funct. Genom. 2019, 18, 377–394. [Google Scholar] [CrossRef]
- Astorga, M.P. Genetic considerations for mollusk production in aquaculture: Current state of knowledge. Front. Genet. 2014, 5, 435. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, H.; Sun, T.; Li, X.; Lv, G.; Bai, Z.; Li, J. Genomic selection for improvement of growth traits in triangle sail mussel (Hyriopsis cumingii). Aquaculture 2022, 561, 738692. [Google Scholar] [CrossRef]
- Mazurais, D.; Darias, M.; Zambonino-Infante, J.L.; Cahu, C.L. Transcriptomics for understanding marine fish larval development. Can. J. Zool. 2011, 89, 599–611. [Google Scholar] [CrossRef]
- Li, H.; Zhang, B.; Huang, G.; Liu, B.; Fan, S.; Zhang, D.; Yu, D. Differential gene expression during larval metamorphic development in the Pearl Oyster, Pinctada fucata, based on transcriptome analysis. Int. J. Genom. 2016, 2016, 2895303. [Google Scholar] [CrossRef]
- Song, H.; Yu, Z.-L.; Sun, L.-N.; Xue, D.-X.; Zhang, T.; Wang, H.-Y. Transcriptomic analysis of differentially expressed genes during larval development of Rapana venosa by digital gene expression profiling. Genes Genomes Genet. 2016, 6, 2181–2193. [Google Scholar]
- Klein, A.; Zhao, M.; Motti, C.A.; Cummins, S. Gene expression analysis of the giant triton snail, Charonia tritonis, during larval development. In Report Prepared for Reef2050 Grant Id: 3600000775; Australian Institute of Marine Science: Townsville, Australia, 2019; p. 18. [Google Scholar]
- Fioroni, P. Larval organs, larvae, metamorphosis and types of development of Mollusca, a comprehensive review. Zool. Jahrbcher Abt. Anat. Ontog. Tiere 1982, 108, 375–420. [Google Scholar]
- Scheltema, R.S. The dispersal of larvae of shoal-water benthic invertebrate species over long distances by ocean currents. In Fourth European Marine Biology Symposium, Bangor, North Wales, UK, 7–28 September 1969; Cambridge University Press: Cambridge, UK, 1971. [Google Scholar]
- Pechenik, J.A. Growth and energy balance during the larval lives of three prosobranch gastropods. J. Mar. Biol. Ecol. 1980, 44, 1–28. [Google Scholar] [CrossRef]
- Scheltema, R.S. Larval dispersal as a means of genetic exchange between geographically separated populations of shallow water benthic marine gastropods. Biol. Bull. 1971, 140, 284–322. [Google Scholar] [CrossRef]
- Strathmann, M.F.; Strathmann, R.R. An extraordinary long larval duration of 4.5 years from hatching to metamorphosis for teleplanic veligers of Fusitriton oregonensis. Biol. Bull. 2007, 213, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Motti, C.A.; Cummins, S.; Armstrong, T.; Barker, T.; Hillberg, A.; Schlawinsky, M.; Thomas-Hall, P. Charonia tritonis larval rearing: Progress report Jan 2018. In Report Prepared for Reef2050 Grant Id: 3600000775; Australian Institute of Marine Science: Townsville, Australia, 2018; p. 43. [Google Scholar]
- Hadfield, M.G. Metamorphosis in marine molluscan larvae: An anlaysis of stimulus and response. In Settlement and Metamorphosis of Marine Invertebrate Larvae; Chia, F., Rice, M., Eds.; Elsevier: North-Hollad, The Netherlands, 1978; pp. 165–175. [Google Scholar]
- Hadfield, M.G.; and Strathmann, M.F. Variability, flexibility and plasticity in life histories of marine invertebrates. Oceanol. Acta 1996, 19, 323–334. [Google Scholar]
- Lebour, M.V. The eggs and larvae of some prosobranchs froum Bermuda. Proc. Zool. Soc. Lond. 1945, 114, 462–489. [Google Scholar] [CrossRef]
- Scheltema, R.S. Biological interactions determining larval settlement in marine invertebrates. Thalass. Jugosl. 1974, 10, 263–296. [Google Scholar]
- Pechenik, J.A.; Scheltema, R.S.; Eyster, L.S. Growth stasis and limited shell calcification in larvae of Cymatium pathenopeum during trans-Atlantic transport. Science 1984, 224, 1097–1099. [Google Scholar] [CrossRef]
- Richter, G. Die gehauseentwicklung bei den Larven der Cymatiiden (Prosobranchia: Tonnacea). Arch. Molluskenkd. Senckenberg. Nat. Ges. 1984, 115, 125–141. [Google Scholar]
- Scheltema, R.S. Long distance dispersal by planktonic larval of shoal-water benthic invertebrates among Pacific Islands. Bull. Mar. Sci. 1986, 39, 241–256. [Google Scholar]
- Hadfield, M.G.; Carpizo-Ituarte, E.J.; del Carmen, K.; Nedved, B.T. Metamorphic competence, a major adaptive convergence in marine invertebrate larvae. Am. Zool. 2001, 41, 1123–1131. [Google Scholar] [CrossRef]
- Dalesman, S.; Rundle, S.D.; Coleman, R.A.; Cotton, P.A. Cue association and antipredator behaviour in a pulmonate snail, Lymnaea stagnalis. Anim. Behav. 2006, 71, 789–797. [Google Scholar] [CrossRef]
- Lesoway, M.P.; Page, L.R. Growth and differentiation during delayed metamorphosis of feeding gastropod larvae: Signatures of ancestry and innovation. Mar. Biol. 2008, 153, 723–734. [Google Scholar] [CrossRef]
- Page, L.R. Molluscan larvae: Pelagic juveniles or slowly metamorphosing larvae. Biol. Bull. 2009, 216, 216–225. [Google Scholar] [CrossRef]
- Martel, A.L.; Tremblay, R.; Toupoint, N.; Olivier, F.; Myrand, B. Veliger size at metamorphosis and temporal variability in prodissoconch II morphometry in the blue mussel (Mytilius edulis): Potential impact on recruitment. J. Shellfish. Res. 2014, 33, 443–455. [Google Scholar] [CrossRef]
- Ritson-Williams, R.; Shjegstad, S.M.; Paul, V.J. Larval metamorphosis of Phestilla spp. in response to waterborne cues from corals. J. Exp. Mar. Biol. Ecol. 2009, 375, 84–88. [Google Scholar] [CrossRef]
- Govan, H. Cymatium Muricinum and Other Ranellid Gastropods: Major Predators of Cultured Tridacnid Clams; Intern Centre for Living Aquatic Resources Management: Manila, Philippines, 1995. [Google Scholar]
- Heslinga, G.A.; Watson, T.C.; Isamu, T. Cultivation of giant clams: Beyond the hatchery. In The First Asian Fisheries Forum; Maclean, J., Dizon, L., Hosillos, L., Eds.; Asian Fisheries Society: Manila, Philipinnes, 1986; pp. 53–58. [Google Scholar]
- Bornancin, L.; Bonnard, I.; Mills, S.C.; Banaigs, B. Chemical mediation as a structuring element in marine gastropod predator-prey interactions. Nat. Prod. Rep. 2017, 34, 644–676. [Google Scholar] [CrossRef] [PubMed]
- Manríquez, P.H.; Navarrete, S.A.; Rosson, A.; Castilla, J.C. Settlement of the gastropod Concholepas concholepas on shells of conspecific adults. J. Mar. Biol. Assoc. UK 2004, 84, 651–658. [Google Scholar] [CrossRef]
- Cahill, A.E.; Koury, S.A. Larval settlement and metamorphosis in a marine gastropod in response to multiple conspecific cues. PeerJ 2016, 4, e2295. [Google Scholar] [CrossRef] [PubMed]
- Padilla, D.K.; McCann, M.J.; Glenn, M.M.; Hooks, A.P.; Shumway, S.E. Effect of food on metamorphic competence in the model system Crepidula fornicata. Biol. Bull. 2014, 227, 242–251. [Google Scholar] [CrossRef]
- Pechenik, J.A.; Heyman, W.D. Using KCl to determine size at competence for larvae of the marine gastropod Crepidula fornicata. J. Mar. Biol. Ecol. 1987, 112, 27–38. [Google Scholar] [CrossRef]
- Penniman, J.R.; Doll, M.K.; Pires, A. Neural correlates of settlement in veliger larvae of the gastropod, Crepidula fornicata. Invertebr. Biol. 2013, 132, 14–26. [Google Scholar] [CrossRef]
- Taris, N.; Comtet, T.; Stolba, R.; Lasbleiz, R.; Pechenik, J.A.; Viard, F. Experimental induction of larval metamorphosis by a naturally-produced halogenated compound (bromomethane) in the invasive mollusc Crepidula fornicata. J. Mar. Biol. Ecol. 2010, 393, 71–77. [Google Scholar] [CrossRef]
- Boettcher, A.A.; Dyer, C.; Casey, J.; Targett, N.M. Hydrogen peroxide induced metamorphosis of queen conch, Strombus gigas: Tests at the commercial scale. Aquaculture 1997, 148, 247–258. [Google Scholar] [CrossRef]
- Boettcher, A.A.; Targett, N.M. Role of chemical inducers in larval metamorphosis of Queen conch, Strombus gigas: Relationship to other marine invertebrate systems. Biol. Bull. 1998, 194, 132–142. [Google Scholar] [CrossRef]
- Davis, M.; Heyman, W.D.; Harvey, W.; Withstandley, C.A. A comparison of two inducers, KCl and Laurencia extracts and techniques for the commercial scale induction of metamorphosis in queen conch, Strombus gigas larvae. J. Shellfish. Res. 1990, 9, 67–73. [Google Scholar]
- Cob, Z.C.; Arshad, A.; Bujang, J.S.; Muda, W.L.W.; Ghaffar, M.A. Metamorphosis induction of the dog conch Strombus canarium using cues associated with conch nursery habitat. J. Appl. Sci. 2010, 10, 628–635. [Google Scholar] [CrossRef]
- Siddall, S.E. Biological and economic outlook for hatchery production of queen conch. In Proceedings of the 35th Gulf and Caribbean Fisheries Institute, Miami, FL, USA, 11–13 November 1982; pp. 46–53. [Google Scholar]
- Davis, M.; Stoner, A.W. Trophic cues induce metamorphosis of queen conch larvae (Strombus gigas). J. Exp. Mar. Biol. Ecol. 1994, 180, 83–102. [Google Scholar] [CrossRef]
- Kang, K.H.; Kim, J.M. The predation of trumpet shell, Charonia sp. on eight different marine invertebrate species. Aquac. Res. 2004, 35, 1202–1206. [Google Scholar] [CrossRef]
- Searcy-Bernal, R.; Anguiano-Beltrán, C. Optimizing the concentration of gamma-aminobutyric acid (GABA) for inducing larval metamorphosis in the Red Abalone Haliotis rufscens (Mollusca: Gastropoda). J. World Aquac. Soc. 1998, 29, 463–470. [Google Scholar] [CrossRef]
- Huggett, M.J.; de Nys, R.; Williamson, J.E.; Heasman, M.; Steinberg, P.D. Settlement of larval blacklip abalone, Haliotis rubra, in response to green and red algae. Mar. Biol. 2005, 147, 1155–1163. [Google Scholar] [CrossRef]
- Li, H.; Lin, W.; Zhang, G.; Cai, Z.; Cai, G.; Chang, Y.; Xing, K. Enhancement of larval settlement and metamorphosis through biological and chemical cues in the abalone Haliotis diversicolor supertexta. Aquaculture 2006, 258, 416–423. [Google Scholar] [CrossRef]
