Diversity and Distribution of Culturable Thermus Species in Terrestrial Hot Springs of Southwestern Yunnan Province in China
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling Area and Sample Collection
2.2. Thermus Strain Isolation and Culture Conditions
2.3. DNA Extraction and PCR Amplifications
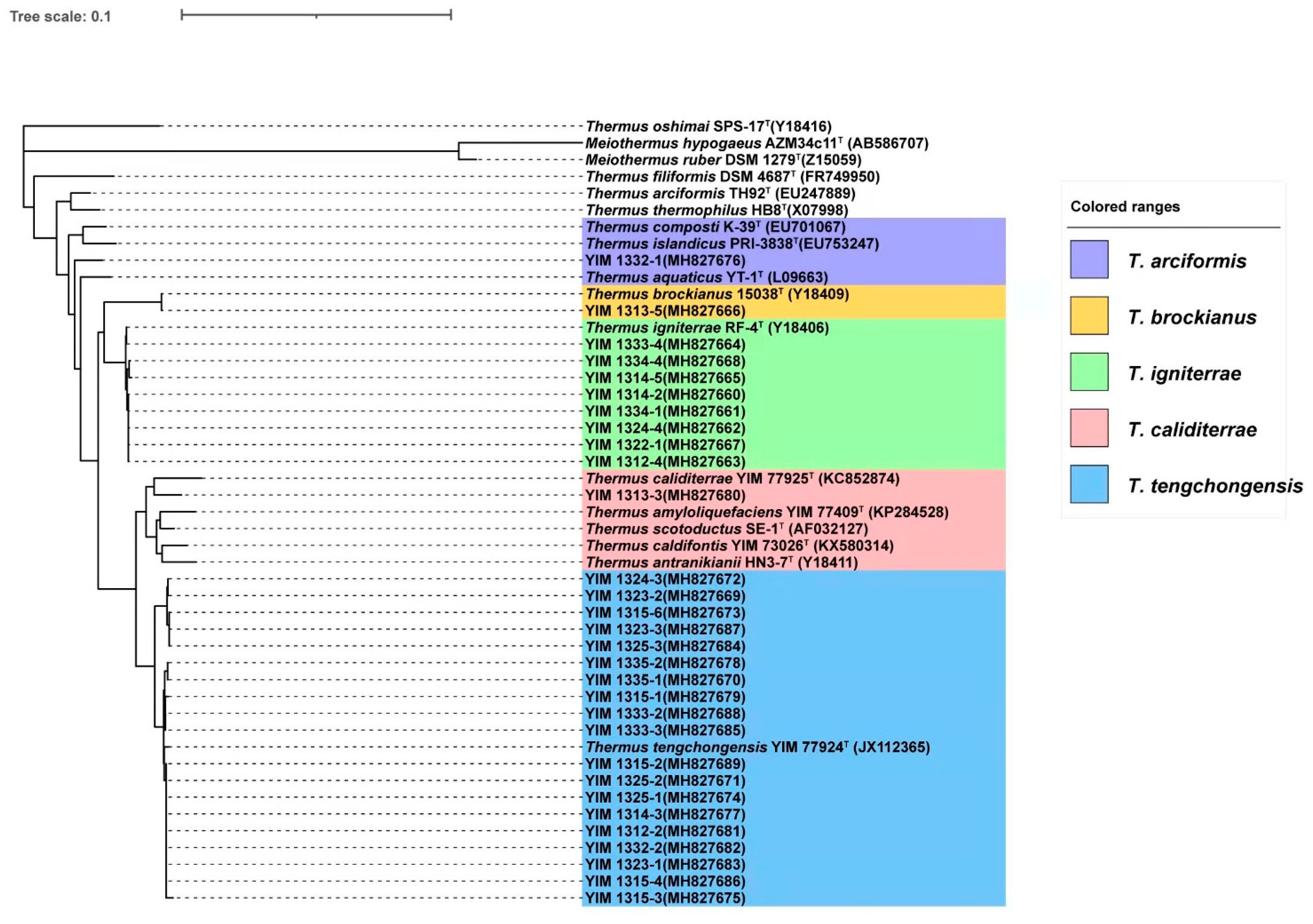
2.4. Sequence Classification and Phylogenetic Analysis
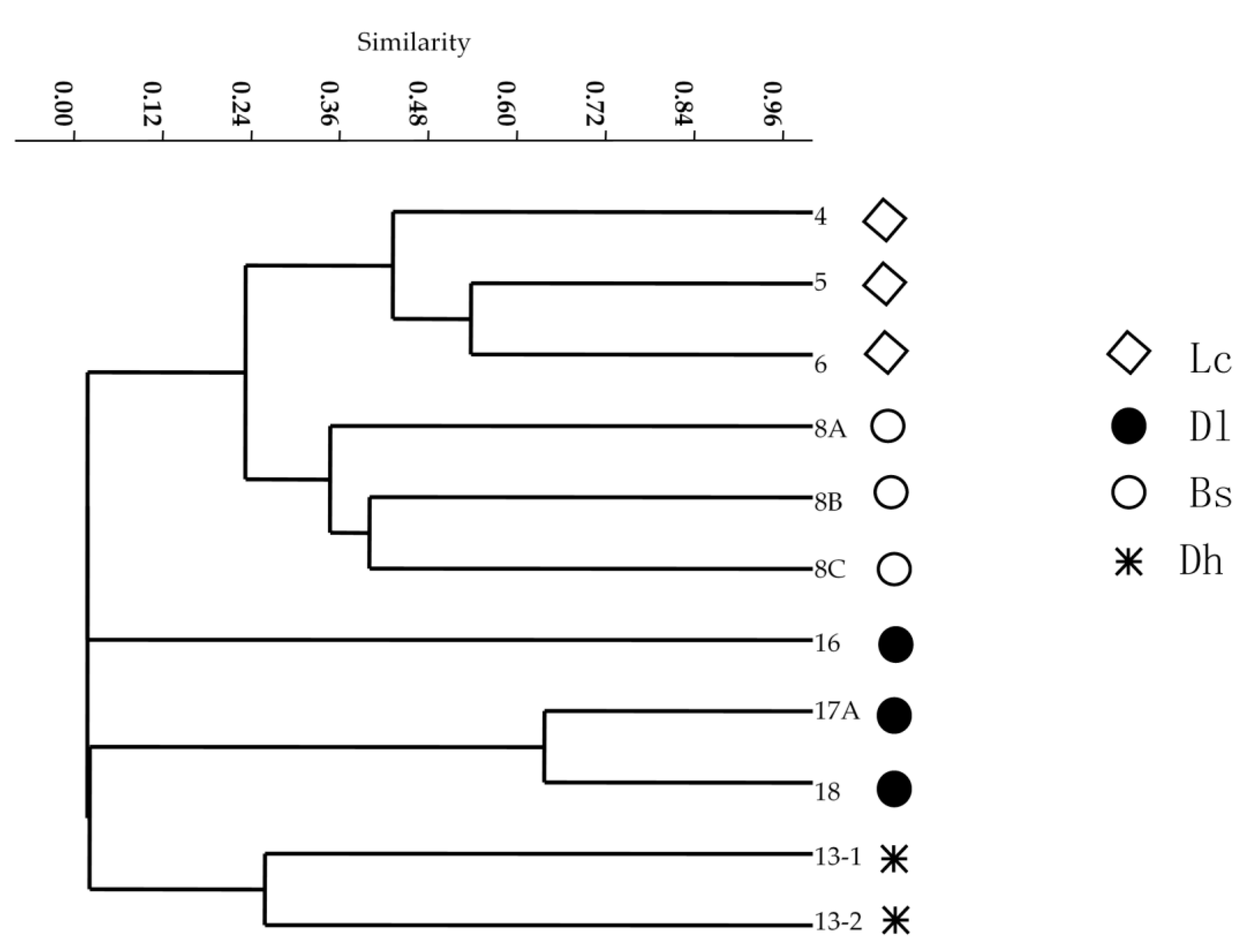
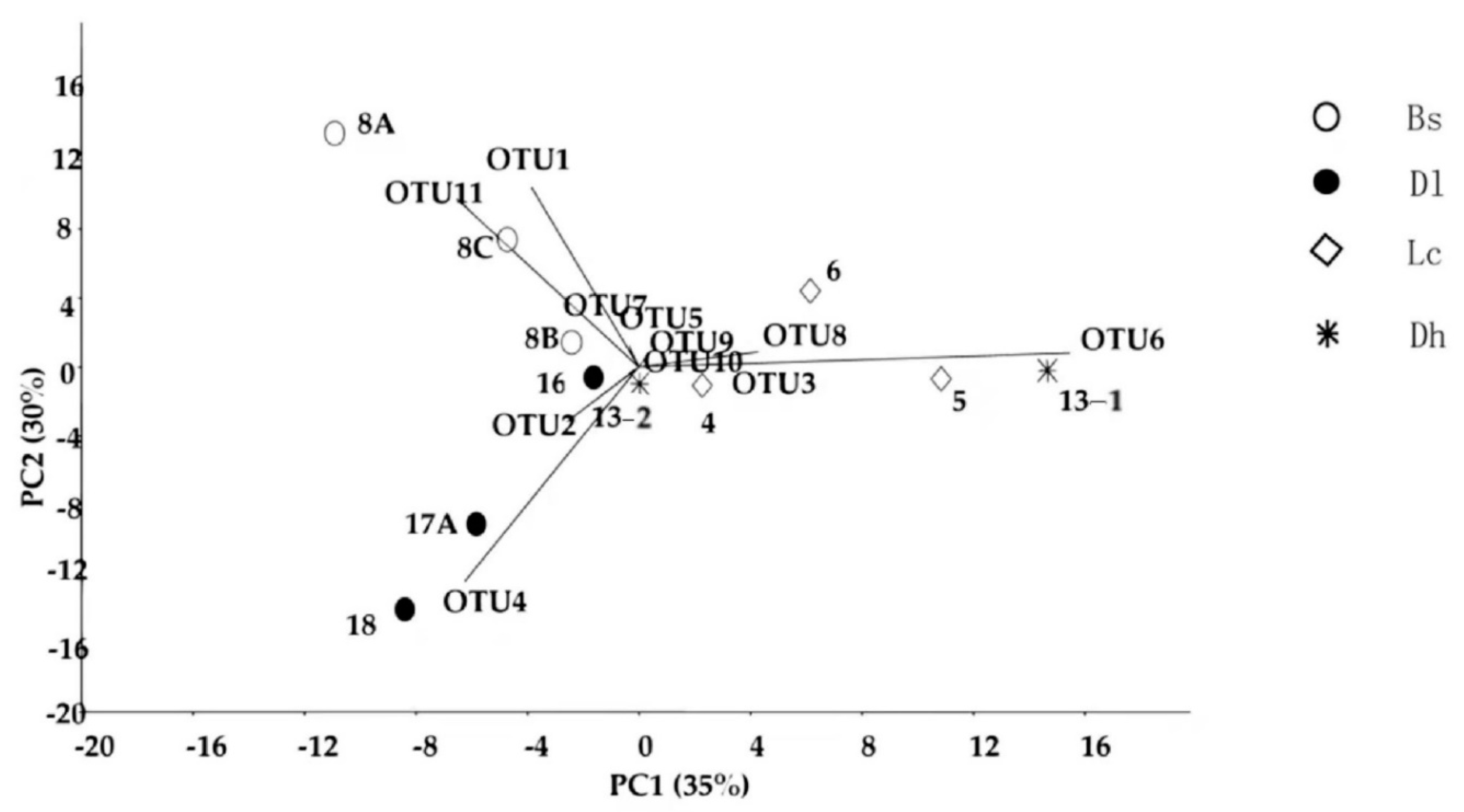
2.5. OTU Assignment and Community Analysis
3. Results
3.1. Screening of the Culturable Thermus-Like Strains from the Four Geothermal Regions
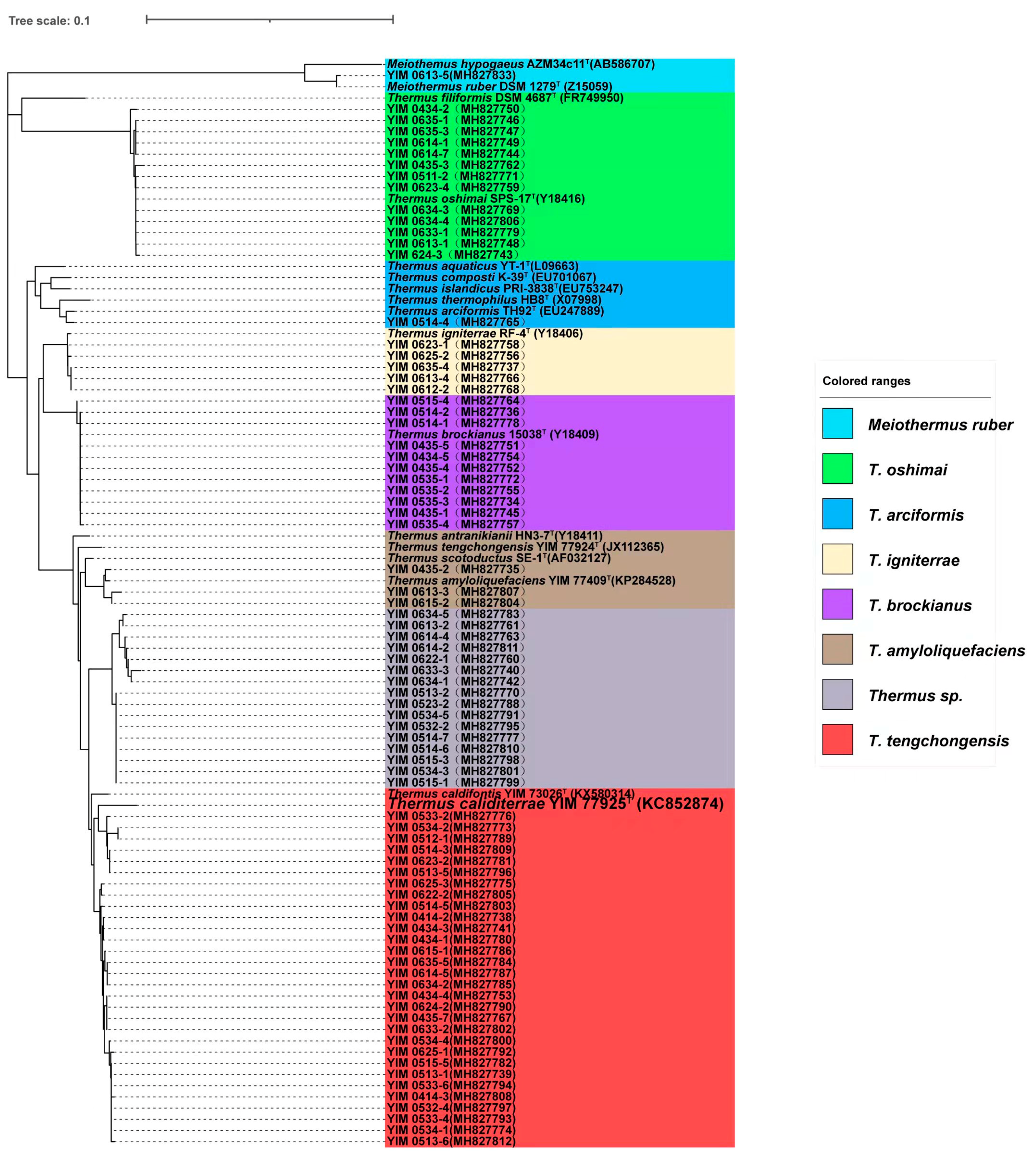
3.2. Species of Culturable Thermus-Like Bacteria in the Lincang Geothermal Region
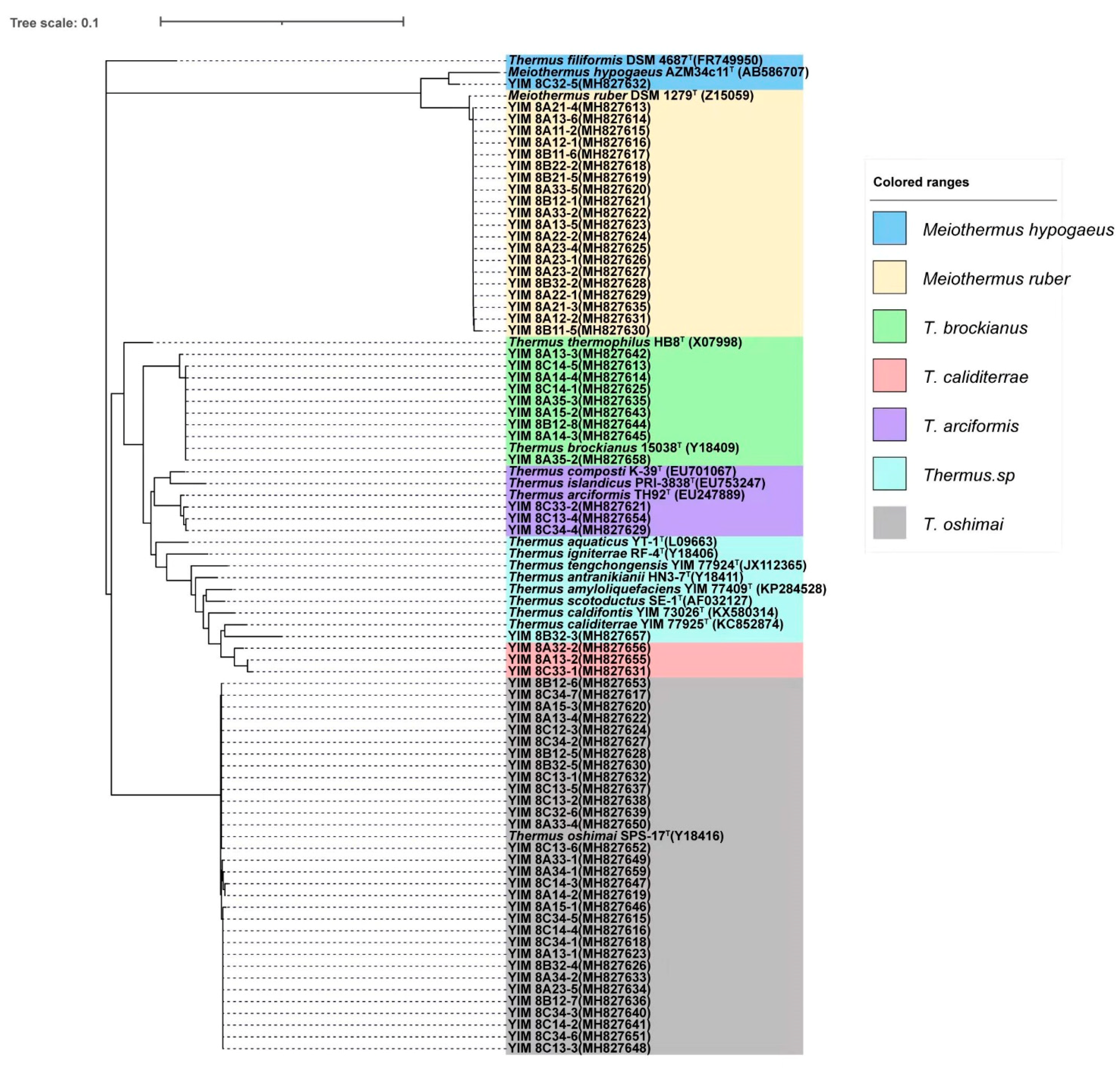
3.3. Species of Culturable Thermus-Like Bacteria in the Baoshan Geothermal Region
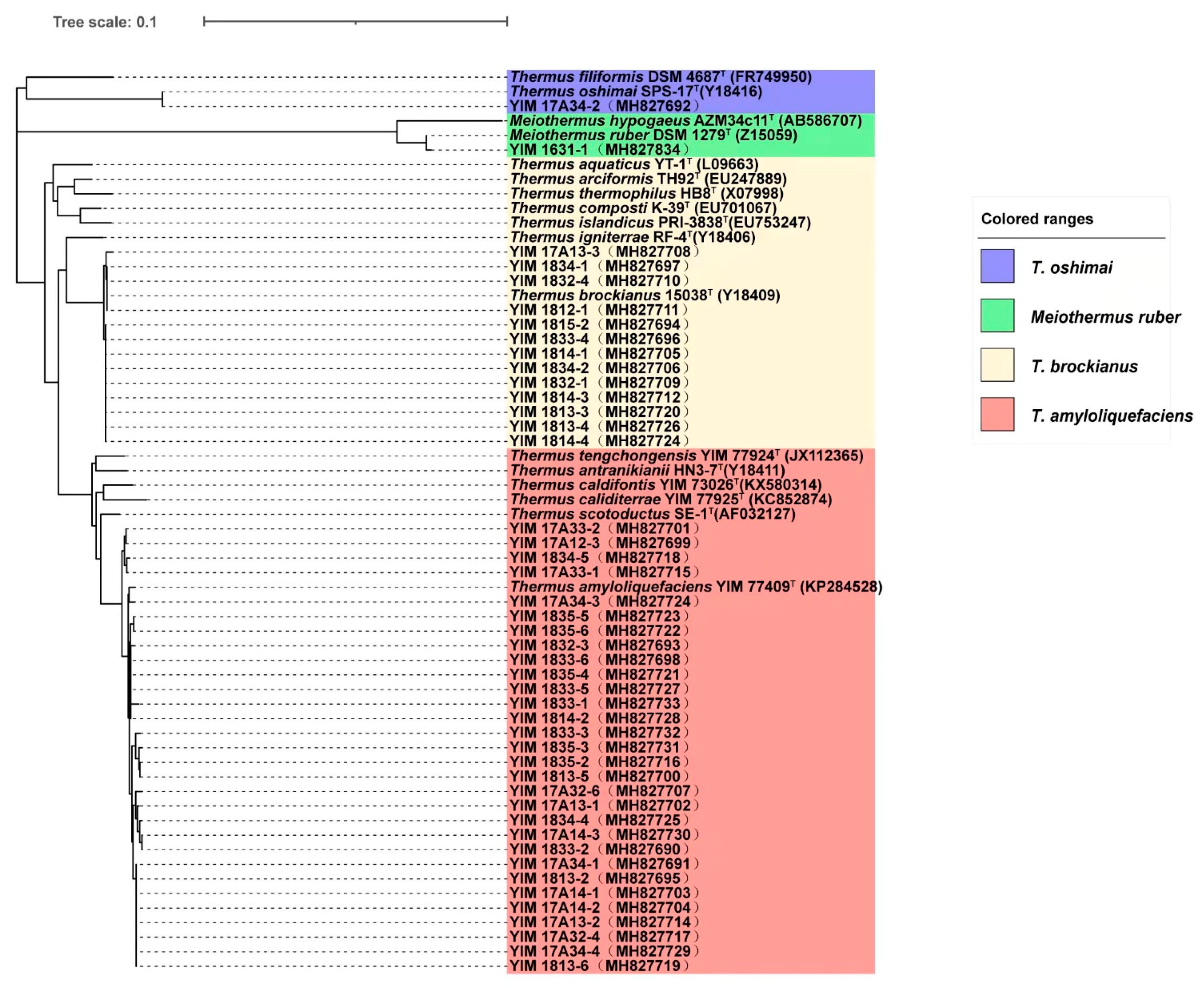
3.4. Species of Culturable Thermus-Like Bacteria in the Dali Geothermal Region
3.5. Species of Culturable Thermus-Like Bacteria in the Dehong Geothermal Region
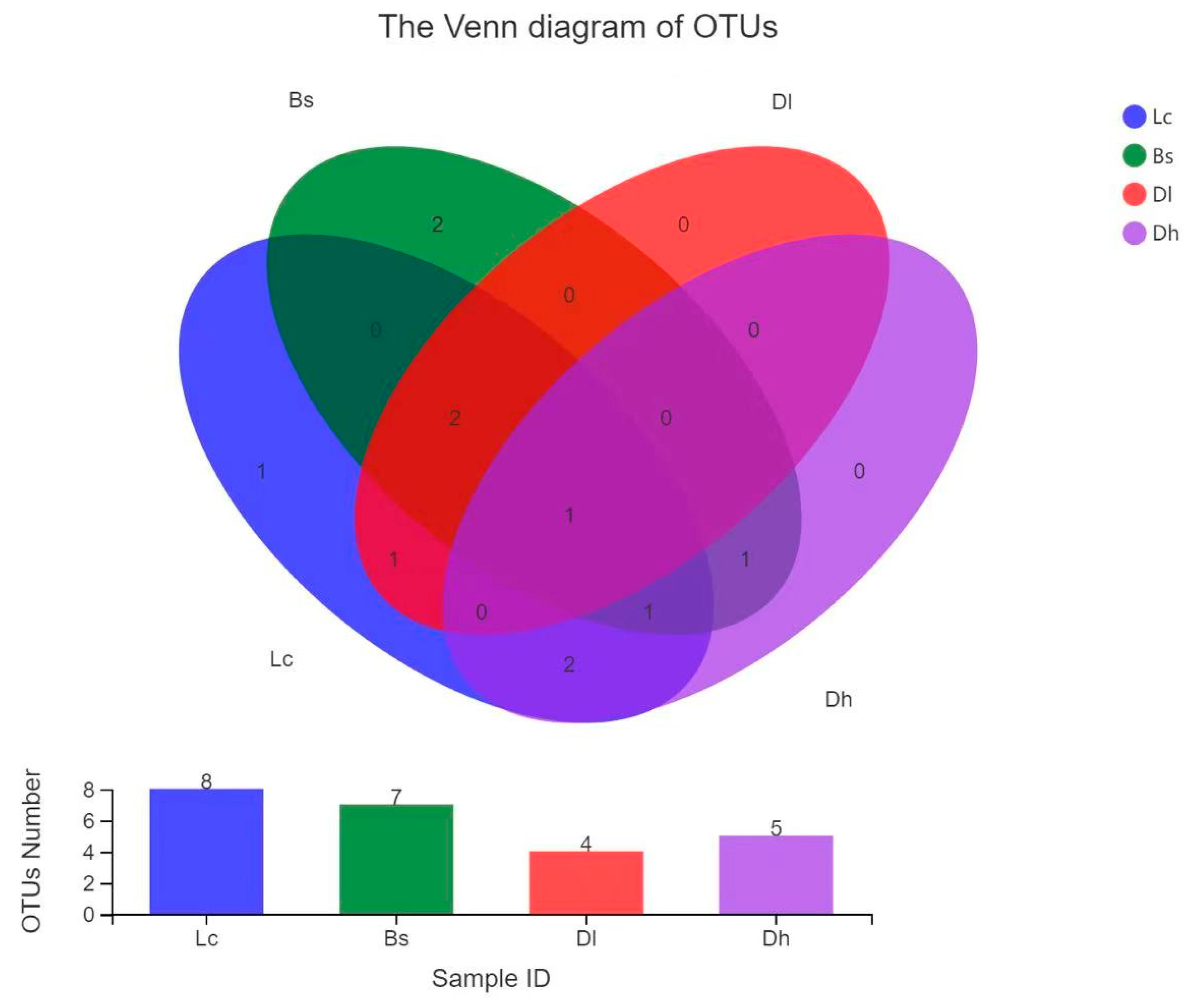
3.6. Distribution of Culturable Thermus-Like Bacteria in the Four Geothermal Regions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, C.L.; Ye, Q.; Huang, Z.; Li, W.; Chen, J.; Song, Z.; Zhao, W.; Bagwell, C.; Inskeep, W.P.; Ross, C.; et al. Global occurrence of archaeal amoA genes in terrestrial hot springs. Appl. Environ. Micobiol. 2008, 74, 6417–6426. [Google Scholar] [CrossRef]
- Pearson, A.; Pi, Y.; Zhao, W.; Li, W.; Li, Y.; Inskeep, W.; Perevalova, A.; Romanek, C.; Li, S.; Zhang, C.L. Factors controlling the distribution of archaeal tetraethers in terrestrial hot springs. Appl. Environ. Micobiol. 2008, 74, 3523–3532. [Google Scholar] [CrossRef]
- Jiang, H.; Huang, Q.; Dong, H.; Wang, P.; Wang, F.; Li, W.; Zhang, C. RNA-based investigation of ammonia-oxidizing archaea in hot springs of Yunnan Province, China. Appl. Environ. Micobiol. 2010, 76, 4538–4541. [Google Scholar] [CrossRef]
