The Placenta of Physcomitrium patens: Transfer Cell Wall Polymers Compared across the Three Bryophyte Groups
Abstract
1. Introduction
2. Materials and Methods
2.1. Gametophyte Culture
2.2. Preparation for Transmission Electron Microscopy
2.3. Immunogold Labeling
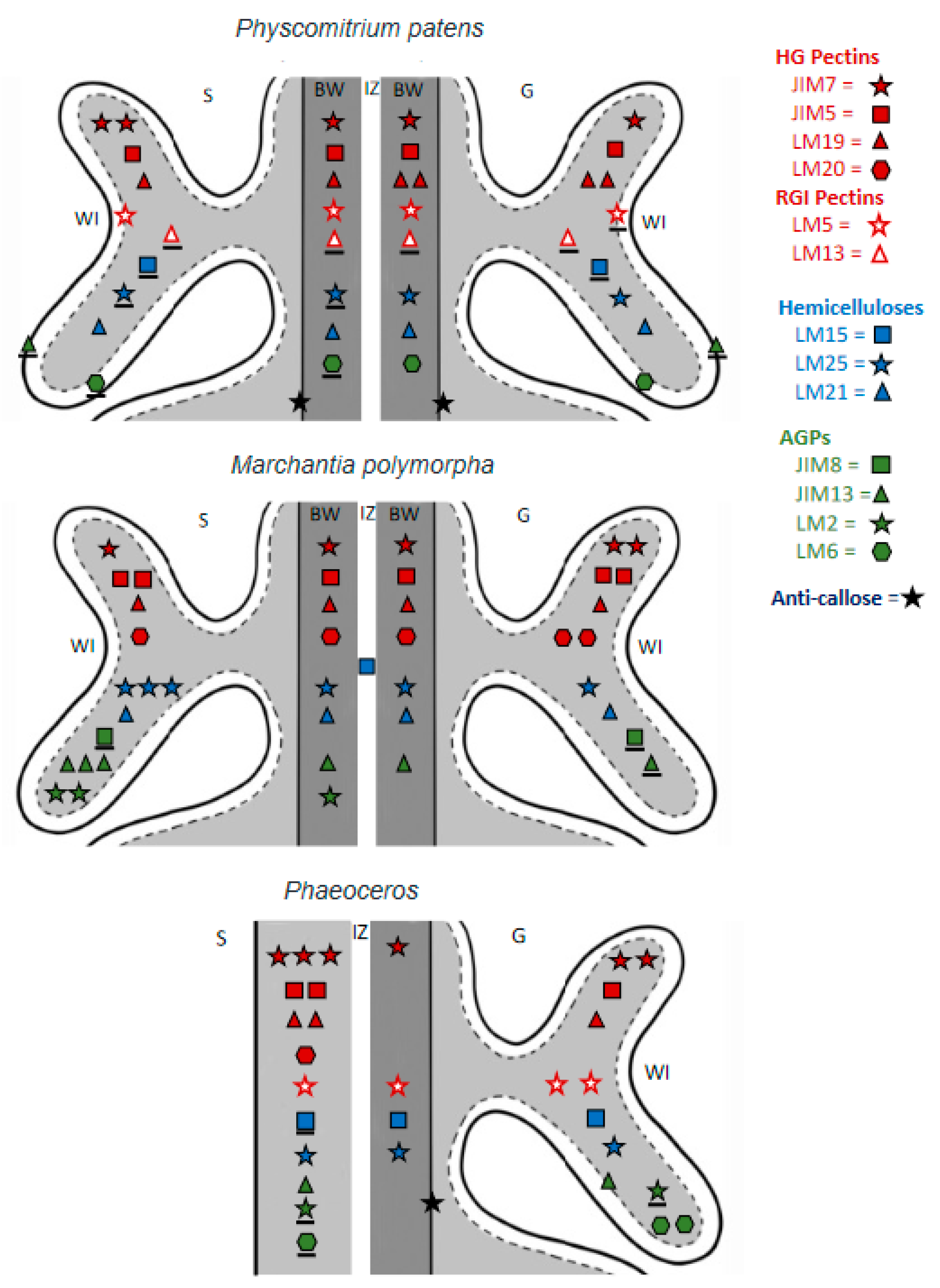
2.4. Scoring Label Intensity
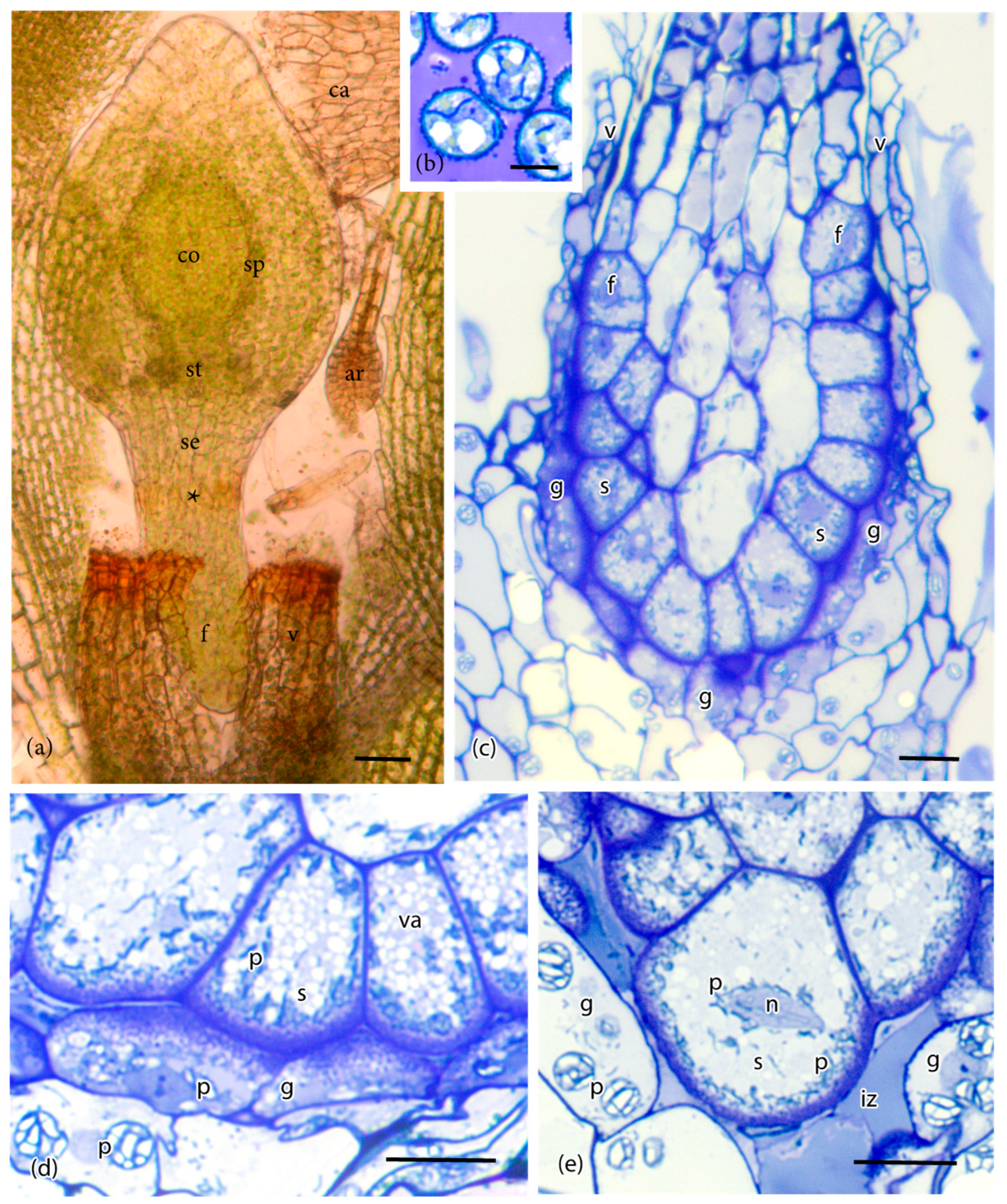
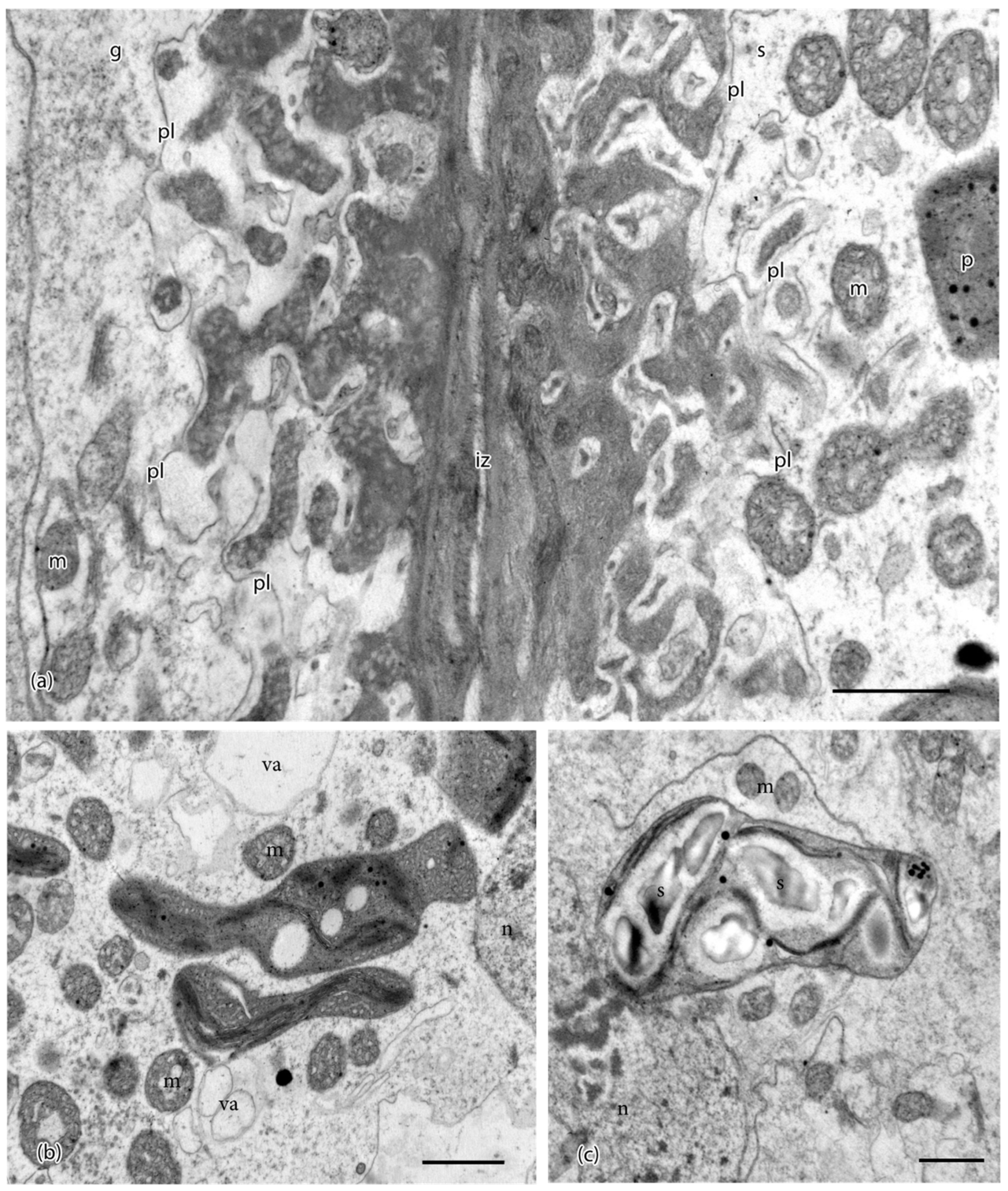
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ligrone, R.; Duckett, J.G.; Renzaglia, K.S. The Gametophyte-Sporophyte Junction in Land Plants. Adv. Bot. Res. 1993, 19, 231–318. [Google Scholar] [CrossRef]
- Ligrone, R.; Duckett, J.G.; Renzaglia, K.S. The origin of the sporophyte shoot in land plants: A bryological perspective. Ann. Bot. 2012, 110, 935–941. [Google Scholar] [CrossRef]
- Gunning, B.E.; Pate, J.S. “Transfer cells” plant cells with wall ingrowths, specialized in relation to short distance transport of solutes—Their occurrence, structure, and development. Protoplasma 1969, 68, 107–133. [Google Scholar] [CrossRef]
- Thompson, R.D.; Hueros, G.; Becker, H.-A.; Maitz, M. Development and functions of seed transfer cells. Plant Sci. 2001, 160, 775–783. [Google Scholar] [CrossRef]
- Henry, J.S.; Ligrone, R.; Vaughn, K.C.; Lopez, R.A.; Renzaglia, K.S. Cell wall polymers in the Phaeoceros placenta reflect devel-opmental and functional differences across generations. Bryophyt. Divers. Evol. 2021, 43, 265–283. [Google Scholar]
- McCurdy, D.W.; Patrick, J.W.; Offler, C.E. Wall ingrowth formation in transfer cells: Novel examples of localized wall deposition in plant cells. Curr. Opin. Plant Biol. 2008, 11, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Talbot, M.J.; Franceschi, V.R.; McCurdy, D.W.; Offler, C.E. Wall ingrowth architecture in epidermal transfer cells of Vicia faba cotyledons. Protoplasma 2001, 215, 191–203. [Google Scholar] [CrossRef]
- Pate, J.S.; Gunning, B.E.S.; Milliken, F.F. Function of transfer cells in the nodal regions of stems, particularly in relation to the nutrition of young seedlings. Protoplasma 1970, 71, 313–334. [Google Scholar] [CrossRef]
- Cochrane, M.P.; Duffus, C.M. The nucellar projection and modified aleurone in the crease region of developing caryopses of barley (Hordeum vulgare L. var. distichum). Protoplasma 1980, 103, 361–375. [Google Scholar] [CrossRef]
- Zee, S.-Y.; O’Brien, T.P. Aleurone Transfer Cells and other Structural Features of the Spikelet of Millet. Aust. J. Biol. Sci. 1971, 24, 391–395. [Google Scholar] [CrossRef][Green Version]
- Patrick, J.W.; Offler, C.E. Compartmentation of transport and transfer events in developing seeds. J. Exp. Bot. 2001, 52, 551–564. [Google Scholar] [CrossRef] [PubMed]
- Tegeder, M.; Wang, X.-D.; Frommer, W.; Offler, C.E.; Patrick, J.W.B. Sucrose transport into developing seeds of Pisum sativum L. Plant J. 1999, 18, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Faraday, C.D.; Thompson, W.W. Structural aspects of the salt glands of the Plumbaginaceae. J. Exp. Bot. 1986, 37, 461–470. [Google Scholar] [CrossRef]
