Trade-Off Relationships of Leaf Functional Traits of Lycium ruthenicum in Response to Soil Properties in the Lower Reaches of Heihe River, Northwest China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sampling Protocol and Community Characteristics
2.3. Determination of Leaf Water Physiological and Stoichiometric Traits
2.4. Measurement of Soil Moisture, Salinity and Ion Contents
2.5. Statistical Analysis
3. Results
3.1. Leaf Functional Traits in Different Populations of L. ruthenicum
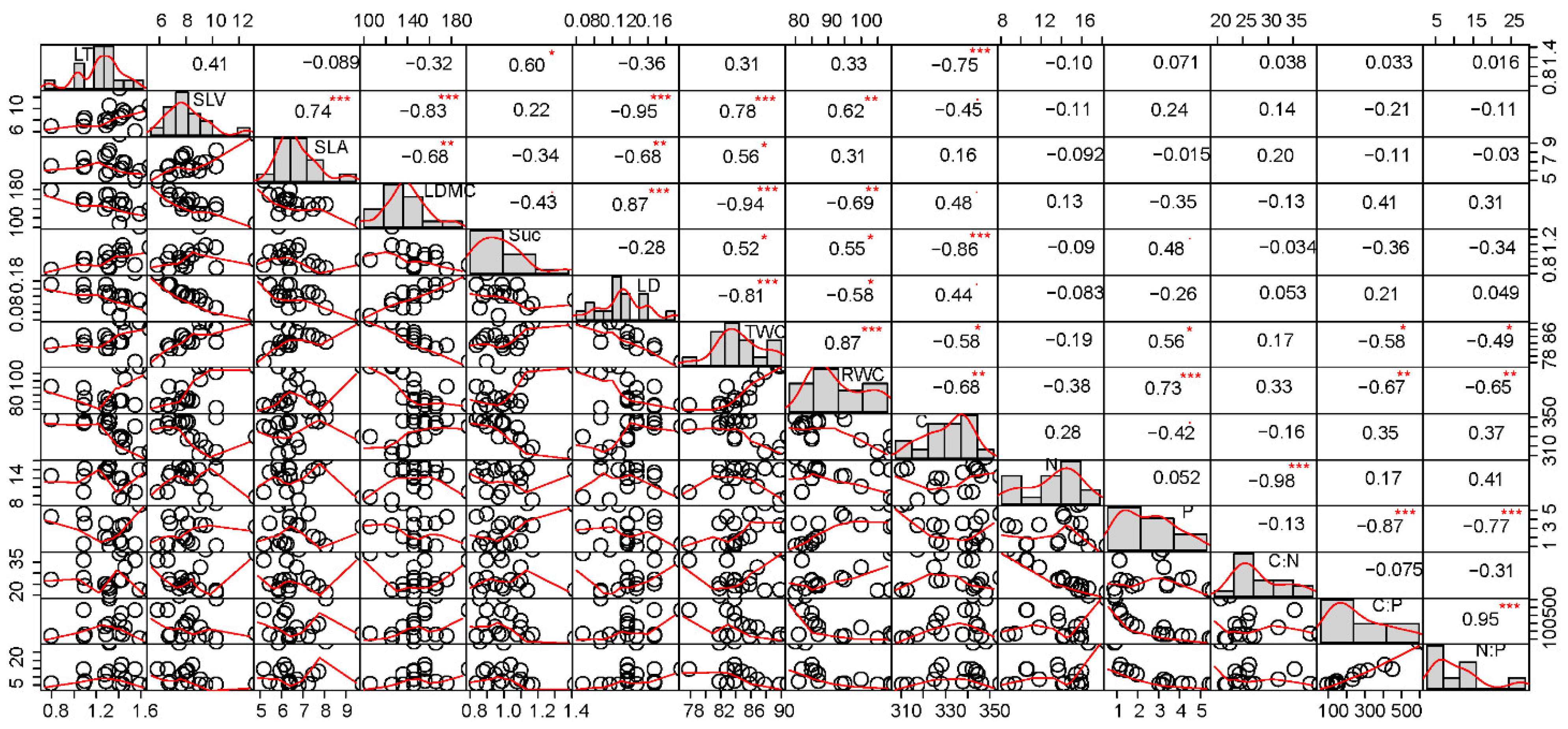
3.2. Correlation between Leaf Functional Traits of L. ruthenicum in Eight Habitats
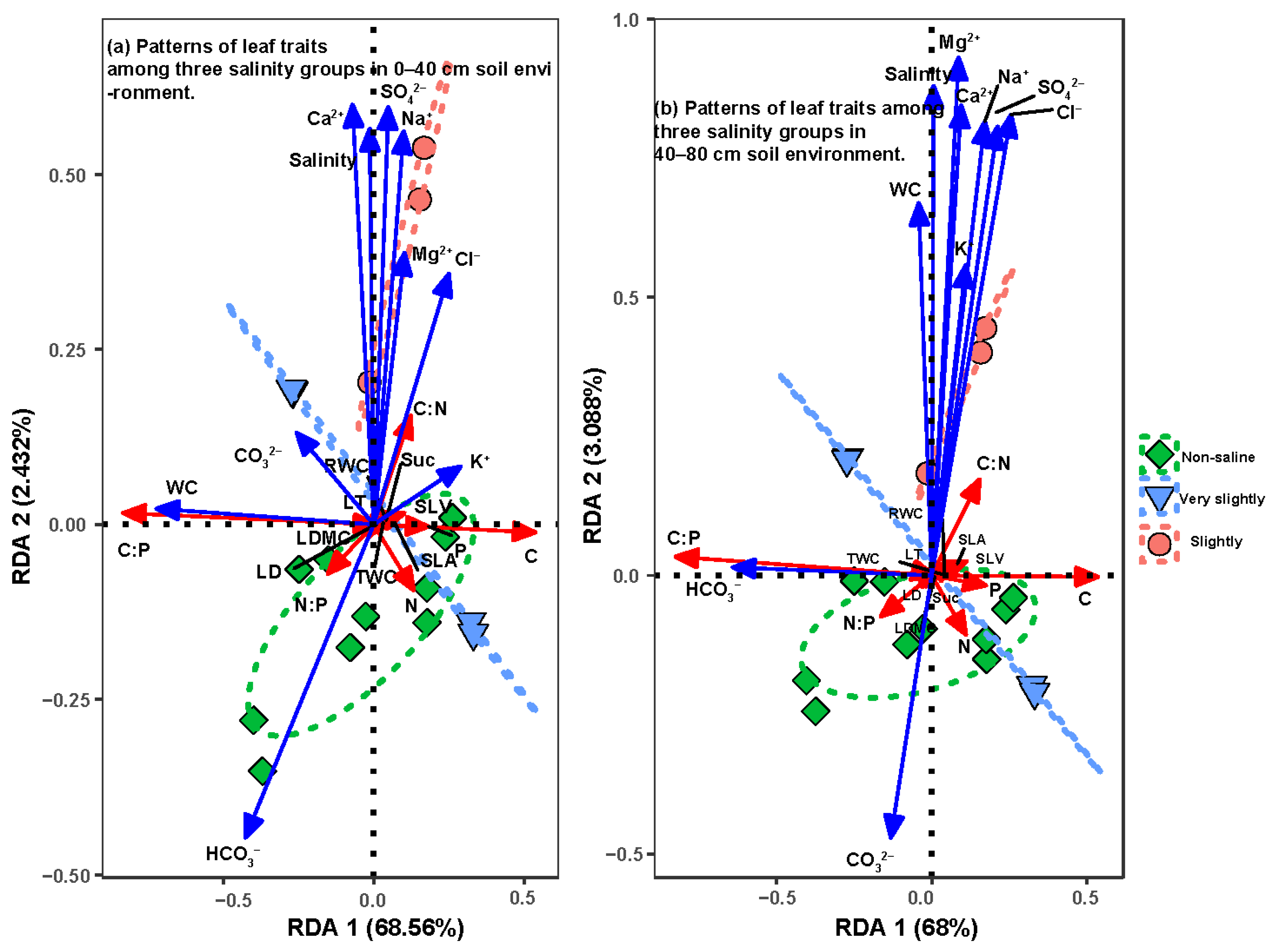
3.3. RDA of Leaf Functional Traits in Soil Moisture and Salinity Properties
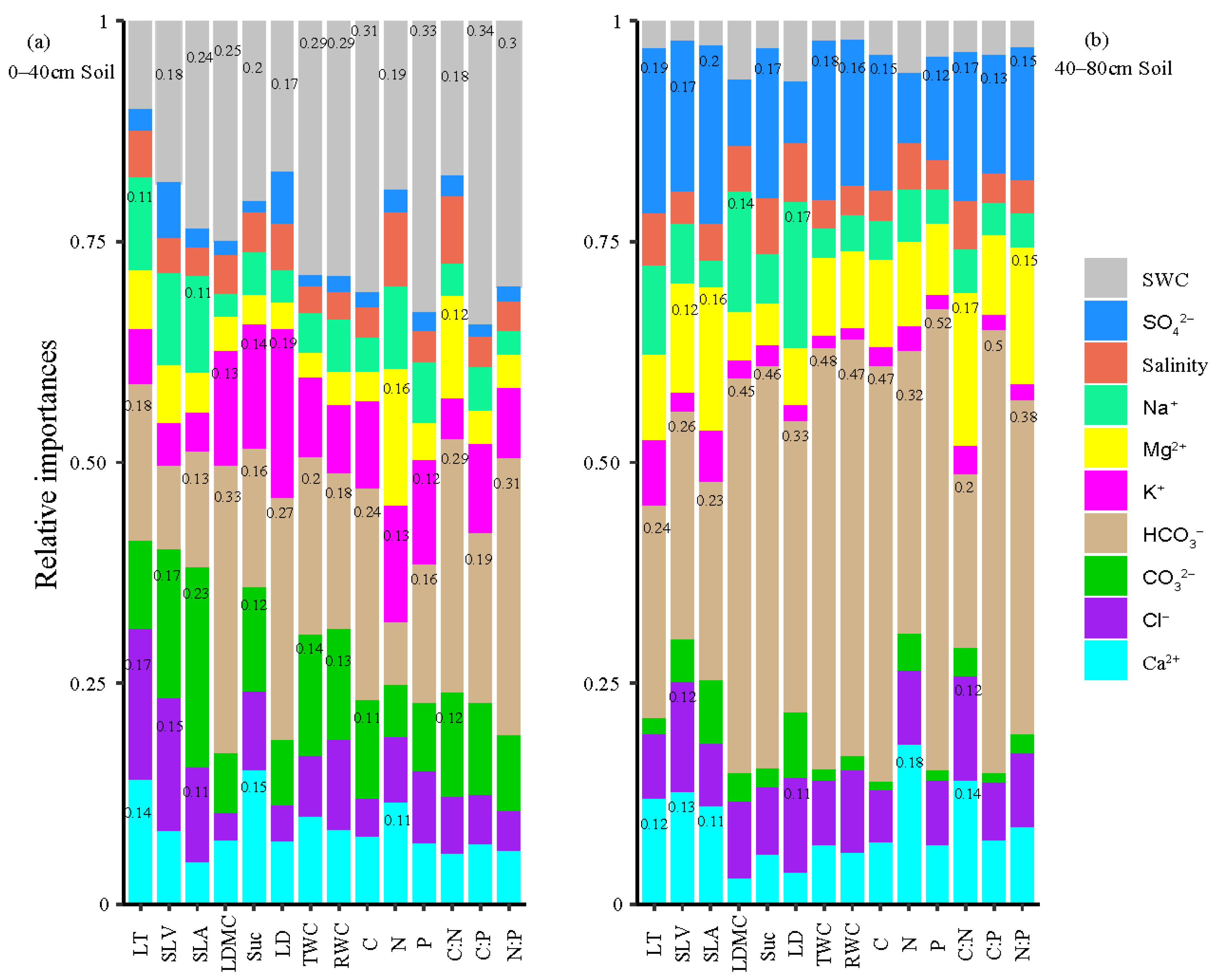
3.4. Relative Importance of Soil Factors to Leaf Functional Traits Variation
4. Discussion
4.1. Variations of L. ruthenicum Leaf Functional Traits in the Lower Reaches of Heihe River
4.2. Trade-off Relationships between Leaf Functional Traits of L. ruthenicum
4.3. To What Extent Does Soil Moisture and Salinity Affect Leaf Functional Traits?
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Violle, C.; Navas, M.L.; Vile, D.; Kazakou, E.; Fortunel, C.; Hummel, I.; Garnier, E. Let the concept of trait be functional. Oikos 2007, 116, 882–892. [Google Scholar] [CrossRef]
- Perrino, E.V.; Brunetti, G.; Farrag, K. Plant communities of multi-metal contaminated soils: A case study in National Park of Alta Murgia (Apulia Region-Southern Italy). Int. J. Phytoremediation 2014, 16, 871–888. [Google Scholar] [CrossRef] [PubMed]
