Abstract
The Biodiversity and Bioindicators research group (BiBIO), based at the Natural Sciences Museum of Granollers, has coordinated four long-term faunal monitoring programmes based on citizen science over more than two decades in Catalonia (NE Spain). We summarize the historical progress of these programmes, describing their main conservation outputs, the challenges overcome, and future directions. The Catalan Butterfly Monitoring Scheme (CBMS) consists of a network of nearly 200 recording sites where butterfly populations have been monitored through visual censuses along transects for nearly three decades. This programme provides accurate temporal and spatial changes in the abundance of butterflies and relates them to different environmental factors (e.g., habitat and weather conditions). The Bat Monitoring Programme has progressively evolved to include passive acoustic monitoring protocols, as well as bat box-, underground- and river-bat surveys, and community ecological indices have been developed to monitor bat responses at assemblage level to both landscape and climatic changes. The Monitoring of common small mammals in Spain (SEMICE), a common small mammal monitoring programme with almost 80 active live-trapping stations, provides information to estimate population trends and has underlined the relevance of small mammals as both prey (of several predators) and predators (of insect forest pests). The Dormouse Monitoring Programme represents the first monitoring programme in Europe using specific nest boxes for the edible dormouse, providing information about biological and demographic data of the species at the southern limit of its distribution range. The combination and complementarity of these monitoring programmes provide crucial data to land managers to improve the understanding of conservation needs and develop efficient protection laws.
1. Introduction
As biodiversity is becoming increasingly threatened by global change, robust methods for measuring current trends of wild faunal populations are urgently needed. Monitoring programmes aimed at recording biodiversity changes over time have become indispensable tools for biodiversity conservation [1]. However, long-term monitoring schemes generally depend on very demanding and challenging sampling efforts, given the large spatial and temporal resolutions of data collection. Citizen Science (hereafter CS), has increasingly been identified as a popular solution, as it is based on the work of large networks of volunteers for collecting big data that otherwise would be unfeasible to assemble [2]. Despite the term being coined in the 1990s [3], and the fact that much of our current understanding of the natural world has historically been collected by members of the general public [4], the development of CS projects increased continuously during the first decade of the 21st century [5]. Today, due to technological advancements, Internet platforms and social media, a wider range of interested people is willing to collaborate in CS projects [6]. In fact, in recent years, CS programmes have taken a predominant role in monitoring biodiversity and providing essential data for its conservation [7,8] by engaging thousands of individuals who are not trained as scientists to obtain large amounts of data that are then scientifically analysed [4].
However, researchers and conservationists only superficially understand the unique motivations of the volunteers and the wide range of obstacles and barriers affecting their participation in CS. In recent decades, researchers from a broad diversity of disciplines have worked intensively on the engagement of participants and promoting partnerships with local entities focused on incentives towards CS [9]. Volunteers are motivated by a complex framework of factors that change geographically and temporally, at both the personal (e.g., altruism, egoism, collectivism) and scientific level (e.g., conservation needs, novelty). These factors are decisive and pivotal for promoting CS among the general public and increasing the cost efficiency of any scientific research [9].
Among the general barriers hindering CS worldwide, the inconsistency of data quality, the need for intense training and capacitation of volunteers, the fact that not all biodiversity science is well-suited for CS, and the general lack of financial support were highlighted as major limitations, among many others, in [10]. All of these should be accurately assessed and considered when any institution aims to design and establish a successful CS project. To achieve full potential as soon as possible, several guidelines and steps have already been suggested, including specific project design, protocol development, recruitment and training of volunteers, the establishment of coordinators and evaluators, data analysis and interpretation, communication strategy, educational materials preparation, and realistic budget coverage [11]. Indeed, while many citizen science projects emerge yearly almost in every country, many of them perish, and only a few of them endure, providing long-term datasets that can be effectively used to analyse population trends at both local and global scales.
Some disciplines, such as ornithology and astronomy, have historically embraced prosperous and permanent volunteer involvement. Fortunately, other research fields are currently emerging, providing new and variegated opportunities for the public to engage. Combined with the evolution of new technologies and available means of analysis for big data, the opportunities for CS have dramatically increased worldwide. However, the rise of computer-aided techniques (AI image classification algorithms) and online platforms (e.g., eBird—www.ebird.org—or iNaturalist—www.inaturalist.org) has been so rapid and disorganized that new problems such as data input homogenization or duplication of public platform services might also compromise the feasibility of CS projects. Therefore, efforts towards compilation, standardization and regularization are required if multiple information sources are to be used [12]. In Europe, bird and butterfly CS projects have brought together a large group of naturalists and volunteers, resulting in extensive monitoring programmes following standard methodologies that allow robust analysis to be undertaken at the continental scale [13,14,15]. For example, these CS projects gather up to hundreds of daily reports per country [16] and provide reliable data for assessing the effects of the European Union’s Natura 2000 network of protected areas [17]. Most importantly, these data have been used to build biodiversity indicators and identify the most relevant drivers of population declines and those habitats that face the most severe conservation problems [13,18]. Some of these indicators have recently been adopted as general indicators of the state of biodiversity by the European Community (e.g., the Farmland Bird Indicator and the Grassland Butterfly Indicator [19]). The potential of citizen science for engaging society in biodiversity conservation has recently been recognized in Europe through several directives [20,21,22].
Although understanding the broad patterns in biodiversity is essential, information at much finer scales is generally requested by local governments and land managers for developing efficient protection laws of protected areas (e.g., Red Data lists). These needs have often resulted in local governments being officially engaged in CS projects. In Catalonia (NE Spain), for instance, almost 60 CS projects are active nowadays, some of them aimed at monitoring biodiversity at this regional level (Aparador Virtual d’Iniciatives de Ciència Ciutadana a Catalunya, www.mediambient.gencat.cat (accessed on 15 April 2021)). Mirroring the European situation, the oldest and most extensive CS biodiversity projects in Catalonia are focused on bird and butterfly populations. However, there has also been an increased awareness that monitoring schemes for other taxa are currently needed to assemble a comprehensive assessment of biodiversity status and trends [23].
Since the beginning of biodiversity monitoring in Spain, the Natural Sciences Museum of Granollers (hereafter NSMG) has played a central role. The NSMG holds the BiBio Research Group, which coordinates four CS programmes, targeting butterflies, common small mammals, bats and arboreal mammals. These taxa have long been recognized as bioindicator groups, for landscape modifications, habitat degradation, climate change and environmental pollution, for instance, owing to their relevant ecological role in terrestrial ecosystems [24,25,26,27,28,29,30,31], which makes monitoring programmes focused on these groups especially valuable. While the use of a standardized sampling method for butterflies throughout Europe has made it possible to calculate relevant bioindicators at the continental scale [19], this was not the case for the other monitored groups in the earliest stages. In the case of bats, the development of bioacoustics has opened new possibilities for monitoring populations of either elusive or cryptic species. In the case of small mammals, a plethora of available sampling methods precluded a widespread standardization of monitoring programmes at large geographical scales. For both bats and small mammals, we have proposed in recent decades the implementation of new standardized sampling methods for monitoring their populations, since no or few other monitoring programmes are yet available at the European scale.
Implementing volunteer-based research using CS requires careful planning and organization due to its multiple and complex structure. Here we summarize the historical progress of four concurrent CS programmes (focused on terrestrial mammals and butterflies) led by the NSMG since the end of the last century in the Mediterranean basin, one of the most important European biodiversity hotspots. We describe their main conservation outputs in terms of scientific publications, reports and influences in landowners or local land management, synergies between the schemes and other international research groups, overcome challenges during the establishment and the growth of the CS projects, as well as the future directions, with the aim that our experience will present a useful baseline and body of experience for other groups with similar projects and initiatives in Europe and beyond. Our programmes exhibited some sampling biases regarding the methods used, the detectability of species, and the experience of surveyors, which may hamper the results obtained and their interpretation, and so we offer some solutions to overcome these problems. Furthermore, our programmes have identified serious problems related to biodiversity conservation, and we present some examples.
2. Materials and Methods
2.1. Citizen Science Schemes Geographical Coverage
The monitoring schemes described below are circumscribed to the region of Catalonia (NE Spain) (Figure 1), in the NW of the Mediterranean Basin. The region is topographically and climatically highly diverse, with elevations between sea level and 3143 m a.s.l. and local climates ranging from Mediterranean xeric to alpine [32]. While all the programmes started in Catalonia, some of them are also included in sampling surveys at the national scale (mainly Spain), and some of them now contribute to international monitoring networks (CBMS; e.g., [17,19]).
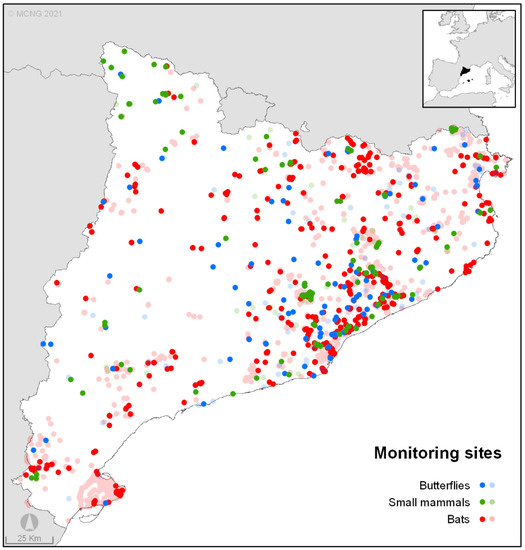
Figure 1.
Sampling stations for the three main taxa monitored by MCNG during 2020 (solid colours) and historical (light colours) in Catalonia (NE Spain).
