The Morphology, Ultrastructure and Molecular Phylogeny of a New Freshwater Heterolobose Amoeba Parafumarolamoeba stagnalis n. sp. (Vahlkampfiidae; Heterolobosea)
Abstract
1. Introduction
2. Materials and Methods
2.1. Clone Isolation, Microscopy, and Laboratory Experiments
2.2. DNA Sequencing and Phylogenetic Analysis
3. Results
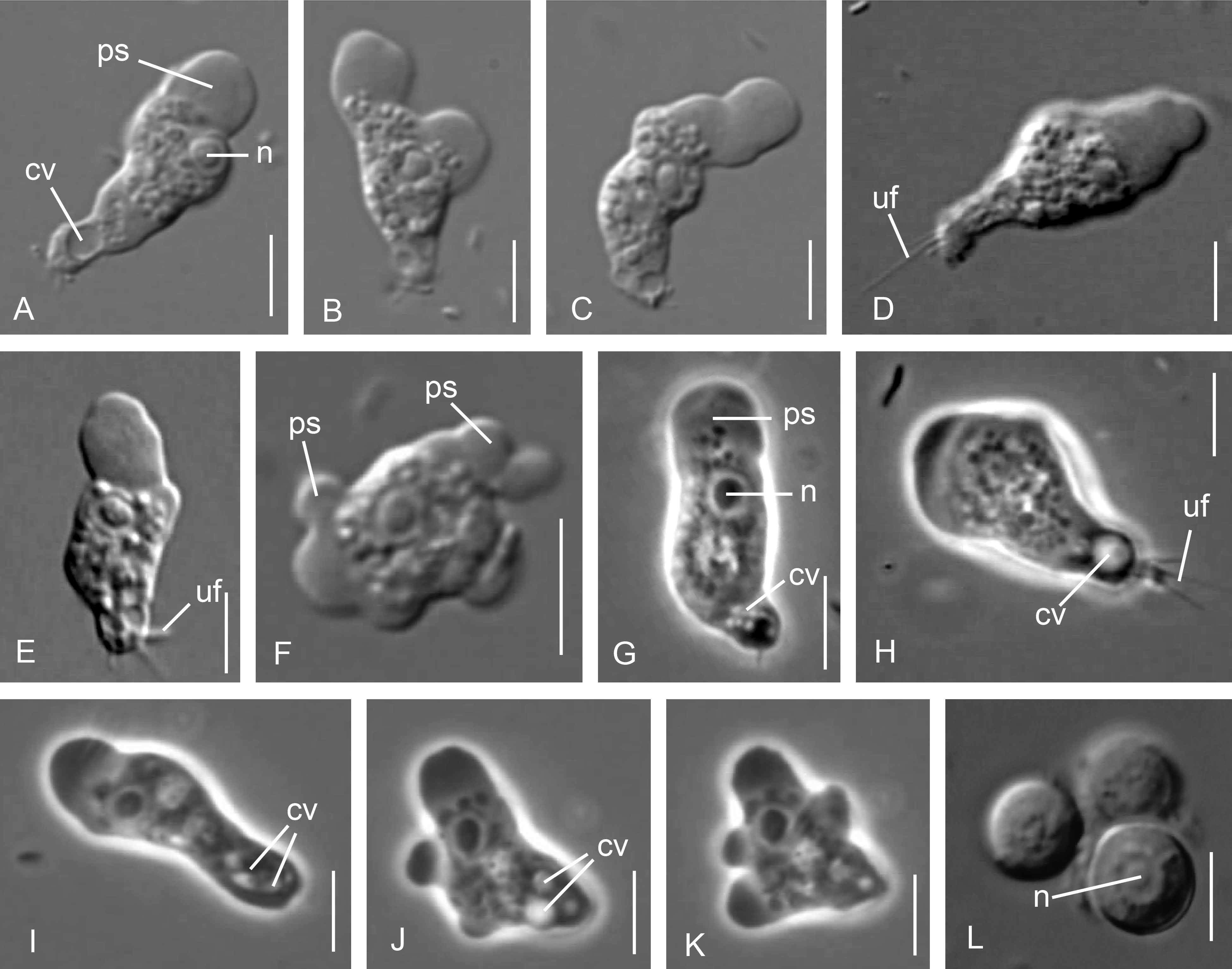
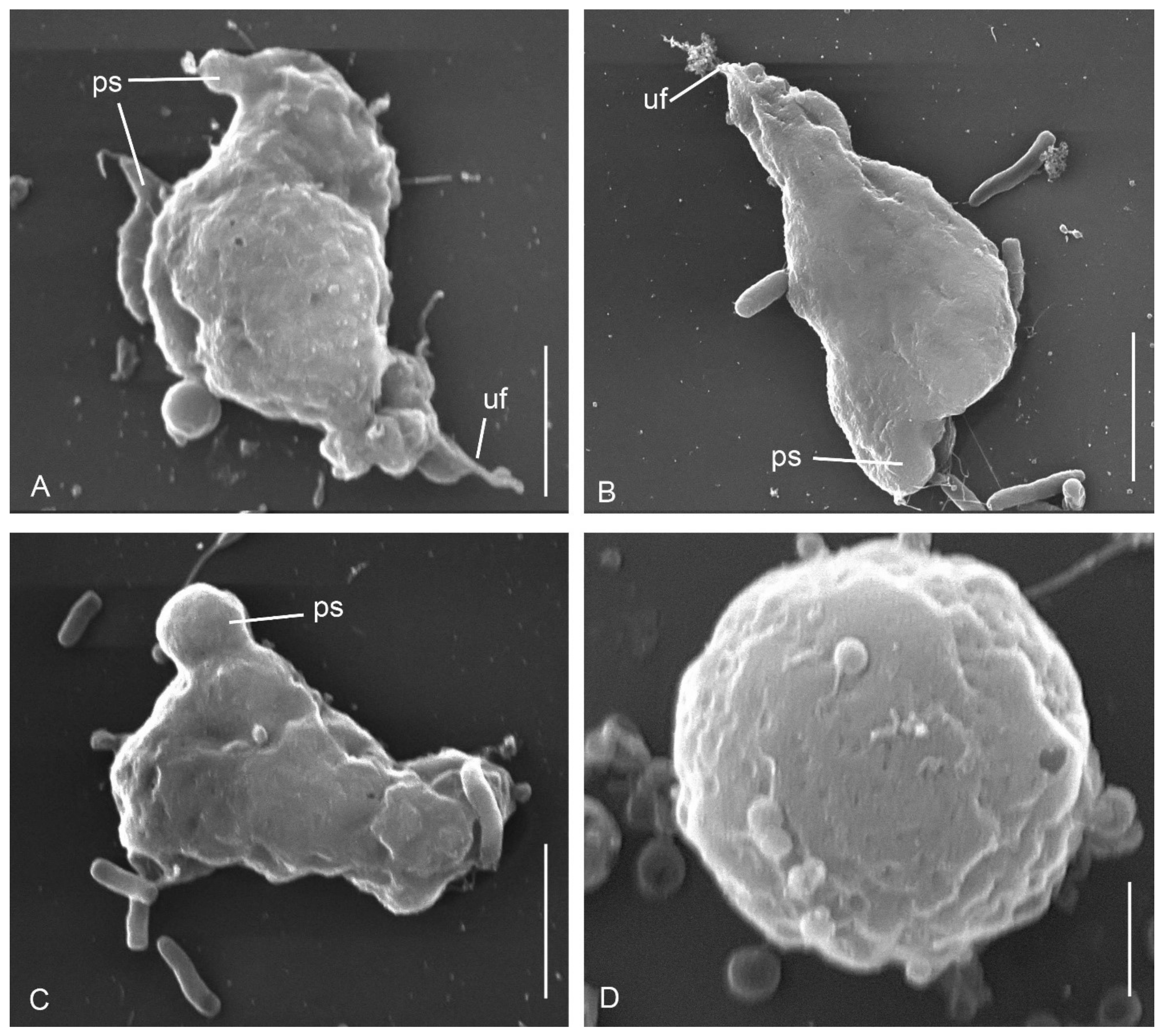
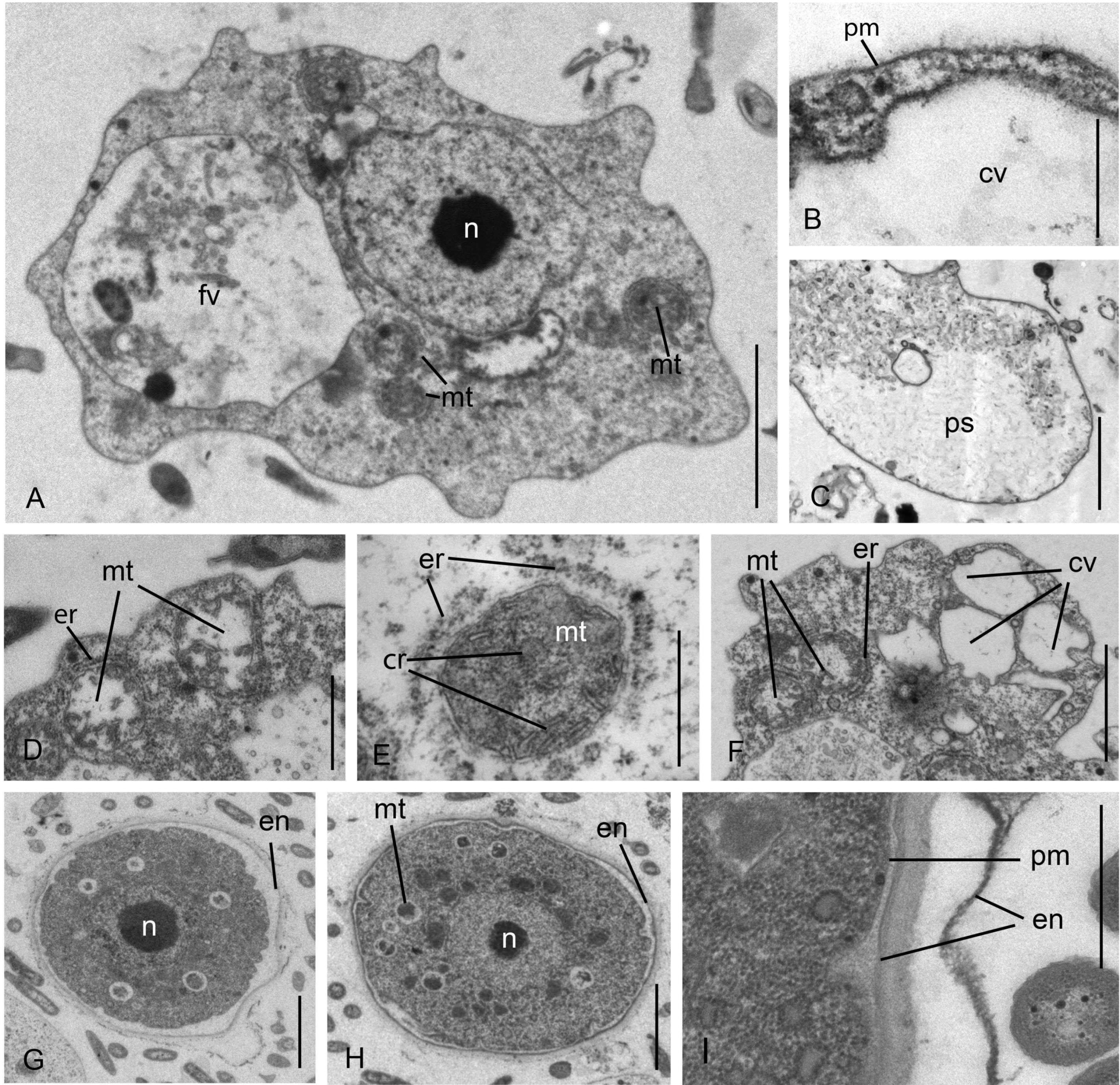
3.1. Cell Morphology
Parafumarolamoeba stagnalis n. sp.
