Four-Year Field Survey of Black Band Disease and Skeletal Growth Anomalies in Encrusting Montipora spp. Corals around Sesoko Island, Okinawa
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. BBD and GAs Prevalence (2017–2020 Field Surveys) on Encrusting Montipora Colonies
2.3. BBD Progression Rates
2.4. Montipora Colony Counts and Coverage
2.5. Statistical Analyses
2.6. Environmental Parameters
2.6.1. In-Situ Data
2.6.2. Publicly Available Japanese Meteorological Association (JMA) Data
2.7. Types of Data Collected
3. Results
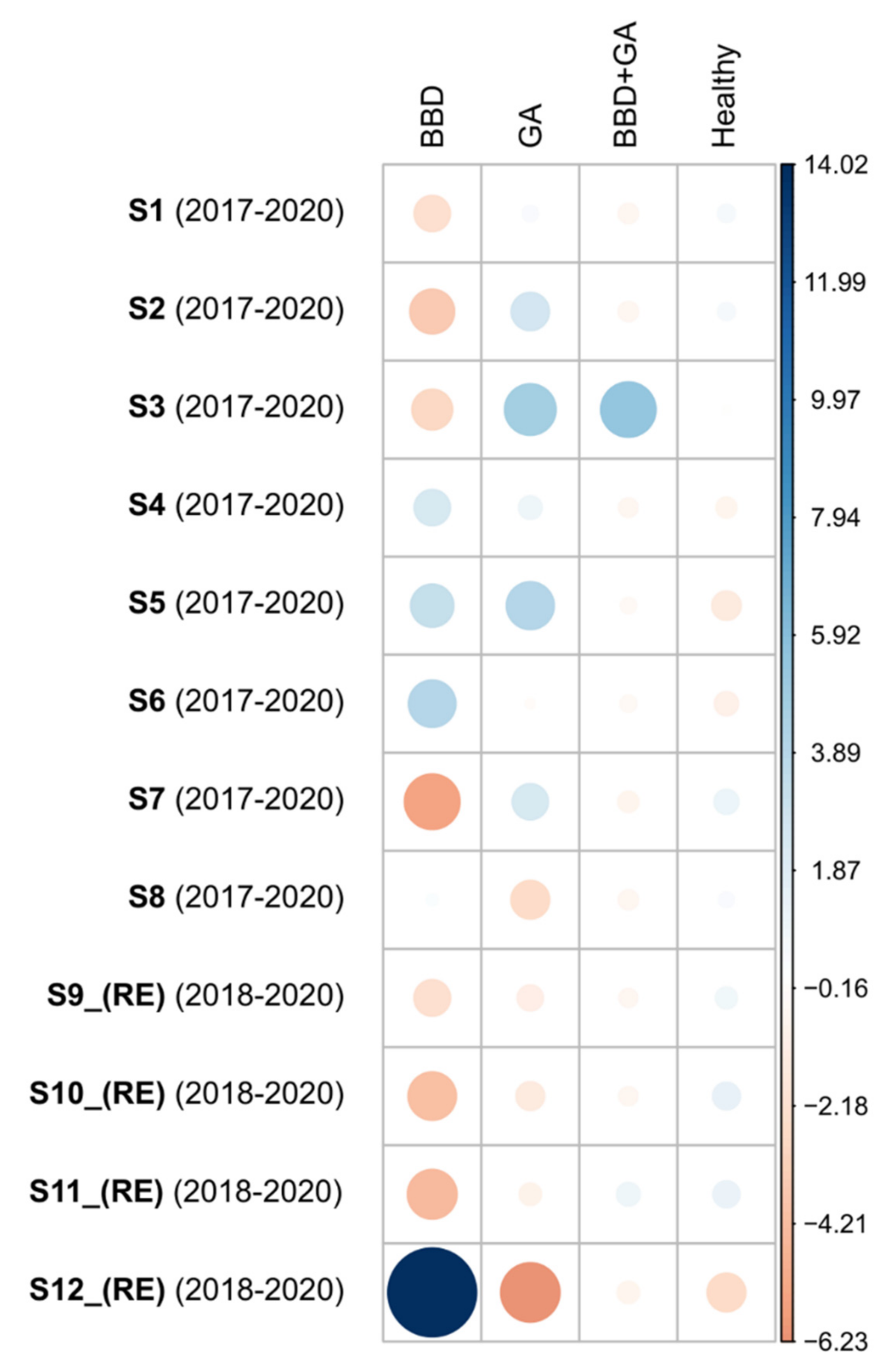
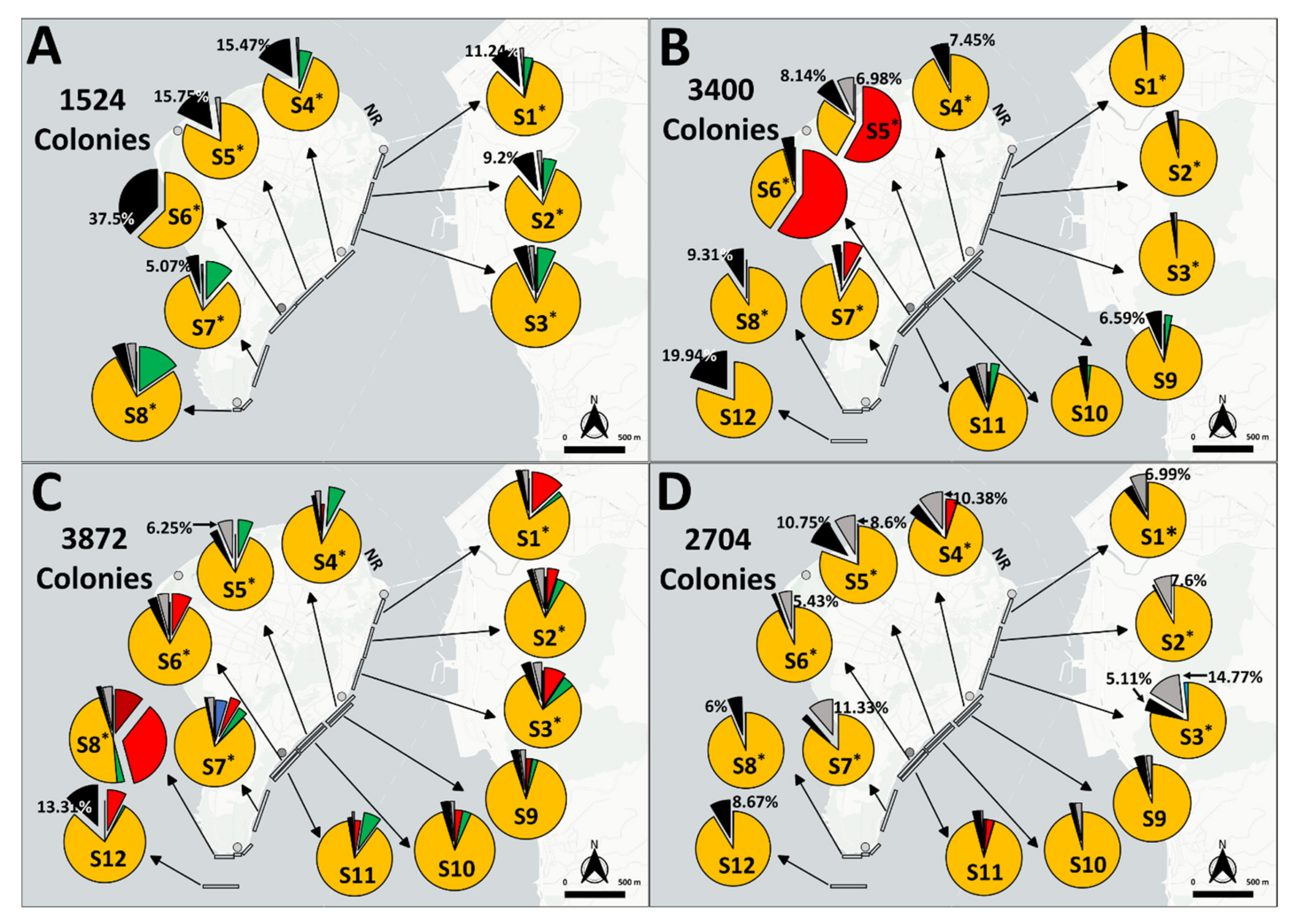
3.1. BBD Prevalences
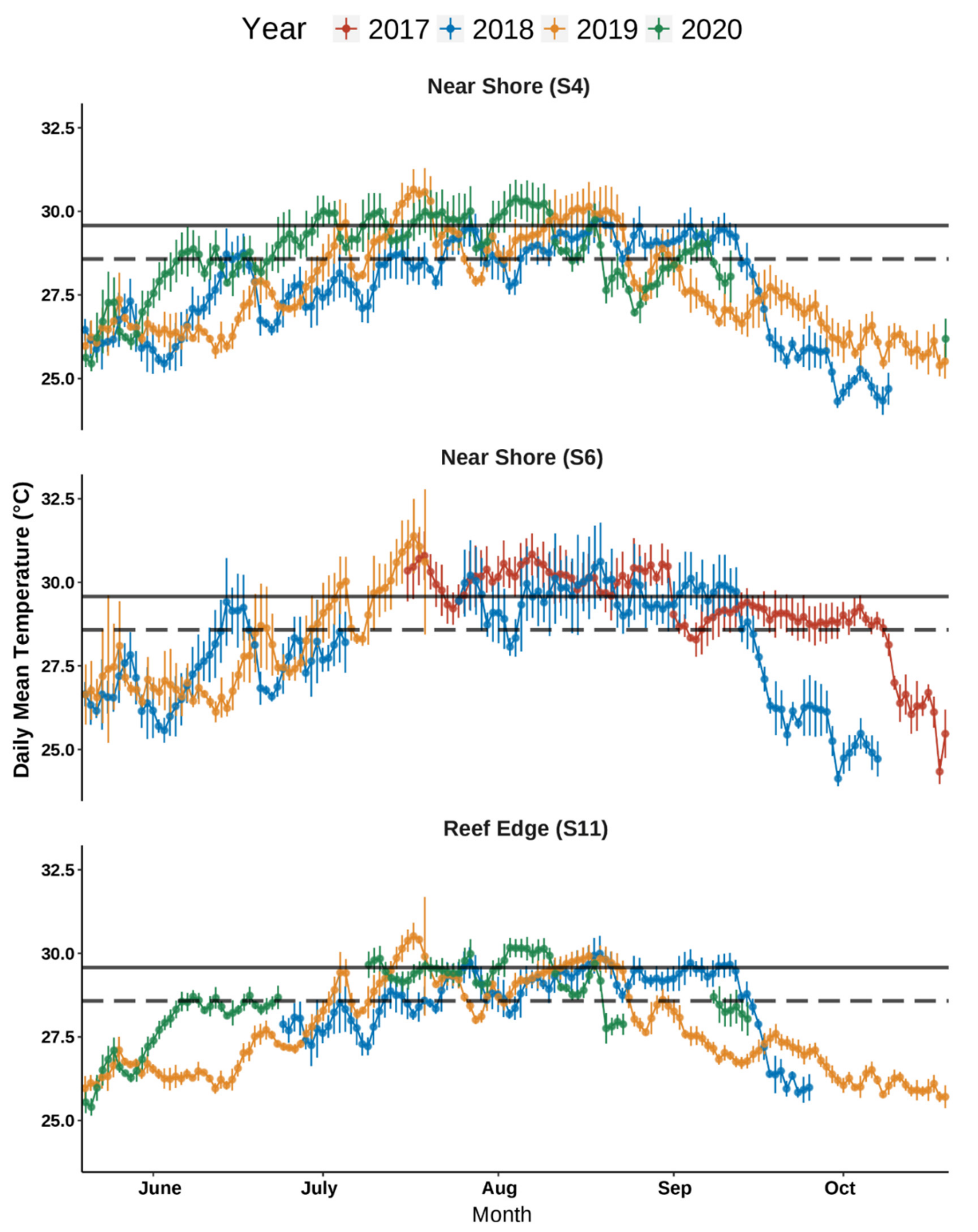
3.2. BBD Progression and Seawater Temperatures
3.3. GAs Prevalence
3.4. Abundance (Colony Count) and Percent Coverage
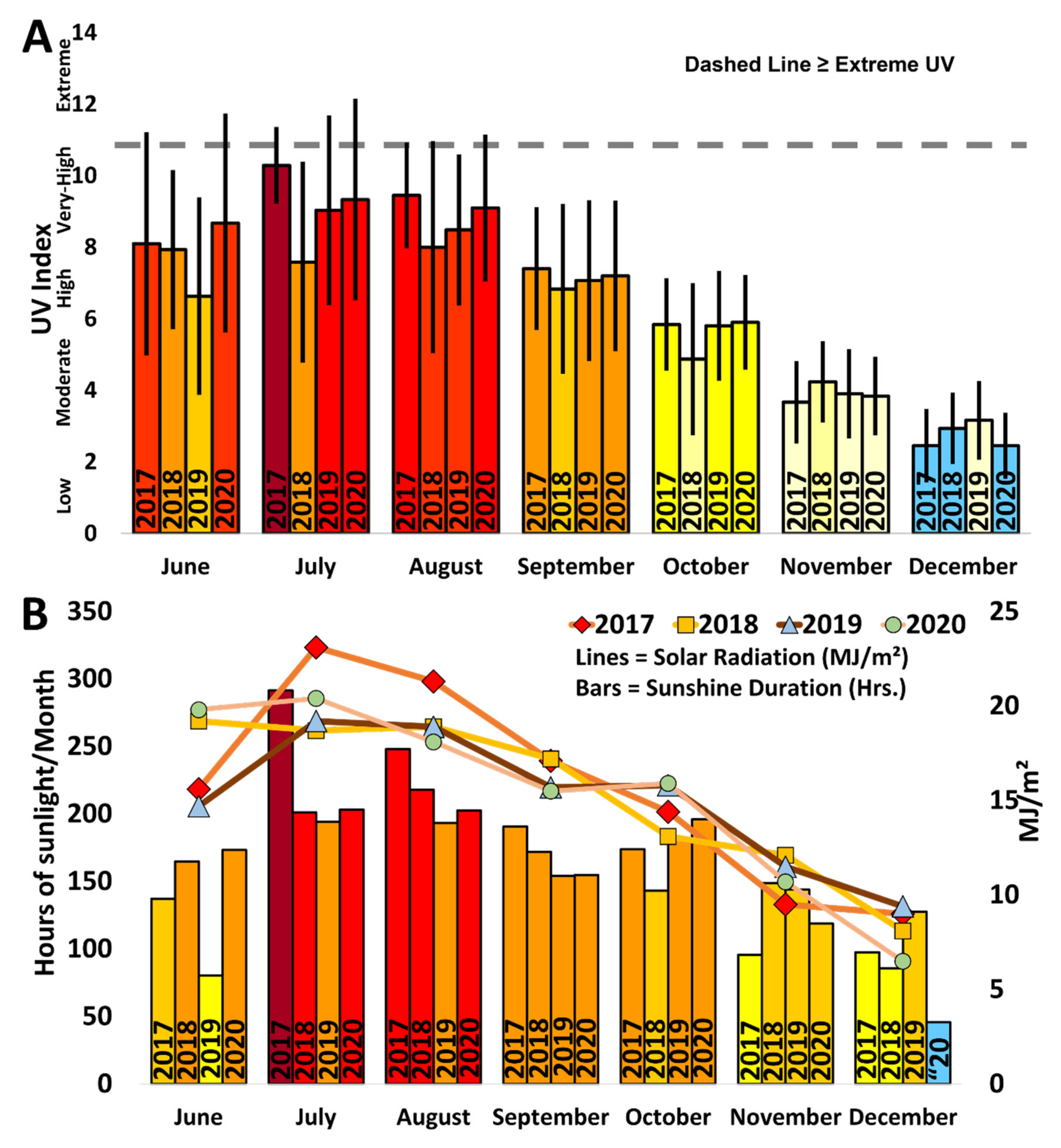
3.5. Environmental Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sutherland, K.P.; Porter, J.W.; Torres, C. Disease and immunity in Caribbean and Indo-Pacific zooxanthellate corals. Mar. Ecol. Prog. Ser. 2004, 266, 273–302. [Google Scholar] [CrossRef]
- Lafferty, K.D.; Porter, J.W.; Ford, S.E. Are diseases increasing in the ocean? Annu. Rev. Ecol. Evol. Syst. 2004, 35, 31–54. [Google Scholar] [CrossRef]
- Yamashiro, H.; Yamamoto, M.; van Woesik, R. Tumor formation on the coral Montipora informis. Dis. Aquat. Org. 2000, 41, 211–217. [Google Scholar] [CrossRef][Green Version]
- Sato, Y.; Bourne, D.G.; Willis, B.L. Dynamics of seasonal outbreaks of black band disease in an assemblage of Montipora species at Pelorus Island (Great Barrier Reef, Australia). Proc. Royal Soc. B. 2009, 276, 2795–2803. [Google Scholar] [CrossRef]
- Wada, N.; Mano, N.; Yanagisawa, Y.; Mori, T. Occurrence of coral diseases at Akajima, Okinawa, Japan in 2010 and 2011. Galaxea J. Coral Reef Stud. 2017, 19, 35–44. [Google Scholar] [CrossRef][Green Version]
- Huang, C.Y.; Hwang, J.S.; Yamashiro, H.; Tang, S.-L. Spatial and cross-seasonal patterns of coral diseases in reefs of Taiwan: High prevalence and regional variation. Dis. Aquat. Org. 2021, 146, 145–156. [Google Scholar] [CrossRef]
- Aini, S.N.; Tang, S.-L.; Yamashiro, H. Monthly progression rates of the coral killing sponge Terpios hoshinota in Sesoko Island, Okinawa, Japan. Coral Reefs 2021, 40, 973–981. [Google Scholar] [CrossRef]
- Veron, J.E.N. Corals of The World. 3 Volumes; Australian Institute of Marine Science: Townsville, Australia, 2000; p. 1382. [Google Scholar]
- Nishihira, M. Hermatypic corals of Japan. In Coral Reefs of Japan; Tsuchiya, M., Nadaoka, K., Kayanne, H., Yamano, H., Eds.; Ministry of the Environment and Japanese Coral Reef Society: Tokyo, Japan, 2004; pp. 10–19. [Google Scholar]
