Abstract
Assemblages of adult Odonata were studied in four intermittent karst rivers encompassing macrophyte-rich (MRH) and macrophyte-poor habitats (MPH) in southern Europe, where temporary lotic habitats are the predominant freshwater type but are still understudied. With a total of 25 recorded species, the studied habitats support species-rich Odonata assemblages, as already shown for intermittent rivers in the Mediterranean. Aquatic macrophyte abundance, conductivity, and water velocity are the most significant determinants of Odonata assemblages in the studied IRES. MRH promote higher Odonata abundance and the taxonomic and functional diversity of their assemblages compared to the MPH. Odonata assemblages in MRH are characterized by higher values of body size and a higher share of species preferring lentic and temporary hydrological conditions. Moreover, their assemblages are characterized by various patterns of nymphal development and drought resilience strategies. In contrast, MPH are preferred by lotic species, with nymphal development all year round and with no specific drought-resisting strategies. Our results contribute to the knowledge of diversity and ecological requirements of dragonflies and damselflies in IRES habitats, which could provide scientific background for future conservation activities and bioassessment protocols of such habitats and their biota.
1. Introduction
Approximately half of the running waters worldwide do not have continuous flow of surface water throughout the year and thus are categorized as temporary or non-perennial [1,2]. In the Mediterranean region of Europe, such rivers and streams are the predominant type of freshwater lotic habitats, due to dry climatic conditions, climate change, and land-use development. Non-perennial habitats are characterized by a wide range of hydrological regimes and can be categorized as intermittent (cease to flow seasonally or occasionally), ephemeral (flow only due to precipitation or snowmelt events), or episodic (flow primarily after heavy rainfall events) [3,4]. Here, we focus on intermittent rivers and streams (IRES), hydrologically highly dynamic and complex freshwater ecosystems that periodically cease to flow and run dry. Within such systems, three different flow categories can be distinguished: lotic (flowing), lentic phase (isolated pools), and dry riverbed, with the latter two being present during the dry periods [5,6].
Due to increasing anthropogenic pressures (e.g., river regulation, water abstraction, and pollution) and the global climate change, the Mediterranean basin is one of the most vulnerable regions in the world [7].The flow regimes of the IRES are rapidly changing, and the extent and intensity of dry periods in the IRES are expected to increase in the forthcoming future [6,8], which will also lead to serious water availability problems in the Mediterranean area [7]. Additionally, large parts of this area are densely populated, increasing the demand for irrigation and drinking water. The negative consequences of water abstraction and regulation are reflected in river hydrology modifications, with intensified drought effects [9]. Over the past few decades, water abstraction and impoundment have even caused many previously perennial rivers to become intermittent [10,11]. This trend is expected to continue in the near future [12], which will surely lead to irreversible changes in biological communities [13].
As IRES cover more than half of the global river network, it is essential to understand their contribution to biotic diversity at both local and landscape scales [5,6]. During the past decade, many studies have investigated and highlighted the importance of flow permanence for the composition and structure of aquatic macroinvertebrate assemblages [14,15,16]. Nevertheless, there are still large gaps regarding the environmental drivers that shape their diversity and composition in intermittent lotic habitats. Consequently, intermittent rivers and streams are still not included in biomonitoring programs in the majority of EU countries [8]. Yet, it is worth mentioning that in Croatia, there are two intermittent river/stream types in the National River Typology [17] with defined ecological status classes. In order to provide a scientific background for the development of widely applicable bioassessment methodology of IRES, it is essential to conduct further studies on the effect of flow intermittency on all aspects of stream ecology.
Odonata are an amphibious insect order (with aquatic nymphs and terrestrial adults) widely used as ecological indicators of freshwater ecosystem health [18,19,20,21]. Many studies showed that their assemblages are highly influenced by physicochemical water conditions [22,23,24,25,26] but even more importantly by habitat’s morphology and structure (e.g., bottom substrate and structure of aquatic vegetation) [27,28,29,30,31,32,33]. Many Odonata species have life-history adaptations that enable them to occupy temporary habitats, such as desiccation-tolerant eggs or fast larval growth [34,35,36]. Nymphs of some large Odonata species can also use damp sediment beneath the stones for aestivation [37]. For those species whose drought-resisting abilities are low, perennial lotic habitats and pools [37] as well as artificial reservoirs [38] in the vicinity of IRES were shown to be suitable refuge sites during dry periods.
To improve our knowledge about the aquatic insect communities in IRES, we studied the assemblages of adult Odonata in karst intermittent rivers in the Dinaric Western Balkans ecoregion [39]. The Dinaric Alps extend over approximately 60,000 km2 and are the largest continuous karst landscape in Europe [40]. Karst is a set of morphological, hydrological, and hydrogeological terrain features built of water-soluble rock. The Dinaric Western Balkans area is characterized by an extremely complex hydrological network [41] and extraordinary diversity of biota [42], yet it is still greatly understudied. Although Odonata are considered to be among the well-studied aquatic insect orders [43], their ecological requirements in karst rivers and streams are very poorly known [22,44], especially in intermittent habitats. Therefore, the main objectives of this study were (i) to compare Odonata assemblages (species richness, diversity, and abundance) in two focal habitat types: macrophyte rich and macrophyte poor, in the Mediterranean intermittent karst rivers; (ii) to examine the functional diversity of Odonata assemblages and detect changes in functional traits; and (iii) to determine the main environmental drivers that shape these assemblages. We hypothesize that the structure and abundance of aquatic macrophyte vegetation are the main environmental drivers shaping Odonata assemblages in the studied karst intermittent rivers, where macrophyte-rich habitats support the higher taxonomic and functional diversity of Odonata.
2. Materials and Methods
2.1. Study Area
The study was conducted in the Dinaric Western Balkan ecoregion (ER5) of Croatia [39]. Our study encompassed four Mediterranean karst intermittent rivers belonging to the Adriatic Sea basin: the Krčić, Čikola, Miljašić Jaruga, and Guduča Rivers (Figure 1). The Krčić River catchment covers 157 km2. Its source is located at the foot of the Dinara Mountain, near the town of Knin. The river flows for 10.5 km and ends with a 40 m high waterfall, which contributes to the forming of the Krka River [45]. The average annual discharge values for the period 1982–1990 for the Krčić River were 3.93 m3/s [46]. The catchment area of the Čikola River is approximately 300 km2. Its spring is located near Mirlović Polje village. The river runs for 39 km, ending as a tributary of the Krka River near Nos Kalik village [47,48]. The mean annual discharge for the Drniš hydrological station during 2003–2017 was 5.0 m3/s [48]. The Miljašić Jaruga River is a part of the Bokanjac-Poličnik catchment area of 244.51 km2 [49]. It springs near Suhovare village and flows for 25 km to its mouth in the Adriatic Sea near the town of Nin [50]. The mean annual discharge for the Boljkovac-Miljašić Jaruga hydrological station for the period 1961–2009 was 0.85 m3/s [51]. The Bribišnica River belongs to the Prokljan Lake catchment area, which amounts to 596.22 km2. It springs on the west side of the Bribirska Glavica hill. Near the Lađevci bridge, the river becomes the torrential Guduča River, which flows for seven more kilometers and runs into the Prokljan Lake [49]. The Guduča River generally carries on less than 1 m3/s of water [52]. Throughout the text, this river is referred to as the Guduča River.
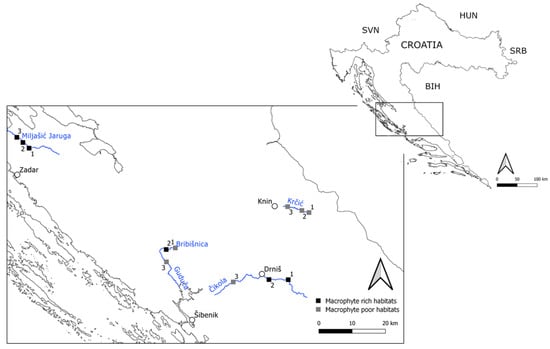
Figure 1.
Geographical position of the four studied intermittent karst rivers located in the Croatian part of the ER5. Legend: SVN—Slovenia, BIH—Bosnia and Herzegovina, SRB—Serbia, HUN—Hungary.
Our study was conducted at a total of 12 study sites (three sites per river) (Figure 1). At each site, we analyzed the vegetation structure, Odonata assemblages, and measured physicochemical water parameters.