- Harder, L.D.; Strelin, M.M.; Clocher, I.C.; Kulbaba, M.W.; Aizen, M.A. The dynamic mosaic phenotypes of flowering plants. New Phytol. 2019, 224, 1021–1034. [Google Scholar] [CrossRef] [Green Version]
- Perrino, E.V.; Valerio, F.; Gannouchi, A.; Trani, A.; Mezzapesa, G. Ecological and plant community implication on essential oils composition in useful wild officinal species: A pilot case study in Apulia (Italy). Plants 2021, 10, 574. [Google Scholar] [CrossRef] [PubMed]
- Bernhardt-Römermann, M.; Römermann, C.; Nuske, R.; Parth, A.; Klotz, S.; Schmidt, W.; Stadler, J. On the identification of the most suitable traits for plant functional trait analyses. Oikos 2008, 117, 1533–1541. [Google Scholar] [CrossRef]
- Westoby, M.; Wright, I.J. Land-plant ecology on the basis of functional traits. Trends Ecol. Evol. 2006, 21, 261–268. [Google Scholar] [CrossRef]
- Reich, P.B. The world-wide ‘fast-slow’ plant economics spectrum: A traits manifesto. J. Ecol. 2014, 102, 275–301. [Google Scholar] [CrossRef]
- Ivanova, L.A.; Zolotareva, N.V.; Ronzhina, D.A.; Podgaevskaya, E.N.; Migalina, S.V.; Ivanov, L.A. Leaf functional traits of abundant species predict productivity in three temperate herbaceous communities along an environmental gradient. Flora 2018, 239, 11–19. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.C.; Diemer, M. World-wide leaf economics spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef]
- Niu, K.; Zhang, S.; Zhao, B.; Du, G. Linking grazing response of species abundance to functional traits in the Tibetan alpine meadow. Plant Soil 2010, 330, 215–223. [Google Scholar] [CrossRef]