2.2. Sampling Scheme Design and Description
The Catalan Butterfly Monitoring Scheme or CBMS (www.catalanbms.org): Butterflies are monitored using the standardized methodology originally developed in the United Kingdom (i.e., Pollard walks; [33]), which has been adopted as a standard in similar schemes throughout Europe [14]. At each location, weekly counts along fixed transects start on March 1 and finish on September 26, spanning a total of 30 weeks. Butterflies are counted in a 5 × 5 m area (2.5 m to each side and 5 m in front of the recorder), always under good weather conditions. The transect route is divided into a variable number of sections, each one corresponding to a different habitat type. An innovative aspect of this programme is that botanical characterizations of butterfly transects (performed every six years by a botanist) have been used both to derive a preference index of each butterfly species (i.e., open vs. closed habitats) and to record changes in the plant communities at individual sites during the butterfly recording period for understanding how butterfly communities respond to landscape changes. Furthermore, butterfly flower interactions recorded while doing the counts allowed us to accumulate information of nectar sources and to use this information to analyse long term mutualistic (pollinator) network interactions between these two taxa.
Bat Monitoring Programme (www.batmonitoring.org): This CS initiative includes four different protocols: ChiroHabitats, ChiroRivers, ChiroBoxes and ChiroRoosts. Each of them was specifically designed to monitor a particular group of bat species, either forest-dwelling, cave-dwelling or urban-dwelling species. The combination of all four monitoring programmes provides a complete image of the health status of all bat species populations and compensates for the sampling biases that exist in each methodology. The ChiroHabitats represents a bat monitoring protocol in foraging sites through passive acoustic surveys. It is aimed at surveilling bat populations of most species in their foraging sites using passive ultrasound detectors. Permanent sampling stations are sampled either only in summer during seven continuous nights (summer approach) or on one single night per season (extended approach). One-time sampling stations are only surveyed once for species distribution data. The ChiroRivers is a specific monitoring protocol for trawling bat species in aquatic habitats, used as indicators of water and riparian forest quality. It is a simple monitoring protocol that needs to be carried out in summer (June–August) and quantifies the activity of trawling bats using a flashlight in calm riparian waters. It has been adapted from the British Waterway Survey, led by the Bat Conservation Trust (UK). The ChiroBoxes are especially useful for monitoring fissure-dwelling bats using a network of bat boxes. Bat boxes are a compensatory measure, and therefore should not be used as a definitive replacement for natural roosts but are extremely useful to monitor the phenology of some bat species (including the long-distance migratory species). They are checked once per season yearly. Finally, with the ChiroRoosts we can efficiently monitor cave-dwelling bats (those that are otherwise difficult to detect) in natural (e.g., caves, rock shelters or trees) and artificial roosts (e.g., mines, tunnels, roofs, stone or cabins). Each roost is evaluated by an expert and the specific protocol for monitoring bats is case-specific and designed according to their structure and feasibility (e.g., infrared or thermal video recording, acoustic surveys, emergence counts). In this case, most of the roosts have been only surveyed once for species occurrence data. The Bat Monitoring Programme provides an innovative and pioneering open platform where information collected under different standardized protocols and specific technologies converges into a single database that can be used to address conservation problems using a wide range of different approaches, therefore contributing significantly to avoid inherent bat sampling biases. The project is largely based on the use of technology for conservation (bioacoustics, infrared imaginary, among others), representing an inflection point in terms of long-term bat monitoring schemes worldwide.
SEMICE (www.semice.org): This programme is aimed at obtaining indices of relative abundance of highly detectable common small mammal species, and is based on live-trapping plots; each plot represents a 6 × 6 trapping grid consisting of 18 Sherman traps (Sherman folding small animal trap; 23 × 7.5 × 9 cm; H. B. Sherman Traps Inc., Tallahassee, FL, USA), and 18 Longworth traps (Penlon Ltd., Oxford, UK), alternated in position and spaced 15 m apart, that are set in the field for three consecutive nights [34,35]. At new stations, Longworth traps have been progressively substituted by Heslinga traps (www.heslingatraps.eu (accessed on 15 April 2021)), due to their lower price and higher performance (higher efficacy, strength, and durability). Every sampling session is repeated twice per year (spring/summer and autumn). Individuals captured are marked with permanent ear/tags in rodents and with fur clips in the case of shrews.
DORMOUSE Project (www.dormice.org): The dormouse CS project includes two different protocols: one for experienced volunteers, to obtain robust information from quantitative data (capture-recapture), and another for early volunteers aimed at gathering qualitative (presence–absence) data without requiring handling of dormice. This programme is based on specifically designed nest box sampling (30 × 15 × 15 cm, with a 5-cm entry hole), attached to trees at a height of approximately 3 m above ground in deciduous forests [36]. Nest boxes are set 25–30 m apart in a grid (5 × 4 nest boxes) or line (six nest boxes). A monthly check is carried out from July to October between the 15th and 25th of each month, coinciding with the period of highest activity of the species. There is an additional cleaning survey during June to clean and repair the nest boxes if needed. In some sampling localities (southernmost populations) they are checked every two weeks from July to November using advanced-protocol surveys [37]. By marking all individuals with ear tags, we have gathered the first 10-year-long dataset of capture-recapture information with information of juvenile recruitment, reproductive success, individual fitness and demography trends (survival, fertility and population growth rate). Concerning small mammal monitoring in Europe (Eumon portal for Biodiversity Monitoring in Europe: http://eumon.ckff.si (accessed on 15 June 2021)), our programmes are innovative in developing our own efficient cost–benefit sampling methodologies for implementing monitoring at large temporal (>10 years) and spatial scales (region and country).
All projects have specifically designed websites where the collaborators can upload/download their data and explore population trends at the regional and local levels, and training materials are available for download. These websites offer a broader diversity of options in terms of data management and visualization (particularly for the taxa) than any other CS platform nowadays.
2.3. Monitoring Programme Establishment and Historical Trends
As an internal evaluation of any institution carrying out CS projects, it is essential to evaluate the success of the monitoring network for every programme with respect to the number of stations established and the number of species/individuals recorded over the years. This is a necessary step for ascertaining whether these monitoring programmes are able to record a relevant number of the species present in the sampling stations, and hence, to assess whether they are good candidates for biodiversity monitoring. Here we present the global and historical assessment of our four programmes, undertaken at the end of 2020. Additionally, to evaluate the participation trends of all CS programmes, we calculated the annual rate of change of sampling stations and their trend by means of Spearman rank correlations.
2.4. Sources of Sampling Bias in Species Detectability and Trend Calculation
Different sources of sampling bias might hinder the results of any monitoring scheme, and therefore it is imperative to check them all during analysis [21,38,39,40,41]. Although richness and abundance are two classic variables for assessing biodiversity, it is extremely unlikely that all suitable species will occur at a certain place and moment, and therefore any survey will always exhibit several biases due to different biotic and abiotic factors (e.g., climatic conditions, devices sensitivity, predators presence, among many others) [38]. All monitoring programmes and protocols need to assess their species detectability in order to avoid false negatives, as well as their accuracy in species detection in order to avoid false positives [42].
Here we identify and assess all sorts of sampling biases we have found since the establishment of the programmes for all the taxa and suggest some guidelines for reducing or eliminating them.
2.5. Scientific Questions for Tracking Biodiversity Change
One of the main goals of monitoring programmes to track changes in global biodiversity and relate these changes to environmental degradation or recovery. Some Essential Biodiversity Variables (EBVs) were described as a minimum set of measurements by the GEO BON group [43] for studying, reporting and managing biodiversity change, focusing on the status and trend in elements of biodiversity. Later, [7] further developed the contents of these groups of variables by suggesting a set of 23 EBVs. To test whether the information from our monitoring schemes makes it possible to track biodiversity change efficiently, we evaluated which of these EBVs could be derived from our datasets.
Independently, we also summarize and describe the scientific outputs that have been published using the datasets from these four monitoring programmes, highlighting the main improvements in terms of land management impact, scientific knowledge contributions and external collaborations.
2.6. Programme Stability and Internal Structure
Finally, monitoring programmes must be robust and stable in order to endure and persist over time, independently of any economic crises, government changes and/or political decisions. Then, the structure and logistical organization of the project becomes a crucial aspect of their success. These programmes benefit from the engagement of non-professionals [44], making them a cost-effective solution in the long term [11]. However, collaborators need to be trained by both professionals and experienced volunteers, either by sharing field campaigns or attending some guidance courses, until they can oversee their sampling stations. In this review, we also assess these characteristics as applied to the monitoring programmes led by BiBio and accurately describe their organisational structure. All collaborators were assigned to one of three different profiles: (1) internal coordinators and collaborators who are paid for their contribution to the network; (2) volunteers or amateur naturalists without financial remuneration; and (3) collaborators with profiles as naturalists from public administrations, institutions or companies (e.g., technicians and park rangers) who participate in CS projects during their working hours.
3. Results and Discussion
3.1. Monitoring Programme Establishment and Historical Trends
Since their establishment, these four monitoring programmes have gathered information from a total of 2818 permanent stations in Catalonia (706 currently active) (Figure 1). All monitoring schemes showed positive annual rates of change ( = +9.0%), ranging from 3.5% in ChiroRivers to 13% in SEMICE. The monitoring programme’s time-series average was 17 years (range 5–27 years), with six out of seven used protocols having already produced long series of data (>10 years, Figure 2). The four programmes have been led by experienced researchers, supported by a crew of technicians and coordinators, some giving transversal assistance (e.g., web designers and managers), and have involved roughly 1000 collaborators since the start of the monitoring programmes.