- Bryan, P.J.; Qian, P.Y. Induction of larval attachment and metamorphosis in the abalone Haliotis diversicolor. J. Exp. Mar. Biol. Ecol. 1998, 223, 39–51. [Google Scholar] [CrossRef]
- Morse, D.E. Recent progress in larval settlement and metamorphosis: Closing the gaps between molecular biology and ecology. Bull. Mar. Sci. 1990, 46, 465–483. [Google Scholar]
- Stewart, P.; Soonklang, N.; Stewart, M.J.; Wanichanon, C.; Hanna, P.J.; Poomtong, T.; Sobhon, P. Larval settlement of the tropical abalone, Haliotis asinina Linnaeus, using natural and artificial chemical inducers. Aquac. Res. 2008, 39, 1181–1189. [Google Scholar] [CrossRef]
- Sawatpeera, S.; Krauatrachue, M.; Sonchaeng, P.; Upatham, S.; Rojanasarampkit, T. Settlement and early growth of abalone larvae Haliotis asinina in response to the presence of diatoms. Veliger 2004, 47, 91–99. [Google Scholar]
- Pires, A.; Croll, R.P.; Hadfield, M.G. Catecholamines modulate metamorphosis in the opisthobranch gastropod Phestilla sibogae. Biol. Bull. 2000, 198, 319–331. [Google Scholar] [CrossRef]
- Avila, C.; Tamse, C.T.; Kuzirian, A.M. Induction of metamorphosis in Hermissenda crassicornislarvae (Molluscs: Nudibranchia) by GABA, choline and serotonin. Invertebr. Reprod. Dev. 1996, 29, 127–141. [Google Scholar] [CrossRef]
- Lambert, W.; Todd, C. Evidence for a water-borne cue inducing metamorphosis in the dorid nudibranch mollusc Adalaria proxima (Gastropoda: Nudibranchia). Mar. Biol. 1994, 120, 265–271. [Google Scholar] [CrossRef]
- Lambert, W.J.; Todd, C.D.; Hardege, J.D. Partial characterization and biological activity of a metamorphic inducer of the dorid nudibranch Adalaria proxima (Gastropoda: Nudibranchia). Invertebr. Biol. 1997, 116, 71. [Google Scholar] [CrossRef]
- Zhang, G.; Xu, M.; Zhang, C.; Jia, H.; Zhang, H.; He, M.; Liu, W. Comparative transcriptomic and expression profiles between the foot muscle and mantle tissues in the giant triton snail Charonia tritonis. Front. Physiol. 2021, 12, 632518. [Google Scholar] [CrossRef]
- Laxton, J.H. Shell growth in some New Zealand Cymatiidae (Gastropoda: Prosobranchia). J. Exp. Mar. Biol. Ecol. 1970, 4, 250–260. [Google Scholar] [CrossRef]
- Perron, F.E.; Heslinga, G.A.; Fagolimul, J.O. The gastropod Cymatium muricinum, a predator on juvenile tridacnid clams. Aquaculture 1985, 48, 211–221. [Google Scholar] [CrossRef]
- Vermeij, G.J.; Signor, P.W. The geographic, taxonomic and temporal distribution of determinate growth in marine gastropods. Biol. J. Linn. Soc. Lond. 1992, 47, 233–247. [Google Scholar] [CrossRef]
- Hombre, S.E.; Gonzalez, J.B.; Baguinbin, D.M.; Balisco, R.A.T.; Dolorosa, R.G. Preliminary checklist of marine gastropods and bivalves in the Kalayaan Island group Palawan, Western Philippines. Philipp. J. Syst. Biol. 2016, 10, 25–34. [Google Scholar]
- Salm, R.V. Conservation of Marine Resources in Seychelles. In Report to International Union for Conservation of Nature and Natural Resources; United Nations Environment Program: Nairobi, Kenya, 1978; p. 52. [Google Scholar]
- Council of Europe. CETS No. 104 Convention on the Conservation of European Wildlife and Natural Habitats. In European Treaty Series; Council of Europe: Bern, Switzerland, 1979; pp. 1–26. [Google Scholar]
- European Community. Council Decision of 22 October on concluding the protocol concerning specially protected areas and biological diversity in the Mediterranean and on accepting the annexes to that protocol Barcelona Convention Official 14-12-1999. J. Eur. Communities 1999, 322, 1–17. [Google Scholar]
- Cavallaro, M.; Navarra, E.; Danze, A.; Danze, G.; Muscolino, D.; Giarratana, F. Mediterranean triton Charonia lampas lampas (Gastropoda: Caenogastropoda): Report on captive breeding. Acta Adriat. 2016, 57, 263–272. [Google Scholar]
- Marler, G.; Marler, L. Triton’s triumph. Sea Front. 1982, 28, 354–356. [Google Scholar]
- McClanahan, T.R. Kenyan coral reef-associated gastropod fauna: A comparison between protected and unprotected reefs. Mar. Ecol. Prog. Ser. 1989, 53, 11–20. [Google Scholar] [CrossRef]
- Chesher, R.; Poulsen, A. A key predator on coral reefs a favoured prey for shell collector. Giant Triton Aust. Shell News 1993, 83, 1–2. [Google Scholar]
- Sapp, J. What Is Natural? Coral Reef Crisis; Oxford University Press: Oxford, UK, 1999. [Google Scholar]
- Paterson, J.C.; Poulsen, A.L. A study of Charonis tritonis on the Great Barrier Reef. Report to the Great Barrier Reef Marine Park Authority. 1988. Available online: https://charonia.wordpress.com/report-to-gbrmpa-1988/ (accessed on 15 March 2021).
- Poulsen, A.L. Coral reef gastropods—A sustainable resource? Pac. Conserv. Biol. 1995, 2, 142–145. [Google Scholar] [CrossRef]
- Moon, S.; (Association of Marine Park Tourism Operators, Cairns, Australia). Personal Communications, 2016.
- Chesher, D.P. Destruction of Pacific corals by the sea star Acanthaster planci. Science 1969, 165, 280–283. [Google Scholar] [CrossRef]
- Tropical Marine Mollusc Programme. Workshop of the Tropical Marine Mollusc Programme. In Research Bulletin (Sun Chiwawitthaya Thang Thale Phuket), 12–18 August 1991; Phuket Marine Biological Center: Wichit, Thailand, 1991. [Google Scholar]
- Stoner, A.W. Evidence for a significant decline in Queen Conch in the Bahamas, including the population in a marine protected area. In Proceedings of the 65th Gulf and Caribbean Fish Institute, Santa Marta, Colombia, 5–9 November 2012; pp. 349–361. [Google Scholar]
- Stoner, A.W.; Davis, M.; Bull, U.S. Experimental outplanting of juvenile queen conch, Strombus gigas: Comparisons of wild and hatchery-reared stocks. Fish 1994, 92, 390–411. [Google Scholar]
- Stoner, A.W.; Ray-Culp, M. Evidence for Allee effects in an overharvested marine gastropod: Density dependent mating and egg production. Mar. Ecol. Prog. Ser. 2000, 202, 297–302. [Google Scholar] [CrossRef]
- Antonelli, P.J.; Kazarinoff, N.D. Starfish predation of a growing coral reef community. J. Theor. Biol. 1984, 107, 667–684. [Google Scholar] [CrossRef]
- Bradbury, R.H.; Hammond, L.S.; Moran, P.J.; Reichelt, R.E. Coral reef communities and the crown-of-thorns starfish: Evidence for quantitatively stable cycles. J. Theor. Biol. 1985, 113, 69–80. [Google Scholar] [CrossRef]
- McCallum, H.I. Predator regulation of Acanthaster planci. J. Theor. Biol. 1987, 127, 207–220. [Google Scholar] [CrossRef]
- Morello, E.B.; Plagányi, É.; Babcock, R.C.; Sweatman, H.; Hillary, R.; Punt, A.E. Model to manage and reduce crown-of-thorns starfish outbreaks. Mar. Ecol. Prog. Ser. 2014, 512, 167–183. [Google Scholar] [CrossRef]
- Great Barrier Reef Marine Park Authority. Great Barrier Reef Marine Park Regulations 2019: F2021L01190; Office of Parliamentary Counsel: Canberra, Australia, 2019.
- Nautical Crush Trading. Available online: https://www.nauticalcrushtrading.com/triton-shell-1-triton-seashell-large-9–10-for-decor/ (accessed on 31 August 2022).
- Chesher, R.H. Charonia Tritonis; Proposal for listing in Schedule II of CITES; Commonwealth of Australia: Canberra, Australia, 1993. [Google Scholar]
- Convention in Trade in Endangered Species. Inclusion of the Giant Triton Charonia Tritonis on Appendix II of the Convention in Trade in Endangered Species (CITES); Appendix 2; Commonwealth of Australia: Canberra, Australia, 1994. [Google Scholar]
- Rosser, A.R.; Haywood, M.J. Guidance for CITES Scientific Authorities: Checklist to Assist in Making Non-Detriment Findings for Appendix II Exports; International Union for Conservation of Nature: Gland, Switzerland; Cambridge, UK, 2002; p. 146. [Google Scholar]
- Kay, E.A. The Conservation Biology of Molluscs. In Occasional Paper of the Survival Commission No 9; International Union for Conservation of Nature: Gland, Switzerland, 1995; p. 81. [Google Scholar]
- Weis, A.; Dunning, M.; Gaffney, P. Ecological assessment of Queensland’s Marine Specimen Shell Collection Fishery. In A Report to the Australian Government Department of the Environment and Heritage on the Ecological Sustainable Management of a Small Scale Highly Selective Hand and Shell Dredge Collection Fishery; Queensland Government Department of Primary Industries and Fisheries: Brisbane, Australia, 2004; p. 30. [Google Scholar]
- United Nations Environment Program. Decision IG.19/12. Annex II. Amendments of the list of Annexes II and III of the Protocol concerning Specially Protected Areas and Biological Diversity in the Mediterranean; United Nations Environment Program: Brussels, Belgium, 2009; Available online: https://wedocs.unep.org/bitstream/handle/20.500.11822/10181/09ig19_08_annex2_19_12_eng.pdf (accessed on 6 May 2021).
- Fisheries Act 2014; Laws of Seychelles; ILO: Geneva, Switzerland, 2014; pp. 1–29.
- Palawan Council for Sustainable Development. Approving the 2014 updated list of terrestrial and marine wildlife in Palawan and their categories pursuant to Republic Act 9147, otherwise known as the Wildlife Resources Conservation and Protection Act of 2001. In Palawan Council for Sustainable Development Resolution No. 15–521; Republic of the Philippines Republic Act 7611: Palau, Philippines, 2015; pp. 1–18. [Google Scholar]
- PCSD. Illegal Traders of Endangered Wildlife, Caught. Available online: https://pcsd.gov.ph/3323/ (accessed on 1 November 2021).
- Convention on the Conservation of European Wildlife and Natural Habitats Standing Committee. Presumed large-scale exploitation and marketing of protected marine shelled molluscs in Greece. In Proceedings of the Convention on the Conservation of European Wildlife and Natural Habitats. Standing Committee 38th Meeting, Strasbourg, France, 27–30 November 2018.