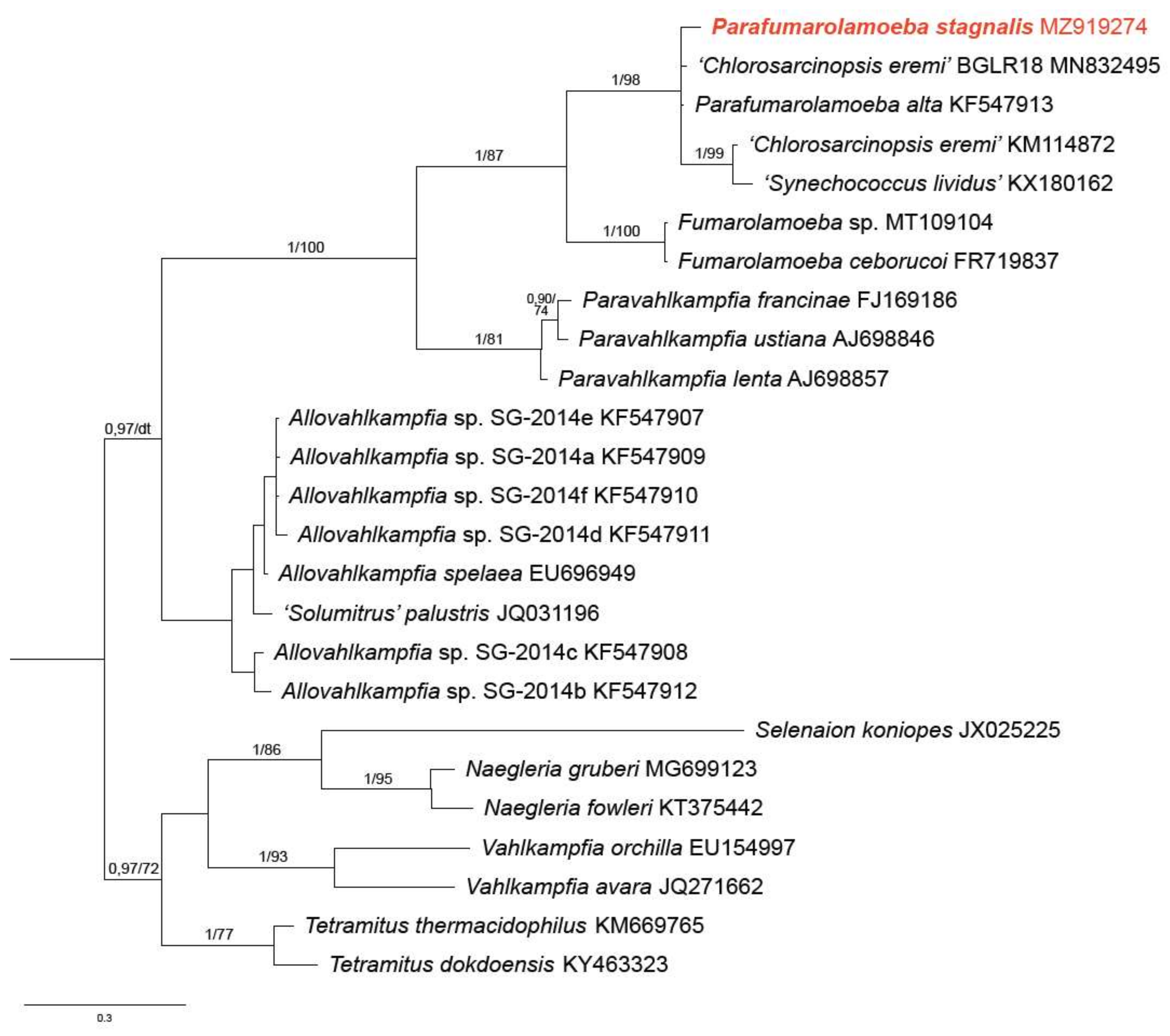
3.2. Phylogenetic Analysis
4. Discussion
Taxonomic Summary
Parafumarolamoeba stagnalis n. sp.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Visvesvara, G.S.; Moura, H.; Schuster, F.L. Pathogenic and opportunistic free-living amoebae: Acanthamoeba spp., Balamuthia mandrillaris, Naegleria fowleri, and Sappinia diploidea. FEMS Immunol. Med. Microbiol. 2007, 50, 1–26. [Google Scholar] [CrossRef]
- Lee, J. De novo formation of basal bodies during cellular differentiation of Naegleria gruberi: Progress and hypotheses. Semin. Cell Dev. Biol. 2010, 21, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.J.; Cursons, R.T.; Keys, E.A. Amoebae from Antarctic Soil and Water. Appl. Environ. Microbiol. 1982, 44, 491–493. [Google Scholar] [CrossRef]
- Ettinger, M.R.; Webb, S.R.; Harris, S.A.; McIninch, S.P.; Garman, G.C.; Brown, B.L. Distribution of free-living amoebae in James River, Virginia, USA. Parasitol. Res. 2003, 89, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Garstecki, T.; Arndt, H. Seasonal abundances and community structure of benthic rhizopods in shallow lagoons of the Southern Baltic Sea. Europ. J. Protistol. 2000, 36, 103–115. [Google Scholar] [CrossRef]
- Page, F.C.; Siemensma, F.J. Nackte Rhizopoda und Heliozoea (Protozoenfauna Band 2); Gustav Fischer Verlag: Stuttgart, NY, USA, 1991; pp. 3–170. [Google Scholar]
- Hinkle, G.; Sogin, M.L. The evolution of the Vahlkampfiidae as deduced from 16S-like ribosomal RNA analysis. J. Eukaryot. Microbiol. 1993, 40, 599–603. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.; De Jonckheere, J.F. A reevaluation of the amoeba genus Vahlkampfia based on SSU rDNA sequences. Eur. J. Protistol. 1999, 35, 49–54. [Google Scholar] [CrossRef]
- Brown, S.; De Jonckheere, J.F. Isolation of a new vahlkampfiid amoeba from soil: Paravahlkampfia lenta n. sp. Eur. J. Protistol. 2004, 40, 289–294. [Google Scholar] [CrossRef]
- De Jonckheere, J.F.; Brown, S. The identification of vahlkampfiid amoebae by ITS sequencing. Protist 2005, 156, 89–96. [Google Scholar] [CrossRef]
- Geisen, S.; Bonkowski, M.; Zhang, J.; De Jonckheere, J.F. Heterogeneity in the genus Allovahlkampfia and the description of the new genus Parafumarolamoeba (Vahlkampfiidae; Heterolobosea). Eur. J. Protistol. 2015, 51, 335–349. [Google Scholar] [CrossRef]