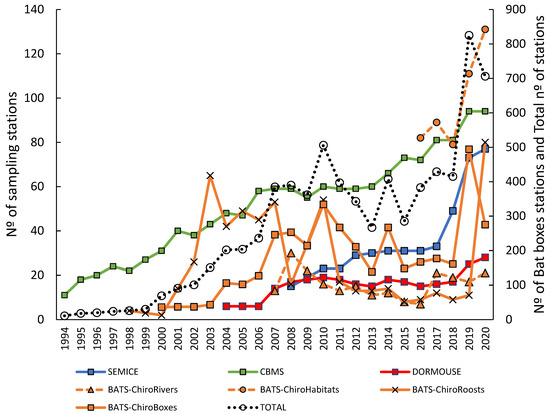
Figure 2.
Number of sampling stations per monitoring programme along the study period (1994–2020). The right axis shows the number of bat boxes (the ChiroBoxes protocol), and the total number of stations.
The CBMS programme has been active from 1994 to the present, with more than 180 stations created, and 94 active stations today. During these 27 years, we have counted close to 3,000,000 individuals of 189 butterfly species, 94% of those present in Catalonia. The average number of species detected per sampling station is 62.0 ± 23.3 (range 10–81), with an average number of 1972.4 ± 1371.9 (range 143–8925) individuals censused. The annual growth rate of the network is 8%, with a significant positive trend along the years (rS = 0.98, p < 0.001, n = 27).
The Bat Monitoring Programme is the youngest and the most structurally complex, including four different protocols. It was recently released as a full CS programme (2018). However, equivalent datasets were collected for a longer period (since 2015), as it was run by researchers using the same standardized methodologies. We have recorded a total of >2,500,000 bat passes using acoustic devices, installed and inspected 1414 bat boxes (>40,000 individuals found), and inspected more than 500 under- and over-ground roosts, including some with long-term monitoring data and endangered species. This last project (ChiroRoosts) has obtained information on all the species currently present in the area (29 species). All four projects show positive annual rates of change along the years (from 3.5 to 12.7%), but only two projects show overall positive trends due to strong heterogeneity of sampling stations between years.
The SEMICE programme has been active from 2008 to the present, with more than a hundred stations created, 77 of which are currently active. During these 13 years, we have trapped 9934 individuals of 18 small mammal species, 78% of the species present and detectable with the sampling method used. The average number of species detected per sampling station is 1.9 ± 1.1 (range 0–6), with an average number of 12.1 ± 12.5 (range 0–75) individuals captured. The annual growth rate of the network is 13%, with a significant positive trend (rS = 0.99, p < 0.001, n = 13).
The Dormouse programme has been active from 2004 to the present, with 28 stations active (out of 44). During these 17 years, we have trapped 888 individuals of dormouse and 512 individuals of other three forest-dwelling species (almost all Apodemus flavicollis, but also Eliomys quercinus and Rattus rattus). The average number of species detected per line of nest boxes is 0.68 ± 0.59 (range 0–2), with an average number of 1.6 ± 2.6 (range 0–14) individuals captured. The average number of species detected per plot of nest boxes is 1.60 ± 0.58 (range 0–2), with an average number of 18.2 ± 19.3 (range 0–96) individuals captured. The annual growth rate of the network is 6%, with a significant positive trend (rS = 0.60, p < 0.01, n = 17).
3.2. Sources of Sampling Bias in Species Detectability and Trend Calculation
Sampling biases (e.g., species detectability, surveyor experience) need to be openly acknowledged during all the phases of the monitoring programme (project design, data acquisition, resulting analyses and communication), and need to be treated accurately in order to avoid misinterpretation of the results. Here we list the most critical biases identified during the last few decades of experience with the four monitoring programmes:
CBMS programme: As a common methodology used throughout Europe (i.e., Pollard walks), there have been some attempts to delve into various aspects related to the detectability of species during censuses [45]. It has been suggested that about one third of individual butterflies are missed from the censuses, but the method still provides a good measure of the relative abundance of species over time. Attempts to increase reliability in count data would make the method impractical for a CS project [45]. Furthermore, rarefaction analysis showed that the butterfly transect method provides highly complete assessments of the local fauna in Europe [46].
We have developed more complex statistical approaches for calculating species indices of abundance correcting for species detectability, surveyor experience, and missing counts. The ‘regional GAM’ method [14] greatly improves the accuracy of abundance indices, allowing data from different sites from a common climatic region to compensate for missing counts at a particular site. The CBMS has adopted this method for the calculation of annual abundance indices, making trend estimates more reliable. The Bayesian approach, correcting for the detectability of the species and the surveyor experience, provided very similar results to those obtained with the standard method [47], but population estimates gained in accuracy by adding the detection probability—which increased in time—due to the improvement of identification skills of volunteers since the start of the programme [48].
Bat monitoring programme: Bat functional guilds and species do not respond equally to the sampling techniques used, and while there are some species easily detectable with most techniques (harp trapping, mist-netting or acoustic surveys), others are highly elusive and difficult to study. For example, some species are easy to spot inside their roosts, while others become undetectable while roosting well inside the crevices and cracks. In our monitoring schemes, we include specific methodologies to overcome these biases, such as the video recording of the emergence with synchronized ultrasound detectors and infrared lights or a combination of techniques in bat surveys in foraging habitats. Because bioacoustics is gaining momentum worldwide, special care must be taken in acoustic surveys; the availability of detectors and microphones in the market has been increasing dramatically in recent last decades, but acoustic surveys are somewhat difficult to compare, as each detector is optimized under certain conditions and for specific aims, having quite different sensitivities [49]. We recommend using only one or two models (one economic version for volunteers and another more advanced version for professionals), because direct side-by-side comparisons between detectors’ performance is crucial for modelling any data resulting from various devices collected from different sources [40]. We also recommend avoiding both temporal and spatial interspecific comparisons and designing the monitoring surveys to unravel only intraspecific patterns (i.e., comparisons between sites or times within the same species) [38]. In general, a combination of methods to survey the complete bat assemblages is highly encouraged [50,51]. However, surveyor experience and identification confidence are crucial for ensuring the reliability of the collected data, and capacity training and specific courses are encouraged. We recommend offering several courses all year round to maintain interest and engagement in the project and to classify the participants according to their level. The validation process can be complemented by a post-validation system after participants have uploaded their data to the website. In any case, the protocol guidelines must be short, clear, direct and standardized over a long time, with few modifications and amendments. Finally, taking note of the climatic and environmental conditions for all the surveys has become a mandatory requirement for all of the protocols in order to properly model species response to habitat modification or time [39].
Small mammal programmes: Monitoring small mammal biodiversity is hampered by the lack of “universal” sampling protocols that preclude the application of standardized monitoring programs [34]. This is because small mammals are a hyper-diverse group, with species displaying different sizes, microhabitat selection, and behaviour (fossorial, arboreal), and no single technique can sample all species within a group with the same degree of accuracy [35]. Indirect sampling techniques (i.e., the diet of small mammal predators) have been highlighted as relevant for species inventories [52,53], but live trapping has been suggested as the key technique for population monitoring [54]; this latter was the technique used in the SEMICE programme. Additionally, several authors suggest using a combination of sampling techniques to account for trappability differences [55], to restrict monitoring to the most captured species [25], and to estimate detectability and correct for its effects on species occupancy [42]. Indeed, our studies confirmed that the trap models used (medium Sherman and Longworth) could be employed interchangeably—without relevant biases—in small mammal community assessments where large species are infrequent [34,35]. Small mammal communities in the study area are mostly composed of small species (i.e., Apodemus sylvaticus), and our analyses further confirmed that they were highly detectable with the sampling methods used. Hence, differences in detectability hardly affected estimates of occupancy for common small mammal species. We recommend restricting the monitoring to the common species, since they produce powerful population estimates and trends owing to the fact that population changes of common species can be measured with greater precision than those of rare species [56]. We also recommend using these two trap models in combination to compensate for the trap model specificities observed. However, different trap models should be used for the particular small mammal communities studied. In the case of dormouse, standard small mammal techniques (i.e., trapping) produce underestimates of abundance, and other sampling methods are recommended [57]. Indeed, SEMICE live trapping grids share the same habitat with dormouse nest boxes in some areas, but dormice were never trapped despite occupying the boxes placed 3 m high on the trees. In that latter case, nest boxes need to be placed in grids—rather than in lines—if population density estimates are necessary [58]. The use of lines of nest boxes provides biased information regarding densities, and suffers from the same biases as lines of live traps [54]. Finally, monitoring on the basis of citizen science must pay special attention to the standardization of variations in surveyors’ sampling and identification skill. Despite species detectability being dependent on the specificity of the trap models used and independent of the surveyor, skills are necessary for setting traps, and for handling and identifying captures. Incorrectly setting trapping devices reduces the number of traps available and will result in biased estimates of species abundance [59]. However, our results show low overall sampling inaccuracies that were independent of the experience of surveyors [60]. On the other hand, attempts to sex individuals captured increased with surveyor experience (especially in the case of shrews), and training resources will be necessary to ensure that surveyors obtain the skill levels required [61].
3.3. Scientific Questions for Tracking Biodiversity Change
The CBMS programme has detected 189 out of 201 species present in Catalonia (94%); the Bat Monitoring Programme has recorded 25 species out of the 30 bat species occurring in the territory (15 spp./phonic groups in the ChiroHabitats, 10 spp. in the ChiroBoxes and 23 spp. in the ChiroRoosts); and the SEMICE has found 18 species out of 23 detectable by the used methodology (78%). Finally, although the dormouse programme provides detailed information on the population demography of this species, it also provides additional information about other tree-dwelling small mammals.
All programmes are aimed at obtaining detailed information about species distribution, abundance, and population structure. The four monitoring programmes provide information on 9 to 11 variables out of the 23 EBVs defined in Table 1 as primary targets (39–47%), and additional information on 1 to 5 EBVs as secondary targets (4–22%). All programmes also provide information on habitat structure/composition by routinely incorporating vegetation inventories by visual inspection (once every six years in CBMS) and land-use information available on the Internet (i.e., LiDAR).