- Motti, C. The shells of captive Charonia tritonis snails were observed to be biofouled to various degrees with organisms including crustose corraline algae and boring sponges. Extensive pitting was observed in the older whorls, some pitting was observed by X-ray in the more recent whorls. In captivity, observations have revealed CoTS that initially survive an incomplete C. tritonis attack ultimately perish within 12–48 h if the proboscis has penetrated the outer skin. Unpublished data. 2022. [Google Scholar]
- Stefaniak, L.M.; McAtee, J.; Shulman, M.J. The costs of being bored: Effects of a clionid sponge on the gastropod Littorina littorea (L). J. Exp. Mar. Biol. Ecol. 2005, 327, 103–114. [Google Scholar] [CrossRef]
- Noisette, F.; Comtet, T.; Legrand, E.; Bordeyne, F.; Davoult, D.; Martin, S. Does encapsulation protect embryos from the effects of ocean acidification? The example of Crepidula fornicata. PLoS ONE 2014, 9, e93021. [Google Scholar] [CrossRef] [PubMed]
- Padilla, D.K.; Charifson, D.; Liguori, A.; McCarty-Glenn, M.; Rosa, M.; Rugila, A. Factors affecting gastropod larval development and performance: A systematic review. J. Shellfish. Res. 2018, 37, 851–867. [Google Scholar] [CrossRef]
- Bogan, S.N.; Mcmahon, J.B.; Pechenik, J.A.; Pires, A. Legacy of multiple stressors: Responses of gastropod larvae and juveniles to ocean acidification and nutrition. Biol. Bull. 2019, 236, 159–173. [Google Scholar] [CrossRef] [PubMed]
- Pechenik, J.A.; Tyrell, A.S. Larval diet alters larval growth rates and post-metamorphic performance in the marine gastropod Crepidula fornicata. Mar. Biol. 2015, 162, 1597–1610. [Google Scholar] [CrossRef]
- Lu, J.; Shi, Y.; Wang, S.; Chen, H.; Cai, S.; Feng, J. NMR-based metabolomic analysis of Haliotis diversicolor exposed to thermal and hypoxic stresses. Sci. Total Environ. 2016, 545–546, 280–288. [Google Scholar] [CrossRef]
- Coleman, D.W.; Byrne, M.; Davis, A.R. Molluscs on acid: Gastropod shell repair and strength in acidifying oceans. Mar. Ecol. Prog. Ser. 2014, 509, 203–211. [Google Scholar] [CrossRef]
- Harvey, B.P.; Agostini, S.; Wada, S.; Inaba, K.; Hall-Spencer, J.M. Dissolution: The Achilles’ heel of the triton shell in an acidifying ocean. Front. Mar. Sci. 2018, 5, 371. [Google Scholar] [CrossRef]
- Guiden, P.W.; Bartel, S.L.; Byer, N.W.; Shipley, A.A.; Orrock, J.L. Predator-prey interactions in the anthropocene: Reconciling multiple aspects of novelty. Trends Ecol. Evol. 2019, 34, 616–627. [Google Scholar] [CrossRef] [PubMed]
- Modica, M.V.; Russini, V.; Fassio, G.; Oliverio, M. Do larval types affect genetic connectivity at sea? Testing hypothesis in two sibling marine gastropods with contrasting larval development. Mar. Environ. Res. 2017, 127, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Crocetta, F.; Caputi, L.; Paz-Sedano, S.; Tanduo, V.; Vazzana, A.; Oliverio, M. High genetic connectivity in a gastropod with long-lived planktonic larvae. J. Molluscan Stud. 2020, 86, 42–55. [Google Scholar] [CrossRef]
- Dwiono, S.A.P.; Makaputi, P.C.; Pradina, D.A. A hatchery for the topshell (T. niloticus) in Eastern Indonesia. In Trochus: Status, Hatchery Practice and Nutrition. ACIAR Proceedings No. 79; Lee, C., Lynch, P., Eds.; Australian Centre for International Agricultural Research: Canberra, Australia, 1997; pp. 33–37. [Google Scholar]
- Guo, X.; Ford, S.E.; Zhang, F. Molluscan aquaculture in China. J. Shellfish. Res. 1999, 18, 19–31. [Google Scholar]
- Davis, M. Queen conch (Strombus gigas) culture techniques for research, stock enhancement and grow-out markets. In Recent Advances in Marine Biotechnology, Seaweeds and Invertebrates, Aquaculture Part A Seaweeds and Invertebrates; Fingerman, M., Nagabhushanam, R., Eds.; Science Publishers: Endfield, CT, USA, 2000; Volume 4. [Google Scholar]
- Dwiono; Pradina, S.A.P.; Makatipu, P.C. Spawning and seed production of the green snail (Turbo marmoratus L.) in Indonesia. SPC Trochus Inf. Bull. 2001, 7, 9–13. [Google Scholar]
- Katsanevakis, S.; Lefkaditou, E.; Galinou-Mitsoudi, S.; Koutsoubas, D.; Zenetos, A. Molluscan species of minor commercial interest in the Hellenic seas: Distribution, exploitation and conservation status. Mediterr. Mar. Sci. 2008, 9, 77–118. [Google Scholar] [CrossRef]
- Laughlin, R.A.; Weil, E. Queen conch mariculture and restoration in the Archipiélago de Los Roques: Preliminary results. In Proceedings of the 35th Gulf and Caribbean Fisheries Institute, Miami, FL, USA, 11–13 November 1982; pp. 64–72. [Google Scholar]
- Glazer, R.A.; Delgado, G.A.; Kidney, J.A. Estimating Queen Conch (Strombus gigas) home ranges using acoustic telemetry: Implications for the design of marine fishery reserves. Gulf Caribb. Res. 2003, 14, 79–89. [Google Scholar] [CrossRef]
- Spring, A.; Davis, M. Recommendations for culturing juvenile Queen Conch, Strombus gigas, for restocking and commercial purposes. In Proceedings of the 58th Gulf and Caribbean Fish Institute, San Andres, Colombia, 7–11 November 2005; pp. 781–787. [Google Scholar]
- Delgado, G.A.; Glazer, R.A. Interactions between translocated and native queen conch Strombus gigas: Evaluating a restorative strategy. Endanger. Species Res. 2007, 3, 259–266. [Google Scholar]
- McCarthy, K. A Review of Queen Conch (Strombus gigas) Life History; Sustainable Fisheries Division, Ed.; SEDAR 14DW4; National Marine Fishery Service: Miami, FL, USA, 2008. [Google Scholar]
- Crowe, T.P.; Lee, C.L.; McGuinness, K.A.; Amos, M.J.; Dangeubun, J.; Dwiono, S.A.P.; Makatipu, P.C.; Manuputty, J.; N’guyen, F.; Pakoa, K.; et al. Experimental evaluation of the use of hatchery-reared juveniles to enhance stocks of the topshell Trochus niloticus in Australia, Indonesia and Vanuatu. Aquaculture 2002, 206, 175–197. [Google Scholar] [CrossRef]
- Hoang, D.H.; Tuan, V.S.; Hoa, N.X.; Sang, H.M.; Lu, H.D.; Tuyen, H.T. Experiments on using hatchery-reared Trochus niloticus juveniles for stock enhancement in Vietnam. SPC Trochus Inf. Bull. 2007, 13, 13–18. [Google Scholar]
- Ridlon, A.D.; Wasson, K.; Waters, T.; Adams, J.; Donatuto, J.; Fleener, G.; Froehlich, H.; Govender, R.; Kornbluth, A.; Lorda, J.; et al. Conservation aquaculture as a tool for imperiled marine species: Evaluation of opportunities and risks for Olympia oysters, Ostrea lurida. PLoS ONE 2021, 16, e0252810. [Google Scholar] [CrossRef] [PubMed]
- FAO. The state of world fisheries and aquaculture 2016. In Contributing to Food Security and Nutrition for All; Food and Agriculture Organization of the United Nations: Rome, Italy, 2016; p. 200. [Google Scholar]
- Castell, L. Gastropod Molluscs. In Aquaculture: Farming Aquatic Animals and Plants; Lucas, J.S., Southgate, P.C., Eds.; Blackwell Publishing Ltd.: Oxford, UK, 2012; pp. 567–582. [Google Scholar]
- Nash, W.J. Hatchery Production of Trochus (Trochus niloticus) in Vanuatu: A Review of the Existing Facilities and a Manual of Rearing Techniques Appropriate for a Small-Scale Hatchery; Food and Agriculture Organisation: Tasmania, Australia, 1989. [Google Scholar]
- Chaitanawisuti, N.; Kritsanapuntu, A. Growth and production of hatchery-reared juvenile spotted babylon Babylonia areolata Link 1807 cultured to marketable size in intensive lowthrough and semi-closed recirculating water systems. Aquac. Res. 2000, 31, 415–419. [Google Scholar] [CrossRef]
- Chaitanawisuti, N.; Kritsanapuntu, A.; Natsukari, Y.; Kathinmai, S. Effects of different types of substrate on growth and survival of juvenile spotted babylon, Babylonia areolata Link 1807 reared to marketable size in a flow-through seawater system. Asian Fish. Sci. 2001, 14, 279–284. [Google Scholar] [CrossRef]
- Clarke, P.J.; Komatsu, T. Successful culture and release of trochus in Solomon Islands. SPC Trochus Inf. Bull. 2001, 8, 11–14. [Google Scholar]
- Amos, M.J.; Purcell, S.W. Evaluation of strategies for intermediate culture of Trochus niloticus (Gastropoda) in sea cages for restocking. Aquaculture 2003, 218, 235–249. [Google Scholar] [CrossRef]
- Hall, M.R.; Bose, U.; Cummins, S.F.; Motti, C.A.; Wang, T.; Zhao, M.; Roberts, R.; Smith, M.; Rotgans, B.A.; Wyeth, R.C.; et al. The Crown-of-Thorns Secretome: Towards a Control Technology; Australian Government Department of the Environment: Townsville, Australia, 2016; pp. 1–312. [Google Scholar]
- Franz, D.R. Opisthobranch culture. In Culture of Marine Invertebrates; Smith, W., Chanley, M., Eds.; Plenum Press: New York, NY, USA, 1971; pp. 245–256. [Google Scholar]
- Bertram, D.; Strathmann, R. Effects of maternal and larval nutrition on growth and form of planktotrophic larvae. Ecology 1998, 79, 315–327. [Google Scholar] [CrossRef]
- Seon, S.C.; Kim, J.M.; Jung, C.-G.; Yun, S.J.; Kang, K.H. Influence of water temperature on spawning induction, larval and spat rearing of trumpet shell, Charonia lampas sauliae. Korean J. Malacol. 2005, 21, 107–111. [Google Scholar]
- Kang, K.; Kim, M.; Hong, H.; Cha, G.; Sui, Z. Feeding broodstocks different starfish diets affect growth and survival of larvae of trumpet shell (Charonia lampas sauliae Reeve 1844). J. Ocean. Univ. China 2016, 15, 861–865. [Google Scholar] [CrossRef]
