Presence and Biomass Information Extraction from Highly Uncertain Data of an Experimental Low-Range Insect Radar Setup
Abstract
:1. Introduction
2. Materials and Methods
2.1. Radar Device
- configuration of radar parameters (such as the operation mode or the frequency range);
- accessing internal radar parameters, such as the current frontend temperature or the transmission power;
- triggering a measurement process and accessing the generated data;
- starting high-level applications (e.g., the human tracker algorithm).
2.2. Conditions of Experiments
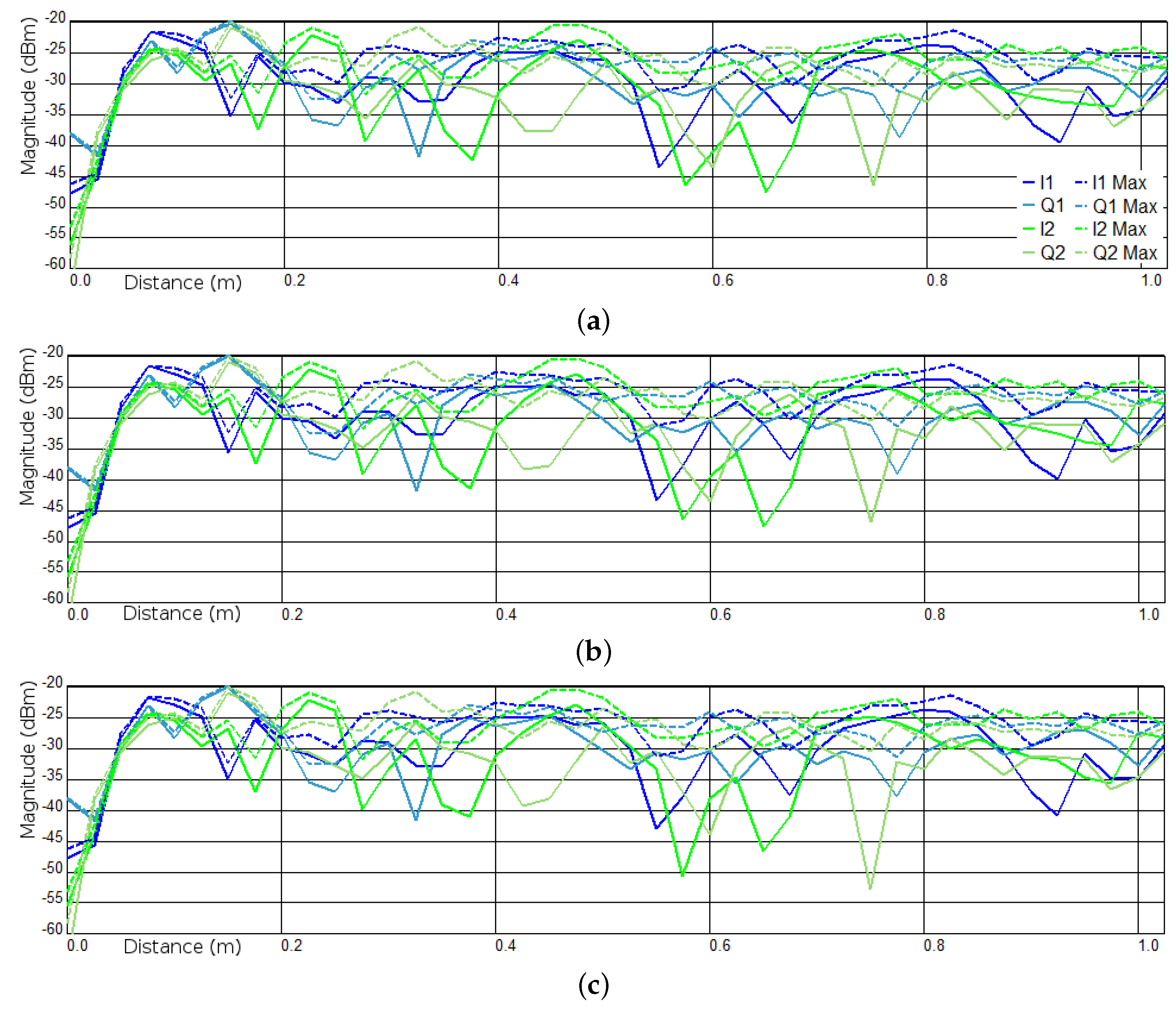
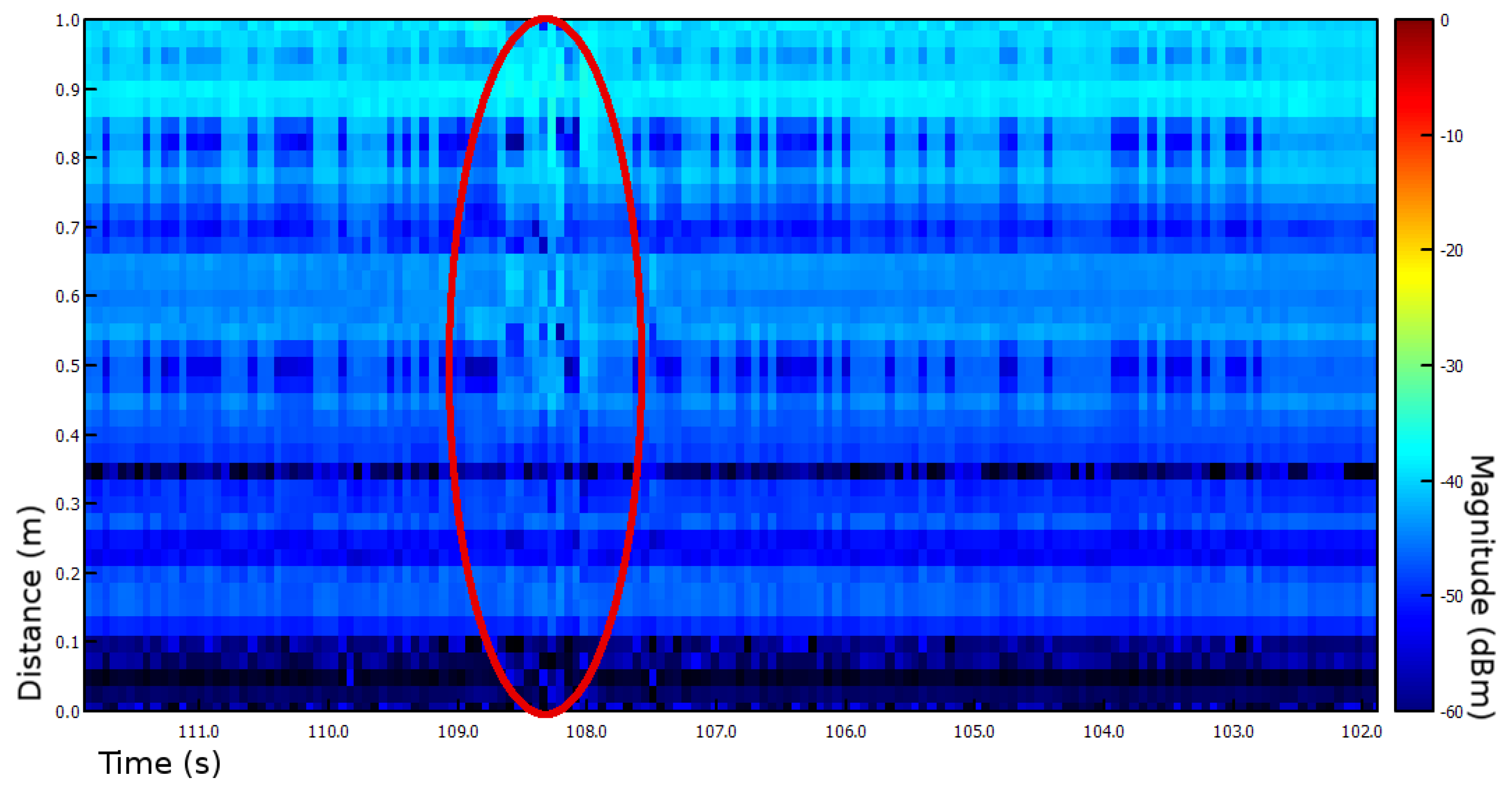
2.3. Sum of Sequential Absolute Magnitude Differences for Uncertainty Reduction
3. Results
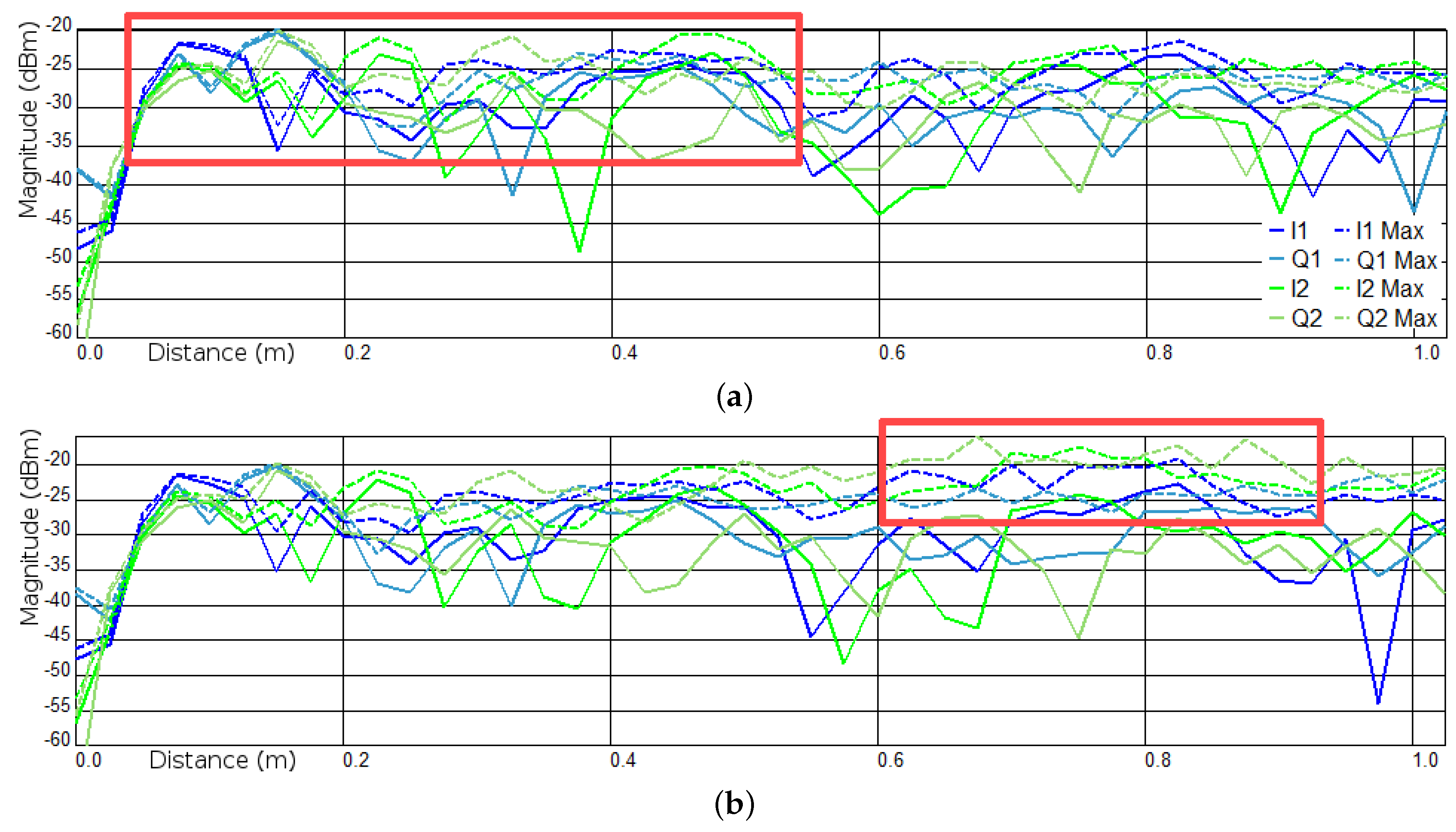
3.1. Experiments I and II—Presence Detection
3.2. Experiment III—Biomass Estimation
3.3. Resulting Table
3.4. Low Possibility of Insect Range Estimation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
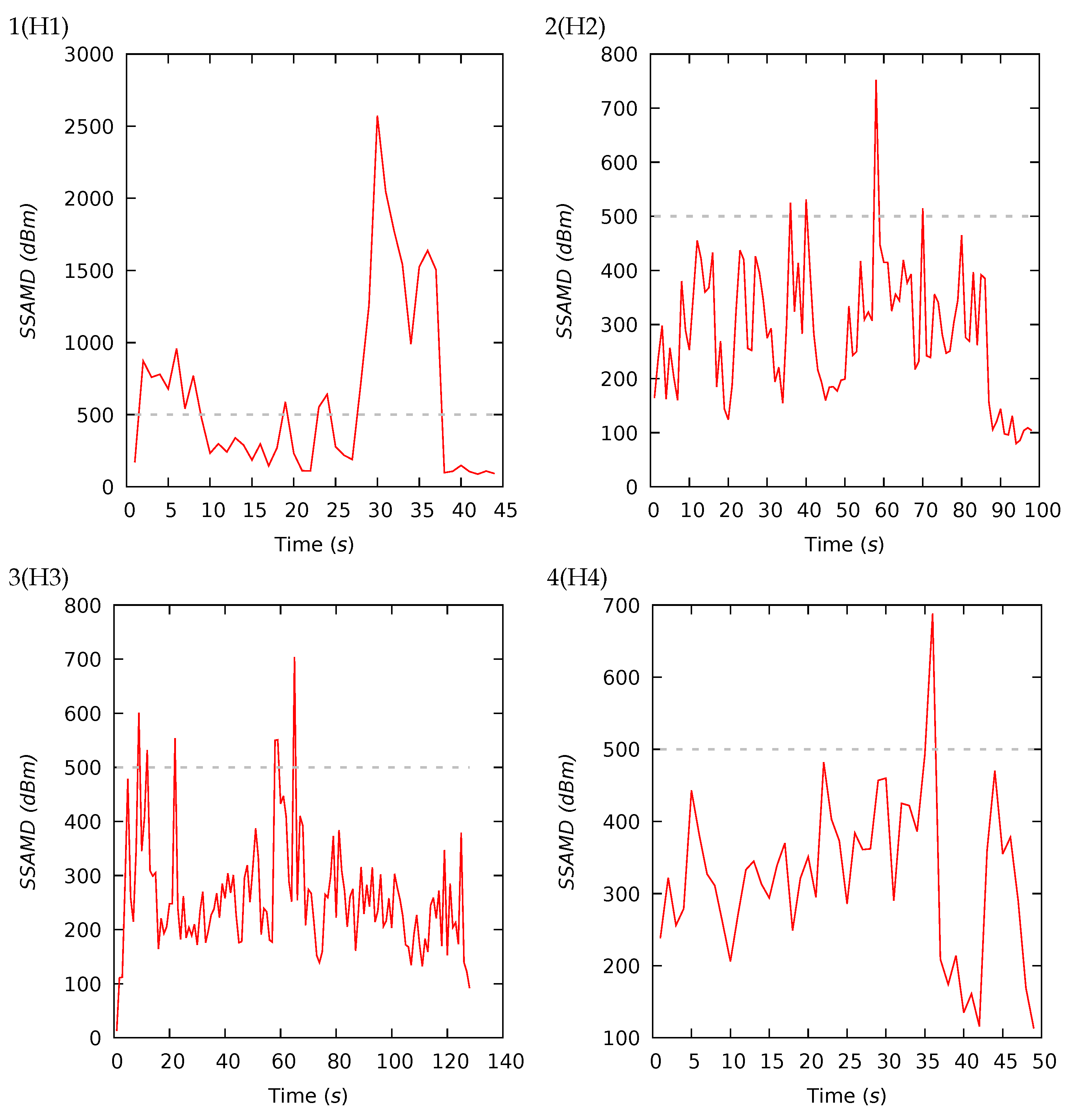
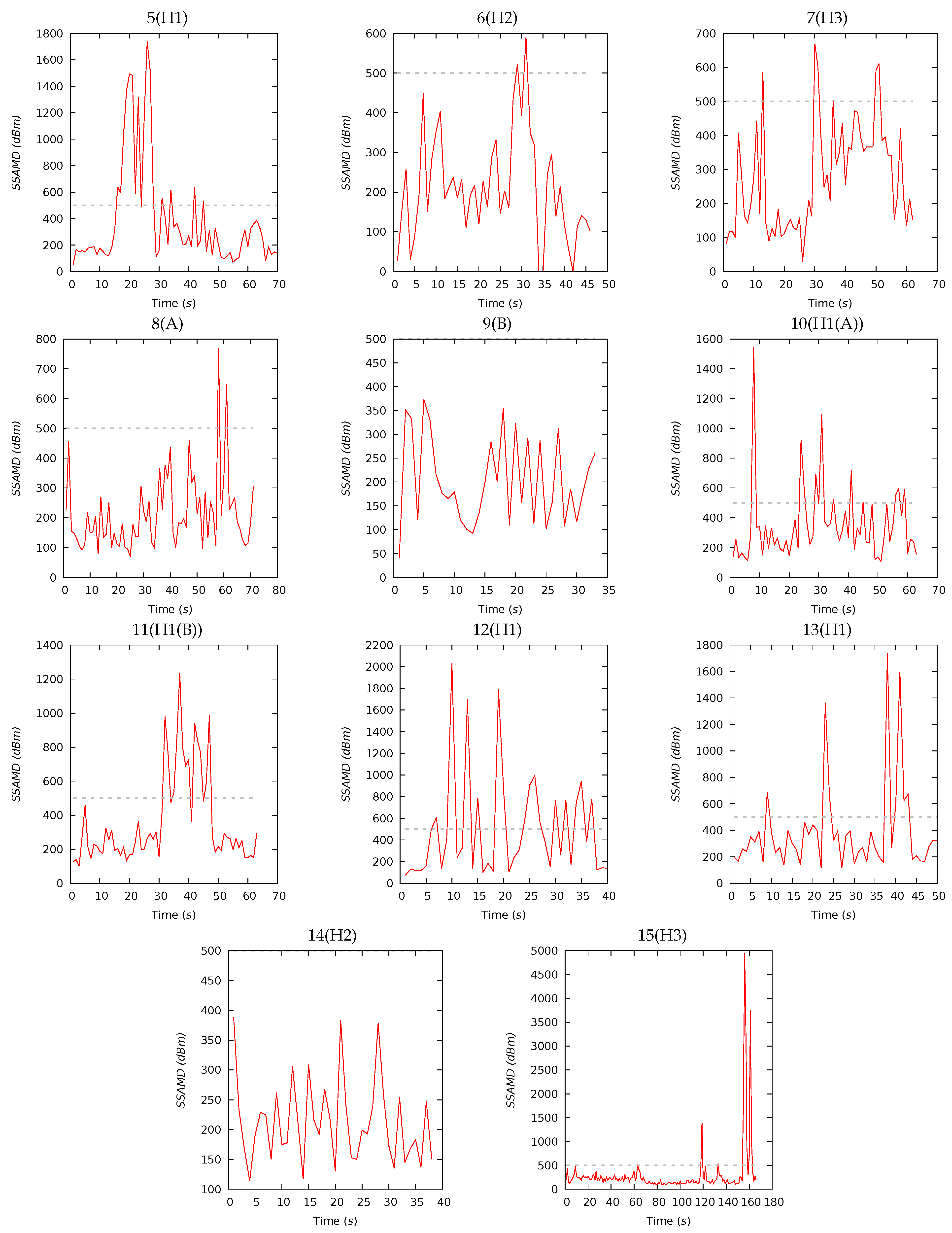
Appendix A. Experiment Details and Notes