Table 1.
Essential Biodiversity Variables (EBVs) covered by every monitoring programme as primary and secondary targets, defined as in [7].
The scientific output and knowledge compiled in recent years has been noticeable in the case of the CBMS, which has produced a total of 43 papers in peer-reviewed journals, followed by the Bat Monitoring Programme (16), the SEMICE (8), and the Dormouse project (2).
The CBMS has made significant contributions to knowledge on the effect of global change on Mediterranean butterflies, including the effects of climate change on phenology and population dynamics [62,63,64,65], the significance of current landscape changes on butterfly communities [66,67,68] and, more generally, a robust assessment of butterfly trends at the regional scale [47,69,70]. From a practical perspective, data from the CBMs have been used to evaluate the effects of several managing practices [68,71,72]. Moreover, the assembled dataset has provided invaluable information in various autoecological studies [73,74,75,76] and has also been used in combination with data from other similar schemes to reveal ecological patterns and responses at the continental level [13,77,78]. The CBMS also contributes to biodiversity conservation at the European level, for instance, by providing data for the European Grassland Butterfly Indicator [19] and the broad assessment of the efficiency of the Natura 2000 network on butterfly and bird conservation [17].
The Bat Monitoring Programme has focused most of its efforts on standardizing acoustic protocols for monitoring bats in foraging grounds and improving the interpretation of recorded bioacoustics datasets [79] as well its implementation to efficiently monitor some cave-dwelling bats [80]. This has been especially noteworthy regarding the ChiroHabitats protocol, as we especially adapted and tested new ecological indicators to relate changes in bat assemblages to climate and landscape change [31]. Due to the implementation of acoustic protocols as well as the installation and monitoring of bat boxes in agroforest landscapes, this programme also provides useful information for fostering eco-friendly practices in agricultural circles. We have provided strong evidence of the benefits of organic olive farming for the conservation of gleaning bats in a Mediterranean agroforest landscape [81], and the impact of common bat species in suppressing notorious insect pest species in rice paddies such as chironomids and mosquitoes [82], the rice water weevil [83] and the rice borer [84]; these last studies were carried out in the Ebro Delta, where much bat research is clearly lacking [85]. Regarding the use of bat boxes, this programme also provides guidelines for their installation in a wide range of habitats [86,87,88], and in terms of bioindication and ecosystem health. Specifically for trawling bats, we also tested the reliability of using them as indicators of water and riparian forest health [89]. At another level, natural history information—inventories as well as descriptive studies—has always complemented our research [90,91,92]. The datasets compiled throughout all these years are sent regularly to the local government and institutional representatives and have contributed significantly to both habitat and fauna local law development, improving species conservation plans and directives. International collaborations have been established on several occasions with the aim of standardizing methodologies at a broader scale, following examples from other taxa.
The SEMICE programme was inspired by monitoring schemes that emerge during the end of the last century in Great Britain [54]. Since we implemented a new methodology, our scientific contributions were aimed at testing the reliability of data obtained concerning sampling biases [34], interpreting population trends of common small mammal species [35], and evaluating the quality of data obtained with respect to the experience of collaborators [60]. More recently, we have investigated synergies with other groups, by analysing the association between small mammals populations and the pest outbreaks of the Gypsy moth Lymantria dispar [93], and establishing the role of small mammals on the demography of generalist predators [94].
The DORMOUSE programme is the first monitoring focused on this species in Europe and allowed the expansion of knowledge of the edible dormouse (Glis glis) and provided new data regarding its distribution through a simple, specific, and efficient methodology using nest boxes [36]. Our long-term monitoring allowed the collection of temporal and spatial variations in presence, activity and reproduction, in response to habitat impacts as destructions and forest fragmentation, but also regarding the climatic change effects on this species and their populations [37]. The monitoring has contributed to the expansion of knowledge of the hibernation mechanisms in mammals [95].
All of these programmes have provided valuable academic contributions, in collaboration with universities (4 PhD and 17 master/bachelor theses). In fact, the data gathered by the NSMG were recently used in the diagnosis of the “Nature Status” in Catalonia [23].
3.4. Programme Stability and Internal Structure
Field sampling effort: To develop a stable monitoring protocol that will endure over time, it needs to be robust and provide its participants a long-lasting motivation that will not vanish. The different programmes require low to moderate effort from the participants. There are some protocols requiring low effort (BAT-ChiroRoosts and BAT-ChiroRivers: <2 days/year), some requiring intermediate effort (SEMICE and DORMOUSE: <10 days/year), and some requiring high effort (Butterflies: >30 days/year). To collaborate in some of them, a high degree of naturalist skill is strictly required (i.e., CBMS due to the identification skills necessary to classify the species, or the case of small mammal protocols, as all collaborators need to handle animals for marking). In the case of bats, however, no protocol involves species handling. The need for handling individuals through field surveys obviously requires a higher degree of skill, which might hamper the collaboration of some volunteers, but at the same time, it generally provides greater and more intense personal experiences and represents a direct appeal for participation. To overcome these challenges, training courses and specific capacitation are continuously offered, and several dissemination activities are also scheduled all year round (Table 2).

Table 2.
General information about the four monitoring programmes.
Interactive platforms: Optimally, collaborators should have access to learning materials and protocols, collect and upload data online into web databases, and download and view their results using interactive graphs and maps [2]. Interactive platforms and active groups of volunteers contribute greatly to project stability and endurance. As demonstrated by the exponential increase in participation and the clear gain in external visibility in recent years, we have experienced a remarkable improvement in programme performance since the update of the websites and the release of social networks. We recommend committing a relatively large effort and budget to organizing outreach activities and designing a clear roadmap for reaching the potential target of any CS programme effectively.
Programme budget: The budget that supports these monitoring programmes is also crucial to keep it operating for a long time. However, the four monitoring programmes are quite different regarding the budget necessary to provide the material required to establish a sampling station. While the SEMICE is based on setting up live-trapping grids, and the budget is generally only attainable under a professional scheme (traps acquired by an institution) or by lending the materials to volunteers, other programmes require moderate to no investment at all for collaborators. For example, to participate in the CBMS, only a butterfly net is necessary, and to participate in BAT-ChiroRivers, only a torch is required to sample the rivers at night.
Programme chart and coordination: Finally, in terms of coordination teams and the programme chart, the proportion of paid-job positions within each programme greatly affects their capacity to prevail. Altogether, almost half of the stations are in charge of professionals (49% with cost to the projects) and 37% are overseen by volunteers. The contribution of volunteers to the monitoring schemes is heterogeneous (Figure 3), from less than 20% of stations in SEMICE and Bat-ChiroBoxes, to close to 80% of the stations in Bat-ChiroHabitats and Bat-ChiroRivers, with intermediate values in CBMS and the Dormouse project (40%). Despite volunteer-based monitoring being a potential solution for maintaining time-consuming and expensive data collection [1], our monitoring schemes include a substantial portion based on professional staff, in which experienced scientists are hired by or involved in the different projects. Some authors consider that a CS programme must include half of the stations monitored by volunteers [7], and in our case, only three out of four protocols within the bat programme meet this condition. The other three programmes are below 50%. This imbalance is only possible because the projects are subsidized by the Catalan Administration. Although these subsidized models may be in danger when economic crises occur, they have overcome the economic difficulties resulting from the two most recent crises (2008–2013 and 2020–2021).
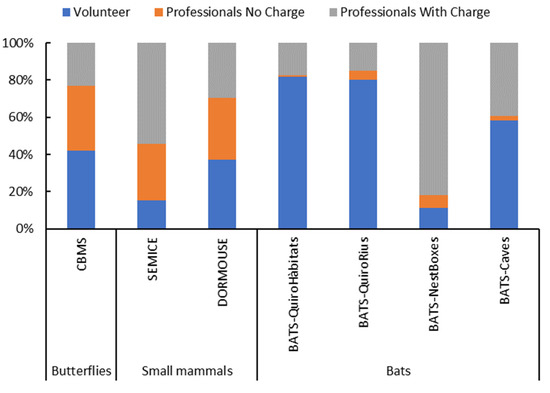
Figure 3.
Contribution of the three classes of collaborators to the maintenance of the monitoring stations during the year 2020.
Scientist–volunteer interaction: The citizen science programmes described here fit into the contributory model, in which protocols and research questions are conceived by the scientists but supported by the volunteers who collect the data [96]. Despite that research questions and protocols can be formulated under the leading roles of experienced scientists, recording data by non-expert volunteers can be subject to some biases and need to be verified by experts [2]. Accurate training and the use of standardized methods are crucial to the success of volunteer-based surveys [97], but the quality of information recorded somehow depends on volunteer skills. In this regard, some monitoring programmes need high detection/identification skills and quality of information can be directly associated with the experience of people recording data [48]. Some of our monitoring programmes are based on highly detectable common species to avoid misidentification problems, and hence, reducing biases in occupancy estimation and population trends [35,60].
The use of remote sensing and technology for monitoring (e.g., bat detectors) can mitigate these problems [96], since the information can be obtained without the intervention of the volunteers and later verified, if necessary, for quality control [98]. A similar problem can be observed when using mechanical devices for monitoring (e.g., live traps), in which species detectability is intrinsic to trap devices and species behaviour and, therefore, independent of the observer [60]. However, all devices need to be appropriately placed to avoid sampling inaccuracies, and skills are necessary for handling, marking, and identifying individuals after releasing them from the traps.