- Kingsford, M.J.; Leis, J.M.; Shanks, A.; Lindeman, K.C.; Morgan, S.G.; Pineda, J. Sensory environments, larval abilities and local self-recruitment. Bull. Mar. Sci. 2002, 70, 309–340. [Google Scholar]
- Hay, M.E. Marine chemical ecology: Chemical signals and cues structure marine populations, communities, and ecosystems. Annu. Rev. Mar. Sci. 2009, 1, 193–212. [Google Scholar] [CrossRef]
- Hadfield, M.G. Biofilms and marine invertebrate larvae: What bacteria produce that larvae use to choose settlement sites. Annual Review of Marine Science 2011, 3, 453–470. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, B.; Kohn, A.B.; Nahir, B.; McFadden, C.; Moroz, L. Complete DNA sequence of the mitochondrial genome of the sea-slug, Aplysia californica: Conservation of the gene order in Euthyneura. Mol. Phylogenet. Evol. 2006, 38, 459–469. [Google Scholar] [CrossRef]
- Spade, D.J.; Griffitt, R.J.; Liu, L.; Brown-Peterson, N.J.; Kroll, K.J.; Feswick, A.; Glazer, R.A.; Barber, D.S.; Denslow, N.D. Queen conch (Strombus gigas) testis regresses during the reproductive season at nearshore sites in the Florida Keys. PLoS ONE 2010, 5, e12737. [Google Scholar] [CrossRef] [PubMed]
- Márquez, E.; Landínez-García, R.M.; Ospina-Guerrero, S.P.; Segura, J.A.; Prada, M.; Castro, E.; Correa, J.L.; Borda, C. Genetic analysis of Queen Conch Strombus gigas from the Southwest Caribbean. In Proceedings of the 65th Gulf and Caribbean Fisheries Institute, Santa Marta, Colombia, 5–9 November 2012; pp. 410–416. [Google Scholar]
- Simakov, O.; Marletaz, F.; Cho, S.-J.; Edsinger-Gonzales, E.; Havlak, P.; Hellsten, U.; Kuo, D.-H.; Larsson, T.; Lv, J.; Arendt, D.; et al. Insights into bilaterian evolution from three spiralian genomes. Nature 2013, 493, 526–531. [Google Scholar] [CrossRef]
- Dominguez-Perez, D.; Lippolis, J.; Dennis, M.; Miller, B.; Tiley, K.; Vasconcelos, V.; de Almeida, A.M.; Campos, A. The queen conch (Lobatus gigas) proteome: A valuable tool for biological studies in marine gastropods. Protein J. 2019, 38, 628–639. [Google Scholar] [CrossRef]
- Tollrian, R.; Harvell, C.D. The Ecology and Evolution of Inducible Defenses; Princeton University Press: Princeton, NJ, USA, 1999. [Google Scholar]
- Khater, M.; Murariu, D.; Gras, R. Predation risk tradeoffs in prey: Effects on energy and behaviour. Theor. Ecol. 2016, 9, 251–268. [Google Scholar] [CrossRef]
- Motti, C.A.; Bose, U.; Roberts, R.E.; McDougall, C.; Smith, M.K.; Hall, M.R.; Cummins, S.F. Chemical ecology of chemosensation in Asteroidea: Insights towards management strategies of pest species. J. Chem. Ecol. 2018, 44, 147–177. [Google Scholar] [CrossRef]
- Moran, P.J. The Acanthaster phenomenon. Annu. Rev. Oceanogr. Mar. Biol. 1986, 24, 379–480. [Google Scholar]
- Birkeland, C.; Lucas, J. Acanthaster planci: Major Management Problems of Coral Reefs; CRC Press: Boca Raton, FL, USA, 1990. [Google Scholar]
- Glynn, P.W. An amphinomid worm predator of the crown-of-thorns sea star and general predation on asteroids in eastern and western Pacific coral reefs. Bull. Mar. Sci. 1984, 35, 54–71. [Google Scholar]
- Bos, A.R.; Gumanao, G.S.; Salac, F.N. A newly discovered predator of the crown-of-thorns starfish. Coral Reefs 2008, 27, 581. [Google Scholar] [CrossRef]
- Bos, A.R.; Mueller, B.; Gumanao, G.S. Feeding biology and symbiotic relationships of the corallimorpharian Paracorynactis hoplites (Anthozoa: Hexacorallia). Raffles Bull. Zool. 2011, 59, 245–250. [Google Scholar]
- Glynn, P.W. Acanthaster population regulation by a shrimp and a worm. In Proceedings 4th International Coral Reef Symposium, 18–22 May 1981; Marine Science Center, University of the Philippines: Manila, Philippines, 1982; pp. 607–612. [Google Scholar]
- Pearson, R.G.; Endean, R. A preliminary study of the coral predator Acanthaster planci on the Great Barrier Reef. Qld Fish. Branch Fish. Notes 1969, 3, 27–55. [Google Scholar]
- Endean, R. Population explosions of Acanthaster planci and associated destruction of hermatypic corals in the Indo-west Pacific region. In Biology and Geology of Coral Reefs; Jones, O., Endean, R., Eds.; Academic Press: New York, NY, USA, 1973; pp. 389–438. [Google Scholar]
- Ormond, R.F.G.; Campbell, A.C.; Head, S.H.; Moore, R.J.; Rainbow, P.R.; Saunders, A.P. Formation and breakdown of aggregations of the Crown-of-Thorns starfish, Acanthaster planci (L.). Nature 1973, 246, 167–168. [Google Scholar] [CrossRef]
- Alcala, A.C. The sponge crab Dromidiopsis dormia as a predator of the crown of thorns starfish. Siliman J. 1974, 21, 174. [Google Scholar]
- Brown, T.; Willey, K. Crown of Thorns: The Death of the Great Barrier Reef? Angus and Robertson: Sydney, Australia, 1972. [Google Scholar]
- Glynn, P.W. Interactions between Acanthaster and Hymenocera in the field and laboratory. In Proceedings of the 3rd International Coral Reef Symposium, Miami, FL, USA, May 1977; pp. 209–215. [Google Scholar]
- Owens, D. Acanthaster planci starfish in Fiji: Survey of incidence and biological studies. Fiji Agric. J. 1971, 33, 15–23. [Google Scholar]
- Wilson, B.R.; Marsh, L.M. Seasonal behaviour of a ‘normal’ population of Acanthaster in Western Australia. In Proceedings of the Crown-of-Thorns Starfish Seminar, 6th September 1974; Australian Government Publishing Service: Brisbane, Australia, 1975; pp. 167–179. [Google Scholar]
- Endean, R. Destruction and recovery of coral reef communities. In Biology and Geology of Coral Reefs; Jones, O., Endean, R., Eds.; Academic Press: New York, NY, USA, 1976; pp. 215–254. [Google Scholar]
- Ormond, R.; Bradbury, R.; Bainbridge, S.; Fabricius, K.; Keesing, J.; de Vantier, L.; Medlay, A.; Steven, A. Test of a model of regulation of crown-of-thorns starfish by fish predators. In Acanthaster and the Coral Reef: A Theoretical Perspective; Bradbury, R., Ed.; Springer: Heidelberg, Germany, 1990; pp. 189–207. [Google Scholar]
- Keesing, J.K.; Halford, A.R. Field measurement of survival rates of juvenile Acanthaster planci: Techniques and preliminary results. Mar. Ecol. Prog. Ser. 1992, 85, 107–114. [Google Scholar] [CrossRef]
- Sweatman, H.P.A. A field study of fish predation on juvenile crown-of-thorns starfish. Coral Reefs 1995, 14, 47–53. [Google Scholar] [CrossRef]
- Kroon, F.; (Australian Institute of Marine Science, Townsville, Australia). Personal Communications, Adult pufferfish (Arothron hispidus) were observed attacking and feeding on juvenile CoTS in aquaria studies. 2017.
- Godoy, D. (Pacific Marine Group, Townsville, Australia). Personal Communications, Observations of Charonia tritonis feeding on Crown-of-Thorns starfish and Linckia sp. on the Great Barrier Reef. 2020.
- Hughes, R.N. A Functional Biology of Marine Gastropods; Croom Helm: London, UK, 1986. [Google Scholar]
- Littlewood, D.T.J. Pests and predators of cultivated mangrove oysters. In Oyster Culture in the Caribbean; Newkirk, G.F., Field, B.A., Eds.; International Mollusc Culture Research Centre: Halifax, NS, Canada, 1991; pp. 109–146. [Google Scholar]
- Endean, R. Aspects of molluscan pharmacology. In Chemical Zoology. Mollusca; Flor-Kin, M., Scheer, N.Y., Eds.; Academic Press: New York, NY, USA, 1972; pp. 421–466. [Google Scholar]
- Hall, M. Playing on Fears: Exploring the Use of the Pacific Triton for Mitigating Crown-of-Thorns Starfish Outbreaks. Australian Institute of Marine Science (AIMS). Available online: http://www.aims.gov.au/docs/media/featured-content.html/-/asset_publisher/Ydk18I5jDwF7/content/playing-on-fears-exploring-the-use-of-the-pacific-triton-for-mitigating-crown-of-thorns-starfish-outbreaks (accessed on 6 June 2016).
- Mackie, A.M.; Singh, H.T.; Fletcher, T.C. Studies on the cytolytic effects of seastar (Marthasterias glacialis) saponins and synthetic surfactants in the plaice Pleuronectes platessa. Mar. Biol. 1975, 29, 307–314. [Google Scholar] [CrossRef]
- Andersson, L.; Bohlin, L.; Iorizzi, M.; Riccio, R.; Minale, L.; Moreno-López, W. Biological activity of saponins and saponin-like compounds from starfish and brittle-stars. Toxicon 1989, 27, 179–188. [Google Scholar] [CrossRef]
- Francis, G.; Kerem, Z.; Makkar, H.P.; Becker, K. The biological action of saponins in animal systems: A review. Br. J. Nutr. 2002, 88, 587–605. [Google Scholar] [CrossRef]
- Sharmin, F.; Koyama, T.; Koyama, H.; Ishizaki, S. Cholesterol-binding ability of saponin from Japanese starfish. J. Food Sci. Technol. 2020, 58, 3056–3064. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, I.; Kobayashi, M. Saponin sapogenol XXVI. Steroidal saponins from the starfish Acanthaster planci L. (crown of thorns). Structue of major saponin thornasterosdie A. Chem. Pharm. Bull. 1978, 26, 1864–1873. [Google Scholar] [CrossRef][Green Version]
- Lucas, J.S.; Hart, R.J.; Howden, M.E.; Salathe, R. Saponins in eggs and larvae of Acanthaster planci (L.) (Asteroidea) as chemical defences against planktivorous fish. J. Exp. Mar. Biol. Ecol. 1979, 40, 155–165. [Google Scholar] [CrossRef]
- Montgomery, J.; Carlton, G.; Bodznick, D. Error-driven motor learning in fish. Biol. Bull. 2002, 203, 238–239. [Google Scholar] [CrossRef]