Appendix A.1. Preliminary Tests
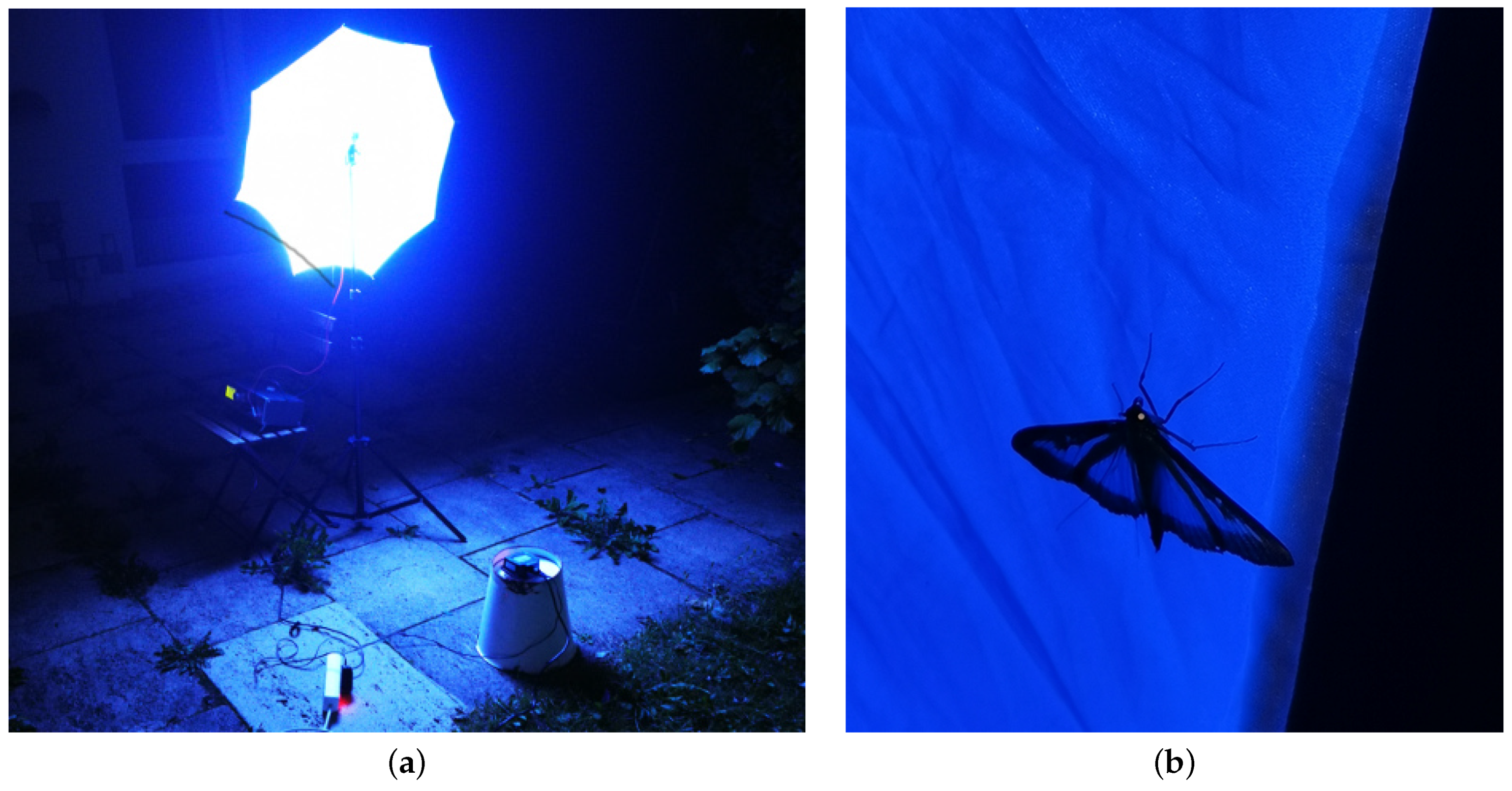
Appendix A.2. Experiment I

Appendix A.3. Experiment II
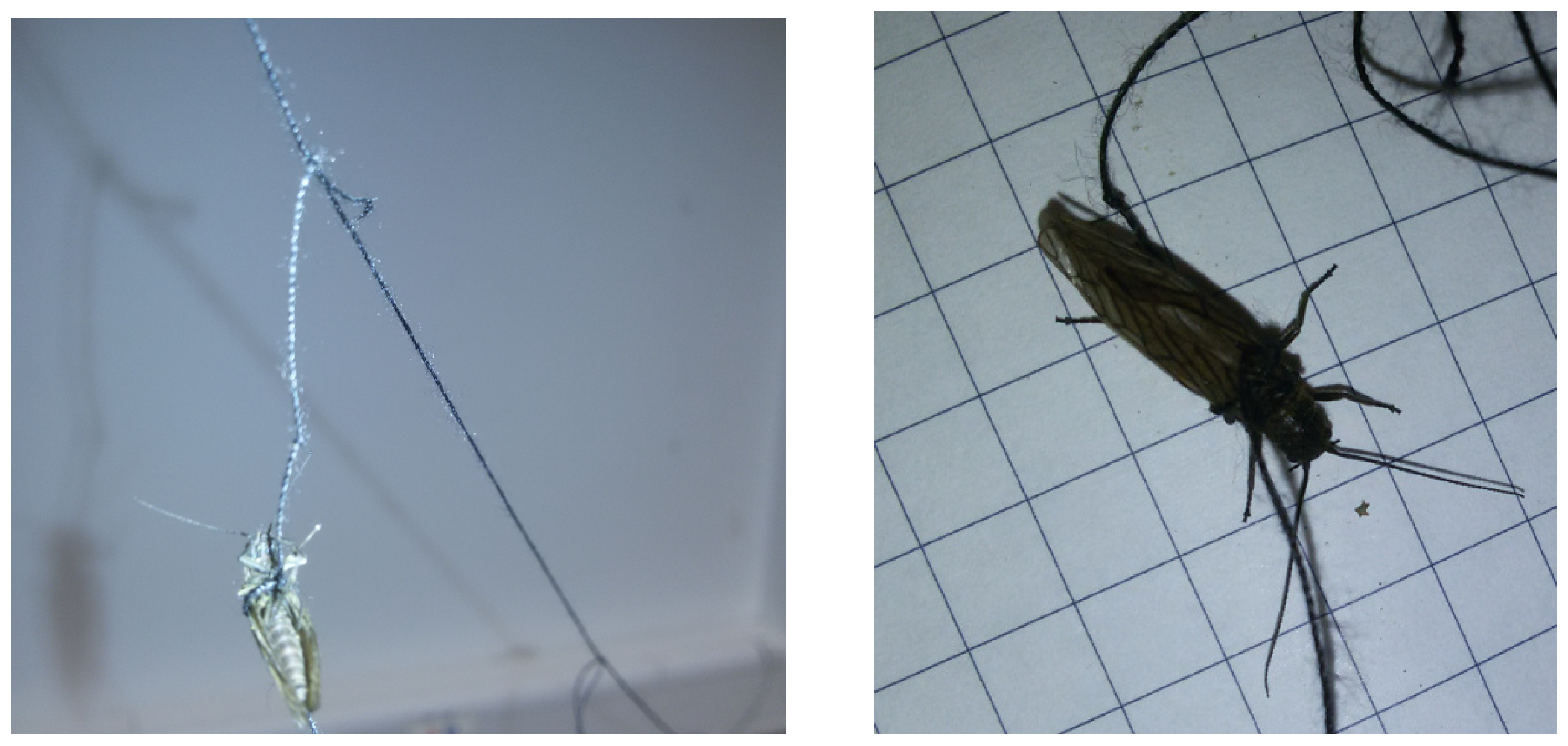


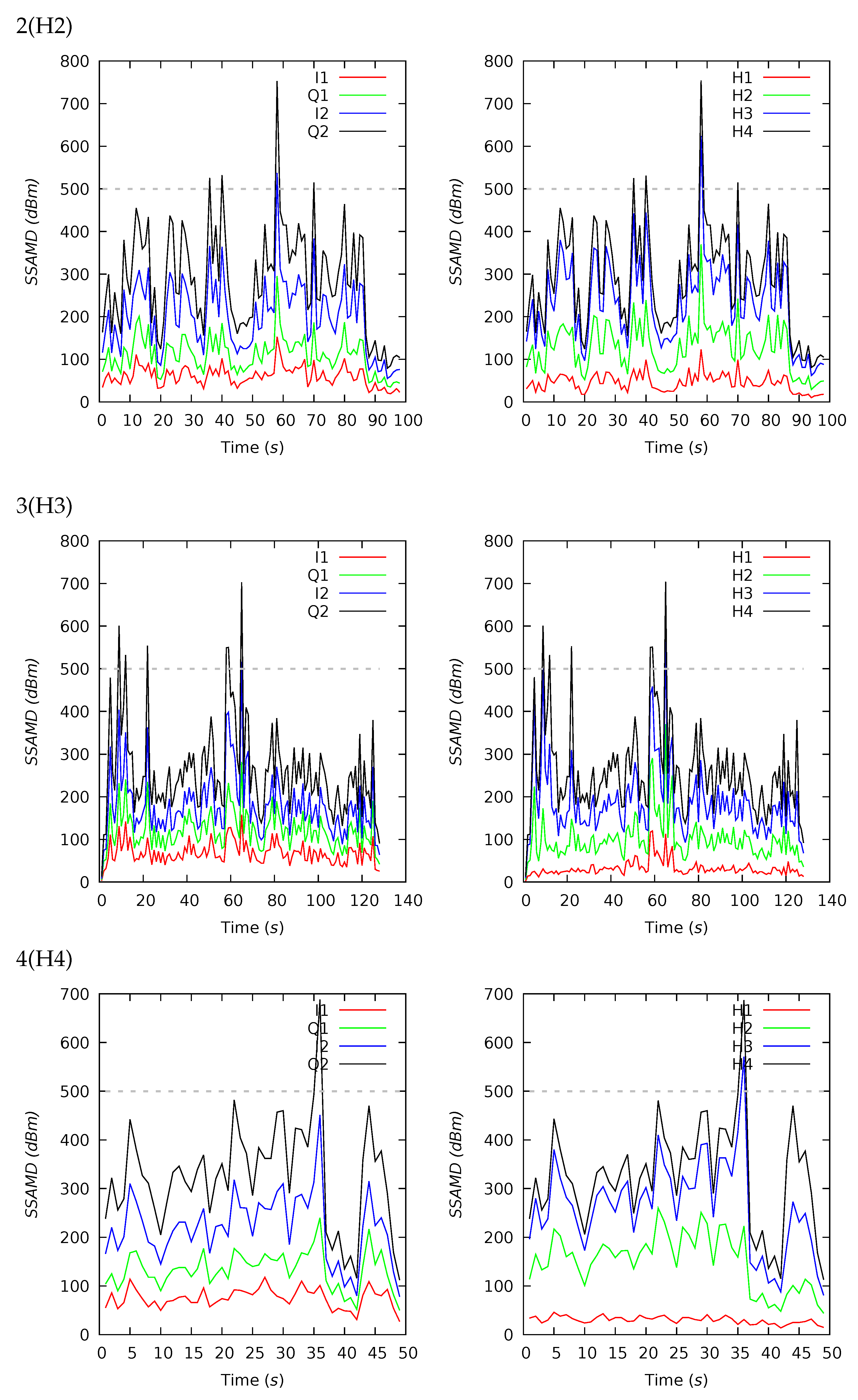
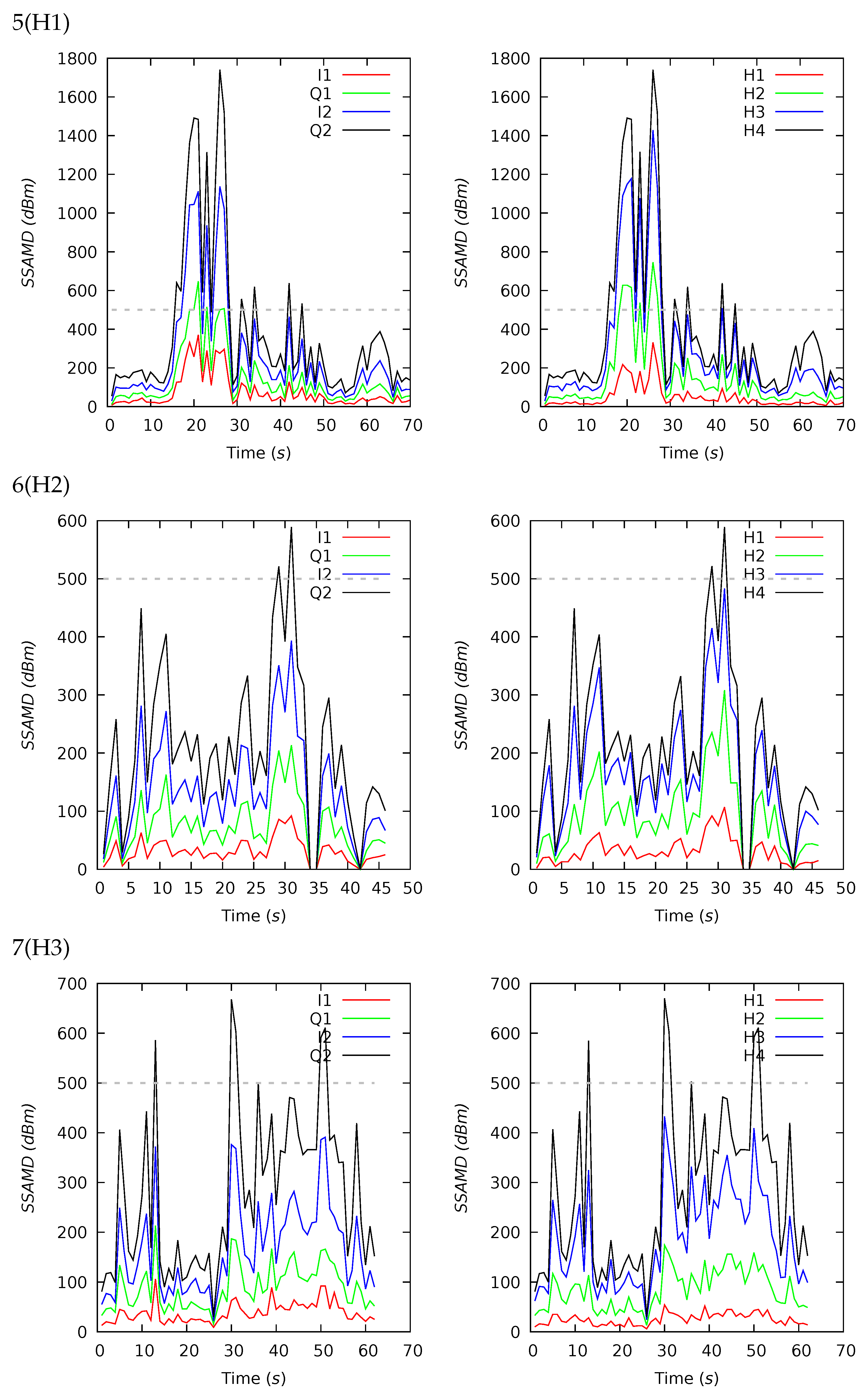
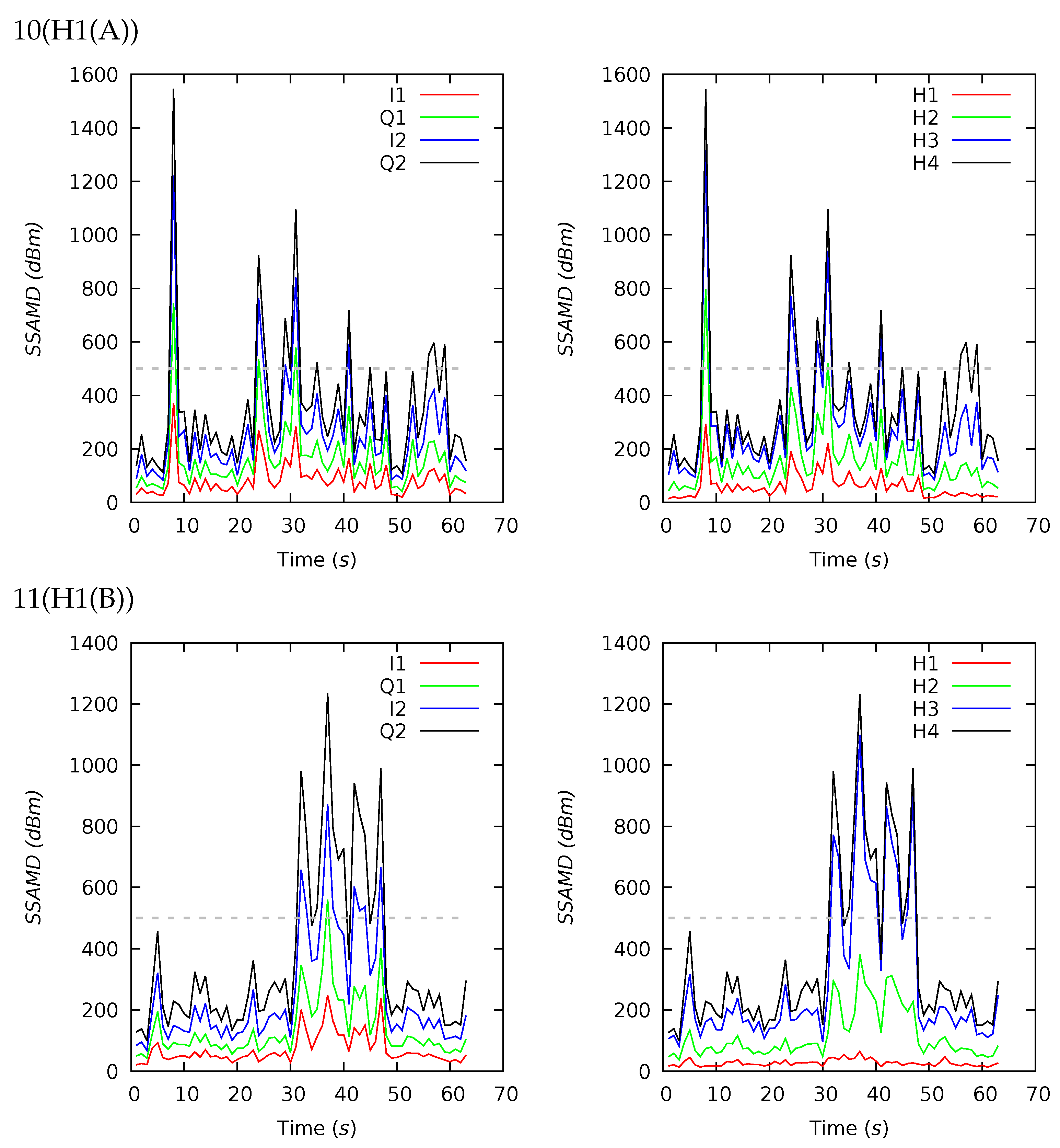
Appendix B. Channel and Height Level Contributions

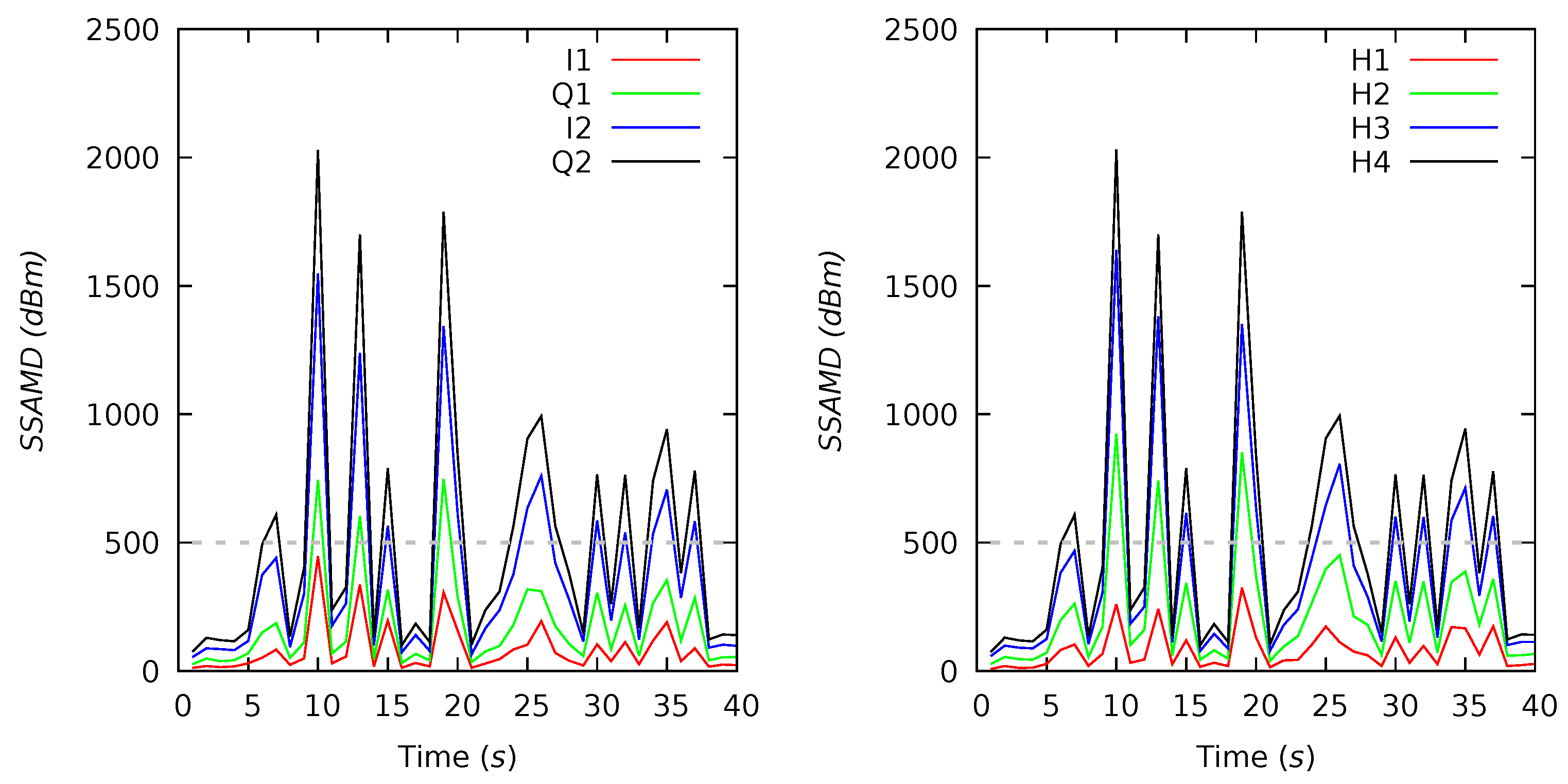
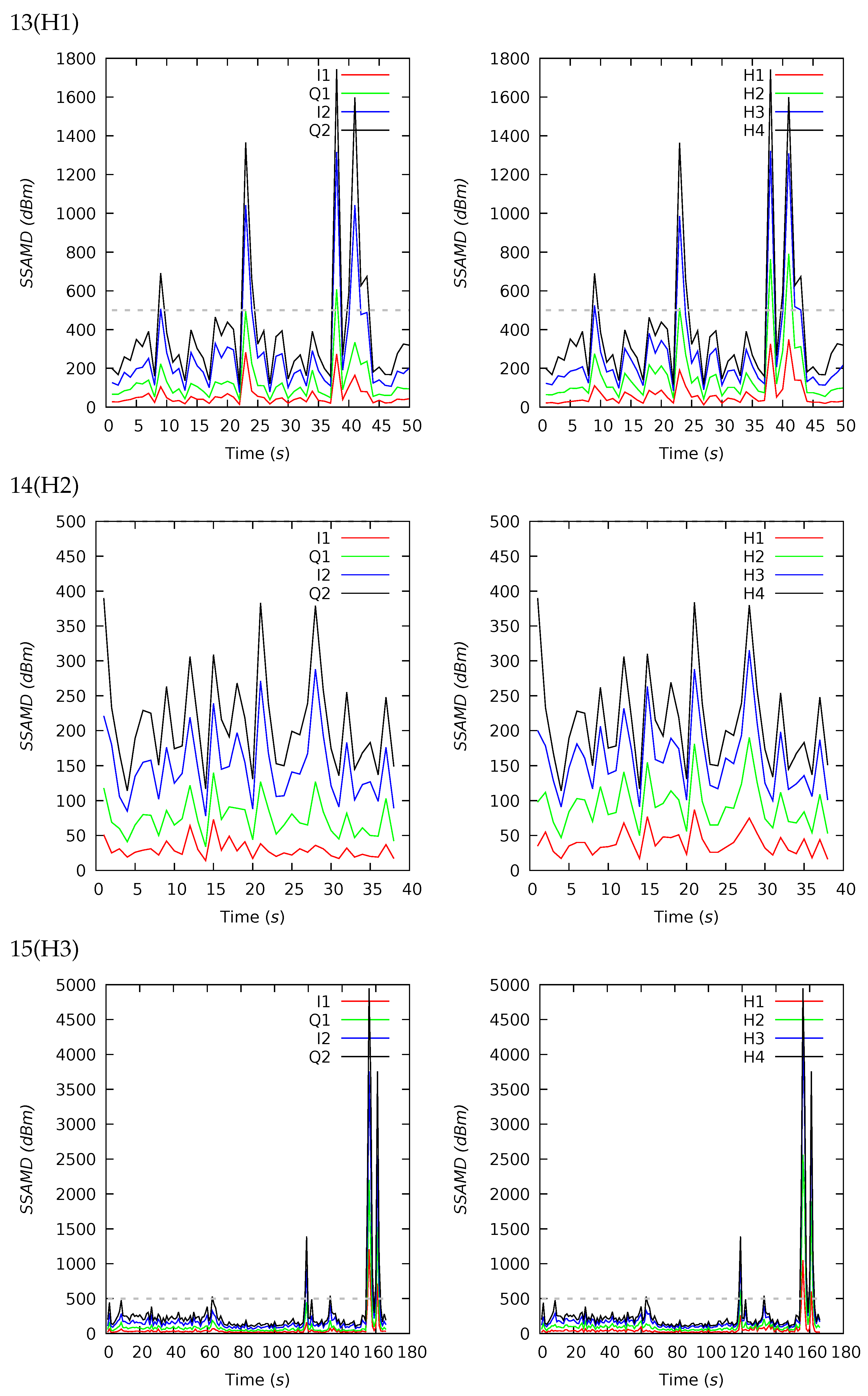
Appendix C. Radar Software Installation and Configuration
- sudo apt-get install wine64 winetricks \