2.2. Vegetation Analysis
During our third sampling event (30 June/1 July 2021), at each study site, we conducted a macrophyte vegetation survey (aquatic vascular plants and bryophytes) that included the assessment of species coverage and abundance. The sampling plot size was approximately 100 m2. The assessment of macrophyte species coverage and abundance was performed using the expanded, nine-degree Braun–Blanquet scale (+ = up to 5 individuals; 1 = up to 50 individuals; 2m = more than 50 individuals; 2a = coverage between 5 and 15%; 2b = coverage between 15 and 25%; 3 = coverage between 25 and 50%; 4 = coverage between 50 and 75%; 5 = coverage over 75%) [53,54,55]. The assessment included both aquatic vascular plants and aquatic bryophytes, for which the cumulative plant coverage of each group was calculated. Additionally, a cumulative plant coverage was calculated separately for lower (<30 cm) and higher (>30 cm) aquatic vascular plants.
2.3. Environmental Variables
In April 2021, when all four rivers were flowing (i.e., in the lotic phase), the following environmental parameters were measured at each study site: water temperature, dissolved oxygen concentration and saturation (using the oximeter WTW Oxi 330/SET), conductivity (using the conductivity meter WTW LF 330), pH (using the pH-meter WTW pH 330), water width and depth (using a hand meter/measuring tape), and water velocity (using the SonTek Flow Tracker). At each site, the parameters were measured at three equally spaced points in a transect from the shoreline to the center of the river, perpendicular to the river flow. Additionally, at those same points at each sampling site, triplicate 1 L water samples were taken for the laboratory analysis of the chemical parameters (alkalinity, chemical oxygen demand, concentrations of nitrites, nitrates, and orthophosphates) using Standard Analytical Procedure [56]. At the visited study sites, substrates were composed mostly of fine sediment (silt, mud), lithal (stones, gravel), and aquatic vegetation.
2.4. Odonata Sampling
Odonata adults were investigated between the end of May and beginning of July 2021, every two weeks, during a total of three sampling events (30 May/1 June, 14 June/15 June, 30 June/1 July 2021). At each river, three sites were visited at each sampling event (Figure 2); the first site was the closest to the river source, while the third was the most distant. At each study site, Odonata were investigated along a 200 m transect during the period of 45 min (until no additional species were detected). Species flying or perching within ≈5 m of the transect route were documented and counted (high abundances of damselflies were estimated instantaneously). The surveys were conducted on sunny days, between 10 a.m. and 4 p.m. Adults were mostly observed visually, identified by eye, or using close focusing binoculars. Some species were sampled using an entomological net (e.g., those from the genus Sympetrum). Collected individuals were identified in the field, photographed, and released. Surveys of all sites were conducted on foot by the same observer (M. V.). Taxonomy follows Ref. [57].

Figure 2.
Examples of the sites where Odonata were studied at four intermittent karst rivers (Croatia): sites characterized by poorly developed aquatic vegetation: (a) the Krčić River (Site 3), (b) the Guduča River (Site 1), (c) the Čikola River (Site 3); sites with well-developed aquatic vegetation: (d) the Čikola River (Site 2), (e) the Guduča River (Site 3), (f) the Miljašić Jaruga River (Site 1).
2.5. Data Analyses
Among a total of 12 study sites, six macrophyte-rich (MRH) and six macrophyte-poor habitats (MPH) were identified based on their aquatic macrophyte species richness and abundance (>10% habitat covered with macrophytes in MRH, <10% in MPH). Species richness and abundance data were tested for normality using the Shapiro–Wilk test in SPSS Statistics ver. 27.0 [58]. Then, aquatic macrophytes (total) and vascular plants (low and high) were analyzed with respect to species richness and abundance in two focal habitats: MPH vs. MRH, using Mann–Whitney U-tests in SPSS Statistics ver. 27.0 [58]. Differences in physicochemical water parameters between the MPH and MRH were tested using generalized linear mixed models (GLMMs). In all the constructed models, we included sites (level 1) nested within macrophyte vegetation type (level 2) with sampling events as repeated measures. For all the physicochemical parameters, we applied the gamma distribution with log link function. The macrophyte vegetation type was used as a fixed effect in all models. To account for the variation introduced by potential differences among sampling sites and events, sites and sampling events were included in all models as random effects, with first-order autoregressive (AR1) covariance type, which was assumed for repeated measures over time [59]. Pairwise contrasts of estimated means were applied using a least significant difference (LSD) post hoc test. We constructed a full model for each target physicochemical variable, as recommended by [60]. The above-mentioned analyses were performed in SPSS Statistics ver. 27.0 [58].
Assemblage metrics: diversity (Shannon diversity index, H′, Simpson diversity index, 1 − λ), species richness (S) and abundance (N), were calculated for Odonata assemblages at each study site in each of the two habitat types (MPH and MRH). In community ecology, it is common to use several diversity indices differing by their sensitivity to rare or common species, i.e., the most commonly used Shannon diversity index is disproportionately sensitive to the rare species, while the Simpson diversity index is disproportionately sensitive to the most common species (see in [61]). Prior to the analysis, assemblage data were tested for normality using the Shapiro–Wilk test in SPSS Statistics ver. 27.0 [58]. The similarity of Odonata assemblages between the two habitats was examined using hierarchical cluster analysis (HCA) based on the Bray–Curtis similarity matrix in Primer 6.0 [62]. Species data were log (x + 1) transformed prior to the HCA. Study sites with no Odonata records were excluded from the HCA. To evaluate the differences in Odonata assemblage metrics between the two habitats, a set of generalized linear mixed models (GLMMs) was constructed. In the construction of all models, we used the same approach as for physicochemical parameters. For species richness and abundance, Poisson distribution was applied, while for diversity indices, gamma distribution was used, with the log link function. The significance of the models was tested using the least significant difference (LSD) post hoc test.
To quantify the functional diversity of Odonata assemblages, a total of 17 functional traits from four groups were used: (i) body size, (ii) nymphal development (all year, mainly in spring, mainly in summer, mainly in autumn, mainly in winter, unknown), (iii) hydrological preference (eupotamon—main channel and connected side arms; parapotamon—side arms connected only at the downstream end at mean water levels; plesiopotamon—no connectivity with the main channel at the mean water level, including lakes, where coverage by macrophytes does not exceed 20%; palaeopotamon—no connectivity with the main channel at mean water levels, including lakes and pools, where coverage by macrophytes exceeds 20%; temporary water bodies—temporary pools, where the water level is primarily dependent on ground water levels) and (iv) drought resilience form (no resilience strategy against droughts, egg diapause—resisting in the egg stage; nymph diapause—resisting in the nymphal stage; adult diapause—resisting in the adult stage; unknown resilience strategy) (retracted from Refs. [57,63,64]) (Appendix A).
The functional diversity of Odonata assemblages was quantified using the Rao quadratic diversity (RaoQ) coefficient, which is a measure of trait convergence or divergence patterns compared to random expectation. Community weighted mean (CWM) values were calculated for each functional trait in Odonata assemblages to quantify shifts in mean trait values within the assemblages, resulting from environmental selection for certain functional trait categories [65]. RaoQ and CWM values were calculated in CANOCO version 5.11 package [66]. Prior to the analysis, functional data were tested for normality using the Shapiro–Wilk test in SPSS Statistics ver. 27.0 [58]. Differences in the RaoQ coefficient and trait CWM values between the two habitats were tested using a generalized linear mixed model (GLMM). We used a gamma distribution for each variable with log link function. We used the same approach as for physicochemical parameters and assemblage metrics to construct the models and test their significance.
The relationship between Odonata assemblages and environmental variables was tested using canonical correspondence analysis (CCA). Odonata represented by fewer than 20 individuals were omitted from the CCA, and a total of 17 species was used in the analysis. To assess the influence of environmental factors on the spatial distribution of CWM values of functional traits in Odonata assemblages, redundancy analysis (RDA) was used. All the recorded species were included in the RDA. A total of six statistically significant environmental variables (water temperature, velocity, hardness, conductivity, abundance of vascular macrophytes, and bryophytes) were included in the CCA and RDA. Prior to the RDA, Odonata abundances were centered and standardized by the average functional traits, while they were log (x + 1) transformed prior to the CCA. To test the relationship between trait or species composition and environmental variables, a Monte Carlo test using 499 permutations (p < 0.05) was performed. These analyses were performed in the CANOCO version 5.11 package [66].
3. Results
3.1. Vegetation Analysis
Vascular macrophyte species richness and abundance were significantly higher in MRH (Table 1) compared to the MPH. The same pattern was observed for species richness and the abundance of low and high vascular plants (Table 1). In MRH, tall (e.g., Phragmites australis (Cav.) Steud., Scirpus lacustris L., Cyperus longus L., Typha angustifolia L.) and low vascular plants (e.g., Lythrum salicaria L., Mentha aquatica L., Alisma plantago-aquatica L., Agrostis stolonifera L.) are intermixed in mosaic assemblages (Figure 2d–f).