- Veron, J.E.N. Conservation of biodiversity: A critical time for the hermatypic corals of Japan. Coral Reefs 1992, 11, 13–21. [Google Scholar] [CrossRef]
- Aeby, G.S. Baseline levels of coral disease in the Northwestern Hawaiian Islands. Atoll Res. Bull. 2006, 543, 471–488. [Google Scholar]
- Aeby, G.S.; Santavy, D.L. Factors affecting susceptibility of the coral Montastraea faveolata to black-band disease. Mar. Ecol. Prog. Ser. 2006, 318, 103–110. [Google Scholar] [CrossRef]
- Aeby, G.S.; Ross, M.; Williams, G.J.; Lewis, T.D.; Work, T.M. Disease dynamics of Montipora white syndrome within Kaneohe Bay, Oahu, Hawaii: Distribution, seasonality, virulence, and transmissibility. Dis. Aquat. Org. 2010, 91, 1–8. [Google Scholar] [CrossRef]
- Aeby, G.S.; Work, T.M.; Runyon, C.M.; Shore-Maggio, A.; Ushijima, B.; Videau, P.; Beurmann, S.; Callahan, S.M. First record of black band disease in the Hawaiian archipelago: Response, outbreak status, virulence, and a method of treatment. PLoS ONE 2015, 10, e0120853. [Google Scholar] [CrossRef]
- Palmer, C.V.; Gates, R.D. Skeletal eroding band in Hawaiian corals. Coral Reefs 2010, 29, 469. [Google Scholar] [CrossRef][Green Version]
- Jones, R.J.; Bowyer, J.; Hoegh-Guldberg, O.; Blackall, L.L. Dynamics of a temperature-related coral disease outbreak. Mar. Ecol. Prog. Ser. 2004, 281, 63–77. [Google Scholar] [CrossRef]
- Johan, O.; Zamany, N.P.; Smith, D.; Sweet, M.J. Prevalence and incidence of black band disease of scleractinian corals in the Kepulauan Seribu Region of Indonesia. Diversity 2016, 8, 11. [Google Scholar] [CrossRef]
- McClanahan, T. Coral bleaching, diseases, and mortality in the western Indian Ocean. In Coral Health and Disease; Rosenberg, E., Loya, Y., Eds.; Springer: Berlin/Heidelberg, Germany, 2004; pp. 157–176. [Google Scholar]
- Thinesh, T.; Diraviya-Raj, K.; Mathews, G.; Patterson-Edward, J.K. Coral diseases are major contributors to coral mortality in Shingle Island, Gulf of Mannar, southeastern India. Dis. Aquat. Org. 2013, 106, 69–77. [Google Scholar] [CrossRef]
- Diraviya-Raj, K.; Aeby, G.S.; Mathews, G.; Bharath, M.S.; Rajesh, S.; Laju, R.L.; Arasamuthu, A.; Kumar, P.D.; Patterson-Edward, J.K. Patterns in the abundance of fish and snail corallivores associated with an outbreak of acute tissue loss disease on the reefs of Vaan Island in the Gulf of Mannar, India. In Proceedings of the 13th International Coral Reef Symposium at Honolulu, Honolulu, HI, USA, 19–24 June 2016. [Google Scholar]
- Diraviya-Raj, K.; Selva-Bharath, M.; Mathews, G.; Aeby, G.S.; Patterson-Edward, J.K. Coral-killing sponge Terpios hoshinota invades the corals of Gulf of Mannar, Southeast India. Curr. Sci. 2018, 114, 1117–1119. [Google Scholar] [CrossRef]
- Yamashiro, H. Corals of the Unknown World; Seizando-Shoten Publishing Co. Ltd.: Tokyo, Japan, 2016; p. 165. (In Japanese) [Google Scholar]