Although monitoring rich faunal communities can be challenging at first and demands high degrees of skill and time, our experience shows that this problem can be perfectly circumvented by well-designed CS programmes. The CBMS programme has the longest series of data (from 1994 to the present), being supported by many volunteers and yielding an important research output (Table 2) despite the inherent difficulty of sampling highly diverse butterfly communities. Volunteers find a rewarding challenge in incorporating new species records into their stations as they improve their ability to detect and identify over time. This scheme has strong data quality control, providing full support to collaborators in the field (extremely helpful for species identification). For example, in order to avoid inaccuracies, the CBMS does not consider the data collected during the first year by inexperienced volunteers (considered as a year of training). Albeit less demanding in terms of necessary skills, monitoring poor communities (e.g., small mammals), especially in years with low capture rates (sometimes no captures for three days), can be discouraging for volunteers, leading to abandonment. This could be a reason the SEMICE programme maintained the lowest proportion of stations led by volunteers.
3.5. Serious Problems in Biodiversity Conservation in Protected Areas in Catalonia: The Complexity of Spatial Scale and Stakeholder Agreement and Commitment
Our monitoring programmes are incorporated as a source of information for conservation planning in protected areas in Catalonia and, more generally, in the whole region [23]. One of the main conservation problems that our monitoring programmes have identified is the loss of biodiversity associated with afforestation [67], together with the lack of mature forests [99], a natural process resulting from the loss of traditional land uses and land abandonment in natural areas [100]. Fighting against this natural process is challenging and can be considered a serious problem without a clear solution [101]. To efficiently reverse this landscape transformation trend falls far beyond the capacity of the managers of protected areas, even considering the increasing impact of wildfires in the present context of climate change [100]. New socio-economic conditions oppose the reversal of this widespread pattern in the Mediterranean Basin.
A wide consensus is needed to effectively conserve biodiversity in protected areas, involving all relevant stakeholders in decision making. Although this democratic form of governance of protected areas has been implemented in many Catalan natural parks since their creation (some of them in the early 1980s), the lack of communication among a large number of stakeholders (up to 200 in some cases) and mistrust in participatory processes are undermining the confidence of participants [102]. This means that, in the end, decisions are mostly taken in a top-down manner, only on the basis of the intervention of higher levels of the government (politicians and public land managers), which can be discouraging for the widespread participation of stakeholders.
A particular case of disagreement between different stakeholders was the application of the aerial treatment of Bacillus thuringiensis to control the recent Gypsy moth (Lymantria dispar) outbreak in the forests of Montnegre Natural Park. The social concern of the forest owners regarding the safety of their properties led to an action implemented by the Catalan Government that was strongly discouraged by scientists [93]. As predicted, the measure was unsuccessful, and a complex system of natural enemies completely regulated the pest without the need for drastic and costly actions affecting other components of biodiversity.
The last financial crisis represented a significant decline of public resources devoted to biodiversity conservation and a need to generate own resources for the maintenance and management of protected areas [103]. Hence, an emphasis on the economic profitability of nature has been claimed under this new general context of budget scarcity, and the creation of new financing mechanisms to circumvent this problem are welcome and encouraged [103]. Our monitoring programmes involve participants engaged in ecotourism projects, which not only return clear economic benefits to the owners, but also help in the long-term maintenance of the network of monitoring sites. Indeed, our regular field sampling proved to be the easiest way of looking at local fauna by visitors in protected areas without creating additional disturbance. This was also another way of involving private stakeholders in the need for biodiversity conservation.
3.6. Current Challenges, Recommendations, and Future Opportunities
Biodiversity is essential for healthy ecosystems and beneficial for humans, as it delivers much-needed ecosystem services [104]. However, global change is currently threatening biodiversity, and thus, it is imperative to develop effective systems to measure biodiversity trends. Data are needed to reflect negative regional trends that can be tackled with adequate policies. Local governments can implement such policies in many cases, and therefore, they play a key role in the conservation of biodiversity. As a first step, governments can support initiatives for gathering good quality data on biodiversity trends, which, as shown in this paper, can be obtained through CS projects. The CS programme can be developed by relatively small institutions such as the NSMG if the structure is sound and robust, and the potential target well established. Hereby, we provide a list of current recommendations to establish and develop CS programmes at local scale and future directions for CS:
- -
- The selection of potential collaborators within the general public is a key element for the programme design.
- -
- Outreach and communication must be effective and follow the current channels that participants use during the daily lives. News, courses, and specific training must be provided.
- -
- The sampling effort must be scaled to the participants’ possibilities but adapted to the scientific question to acquire good-quality data.
- -
- The motivations driving a volunteer’s participation in CS are crucial to keeping it operative in the long term and must be reinforced throughout the whole period with clear feedback and integrative activities with all the participants.
- -
- The programmes must have a minimum budget to cover the permanent scientific team that is responsible coordinating, analysing and establishing communication with the volunteers, as well as for its web design and management.
- -
- Data storage must follow international standards in order to provide good-quality data that can be used overseas or combined in international data repositories.
- -
- Multidisciplinary projects or the combination of multiple CS boost the participation of volunteers simultaneously and strengthen the network of collaborators.
- -
- Scientific publications contribute to the validity and integrity of the project.
- -
- Sampling biases (e.g., species detectability, participant experience or methodological sensibility) need to be openly acknowledged during project design, data acquisition and the resulting analyses and treated accurately in order to avoid misinterpretation of results.
- -
- The use of technology and remote sensing and specific intense training for highly skilled programmes might overcome several of these sampling biases.
- -
- Owing to budget scarcity of public resources for biodiversity conservation, the commitment of private stakeholders—obtaining economic profit from nature—is necessary to guarantee long-term monitoring networks.
Author Contributions
Conceptualization, I.T., A.L.-B., C.S. and L.F.; methodology, I.T., A.L.-B., C.S., L.F. and C.F.; software, F.P., X.P.-M.; validation, I.T., A.L.-B., C.S. L.F. and C.F.; formal analysis, I.T., A.L.-B., C.S. and L.F.; investigation, I.T., A.L.-B., C.S., L.F., C.F., C.B., A.C., D.L.-B., M.M., S.M., J.M., F.P., X.P.-M. and C.T.-C.; data curation, J.M., F.P. and X.P.-M.; writing—original draft preparation, I.T., A.L.-B., C.S. and L.F.; writing—review and editing, all authors; visualization, I.T., A.L.-B., C.S. and L.F.; supervision, C.F., A.A.; project administration, C.F., A.A.; funding acquisition, C.F. and A.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Catalan Government (Generalitat de Catalunya, DB201804) and the Barcelona Provincial Council (Diputació de Barcelona: reference numbers 2015/3456 and 2019/0007297).
Institutional Review Board Statement
Investigations regarding small mammals and bats followed the ethical guidelines for the use of wild mammals in research and education [105].