Table 1.
Vegetation analysis of macrophyte-poor and macrophyte-rich habitats of four intermittent karst rivers (Croatia), with mean values ± standard error per habitat type (n = 6, for each habitat type). Different letters indicate a significant difference between the habitats (Mann–Whitney U-test, p < 0.01). Legend: LM—low macrophytes, HM—high macrophytes.
On the other hand, aquatic bryophytes were more species rich and abundant at MPH (Table 1), with bryophyte species such as Cinclidotus aquaticus (Hedw.) Bruch et Schimp., C. fontinaloides (Hedw.) P. Beauv., Cratoneuron filicinum (Hedw.) Spruce, Fissidens crassipes Wilson ex Bruch et Schimp., and Rhynchostegium riparioides (Hedw.) Cardot predominating. Those habitats are characterized by low abundance and low number (solely six taxa overall) of macrophyte vascular species (Figure 2a–c).
3.2. Environmental Variables
Alkalinity, water hardness, conductivity, water temperature, and velocity differed significantly between the MPH and MRH (Table 2). MPH were characterized by significantly lower water temperature, hardness, conductivity and alkalinity, and significantly higher water velocity compared to the MRH (Table 2). The other measured parameters did not significantly differ between the two habitat types (MPH and MRH) (Table 2).

Table 2.
Physicochemical properties of water measured in macrophyte-poor and macrophyte-rich habitats of four intermittent karst rivers (Croatia), with mean values ± standard error per habitat type (n = 18, for each habitat type). GLMM (full model) output shows differences in physicochemical water properties between the habitats. For all the physicochemical parameters, the gamma distribution with log link function was applied. Macrophyte vegetation was used as a fixed effect, while sites were included as a random effect. Statistically significant effects obtained from the least significant difference post hoc test (p < 0.05) are reported in bold. Legend: F—F statistic, d.f. 1—degrees of freedom, d.f. 2—denominator degrees of freedom, MPH—macrophyte-poor habitats, MRH—macrophyte-rich habitats.
3.3. Odonata Species Occurrence
In total, 25 Odonata species were recorded (Table 3). Overall, the most numerous species was Platycnemis pennipes (Pallas, 1771), which was also most frequently recorded at MRH. Calopteryx virgo (Linnaeus, 1758) was the most numerous species at MPH (Table 3). Species recorded in low numbers (less than 20 individuals) were Sympetrum fonscolombii (Selys, 1840), S. sanguineum (Müller, 1764), S. meridionale (Selys, 1841), Cordulegaster heros Theischinger, 1979, Aeshna affinis Vander Linden, 1820, Crocothemis erythraea (Brullé, 1832), Orthetrum cancellatum (Linnaeus, 1758), and Somatochlora meridionalis Nielsen, 1935 (Table 3).

Table 3.
Odonata recorded in macrophyte-poor and macrophyte-rich habitats of four intermittent karst rivers (Croatia). Species are represented by the total number of individuals (N) and frequency (%). Species codes are those used in the CCA.
3.4. Odonata Assemblages and Their Functional Diversity
Odonata species richness and diversity were five to seven times significantly higher in MRH than in the MPH (Table 4, Figure 3a,c,d). Furthermore, Odonata abundance was over nine times significantly higher in MRH than in the MPH (Table 4, Figure 3b). Moreover, the results of the cluster analysis revealed clear separation of MPH and MRH, with low similarity of their respective Odonata assemblages, accounting for less than 5% (Figure 4).

Table 4.
GLMM (full model) output showing differences in Odonata assemblage metrics, functional diversity, and community weighted mean (CWM) values of functional traits between macrophyte-poor and macrophyte-rich habitats of four intermittent karst rivers (Croatia). Poisson distribution was applied for species richness and abundance, while for diversity indices and functional traits, gamma distribution was used with log link function. Vegetation was used as a fixed effect, with sites and months as random effects. Statistically significant effects obtained from the least significant difference post hoc test (p < 0.05) are reported in bold. Legend: F—F statistic, d.f. 1—degrees of freedom, d.f. 2—denominator degrees of freedom.
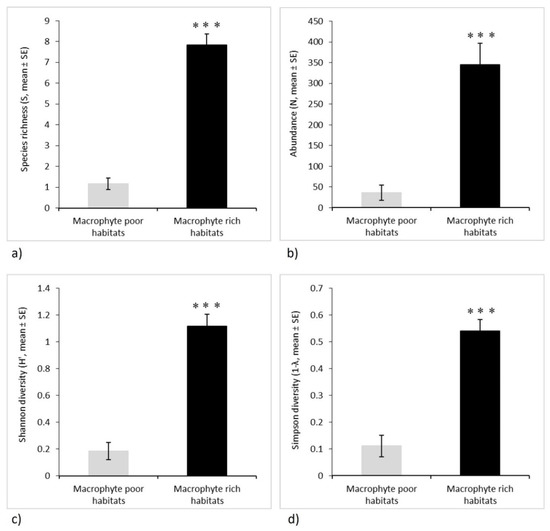
Figure 3.
Odonata assemblages in macrophyte-poor and macrophyte-rich habitats of four intermittent karst rivers (Croatia). Each assemblage metric is shown by mean and standard error: (a) species richness (S), (b) abundance (N), (c) Shannon diversity index (H′), (d) Simpson diversity index (1 − λ). Asterisk indicates a significant difference between the habitats (GLMM including sites as fixed factors and months as repeated measures, least significant difference post hoc test, p < 0.001). Poisson distribution was applied for species richness and abundance, while for diversity indices, gamma distribution was used with log link function.
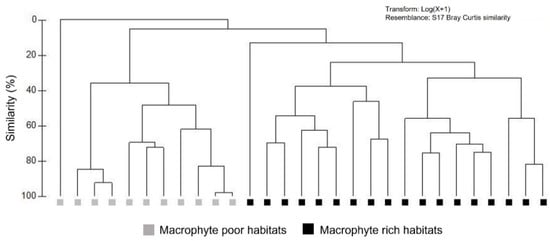
Figure 4.
Cluster analysis of Odonata assemblages in MPH and MRH of four intermittent karst rivers (Croatia), based on Bray–Curtis similarity coefficient and species’ log (x + 1)-transformed abundances. Study sites with no Odonata records were excluded from the analysis.
Functional diversity (RaoQ) was also significantly higher at MRH (Table 4, Figure 5a). Odonata assemblages inhabiting MRH are characterized by significantly higher CWM values of body size compared to the assemblages at MPH. In such habitats, other groups of functional traits were shown to be more diverse compared to those at MPH (Table 4, Figure 5). At MRH, a similar share of species with nymphal development all year, in spring and summer were recorded, while in MPH, species with nymphal development all year dominate (Figure 5). At MRH, we recorded a higher share of species with a preference for lentic hydrological conditions (plesiopotamon, palaeopotamon) and temporary water bodies, while those with a preference for eupotamon (lotic hydrological conditions) dominate at MPH. Finally, at MRH, we recorded a higher number of species with certain strategies to drought resilience (especially with egg diapause), while at MPH, mainly species with no resilience strategy against droughts were recorded (Table 4, Figure 5).
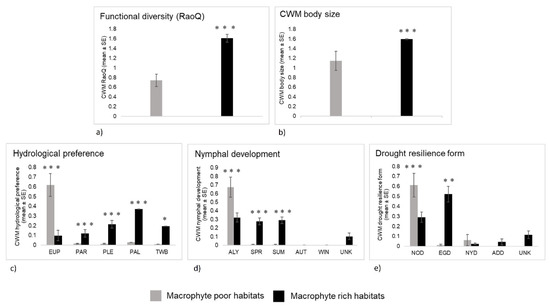
Figure 5.
Odonata functional traits in macrophyte-poor and macrophyte-rich habitats of four intermittent karst rivers (Croatia) shown as mean and standard error: (a) functional diversity (RaoQ), (b) CWM body size, (c) hydrological preference, (d) nymphal development, (e) drought resilience form. Asterisk indicates a significant difference between the habitats (*** = p < 0.001), (** = p < 0.01), (* = p < 0.05). Legend: Hydrological preference: EUP—eupotamon, PAR—parapotamon, PLE—plesiopotamon, PAL—palaeopotamon, TWB—temporary water bodies. Nymphal development: ALY—nymphal development all year, SPR—nymphal development mainly in spring, SUM—nymphal development mainly in summer, AUT—nymphal development mainly in autumn, WIN—nymphal development mainly in winter, UNK—unknown nymphal development. Drought resistance forms: NOD—no resilience strategy against droughts; EGD—egg diapause, NYD—nymph diapause, ADD—adult diapause, UNK—unknown resilience strategy.