- Díaz, S.; Hodgson, J.G.; Thompson, K.; Cabido, M. The plant traits that drive ecosystems: Evidence from three continents. J. Veg. Sci. 2004, 15, 295–304. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.L.; Zhao, G.X.; Li, M.; Zhang, M.T.; Zhang, L.F.; Zhang, X.F.; An, L.Z.; Xu, S.J. C:N:P stoichiometry and leaf traits of halophytes in an arid saline environment, Northwest China. PLoS ONE 2015, 10, e0119935. [Google Scholar] [CrossRef] [Green Version]
- Li, J.Y.; Zhao, C.Y.; Li, J.; Yan, Y.Y.; Yu, B.; Han, M. Growth and leaf gas exchange in Populus euphratica across soil water and salinity gradients. Photosynthetica 2013, 51, 321–329. [Google Scholar] [CrossRef]
- Lu, Y.W.; Miao, X.L.; Song, Q.Y.; Peng, S.M.; Duan, B.L. Morphological and ecophysiological plasticity in dioecious plant Populus tomentosa under drought and alkaline stresses. Photosynthetica 2018, 56, 1353–1364. [Google Scholar] [CrossRef]
- Shabala, S.; Munns, R. Salinity stress: Physiological constraints and adaptive mechanisms. Plant Stress Physiol. 2012, 1, 59–93. [Google Scholar]
- Flowers, T.J.; Colmer, T.D. Salinity tolerance in halophytes. New Phytol. 2008, 179, 945–963. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Li, Y. Land exploitation resulting in soil salinization in a desert-oasis ecotone. Catena 2013, 100, 50–56. [Google Scholar] [CrossRef]
- Rodríguez, P.; Torrecillas, A.; Morales, M.A.; Ortuno, M.F.; Sánchez-Blancoa, M.J. Effects of NaCl salinity and water stress on growth and leaf water relations of Asteriscus maritimus plants. Environ. Exp. Bot. 2005, 53, 113–123. [Google Scholar] [CrossRef]
- Sun, X.; Gao, Y.; Wang, D.; Chen, J.; Zhang, F.; Zhou, J.; Yan, X.; Li, Y. Stoichiometric variation of halophytes in response to changes in soil salinity. Plant Biol. 2017, 19, 360–367. [Google Scholar] [CrossRef]