- Prokofeva, N.G.; Chaikina, E.L.; Kicha, A.A.; Ivanchina, N.V. Biological activities of steroid glycosides from starfish. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2003, 134, 695–701. [Google Scholar] [CrossRef]
- Podolak, I.; Galanty, A.; Sobolewska, D. Saponins as cytotoxic agents: A review. Phytochem. Rev. 2010, 9, 425–474. [Google Scholar] [CrossRef]
- Thakur, M.; Melzig, M.F.; Fuchs, H.; Weng, A. Chemistry and pharmacology of saponins: Special focus on cytotoxic properties. Bot. Targets Ther. 2011, 1, 19–29. [Google Scholar]
- Van Dyck, S.; Caulier, G.; Todesco, M.; Gerbaux, P.; Fournier, I.; Wisztorski, M.; Flammang, P. The triterpene glycosides of Holothuria forskali: Usefulness and efficiency as a chemical defense mechanism against predatory fish. J. Exp. Biol. 2011, 214, 1347–1356. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Yasumoto, T. Confirmation of saponin as a toxic principle of starfish. Bull. Jpn. Soc. Sci. Fish. 1960, 26, 1132–1138. [Google Scholar] [CrossRef]
- Mackie, A.M.; Singh, H.T.; Owen, J.M. Studies on the distribution, biosynthesis and function of steroidal saponins in echinoderms. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1977, 56, 9–14. [Google Scholar] [CrossRef]
- Komori, T. Toxins from the starfish Acanthaster planci and Asterina pectinifera. Toxicon 1997, 35, 1537–1548. [Google Scholar] [CrossRef]
- Narita, H.; Nara, M.; Baba, K.; Ohgami, H.; Ai, T.K.; Noguchi, T.; Hashimoto, K. Effect of feeding a trumpet shell, Charonia sauliae, with toxic starfish. Food Hyg. Saf. Sci. 1984, 25, 251–255. [Google Scholar] [CrossRef]
- Fukuda, M.; Egami, F. b-Xylosidase from the liver of Charonia lampas II. b-Xylosidase and b-glucosidase. J. Biochem. 1969, 66, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Butters, T.D.; Scudder, P.; Rotsaert, J.; Petursson, S.; Fleet, G.W.J.; Willenbrock, F.W.; Jacob, G.S. Purification to homogeneity of Charonia lampas a-fucosidase by using sequential ligand-affinity chromatography. Biochem. J. 1991, 279, 189–195. [Google Scholar] [CrossRef]
- Teshima, S.; Kanazawa, A.; Hyodo, S.; Ando, T. Sterols of the triton, Charonia tritonis. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1979, 64, 225–228. [Google Scholar] [CrossRef]
- Broom, M.J. A preliminary investigation into prey species preference by the tropical gastropods Natica maculosa and Thais carnifera. J. Molluscan Stud. 1983, 49, 43–52. [Google Scholar] [CrossRef]
- Reichelt, R.E.; Kohn, A.J. Feeding and distribution of predatory gastropods on some Great Barrier Reef platforms. In Proceedings of the 5th International Coral Reef Congress, Tahiti, French Polynesia, 27 May–1 June 1985; pp. 191–196. [Google Scholar]
- Jory, D.E.; Carriker, M.R.; Iversen, E.S. Preventing predation in molluscan aquaculture: An overview. J. World Maric. Soc. 1984, 15, 421–432. [Google Scholar] [CrossRef]
- Gutiérrez, R.M.; Gallardo, C.S. Prey attack, food preference and growth in juveniles of the edible muricid snail, Chorus giganteus. Aquaculture 1999, 174, 69–79. [Google Scholar] [CrossRef]
- Murdoch, W.W. Switching in general predators: Experiments on predator specificity and stability of prey populations. Ecol. Monogr. 1969, 39, 335–354. [Google Scholar] [CrossRef]
- Russo, G.F.; Fasulo, G.; Toscano, A.; Toscano, F. On the presence of triton species (Charonia spp.) (Mollusca, Gastropoda) in the Mediterranean Sea: Ecological considerations. Boll. Malacol. 1990, 26, 91–104. [Google Scholar]
- Doxa, C.K.; Papadakis, I.; Kentouri, M.; Divanach, R. Feeding preference of the Giant triton (Charonia tritonis variegata) and its contribution to the conservation of the marine environment. In AQUA 2006: Linking Tradition & Technology; World Aquaculture Society: Florence, Italy; Baton Rouge, LA, USA, 2006; p. 263. [Google Scholar]
- Kisch, B.S. Further note on Charonia lampas. J. Conchol. 1952, 23, 266. [Google Scholar]
- Kisch, B.S. Further observations on Charonia lampas. J. Conchol. 1949, 23, 84. [Google Scholar]
- Doxa, C.K.; Kentouri, M.; Divanach, P. Feeding of Charonia sequenzae (Arada & Benoit, 1870) on natural prey and alternative foods. J. Molluscan Stud. 2012, 79, 76–78. [Google Scholar]
- Birkeland, C. The faustian traits of crown-of-thorns starfish. Am. Sci. 1989, 77, 155–163. [Google Scholar]
- Birkeland, C. The influence of echinoderms on coral reef communities. In Echinoderm Studies; Jangoux, M., Lawrence, J., Eds.; Balkema: Rotterdam, The Netherlands, 1989; pp. 1–77. [Google Scholar]
- Trussell, C.C.; Ewanchuk, P.J.; Bertness, M.D. Trait-mediated effects in rocky intertidal food chains: Predator risk cues alter prey feeding rates. Ecology 2003, 84, 629–640. [Google Scholar] [CrossRef]
- Turner, A.M.; Mittelbach, G.G. Predator avoidance and community structure: Interactions among piscivores, planktivores and plankton. Ecology 1990, 71, 2241–2254. [Google Scholar] [CrossRef]
- Legault, C.; Himmelman, J.H. Relation between escape behaviour of benthic invertebrates and the risk of predation. J. Exp. Mar. Biol. Ecol. 1993, 170, 55–74. [Google Scholar] [CrossRef]
- Soluk, D.A. Multiple predator effects: Predicting combined functional response of stream fish and invertebrate predators. Ecology 1993, 74, 219–225. [Google Scholar] [CrossRef]
- Dodson, S.I.; Crowl, T.A.; Peckarsky, B.L.; Kats, L.B.; Covich, A.P.; Culp, J.M. Non-visual communication in freshwater benthos: An overview. J. N. Am. Benthol. Soc. 1994, 13, 268–282. [Google Scholar] [CrossRef]
- Swisher, B.J.; Soluk, D.A.; Wahl, D.H. Non-additive predation in littoral habitats: Influences of habitat complexity. Oikos 1998, 81, 30–37. [Google Scholar] [CrossRef]
- Bruno, J.F.; Bertness, M.D. Habitat modification and facilitation in benthic marine communities. In Marine Community Ecology; Bertness, M.D., Gaines, S.D., Hay, M.E., Eds.; Sinauer Associates: Sunderland, MA, USA, 2001. [Google Scholar]
- Werner, E.; Peacor, S. A review of trait-mediated indirect interactions in ecological communities. Ecology 2003, 5, 1083–1100. [Google Scholar] [CrossRef]
- Abrams, P.A. Habitat choice in predator-prey systems: Spatial instability due to interacting adaptive movements. Am. Nat. 2007, 169, 581–594. [Google Scholar] [CrossRef]
- Ferrer, R.P.; Zimmer, R. Community ecology and the evolution of molecules of keystone significance. Biol. Bull. 2012, 223, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, R.P.; Zimmer, R.K. Molecules of keystone significance: Crucial agents in ecology and resource management. Bioscience 2013, 63, 428–438. [Google Scholar] [CrossRef]
- Murray, J.A.; Wyeth, R.C. Introduction to the symposium-chemicals that organize ecology: Towards a greater integration of chemoreception, neuroscience organismal biology and chemical ecology. Integr. Comput. Biol. 2015, 55, 444–446. [Google Scholar] [CrossRef]
- Tewfik, A. Losing the Shell Game: Consequences of Seascapes without Predatory Gastropods. In Proceedings of the 67th Gulf and Caribbean Fisheries Institute, Christ Church, Barbados, 3–7 November 2014; pp. 331–338. [Google Scholar]
- Brown, K.M.; Alexander, J.E. Group foraging in a marine gastropod predator: Benefits and costs to individuals. Mar. Ecol. Prog. Ser. 1994, 112, 97–105. [Google Scholar] [CrossRef]
- Abrams, P.A. Implications of dynamically variable traits for identifying, classifying and measuring direct and indirect effects in ecological communities. Am. Nat. 1995, 146, 112–134. [Google Scholar] [CrossRef]
- Schmitz, O.J.; Berckerman, A.; O’Brien, K.M. Behaviourally mediated trophic cascades: Effects of predation risk on food web interactions. Ecology 1997, 78, 1388–1399. [Google Scholar] [CrossRef]
- Pinnegar, J.K.; Polunin, N.V.C.; Francour, P.; Badalamenti, F.; Chemello, R.; Harmelin-Vivien, M.L.; Hereu, B.; Milazzo, M.; Zabala, M.; D’anna, G.; et al. Trophic cascades in benthic ecosystems: Lessons for fisheries and protected-marine areas. Environ. Conserv. 2000, 27, 179–200. [Google Scholar] [CrossRef]
- Bernot, R.; Turner, A. Predatory identity and trait-mediated indirect effects in a littoral food web. Oecologia Aquat. 2001, 129, 139–146. [Google Scholar] [CrossRef]
- Dill, L.M.; Heithaus, M.R.; Walter, C.J.s. Behaviourally mediated indirect interactions in marine communities and their conservation implications. Ecology 2003, 84, 1151–1157. [Google Scholar] [CrossRef]
- Witman, J.D.; Genovese, S.J.; Bruno, J.F.; McLaughlin, J.W.; Pavlin, B.I. Massive prey recruitment and the control of rocky subtidal communities on large spatial scales. Ecol. Monogr. 2003, 73, 441–462. [Google Scholar] [CrossRef][Green Version]
- Bolnick, D.I.; Preisser, E.L. Resource competition modifies the strength of trait-mediated predator-prey interactions: A meta-analaysis. Ecology 2005, 86, 2771–2779. [Google Scholar] [CrossRef]
- Toscano, B.J.; Griffen, B.D. Trait-mediated functional responses: Predator behavioural type mediates prey consumption. J. Anim. Ecol. 2014, 83, 1469–1477. [Google Scholar] [CrossRef]
- Hall, A.E.; Kingsford, M.J. Variation in the population demographics of Scolopsis bilineatus in response to predators. Coral Reefs 2016, 35, 1173–1185. [Google Scholar] [CrossRef]