- Keeling, P.J.; Poulson, N.; McFadden, G.I. Phylogenetic diversity of parabasalian symbionts from termites, including the phylogenetic position of Pseudotrypanosoma and Trichonympha. J. Eukaryot. Microbiol. 1998, 45, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Gordon, D.; Green, P. Consed: A graphical editor for next-generation sequencing. Bioinformatics 2013, 29, 2936–2937. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Capella-Gutierrez, S.; Silla-Martínez, J.M.; Gabaldón, T. trimAl: A tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 2009, 25, 1972–1973. [Google Scholar] [CrossRef]
- Ronquist, F.; Huelsenbeck, J. MRBAYES 3: Bayesian Phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef]
- Nguyen, L.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A Fast and Effective Stochastic Algorithm for Estimating Maximum-Likelihood Phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- De Jonckheere, J. A Century of Research on the Amoeboflagellate Genus Naegleria. Acta Protozool. 2002, 41, 309–342. [Google Scholar]
- Walochnik, J.; Mulec, J. Free-living Amoebae in Carbonate Precipitating Microhabitats of Karst Caves and a New Vahlkampfiid Amoeba, Allovahlkampfia spelaea gen. nov., sp. nov. Acta Protozool. 2009, 48, 25–33. [Google Scholar]
- Brown, M.W.; Silberman, J.D.; Spiegel, F.W. A contemporary evaluation of the acrasids (Acrasidae, Heterolobosea, Excavata). Eur. J. Protistol. 2012, 48, 103–123. [Google Scholar] [CrossRef]
- Smirnov, A.V.; Fenchel, T. Vahlkampfia anaerobica n. sp., Vannella peregrinia n. sp. (Rhizopoda)—Anaerobic amoebae from a marine sediment. Arch. Protistenk. 1996, 147, 189–198. [Google Scholar] [CrossRef]
- Page, F.C. An Illustrated Key to Freshwater and Soil Amoebae with Notes on Cultivation and Ecology; Freshwater Biological Association: Ambleside, UK, 1976; 155p. [Google Scholar]
- Anderson, O.R.; Rogerson, A.; Hannah, F. Three new limax amoebae isolated from marine surface sediments. Vahlkampfia caledonica n. sp., Saccamoeba marina n. sp. and Hartmannella vacuolata n. sp. J. Eukaryot. Microbiol. 1997, 44, 33–42. [Google Scholar]
- Visvesvara, G.S.; Spiram, R.; Qvarnstrom, Y.; Bandyopadhyay, K.; Da Silva, A.J.; Pieniazek, N.J.; Cabral, G.A. Paravahlkampfia francinae n.sp. Masquerading as an Agent of Primary Amoebic Meningoencephalitis. J. Eukaryot. Microbiol. 2009, 56, 357–366. [Google Scholar] [CrossRef]
- Page, F.C. The limax amoebae, comparative fine structure of the Hartmannellidae (Lobosea) and further comparisons with the Vahlkampfiidae (Heterolobosea). Protistologica 1985, 21, 361–383. [Google Scholar]