- Richardson, L.L. Black band disease. In Coral Health and Disease; Rosenberg, E., Loya, Y., Eds.; Springer: Berlin, Heidelberg, Germany, 2004; pp. 325–336. [Google Scholar]
- Biodiversity Centre of Japan. The Annual Report of the “Monitoring Sites 1000” in Japan: Coral Reefs. Biodiversity Center of Japan; Ministry of the Environment of Japan: Tokyo, Japan, 2003. (In Japanese) [Google Scholar]
- Sato, T. A report on coral disease prevalence at the Sekisei Lagoon, Okinawa, Japan. Lagoon 2006, 9, 13–14. (In Japanese) [Google Scholar]
- Casareto, B.E. Studies on water quality and bacteria composition in corals showing ‘white syndrome’ signs at Sekisei Lagoon, South Ishigaki Island, Okinawa, Japan. Lagoon 2008, 11, 2–5. [Google Scholar]
- Yasuda, N.; Hidaka, M. Cellular kinetics in growth anomalies of the scleractinian corals Porites australiensis and Montipora informis. Dis. Aquat. Org. 2012, 102, 1–11. [Google Scholar] [CrossRef]
- Yasuda, N.; Nakano, Y.; Yamashiro, H.; Hidaka, M. Skeletal structure, and progression of growth anomalies in Porites australiensis in Okinawa, Japan. Dis. Aquat. Org. 2012, 12, 237–247. [Google Scholar] [CrossRef]
- Muko, S.; Nadaoka, K. Ecological disturbances and their relative impacts on coral cover: Analysis of monitoring data in Sekisei lagoon (Okinawa, Japan). Bull. Mar. Sci. 2020, 96, 195–216. [Google Scholar] [CrossRef]
- Weil, E.; Irikawa; Casareto, B.; Suzuki, Y. Extended geographic distribution of several Indo-Pacific coral reef diseases. Dis. Aquat. Org. 2012, 98, 163–170. [Google Scholar] [CrossRef]
- Chen, C.C.M.; Bourne, D.G.; Drovandi, C.C.; Mengersen, K.; Willis, B.L.; Caley, M.J.; Sato, Y. Modelling environmental drivers of black band disease outbreaks in populations of foliose corals in the genus Montipora. PeerJ 2017, 5, e3438. [Google Scholar] [CrossRef][Green Version]
- Frias-Lopez, J.; Klaus, J.S.; Bonheyo, G.T.; Fourke, B.W. Bacterial community associated with Black band disease in Corals. Appl. Environ. Microbiol. 2004, 70, 5955–5962. [Google Scholar] [CrossRef]
- Miller, A.W.; Richardson, L.L. A meta-analysis of 16S rRNA gene clone libraries from the polymicrobial black band disease of corals. FEMS Microb. Ecol. 2011, 75, 231–241. [Google Scholar] [CrossRef]
- Sato, Y.; Civiello, M.; Bell, S.C.; Willis, B.L.; Bourne, D.G. Integrated approach to understanding the onset and pathogenesis of black band disease in corals. Environ. Microbiol. 2016, 18, 752–765. [Google Scholar] [CrossRef]
- Frias-Lopez, J.; Bonheyo, G.T.; Jin, Q.; Fourke, B.W. Cyanobacteria associated with coral black band disease in Caribbean and Indo-Pacific reefs. Appl. Environ. Microbiol. 2003, 69, 2409–2413. [Google Scholar] [CrossRef]