- Song, Z.; Zhi, X.; Li, W.; Zhang, C.; Dong, H. Actinobacterial Diversity in Hot Springs in Tengchong (China), Kamchatka (Russia), and Nevada (USA). Geomicrobiol. J. 2009, 26, 256–263. [Google Scholar] [CrossRef]
- Song, Z.Q.; Chen, J.Q.; Jiang, H.C.; Zhou, E.M.; Tang, S.K.; Zhi, X.Y.; Zhang, L.X.; Zhang, C.L.L.; Li, W.J. Diversity of Crenarchaeota in terrestrial hot springs in Tengchong, China. Extremophiles 2010, 14, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.Q.; Wang, F.P.; Zhi, X.Y.; Chen, J.Q.; Zhou, E.M.; Liang, F.; Xiao, X.; Tang, S.K.; Jiang, H.C.; Zhang, C.L.; et al. Bacterial and archaeal diversities in Yunnan and Tibetan hot springs, China. Environ. Microbiol. 2013, 15, 1160–1175. [Google Scholar] [CrossRef] [PubMed]
- Pagaling, E.; Grant, W.D.; Cowan, D.A.; Jones, B.E.; Ma, Y.; Ventosa, A.; Heaphy, S. Bacterial and archaeal diversity in two hot spring microbial mats from the geothermal region of Tengchong, China. Extremophiles 2012, 16, 607–618. [Google Scholar] [CrossRef]
- Hou, W.; Wang, S.; Dong, H.; Jiang, H.; Briggs, B.R.; Peacock, J.P.; Huang, Q.; Huang, L.; Wu, G.; Zhi, X.; et al. A comprehensive census of microbial diversity in hot springs of Tengchong, Yunnan Province China using 16S rRNA gene pyrosequencing. PLoS ONE 2013, 8, e53350. [Google Scholar] [CrossRef] [PubMed]
- Briggs, B.R.; Brodie, E.L.; Tom, L.M.; Dong, H.; Jiang, H.; Huang, Q.; Wang, S.; Hou, W.; Wu, G.; Huang, L.; et al. Seasonal patterns in microbial communities inhabiting the hot springs of Tengchong, Yunnan Province, China. Environ. Microbiol. 2014, 16, 1579–1591. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; He, H.; Zhang, C.; Yu, Y.; Qiu, G. Isolation and characterization of YNTC-1, a novel Alicyclobacillus sentainesis strain. J. Cent. South Univ. Technol. 2008, 15, 508–514. [Google Scholar] [CrossRef]
- Ding, J.; Zhang, R.; Yu, Y.; Jin, D.; Liang, C.; Yi, Y.; Zhu, W.; Xia, J. Novel acidophilic, thermophilic iron and sulfur-oxidizing archaeon isolate from a hot spring in Tengchong, Yunnan, China. Braz. J. Microbiol. 2011, 42, 514–525. [Google Scholar] [CrossRef]