3.5. Odonata Species and Functional Traits Related to Environmental Variables
Odonata assemblages clearly differed with respect to two habitat types, as shown by statistically significant results of the CCA (explanatory variables accounted for 52.86%; F ratio = 3.9, p = 0.002). The first two ordination axes (eigenvalues 0.616 and 0.333) explained 40.62% of the variation (Figure 6). Axis 1 was related to conductivity (R = −0.672), water velocity (R = 0.616), and vascular macrophytes (R = −0.433), and axis 2 was related to bryophytes (R = 0.684) (Figure 6). These results indicate a strong separation between the MPH and MRH, with the latter mostly positioned to the left of zero, which is a pattern governed by higher conductivity and an abundance of vascular macrophytes, while all macrophyte-poor habitats were positioned to the right, in correlation with higher water velocity. Lentic species, such as Anax imperator (Selys, 1839), Brachytron pratense (Müller, 1764), and Chalcolestes viridis (Vander Linden, 1825) were positively associated with MRH, while the lotic ones such as Onychogomphus forcipatus (Linnaeus, 1758), Calopteryx splendens (Harris, 1782), and C. virgo were abundant in MPH (Figure 6).
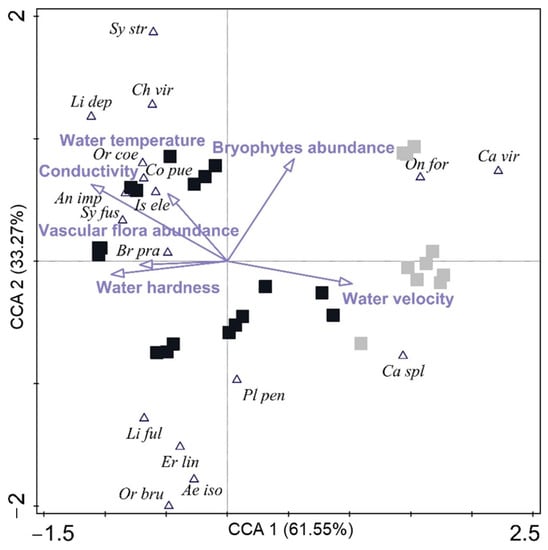
Figure 6.
CCA ordination triplot on standardized and log (x + 1) transformed data of 17 Odonata species and six environmental variables recorded at four intermittent karst rivers (Croatia). Taxa codes (triangles) are presented in Table 3. Black squares represent macrophyte-rich habitats, and grey squares represent macrophyte-poor habitats.
Statistically significant results of RDA showed that Odonata functional traits differed with respect to the studied habitat types (explanatory variables accounted for 61.39%; F ratio = 5.8, p = 0.002). The first two ordination axes (eigenvalues 0.457 and 0.101) explained 55.75% of the variation (Figure 7). The first ordination axis is correlated mainly with macrophytes (vascular macrophytes (R = −0.834) and bryophyte abundance (R = 0.853), and the second axis is correlated mainly with water velocity (R = −0.382), once again indicating a strong separation of MRH (with abundant vascular macrophytes) and MPH (with higher water velocity).
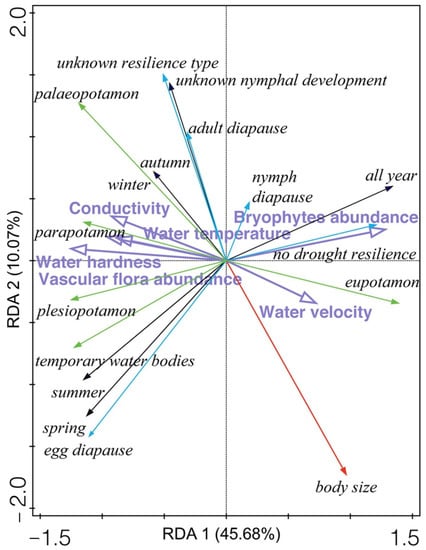
Figure 7.
Redundancy analysis (RDA) ordination biplot showing the relationships between Odonata functional traits (CWM body size—red arrow, CWM hydrological preferences—green arrows, CWM nymphal development—black arrows, CWM drought resilience form—blue arrows) and six significant environmental variables (purple arrows) at four intermittent karst rivers (Croatia).
4. Discussion
With a total of 25 recorded species (37% of Croatian, 15% of European Odonata fauna) [67,68], our results indicate rather high Odonata species richness in the studied intermittent karst rivers, similar to previous studies on IRES in the Mediterranean area [69,70,71]. Although ephemeral and intermittent Mediterranean streams generally have different and less diverse macroinvertebrate communities compared to the perennial ones [16,70,72,73], previous studies observed a shift in community structure with changing hydrology. Lotic diversity tends to decrease with increasing flow intermittence, but in such habitats, there is often a compensation with an increase in lentic diversity, including Odonata [6,70]. This is not surprising, as higher numbers of European Odonata prefer lentic habitats or lotic ones with low water velocity, which are characterized by higher habitat heterogeneity [57,74]. One of the species recorded in our study, C. heros, is of international conservation concern. The species is endemic to Central and Southeastern Europe [57,75], and it is one of the eight near-threatened (NT) European Odonata [75]. It is indicative of pristine lotic habitats and thus is listed in Annexes II and IV of the EU Habitat Directive and in Annex I of the Bern Convention [75], implying that its habitats should not be altered [76]. The occurrence of C. heros in the studied IRES indicates the potential conservation value of such habitats.
The current study shows that Odonata assemblages in the studied intermittent rivers are highly influenced by habitat features, such as the structure and abundance of aquatic macrophytes, and physicochemical water parameters, particularly conductivity and water velocity. Physicochemical water properties and aquatic vegetation composition are strongly mutually influenced [77,78]. Our results corroborate the results of previous studies showing that the diversity of vascular macrophyte assemblages increases with decreasing water velocity, while bryophytes are generally associated with more turbulent water flow [78,79]. High abundances of vascular macrophytes lead to the increase in flow resistance and sedimentation of organic debris, which results in reduced water velocity, increased conductivity, and produces lentic conditions in a particular habitat [80,81]. In IRES, the occurrence of lentic conditions within a stream or river is enhanced by the absence of high flow periods that could limit the growth of vascular macrophytes [78,82].
Our study shows that abundant and diverse macrophyte vegetation promotes increased abundance, species richness, taxonomic, and functional diversity of Odonata assemblages in intermittent karst rivers. This corroborates the results of previous studies frequently demonstrating close relationship between Odonata and aquatic vegetation [31,32,33,83,84,85,86,87]. Odonata require aquatic vegetation to complete key stages of their life cycle, using it as shelter and hunting ground (both as nymphs and adults) [34,88,89], for emergence [90], perching, thermoregulation, and oviposition in the adult stage [34,91,92]. The presence of water and emergent macrophyte vegetation are the most important visual cues for adult habitat selection [93,94], yet they respond more to the structural variety of macrophytes than to macrophyte species composition [34,95].
Odonata assemblages in macrophyte-rich habitats showed higher values of body size, which is probably due to the preference of the recorded Anisoptera (e.g., Aeshna isoceles (Müller, 1767), A. imperator, B. pratense) toward such habitats. This preference is reflected in their higher species richness and abundance in macrophyte-rich habitats compared to macrophyte poor ones. Due to the lower water velocity and higher abundance of vascular macrophytes, such habitats had numerous lentic sections along the river course, and consequently a higher share of species with the preference for lentic hydrological conditions (such as Sympecma fusca (Vander Linden, 1820), A. isoceles, and B. pratense) and the species frequently occurring in temporary water bodies (such as C. viridis, S. meridionale, and Libellula depressa Linnaeus, 1758) [63,64]. During the fieldwork, we also observed high abundances of teneral individuals of many of the recorded lentic Zygoptera species (e.g., C. viridis, S. fusca), as well as the reproductive behavior of many of the lentic Anisoptera and Zygoptera, e.g., copulation and oviposition. Therefore, those species are very likely to complete their life cycle in macrophyte-rich intermittent rivers. Nevertheless, we strongly recommend future studies to be focused on systematic nymph-focused research. On the other hand, macrophyte-poor habitats were characterized by more turbulent water flow, and their assemblages consisted predominantly of lotic species (such as C. virgo, O. forcipatus, and C. heros) [63,64].