- Hutabarat, P.U.B.; Nguyen, X.H.; Suda, S. Black Band disease-related (BBD) cyanobacterium from Okinawan corals. J. Appl. Phycol. 2018, 30, 3197–3203. [Google Scholar] [CrossRef]
- Sato, Y.; Willis, B.L.; Bourne, D.G. Successional changes in bacterial communities during the development of black band disease on the reef coral, Montipora hispida. ISME J. 2010, 4, 207–214. [Google Scholar] [CrossRef]
- Sato, Y.; Bourne, D.G.; Willis, B.L. Effects of temperature and light on the progression of black band disease on the reef coral, Montipora hispida. Coral Reefs 2011, 30, 753–761. [Google Scholar] [CrossRef]
- Kuehl, K.; Jones, R.; Gibbs, D.; Richardson, L. The roles of temperature and light in black band disease (BBD) progression on corals of the genus Diploria in Bermuda. J. Invertebr. Pathol. 2011, 106, 366–370. [Google Scholar] [CrossRef]
- Boyett, H.V.; Bourne, D.G.; Willis, B.L. Elevated temperature and light enhance progression and spread of black band disease on staghorn corals of the Great Barrier Reef. Mar. Biol. 2007, 151, 1711–1720. [Google Scholar] [CrossRef]
- Peters, E.C.; Halas, J.C.; McCarty, H.B. Calicoblastic neoplasms in Acropora palmata, with a review of reports on anomalies of growth and form in corals. J. Natl. Cancer Inst. 1986, 76, 895–912. [Google Scholar]
- Domart-Coulon, I.J.; Traylor-Knowles, N.; Peters, E.; Elbert, D.; Downs, C.A.; Price, K.; Stubbs, J.; McLaughlin, S.; Cox, E.; Aeby, G.; et al. Comprehensive characterization of skeletal tissue growth anomalies of the finger coral Porites compressa. Coral Reefs 2006, 25, 531–543. [Google Scholar] [CrossRef]
- Preston, S.; Richards, Z. Biological consequences of an outbreak of growth anomalies on Isopora palifera at the Cocos (Keeling) Islands. Coral Reefs 2020, 40, 97–109. [Google Scholar] [CrossRef]
- Stimson, J. Ecological characterization of coral growth anomalies on Porites compressa in Hawai ‘i. Coral Reefs 2011, 30, 133–142. [Google Scholar] [CrossRef]
- Burns, J.H.R.; Takabayashi, M. Histopathology of growth anomaly affecting the coral, Montipora capitata: Implications on biological functions and population viability. PLoS ONE 2011, 6, e28854. [Google Scholar]
- Palmer, C.V.; Baird, A.H. Coral tumor-like growth anomalies induce an immune response and reduce fecundity. Dis. Aquat. Org. 2018, 130, 77–81. [Google Scholar] [CrossRef]
- McClanahan, T.R.; Weil, E.; Maina, J. Strong relationship between coral bleaching and growth anomalies in massive Porites. Glob. Chang. Biol. 2009, 15, 1804–1816. [Google Scholar] [CrossRef]
- Coles, S.L.; Seapy, D.G. Ultra-violet absorbing compounds and tumorous growths on acroporid corals from Bandar Khayran, Gulf of Oman, Indian Ocean. Coral Reefs 1998, 17, 195–198. [Google Scholar] [CrossRef]