- winetricks corefonts d3dx9_36 vcrun2005 vcrun2008 vcrun2010 \
- winhttp
- WINEPREFIX=~/.wine/drive_c/Program\ Files/SenTool_60GHz/ \
- wine \
- /home/$USER/.wine/drive_c/Program\ Files/SenTool_60GHz/StartApp.exe
Appendix D. Resulting Data and Data Processing Software
- for cur in $(find /home/username/RadarLab/RawData \
- -type d -name FD)
- do; tclsh radar.tcl \$cur /tmp/Results \
- ‘echo \$cur|xargs dirname|xargs dirname|sed \’
- ‘s/. *RawData//g; s/\///g’|sed ‘s/\///g’; done
References
- Hochkirch, A. The insect crisis we can’t ignore. Nature 2016, 539, 141. [Google Scholar] [CrossRef] [Green Version]
- Hallmann, C.A.; Sorg, M.; Jongejans, E.; Siepel, H.; Hofland, N.; Schwan, H.; Stenmans, W.; Müller, A.; Sumser, H.; Hörren, T.; et al. More than 75 percent decline over 27 years in total flying insect biomass in protected areas. PLoS ONE 2017, 12, e0185809. [Google Scholar] [CrossRef] [Green Version]
- McGrath, M. Global Insect Decline may See ‘Plague of Pests’. 2019. Available online: https://www.bbc.com/news/science-environment-47198576 (accessed on 20 December 2020).