- Jiang, C.; Liu, Y.; Liu, Y.Y.; Guo, X.; Liu, S.J. Isolation and characterization of ferrous- and sulfur-oxidizing bacteria from Tengchong solfataric region, China. J. Environ. Sci. 2009, 21, 1247–1252. [Google Scholar] [CrossRef]
- Zhang, C.M.; Huang, X.W.; Pan, W.Z.; Zhang, J.; Wei, K.B.; Klenk, H.P.; Tang, S.K.; Li, W.J.; Zhang, K.Q. Anoxybacillus tengchongensis sp. nov. and Anoxybacillus eryuanensis sp. nov., facultatively anaerobic, alkalitolerant bacteria from hot springs. Int. J. Syst. Evol. Microbiol. 2011, 61, 118–122. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, F.; Liu, X.L.; Dong, X.Z. Thermosyntropha tengcongensis sp. nov., a thermophilic bacterium that syntrophically degrades long-chain fatty acids. Int. J. Syst. Evol. Microbiol. 2012, 62, 759–763. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.J.; Lin, L.B.; Zhang, L.L.; Zhang, J.; Tang, S.K.; Wei, Y.L.; Li, W.J. Laceyella sediminis sp. nov., a thermophilic bacterium isolated from a hot spring in China. Int. J. Syst. Evol. Microbiol. 2012, 62, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Hedlund, B.P.; Cole, J.K.; Williams, A.J.; Hou, W.; Zhou, E.; Li, W.; Dong, H. A review of the microbiology of the Rehai geothermal field in Tengchong, Yunnan Province, China. Geosci. Front. 2012, 3, 273–288. [Google Scholar] [CrossRef]
- Yu, T.T.; Yao, J.C.; Ming, H.; Yin, Y.R.; Zhou, E.M.; Liu, M.J.; Tang, S.K.; Li, W.J. Thermus tengchongensis sp. nov., isolated from a geothermally heated soil sample in Tengchong, Yunnan, south-west China. Antonie Leeuwenhoek 2013, 103, 513–518. [Google Scholar] [CrossRef]
- Ming, H.; Yin, Y.R.; Li, S.; Nie, G.X.; Yu, T.T.; Zhou, E.M.; Liu, L.; Dong, L.; Li, W.J. Thermus caliditerrae sp. nov., a novel thermophilic species isolated from a geothermal area. Int. J. Syst. Evol. Microbiol. 2014, 64, 650–656. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.T.; Ming, H.; Yao, J.C.; Zhou, E.M.; Park, D.J.; Hozzein, W.N.; Kim, C.J.; Wadaan, M.A.; Li, W.J. Thermus amyloliquefaciens sp. nov., isolated from a hot spring sediment sample. Int. J. Syst. Evol. Microbiol. 2015, 65, 2491–2495. [Google Scholar] [CrossRef]