In macrophyte-rich habitats, we recorded a higher number of species with certain drought resilience strategies, especially egg diapause (such as in C. viridis, A. affinis, and most of the recorded Sympetrum species). In addition, Odonata assemblages in such habitats consisted of species whose nymphs develop all year round or specifically in spring or summer. After the oviposition into the waterbody, in order to survive harsh environmental conditions such as droughts, eggs may go through a diapause, or they begin to develop immediately into the aquatic nymphs. In intermittent habitats, rapid growth is crucial, as the nymphs must develop rapidly to emerge into aerial adults before the habitat dries out. Such nymphs generally have faster development that occurs within weeks after oviposition [96]. Therefore, drought resilience strategies and nymphal development are most likely closely related traits in the studied Odonata assemblages. Due to relatively high drought resilience, Odonata are often amongst the dominant and relatively diverse taxa in the Mediterranean intermittent rivers and streams [70,72,97]. In contrast, macrophyte-poor habitats in our study were characterized mainly by the species with all year-round nymphal development and with no drought resilience strategies [63,64]. However, for some of those species, drought-survival strategies and mechanisms may be insufficiently known (e.g., C. splendens, C. heros) [69,98], or their occurrence in the studied IRES could be the result of the adult search for food resources (e.g., S. meridionalis, O. forcipatus) [69,99]. Thus, their occurrence in IRES should be confirmed with future, nymph-focused studies.
5. Conclusions
The current study revealed high Odonata species richness in karst IRES ecosystems. One of the recorded species is of international conservation concern, indicating the potential conservation value of IRES habitats. Habitats with well-developed aquatic macrophytes promote higher abundance as well as the taxonomic and functional diversity of Odonata assemblages compared to the habitats with poorly developed macrophytes. In addition to aquatic vegetation, physicochemical water properties, particularly conductivity and water velocity, are shown to be amongst the most significant determinants of Odonata assemblages in the studied IRES. To define adequate conservation measures for habitats and the species they support, it is crucial to understand species diversity patterns related to the quality of their environment [100]. Therefore, the current study represents an interesting contribution to our knowledge of Odonata diversity and their ecological requirements in intermittent karst rivers in the Mediterranean. These results also provide some new insights that could be useful for sampling protocol development and the bioassessment of IRES.
Author Contributions
Conceptualization, M.V. and A.B.; methodology, M.V. and A.B.; formal analysis, A.B., M.V. and V.Š.; investigation, M.V.; resources, A.B. and R.M.K.; data curation, M.V., A.B., F.R., M.R., R.M.K., V.Š. and L.R.; writing—original draft preparation, M.V. and A.B.; writing—review and editing, M.V., A.B., F.R., M.R., R.M.K., V.Š. and L.R; visualization, M.V., A.B. and L.R.; supervision, M.V. and A.B.; project administration, M.V. and A.B.; funding acquisition, A.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Croatian Scientific Foundation, DinDRY to A.B., grant number UIP-2020-02-5385.
Institutional Review Board Statement
The study was approved by The Ministry of Economy and Sustainable Development of the Republic of Croatia with a permission for investigating in the protected area and for sampling protected taxa (permission number 640-01/21-01/7).
Data Availability Statement
Data are available from the corresponding author upon request. The data are not publicly available [due to the authors’ policy of saving unpublished data for future publications].
Acknowledgments
We kindly thank Vladimir Bartovsky and Marija Starčević for help with the sampling. Zlatko Mihaljević and employers of the Public Institution Nature of the Šibenik-Knin County for their help with study site selection. Ivka Štefanić and Vesna Gulin are thanked for their help with the laboratory analysis of the water samples. Finally, three anonymous reviewers are thanked for their useful comments and suggestions that helped to improve this paper.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
Four groups of functional traits characteristic for the recorded Odonata species (body size according to Ref. [57]), other functional traits adapted from Refs. [63,64]). Legend: eupotamon = main channel and connected side arms; parapotamon = side arms connected only at the downstream end at mean water levels, plesiopotamon (including lakes) = no connectivity with the main channel at mean water levels; coverage by macrophytes does not exceed 20%, palaeopotamon (incl. pools, ponds) = no connectivity with the main channel at mean water levels; coverage by macrophytes exceeds 20%.
Table A1.
Four groups of functional traits characteristic for the recorded Odonata species (body size according to Ref. [57]), other functional traits adapted from Refs. [63,64]). Legend: eupotamon = main channel and connected side arms; parapotamon = side arms connected only at the downstream end at mean water levels, plesiopotamon (including lakes) = no connectivity with the main channel at mean water levels; coverage by macrophytes does not exceed 20%, palaeopotamon (incl. pools, ponds) = no connectivity with the main channel at mean water levels; coverage by macrophytes exceeds 20%.
| Species Name | Functional Traits | |||
|---|---|---|---|---|
| Average Body Size (mm) | Hydrological Preference | Nymphal Development | Drought Resilience Form | |
| Calopteryx splendens (Harris, 1782) | 46.5 | Lotic (eupotamon, parapotamon) | All year | No drought resilience |
| Calopteryx virgo (Linnaeus, 1758) | 47.0 | Lotic (eupotamon) | All year | No drought resilience |
| Chalcolestes viridis (Vander Linden, 1825) | 43.5 | Predominantly lentic (plesiopotamon, palaeopotamon), but can occur in lotic habitats. Recorded from temporary waterbodies. | Spring, summer | Egg diapause |
| Sympecma fusca (Vander Linden, 1820) | 36.5 | Predominantly lentic (plesiopotamon, palaeopotamon), but can occur in lotic habitats. Recorded from temporary waterbodies. | Mainly in summer | Adult diapause |
| Ischnura elegans (Van der Linden, 1820) | 32.0 | Eurytopic. Recorded from temporary waterbodies | All year | No drought resilience |
| Coenagrion puella (Linnaeus, 1758) | 34.0 | Predominantly lentic (plesiopotamon, palaeopotamon), but can occur in lotic habitats. Recorded from temporary waterbodies. | All year | No drought resilience |
| Erythromma lindenii (Selys, 1840) | 33.0 | Predominantly lentic (plesiopotamon, palaeopotamon), but can occur in lotic habitats | Unknown | Unknown |
| Platycnemis pennipes (Pallas, 1771) | 36.0 | Predominantly lotic (eupotamon, parapotamon) but can occur in lentic habitats | All year | No drought resilience |
| Aeshna affinis Vander Linden, 1820 | 61.5 | Lentic (palaeopotamon). Recorded from temporary waterbodies | Spring, summer | Egg diapause |
| Aeshna isoceles (Müller, 1767) | 64.0 | Lentic (plesiopotamon, palaeopotamon) | All year | No drought resilience |
| Anax imperator (Selys, 1839) | 75.0 | Predominantly lentic (plesiopotamon, palaeopotamon), but can occur in lotic habitats | All year | No drought resilience |
| Brachytron pratense (Müller, 1764) | 58.5 | Predominantly lentic (plesiopotamon, palaeopotamon), but can occur in lotic habitats | All year | No drought resilience |
| Onychogomphus forcipatus (Linnaeus, 1758) | 48.0 | Lotic (eupotamon, parapotamon) | All year | No drought resilience |