- Berry, A.M.; McIntyre, L.; McCully, M.E. Fine structure of root hair infection leading to nodulation in the Frankia–Alnus sym-biosis. Can. J. Bot. 1986, 64, 292–305. [Google Scholar] [CrossRef]
- Gunning, B.E.S.; Pate, J.S.; Minchin, F.R.; Marks, I. Quantitative aspects of transfer cell structure in relation to vein loading in leaves and solute transport in legume nodules. Symp. Soc. Exp. Biol. 1974, 28, 87–126. [Google Scholar]
- Regmi, K.C.; Li, L.; Gaxiola, R.A. Alternate Modes of Photosynthate Transport in the Alternating Generations of Physcomitrella patens. Front. Plant Sci. 2017, 8, 1956. [Google Scholar] [CrossRef] [PubMed]
- Courtice, G.R.M.; Ashton, N.W.; Cove, D.J. Evidence for the restricted passage of metabolites into the sporophyte of the moss Physcomitrella patens (Hedw.) Br. Eur. J. Bryol. 1978, 10, 191–198. [Google Scholar] [CrossRef]
- Henry, J.S.; Lopez, R.A.; Renzaglia, K.S. Differential localization of cell wall polymers across generations in the placenta of Marchantia polymorpha. J. Plant Res. 2020, 133, 911–924. [Google Scholar] [CrossRef]
- Renzaglia, K.S.; Lopez, R.A.; Henry, J.S.; Flowers, N.D.; Vaughn, K.C. Transmission Electron Microscopy of Centrioles, Basal Bodies and Flagella in Motile Male Gametes of Land Plants. Bio-Protocol 2017, 7. [Google Scholar] [CrossRef]
- Lopez, R.A.; Mansouri, K.; Henry, J.S.; Flowers, N.D.; Vaughn, K.C.; Renzaglia, K.S. Immunogold localization of molecular constituents associated with basal bodies, flagella, and extracellular matrices in male gametes of land plants. Bio-Protocol 2017, 7, e2599. [Google Scholar] [CrossRef]
- Willats, W.G.; Limberg, G.; Buchholt, H.C.; van Alebeek, G.-J.; Benen, J.; Christensen, T.M.; Visser, J.; Voragen, A.; Mikkelsen, J.D.; Knox, P. Analysis of pectic epitopes recognised by hybridoma and phage display monoclonal antibodies using defined oligosaccharides, polysaccharides, and enzymatic degradation. Carbohydr. Res. 2000, 327, 309–320. [Google Scholar] [CrossRef]
- Knox, P.; Linstead, P.J.; King, J.; Cooper, C.; Roberts, K. Pectin esterification is spatially regulated both within cell walls and between developing tissues of root apices. Planta 1990, 181, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Verhertbruggen, Y.; Marcus, S.E.; Haeger, A.; Ordaz-Ortiz, J.J.; Knox, P. An extended set of monoclonal antibodies to pectic homogalacturonan. Carbohydr. Res. 2009, 344, 1858–1862. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.; Seymour, G.; Knox, J.P. Localization of Pectic Galactan in Tomato Cell Walls Using a Monoclonal Antibody Specific to (1[->]4)-β-D-Galactan. Plant Physiol. 1997, 113, 1405–1412. [Google Scholar] [CrossRef] [PubMed]
- Moller, I.; Sørensen, I.; Bernal, A.; Blaukopf, C.; Lee, K.; Øbro, J.; Pettolino, F.; Roberts, A.; Mikkelsen, J.D.; Knox, P.; et al. High-throughput mapping of cell-wall polymers within and between plants using novel microarrays. Plant J. 2007, 50, 1118–1128. [Google Scholar] [CrossRef]