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are partially publicly available due to the current development of an online sharing platform. Sampling locations are already available at https://www.bibio.org/explore/ (accessed on 15 September 2021). The rest of the data (samples and counts) can be requested to the Natural Sciences Museum of Granollers by indicating the desired geographical extension and time span.
Acknowledgments
We are grateful to the thousand collaborators involved in the monitoring programmes along the years. All programmes were also supported by the Centre de la Neu i la Muntanya d’Andorra (CENMA). The CBMs project was supported by Observatori Socioambiental de Menorca (OBSAM). The Bat projects were supported by Recaredo-Celler Credo wineries (Penedès) since 2013. Small mammal projects were supported by Fundación Biodiversidad (2015), Ministerio de Medio Ambiente de España, Conselh Generau d’Aran, and SECEM (Sociedad Española para la Conservación y Estudio de los Mamíferos). All monitoring programmes obtained the scientific and legal permissions of the Spanish Administration.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Magurran, A.E.; Baillie, S.R.; Buckland, S.T.; Dick, J.M.P.; Elston, D.A.; Scott, E.M.; Smith, R.I.; Somerfield, P.J.; Watt, A.D. Long-term datasets in biodiversity research and monitoring: Assessing change in ecological communities through time. Trends Ecol. Evol. 2010, 25, 574–582. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, J.L.; Zuckerberg, B.; Bonter, D.N. Citizen Science as an Ecological Research Tool: Challenges and Benefits. Annu. Rev. Ecol. Evol. Syst. 2010, 41, 149–172. [Google Scholar] [CrossRef]
- Cooper, C.B.; Shirk, J.; Zuckerberg, B. The invisible prevalence of citizen science in global research: Migratory birds and climate change. PLoS ONE 2014, 9. [Google Scholar] [CrossRef]
- Bonney, R.; Shirk, J.L.; Phillips, T.B.; Wiggins, A.; Ballard, H.L.; Miller-Rushing, A.J.; Parrish, J.K. Next steps for citizen science. Science 2014, 343, 1436–1437. [Google Scholar] [CrossRef] [PubMed]
- Kosmala, M.; Wiggins, A.; Swanson, A.; Simmons, B. Assessing data quality in citizen science. Front. Ecol. Environ. 2016, 14, 551–560. [Google Scholar] [CrossRef]
- Wagenknecht, K.; Woods, T.; Sanz, F.G.; Gold, M.; Bowser, A.; Rüfenacht, S.; Ceccaroni, L.; Piera, J. EU-Citizen.Science: A Platform for Mainstreaming Citizen Science and Open Science in Europe. Data Intell. 2021, 3, 136–149. [Google Scholar] [CrossRef]
- Chandler, M.; See, L.; Copas, K.; Bonde, A.M.Z.; López, B.C.; Danielsen, F.; Legind, J.K.; Masinde, S.; Miller-Rushing, A.J.; Newman, G.; et al. Contribution of citizen science towards international biodiversity monitoring. Biol. Conserv. 2017, 213, 280–294. [Google Scholar] [CrossRef]
- Pocock, M.J.O.; Chandler, M.; Bonney, R.; Thornhill, I.; Albin, A.; August, T.; Bachman, S.; Brown, P.M.J.; Cunha, D.G.F.; Grez, A.; et al. A Vision for Global Biodiversity Monitoring with Citizen Science. In Advances in Ecological Research; Elsevier Ltd.: Oxford, UK, 2018; Volume 59, pp. 169–223. [Google Scholar] [CrossRef]
- Rotman, D.; Preece, J.; Hammock, J.; Procita, K.; Hansen, D.; Parr, C.; Lewis, D.; Jacobs, D. Dynamic changes in motivation in collaborative citizen-science projects. In Proceedings of the ACM Conference on Computer Supported Cooperative Work, CSCW, Seattle, WA, USA, 11–15 February 2012; pp. 217–226. [Google Scholar] [CrossRef]
- Burgess, H.K.; DeBey, L.B.; Froehlich, H.E.; Schmidt, N.; Theobald, E.J.; Ettinger, A.K.; HilleRisLambers, J.; Tewksbury, J.; Parrish, J.K. The science of citizen science: Exploring barriers to use as a primary research tool. Biol. Conserv. 2017, 208, 113–120. [Google Scholar] [CrossRef]
- Bonney, R.; Cooper, C.B.; Dickinson, J.; Kelling, S.; Phillips, T.; Rosenberg, K.V.; Shirk, J. Citizen science: A developing tool for expanding science knowledge and scientific literacy. Bioscience 2009, 59, 977–984. [Google Scholar] [CrossRef]
- Sturm, U.; Schade, S.; Ceccaroni, L.; Gold, M.; Kyba, C.; Claramunt, B.; Haklay, M.; Kasperowski, D.; Albert, A.; Piera, J.; et al. Defining principles for mobile apps and platforms development in citizen science. Res. Ideas Outcomes 2017, 3, e21283. [Google Scholar] [CrossRef]
- Devictor, V.; van Swaay, C.; Brereton, T.; Brotons, L.; Chamberlain, D.; Heliölä, J.; Herrando, S.; Julliard, R.; Kuussaari, M.; Lindström, Å.; et al. Differences in the climatic debts of birds and butterflies at a continental scale. Nat. Clim. Chang. 2012 22 2012, 2, 121–124. [Google Scholar] [CrossRef]
- Schmucki, R.; Pe’er, G.; Roy, D.B.; Stefanescu, C.; Van Swaay, C.A.M.; Oliver, T.H.; Kuussaari, M.; Van Strien, A.J.; Ries, L.; Settele, J.; et al. A regionally informed abundance index for supporting integrative analyses across butterfly monitoring schemes. J. Appl. Ecol. 2016, 53, 501–510. [Google Scholar] [CrossRef]
- Stephens, P.A.; Mason, L.R.; Green, R.E.; Gregory, R.D.; Sauer, J.R.; Alison, J.; Aunins, A.; Brotons, L.; Butchart, S.H.M.; Campedelli, T.; et al. Consistent response of bird populations to climate change on two continents. Science 2016, 352, 84–87. [Google Scholar] [CrossRef]
- Basile, M.; Russo, L.F.; Russo, V.G.; Senese, A.; Bernardo, N. Birds seen and not seen during the COVID-19 pandemic: The impact of lockdown measures on citizen science bird observations. Biol. Conserv. 2021, 256, 109079. [Google Scholar] [CrossRef]
- Pellissier, V.; Schmucki, R.; Pe’er, G.; Aunins, A.; Brereton, T.M.; Brotons, L.; Carnicer, J.; Chodkiewicz, T.; Chylarecki, P.; del Moral, J.C.; et al. Effects of Natura 2000 on nontarget bird and butterfly species based on citizen science data. Conserv. Biol. 2020, 34, 666–676. [Google Scholar] [CrossRef] [PubMed]
- Gregory, R.D.; van Strien, A.; Vorisek, P.; Meyling, A.W.G.; Noble, D.G.; Foppen, R.P.; Gibbons, D.W. Developing indicators for European birds. Philos. Trans. R. Soc. B Biol. Sci. 2005, 360, 269–288. [Google Scholar] [CrossRef] [PubMed]
- Van Swaay, C.A.M.; Dennis, E.; Schmucki, R.; Sevilleja, C.; Balalaikins, M.; Botham, M.; Bourn, N.; Brereton, T.; Cancela, J.P.; Carlisle, B.; et al. The EU Butterfly Indicator for Grassland Species: 1990–2017; Technical Report; Butterfly Conservation Europe: Wageningen, The Netherlands, 2019; pp. 1–23. [Google Scholar]
- European Commission. Natura 2000. The Action Plan: For nature, people and the economy. Nat. Biodivers. Newsl. 2017, 16. [Google Scholar]
- European Commission. EU Actions to Improve Environmental Compliance and Governance; COM(2018) 10 Final; European Commission: Brussels, Belgium; Luxembourg, 2018. [Google Scholar]
- European Commission. EU Pollinators Initiative, SWD(2018) 302 Final—SWD(2018); European Commission: Brussels, Belgium; Luxembourg, 2018. [Google Scholar]
- Brotons, L.; Pou, N.; Herrando, S.; Bota, G.; Villero, D.; Garrabou, J.; Ordóñez, J.L.; Anton, M.; Gual, G.; Recoder, L.; et al. Estat de la Natura a Catalunya 2020. Dep. Medi Ambient Sostenibilitat 2020, 1–56. [Google Scholar]
- Thomas, J.A. Monitoring change in the abundance and distribution of insects using butterflies and other indicator groups. Philos. Trans. R. Soc. B Biol. Sci. 2005, 360, 339–357. [Google Scholar] [CrossRef]
- Solari, S.; Rodriguez, J.J.; Vivar, E.; Velazco, P.M. A framework for assessment and monitoring of small mammals in a lowland tropical forest. Environ. Monit. Assess. 2002, 76, 89–104. [Google Scholar] [CrossRef]
- Jones, G.; Jacobs, D.S.; Kunz, T.H.; Wilig, M.R.; Racey, P.A. Carpe noctem: The importance of bats as bioindicators. Endanger. Species Res. 2009, 8, 93–115. [Google Scholar] [CrossRef]
- Avenant, N. The potential utility of rodents and other small mammals as indicators of ecosystem “integrity” of South African grasslands. Wildl. Res. 2011, 38, 626–639. [Google Scholar] [CrossRef]
- Drăgoi, C.I.; Faur, M. Monitoring dormice (Gliridae) populations as a method of evaluating the efficiency of biodiversity management tools in Grădiştea Muncelului—Cioclovina Nature Park. Acta Zool. Bulg. 2013, 5, 143–146. [Google Scholar]
- Syaripuddin, K.; Sing, K.W.; Wilson, J.J. Comparison of butterflies, bats and beetles as bioindicators based on four key criteria and DNA barcodes. Trop. Conserv. Sci. 2015, 8, 138–149. [Google Scholar] [CrossRef]
- Naderi, M.; Farashi, A.; Markov, G. Exploring contents of lead and cadmium in tissues of fat dormouse Glis glis (Linnaeus, 1766) (Rodentia: Gliridae) for use in monitoring of environmental pollutants in the Southern Caspian Coast Forests, Iran. Acta Zool. Bulg. 2017, 69, 61–64. [Google Scholar]
- Tuneu-Corral, C.; Puig-Montserrat, X.; Flaquer, C.; Mas, M.; Budinski, I.; López-Baucells, A. Ecological indices in long-term acoustic bat surveys for assessing and monitoring bats’ responses to climatic and land-cover changes. Ecol. Indic. 2020, 110, 105849. [Google Scholar] [CrossRef]