| Cordulegaster heros Theischinger, 1979 | 80.5 | Lotic (eupotamon) | All year | No drought resilience |
| Somatochlora meridionalis Nielsen, 1935 | 52.5 | Lotic (eupotamon, parapotamon) | All year | No drought resilience |
| Libellula depressa Linnaeus, 1758 | 43.5 | Predominantly lentic (plesiopotamon, palaeopotamon), but can occur in lotic habitats. Recorded from temporary waterbodies. | All year | Nymph diapause |
| Libellula fulva Müller, 1764 | 43.5 | Predominantly lotic (eupotamon, parapotamon) but can occur in lentic habitats | All year | Nymph diapause |
| Orthetrum cancellatum (Linnaeus, 1758) | 47.0 | Predominantly lotic (eupotamon, parapotamon) but can occur in lentic habitats | All year | No drought resilience |
| Orthetrum coerulescens (Fabricius, 1798) | 40.5 | Predominantly lotic (eupotamon, parapotamon) but can occur in lentic habitats. Recorded from temporary waterbodies | All year | Nymph diapause |
| Orthetrum brunneum (Fonscolombe, 1837) | 45.0 | Lotic (eupotamon, parapotamon) | All year | Unknown |
| Sympetrum sanguineum (Müller, 1764) | 36.5 | Predominantly lentic (plesiopotamon, palaeopotamon), but can occur in lotic habitats. Recorded from temporary waterbodies. | Spring, summer | Egg diapause |
| Sympetrum fonscolombii (Selys, 1840) | 36.5 | Eurytopic. Recorded from temporary waterbodies | All year | Unknown |
| Sympetrum striolatum (Charpentier, 1840) | 39.5 | Eurytopic. Recorded from temporary waterbodies | All year | Egg diapause |
| Sympetrum meridionale (Selys, 1841) | 37.5 | Lentic (palaeopotamon). Recorded from temporary waterbodies | Spring, summer | Egg diapause |
| Crocothemis erythraea (Brullé, 1832) | 40.5 | Predominantly lentic (plesiopotamon, palaeopotamon), but can occur in lotic habitats | All year | No drought resilience |
References
- Lytle, D.A.; Poff, N.L. Adaptation to natural flow regimes. Trends Ecol. Evol. 2004, 19, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Datry, T.; Bonada, N.; Boulton, A. Intermittent Rivers and Ephemeral Streams: Ecology and Management, 1st ed.; Academic Press: Cambridge, MA, USA, 2017. [Google Scholar]
- McDonοugh, O.T.; Hosen, J.D.; Palmer, M.A. Temporary streams: The hydrology, geography and ecology of non-perennially flowing waters. In River Ecosystems: Dynamics, Management and Conservation, 1st ed.; Elliot, H.S., Martin, L.E., Eds.; Nova Science Publ. Inc.: New York, NY, USA, 2011; pp. 259–289. [Google Scholar]
- Arthington, A.H.; Bernardo, J.M.; Ilhèu, M. Temporary rivers: Linking ecohydrology, ecological quality and reconciliation ecology. River Res. Appl. 2014, 30, 1209–1215. [Google Scholar] [CrossRef]
- Tockner, K.; Robinson, C.T.; Uehlinger, U. Rivers of Europe, 1st ed.; Academic Press: Cambridge, MA, USA, 2009. [Google Scholar]
- Larned, S.T.; Datry, T.; Arscott, D.B.; Tockner, K. Emerging concepts in temporary-river ecology. Freshw. Biol. 2010, 55, 717–738. [Google Scholar] [CrossRef]
- Vörösmarty, C.; McIntyre, P.; Gessner, M.; Dudgeon, D.; Prusevich, A.; Green, P.; Glidden, S.; Bunn, S.E.; Sullivan, C.A.; Reidy Liermann, A.; et al. Global threats to human water security and river biodiversity. Nature 2010, 468, 334. [Google Scholar] [CrossRef]
- Stubbington, R.; Chadd, R.; Cid, N.; Csabai, Z.; Miliša, M.; Morais, M.; Munné, A.; Pařil, P.; Pešić, V.; Tziortzis, I.; et al. Biomonitoring of intermittent rivers and ephemeral streams in Europe: Current practice and priorities to enhance ecological status assessments. Sci. Total Environ. 2018, 618, 1096–1113. [Google Scholar] [CrossRef] [PubMed]
- Boix, D.; García-Berthou, E.; Gascón, S.; Benejam, L.L.; Tornés, E.; Sala, J.; Benito, J.; Munné, A.; Solà, C.; Sabater, S. Response of community structure to sustained drought in Mediterranean rivers. J. Hydrol. 2010, 383, 135–146. [Google Scholar] [CrossRef]
- Gleick, P.H. Global freshwater resources: Soft-path solutions for the 21st century. Science 2003, 302, 1524–1528. [Google Scholar] [CrossRef] [PubMed]
- Shanafield, M.; Bourke, S.A.; Zimmer, M.A.; Costigan, K.H. An overview of the hydrology of non-perennial rivers and streams. WIREs Water 2021, 8, 1–25. [Google Scholar] [CrossRef]
- Döll, P.; Schmied, H.M. How is the impact of climate change on river flow regimes related to the impact on mean annual runoff? A global-scale analysis. Environ. Res. Lett. 2012, 7, 14–37. [Google Scholar] [CrossRef]
- Datry, T.; Larned, S.T.; Tockner, T. Intermittent rivers: A challenge for freshwater ecology. BioScience 2014, 64, 229–235. [Google Scholar] [CrossRef]
- del Rosario, R.B.; Resh, V.H. Invertebrates in intermittent and perennial streams: Is the hyporheic zone a refuge from drying? J. N. Am. Benthol. Soc. 2000, 19, 680–696. [Google Scholar] [CrossRef]
- Bonada, N.; Zamora-Munoz, C.; Rieradevall, M.; Prat, N. Ecological profiles of caddisfly larvae in Mediterranean streams: Implications for bioassessment methods. Environ. Pollut. 2004, 132, 509–521. [Google Scholar] [CrossRef]
- Argyroudi, A.; Chatzinikolaou, Y.; Poirazidis, K.; Lazaridou, M. Do intermittent and ephemeral Mediterranean rivers belong to the same river type? Aquat. Ecol. 2009, 43, 465–476. [Google Scholar]
- Government of the Republic of Croatia (GRC). Directive on Water Classification. Available online: https://www.irb.hr/ (accessed on 28 November 2021).
- Golfieri, B.; Hardersen, S.; Maiolini, B.; Surian, N. Odonates as indicators of the ecological integrity of the river corridor: Development and application of the Odonate River Indec (ORI) in northern Italy. Ecol. Indic. 2016, 61, 234–247. [Google Scholar] [CrossRef]
- Martín, R.; Maynou, X. Dragonflies (Insecta: Odonata) as indicators of habitat quality in Mediterranean streams and rivers in the province of Barcelona (Catalonia, Iberian Peninsula). Int. J. Odonatol. 2016, 19, 107–124. [Google Scholar] [CrossRef]
- Oliveira-Junior, J.M.B.; Shimano, Y.; Gardner, T.A.; Hughes, R.M.; De Marco, P.; Juen, L. Neotropical dragonflies (Insecta: Odonata) as indicators of ecological condition of small streams in the eastern Amazon. Austral Ecol. 2015, 40, 733–744. [Google Scholar] [CrossRef]
- O’Malley, Z.G.; Compson, Z.G.; Orlofske, J.M.; Baird, D.J.; Curry, R.A.; Monk, W.A. Riparian and in-channel habitat properties linked to dragonfly emergence. Sci. Rep. 2020, 10, 17665. [Google Scholar] [CrossRef]
- Vilenica, M. Ecological traits of dragonfly (Odonata) assemblages along an oligotrophic Dinaric karst hydrosystem. Ann. Limnol. -Int. J. Lim. 2017, 53, 377–389. [Google Scholar] [CrossRef]
- Vilenica, M.; Kerovec, M.; Pozojević, I.; Mihaljević, Z. Odonata assemblages in anthropogenically impacted lotic habitats. J. Limnol. 2020, 80, 1–9. [Google Scholar] [CrossRef]
- Brito, J.; Calvão, L.; Cunha, E.; Maioli, L.; Barbirato, M.; Rolim, S.; Juen, L. Environmental variables affect the diversity of adult damselflies (Odonata: Zygoptera) in western Amazonia. Int. J. Odonatol. 2021, 24, 108–121. [Google Scholar] [CrossRef]
- Adu, B.W.; Amusan, B.O.; Oke, T.O. Assessment of the water quality and Odonata assemblages in three waterbodies in Ilara-Mokin, south-western Nigeria. Int. J. Odonatol. 2019, 22, 101–114. [Google Scholar] [CrossRef]
- Oliveira-Junior, J.M.B.; Juen, L. Structuring of dragonfly communities (insecta: Odonata) in Eastern Amazon: Effects of environmental and spatial factors in preserved and altered streams. Insects 2019, 10, 322. [Google Scholar] [CrossRef]
- Brito, J.S.; Michelan, T.S.; Juen, L. Aquatic macrophytes are important substrates for Libellulidae (Odonata) larvae and adults. Limnology 2021, 22, 139–149. [Google Scholar] [CrossRef]
- McAbendroth, L.; Ramsay, P.M.; Foggo, A.; Rundle, S.D.; Bilton, D.T. Does macrophyte fractal complexity drive invertebrate diversity, biomass and body size distributions? Oikos 2005, 111, 279–290. [Google Scholar] [CrossRef]