- Morgan, S.G.; Gavem, S.A.; Lipus, A.C.; Grabiel, M.; Miner, B.G. Trait-mediated indirect interactions among residents of rocky shore tidepools. Mar. Ecol. Prog. Ser. 2016, 552, 31–46. [Google Scholar] [CrossRef][Green Version]
- Ng, G.; Gaylord, B. The legacy of predators: Persistence of trait-mediated indirect effects in an intertidal food chain. J. Exp. Mar. Biol. Ecol. 2020, 530–531, 151416. [Google Scholar] [CrossRef]
- Luttbeg, B.; Kerby, J.L. Are scared prey as good as dead? Trends Ecol. Evol. 2005, 20, 416–418. [Google Scholar] [CrossRef]
- Peckarsky, B.L.; Abrams, P.A.; Bolnick, D.I.; Dill, L.M.; Grabowski, J.H.; Luttbeg, B.; Orrock, J.L.; Peacor, S.D.; Preisser, E.L.; Schmitz, O.J.; et al. Revisiting the classics: Considering nonconsumptives effects in textbook examples of predator-prey interactions. Ecology 2008, 89, 2416–2425. [Google Scholar] [CrossRef] [PubMed]
- Peckarsky, B.L.; Kerans, B.L.; McIntosh, A.R.; Taylor, B.W. Predator effects on prey population dynamics in open systems. Oecologia 2008, 156, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Orrock, J.L.; Sih, A.; Dill, L.M.; Grabowski, J.H.; Peacor, S.D.; Peckarsky, B.L.; Preisser, E.L.; Vonesh, J.R.; Werner, E.E. Predator effects in predator-free space: The remote effects of predators on prey. Open Ecol. J. 2010, 3, 22–30. [Google Scholar] [CrossRef]
- Paterson, R.A.; Pritchard, D.W.; Cick, J.T.A.; Alexander, M.E.; Hatcher, M.J.; Dunn, A.M. Predator cue studies reveal strong trait-mediated effects in communities despite variation in experimental designs. Anim. Behav. 2013, 86, 1301–1313. [Google Scholar] [CrossRef]
- Schmitz, O.J.; Krivan, K.; Ovadia, O. Trophic cascades: The primacy of trait-mediated indirect interactions. Ecol. Lett. 2004, 7, 153–163. [Google Scholar] [CrossRef]
- Turner, A.M.; Bernot, R.J.; Boes, C.M. Chemical cues modify speciesinteractions: The ecological consequences of predator avoidance by freshwater snails. Oikos 2000, 88, 148–158. [Google Scholar] [CrossRef]
- Preisser, E.L.; Bolnick, D.I.; Benard, M.F. Scared to death? The effects of intimidation and consumption in predator-prey interactions. Ecology 2005, 86, 501–509. [Google Scholar] [CrossRef]
- Dill, L.M. Animal decision making and its ecological consequences: The future of aquatic ecology and behaviour. Can. J. Zool. 1987, 65, 803–811. [Google Scholar] [CrossRef]
- Fletcher, W.J. Interactions among subtidal Australian sea urchins, gastropods and algae: Effects of experimental removals. Ecol. Monogr. 1987, 57, 89–109. [Google Scholar] [CrossRef]
- Shurin, J.B.; Borer, E.T.; Seabloom, E.W.; Anderson, K.; Blanchette, C.A.; Broitman, B.; Cooper, S.D.; Halpern, B.S. A cross-ecosystem comparison of the strength of trophic cascades. Ecol. Lett. 2002, 5, 785–791. [Google Scholar] [CrossRef]
- Schultz, J.A.; Cloutier, R.N.; Côte, I. Evidence for a trophic cascade on rock reefs following sea star mass mortality in British Columbia. PeerJ 2016, 4, e1980. [Google Scholar] [CrossRef] [PubMed]
- Freeman, A. Size-dependent trait-mediated indirect interactions among sea urchin herbivores. Behav. Ecol. 2005, 17, 182–187. [Google Scholar] [CrossRef]
- Preisser, E.L.; Bolnick, D.I. The many faces of fear: Comparing the pathways and impacts of nonconsumptive predator effects on prey populations. PLoS ONE 2008, 3, e2465. [Google Scholar] [CrossRef] [PubMed]
- Deletre, E.; Schatz, B.; Bourguet, D.; Chandre, F.; Williams, L.; Ratnadass, A.; Martin, T. Prospects for repellent in pest control: Current developments and future challenges. Chemoecology 2016, 26, 127–142. [Google Scholar] [CrossRef]
- Atalah, J.; Hopkins, G.A.; Fletcher, L.M.; Castinel, A.; Forrest, B.M. Concepts for biocontrol in marine environments: Is there a way forward? Manag. Biol. Invasions 2015, 6, 1–12. [Google Scholar] [CrossRef]
- Lima, S.L.; Bednekoff, P.A. Temporal variation in danger drives antipredator behaviour: The predation risk allocation hypothesis. Am. Nat. 1999, 153, 649–659. [Google Scholar] [CrossRef]
- Lima, S.L. Putting predators back into behavioral predator-prey interactions. Trends Ecol. Evol. 2002, 17, 70–75. [Google Scholar] [CrossRef]
- Fryxell, J.M.; Mosser, A.; Sinclair, A.R.; Packer, C. Group formation stabilizes predator-prey dynamics. Nature 2007, 449, 1041–1043. [Google Scholar] [CrossRef]
- Ferrari, M.C.; Sih, A.; Chivers, D.P. The paradox of risk allocation: A review and prospectus. Anim. Behav. 2009, 78, 579–585. [Google Scholar] [CrossRef]
- Khater, M.; Murariu, D.; Gras, R. Contemporary evolution and genetic change of prey as a response to predator removal. Ecol. Inform. 2014, 22, 13–22. [Google Scholar] [CrossRef]
- Zimmer, R.K.; Butman, C.A. Chemical signaling processes in the marine environment. Biol. Bull. 2000, 198, 168–187. [Google Scholar] [CrossRef]
- Buskirk, J.V.; Krügel, A.; Kunz, J.; Miss, F.; Stamm, A. The rate of degradation of chemical cues indicating predation risk: An experiment and review. Ethology 2014, 120, 942–949. [Google Scholar] [CrossRef]
- Gerlach, G.; Atema, J.; Kingsford, M.; Black, K.; Miller-Sims, V. Smelling home can prevent dispersal of reef fish larvae. Proc. Natl. Acad. Sci. USA 2007, 104, 858–863. [Google Scholar] [CrossRef]
- Atema, J.; Brönmark, C.; Hansson, L.A. Aquatic odor dispersal fields: Opportunities and limits of detection, communication and navigation. In Chemical Ecology in Aquatic Systems; Brönmark, C., Hansson, L.A., Eds.; Oxford University Press: Oxford, UK, 2012; pp. 1–18. [Google Scholar]
- Kats, L.B.; Dill, L.M. The scent of death: Chemosensory assessment of predation risk by prey animals. Ecoscience 1998, 5, 361–394. [Google Scholar] [CrossRef]
- Mirza, R.S.; Chiver, D.P. Learned recognition of heterospecific alarm signals: The importance of a mixed predator diet. Ethology 2001, 107, 1007–1018. [Google Scholar] [CrossRef]
- McCarthy, T.M.; Dickey, B.F. Mediated effects of injured prey on behavior of both prey and predators. Behavior 2002, 139, 585–602. [Google Scholar]
- Gras, R.; Devaurs, D.; Wozniak, A.; Aspinall, A. An individual based evolving predator-prey ecosystem simulation using fuzzy cognitive map as behavior model. Artif. Life 2009, 15, 423–463. [Google Scholar] [CrossRef] [PubMed]
- Wicher, D. Functional and evolutionary aspects of chemoreceptors. Front. Cell. Neurosci. 2012, 6, 48. [Google Scholar] [CrossRef]
- Beirner, B.P. Biological control and its potential. World Rev. Pest Control 1967, 6, 7–20. [Google Scholar]
- Lafferty, K.; Kuris, A. Biological control of marine pests. Ecology 1996, 77, 1989–2000. [Google Scholar] [CrossRef]
- Snyder, N.F.R.; Derrickson, S.R.; Beissinger, S.R.; Wiley, J.W.; Smith, T.B.; Toone, W.D.; Miller, B. Limitations of captive breeding in endangered species recovery. Conserv. Biol. Pract. 1996, 10, 338–348. [Google Scholar] [CrossRef]
- Kroon, F.J.; Barneche, D.R.; Emslie, M.J. Fish predators control outbreaks of Crown-of-Thorns Starfish. Nat. Commun. 2021, 12, 6986. [Google Scholar] [CrossRef]
- Zhou, Y.; Pan, J. A preliminary study on biological control of Cymatium, a predator of Pinctada martensis. J. Trop. Oceanol. 2001, 3, 20–22. [Google Scholar]
- Malavé, C.; Freites, L.; Lodeiros, C.; Mendoza, J.; Troccoli, L.; Dale, A.W. Annual recruitment, predation rates and biocontrol of Linatella caudata (Mollusca: Gastropoda) in suspended enclosure culture of the pearl oyster Pinctada imbricata. Aquaculture 2012, 354–355, 75–83. [Google Scholar] [CrossRef]
- Delgado, G.A.; Sharp, W.C. Capitalizing on an ecological process to aid coral reef ecosystem restoration: Can gastropod trophodynamics enhance coral survival? Coral Reefs 2020, 39, 319–330. [Google Scholar] [CrossRef]
- Holland, B.S.; Chock, T.; Lee, A.; Sugiura, S. Tracking behavior in the snail Euglandina rosea: First evidence of preference for endemic vs. Biocontrol target pest species in Hawaii. Am. Malacol. Bull. 2012, 30, 153–157. [Google Scholar] [CrossRef]
- Cowie, R.H. Can snails ever be effective and safe biocontrol agents? Int. J. Pest Manag. 2001, 47, 23–40. [Google Scholar] [CrossRef]
- Cunha, R.L.; Grande, C.; Zardoya, R. Neogastropod phylogenetic relationships based on entire mitochondrial genomes. BMC Evol. Biol. 2009, 9, 210. [Google Scholar] [CrossRef]
- Xiong, G.; Wang, X.Q.; Kang, L.; Ma, X.; Zhu, D.L.; Wang, L.M.; Wu, Q.S.; Zeng, Z.N. The complete mitochondrial genome of the Babylonia areolata. Mitochondrial DNA Part A DNA Mapp. Seq. Anal. 2016, 27, 645–646. [Google Scholar]
- Chen, P.-W.; Hsiao, S.-T.; Chen, K.-S.; Tseng, C.-T.; Wu, W.-L.; Hwang, D.-F. Mitochondrial DNA sequence of Conus textile (Neogastropoda: Conidae). Mitochondrial DNA Part B Resour. 2016, 1, 508–509. [Google Scholar] [CrossRef]
- He, Z.P.; Dai, X.B.; Zhang, S.; Zhi, T.T.; Lun, Z.R.; Wu, Z.D.; Yang, T.B. Complete mitochondrial genome of the giant African snail, Achatina fulica (Mollusca: Achatinidae): A novel location of putative control regions (CR) in the mitogenome within Pulmonate species. Mitochondrial DNA Part A DNA Mapp. Seq. Anal. 2016, 27, 1084–1085. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Qiu, J.W. Complete mitochondrial genome of the giant ramshorn snail Marisa cornuarietis (Gastropoda: Ampullariidae). Mitochondrial DNA Part A DNA Mapp. Seq. Anal. 2016, 27, 1734–1735. [Google Scholar]