- Andersson, E.R.; Stewart, J.A.; Work, T.M.; Woodley, C.M.; Schock, T.B.; Day, R.D. Morphological, elemental, and boron isotopic insights into pathophysiology of diseased coral growth anomalies. Sci. Rep. 2020, 10, 8252. [Google Scholar] [CrossRef]
- Andersson, E.R.; Day, R.D.; Work, T.M.; Anderson, P.E.; Woodley, C.M.; Schock, T.B. Identifying metabolic alterations associated with coral growth anomalies using 1H NMR metabolomics. Coral Reefs 2021, 1–15. [Google Scholar] [CrossRef]
- Zvuloni, A.; Artzy-Randrup, Y.; Stone, L.; Kramarsky-Winter, E.; Barkan, R.; Loya, Y. Spatio-temporal transmission patterns of Black-Band disease in a coral community. PLoS ONE 2009, 4, e4993. [Google Scholar] [CrossRef]
- Irikawa, A.; Casareto, B.E.; Suzuki, Y.; Agostini, S.; Hidaka, M.; van Woesik, R. Growth anomalies on Acropora cytherea corals. Mar. Pollut. Bull. 2011, 62, 1702–1707. [Google Scholar] [CrossRef]
- Yamashiro, H.; Mikame, Y.; Suzuki, H. Localized outbreak of attached diatoms on the coral Montipora due to low-temperature stress. Sci. Rep. 2012, 2, 1–4. [Google Scholar] [CrossRef]
- Das, R.R.; Yamashiro, H. Corals dominate monofilament lines in Sesoko Island, Japan. Curr. Sci. 2018, 114, 730–731. [Google Scholar]
- Wada, N.; Ohdera, A.; Mano, N. Coral disease in Japan. In Coral Reef Studies of Japan; Iguchi, A., Hongo, C., Eds.; Springer: Singapore, 2018; pp. 41–62. [Google Scholar]
- Takeuchi, I.; Yamashiro, H. Large Porites microatoll found by aerial survey at Sesoko Island, Okinawa, Japan. Coral Reefs 2017, 36, 1317. [Google Scholar] [CrossRef]
- Kubomura, T.; Boo, W.H.; Reimer, J.D. Investigation incidence and possible cause of pink and purple pigmentation response in hard coral genus Porites around Okinawajima Island, Japan. Reg. Stud. Mar. Sci. 2020, 41, 101569. [Google Scholar] [CrossRef]
- Takeuchi, I.; Yamashiro, H. Bird-view of the reef flat off Sesoko Station on the east coast of Sesoko Island, Okinawa, Japan obtained by aerial survey. Galaxea J. Coral Reef Stud. 2021. [Google Scholar] [CrossRef]
- Beijbom, O.; Edmunds, P.J.; Kline, D.I.; Mitchell, B.G.; Kriegman, D. Automated annotation of coral reef survey images. In Proceedings of the 2012 IEEE Conference on Computer Vision and Pattern Recognition (IEEE), Providence, RI, USA, 16–21 June 2012; pp. 1170–1177. [Google Scholar]
- Perneger, T.V. What’s wrong with Bonferroni adjustments. BMJ 1998, 316, 1236. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for statistical COMPUTING. 2020. R Foundation for Statistical Computing: Vienna, Austria. Available online: https://www.R-project.org/ (accessed on 1 October 2021).
- Fox, J.; Weisberg, S. An {R} Companion to Applied Regression, 3rd ed.; Sage: Thousand Oaks, CA, USA, 2019; Available online: https://socialsciences.mcmaster.ca/jfox/Books/Companion/ (accessed on 1 October 2021).