- Didham, R.K.; Basset, Y.; Collins, C.M.; Leather, S.R.; Littlewood, N.A.; Menz, M.H.M.; Müller, J.; Packer, L.; Saunders, M.E.; Schönrogge, K.; et al. Interpreting insect declines: Seven challenges and a way forward. Insect Conserv. Divers. 2020, 13, 103–114. [Google Scholar] [CrossRef] [Green Version]
- Petrovskii, S.; Petrovskaya, N.; Bearup, D. Multiscale approach to pest insect monitoring: Random walks, pattern formation, synchronization, and networks. Phys. Life Rev. 2014, 11, 467–525. [Google Scholar] [CrossRef]
- Gressitt, J.L.; Gressitt, M.K. An improved Malaise trap. Pac. Insects 1962, 4, 87–90. Available online: http://hbs.bishopmuseum.org/pi/pdf/4(1)-87.pdf (accessed on 20 September 2021).
- Southwood, E. Ecological Methods with Particular Reference to the Study of Insect Populations; Barnes and Noble: New York, NY, USA, 1966; 409p. [Google Scholar]
- Gunstream, S.E.; Chew, R.M. A Comparison of Mosquito Collection by Malaise and Miniature Light Traps1. J. Med. Entomol. 1967, 4, 495–496. [Google Scholar] [CrossRef]
- Owen, D.F. Species diversity and seasonal abundance in tropical Sphingidae (Lepidoptera). Proc. R. Entomol. Soc. Lond. Ser. A Gen. Entomol. 1969, 44, 162–168. [Google Scholar] [CrossRef]
- Shimoda, M.; Honda, K. Insect reactions to light and its applications to pest management. Appl. Entomol. Zool. 2013, 48, 413–421. [Google Scholar] [CrossRef] [Green Version]
- Yao, Q.; Lv, J.; Liu, Q.J.; Diao, G.Q.; Yang, B.J.; Chen, H.M.; Tang, J. An Insect Imaging System to Automate Rice Light-Trap Pest Identification. J. Integr. Agric. 2012, 11, 978–985. [Google Scholar] [CrossRef]
- White, P.J.T.; Glover, K.; Stewart, J.; Rice, A. The Technical and Performance Characteristics of a Low-Cost, Simply Constructed, Black Light Moth Trap. J. Insect Sci. 2016, 16, 25. [Google Scholar] [CrossRef] [Green Version]
- Bjerge, K.; Nielsen, J.B.; Videbæk Sepstrup, M.; Helsing-Nielsen, F.; Høye, T.T. An Automated Light Trap to Monitor Moths (Lepidoptera) Using Computer Vision-Based Tracking and Deep Learning. Sensors 2021, 21, 343. [Google Scholar] [CrossRef] [PubMed]
- Ruczyński, I.; Hałat, Z.; Zegarek, M.; Borowik, T.; Dechmann, D.K.N. Camera transects as a method to monitor high temporal and spatial ephemerality of flying nocturnal insects. Methods Ecol. Evol. 2019, 11, 294–302. [Google Scholar] [CrossRef]
- Pádua, L.; Adão, T.; Sousa, A.; Peres, E.; Sousa, J.J. Individual Grapevine Analysis in a Multi-Temporal Context Using UAV-Based Multi-Sensor Imagery. Remote Sens. 2020, 12, 139. [Google Scholar] [CrossRef] [Green Version]
- Song, B.; Park, K. Verification of Accuracy of Unmanned Aerial Vehicle (UAV) Land Surface Temperature Images Using In-Situ Data. Remote Sens. 2020, 12, 288. [Google Scholar] [CrossRef] [Green Version]
- Crawford, A. Radar reflections in the lower atmosphere. Proc. Inst. Radio Eng. 1949, 37, 404–405. [Google Scholar]
- Riley, J. Radar as an Aid to the Study of Insect Flight. In A Handbook on Biotelemetry and Radio Tracking; Pergamon Press: Oxford, UK, 1980; pp. 131–140. [Google Scholar] [CrossRef]
- Vaughn, C.R. Birds and insects as radar targets: A review. Proc. IEEE 1985, 73, 205–227. [Google Scholar] [CrossRef]
- Rainey, R.C. Observation of Desert Locust Swarms by Radar. Nature 1955, 175, 77. [Google Scholar] [CrossRef]
- Hao, Z.; Drake, V.A.; Sidhu, L.; Taylor, J.R. Locust displacing winds in eastern Australia reassessed with observations from an insect monitoring radar. Int. J. Biometeorol. 2017, 61, 2073–2084. [Google Scholar] [CrossRef]
- Smith, A.; Riley, J. Signal processing in a novel radar system for monitoring insect migration. Comput. Electron. Agric. 1996, 15, 267–278. [Google Scholar] [CrossRef]
- Chapman, J.; Smith, A.; Woiwod, I.; Reynolds, D.; Riley, J. Development of vertical-looking radar technology for monitoring insect migration. Comput. Electron. Agric. 2002, 35, 95–110. [Google Scholar] [CrossRef]
- Chapman, J.W.; Reynolds, D.R.; Smith, A.D. Vertical-Looking Radar: A New Tool for Monitoring High-Altitude Insect Migration. BioScience 2003, 53, 503–511. [Google Scholar] [CrossRef] [Green Version]
- Mascanzoni, D.; Wallin, H. The harmonic radar: A new method of tracing insects in the field. Ecol. Entomol. 1986, 11, 387–390. [Google Scholar] [CrossRef]
- Riley, J.; Smith, A. Design considerations for an harmonic radar to investigate the flight of insects at low altitude. Comput. Electron. Agric. 2002, 35, 151–169. [Google Scholar] [CrossRef]
- Colpitts, B.; Boiteau, G. Harmonic Radar Transceiver Design: Miniature Tags for Insect Tracking. IEEE Trans. Antennas Propag. 2004, 52, 2825–2832. [Google Scholar] [CrossRef]
- Psychoudakis, D.; Moulder, W.; Chen, C.; Zhu, H.; Volakis, J.L. A Portable Low-Power Harmonic Radar System and Conformal Tag for Insect Tracking. IEEE Antennas Wirel. Propag. Lett. 2008, 7, 444–447. [Google Scholar] [CrossRef]
- Drake, V.; Reynolds, D. Radar Entomology: Observing Insect Flight and Migration; CAB International: Wallingford, UK, 2012. [Google Scholar]
- Chapman, J.W.; Drake, V.A.; Reynolds, D.R. Recent Insights from Radar Studies of Insect Flight. Annu. Rev. Entomol. 2011, 56, 337–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shamoun-Baranes, J.; Nilsson, C.; Bauer, S.; Chapman, J. Taking radar aeroecology into the 21st century. Ecography 2019, 42, 847–851. [Google Scholar] [CrossRef]
- Noskov, A.; Bendix, J.; Friess, N. A Review of Insect Monitoring Approaches with Special Reference to Radar Techniques. Sensors 2021, 21, 1474. [Google Scholar] [CrossRef] [PubMed]
- Jansson, S.; Malmqvist, E.; Brydegaard, M.; Akesson, S.; Rydell, J. A Scheimpflug lidar used to observe insect swarming at a wind turbine. Ecol. Indic. 2020, 117, 106578. [Google Scholar] [CrossRef]
- Rankin, G.A.; Bui, L.Q.; Tirkel, A.Z.; Le Marshall, N. Radar imaging: Conventional and MIMO. In Proceedings of the 2012 Fourth International Conference on Communications and Electronics (ICCE), Hue, Vietnam, 1–3 August 2012; pp. 171–176. [Google Scholar] [CrossRef]
- IMST. IMST Sentire Radar Module 24 GHz sR-1200 Series: User Manual; Version 1.3; IMST GmbH: Kamp-Lintfort, Germany, 2017; Available online: https://imst.de/ (accessed on 20 September 2021).
- Kirkhorn, J. Introduction to IQ-Demodulation of RF-Data; IFBT, NTNU: Trondheim, Norway, 1999. [Google Scholar]
- Mendelson, G. All You Need to Know about Power over Ethernet (PoE) and the IEEE 802.3 af Standard; PowerDsine Ltd.: Hod HaSharon, Israel, 2004. [Google Scholar]
- Noskov, A. Low-Range FMCW Insect Radar—Lab Experiments Data, Results, and Data Processing Software. 2021. Available online: https://doi.org/10.5281/zenodo.5035849 (accessed on 20 September 2021).