- Metzger, M.J.; Bunce, R.G.H.; Jongman, R.H.G.; Mücher, C.A.; Watkins, J.W. A climatic stratification of the environment of Europe. Glob. Ecol. Biogeogr. 2005, 14, 549–563. [Google Scholar] [CrossRef]
- Pollard, E.; Yates, T.J. Monitoring Butterflies for Ecology and Conservation. The British Butterfly Monitoring Scheme; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1993; p. 274. [Google Scholar]
- Torre, I.; Freixas, L.; Arrizabalaga, A.; Díaz, M. The efficiency of two widely used commercial live-traps to develop monitoring protocols for small mammal biodiversity. Ecol. Indic. 2016, 66, 481–487. [Google Scholar] [CrossRef]
- Torre, I.; Raspall, A.; Arrizabalaga, A.; Díaz, M. SEMICE: An unbiased and powerful monitoring protocol for small mammals in the Mediterranean Region. Mamm. Biol. 2018, 88, 161–167. [Google Scholar] [CrossRef]
- Freixas, L.; Pertierra, D.; Torre, I.; Arrizabalaga, A. Seguimiento de las poblaciones de lirón gris (Glis glis) en el NE de la Península Ibérica. Galemys 2011, 22, 105–111. [Google Scholar]
- Ferrandiz-Rovira, M.; Freixas, L.; Torre, I.; Míguez, S.; Arrizabalaga, A. Male-biased litter sex ratio in the southernmost Iberian population of edible dormouse: A strategy against isolation? Anim. Biol. 2016, 66, 415–425. [Google Scholar] [CrossRef]
- Meyer, C.F.J.; Aguiar, L.M.S.; Aguirre, L.F.; Baumgarten, J.; Clarke, F.M.; Cosson, J.-F.; Villegas, S.E.; Fahr, J.; Faria, D.; Furey, N.; et al. Accounting for detectability improves estimates of species richness in tropical bat surveys. J. Appl. Ecol. 2011, 48, 777–787. [Google Scholar] [CrossRef]
- Perks, S.J.; Goodenough, A.E. Abiotic and spatiotemporal factors affect activity of European bat species and have implications for detectability for acoustic surveys. Wildl. Biol. 2020, 2020. [Google Scholar] [CrossRef]
- Gorresen, P.M.; Miles, A.C.; Todd, C.M.; Bonaccorso, F.J.; Weller, T.J. Assessing Bat Detectability and Occupancy with Multiple Automated Echolocation Detectors. J. Mammal. 2008, 89, 11–17. [Google Scholar] [CrossRef]
- Hyzy, B.A.; Russell, R.E.; Silvis, A.; Ford, W.M.; Riddle, J.; Russell, K. Occupancy and Detectability of Northern Long-eared Bats in the Lake States Region. Wildl. Soc. Bull. 2020, 44, 732–740. [Google Scholar] [CrossRef]
- Mackenzie, D.L.; Nichols, J.D.; Royle, J.A.; Pollock, K.H.; Bailey, L.L.; Hines, J.E. Occupancy Estimation and Modeling Inferring Patterns and Dynamics of Species Occurrence; Academic Press: Oxford, UK, 2018. [Google Scholar]
- Pereira, H.M.; Ferrier, S.; Walters, M.; Geller, G.N.; Jongman, R.H.G.; Scholes, R.J.; Bruford, M.W.; Brummitt, N.; Butchart, S.H.M.; Cardoso, A.C.; et al. Essential biodiversity variables. Science 2013, 339, 277–278. [Google Scholar] [CrossRef] [PubMed]
- García, M.B.; Silva, J.L.; Pardo, I.; Gómez, D.; Tejero, P. Tracking the long-term dynamics of plant diversity in Northeast Spain with a network of volunteers and rangers. Reg. Environ. Chang. 2019, 19, 391–401. [Google Scholar] [CrossRef]
- Isaac, N.J.B.; Cruickshanks, K.L.; Weddle, A.M.; Rowcliffe, J.M.; Brereton, T.M.; Dennis, R.L.H.; Shuker, D.M.; Thomas, C.D. Distance sampling and the challenge of monitoring butterfly populations. Methods Ecol. Evol. 2011, 2, 585–594. [Google Scholar] [CrossRef]
- Zhang, C.; Harpke, A.; Kühn, E.; Páramo, F.; Settele, J.; Stefanescu, C.; Wiemers, M.; Zhang, Y.; Schweiger, O. Applicability of butterfly transect counts to estimate species richness in different parts of the palaearctic region. Ecol. Indic. 2018, 95, 735–740. [Google Scholar] [CrossRef]
- Melero, Y.; Stefanescu, C.; Pino, J. General declines in Mediterranean butterflies over the last two decades are modulated by species traits. Biol. Conserv. 2016, 201, 336–342. [Google Scholar] [CrossRef]
- Jiguet, F. Method learning caused a first-time observer effect in a newly started breeding bird survey. Bird Study 2009, 56, 253–258. [Google Scholar] [CrossRef]
- Adams, A.M.; Jantzen, M.K.; Hamilton, R.M.; Fenton, M.B. Do you hear what I hear? Implications of detector selection for acoustic monitoring of bats. Methods Ecol. Evol. 2012, 3, 992–998. [Google Scholar] [CrossRef]
- Flaquer, C.; Torre, I.; Arrizabalaga, A. Comparison of sampling methods for inventory of bat communities. J. Mammal. 2007, 88, 526–533. [Google Scholar] [CrossRef]
- Lintott, P.R.; Fuentes-Montemayor, E.; Goulson, D.; Park, K.J. Testing the effectiveness of surveying techniques in determining bat community composition within woodland. Wildl. Res. 2014, 40, 675–684. [Google Scholar] [CrossRef]
- Torre, I.; Arrizabalaga, A.; Flaquer, C. Three methods for assessing richness and composition of small mammal communities. J. Mammal. 2004, 85, 524–530. [Google Scholar] [CrossRef]
- Torre, I.; Arrizabalaga, A.; Freixas, L.; Ribas, A.; Flaquer, C.; Diaz, M. Using scats of a generalist carnivore as a tool to monitor small mammal communities in Mediterranean habitats. Basic Appl. Ecol. 2013, 14, 155–164. [Google Scholar] [CrossRef]
- Flowerdew, J.R.; Shore, R.F.; Poulton, S.M.C.; Sparks, T.H. Live trapping to monitor small mammals in Britain. Mamm. Rev. 2004, 34, 31–50. [Google Scholar] [CrossRef]
- Fonturbel, F.E. A methodological approach to assess the small mammal community diversity in the temperate rainforest of Patagonia. Mamm. Biol. 2010, 75, 294–301. [Google Scholar] [CrossRef]
- Battersby, J.E.; Greenwood, J.J.D. Monitoring terrestrial mammals in the UK: Past, present and future, using lessons from the bird world. Mamm. Rev. 2004, 34, 3–29. [Google Scholar] [CrossRef]
- Kryštufek, B.; Hudoklin, A.; Pavlin, D. Population biology of the edible dormouse Glis glis in a mixed montane forest in central Slovenia over three years. Acta Zool. Acad. Sci. Hung. 2003, 49, 99–108. [Google Scholar]
- Bright, P.W.; Morris, P.; Mitchell-Jones, T. The Dormouse Conservation Handbook, 2nd ed.; English Nature: Peterborough, UK, 2006.
- Beauvais, G.P.; Buskirk, S.W. Modifying estimates of sampling effort to account for sprung traps. Wildl. Soc. Bull. 1999, 27, 39–43. [Google Scholar] [CrossRef]
- Torre, I.; Raspall, A.; Arrizabalaga, A.; Díaz, M. Evaluating trap performance and volunteers’ experience in small mammal monitoring programs based on citizen science: The SEMICE case study. Mamm. Biol. 2019, 95, 26–30. [Google Scholar] [CrossRef]
- Barlow, K.E.; Briggs, P.A.; Haysom, K.A.; Hutson, A.M.; Lechiara, N.L.; Racey, P.A.; Walsh, A.L.; Langton, S.D. Citizen science reveals trends in bat populations: The National Bat Monitoring Programme in Great Britain. Biol. Conserv. 2015, 182, 14–26. [Google Scholar] [CrossRef]
- Stefanescu, C.; Peñuelas, J.; Filella, I. Effects of climatic change on the phenology of butterflies in the northwest Mediterranean Basin. Glob. Chang. Biol. 2003, 9, 1494–1506. [Google Scholar] [CrossRef]
- Donoso, I.; Stefanescu, C.; Martínez-Abraín, A.; Traveset, A. Phenological asynchrony in plant–butterfly interactions associated with climate: A community-wide perspective. Oikos 2016, 125, 1434–1444. [Google Scholar] [CrossRef]
- Herrando, S.; Titeux, N.; Brotons, L.; Anton, M.; Ubach, A.; Villero, D.; García-Barros, E.; Munguira, M.L.; Godinho, C.; Stefanescu, C. Contrasting impacts of precipitation on Mediterranean birds and butterflies. Sci. Rep. 2019, 9, 5680. [Google Scholar] [CrossRef]
- Colom, P.; Traveset, A.; Carreras, D.; Stefanescu, C. Spatio-temporal responses of butterflies to global warming on a Mediterranean island over two decades. Ecol. Entomol. 2021, 46, 262–272. [Google Scholar] [CrossRef]
- Lee, M.S.; Comas, J.; Stefanescu, C.; Albajes, R. The Catalan butterfly monitoring scheme has the capacity to detect effects of modifying agricultural practices. Ecosphere 2020, 11, e03004. [Google Scholar] [CrossRef]
- Ubach, A.; Páramo, F.; Gutiérrez, C.; Stefanescu, C. Vegetation encroachment drives changes in the composition of butterfly assemblages and species loss in Mediterranean ecosystems. Insect Conserv. Divers. 2020, 13, 151–161. [Google Scholar] [CrossRef]
- Colom, P.; Traveset, A.; Stefanescu, C. Long-term effects of abandonment and restoration of Mediterranean meadows on butterfly-plant interactions. J. Insect Conserv. 2021, 25, 383–393. [Google Scholar] [CrossRef]
- Stefanescu, C.; Carnicer, J.; Penuelas, J. Determinants of species richness in generalist and specialist Mediterranean butterflies: The negative synergistic forces of climate and habitat change. Ecography (Cop.) 2011, 34, 353–363. [Google Scholar] [CrossRef]
- Stefanescu, C.; Torre, I.; Jubany, J.; Paramo, F. Recent trends in butterfly populations from north-east Spain and Andorra in the light of habitat and climate change. J. Insect Conserv. 2011, 15, 83–93. [Google Scholar] [CrossRef]
- Stefanescu, C.; Peñuelas, J.; Filella, I. Butterflies highlight the conservation value of hay meadows highly threatened by land-use changes in a protected Mediterranean area. Biol. Conserv. 2005, 126, 234–246. [Google Scholar] [CrossRef]