- Marcus, S.E.; Verhertbruggen, Y.; Hervé, C.; Ordaz-Ortiz, J.J.; Farkas, V.; Pedersen, H.L.; Willats, W.G.T.; Knox, J.P. Pectic homogalacturonan masks abundant sets of xyloglucan epitopes in plant cell walls. BMC Plant Biol. 2008, 8, 60. [Google Scholar] [CrossRef]
- Pedersen, H.L.; Fangel, J.U.; McCleary, B.; Ruzanski, C.; Rydahl, M.G.; Ralet, M.-C.; Farkas, V.; von Schantz, L.; Marcus, S.E.; Andersen, M.C.; et al. Versatile High Resolution Oligosaccharide Microarrays for Plant Glycobiology and Cell Wall Research. J. Biol. Chem. 2012, 287, 39429–39438. [Google Scholar] [CrossRef]
- Marcus, S.E.; Blake, A.W.; Benians, T.A.S.; Lee, K.J.D.; Poyser, C.; Donaldson, L.; Leroux, O.; Rogowski, A.; Petersen, H.L.; Boraston, A.; et al. Restricted access of proteins to mannan polysaccharides in intact plant cell walls. Plant J. 2010, 64, 191–203. [Google Scholar] [CrossRef]
- Cornuault, V.; Buffetto, F.; Rydahl, M.; Marcus, S.E.; Torode, T.; Xue, J.; Crépeau, M.-J.; Faria-Blanc, N.; Willats, W.G.T.; Du Pree, P.; et al. Monoclonal antibodies indicate low-abundance links between heteroxylan and other glycans of plant cell walls. Planta 2015, 242, 1321–1334. [Google Scholar] [CrossRef]
- Yates, E.A.; Valdor, J.-F.; Haslam, S.M.; Morris, H.R.; Dell, A.; Mackie, W.; Knox, P. Characterization of carbohydrate structural features recognized by anti-arabinogalactan-protein monoclonal antibodies. Glycobiology 1996, 6, 131–139. [Google Scholar] [CrossRef]
- Willats, W.; Marcus, S.E.; Knox, P. Generation of a monoclonal antibody specific to (1→5)-α-l-arabinan. Carbohydr. Res. 1998, 308, 149–152. [Google Scholar] [CrossRef]
- Pennell, R.I.; Janniche, L.; Kjellbom, P.; Scofield, G.N.; Peart, J.M.; Roberts, K. Developmental Regulation of a Plasma Membrane Arabinogalactan Protein Epitope in Oilseed Rape Flowers. Plant Cell 1991, 3, 1317–1326. [Google Scholar] [CrossRef] [PubMed]
- Smallwood, M.; Yates, E.A.; Willats, W.G.; Martin, H.; Knox, P. Immunochemical comparison of membrane-associated and secreted arabinogalactan-proteins in rice and carrot. Planta 1996, 198, 452–459. [Google Scholar] [CrossRef]
- Meikle, P.; Bonig, I.; Hoogenraad, N.J.; Clarke, A.E.; Stone, B.A. The location of (1→3)-β-glucans in the walls of pollen tubes of Nicotiana alata using a (1→3)-β-glucan-specific monoclonal antibody. Planta 1991, 185, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Smallwood, M.; Beven, A.; Donovan, N.; Neill, S.J.; Peart, J.; Roberts, K.; Knox, P. Localization of cell wall proteins in relation to the developmental anatomy of the carrot root apex. Plant J. 1994, 5, 237–246. [Google Scholar] [CrossRef]
- Schmid, R.; Crum, H. Structural Diversity of Bryophytes; University of Michigan Herbarium: Ann Arbor, MI, USA, 2001; p. 379. [Google Scholar]