- Zhang, X.Q.; Ying, Y.; Ye, Y.; Xu, X.W.; Zhu, X.F.; Wu, M. Thermus arciformis sp. nov., a thermophilic species from a geothermal area. Int. J. Syst. Evol. Microbiol. 2010, 60, 834–839. [Google Scholar] [CrossRef]
- Brock, T.D.; Freeze, H. Thermus aquaticus gen. nov. and sp. nov., a nonsporulating extreme thermophile. J. Bacteriol. 1969, 98, 289–297. [Google Scholar] [CrossRef] [PubMed]
- da Costa, M.S.; Rainey, F.A.; Nobre, M.F. The genus Thermus and relatives. In The Prokaryotes: A Handbook on the Biology of Bacteria, 3rd ed.; Dworkin, M., Falkow, S., Rosenberg, E., Schleifer, K.H., Stackebrandt, E., Eds.; Springer: New York, NY, USA, 2006; Volume 7, pp. 797–812. [Google Scholar]
- Williams, R.A.D.; Smith, K.E.; Welch, S.G.; Micallef, J.; Sharp, R.J. DNA Relatedness of Thermus strains, description of Thermus brockianus sp. nov. and proposal to reestablish Thermus thermophilus (Oshima and Imahori). Int. J. Syst. Bacteriol. 1995, 45, 495–499. [Google Scholar] [CrossRef]
- Lioliou, E.E.; Pantazaki, A.A.; Kyriakidis, D.A. Thermus thermophilus genome analysis: Benefits and implications. Microbial. Cell Fact. 2004, 3, 1723–1727. [Google Scholar]
- Balkwill, D.L.; Keift, T.L.; Tsukuda, T.; Kostandarithes, H.M.; Onstott, T.C.; Macanaughton, S.; Bownas, J.; Fredrickson, F. Identification of iron-reducing Thermus strains as Thermus scotoductus. Extremophiles 2004, 8, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Opperman, D.J.; van Heerden, E. Aerobic Cr(VI) reduction by Thermus scotoductus strain SA-01. J. App. Microbiol. 2007, 103, 1907–1913. [Google Scholar] [CrossRef]
- Lin, L.; Chen, C.; Peng, Q.; Ben, K.; Zhou, Z. Thermus rehai sp. nov., isolated from Rehai of Tengchong, Yunnan Province, China. J. Basic Microbiol. 2002, 42, 337–344. [Google Scholar] [CrossRef]