- Rong, Q.Q.; Liu, J.T.; Cai, Y.P.; Lu, Z.H.; Zhao, Z.Z.; Yue, W.C.; Xia, J.X. Leaf carbon, nitrogen and phosphorus stoichiometry of Tamarix Chinensis (Lour) in the Laizhou Bay coastal wetland, China. Ecol. Eng. 2015, 76, 57–65. [Google Scholar] [CrossRef]
- Fu, A.H.; Chen, Y.N.; Li, W.H. Water use strategies of the desert riparian forest plant community in the lower reaches of Heihe River Basin, China. Sci. China Earth Sci. 2014, 57, 1293–1305. [Google Scholar] [CrossRef]
- Rasband, W.S.; Image, J. National Institutes of Health, Bethesda. 1997–2016. Available online: https://imagej.nih.gov/ij/ (accessed on 1 September 2018).
- Li, S.J.; Su, P.X.; Zhang, H.N.; Zhou, Z.J.; Xie, T.T.; Shi, R.; Gou, W. Distribution patterns of desert plant diversity and relationship to soil properties in the Heihe River Basin, China. Ecosphere 2018, 9, e02355. [Google Scholar] [CrossRef]
- Regional Salinity Laboratory (US). Diagnosis and improvement of saline and alkali soils. In USDA Handbook No. 60.; United States Department of Agriculture: Washington, DC, USA, 1954. [Google Scholar]
- Cornelissen, J.H.C.; Lavorel, S.; Garnier, E.; Díaz, S.; Buchmann, N.; Gurvich., D.E.; Reich, P.B.; ter Steege, H.; Morgan, H.D.; van der Heijden, M.G.A.; et al. A handbook of protocols for standardized and easy measurement of plant functional traits worldwide. Aust. J. Bot. 2003, 51, 335–380. [Google Scholar] [CrossRef] [Green Version]
- Brian, G.; Peterson, P.C. Performance Analytics: Econometric Tools for Performance and Risk Analysis. R package version 1.5.2. 2018. Available online: https://CRAN.R-project.org/package=PerformanceAnalytics (accessed on 1 September 2018).