- Page, F.C.; Blanton, R.L. The Heterolobosea (Sarcodina: Rhizopoda), a new class uniting the Schizopyrenida and the Acrasidae (Acrasida). Protistologica 1985, 21, 121–132. [Google Scholar]
- De Jonckheere, J.; Murase, J.; Opperdoes, F.R. A New Thermophilic Heterolobosean Amoeba, Fumarolamoeba ceborucoi, gen. nov., sp. nov., Isolated Near a Fumarole at a Volcano in Mexico. Acta Protozool. 2011, 50, 41–48. [Google Scholar]
- Valster, R.M.; Wullings, B.A.; van den Berg, R.; van der Kooij, D. Relationships between free-living protozoa, cultivable Legionella spp., and water quality characteristics in three drinking water supplies in the Caribbean. Appl. Environ. Microbiol. 2011, 77, 7321–7328. [Google Scholar] [CrossRef]
- Valster, R.M.; Wullings, B.A.; van der Kooij, D. Detection of protozoan hosts for Legionella pneumophila in engineered water systems by using a biofilm batch test. Appl. Environ. Microbiol. 2010, 76, 7144–7153. [Google Scholar] [CrossRef]
- Valster, R.M.; Wullings, B.A.; Bakker, G.; Smidt, H.; van der Kooij, D. Free-living protozoa in two unchlorinated drinking water supplies, identified by phylogenic analysis of 18S rRNA gene sequences. Appl. Environ. Microbiol. 2009, 75, 4736–4746. [Google Scholar] [CrossRef][Green Version]
- Farhat, M.; Moletta-Denat, M.; Frère, J.; Onillon, S.; Trouilhé, M.; Robine, E. Effects of disinfection on Legionella spp., eukarya, and biofilms in a hot water system. Appl. Environ. Microbiol. 2012, 78, 6850–6858. [Google Scholar] [CrossRef]
- De Vera, G.A.; Gerrity, D.; Stoker, M.; Frehner, W.; Wert, E. Impact of upstream chlorination on filter performance and microbial community structure of GAC and anthracite biofilters. Environ. Sci. Water Res. Technol. 2018, 4, 1133–1144. [Google Scholar] [CrossRef]
- Berrilli, F.; Di Cave, D.; Novelletto, A.; Montalbano Di Filippo, M. PCR-based identification of thermotolerant free-living amoebae in Italian hot springs. Eur. J. Protistol. 2021, 80, 125812. [Google Scholar] [CrossRef]
- Amaral Zettler, L.A.; Gomez, F.; Zettler, E.; Keenan, B.G.; Amils, R.; Sogin, M.L. Microbiology: Eukaryotic diversity in Spain’s River of Fire. Nature 2002, 417, 137. [Google Scholar] [CrossRef]
- Amaral-Zettler, L.A.; Zettler, E.R.; Theroux, S.M.; Palacios, C.; Aguilera, A.; Amils, R. Microbial community structure across the tree of life in the extreme Río Tinto. ISME J. 2011, 5, 42–50. [Google Scholar] [CrossRef]

| Species | Locomotive form Length (µm) | Locomotive form Width (µm) | Limax Morphology | Uroidal Filaments | Nucleolus/Nucleus Size Ratio | Cyst Characteristics | Growth Temperature, °C | Source |