- Lin, L.; Zhang, J.; Wei, Y.; Chen, C.; Peng, Q. Phylogenetic analysis of several Thermus strains from Rehai of Tengchong, Yunnan, China. Can. J. Microbiol. 2005, 51, 881–886. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Guo, C.L.; Wang, T.; Zhu, W.; Zhang, D.H.; Cui, X.L.; Xu, L.H.; Peng, Q. The phylotype of Thermus from the rehai geothermal area, Tengchong China. J. Microbiol. 2003, 41, 152–156. [Google Scholar]
- Cui, X.L.; Mao, P.H.; Zeng, M.; Li, W.J.; Zhang, L.P.; Xu, L.H.; Jiang, C.L. Streptimonospora salina gen. nov., sp. nov., a new member of the family Nocardiopsaceae. Int. J. Syst. Evol. Microbiol. 2001, 51, 357–363. [Google Scholar] [CrossRef]
- Lane, D.J. 16S/23S rRNA sequencing. In Nucleic Acid Techniques in Bacterial Systematics; Stackebrandt, E., Goodfellow, M., Eds.; Wiley: Hoboken, NJ, USA, 1991; pp. 115–175. [Google Scholar]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis Version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef]
- Kimura, M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar]
- Thompson, J.D.; Gibson, T.J.; Plewniak, F.; Jeanmougin, F.; Higgins, D.G. The CLUSTAL_X windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997, 25, 4876–4882. [Google Scholar] [CrossRef]
- Schloss, P.D.; Handelsman, J. Introducing DOTUR, a computer program for defining operational taxonomic units and estimating species richness. Appl. Environ. Microbiol. 2005, 71, 1501–1506. [Google Scholar] [CrossRef]
- McLellan, S.L.; Huse, S.M.; Mueller-Spitz, S.R.; Andreishcheva, E.N.; Sogin, M.L. Diversity and population structure of sewage-derived microorganisms in wastewater treatment plant influent. Environ. Microbiol. 2010, 12, 378–392. [Google Scholar] [CrossRef]
- Qian, P.; Wang, Y.; Lee, O.O.; Lou, S.C.; Yang, J.; Lafi, F.F.; Al-Suwailem, A.; Wong, T.Y. Vertical stratification of microbial communities in the Red Sea revealed by 16S rDNA pyrosequencing. ISME J. 2010, 5, 507–518. [Google Scholar] [CrossRef]