- Grömping, U. Relative importance for linear regression in R: The package relaimpo. J. Stat. Softw. 2006, 17, 1–27. [Google Scholar] [CrossRef] [Green Version]
- Wright, I.J.; Westoby, M.; Reich, P.B. Convergence towards higher leaf mass per area in dry and nutrient-poor habitats has different consequences for leaf life span. J. Ecol. 2002, 90, 534–543. [Google Scholar] [CrossRef] [Green Version]
- Burns, K.C. Patterns in specific leaf area and the structure of a temperate heath community. Divers. Distrib. 2004, 10, 105–112. [Google Scholar] [CrossRef]
- Saura-Mas, S.; Lloret, F. Leaf and shoot water content and leaf dry matter content of Mediterranean woody species with different post-fire regenerative strategies. Ann. Bot. 2007, 99, 545–554. [Google Scholar] [CrossRef] [Green Version]
- Westoby, M.; Falster, D.S.; Moles, A.T.; Vesk, P.A.; Wright, I.J. Plant ecological strategies: Some leading dimensions of variation between species. Annu. Rev. Ecol. Syst. 2002, 33, 125–159. [Google Scholar] [CrossRef] [Green Version]
- Wilson, P.J.; Thompson, K.; Hodgson, J.G. Specific leaf area and leaf dry matter content as alternative predictors of plant strategies. New Phytol. 1999, 143, 155–162. [Google Scholar] [CrossRef]
- Koerselman, W.; Meuleman, A.F.M. The vegetation N:P Ratio: A new tool to detect the nature of nutrient limitation. J. Appl. Ecol. 1996, 33, 1441–1450. [Google Scholar] [CrossRef]
- Kleyer, M.; Bekker, R.M.; Knevel, I.C.; Bakker, J.P.; Thompson, K.; Sonnenschein, M.; Poschlod, P.; van Groenendael, J.M.; Klimeš, L.; Klimešová, J.; et al. The LEDA Traitbase: A database of life-history traits of the Northwest European flora. J. Ecol. 2008, 96, 1266–1274. [Google Scholar] [CrossRef]
- Hodgson, J.G.; Montserrat-Martí, G.; Charles, M.; Jones, G.; Wilson, P.; Shipley, B.; Sharafi, M.; Cerabolini, B.E.L.; Cornelissen, J.H.C.; Band, S.R. Is leaf dry matter content a better predictor of soil fertility than specific leaf area? Ann. Bot. 2011, 108, 1337–1345. [Google Scholar] [CrossRef] [Green Version]
- Poorter, L.; Bongers, F. Leaf traits are good predictors of plant performance across 53 rain forest species. Ecology 2006, 87, 1733–1743. [Google Scholar] [CrossRef]
- Han, W.X.; Fang, J.Y.; Guo, D.L.; Zhang, Y. Leaf nitrogen and phosphorus stoichiometry across 753 terrestrial plant species in China. New Phytol. 2005, 168, 377–385. [Google Scholar] [CrossRef]
- Zhao, J.; Xu, F.; Ji, T.; Li, J. A New Spermidine from the Fruits of Lycium ruthenicum. Chem. Nat. Compd. 2014, 50, 880–883. [Google Scholar] [CrossRef]