- Silva, C.V.; Henry, R. Aquatic invertebrates assemblages associated with two floating macrophytes species of contrasting root systems in a tropical wetland. Limnology 2020, 21, 107–118. [Google Scholar] [CrossRef]
- Vilenica, M.; Alegro, A.; Koletić, N.; Mihaljević, Z. New evidence of Lindenia tetraphylla (Vander Linden, 1825) (Odonata, Gomphidae) reproduction at the north-western border of its distribution. Nat. Croat. 2016, 25, 287–294. [Google Scholar] [CrossRef][Green Version]
- Calvão, L.B.; Juen, L.; Oliveira-Junior, J.M.B.; Batista, J.D.; De, M.P.J. Land use modifies Odonata diversity in streams of the Brazilian Cerrado. J. Insect Conserv. 2018, 22, 675–685. [Google Scholar] [CrossRef]
- Huikkonen, I.; Helle, I.; Elo, M. Heterogenic aquatic vegetation promotes abundance and species richness of Odonata (Insecta) in constructed agricultural wetlands. Insect Conserv. Divers. 2019, 13, 374–383. [Google Scholar] [CrossRef]
- Vilenica, M.; Pozojević, I.; Vučković, N.; Mihaljević, Z. How suitable are man-made water bodies as habitats for Odonata? Knowl. Manag. Aquat. Ecosyst. 2020, 421, 1–10. [Google Scholar] [CrossRef]
- Corbet, P.S. Dragonflies: Behavior and Ecology of Odonata; Harley Books: Colchester, UK, 1999. [Google Scholar]
- Stoks, R.; McPeek, M.A. Predators and life histories shape Lestes damselfly assemblages along a freshwater habitat gradient. Ecology 2003, 84, 1576–1587. [Google Scholar] [CrossRef]
- Magnusson, A.K.; Williams, D.D. The roles of natural temporal and spatial variation versus biotic influences in shaping the physicochemical environment of intermittent ponds: A case study. Arch. Hydrobiol. 2006, 165, 537–556. [Google Scholar] [CrossRef]
- Chester, E.T.; Robson, B.J. Drought refuges, spatial scale and recolonisation by invertebrates in non-perennial streams. Freshw. Biol. 2011, 56, 2094–2104. [Google Scholar] [CrossRef]
- Deacon, C.; Samways, M.J.; Pryke, J.S. Aquatic insects decline in abundance and occupy low-quality artificial habitats to survive hydrological droughts. Freshw. Biol. 2019, 64, 1643–1654. [Google Scholar] [CrossRef]
- Illies, J. Limnofauna Europaea, 2nd ed.; Gustav Fischer Verlag: Stuttgart, Germany, 1978. [Google Scholar]
- Mihevc, A.; Zupan-Hajna, N.; Prelovšek, M. Case study from the Dinaric karst of Slovenia. In Introduction to the Dinaric Karst, 1st ed.; Mihevc, A., Prelovšek, M., Zupan Hajna, N., Eds.; Karst Research Institute: Postojna, Slovenia, 2010; pp. 49–66. [Google Scholar]
- Bonacci, O.; Željković, I.; Galić, A. Karst rivers’ particularity: An example from Dinaric karst (Croatia/Bosnia and Herzegovina). Environ. Earth Sci. 2013, 70, 963–974. [Google Scholar] [CrossRef]
- Previšić, A.; Walton, C.; Kučinić, M.; Mitrikeski, P.T.; Kerovec, M. Pleistocene divergence of Dinaric Drusus endemics (Trichoptera, Limnephilidae) in multiple microrefugia within the Balkan Peninsula. Mol. Ecol. 2009, 18, 634–647. [Google Scholar] [CrossRef] [PubMed]
- Suhling, F.; Sahlén, G.; Gorb, S.; Kalkman, V.J.; Dijkstra, K.D.B.; van Tol, J. Order odonata. In Thorp and Covich’s Freshwater Invertebrates, 4th ed.; Thorp, J., Rogers, D.C., Eds.; Academic Press: Cambridge, MA, USA, 2015; pp. 893–932. [Google Scholar] [CrossRef]
- Pešić, V.; Gligorović, B.; Savić, A.; Buczyński, P. Ecological patterns of Odonata assemblages in karst springs in central Montenegro. Knowl. Manag. Aquat. 2017, 418, 1–20. [Google Scholar] [CrossRef]
- Bonacci, O. Hydrological investigations of Dinaric Karst at the Krčić catchment and the River Krka springs (Yugoslavia). J. Hydrol. 1985, 82, 317–326. [Google Scholar] [CrossRef]
- Bonacci, O.; Jukić, D.; Ljubenkov, I. Definition of catchment area in karst: Case of the rivers Krčić and Krka, Croatia. Hydrol. Sci. J. 2006, 51, 682–699. [Google Scholar] [CrossRef][Green Version]
- Bonacci, O.; Terzić, J.; Roje-Bonacci, T. Hidrološka analiza krške rijeke Čikole. Hrvat. Vode 2018, 26, 281–292. [Google Scholar]
- Bonacci, O.; Terzić, J.; Roje-Bonacci, T.; Frangen, T. An intermittent karst river: The case of the Čikola River (Dinaric Karst, Croatia). Water 2019, 11, 2415. [Google Scholar] [CrossRef]
- Brkić, Ž.; Biondić, R.; Pavičić, A.; Slišković, I.; Marković, T.; Terzić, J.; Dukarić, F.; Dolić, M. Određivanje Cjelina Podzemnih Voda na Jadranskom Slivu prema Kriterijima Okvirne Direktive o Vodama EU; Hrvatski Geološki Institute: Zagreb, Croatia, 2006. [Google Scholar]
- Magaš, D. Geographical factors of formation and development of the neolithic settlement Crno Vrilo. In Crno Vrilo, 1st ed.; Marijanović, B., Ed.; University of Zadar: Zadar, Croatia, 2009; pp. 7–23. [Google Scholar]
- Lukač Reberski, J.; Rubinić, J.; Terzić, J.; Radišić, M. Climate change impacts on groundwater resources in the coastal karstic Adriatic area: A case study from the Dinaric karst. Nat. Resour. Res. 2020, 29, 1975–1988. [Google Scholar] [CrossRef]
- Cukrov, N.; Frančišković-Bilinski, S.; Mikac, N.; Roje, V. Natural and anthropogenic influences recorded in sediments from the Krka river estuary (Eastern Adriatic coast), evaluated by statistical methods. Fresnius Environ. Bull. 2008, 17, 855–863. [Google Scholar]
- Barkman, J.J.; Doing, H.; Segal, S. Kritische bemerkungen und vorschläge zur quantitativen vegetationsanalyse. Acta Bot. Neerl. 1964, 13, 394–419. [Google Scholar] [CrossRef]
- Braun-Blanquet, J. Pflanzensoziologie, Grundzüge der Vegetationskunde, 3rd ed.; Springer: Berlin/Heidelberg, Germany, 1964. [Google Scholar]
- Dierschke, H. Pflanzensoziologie: Grundlagen und Methoden, 1st ed.; UTB: Stuttgart, Germany, 1994. [Google Scholar]
- APHA. Standard Methods for the Examination of Water and Wastewater, 18th ed.; American Public Health Association: Washington, DC, USA, 1992. [Google Scholar]
- Dijkstra, K.D.B.; Lewington, R. Field Guide to the Dragonflies of Britain and Europe, 1st ed.; British Wildlife Publishing: Gillingham, UK, 2006. [Google Scholar]
- IBM Corp. IBM SPSS Statistics for Windows, Version 27.0; IBM: Armonk, NY, USA, 2020. [Google Scholar]
- Field, A. Discovering Statistics Using SPSS, 3rd ed.; SAGE Publications: London, UK, 2009. [Google Scholar]
- Thiele, J.; Markussen, B. Potential of GLMM in modelling invasive spread. CAB Rev. Perspect. Agric. Vet. Sci. Nutr. Nat. Resour. 2012, 7, 1–10. [Google Scholar] [CrossRef]
- Jost, L. Partitioning diversity into independent alpha and beta components. Ecology 2007, 88, 2427–2439. [Google Scholar] [CrossRef] [PubMed]
- Clarke, K.R.; Gorley, R.N. Primer V6: User Manual/Tutorial; Plymouth: Auckland, New Zealand, 2006. [Google Scholar]
- Freshwaterecology. Available online: https://www.freshwaterecology.info/fwe_search.php?og=mzb (accessed on 3 November 2021).
- Schmidt-Kloiber, A.; Hering, D. www.freshwaterecology.info—An online tool that unifies, standardises and codifies more than 20,000 European freshwater organisms and their ecological preferences. Ecol. Indic. 2015, 53, 271–282. [Google Scholar] [CrossRef]
- Ricotta, C.; Moretti, M. CWM and Rao’s quadratic diversity: A unified framework for functional ecology. Oecologia 2011, 167, 181–188. [Google Scholar] [CrossRef]
- ter Braak, C.J.F.; Šmilauer, P. Canoco Reference Manual and User’s Guide: Software for Ordination, Version 5.0; Microcomputer Power: Ithaca, NY, USA, 2012. [Google Scholar]
- Kotarac, M.; Šalamun, A.; Vilenica, M. EU Natura 2000 Integration Project: Field Research and Laboratory Processing for Collecting New Inventory Data for Taxonomic Groups: Actinopterygii and Cephalaspidomorphi, Amphibia and Reptilia, Aves, Chiroptera, Decapoda, Lepidoptera, Odonata, Plecoptera–Final Report for the Taxonomic Group Odonata; Ministry of Environmental and Nature Protection: Zagreb, Croatia, 2016.