- Yang, Q.; Liu, S.; Song, F.; Li, H.; Liu, J.; Liu, G.; Yu, X. The mitochondrial genome of Pomacea maculata (Gastropoda: Ampullariidae). Mitochondrial DNA Part A DNA Mapp. Seq. Anal. 2016, 27, 2895–2896. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Chen, Y.; Zhu, S.; Xu, H.; Liu, Y.; Chen, L. The complete mitochondrial genome of Pomacea canaliculata (Gastropoda: Ampullariidae). Mitochondrial DNA Part A DNA Mapp. Seq. Anal. 2016, 27, 884–885. [Google Scholar]
- Liu, C.; Zhang, Y.; Ren, Y.; Wang, H.; Li, S.; Jiang, F.; Yin, L.; Qiao, X.; Zhang, G.; Qian, W. The genome of the golden apple snail Pomacea canaliculata provides insight into stress tolerance and invasive adaptation. GigaScience 2018, 7, 1–13. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, J.E.; Xia, J.; Yang, J.; Guo, J.; Deng, Z.; Luo, M. Comparative characterization of the complete mitochondrial genomes of the three apple snails (Gastropoda: Ampullariidae) and the phylogenetic analyses. Int. J. Mol. Sci. 2018, 19, 3646. [Google Scholar] [CrossRef] [PubMed]
- Sharp, W.; Delgado, G. Predator-prey interactions between the corallivorous snail Coralliophila abbreviata and the carnivorous deltoid rock snail Thais deltoidea. Biol. Bull. 2015, 229, 129–133. [Google Scholar] [CrossRef]
- Barratt, B.I.P.; Berndt, L.A.; Dodd, S.L.; Ferguson, C.M.; Hill, R.L.; Kean, J.M.; Teulon, D.A.J.; Withers, T.M. Biocontrol Information Resource for EPA Applicants. Available online: http://www.b3nz.org/birea/ (accessed on 12 September 2022).
- Symondson, W.O.C.; Sunderland, K.D.; Greenstone, M.H. Can generalist predators be effective biocontrol agents? Annu. Rev. Entomol. 2002, 47, 561–594. [Google Scholar] [CrossRef]
- Brodeur, J. Host specificity in biological control: Insights from opportunistic pathogens. Evol. Appl. 2012, 5, 470–480. [Google Scholar] [CrossRef]
- Hellmann, J.J.; Byers, J.E.; Bierwagen, B.G.; Dukes, J.S. Five potential consequences of climate change for invasive species. Conserv. Biol. 2008, 22, 534–543. [Google Scholar] [CrossRef]
- Cheah, C.A.S.-J.; McClure, M.S. Seasonal synchrony of life cycles between the exotic predator, Pseudoscymnus tsugae (Coleoptera: Coccinellidae) and its prey, the hemlock woolly adelgid Adelges tsugae (Homoptera:Adelgidae). Agric. For. Entomol. 2000, 2, 241–251. [Google Scholar] [CrossRef]
- Webster, D.R.; Weissburg, M.J. The Hydrodynamics of Chemical Cues Among Aquatic Organisms. Annu. Rev. Fluid Mech. 2009, 41, 73–90. [Google Scholar] [CrossRef]
- Motti, C.; Vasile, R.; Robson, B.; Høj, L.; Wang, C.; Craik, D.; Degnan, B.; Degnan, S.; Cummins, S.; Martini, A.; et al. Deployment of semiochemical control agents to manage Crown-of-Thorns starfish populations. In A Report to the Australian Government by the COTS Control Innovation Program; Australian Institute of Marine Science: Townsville, Australia, 2022; p. 80. [Google Scholar]
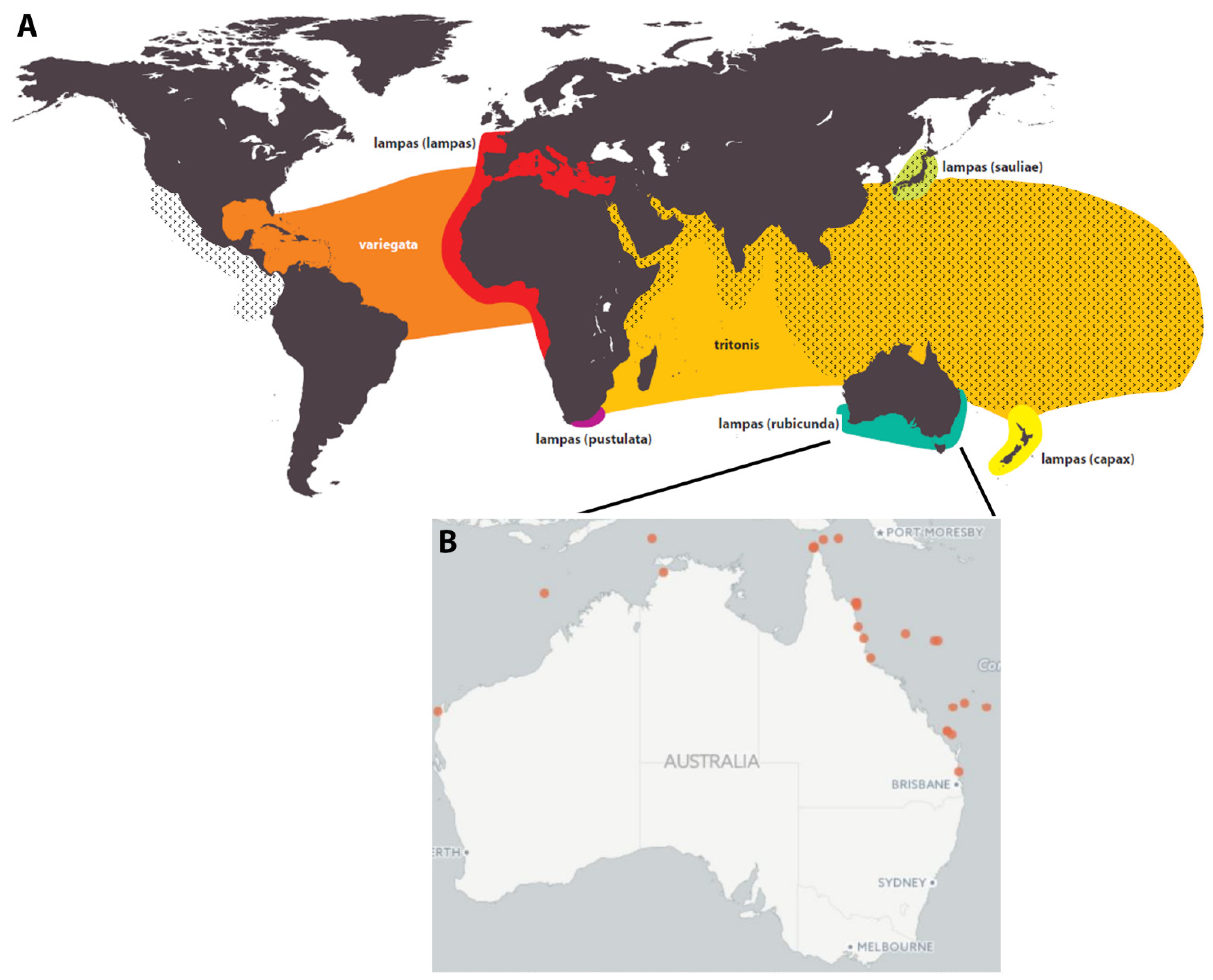
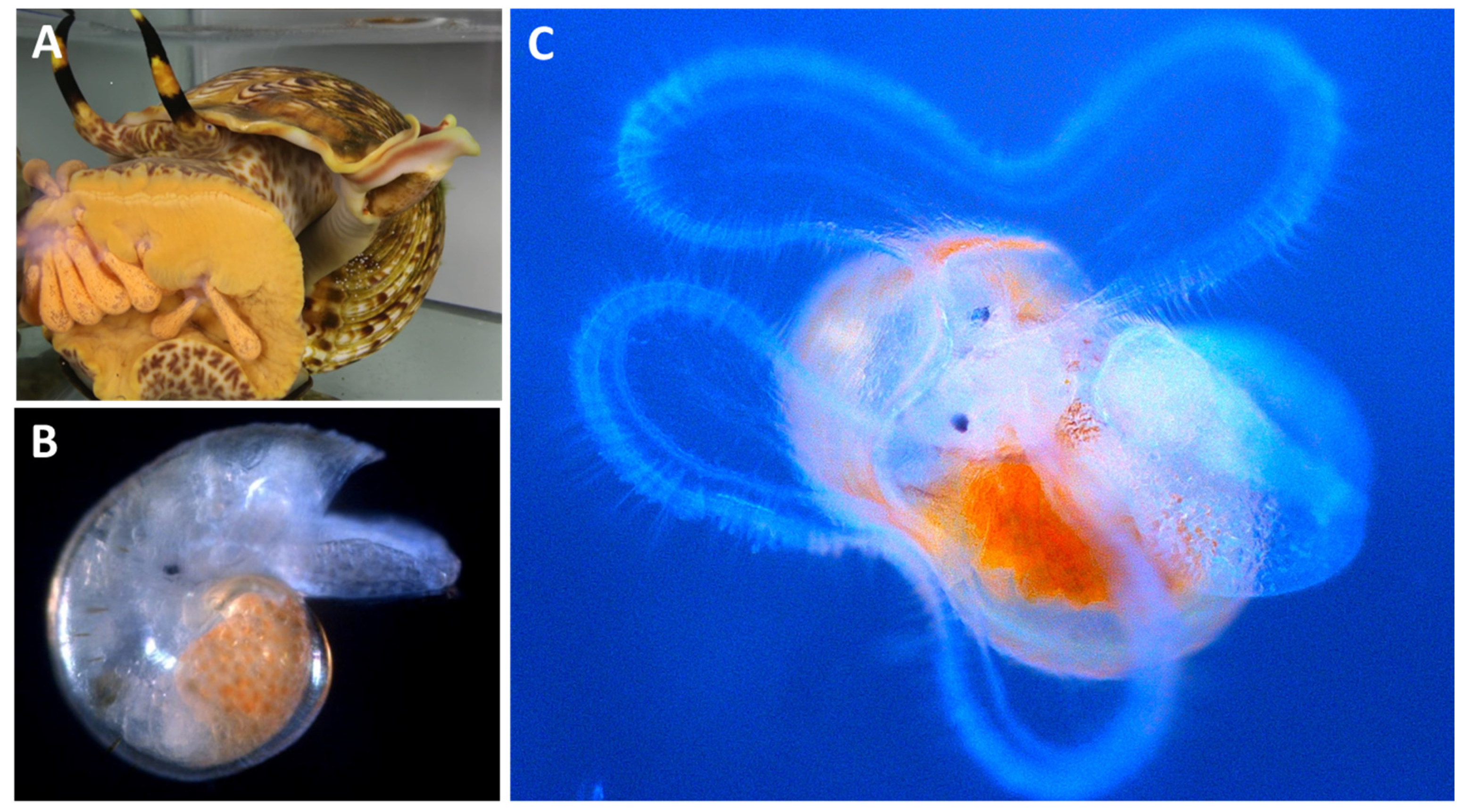
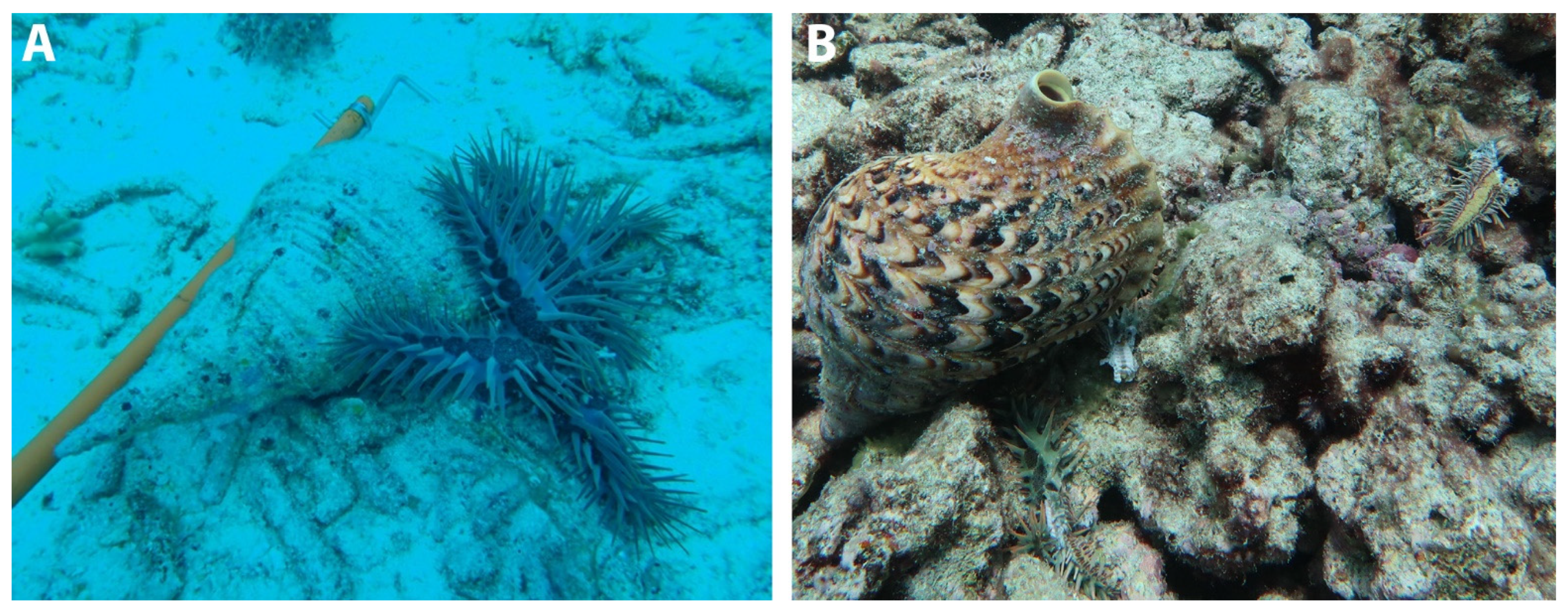
| Scheme | Author(s) | Status | Accepted Name |
|---|---|---|---|
| Charonia lampas | Linnaeus, 1758 | accepted name | Charonia lampas |
| Charonia lampas capax * | Finlay, 1926 | synonym | Charonia lampas |
| Charonia capax euclioides | Finlay, 1926 | synonym | Charonia lampas |
| Charonia crassa | Grateloup, 1847 | synonym | Charonia lampas |
| Charonia euclia | Hedley, 1914 | synonym | Charonia lampas |
| Charonia euclia instructa | Iredale, 1929 | synonym | Charonia lampas |
| Charonia lampas lampas * | Linnaeus, 1758 | synonym | Charonia lampas |
| Charonia lampas macilenta | Kuroda & Habe, 1961 | synonym | Charonia lampas |
| Charonia (lampas) pustulata * | Euthyme, 1889 | synonym | Charonia lampas |
| Charonia lampas sauliae * | Reeve, 1844 | synonym | Charonia lampas |
| Charonia lampas weisbordi | Gibson-Smith, 1976 | synonym | Charonia lampas |
| Charonia lampas ventricose | Grateloup, 1833 | Synonym | Charonia lampas |
| Charonia mirabilis | Parenzan, 1970 | synonym | Charonia lampas |
| Charonia nodifera | Lamarck, 1822 | synonym | Charonia lampas |
| Charonia lampas rubicunda * | Perry, 1811 | synonym | Charonia lampas |
| Charonia powelli | Cotton, 1956 | synonym | Charonia lampas |
| Charonia tritonis | Linnaeus, 1758 | accepted name | Charonia tritonis |
| Charonia variegata | Lamarck, 1816 | accepted name | Charonia variegata |
| Charonia variegata seguenzae * | Aradas & Benoit, 1870 | accepted name | Charonia variegata |
| Charonia tritonis variegata | Lamarck, 1816 | synonym | Charonia variegata |
| Berg (1971) [79] | Nugranad et al. (2000) [80] | Nugranad et al. (2001) [78] | Zhang et al. (2013) [76] | Motti et al. (2019) [25] | |
| Location | Oahu, USA | Phuket, Thailand | Phuket, Thailand | Yongxing Island, China | Townsville, Australia |
| Number of females | 1 | 1 | 5 | 2 | 4 |
| Number of males | At least 1 | - | At least 1 | At least 1 | At least 2 |
| Broodstock diet | Natural diet | CoTS, Culcita novaeguineae, Holothuria atra and Stichopus chloronotus | CoTS, C. novaeguineae, H. atra and S. chloronotus | CoTS and Stichopus horrens | CoTS, Linckia sp. and S. chloronotus |
| Date of reproductive behaviour | Oct | - | Year round | August–September | March-June |
| Temperature of broodstock tank | - | 25.5–33.0 °C | - | - | 23 °C (winter)–30 °C (summer) |
| Copulation until laying (days) | 120–150 | - | 30–60 | 133 | - |
| Duration of spawning (days) | 42–56 | 19 | 60 | 21–35 | - |
| Temperature of egg hatchery | - | - | - | 24 °C | 24.5 °C |
| Total capsules spawned female−1 | 88+ | 50 | 500–1000 | 549–602 | ~400 |
| Egg diameter (µm) | 450–600 | 400–430 | 360–440 | 428 | - |
| Capsule dimensions, H × L (mm) | 25 H × 9 L | 17–39 H × 9–10 L | 17–39 H × 9–10 L | 34 H × 9 L | - |
| Number of eggs per capsule | - | 2000–3400 | 2000–4400 | 2740–3000 | ~2500 |
| Total number of eggs produced | - | ~1.5 × 105 | 1.6 × 106–3.2 × 106 | 1.5 × 106–1.6 × 106 | - |
| Incubation period (days) | 49–56 | - | 35–60 | 55–63 | 52–68 |
| Hatching success of capsules | - | 0% unfertilized | 43–96% | 86–96% | - |
| Veligers per capsule | 1140–1447 | - | 973–1459 | 2046–2110 | - |
| Total veligers produced female−1 | - | - | 0.26 × 106–1.47 × 106 | 1.12 × 106–1.27 × 106 | ~0.8 × 106 |
| Shell length at hatching (µm) | 768–934 | - | 720–925 | 664–700 | 740 |
| Temperature of larval rearing tank | - | - | - | - | 24.5 °C |
| Larval diet | - | - | - | Immediately post hatching: Isochrysis zhanjiangensis, Chaetoceros muelleri and Phaeodactylum tricornutum (1:1:1, 2.0–3.0 × 104 cells mL−1). Two weeks post hatching: formulated brine shrimp flakes (52% protein, 8% crude fat, 5% crude fiber, and 7% moisture) at a rate of 0.3 mg L−1 every other day. | Isochrysis galbana, Diacronema lutheri, Nannochloropsis oceania, Dunaliella sp. |
| Other conditions | 32–34 PSU | 34–36 PSU 0.7 veliger mL−1 | |||
| Settlement | None at 30 days | - | None at 300 days | None at 140 days | None at 83 days |
| Species | Compound | Solution/Dose/Time/ % Metamorphosis | Reference |
|---|---|---|---|
| Concholepas concholepas | Adult conspecific shells covered in barnacles | Up to 4–5 days, 100% | [124] |
| Crepidula fornicata | 20 mM KCl | 50% settlement after 30–50 min | [125] |
| Adult conspecific conditioned water | 40% settlement after 50 min | [125] | |
| Conspecific pedal mucus | 25% settlement after 50 min | [125] | |
| Raise KCl to 20 mM | 55%, Highest settlement in those fed Isochrysis sp. (4 × 105 cells/larva/day) | [126] | |
| Elevated KCl above background by 15–20 mM | 50% within 4 h | [127] | |
| Tested serotonin, dopamine and FMRFamide (10−5 M/L) | Measured whether larvae go up (serotonin) or down (dopamine, FMRFamide) in the water column | [128] | |
| Dibromomethane (DBM) | 90–100% metamorphosis at 5000 ppm, combined DBM and KCl | [129] | |
| Red algae extract, γ-aminobutyric acid (GABA), Hydrogen peroxide | 70–95% metamorphosis | [130,131] | |
| Aliger gigas | Nursery habitat sediment, KCl | [132,133] | |
| Hydrogen peroxide (H2O2) | 100% at 10 h in 50 µM H2O2 | [130] | |
| Extract of Laurencia poiteaui; Phycoerythrins and related protein conjugants | 88% metamorphosis | [132,134,135] | |
| Bromomethane | 90% at 600 ppm | [136] | |
| Haliotis discus hannai, H. rufescens H. diversicolor, H. asinina | conc KCl in normal seawater 9 mM | 40% at 19 mM KCl | [136] |
| 1 × 10−6 M (final) GABA | 37–99% | [137] | |
| Whole Ulva australis and U. compressa and Amphiroa anceps and Corallina officinalis | 0.05–0.1 g wet wt algae or 1 cm2 of 95% cover rock (CCA) added to 5 mL wells in 4 mL of seawater. CCA best (80%) | [138] | |
| Supplemented KCl | 50% in 5–10 mM KCl (supplemented) | [139] | |
| GABA | 40% 10−6 M GABA | [139] | |
| KCl, GABA | >40% 20 mM KCl, >75% 10−6 M GABA | [140] | |
| Biogenic amines | % metamorphosis at 10−6 M of GABA (98%), L-glutamate (80%), L-glutamine (0%), β-alanine (16–68%) | [141] | |