- Ridley, B.L.; O’Neill, M.A.; Mohnen, D. Pectins: Structure, biosynthesis, and oligogalacturonide-related signaling. Phytochemistry 2001, 57, 929–967. [Google Scholar] [CrossRef]
- O’Neill, M.A.; York, W.S.; Roberts, J.A.; Evan, D.; McManus, M.T.; Rose, J.K.C. The Composition and Structure of Plant Primary Cell Walls. Ann. Plant Rev. Online 2018, 1–54. [Google Scholar] [CrossRef]
- Carpita, N.C. Structure and biogenesis of the cell walls of grasses. Annu. Rev. Plant Biol. 1996, 47, 445–476. [Google Scholar] [CrossRef]
- Caffall, K.H.; Mohnen, D. The structure, function, and biosynthesis of plant cell wall pectic polysaccharides. Carbohydr. Res. 2009, 344, 1879–1900. [Google Scholar] [CrossRef] [PubMed]
- Braybrook, S.A.; Jönsson, H. Shifting foundations: The mechanical cell wall and development. Curr. Opin. Plant Biol. 2016, 29, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Cornuault, V.; Buffetto, F.; Marcus, S.E.; Crépeau, M.J.; Guillon, F.; Ralet, M.C.; Knox, P. LM6-M: A high avidity rat monoclonal antibody to pectic α-1, 5-L-arabinan. Bio Rxiv Plant Bio. 2017. [Google Scholar] [CrossRef]
- Verhertbruggen, Y.; Marcus, S.E.; Chen, J.; Knox, J.P. Cell Wall Pectic Arabinans Influence the Mechanical Properties of Arabidopsis thaliana Inflorescence Stems and Their Response to Mechanical Stress. Plant Cell Physiol. 2013, 54, 1278–1288. [Google Scholar] [CrossRef] [PubMed]
- McCartney, L.; Steele-King, C.G.; Jordan, E.; Knox, J.P. Cell wall pectic (1→4)-β-d-galactan marks the acceleration of cell elongation in theArabidopsisseedling root meristem. Plant J. 2003, 33, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.; Milne, J.L.; Ashford, D.; McQueen-Mason, S.J. Cell wall arabinan is essential for guard cell function. Proc. Natl. Acad. Sci. USA 2003, 100, 11783–11788. [Google Scholar] [CrossRef] [PubMed]
- Whitney, S.E.C.; Wilson, E.; Webster, J.; Bacic, A.; Reid, J.S.G.; Gidley, M.J. Effects of structural variation in xyloglucan polymers on interactions with bacterial cellulose. Am. J. Bot. 2006, 93, 1402–1414. [Google Scholar] [CrossRef]
- Chanliaud, E.; Burrows, K.M.; Jeronimidis, G.; Gidley, M.J. Mechanical properties of primary plant cell wall analogues. Planta 2002, 215, 989–996. [Google Scholar] [CrossRef]
- Ordaz-Ortiz, J.J.; Marcus, S.E.; Knox, J.P. Cell Wall Microstructure Analysis Implicates Hemicellulose Polysaccharides in Cell Adhesion in Tomato Fruit Pericarp Parenchyma. Mol. Plant 2009, 2, 910–921. [Google Scholar] [CrossRef]
- Bunterngsook, B.; Eurwilaichitr, L.; Thamchaipenet, A.; Champreda, V. Binding characteristics and synergistic effects of bacterial expansins on cellulosic and hemicellulosic substrates. Bioresour. Technol. 2015, 176, 129–135. [Google Scholar] [CrossRef]
- Lopez, R.A.; Renzaglia, K.S. Multiflagellated sperm cells of Ceratopteris richardii are bathed in arabinogalactan proteins throughout development. Am. J. Bot. 2014, 101, 2052–2061. [Google Scholar] [CrossRef]