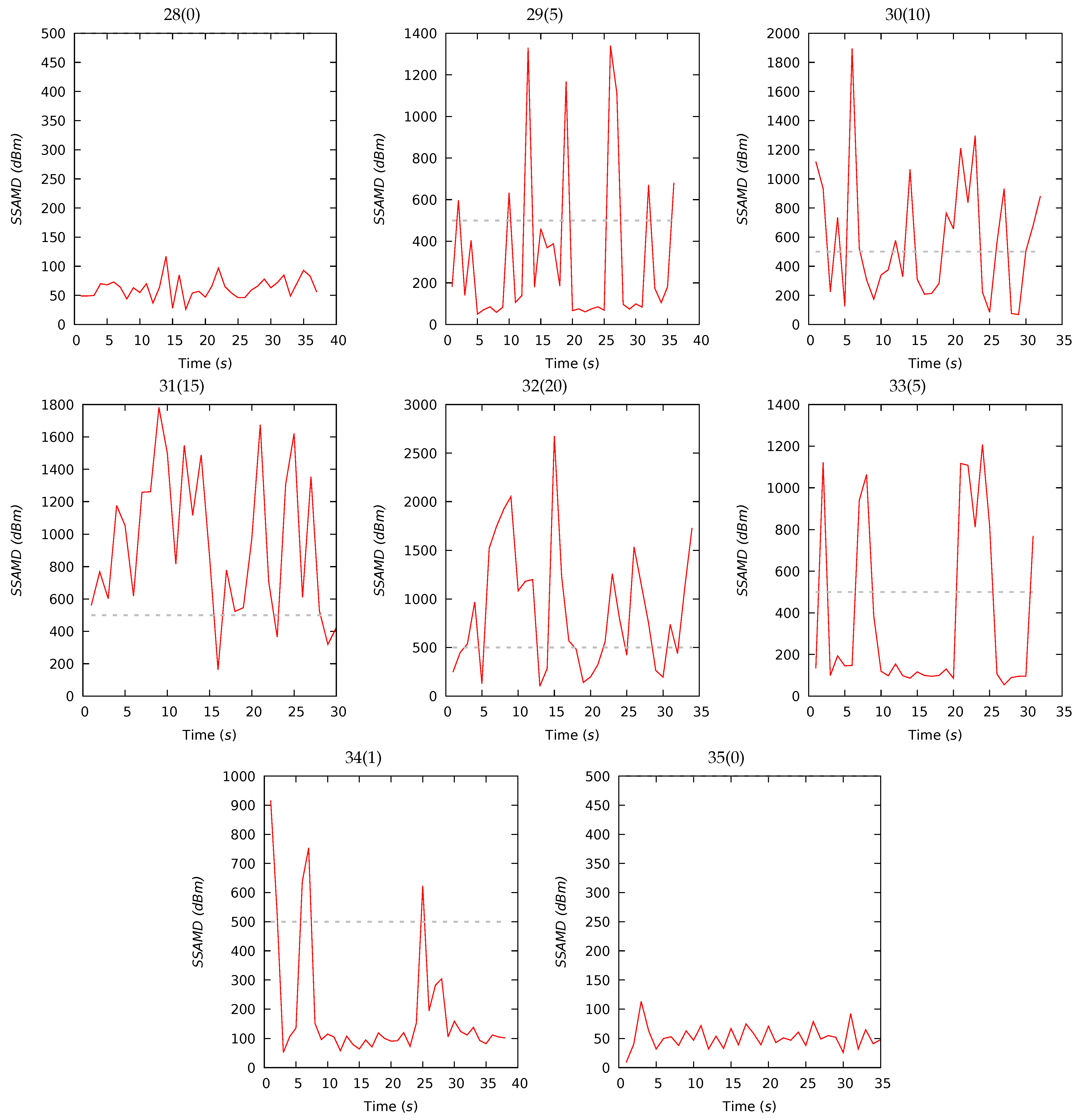
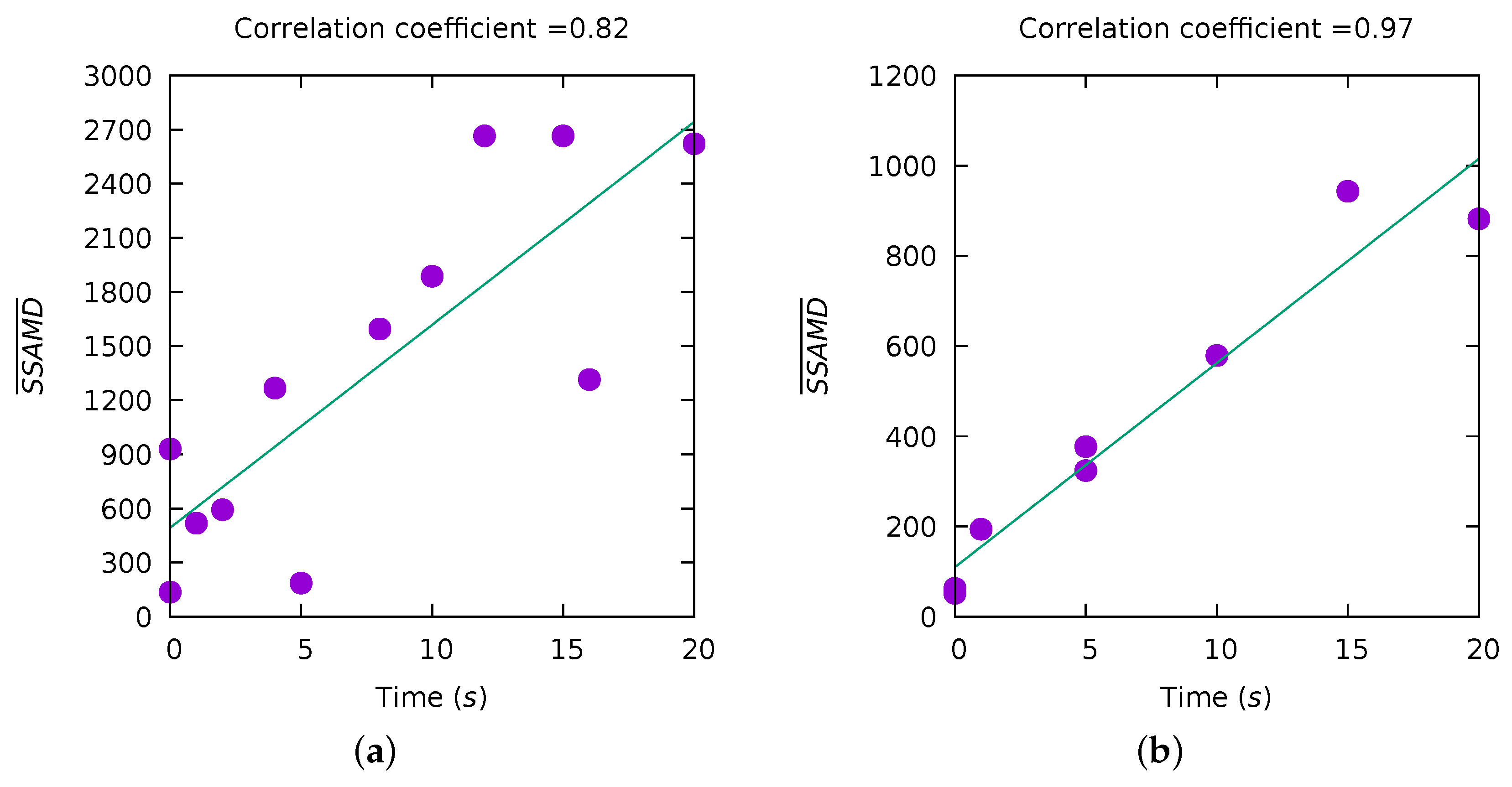
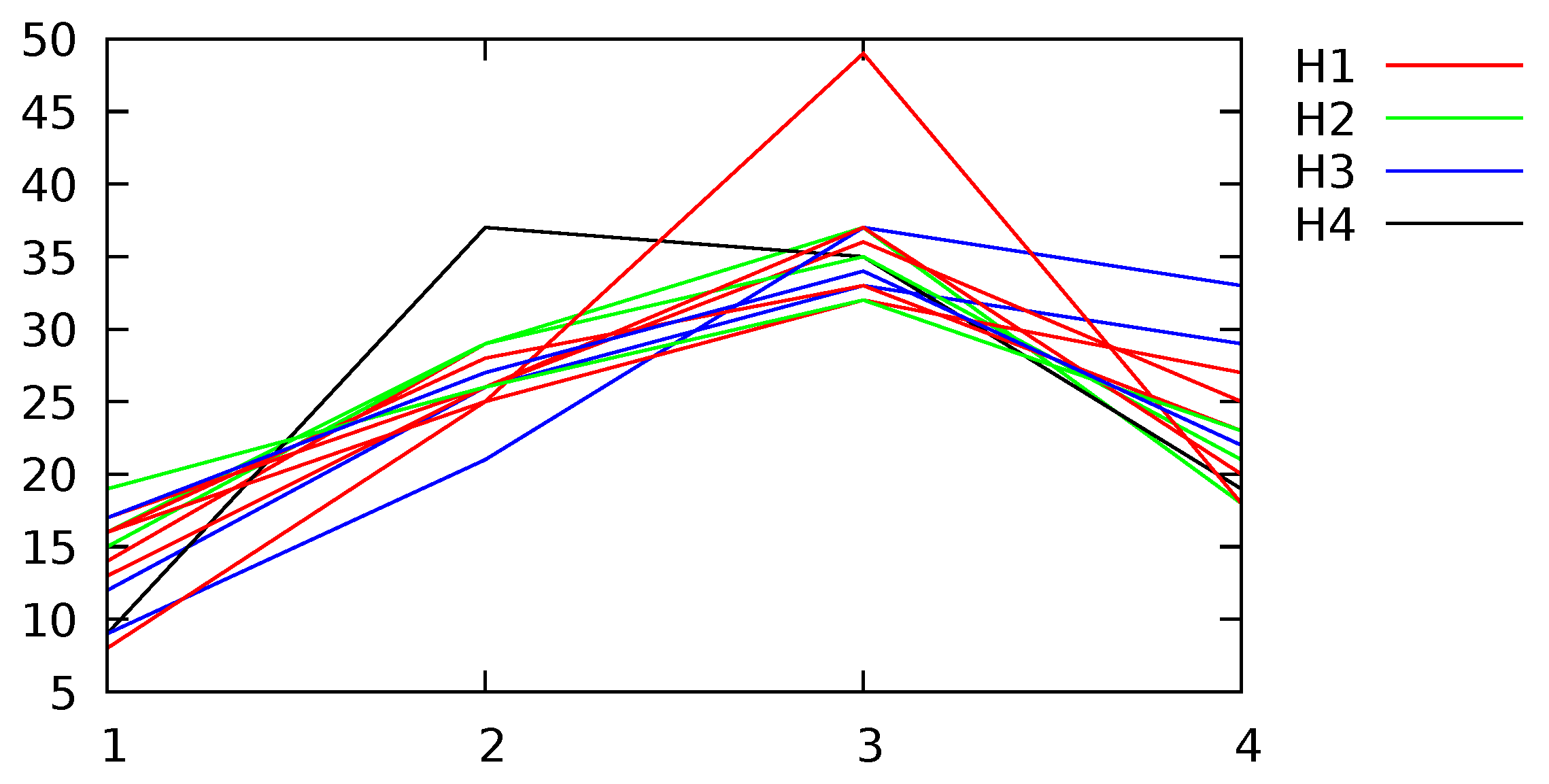
| ID (Desc) | Dur (s) | SSAMD’s Statistics (dBm) | Contribution (%) | Contribution (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Median | StDev | Min | Max | H1 | H2 | H3 | H4 | Q1 | I1 | Q2 | I2 | ||
| Experiment I (Phryganea grandis) | ||||||||||||||
| 1(H1) | 44 | 626 | 319 | 608 | 88 | 2573 | 14 | 29 | 37 | 20 | 15 | 22 | 28 | 35 |
| 2(H2) | 98 | 286 | 279 | 121 | 80 | 752 | 16 | 29 | 37 | 18 | 17 | 21 | 30 | 32 |
| 3(H3) | 128 | 261 | 244 | 105 | 13 | 704 | 12 | 26 | 33 | 29 | 20 | 26 | 31 | 23 |
| 4(H4) | 49 | 325 | 327 | 110 | 113 | 688 | 9 | 37 | 35 | 19 | 18 | 23 | 32 | 27 |
| Experiment II | ||||||||||||||
| Sialis lutaria: | ||||||||||||||
| 5(H1) | 70 | 384 | 207 | 401 | 54 | 1740 | 13 | 26 | 36 | 25 | 17 | 19 | 32 | 32 |
| 6(H2) | 46 | 212 | 190 | 136 | 0 | 589 | 15 | 29 | 35 | 21 | 20 | 15 | 35 | 30 |
| 7(H3) | 62 | 276 | 234 | 157 | 29 | 669 | 9 | 21 | 37 | 33 | 18 | 14 | 39 | 29 |
| Diffuser (no insects): | ||||||||||||||
| 8(A) | 71 | 213 | 183 | 126 | 70 | 770 | 7 | 17 | 34 | 42 | 18 | 14 | 40 | 28 |
| 9(B) | 33 | 203 | 179 | 93 | 41 | 373 | 9 | 19 | 34 | 38 | 19 | 15 | 37 | 29 |
| Sialis lutaria through the diffuser: | ||||||||||||||
| 10(H1(A)) | 63 | 350 | 269 | 246 | 108 | 1545 | 17 | 26 | 37 | 20 | 22 | 24 | 24 | 30 |
| 11(H1(B)) | 63 | 359 | 258 | 264 | 100 | 1233 | 8 | 25 | 49 | 18 | 18 | 20 | 32 | 30 |
| Small fly through the diffuser: | ||||||||||||||
| 12(H1) | 40 | 497 | 318 | 481 | 75 | 2030 | 16 | 28 | 33 | 23 | 19 | 17 | 26 | 38 |
| Single cricket through the diffuser: | ||||||||||||||
| 13(H1) | 50 | 385 | 289 | 338 | 117 | 1742 | 16 | 25 | 32 | 27 | 18 | 14 | 29 | 39 |
| 14(H2) | 38 | 212 | 196 | 71 | 114 | 389 | 19 | 26 | 32 | 23 | 21 | 14 | 30 | 35 |
| 15(H3) | 166 | 303 | 189 | 565 | 98 | 4949 | 17 | 27 | 34 | 22 | 19 | 15 | 31 | 35 |
| Experiment III | ||||||||||||||
| Experiment IIIa—Crickets in the diffuser (shaking): | ||||||||||||||
| 16(0) | 21 | 136 | 131 | 42 | 50 | 238 | 15 | 28 | 38 | 19 | 23 | 15 | 30 | 32 |
| 17(1) | 45 | 518 | 284 | 882 | 103 | 5064 | 16 | 27 | 39 | 18 | 20 | 18 | 31 | 31 |
| 18(2) | 45 | 593 | 275 | 1131 | 115 | 6162 | 14 | 28 | 40 | 18 | 23 | 24 | 25 | 28 |
| 19(4) | 68 | 1267 | 396 | 1752 | 0 | 6429 | 20 | 30 | 35 | 15 | 21 | 23 | 27 | 29 |
| 20(8) | 148 | 1595 | 681 | 1784 | 78 | 6173 | 20 | 30 | 35 | 15 | 21 | 24 | 27 | 28 |
| 21(12) | 133 | 2665 | 2509 | 2070 | 92 | 6533 | 22 | 30 | 33 | 15 | 23 | 25 | 26 | 26 |
| 22(16) | 171 | 1314 | 527 | 1722 | 118 | 6634 | 22 | 29 | 34 | 15 | 23 | 24 | 27 | 26 |