- Boudot, J.P.; Kalkman, V.J. Atlas of the European Dragonflies and Damselflies; KNNV Uitgeverij: Zeist, The Netherlands, 2015. [Google Scholar]
- Hardersen, S. Dragonfly (Odonata) communities at three lotic sites with different hydrological characteristics. Ital. J. Zool. 2008, 75, 271–283. [Google Scholar] [CrossRef]
- Belmar, O.; Velasco, J.; Gutiérrez-Cánovas, C.; Mellado-Díaz, A.; Millán, A.; Wood, P.J. The influence of natural flow regimes on macroinvertebrate assemblages in a semiarid Mediterranean basin. Ecohydrology 2012, 6, 363–379. [Google Scholar] [CrossRef]
- Yalles Satha, A.; Samraoui, B. Environmental factors influencing Odonata communities of three Mediterranean rivers: Kebir-East, Seybouse, and Rhummel wadis, northeastern Algeria. Rev. Ecol. 2017, 72, 314–329. [Google Scholar]
- Bonada, N.; Rieradevall, M.; Prat, N.; Resh, V.H. Benthic macro invertebrate assemblages and macrohabitat connectivity in Mediterranean-climate streams of northern California. J. N. Am. Benthol. Soc. 2006, 25, 32–43. [Google Scholar] [CrossRef]
- Bonada, N.; Rieradevall, M.; Prat, N. Macroinvertebrate community structure and biological traits related to flow permanence in a Mediterranean river network. Hydrobiologia 2007, 589, 91–106. [Google Scholar] [CrossRef]
- Oliveira-Junior, J.M.B.; Dias-Silva, K.; Teodósio, M.A.; Juen, L. The response of neotropical dragonflies (insecta: Odonata) to local and regional abiotic factors in small streams of the amazon. Insects 2019, 10, 446. [Google Scholar] [CrossRef]
- Kalkman, V.J.; Boudot, J.-P.; Bernard, R.; Conze, K.-J.; De Knijf, G.; Dyatlova, E.; Ferreira, S.; Jović, M.; Ott, J.; Riservato, E.; et al. European Red List of Dragonflies; Publications Office of the European Union: Luxembourg City, Luxembourg, 2010. [Google Scholar]
- The IUCN Red List of Threatened Species. Available online: https://doi.org/10.2305/IUCN.UK.2010-1.RLTS.T158700A5263990.en (accessed on 16 November 2021).
- Biudes, J.F.V.; Camargo, A.F.M. Studying the limitant factors to primary production of aquatic macrophytes in Brazil. (Estudos dos Fatores Limitantes à Produção Primária por Macrófitas Aquáticas no Brasil). Oecol. Aust. 2008, 12, 7–19. [Google Scholar] [CrossRef][Green Version]
- Buffagni, A.; Erba, S.; Armanini, D.G. The lentic–lotic character of Mediterranean rivers and its importance to aquatic invertebrate communities. Aquat. Sci. 2010, 72, 45–60. [Google Scholar] [CrossRef]
- Kemp, J.L.; Harper, D.M.; Crosa, G.A. Use of ‘functional habitats’ to link ecology with morphology and hydrology in river rehabilitation. Aquat. Conserv. Mar. Freshw. Ecosyst. 1999, 9, 159–178. [Google Scholar] [CrossRef]
- Pitlo, R.H.; Dawson, F.H. Flow-resistance of aquatic weeds. In Aquatic Weeds, The Ecology and Management of Nuisance Aquatic Vegetation, 1st ed.; Pieterse, A.H., Murphy, K.J., Eds.; Oxford University Press: New York, NY, USA, 1990; pp. 74–84. [Google Scholar]
- Lemly, A.D.; Hilderbrand, R.H. Influence of large woody debris on stream insect communities and benthic detritus. Hydrobiologia 2000, 421, 179–185. [Google Scholar] [CrossRef]
- Johnson, W.C. Tree recruitment and survival in rivers: Influence of hydrological processes. Hydrol. Process 2000, 14, 3051–3074. [Google Scholar] [CrossRef]
- Corbet, P.; Brooks, S. Dragonflies, Collins New Naturalist Library No 106; Collins: London, UK, 2008. [Google Scholar]
- Perron, M.A.C.; Pick, F.R. Stormwater ponds as habitat for Odonata in urban areas: The importance of obligate wetland plant species. Biodivers. Conserv. 2020, 29, 913–931. [Google Scholar] [CrossRef]
- Perron, M.A.C.; Richmond, I.C.; Pick, F.R. Plants, water quality and land cover as drivers of Odonata assemblages in urban ponds. Sci. Total Environ. 2021, 773, 145467. [Google Scholar] [CrossRef]
- Daso, J.M.; Arquisal, I.B.; Yuto, C.M.M.; Mondejar, E.P. Species diversity of Odonata in Bolyok Falls, Naawan, Misamis Oriental, Philippines. Aquac. Aquar. Conserv. Legis. 2021, 14, 664–671. [Google Scholar]
- Holtmann, L.; Brüggeshemke, J.; Juchem, M.; Fartmann, T. Odonate assemblages of urban stormwater ponds: The conservation value depends on pond type. J. Insect Conserv. 2019, 23, 123–132. [Google Scholar] [CrossRef]
- Johansson, F.; Brodin, T. Effects of fish predators and abiotic factors on dragonfly community structure. J. Freshw. Ecol. 2003, 18, 415–423. [Google Scholar] [CrossRef]
- Kriska, G. Dragonflies and damselflies—Odonata. In Freshwater Invertebrates in Central Europe, 1st ed.; Springer: Vienna, Austria, 2013; pp. 194–209. [Google Scholar] [CrossRef]
- Boda, R.; Bereczki, C.; Ortmann-Ajkai, A.; Mauchart, P.; Pernecker, B.; Csabai, Z. Emergence behaviour of the red listed Balkan Goldenring (Cordulegaster heros Theischinger, 1979) in Hungarian upstreams: Vegetation structure affects the last steps of the larvae. J. Insect Conserv. 2015, 19, 547–557. [Google Scholar] [CrossRef]
- Wildermuth, H. Habitat selection and oviposition site recognition by the dragonfly Aeshna juncea (L.): An experimental approach in natural habitats (Anisoptera: Aeshnidae). Odonatologica 1993, 22, 27–44. [Google Scholar]
- Purse, B.V.; Thompson, D.J. Oviposition site selection by Coenagrion mercuriale (Odonata: Coenagrionidae). Int. J. Odonatol. 2009, 12, 257–273. [Google Scholar] [CrossRef]
- Wildermuth, H. Dragonflies recognize the water of rendezvous and oviposition sites by horizontally polarized light: A behavioural field test. Sci. Nat. 1998, 85, 297–302. [Google Scholar] [CrossRef]
- Ward, L.; Mill, P.J. Habitat factors influencing the presence of adult Calopteryx splendens (Odonata: Zygoptera). Eur. J. Entomol. 2005, 102, 47–51. [Google Scholar] [CrossRef]
- Steytler, N.S.; Samways, M.J. Biotope selection by adult male dragonflies (Odonata) at an artifical lake created for insect conservation in South Africa. Biol. Conserv. 1995, 72, 381–386. [Google Scholar] [CrossRef]
- McPeek, M.A. Ecological factors limiting the distributions and abundances of Odonata. In Dragonflies: Model Organisms for Ecological and Evolutionary Research, 1st ed.; Córdoba-Aguilar, A., Ed.; Oxford University Press: Oxford, UK, 2008; pp. 51–62. [Google Scholar]
- Sanchez-Montoya, M.D.; Punti, T.; Suarez, M.L.; Vidal-Abarca, M.D.; Rieradevall, M.; Poquet, J.M.; Zamora-Munoz, C.; Robles, S.; Alvarez, M.; Alba-Tercedor, J.; et al. Concordance between ecotypes and macroinvertebrate assemblages in Mediterranean streams. Freshw. Biol. 2007, 52, 2240–2255. [Google Scholar] [CrossRef]
- Pernecker, B.; Mauchart, P.; Csabai, Z. What to do if streams go dry? Behaviour of Balkan Goldenring (Cordulegaster heros, Odonata) larvae in a simulated drought experiment in SW Hungary. Ecol. Entomol. 2020, 45, 1457–1465. [Google Scholar] [CrossRef]
- Petrovičová, K.; Langraf, V.; David, S.; Krumpálová, Z.; Schlarmannová, J. Distinct Odonata assemblage variations in lentic reservoirs in Slovakia (Central Europe). Biologia 2021. [Google Scholar] [CrossRef]
- Veech, J.A.; Summerville, K.S.; Crist, T.O.; Gering, J.C. The additive partitioning of species diversity: Recent revival of an old idea. Oikos 2002, 99, 3–9. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).