- Scheller, H.V.; Ulvskov, P. Hemicelluloses. Annu. Rev. Plant Biol. 2010, 61, 263–289. [Google Scholar] [CrossRef]
- Dehors, J.; Mareck, A.; Kiefer-Meyer, M.-C.; Menu-Bouaouiche, L.; Lehner, A.; Mollet, J.-C. Evolution of Cell Wall Polymers in Tip-Growing Land Plant Gametophytes: Composition, Distribution, Functional Aspects and Their Remodeling. Front. Plant Sci. 2019, 10, 441. [Google Scholar] [CrossRef]
- Plancot, B.; Gügi, B.; Mollet, J.-C.; Loutelier-Bourhis, C.; Govind, S.R.; Lerouge, P.; Follet-Gueye, M.-L.; Vicré, M.; Alfonso, C.; Nguema-Ona, E.; et al. Desiccation tolerance in plants: Structural characterization of the cell wall hemicellulosic polysaccharides in three Selaginella species. Carbohydr. Polym. 2018, 208, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Torode, T.A.; O’Neill, R.; Marcus, S.E.; Cornuault, V.; Pose-Albacete, S.; Lauder, R.P.; Kracun, S.K.; Rydahl, M.G.; Andersen, M.C.F.; Willats, W.G.T.; et al. Branched Pectic Galactan in Phloem-Sieve-Element Cell Walls: Implications for Cell Mechanics. Plant Physiol. 2017, 176, 1547–1558. [Google Scholar] [CrossRef]
- Lamport, D.T.A.; Varnai, P.; Seal, C. Back to the future with the AGP–Ca2+ flux capacitor. Ann. Bot. 2014, 114, 1069–1085. [Google Scholar] [CrossRef]
- Lamport, D.T.A.; Tan, L.; Held, M.A.; Kieliszewski, M.J. Pollen tube growth and guidance: Occam’s razor sharpened on a molecular arabinogalactan glycoprotein Rosetta Stone. New Phytol. 2018, 217, 491–500. [Google Scholar] [CrossRef]
- Lee, K.J.; Sakata, Y.; Mau, S.-L.; Pettolino, F.; Bacic, A.; Quatrano, R.S.; Knight, C.D.; Knox, J.P. Arabinogalactan Proteins Are Required for Apical Cell Extension in the Moss Physcomitrella patens. Plant Cell 2005, 17, 3051–3065. [Google Scholar] [CrossRef]
- Lopez, R.A.; Renzaglia, K.S. Arabinogalactan proteins and arabinan pectins abound in the specialized matrices surrounding female gametes of the fern Ceratopteris richardii. Planta 2016, 243, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Diet, A.; Link, B.; Seifert, G.J.; Schellenberg, B.; Wagner, U.; Pauly, M.; Reiter, W.-D.; Ringli, C.; Saint-Jore-Dupas, C.; Nebenführ, A.; et al. The Arabidopsis Root Hair Cell Wall Formation Mutantlrx1Is Suppressed by Mutations in theRHM1Gene Encoding a UDP-L-Rhamnose Synthase. Plant Cell 2006, 18, 1630–1641. [Google Scholar] [CrossRef] [PubMed]
- Ringli, C. Monitoring the Outside: Cell Wall-Sensing Mechanisms. Plant Physiol. 2010, 153, 1445–1452. [Google Scholar] [CrossRef]
- Velasquez, S.M.; Salter, J.S.; Dorosz, J.G.; Petersen, B.L.; Estevez, J.M. Recent Advances on the Posttranslational Modifications of EXTs and Their Roles in Plant Cell Walls. Front. Plant Sci. 2012, 3, 93–99. [Google Scholar] [CrossRef]
- Bascom, C.S.; Winship, L.J.; Bezanilla, M. Simultaneous imaging and functional studies reveal a tight correlation between calcium and actin networks. Proc. Natl. Acad. Sci. USA 2018, 115, E2869–E2878. [Google Scholar] [CrossRef]