|---|---|---|---|---|---|---|---|---|
| Parafumarolamoeba stagnalis | 17.9 ± 0.5 | 10.4 ± 0.5 | + | + | 2/3 | d = 5–7 µm round cysts, three-layered envelope | Max 36 | Current study |
| P. alta | 20.9 | 7.9 | + | + | 1/3 | d = 5.7 ± 0.4 µm wrinkled and round | ? | Geisen et al., 2015 |
| Fumarolamoeba ceborucoi | 26 | 13.8 | rarely | - | 1/2 | d = 6.2 µm double wall cysts | Max 51 (not multiply but survives) | De Jonckheere et al., 2011 |
| Vahlkampfia anaerobica | 11–34 | 7 | + | + | ? | no cysts observed | ? | Smirnov et al., 1996 |
| V. avara | 15–33 | 5–11 | + | + | 1/2 | d = 9.7 µm Smooth, gelatinous(‘sticky’) single cystwall | ? | Page, 1967 |
| V. caledonica | 47.4 ± 16.0 | 12.1 ± 3.2 | + | + | 2/5 | no cysts observed | ? | Anderson et al., 2007 |
| Paravahlkampfia. lenta | 37–80 | 11–24 | + | + | 4/5 | d = 18.1 µm smooth, double cystwall, (‘sticky’) outerwall | Max 34 | Brown and De Jonckheere, 2004 |
| P. francinae | 15–25 | 5.9–9.9 | + | + | 3/5 | d = 17.5 µm round, double cyst wall; cysts from older cultures with wrinkled and star-shaped outer cyst walls | Opt 37 | Visvesvara et al., 2009 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borodina, A.S.; Mylnikov, A.P.; Janouškovec, J.; Keeling, P.J.; Tikhonenkov, D.V. The Morphology, Ultrastructure and Molecular Phylogeny of a New Freshwater Heterolobose Amoeba Parafumarolamoeba stagnalis n. sp. (Vahlkampfiidae; Heterolobosea). Diversity 2021, 13, 433. https://doi.org/10.3390/d13090433
Borodina AS, Mylnikov AP, Janouškovec J, Keeling PJ, Tikhonenkov DV. The Morphology, Ultrastructure and Molecular Phylogeny of a New Freshwater Heterolobose Amoeba Parafumarolamoeba stagnalis n. sp. (Vahlkampfiidae; Heterolobosea). Diversity. 2021; 13(9):433. https://doi.org/10.3390/d13090433
Chicago/Turabian StyleBorodina, Anastasia S., Alexander P. Mylnikov, Jan Janouškovec, Patrick J. Keeling, and Denis V. Tikhonenkov. 2021. "The Morphology, Ultrastructure and Molecular Phylogeny of a New Freshwater Heterolobose Amoeba Parafumarolamoeba stagnalis n. sp. (Vahlkampfiidae; Heterolobosea)" Diversity 13, no. 9: 433. https://doi.org/10.3390/d13090433
APA StyleBorodina, A. S., Mylnikov, A. P., Janouškovec, J., Keeling, P. J., & Tikhonenkov, D. V. (2021). The Morphology, Ultrastructure and Molecular Phylogeny of a New Freshwater Heterolobose Amoeba Parafumarolamoeba stagnalis n. sp. (Vahlkampfiidae; Heterolobosea). Diversity, 13(9), 433. https://doi.org/10.3390/d13090433