- Shipley, B.; Lechowicz, M.J.; Wright, I.; Reich, P.B. Fundamental trade-offs generating the worldwide leaf economics spectrum. Ecology 2006, 87, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Morandeira, N.S.; Kandus, P. Plant functional types and trait values in the Paraná River floodplain: Modeling their association with environmental features. Flora-Morphol. Distrib. Funct. Ecol. Plants 2016, 220, 63–73. [Google Scholar] [CrossRef]
- Reich, P.B.; Walters, M.B.; Ellsworth, D.S. From tropics to tundra: Global convergence in plant functioning. Proc. Natl. Acad. Sci. USA 1997, 94, 13730–13734. [Google Scholar] [CrossRef] [Green Version]
- Osmond, C.B.; Austin, M.P.; Berry, J.A.; Billings, W.D.; Boyer, J.S.; Dacey, J.W.H.; Nobel, P.S.; Smith, S.D.; Winner, W.E. Stress physiology and the distribution of plants. Bioscience 1987, 37, 38–48. [Google Scholar] [CrossRef]
- Marenco, R.; Antezana-Vera, S.A.; Nascimento, H. Relationship between specific leaf area, leaf thickness, leaf water content and SPAD-502 readings in six Amazonian tree species. Photosynthetica 2009, 47, 184–190. [Google Scholar] [CrossRef]
- Shipley, B.; Vu, T. Dry matter content as a measure of dry matter concentration in plants and their parts. New Phytol. 2002, 153, 359–364. [Google Scholar] [CrossRef]
- Shipley, B. Structured Interspecific Determinants of Specific Leaf Area in 34 Species of Herbaceous Angiosperms. Funct. Ecol. 1995, 9, 312. [Google Scholar] [CrossRef]
- He, M.; Dijkstra, F.A.; Zhang, K.; Li, X.; Tan, H.; Gao, Y.; Li, G. Leaf nitrogen and phosphorus of temperate desert plants in response to climate and soil nutrient availability. Sci. Rep. 2014, 4, 6932–6939. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Gao, X.; Li, L.; Lu, Y.; Shareef, M.; Huang, C.; Liu, G.; Gui, D.; Zeng, F. Groundwater Depth Affects Phosphorus but Not Carbon and Nitrogen Concentrations of a Desert Phreatophyte in Northwest China. Front. Plant Sci. 2018, 9, 338. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.T.; Stanton, D.; Schmitz, N.; Farquhar, G.; Ball, M. Growth responses of the mangrove Avicennia marina to salinity: Development and function of shoot hydraulic systems require saline conditions. Ann. Bot. 2015, 115, 397–407. [Google Scholar] [CrossRef]
- Chakrabarti, N.; Mukherji, S. Effect of phytohormone pretreatment on nitrogen metabolism in Vigna radiate under salt stress. Bio. Plantarum 2003, 46, 63–66. [Google Scholar] [CrossRef]
- Baki, G.K.A.; Siefritz, F.; Man, H.; Weiner, H.; Kaldenhoff, R.; Kaiser, W.M. Nitrate reductase in Zea mays L. under salinity. Plant Cell Environ. 2000, 23, 515–521. [Google Scholar] [CrossRef]
- Ding, X.; Tian, C.; Zhang, S.; Song, J.; Zhang, F.; Mi, G.; Feng, G. Effects of NO3−-N on the growth and salinity tolerance of Tamarix laxa Willd. Plant Soil 2009, 331, 57–67. [Google Scholar] [CrossRef]