- Length, R.V. Emmeans: Estimated Marginal Means, Aka Least-Squares Means. The R Package Version 1.5.3. 2020. Available online: https://CRAN.R-project.org/packages=emmeans (accessed on 1 October 2021).
- Singh, T.; Ijima, M.; Yasumoto, K.; Sakai, K. Effects of moderate thermal anomalies on Acropora corals around Sesoko Island, Okinawa. PLoS ONE 2019, 14, e0210795. [Google Scholar]
- NOAA Coral Reef Watch. 2019. NOAA Coral Reef Watch Version 3.1 Daily 5km Satellite Regional Virtual Station Time Series Data for Southeast Florida, Mar. 12, 2013-Mar. 11, 2014. College Park, Maryland, USA: NOAA Coral Reef Watch. Available online: https://coralreefwatch.noaa.gov/product/vs/data.php. (accessed on 5 February 2020).
- Willis, B.L.; Page, C.A.; Dinsdale, E.A. Coral disease on the Great Barrier Reef. In Coral Health and Disease; Rosenberg, E., Loya, Y., Eds.; Springer: Berlin/Heidelberg, Germany, 2004; pp. 69–104. [Google Scholar]
- Hadaidi, G.; Ziegler, M.; Shore-Maggio, A.; Jensen, T.; Aeby, G.; Voolstra, C.R. Ecological and molecular characterization of a coral black band disease outbreak in the Red Sea during a bleaching event. PeerJ 2018, 6, e5169. [Google Scholar] [CrossRef]
- Montano, S.; Strona, G.; Seveso, D.; Galli, P. Prevalence, host range, and spatial distribution of black band disease in the Maldivian Archipelago. Dis. Aquat. Org. 2013, 105, 65–74. [Google Scholar] [CrossRef]
- Jordán-Dahlgren, E.; Jordán-Garza, A.G.; Rodríguez-Martínez, R.E. Coral disease prevalence estimation and sampling design. PeerJ 2018, 6, e6006. [Google Scholar] [CrossRef]
- Smith, S.G.; Swanson, D.W.; Chiappone, M.; Miller, S.L.; Ault, J.S. Probability sampling of stony coral populations in the Florida Keys. Environ. Monit. Assess. 2011, 183, 121–138. [Google Scholar] [CrossRef]
- Ruiz-Moreno, D.; Willis, B.L.; Page, A.C.; Weil, E.; Cróquer, A.; Vargas-Angel, B.; Jordan-Garza, A.G.; Jordán-Dahlgren, E.; Raymundo, L.; Harvell, C.D. Global coral disease prevalence associated with sea temperature anomalies and local factors. Dis. Aquat. Org. 2012, 100, 249–261. [Google Scholar] [CrossRef]
- Aeby, G.S.; Williams, G.J.; Franklin, E.C.; Haapkyla, J.; Harvell, C.D.; Neale, S.; Page, C.A.; Raymundo, L.; Vargas-Ángel, B.; Willis, B.L.; et al. Growth anomalies on the coral genera Acropora and Porites are strongly associated with host density and human population size across the Indo-Pacific. PLoS ONE 2011, 6, e16887. [Google Scholar]
- Bruno, J.F.; Selig, E.R.; Casey, K.S.; Page, C.A.; Willis, B.L.; Harvell, C.D.; Sweatmen, H.; Meleny, A.M. Thermal stress and coral cover as drivers of coral disease outbreaks. PLoS Biol. 2007, 5, e124. [Google Scholar] [CrossRef]
- Hazraty-Kari, S.; Tavakoli-Kolour, P.; Das, R.R.; Farhadi, M.; Barkhordari-Ahmadi, A.; Yahyavi, M.; Rezai, H. Baseline assessment of coral diseases in an environmentally extreme environment of the northern Persian Gulf. Mar. Pollut. Bull. 2021, 171, 112707. [Google Scholar] [CrossRef]
- Downs, N.; Parisi, A.V.; Galligan, L.; Turner, J.; Amar, A.; King, R.; Ultra, F.; Butler, H. Solar radiation and the UV index: An application of numerical integration, trigonometric functions, online education, and the modelling process. IJRES 2016, 2, 179–189. [Google Scholar] [CrossRef]