- Samuels, A.L.; Giddings, T.H.; Staehelin, L.A. Cytokinesis in tobacco BY-2 and root tip cells: A new model of cell plate formation in higher plants. J. Cell Biol. 1995, 130, 1345–1357. [Google Scholar] [CrossRef]
- Vaughn, K.C.; Hoffman, J.C.; Hahn, M.G.; Staehelin, L.A. The herbicide dichlobenil disrupts cell plate formation: Immunogold characterization. Protoplasma 1996, 194, 117–132. [Google Scholar] [CrossRef]
- Renzaglia, K.S.; Garbary, D.J. Motile Gametes of Land Plants: Diversity, Development, and Evolution. Crit. Rev. Plant Sci. 2001, 20, 107–213. [Google Scholar] [CrossRef]
- Lopez, R.A.; Renzaglia, K.S. The Ceratopteris (fern) developing motile gamete walls contain diverse polysaccharides, but not pectin. Planta 2018, 247, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Schuette, S.; Wood, A.J.; Geisler, M.; Geisler-Lee, J.; Ligrone, R.; Renzaglia, K.S. Novel localization of callose in the spores of Physcomitrella patens and phylogenomics of the callose synthase gene family. Ann. Bot. 2009, 103, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.-G.; Dai, X.-L.; Zou, H.-M.; Wang, Q.-X. Formation and development of rhizoids of the liverwort Marchantia polymorpha1. J. Torrey Bot. Soc. 2014, 141, 126–134. [Google Scholar] [CrossRef]
- Tang, C.T.C. The Wound Response in Arabidopsis thaliana and Physcomitrella patens. Ph.D. Thesis, Rutgers University-Graduate School-New Brunswick, New Brunswick, NJ, USA, 2007. [Google Scholar]
- Berry, E.A.; Tran, M.L.; Dimos, C.S.; Budziszek, M.J.; Scavuzzo-Duggan, T.; Roberts, A.W. Immuno and Affinity Cytochemical Analysis of Cell Wall Composition in the Moss Physcomitrella patens. Front. Plant Sci. 2016, 7, 248. [Google Scholar] [CrossRef]
- Bopp, M.; Quader, H.; Thoni, C.; Sawidis, T.; Schnepf, E. Filament Disruption in Funaria Protonemata: Formation and Disintegration of Tmema Cells. J. Plant Physiol. 1991, 137, 273–284. [Google Scholar] [CrossRef]
- Renzaglia, K.S.; Lopez, R.A.; Johnson, E.E. Callose is integral to the development of permanent tetrads in the liverwort Sphaerocarpos. Planta 2014, 241, 615–627. [Google Scholar] [CrossRef] [PubMed]
- Renzaglia, K.S.; Lopez, R.A.; Welsh, R.D.; Owen, H.A.; Merced, A. Callose in sporogenesis: Novel composition of the inner spore wall in hornworts. Plant Syst. Evol. 2020, 306, 1–9. [Google Scholar] [CrossRef]
- Radford, J.E.; Vesk, M.; Overall, R.L. Callose deposition at plasmodesmata. Protoplasma 1998, 201, 30–37. [Google Scholar] [CrossRef]
- Clausen, M.H.; Willats, W.G.; Knox, J.P. Synthetic methyl hexagalacturonate hapten inhibitors of anti-homogalacturonan monoclonal antibodies LM7, JIM5 and JIM7. Carbohydr. Res. 2003, 338, 1797–1800. [Google Scholar] [CrossRef]
- Mansouri, K. Comparative Ultrastructure of Apical Cells and Derivatives in Bryophytes, with Special Reference to Plasmodesmata. Ph.D. Thesis, Southern Illinois University at Carbondale, Carbondale, IL, USA, 2012. [Google Scholar]