- Hameed, M.; Ashraf, M.; Ahmad, M.S.A.; Naz, N. Structural and Functional Adaptations in Plants for Salinity Tolerance. In Plant Adaptation and Phytoremediation; Springer Netherlands: Dordrecht, The Netherlands, 2010; pp. 151–170. [Google Scholar]
- Iqbal, N.; Umar, S.; Khan, N. Nitrogen availability regulates proline and ethylene production and alleviates salinity stress in mustard (Brassica juncea). J. Plant Physiol. 2015, 178, 84–91. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Agarwal, R.M. Salinity stress induced alterations in antioxidant metabolism and nitrogen assimilation in wheat (Triticum aestivum L.) as influenced by potassium supplementation. Plant Physiol. Bioch. 2017, 115, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.F.; Feng, Q.; Liu, W.; Si, J.H.; Xi, H.Y.; Chen, L.J. Soil water and salinity in response to water deliveries and the relationship with plant growth at the lower reaches of Heihe River, Northwestern China. Acta Ecol. Sin. 2012, 32, 7009–7701. [Google Scholar]
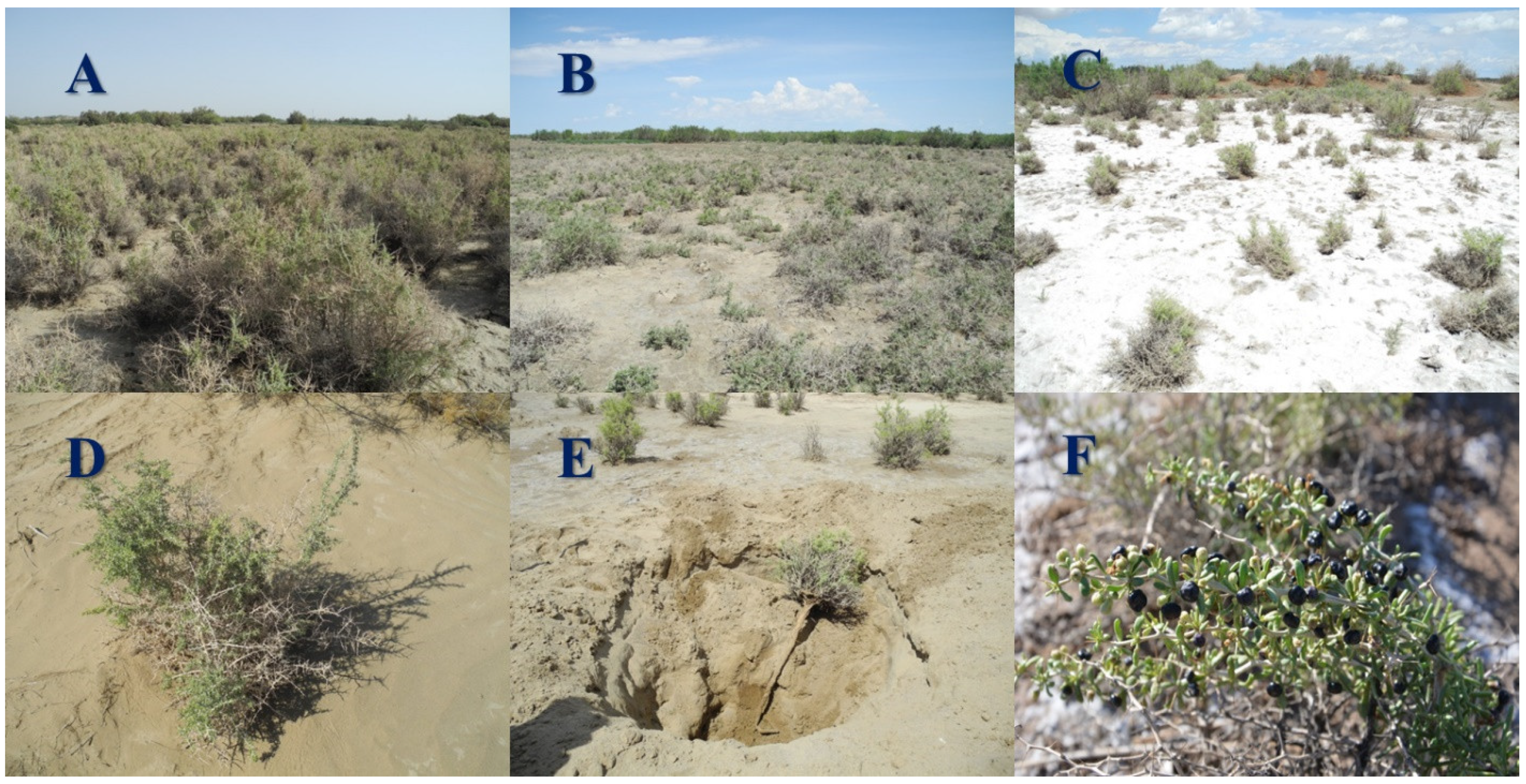
| No. | Types Description of Habitats | Longitude (E) | Latitude (N) | Dominance Index | Evenness Index | Plant Coverage (%) |
|---|---|---|---|---|---|---|
| Ⅰ | Non-saline gravel desert | 101°01′0.6″ | 42°02′9.4″ | 0.70 ± 0.18 bc | 0.54 ± 0.28 ab | 22.42 ± 4.70 abc |
| Ⅱ | Non-saline gravel desert | 101°01′42.4″ | 42°02′7.8″ | 0.66 ± 0.24 bcd | 0.55 ± 0.36 ab | 46.46 ± 8.45 c |
| Ⅲ | Non-saline silt desert | 101°03′13.9″ | 42°01′28.3″ | 0.51 ± 0.13 d | 0.66 ± 0.13 ab | 48.01 ± 7.89 d |
| Ⅳ | Non-saline silt desert | 101°02′42.0″ | 42°03′11.8″ | 0.86 ± 0.21 a | 0.27 ± 0.37 cd | 91.02 ± 12.38 c |
| Ⅴ | Very slightly saline silt desert | 101°02′27.5″ | 42°03′8.0″ | 0.66 ± 0.14 bcd | 0.69 ± 0.20 ab | 37.40 ± 8.79 bc |
| Ⅵ | Non-saline sand desert | 101°16′59.3″ | 42°02′17.8″ | 0.80 ± 0.09 ab | 0.51 ± 0.17 bc | 1.80 ± 0.62 a |
| Ⅶ | Slightly saline silt desert | 101°00′52.5″ | 42°06′56.8″ | 0.94 ± 0.11 a | 0.16 ± 0.26 d | 37.08 ± 6.16 bc |
| Ⅷ | Slightly saline silt desert | 101°00′3.7″ | 42°06′52.0″ | 0.63 ± 0.09 cd | 0.80 ± 0.15 a | 10.15 ± 1.78 ab |
| No. | LT | SLV | SLA | LDMC | Suc | LD | TWC | RWC | C | N | P | C:N | C:P | N:P |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ⅰ | 1.03 ± 0.01 c | 6.69 ± 0.47 bc | 0.007 ± 0.43 a | 141.5 ± 13.4 ab | 0.83 ± 0.03 b | 0.15 ± 0.01 ab | 83.15 ± 0.01 cd | 81.32 ± 0.01 b | 347.5 ± 0.42 a | 13.57 ± 0.06 c | 3.98 ± 0.16 b | 25.79 ± 0.42 c | 85.0 ± 4.05 c | 3.42 ± 0.16 b |
| Ⅱ | 1.14 ± 0.10 bc | 7.00 ± 0.67 bc | 0.006 ± 0.47 a | 147.5 ± 5.1 ab | 0.99 ± 0.04 ab | 0.14 ± 0.01 ab | 82.0 ± 0.01 cd | 78.85 ± 0.03 c | 337.8 ± 0.29 a | 14.84 ± 0.43 b | 3.09 ± 0.00 b | 23.34 ± 0.89 d | 107.5 ± 2.5 c | 4.80 ± 0.14 ab |