- Haapkylä, J.; Unsworth, R.K.F.; Flavell, M.; Bourne, D.G.; Schaffelke, B.; Willis, B.L. Seasonal rainfall and runoff promote coral disease on an inshore reef. PLoS ONE 2011, 6, e16893. [Google Scholar] [CrossRef]
- Muller, E.M.; Leporacci, N.M.; Macartney, K.J.; Shea, A.G.; Crane, R.E.; Hall, E.R.; Ritchie, K.B. Low pH reduces the virulence of black band disease on Orbicella faveolata. PLoS ONE 2017, 12, e0178869. [Google Scholar] [CrossRef]
- Glas, M.S.; Sato, Y.; Ulstrup, K.E.; Bourne, D.G. Biogeochemical conditions determine virulence of black band disease in corals. ISME J. 2012, 6, 1526–1534. [Google Scholar] [CrossRef]
- Cabaitan, P.C.; Yamamoto, H.; Sakai, K. Recovery of corals a decade after thermal stress event at Motobu, Okinawa, Japan: Spatial variability in winners and losers. Galaxea J. Coral Reef Stud. 2012, 14, 27–40. [Google Scholar] [CrossRef]
- Nakamura, T. Mass coral bleaching event in Sekisei lagoon observed in the summer of 2016. Galaxea J. Coral Reef Stud. 2017, 19, 29–40. [Google Scholar] [CrossRef]
- Sakai, K.; Singh, T.; Iguchi, A. Bleaching, and post-bleaching mortality of Acropora corals on a heat-susceptible reef in 2016. PeerJ 2019, 7, e8138. [Google Scholar] [CrossRef]
- Antonius, A. New observations on coral destruction in reefs. In Tenth Meeting of the Association of Island Marine Laboratories of the Caribbean; University of Puerto Rico: Mayaguez, PR, USA, 1973; p. 3. [Google Scholar]
- Roff, G. Earliest record of a coral disease from the Indo-Pacific? Coral Reefs 2016, 35, 457. [Google Scholar] [CrossRef]
- Page, C.; Willis, B. Distribution, host range and large-scale spatial variability in black band disease prevalence on the Great Barrier Reef, Australia. Dis. Aquat. Org. 2006, 69, 41–51. [Google Scholar] [CrossRef]
- Harvell, C.D.; Kim, K.; Burkholder, J.M.; Colwell, R.R.; Epstein, P.R.; Grimes, D.J.; Hofmann, E.E.; Lipp, E.K.; Osterhaus, A.D.M.E.; Overstreet, R.M.; et al. Emerging marine diseases-climate links and anthropogenic factors. Science 1999, 285, 1505–1510. [Google Scholar] [CrossRef]
- Aeby, G.S.; Shore, A.; Jensen, T.; Ziegler, M.; Work, T.; Voolstra, C.R. A comparative baseline of coral disease in three regions along the Saudi Arabian coast of the central Red Sea. PLoS ONE 2021, 16, e0246854. [Google Scholar] [CrossRef]
- Al-Moghrabi, S.M. Unusual black band disease (BBD) outbreak in the northern tip of the Gulf of Aqaba (Jordan). Coral Reefs 2001, 19, 330–331. [Google Scholar] [CrossRef]
- Vega-Thurber, R.L.; Burkepile, D.E.; Fuchs, C.; Shantz, A.A.; Mcminds, R.; Zaneveld, J.R. Chronic nutrient enrichment increases prevalence and severity of coral disease and bleaching. Glob. Chang. Biol. 2014, 20, 544–554. [Google Scholar] [CrossRef]
- Chong-Seng, K.M.; Cole, A.J.; Pratchett, M.S.; Willis, B.L. Selective feeding by coral reef fishes on coral lesions associated with brown band and black band disease. Coral Reefs 2011, 30, 473–481. [Google Scholar] [CrossRef]


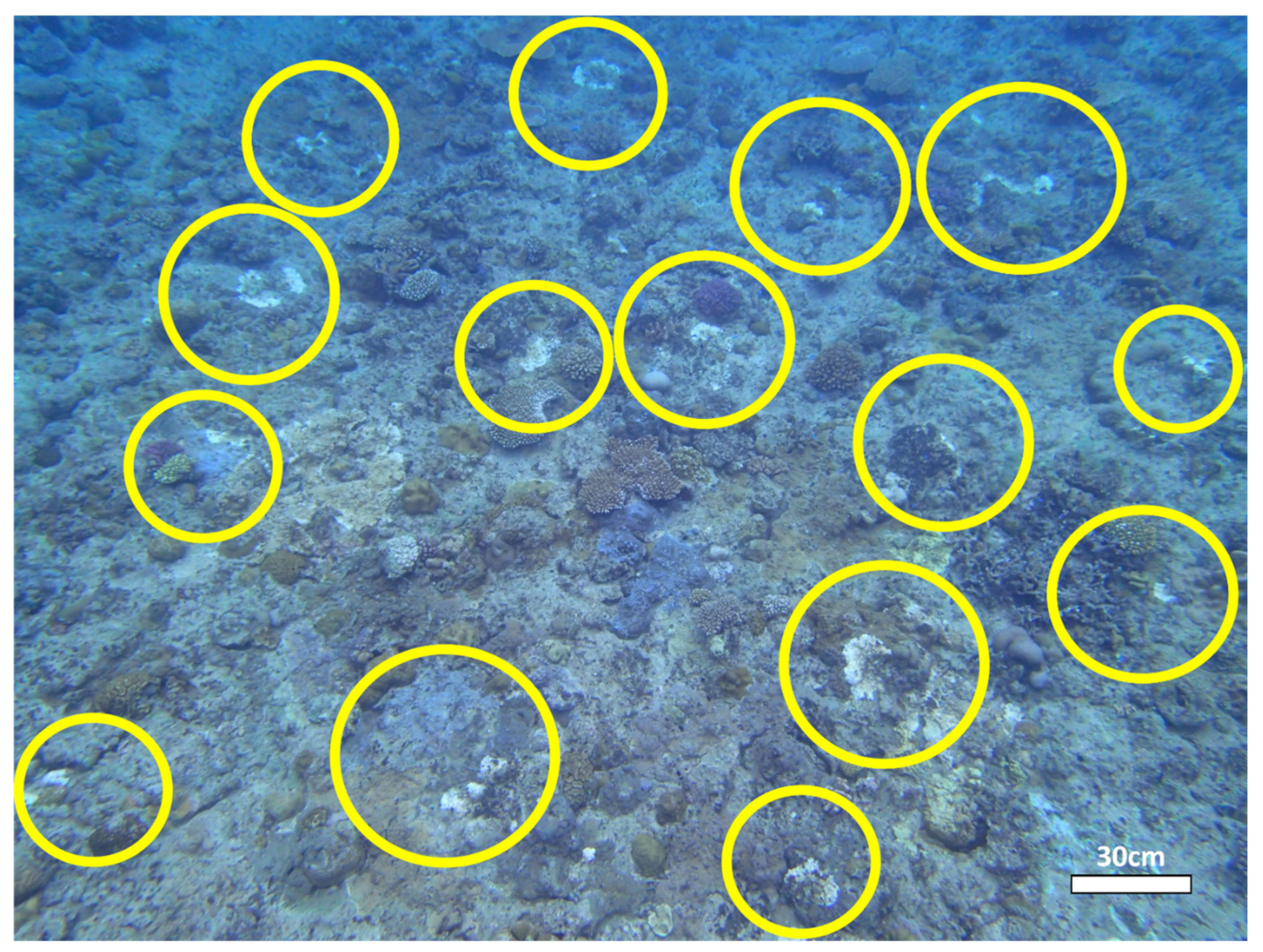

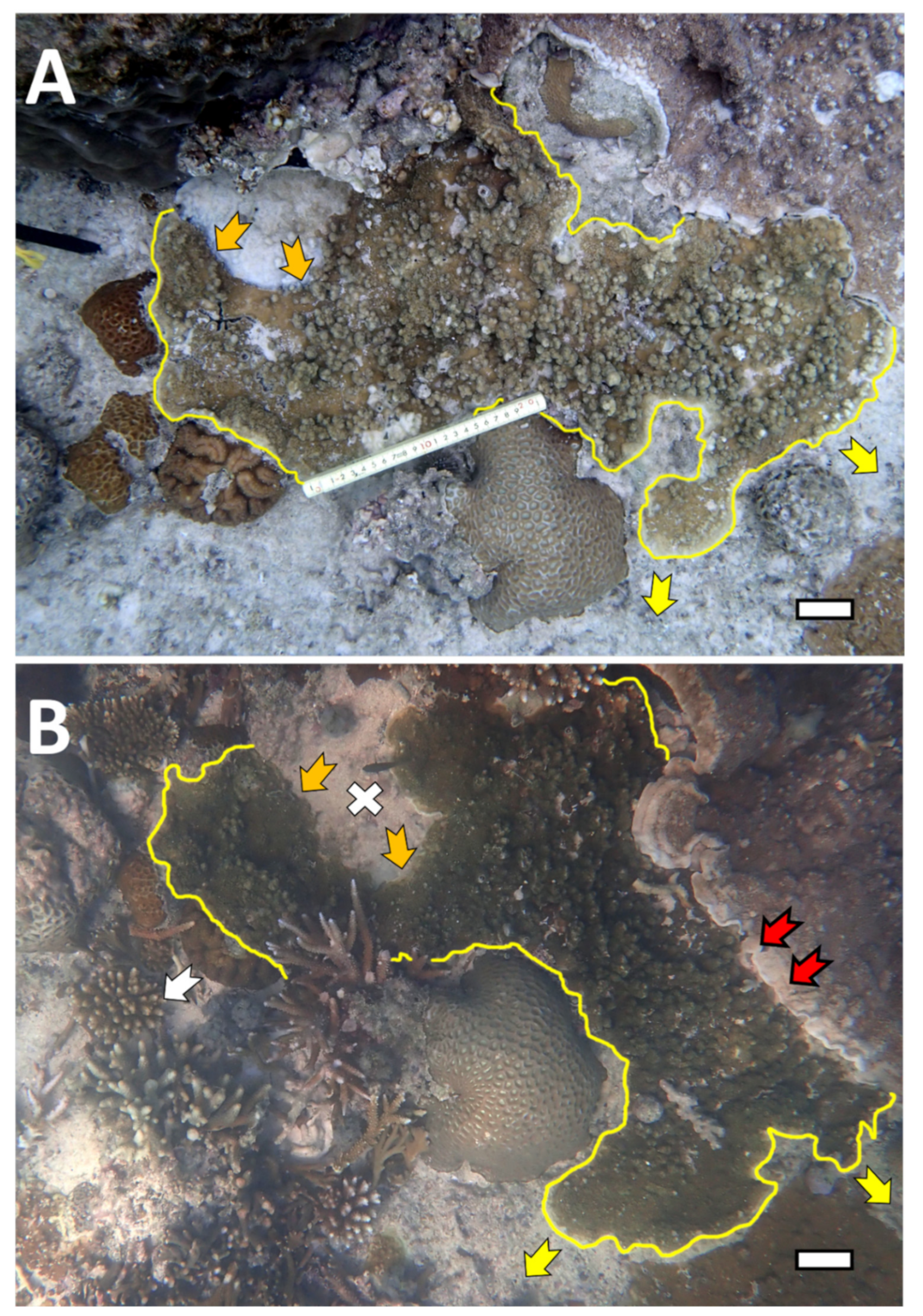

| Coral Condition | Description | Additional References |
|---|---|---|