- Johnson, G.P. Early Embryology of Ceratopteris Richardii and Immunocytochemestry of Placental Transfer Cell Wall Ingrowths. Master’s Thesis, Southern Illinois University, Carbondale, IL, USA, 2008. [Google Scholar]
- Vaughn, K.C.; Talbot, M.J.; Offler, C.E.; McCurdy, D.W. Wall Ingrowths in Epidermal Transfer Cells of Vicia faba Cotyledons are Modified Primary Walls Marked by Localized Accumulations of Arabinogalactan Proteins. Plant Cell Physiol. 2006, 48, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Ligrone, R.; Vaughn, K.C.; Rascio, N. A cytochemical and immunocytochemical analysis of the wall labyrinth apparatus in leaf transfer cells in Elodea canadensis. Ann. Bot. 2011, 107, 717–722. [Google Scholar] [CrossRef]
- Matsunaga, T.; Ishii, T.; Matsumoto, S.; Higuchi, M.; Darvill, A.; Albersheim, P.; O’Neill, M.A. Occurrence of the Primary Cell Wall Polysaccharide Rhamnogalacturonan II in Pteridophytes, Lycophytes, and Bryophytes. Implications for the Evolution of Vascular Plants. Plant Physiol. 2004, 134, 339–351. [Google Scholar] [CrossRef]
- Cornuault, V.; Pose, S.; Knox, J.P. Extraction, texture analysis and polysaccharide epitope mapping data of sequential extracts of strawberry, apple, tomato and aubergine fruit parenchyma. Data Brief 2018, 17, 314–320. [Google Scholar] [CrossRef]
- Lee, K.J.; Sakata, Y.; Mau, S.-L.; Pettolino, F.; Bacic, A.; Quatrano, R.S.; Knight, C.D.; Knox, J.P. Diversity in the distribution of polysaccharide and glyco-protein epitopes in the cell walls of bryophytes: New evidence for multiple evolution of water-conducting cells. New Phytol. 2002, 156, 491–508. [Google Scholar]
- Eeckhout, S.; Leroux, O.; Willats, W.G.T.; Popper, Z.; Viane, R.L.L. Comparative glycan profiling of Ceratopteris richardii ‘C-Fern’ gametophytes and sporophytes links cell-wall composition to functional specialization. Ann. Bot. 2014, 114, 1295–1307. [Google Scholar] [CrossRef][Green Version]
- Popper, Z. Primary Cell Wall Composition of Bryophytes and Charophytes. Ann. Bot. 2003, 91, 1–12. [Google Scholar] [CrossRef]
- Moore, J.P. Understanding drought (and desiccation) tolerance in woody perennials: Lessons from a resurrection plant. S. Afr. J. Bot. 2009, 75, 412–413. [Google Scholar] [CrossRef][Green Version]
- Happ, K.; Classen, B. Happ Arabinogalactan-Proteins from the Liverwort Marchantia polymorpha L., a Member of a Basal Land Plant Lineage, Are Structurally Different to Those of Angiosperms. Plants 2019, 8, 460. [Google Scholar] [CrossRef]
- Nguema-Ona, E.; Moore, J.; Fagerström, A.; Fangel, J.U.; Willats, W.; Hugo, A.; Vivier, M.A. Profiling the main cell wall polysaccharides of tobacco leaves using high-throughput and fractionation techniques. Carbohydr. Polym. 2012, 88, 939–949. [Google Scholar] [CrossRef]
- Gaspar, Y.; Johnson, K.L.; McKenna, J.A.; Bacic, A.; Schultz, C.J. The complex structures of arabinogalactan-proteins and the journey towards understanding function. Plant Mol. Biol. 2001, 47, 161–176. [Google Scholar] [CrossRef]