| Ⅲ | 1.26 ± 0.00 abc | 8.40 ± 1.40 abc | 0.006 ± 1.11 a | 144.7 ± 11.3 ab | 0.89 ± 0.07 b | 0.12 ± 0.02 bc | 82.26 ± 0.01 cd | 78.46 ± 0.02 c | 342.4 ± 0.29 a | 16.92 ± 0.89 a | 1.53 ± 0.91 c | 20.28 ± 0.74 e | 435.8 ± 25.5 a | 17.70 ± 11.13 a |
| Ⅳ | 1.36 ± 0.01 ab | 7.81 ± 0.19 abc | 0.005 ± 0.10 a | 125.0 ± 1.7 abc | 1.03 ± 0.03 ab | 0.13 ± 0.00 bc | 83.13 ± 0.00 cd | 70.41 ± 0.00 c | 324.1 ± 0.12 b | 13.04 ± 0.04 c | 1.01 ± 0.14 c | 26.16 ± 1.85 c | 335.3 ± 11.2 ab | 13.16 ± 1.76 ab |
| Ⅴ | 1.26 ± 0.23 abc | 5.74 ± 0.38 c | 0.005 ± 0.54 a | 197.9 ± 21.0 a | 0.90 ± 0.04 b | 0.17 ± 0.01 a | 79.35 ± 0.02 d | 94.81 ± 0.00 c | 337.6 ± 0.16 a | 9.93 ± 0.04 d | 0.81 ± 0.00 c | 34.34 ± 0.48 b | 414.1 ± 1.8 a | 12.22 ± 0.00 ab |
| Ⅵ | 1.24 ± 0.02 bc | 7.38 ± 0.13 bc | 0.007 ± 0.12 a | 137.9 ± 2.2 abc | 0.87 ± 0.03 b | 0.14 ± 0.00 abc | 84.91 ± 0.00 bc | 90.0 ± 0.00 c | 341.3 ± 0.04 a | 15.07 ± 0.27 b | 1.54 ± 0.11 c | 22.66 ± 0.35 d | 223.3 ± 13.1 bc | 9.87 ± 0.90 ab |
| Ⅶ | 1.58 ± 0.05 a | 9.14 ± 0.64 ab | 0.006 ± 0.24 a | 153.1 ± 7.5 bc | 1.24 ± 0.14 a | 0.11 ± 0.01 bc | 88.37 ± 0.01 ab | 137.35 ± 0.02 a | 308.6 ± 0.12 c | 15.15 ± 0.17 b | 5.45 ± 0.32 a | 20.56 ± 0.30 e | 58.1 ± 1.1 c | 2.79 ±0.20 b |
| Ⅷ | 1.37 ± 0.01 ab | 10.90 ± 1.90 a | 0.008 ± 1.48 a | 151.5 ± 8.5 c | 1.03 ± 0.10 ab | 0.09 ± 0.02 c | 87.95 ± 0.01 a | 130.36 ± 0.01 b | 319.9 ± 0.54 b | 8.43 ± 0.34 e | 2.87 ± 0.00 b | 38.54 ± 1.07 a | 112.3 ± 0.7 c | 2.94 ±0.12 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Gou, W.; Wang, H.; White, J.F.; Wu, G.; Su, P. Trade-Off Relationships of Leaf Functional Traits of Lycium ruthenicum in Response to Soil Properties in the Lower Reaches of Heihe River, Northwest China. Diversity 2021, 13, 453. https://doi.org/10.3390/d13090453
Li S, Gou W, Wang H, White JF, Wu G, Su P. Trade-Off Relationships of Leaf Functional Traits of Lycium ruthenicum in Response to Soil Properties in the Lower Reaches of Heihe River, Northwest China. Diversity. 2021; 13(9):453. https://doi.org/10.3390/d13090453
Chicago/Turabian StyleLi, Shanjia, Wei Gou, Hui Wang, James F. White, Guoqiang Wu, and Peixi Su. 2021. "Trade-Off Relationships of Leaf Functional Traits of Lycium ruthenicum in Response to Soil Properties in the Lower Reaches of Heihe River, Northwest China" Diversity 13, no. 9: 453. https://doi.org/10.3390/d13090453
APA StyleLi, S., Gou, W., Wang, H., White, J. F., Wu, G., & Su, P. (2021). Trade-Off Relationships of Leaf Functional Traits of Lycium ruthenicum in Response to Soil Properties in the Lower Reaches of Heihe River, Northwest China. Diversity, 13(9), 453. https://doi.org/10.3390/d13090453