- Nobre, M.F.; Trüper, H.G.; da Costa, M.S. Transfer of Thermus ruber (Loginova et al. 1984), Thermus silvanus (Tenreiro et al. 1995), and Thermus chliarophilus (Tenreiro et al. 1995) to Meiothermus gen. nov. as Meiothermus ruber comb. nov., Meiothermus silvanus comb. nov., and Meiothermus chliarophilus comb. nov., respectively, and emendation of the genus Thermus. Int. J. Syst. Bacteriol. 1996, 46, 604–606. [Google Scholar]
- Manaia, C.M.; Hoste, B.; Gutierrez, M.C.; Gillis, M.; Ventosa, A.; Kersters, K.; da Costa, M.S. Halotolerant Thermus strains from marine and terrestrial hot springs belong to Thermus thermophilus (ex Oshima and Imahori, 1974) nom. rev. emend. Syst. Appl. Microbiol. 1995, 17, 526–532. [Google Scholar] [CrossRef]
- Hudson, J.A.; Morgan, H.W.; Daniel, R.M. Numerical classification of some Thermus isolates from Icelandic hot springs. Syst. Appl. Microbiol. 1987, 9, 218–223. [Google Scholar] [CrossRef]
- Sharp, R.J.; Williams, R.A.D. Properties of Thermus ruber strains isolated from Icelandic hot springs and DNA-DNA homology of Thermus ruber and Thermus aquaticus. Appl. Environ. Microbiol. 1988, 54, 2049–2053. [Google Scholar] [CrossRef] [PubMed]
- Munster, M.J.; Munster, A.P.; Woodrow, J.R.; Sharp, R.J. Isolation and preliminary taxonomic studies of Thermus strains isolated from Yellowstone National Park, USA. J. Gen. Microbiol. 1986, 132, 1677–1683. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.A.D.; Smith, K.E.; Welch, S.G.; Micallef, J. Thermus oshimai sp. nov., isolated from hot springs in Portugal, Iceland, and the Azores, and comment on the concept of a limited geographical distribution of Thermus species. Int. J. Syst. Bacteriol. 1996, 46, 403–408. [Google Scholar] [CrossRef][Green Version]
- Hedlund, B.P.; Mcdonald, A.I.; Lam, J.; Dodsworth, J.A.; Brown, J.R.; Hungate, B.A. Potential role of Thermus thermophilus and T. oshimai in high rates of nitrous oxide (N2O) production in 80 °C hot springs in the US Great Basin. Geobiology 2011, 9, 471–480. [Google Scholar] [CrossRef]