| Healthy | Colony with normal coloration; no signs of disease. | Sakai et al., 2019; Kubomura et al., 2020 |
| Dead/turf algae | Entire colony dead and/or with turf algae Colony dead. | Yamashiro et al., 2000; Kubomura et al., 2020 |
| Heavily bleached or seriously damaged | Colonies alive, but almost completely bleached, or dead tissue constituting 50% or more of colony surface. | Nakamura, 2017; Sakai et al., 2019; Kubomura et al., 2020 |
| Pale and/or slightly damaged | Colonies showing signs of stress with faded colony color or dead areas below 50% of total colony surface, including presence of fish bites. | Sakai et al., 2019; Kubomura et al., 2020 |
| Black Band Disease (BBD) | Active signs of black cyanobacterial mat present on colony. | Weil et al., 2012; Yamashiro, 2016; Wada et al., 2017 |
| Growth Anomalies (GAs) | Abnormal growth of coral tissue; tissue with GAs may have abnormal coloration. | Yamashiro et al., 2000; Weil et al., 2012; Wada et al., 2017 |
| BBD + GAs | Colony consisting of both GAs and BBD either separately or BBD lesion growing over GAs. |
| Data Collected | Methodology | Period | Location(s)/Site(s) |
|---|---|---|---|
| BBD and GA prevalence | Free swimming | 2017–2020 (June to October) | S1–S8 (2017); S1–S12 (2018–2020) |
| BBD progression | Photograph of permanent plots and tagged colonies | 2020 (June to December) | S4, S6 |
| Colony count and host coverage | 1 m2 quadrats haphazardly placed and photographed | 2020 (July) | S5; S6; S11; S12 |
| Environmental parameters | In-situ and ex-situ HOBO data logger; JMA | 2017–2020 (June to October/December) | S4; S6; S11 (Water Temp Pro V2, Pendant temp/light); Sesoko Station (Pendant temp/light; 2020); UV Index (Nakijin/Motobu area); Solar Radiation (Nago/Naha area) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Das, R.R.; Wada, H.; Masucci, G.D.; Singh, T.; Tavakoli-Kolour, P.; Wada, N.; Tang, S.-L.; Yamashiro, H.; Reimer, J.D. Four-Year Field Survey of Black Band Disease and Skeletal Growth Anomalies in Encrusting Montipora spp. Corals around Sesoko Island, Okinawa. Diversity 2022, 14, 32. https://doi.org/10.3390/d14010032
Das RR, Wada H, Masucci GD, Singh T, Tavakoli-Kolour P, Wada N, Tang S-L, Yamashiro H, Reimer JD. Four-Year Field Survey of Black Band Disease and Skeletal Growth Anomalies in Encrusting Montipora spp. Corals around Sesoko Island, Okinawa. Diversity. 2022; 14(1):32. https://doi.org/10.3390/d14010032
Chicago/Turabian StyleDas, Rocktim Ramen, Haruka Wada, Giovanni Diego Masucci, Tanya Singh, Parviz Tavakoli-Kolour, Naohisa Wada, Sen-Lin Tang, Hideyuki Yamashiro, and James Davis Reimer. 2022. "Four-Year Field Survey of Black Band Disease and Skeletal Growth Anomalies in Encrusting Montipora spp. Corals around Sesoko Island, Okinawa" Diversity 14, no. 1: 32. https://doi.org/10.3390/d14010032
APA StyleDas, R. R., Wada, H., Masucci, G. D., Singh, T., Tavakoli-Kolour, P., Wada, N., Tang, S.-L., Yamashiro, H., & Reimer, J. D. (2022). Four-Year Field Survey of Black Band Disease and Skeletal Growth Anomalies in Encrusting Montipora spp. Corals around Sesoko Island, Okinawa. Diversity, 14(1), 32. https://doi.org/10.3390/d14010032