| GABA, δ-aminovaleric acid (5-AVA), L-glutamic acid, monosodium glutamate (MSG) | 10−1 mM 5-AVA (62% at 6 h) > 10−3 mM GABA (58% at 72 h) > 25 mM MSG (50% at 72 h) > 10−3 mM L-glutamic acid (48% at 72 h). | [142] | |
| 10−3, 10−4, 10−5, 10−6 M GABA | 10−6 M GABA at 2 days, 73% | [143] | |
| 5 spp. Benthic diatoms (Navicula spp. and Nitzschia spp.) | If fed 5 spp., at 2 days 90–94% | [143] | |
| Phestilla sibogae | Catecholamine precursor L-3,4-dihydroxyphenylalanine (L-DOPA) | 20–50-fold increase in dopamine and 2-fold increase in norepinephrine production in 6–9-day larvae, treated with L-DOPA (0.01 mM for 0.5 h) potentiated the frequency of metamorphosis | [144] |
| Hermissenda crassicornis | Ectopleura crocea water soluble secretion; GABA, choline, serotonin, glutamate, K+, Cs+. | induces high proportion of metamorphosis | [145] |
| Adalaria proxima | Presence of Electra pilosa; peptide with low molecular weight (<500 kDa) | [146,147] | |
| Charonia tritonis | Adult conspecific | 5000 L tank with adult conspecific, >10,000 veliger, 0% | [25] |
| Adult conspecific conditioned water | 45 L tank conditioned for 12 h with adult female conspecific, ~2000 veliger, 12 h, 0% | ||
| Conspecific intracapsular fluid | 6-well plates; 6 veliger per well, 20 μL intracapsular fluid added to each well, 12 h, 0% | ||
| Adult prey | 45 L tank, adult CoTS, ~2000 veliger, 12 h 0% | ||
| Adult prey conditioned water | 45 L tank conditioned for 12 h with adult CoTS, ~2000 veliger, 12 h, 0% | ||
| Adult prey mucous | 6-well plates; 6 veliger per well, 20 μL CoTS mucous added to each well, 12 h, 0% | ||
| Juvenile prey | 500 mL tank, 10 x juvenile CoTS, ~2000 veliger, 12 h, 0% | ||
| Environmental cue: crustose coralline algae (CCA) | 6-well plates; 6 veliger per well, CCA chip, 24 h, 0% | ||
| Environmental cue: CCA methanolic extract | 6-well plates; 6 veliger per well, 5, 10 μL mL−1, 12 h, 0% | ||
| Sediment (1–1000 μm) from aquaria (live rock, coral, macroalgae, assemblage of other reef organisms) | 500 mL tank, ~2000 veliger, 12 h, 0% | ||
| Filtered (60 μm mesh) sediment from aquaria (live rock, coral, macroalgae, assemblage of other reef organisms) | 500 mL tank, ~2000 veliger, 12 h, 0% | ||
| Multivitamin | 500 mL roller tank, ~2000 veliger, 0.05 multivitamin capsule, 12 h, 0% | ||
| KCl | 500 mL tank, ~2000 veliger, 10, 20 mM, 12 h, 0% | ||
| Synthetic peptides: Serotonin, GLW-amide, WW-amide, APGW-amide, FRMF-amide, sCAP-amide, FF-amide, FF-amide 2, FV-amide, ADRYSFFGGL, Allotropin, Cerebrin, Conopressin, Myomodulin, KPGW-amide, GnRH, Egg laying hormone, Dopamine, L-DOPA | 6-well plates; 6 veliger per well, 10 μM mL−1, 12 h, 0% |
| Group | Species |
|---|---|
| Bivalvia (44) | |
| Oysters d/f | Ostrea edulis, O. chilensis, O. conchaphila, Magallana gigas, Crassostrea virginica, Saccostrea glomerata |
| Mussels d/f | Mytilus edulis, M. galloprovincialis, M. chilensis, Perna canaliculus, Anodonta cygnea, Aulacomya atra, Choromytilus chorus, Modiolus spp. |
| Scallops d/f | Mizuhopecten yessoensis, Aequipecten opercularis, A. (Agropecten) irradians, Argopecten purpuratus, Mimachlamys varia, Pecten maximus |
| Clams d/f | Mercenaria mercenaria, Corbicula fluminea, Anadara broughtonii, Cyclina sinensis, Venus verrucosa, Donax spp., Mya arenaria, Leukoma staminea, Saxidomus gigantea, Tresus nuttallii |
| Carpet shells d/f | Ruditapes decussatus, Ruditapes philippinarum, Venerupis corrugata, Polititapes rhomboides |
| Razor clams d/f | Sinomovacula spp., Ensis ensis, Panopea abrupta |
| Cockles d/f | Tegillarca granosa, Cerastoderma edule, Cardiidae |
| Pen shell clams d/f | Atrina spp. |
| Gastropoda (+5) | |
| Snails | Rapana spp. c, Babylonia spp. d/c, Buccinum undatum c, Aliger gigas d/h,*, Strombus pugilis d/h, Rochia nilotica h,*, Stromboidea h/d |
| Abalone h | Haliotis rufescens, H. discus, H. tuberculata |
| Cephalopoda (1) | |
| Octopus c | Octopus spp. |
| Taxa (Class) | Species | Reference |
|---|---|---|
| Anthozoa | Stoichactis sp. Paracorynactis hoplites Pseudocorynactis sp. | [164,233,236,237] |
| Polychaeta | Pherecardia striata | [235,238] |
| Gastropoda | Charonia tritonis | [239,240,241,242] |
| Malacostraca | Hymenocera picta Tumidodromia dormia | [235,242,243,244] |
| Actinopterygii | Epinephelus lanceolatus Lethrinus spp. Cheilinus undulatus Arothron hispidus, A. stellatus A. nigropunctatus Balistoides viridescens Pseudobalistes flavimarginatus | [10,164,239,241,245,246,247,248,249,250,251] |
| Species | Target of Control; (N) = Unsuccessful, (Y) = Moderate Success, (P) = Potential | Genome of Gastropod | Mitogenome of Gastropod |
|---|---|---|---|
| Babylonia areolata * | Monoplex pilearis (Y) [346]—mitogenome reported [351] | No | Yes [352] |
| Conus textile * | Monoplex pilearis (Y) [346] | No | Yes [353] |
| Edentulina affinis | Lissachatina fulica (N)—mitogenome reported [354] | No | No |
| Edentulina obesa bulimiformis | Lissachatina fulica (N) | No | No |
| Euglandina rosea | Lissachatina fulica (N), Cornu aspersum (N), Otala lactea (N), Rumina decollata (N), Slugs (N) | No | No |
| Tayloria kibweziensis | Lissachatina fulica (N), Cornu aspersum (N), Otala lactea (N), Rumina decollata (N), Slugs (N) | No | No |
| Tayloria quadrilateralis | Lissachatina fulica (N) | No | No |
| Gonaxis vulcani | Lissachatina fulica (N) | No | No |
| Gulella bicolor | Lissachatina fulica (N), Subulina octona (N) | No | No |
| Gulella wahlbergi | Lissachatina fulica (N) | No | No |
| Marisa cornuarietis ** | Freshwater weeds and snail vectors of schistosomes; Biomphalaria glabrata, Biomphalaria pfeifferi, Bulinus tropicus, Bulinus truncatus, Hydrilla verticillata, Eichhornia crassipes (Y) | No | Yes [355] |
| Melanoides tuberculata ** | Biomphalaria glabrata, Biomphalaria straminea, Biomphalaria havanensis, Biomphalaria peregrina, Biomphalaria helophia (N) | No | No |
| Natalina cafra | Lissachatina fulica (N), Otala lactea (N), Rumina decollata (N), Slugs (N) | No | No |
| Oleacina straminea | Lissachatina fulica (N) | No | No |
| Pomacea glauca ** | Biomphalaria glabrata (N), Pistia stratiotes (N) | No; available for P. canaliculata | No; available for P. canaliculata, P. diffusa & P. maculata [356,357,358,359] |
| Ptychotrema walikalense | Lissachatina fulica (N) | No | No |
| Rumina decollata | Cornu aspersum (N) | No | No |
| Salasiella sp. | Lissachatina fulica (N) | No | No |
| Streptaxis contusus | Lissachatina fulica (N) | No | No |
| Tarebia granifera ** | Biomphalaria havanensis, Biomphalaria peregrina, Biomphalaria helophila (N) | No | No |
| Vasula deltoidea * | Coralliophila galea (Y), [348], C. abbreviata (P) [360] | No | No |
| Characteristic | Definition | Charonia tritonis | Ranking |
|---|---|---|---|
| Narrow host range [362,363] | Generalized predators; preference for the target pest population in the presence of alternate natural prey | Echinoderm specialists; preference for CoTS over other echinoderms not established | ++ |
| Climatic adaptability [364] | Adaptability to the introduced environment, including to environmental extremes | Endemic to GBR | +++ |
| Synchrony with prey life cycle [365] | Should be present when the CoTS juveniles first emerge. | Long-lived; likely decades—unconfirmed | +++ |
| Self-replicating capacity; High reproductive potential with large numbers of offspring. | lays large clusters of capsules—2000 larvae per capsule | ++ | |
| Population growth rates; teleplanic long-lived oceanic larval phase | Likely slow—unconfirmed | + | |
| More than one generation is completed for each generation of the pest | annual spawner on GBR | ++ | |
| Longevity | Likely decades—unconfirmed | +++ | |
| Efficient search ability | Prey detection ability even when prey is scarce | Chemosensory capacity | +++ |
| Short handling time | Higher predator consumption rates equate to greater number of attacks on prey. Small populations of efficient natural enemies may be more effective biocontrol agents than larger populations of less efficient species. Effective biocontrol agents reduce or suppress a pest population below a defined threshold. | Only eat 1–2 CoTS per week | + |
| Survival at low host (prey) density | The type of biocontrol used will depend on several factors for this to be effective | Will prey on other echinoderms in the absence of CoTS | +++ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Motti, C.A.; Cummins, S.F.; Hall, M.R. A Review of the Giant Triton (Charonia tritonis), from Exploitation to Coral Reef Protector? Diversity 2022, 14, 961. https://doi.org/10.3390/d14110961
Motti CA, Cummins SF, Hall MR. A Review of the Giant Triton (Charonia tritonis), from Exploitation to Coral Reef Protector? Diversity. 2022; 14(11):961. https://doi.org/10.3390/d14110961
Chicago/Turabian StyleMotti, Cherie A., Scott F. Cummins, and Michael R. Hall. 2022. "A Review of the Giant Triton (Charonia tritonis), from Exploitation to Coral Reef Protector?" Diversity 14, no. 11: 961. https://doi.org/10.3390/d14110961
APA StyleMotti, C. A., Cummins, S. F., & Hall, M. R. (2022). A Review of the Giant Triton (Charonia tritonis), from Exploitation to Coral Reef Protector? Diversity, 14(11), 961. https://doi.org/10.3390/d14110961