- Stefanescu, C.; Peñuelas, J.; Filella, I. Rapid changes in butterfly communities following the abandonment of grasslands: A case study. Insect Conserv. Divers. 2009, 2, 261–269. [Google Scholar] [CrossRef]
- Stefanescu, C. The nature of migration in the red admiral butterfly Vanessa atalanta: Evidence from the population ecology in its southern range. Ecol. Entomol. 2001, 26, 525–536. [Google Scholar] [CrossRef]
- Carnicer, J.; Stefanescu, C.; Vives-Ingla, M.; López, C.; Cortizas, S.; Wheat, C.; Vila, R.; Llusià, J.; Peñuelas, J. Phenotypic biomarkers of climatic impacts on declining insect populations: A key role for decadal drought, thermal buffering and amplification effects and host plant dynamics. J. Anim. Ecol. 2019, 88, 376–391. [Google Scholar] [CrossRef]
- Hu, G.; Stefanescu, C.; Oliver, T.H.; Roy, D.B.; Brereton, T.; Van Swaay, C.; Reynolds, D.R.; Chapman, J.W. Environmental drivers of annual population fluctuations in a trans-Saharan insect migrant. Proc. Natl. Acad. Sci. USA 2021, 118, e2102762118. [Google Scholar] [CrossRef]
- Stefanescu, C.; Alarcón, M.; Àvila, A. Migration of the painted lady butterfly, Vanessa cardui, to north-eastern Spain is aided by African wind currents. J. Anim. Ecol. 2007, 76, 888–898. [Google Scholar] [CrossRef]
- Suggitt, A.J.; Stefanescu, C.; Páramo, F.; Oliver, T.; Anderson, B.J.; Hill, J.K.; Roy, D.B.; Brereton, T.; Thomas, C.D. Habitat associations of species show consistent but weak responses to climate. Biol. Lett. 2012, 8, 590–593. [Google Scholar] [CrossRef]
- Mills, S.C.; Oliver, T.H.; Bradbury, R.B.; Gregory, R.D.; Brereton, T.; Kühn, E.; Kuussaari, M.; Musche, M.; Roy, D.B.; Schmucki, R.; et al. European butterfly populations vary in sensitivity to weather across their geographical ranges. Glob. Ecol. Biogeogr. 2017, 26, 1374–1385. [Google Scholar] [CrossRef]
- Montauban, C.; Mas, M.; Tuneu-Corral, C.; Wangensteen, O.S.; Budinski, I.; Martí-Carreras, J.; Flaquer, C.; Puig-Montserrat, X.; López-Baucells, A. Bat echolocation plasticity in allopatry: A call for caution in acoustic identification of Pipistrellus sp. Behav. Ecol. Sociobiol. 2021, 75, 70. [Google Scholar] [CrossRef]
- Revilla-Martín, N.; Budinski, I.; Puig-Montserrat, X.; Flaquer, C.; López-Baucells, A. Monitoring cave-dwelling bats using remote passive acoustic detectors: A new approach for cave monitoring. Bioacoustics 2020, 30, 527–542. [Google Scholar] [CrossRef]
- Puig-Montserrat, X.; Mas, M.; Flaquer, C.; Tuneu-Corral, C.; López-Baucells, A. Benefits of organic olive farming for the conservation of gleaning bats. Agric. Ecosyst. Environ. 2021, 313, 107361. [Google Scholar] [CrossRef]
- Puig-Montserrat, X.; Flaquer, C.; Gómez-Aguilera, N.; Burgas, A.; Mas, M.; Tuneu, C.; Marquès, E.; López-Baucells, A. Bats actively prey on mosquitoes and other deleterious insects in rice paddies: Potential impact on human health and agriculture. Pest Manag. Sci. 2020, 76, 3759–3769. [Google Scholar] [CrossRef] [PubMed]
- Montauban, C.; Mas, M.; Wangensteen, O.S.; Sarto i Monteys, V.; Fornós, D.G.; Mola, X.F.; López-Baucells, A. Bats as natural samplers: First record of the invasive pest rice water weevil Lissorhoptrus oryzophilus in the Iberian Peninsula. Crop Prot. 2021, 141, 105427. [Google Scholar] [CrossRef]
- Puig-Montserrat, X.; Torre, I.; López-Baucells, A.; Guerrieri, E.; Monti, M.M.; Ràfols-García, R.; Ferrer, X.; Gisbert, D.; Flaquer, C. Pest control service provided by bats in Mediterranean rice paddies: Linking agroecosystems structure to ecological functions. Mamm. Biol. 2015, 80, 237–245. [Google Scholar] [CrossRef]
- Mas, M.; Flaquer, C.; Rebelo, H.; López-Baucells, A. Bats and wetlands: Synthesising gaps in current knowledge and future opportunities for conservation. Mamm. Rev. 2021, 51, 369–384. [Google Scholar] [CrossRef]
- Bideguren, G.M.; López-Baucells, A.; Puig-Montserrat, X.; Mas, M.; Porres, X.; Flaquer, C. Bat boxes and climate change: Testing the risk of over-heating in the Mediterranean region. Biodivers. Conserv. 2018, 28, 21–35. [Google Scholar] [CrossRef]
- Flaquer, C.; Puig, X.; López-Baucells, A.; Torre, I.; Freixas, L.; Mas, M.; Porres, X.; Arrizabalaga, A. Could overheating turn bat boxes into death traps? Barbastella 2014, 7, 2014. [Google Scholar] [CrossRef]
- López-Baucells, A.; Puig-Montserrat, X.; Torre, I.; Freixas, L.; Mas, M.; Arrizabalaga, A.; Flaquer, C. Bat boxes in urban non-native forests: A popular practice that should be reconsidered. Urban Ecosyst. 2017, 20, 217–225. [Google Scholar] [CrossRef]
- López-Baucells, A.; Casanova, L.; Puig-Montserrat, X.; Espinal, A.; Páramo, F.; Flaquer, C. Evaluating the use of Myotis daubentonii as an ecological indicator in Mediterranean riparian habitats. Ecol. Indic. 2017, 74, 19–27. [Google Scholar] [CrossRef]
- Witsenburg, F.; Clément, L.; López-Baucells, A.; Palmeirim, J.; Pavlinić, I.; Scaravelli, D.; Ševčík, M.; Dutoit, L.; Salamin, N.; Goudet, J.; et al. How a haemosporidian parasite of bats gets around: The genetic structure of a parasite, vector and host compared. Mol. Ecol. 2015, 24, 926–940. [Google Scholar] [CrossRef]
- López-Baucells, A.; Flaquer, C.; Puig-Montserrat, X.; Freixas, L.; Mohamed, L. Actualización del inventario de quirópteros y refugios en Ceuta: Primera cita de Pipistrellus pygmaeus en el norte de África. Barbastella 2012, 5, 43–50. [Google Scholar]
- López-Baucells, A.; Mas, M.; Puig-Montserrat, X.; Flaquer, C. Hypopigmentation in vespertilionid bats: The first record of a leucistic soprano pipistrelle Pipistrellus pygmaeus. Barbastella 2013, 6, 66–72. [Google Scholar] [CrossRef]
- Stefanescu, C.; Soldevila, A.; Gutiérrez, C.; Torre, I.; Ubach, A.; Miralles, M. Explosions demogràfiques de l’eruga peluda del suro, Lymantria dispar (Linnaeus, 1758), als boscos del Montnegre el 2019 i 2020: Possibles causes, impactes i idoneïtat dels tractaments per combatre la plaga. Butlletí Inst. Catalana d’Història Nat. 2020, 84, 267–279. [Google Scholar]
- Oro, D.; Sanz-Aguilar, A.; Carbonell, F.; Grajera, J.; Torre, I. Multi-species prey dynamics influences local survival in resident and wintering generalist predators. Oecologia 2021, in press. [Google Scholar]
- Oro, D.; Freixas, L. Flickering body temperature anticipates criticality in hibernation dynamics. R. Soc. Open Sci. 2021, 8, 201571. [Google Scholar] [CrossRef]
- Parsons, A.W.; Goforth, C.; Costello, R.; Kays, R. The value of citizen science for ecological monitoring of mammals. PeerJ 2018, 2018, e4536. [Google Scholar] [CrossRef] [PubMed]
- Newman, C.; Buesching, C.D.; Macdonald, D.W. Validating mammal monitoring methods and assessing the performance of volunteers in wildlife conservation: “Sed quis custodiet ipsos custodies?”. Biol. Conserv. 2003, 113, 189–197. [Google Scholar] [CrossRef]
- McShea, W.J.; Forrester, T.; Costello, R.; He, Z.; Kays, R. Volunteer-run cameras as distributed sensors for macrosystem mammal research. Landsc. Ecol. 2016, 31, 55–66. [Google Scholar] [CrossRef]
- Coronado, A.; Flaquer, C.; Puig-Montserrat, X.; Barthe, E.; Mas, M.; Arrizabalaga, A.; López-Baucells, A. The role of secondary trees in Mediterranean mature forests for the conservation of the forest-dwelling bat Myotis alcathoe. Are current logging guidelines appropriate? Hystrix Ital. J. Mammal. 2017, 28, 240–246. [Google Scholar] [CrossRef]
- Herrando, S.; Brotons, L.; Antón, M.; Páramo, F.; Villero, D.; Titeux, N.; Quesada, J.; Stefanescu, C. Assessing impacts of land abandonment on Mediterranean biodiversity using indicators based on bird and butterfly monitoring data. Environ. Conserv. 2016, 43, 69–78. [Google Scholar] [CrossRef]
- Mason, T.H.E.; Pollard, C.R.J.; Chimalakonda, D.; Guerrero, A.M.; Kerr-Smith, C.; Milheiras, S.A.G.; Roberts, M.; Ngafack, P.R.; Bunnefeld, N. Wicked conflict: Using wicked problem thinking for holistic management of conservation conflict. Conserv. Lett. 2018, 11, e12460. [Google Scholar] [CrossRef] [PubMed]
- Calvet-Mir, L.; Maestre-Andrés, S.; Molina, J.L.; van den Bergh, J. Participation in protected areas: A social network case study in catalonia, Spain. Ecol. Soc. 2015, 20, 45. [Google Scholar] [CrossRef]
- Maestre-Andrés, S.; Calvet-Mir, L.; Apostolopoulou, E. Unravelling stakeholder participation under conditions of neoliberal biodiversity governance in Catalonia, Spain. Environ. Plan. C Polit. Sp. 2018, 36, 1299–1318. [Google Scholar] [CrossRef]
- Mace, G.M.; Norris, K.; Fitter, A.H. Biodiversity and ecosystem services: A multilayered relationship. Trends Ecol. Evol. 2012, 27, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Sikes, R.S. Animal Care and use Committee of the American Society of Guidelines of the American Society of Mammalogists for the use of wild mammals in research. J. Mammal. 2016, 97, 663–688. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).