- Chung, A.P.; Rainey, F.A.; Valente, M.; Nobre, M.F.; da Costa, M.S. Thermus igniterrae sp. nov. and Thermus antranikianii sp. nov., two new species from Iceland. Int. J. Syst. Evol. Microbiol. 2000, 50, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Kristjánsson, J.K.; Hjöleifsdóttir, S.; Marteinsson, V.; Alfredsson, G.A. Thermus scotoductus, sp. nov., a pigment-producing thermophilic bacterium from hot tap water in Iceland and including Thermus sp. X-1. Syst. Appl. Microbiol. 1994, 17, 44–50. [Google Scholar] [CrossRef]
- Kieft, T.L.; Fredrickson, J.K.; Onstott, T.C.; Gorby, Y.A.; Kostandarithes, H.M.; Bailey, T.J.; Kennedy, D.W.; Li, S.W.; Plymale, A.E.; Spadoni, C.M.; et al. Dissimilatory reduction of Fe (III) and other electron acceptors by a Thermus isolate. Appl. Environ. Microbiol. 1999, 65, 1214–1221. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.A.; Williams, R.A.D.; da Costa, M.S. Numerical taxonomy of Thermus isolates from hot springs in Portugal. Syst. Appl. Microbiol. 1989, 12, 310–315. [Google Scholar] [CrossRef]
| Geothermal Region | Hot Spring | Sample | Sample Temperture (°C) | Sample pH | Isolation Temperature (°C) | Isolation pH |
|---|---|---|---|---|---|---|
| Lincang (Lc) | 4 | 4-1 | 75.3 | 6.92 | 50, 55, 63, 70, 75 | 7.0 |
| 5-1 | / | / | 8.5 | |||
| 5 | 5-2 | 91.5 | 9.20 | |||
| 5-3 | / | / | ||||
| 6 | 6-1 | 93.0 | 8.17 | 8.0 | ||
| 8A | 8-1 | 79.9 | 6.34 | 50, 55, 63, 70, 75 | 7.0 | |
| Baoshan (Bs) | 8-2 | / | / | 50, 55 | 7.0 | |
| 8B | 8-3 | 54.8 | 6.90 | |||
| 8-4 | / | / | ||||
| 8C | 8-5 | 72.7 | 6.62 | 50, 55, 63, 70 | 7.0 | |
| Dali (Dl) | 16 | 16-1 | 56.9 | 7.21 | 50, 55, 63, 70 | 7.0 |
| 17-1 | 71.1 | 6.48 | ||||
| 17A | 17-2 | 74.8 | 6.63 | |||
| 17-3 | / | / | ||||
| 18 | 18-1 | 75.0 | 6.64 | 50, 55, 63, 70, 75 | 7.0 | |
| Dehong (Dh) | 13 | 13-1 | 79.4 | 7.92 | 50, 55, 63, 70, 75 | 8.0 |
| 13-2 | / | / |