| 23(20) | 155 | 2621 | 2028 | 1957 | 252 | 6556 | 21 | 30 | 34 | 15 | 22 | 24 | 26 | 28 |
| 24(15) | 128 | 2665 | 2145 | 1856 | 247 | 6078 | 21 | 30 | 35 | 14 | 22 | 24 | 26 | 28 |
| 25(10) | 166 | 1887 | 865 | 1975 | 135 | 6896 | 21 | 28 | 34 | 17 | 22 | 25 | 26 | 27 |
| 26(5) | 187 | 2033 | 1035 | 1909 | 76 | 6156 | 20 | 29 | 35 | 16 | 23 | 25 | 26 | 26 |
| 27(0) | 202 | 930 | 273 | 1485 | 58 | 5993 | 21 | 27 | 36 | 16 | 22 | 24 | 27 | 27 |
| Experiment IIIb—Crickets in the box (at rest): | ||||||||||||||
| 28(0) | 37 | 63 | 63 | 19 | 26 | 117 | 17 | 23 | 41 | 19 | 25 | 21 | 26 | 28 |
| 29(5) | 36 | 324 | 140 | 379 | 50 | 1340 | 18 | 27 | 38 | 17 | 23 | 20 | 40 | 17 |
| 30(10) | 32 | 579 | 515 | 429 | 69 | 1895 | 14 | 33 | 37 | 16 | 21 | 21 | 31 | 27 |
| 31(15) | 30 | 943 | 838 | 455 | 162 | 1782 | 14 | 30 | 40 | 16 | 17 | 22 | 36 | 25 |
| 32(20) | 34 | 882 | 745 | 648 | 102 | 2676 | 17 | 30 | 39 | 14 | 16 | 20 | 35 | 29 |
| 33(5) | 31 | 377 | 130 | 414 | 54 | 1208 | 12 | 30 | 38 | 20 | 17 | 20 | 39 | 24 |
| 34(1) | 38 | 194 | 110 | 209 | 52 | 916 | 18 | 26 | 41 | 15 | 14 | 14 | 39 | 33 |
| 35(0) | 35 | 52 | 50 | 20 | 9 | 113 | 26 | 21 | 30 | 23 | 21 | 14 | 24 | 41 |
| Overall Statistics by Column | ||||||||||||||
| Mean | 74 | 710 | 469 | 667 | 79 | 2668 | 16 | 27 | 37 | 20 | 20 | 20 | 30 | 30 |
| Median | 46 | 377 | 273 | 401 | 76 | 1740 | 16 | 28 | 36 | 18 | 20 | 21 | 30 | 29 |
| StDev | 54 | 743 | 567 | 692 | 56 | 2441 | 4 | 4 | 4 | 7 | 3 | 4 | 5 | 5 |
| Min | 21 | 52 | 50 | 19 | 0 | 113 | 7 | 17 | 30 | 13 | 14 | 14 | 19 | 17 |
| Max | 202 | 2665 | 2509 | 2070 | 252 | 6896 | 26 | 37 | 49 | 42 | 25 | 26 | 40 | 42 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noskov, A.; Achilles, S.; Bendix, J. Presence and Biomass Information Extraction from Highly Uncertain Data of an Experimental Low-Range Insect Radar Setup. Diversity 2021, 13, 452. https://doi.org/10.3390/d13090452
Noskov A, Achilles S, Bendix J. Presence and Biomass Information Extraction from Highly Uncertain Data of an Experimental Low-Range Insect Radar Setup. Diversity. 2021; 13(9):452. https://doi.org/10.3390/d13090452
Chicago/Turabian StyleNoskov, Alexey, Sebastian Achilles, and Jörg Bendix. 2021. "Presence and Biomass Information Extraction from Highly Uncertain Data of an Experimental Low-Range Insect Radar Setup" Diversity 13, no. 9: 452. https://doi.org/10.3390/d13090452
APA StyleNoskov, A., Achilles, S., & Bendix, J. (2021). Presence and Biomass Information Extraction from Highly Uncertain Data of an Experimental Low-Range Insect Radar Setup. Diversity, 13(9), 452. https://doi.org/10.3390/d13090452