| OTU | Taxon | Hot Spring/Hot Spring Temperature (°C) | Isolaton Temperature (°C) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4/75.3 | 5/91.5 | 6/93.0 | 8A/79.9 | 8B/54.4 | 8C/72.7 | 16/56.9 | 17A/75.3 | 18/71.1 | 13/79.0 | 50 | 55 | 63 | 70 | 75 | ||
| OTU1 | T. oshimai | 2 | 1 | 10 | 10 | 5 | 16 | 1 | 7 | 14 | 19 | 5 | ||||
| OTU2 | T. brockianus | 4 | 7 | 6 | 1 | 2 | 1 | 12 | 1 | 3 | 6 | 13 | 12 | |||
| OTU3 | T. igniterrae | 5 | 8 | 3 | 3 | 5 | 2 | |||||||||
| OTU4 | T. amyloliquefaciens | 1 | 2 | 13 | 17 | 4 | 13 | 9 | 7 | |||||||
| OTU5 | T. arciformis | 1 | 3 | 1 | 1 | 2 | 2 | |||||||||
| OTU6 | T. tengchongensis | 6 | 14 | 10 | 19 | 5 | 13 | 16 | 15 | |||||||
| OTU7 | T. caliditerrae | 2 | 1 | 1 | 1 | 3 | ||||||||||
| OTU8 | T. sp.1 | 9 | 7 | 2 | 4 | 8 | 2 | |||||||||
| OTU9 | T. sp.2 | 1 | 1 | |||||||||||||
| OTU10 | Meio. nypogaeus | 1 | 1 | |||||||||||||
| OTU11 | Meio. ruber | 1 | 20 | 1 | 7 | 7 | 8 | |||||||||
| Total mumber | 13 | 32 | 35 | 38 | 7 | 23 | 1 | 15 | 29 | 30 | 7 | 33 | 68 | 72 | 43 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Xu, C.; Han, L.; Li, C.; Xiao, W.; Cui, X. Diversity and Distribution of Culturable Thermus Species in Terrestrial Hot Springs of Southwestern Yunnan Province in China. Diversity 2021, 13, 455. https://doi.org/10.3390/d13090455
Wang Y, Xu C, Han L, Li C, Xiao W, Cui X. Diversity and Distribution of Culturable Thermus Species in Terrestrial Hot Springs of Southwestern Yunnan Province in China. Diversity. 2021; 13(9):455. https://doi.org/10.3390/d13090455
Chicago/Turabian StyleWang, Yongxia, Canhai Xu, Long Han, Chengpeng Li, Wei Xiao, and Xiaolong Cui. 2021. "Diversity and Distribution of Culturable Thermus Species in Terrestrial Hot Springs of Southwestern Yunnan Province in China" Diversity 13, no. 9: 455. https://doi.org/10.3390/d13090455
APA StyleWang, Y., Xu, C., Han, L., Li, C., Xiao, W., & Cui, X. (2021). Diversity and Distribution of Culturable Thermus Species in Terrestrial Hot Springs of Southwestern Yunnan Province in China. Diversity, 13(9), 455. https://doi.org/10.3390/d13090455