- Majewska-Sawka, A.; Nothnagel, E.A. The Multiple Roles of Arabinogalactan Proteins in Plant Development. Plant Physiol. 2000, 122, 3–10. [Google Scholar] [CrossRef]
- Lamport, D.T.A.; Várnai, P. Periplasmic arabinogalactan glycoproteins act as a calcium capacitor that regulates plant growth and development. New Phytol. 2012, 197, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Offler, C.E.; Patrick, J.W. Transfer cells: What regulates the development of their intricate wall labyrinths? New Phytol. 2020, 228, 427–444. [Google Scholar] [CrossRef]
- Seifert, G.J.; Roberts, K. The Biology of Arabinogalactan Proteins. Annu. Rev. Plant Biol. 2007, 58, 137–161. [Google Scholar] [CrossRef]
- Lamport, D.T.A.; Kieliszewski, M.J.; Showalter, A.M. Salt stress upregulates periplasmic arabinogalactan proteins: Using salt stress to analyse AGP function*. New Phytol. 2005, 169, 479–492. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Motose, H.; Iwamoto, K.; Fukuda, H. Expression and Genome-Wide Analysis of the Xylogen-Type Gene Family. Plant Cell Physiol. 2011, 52, 1095–1106. [Google Scholar] [CrossRef] [PubMed]
- Kremer, C.; Pettolino, F.; Bacic, A.; Drinnan, A. Distribution of cell wall components in Sphagnum hyaline cells and in liverwort and hornwort elaters. Planta 2004, 219, 1023–1035. [Google Scholar] [CrossRef]
- Shibaya, T.; Kaneko, Y.; Sugawara, Y. Involvement of arabinogalactan proteins in protonemata development from cultured cells ofMarchantia polymorpha. Physiol. Plant. 2005, 124, 504–514. [Google Scholar] [CrossRef]
- Shibaya, T.; Sugawara, Y. Involvement of arabinogalactan proteins in the regeneration process of cultured protoplasts of Marchantia polymorpha. Physiol. Plant. 2007, 130, 271–279. [Google Scholar] [CrossRef]
- Shibaya, T.; Sugawara, Y. Induction of multinucleation by β-glucosyl Yariv reagent in regenerated cells from Marchantia polymorpha protoplasts and involvement of arabinogalactan proteins in cell plate formation. Planta 2009, 230, 581–588. [Google Scholar] [CrossRef]
- Lopez-Swalls, R.A. The Special Walls around Gametes in Ceratopteris richardii and Aulacomnium palustre: Using Immunocyto-Chemistry to Expose Structure, Function, and Development. Ph.D. Thesis, Southern Illinois University at Carbondale, Carbondale, IL, USA, 2016. [Google Scholar]
- Dahiya, P.; Brewin, N.J. Immunogold localization of callose and other cell wall components in pea nodule transfer cells. Protoplasma 2000, 214, 210–218. [Google Scholar] [CrossRef]
- Shimamura, M. Marchantia polymorpha: Taxonomy, Phylogeny and Morphology of a Model System. Plant Cell Physiol. 2015, 57, 230–256. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.W.; Roberts, E.M.; Haigler, C.H. Moss cell walls: Structure and biosynthesis. Front. Plant Sci. 2012, 3, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Gunning, B.E. Transfer cells and their roles in transport of solutes in plants. Sci. Prog. 1977, 539–568. [Google Scholar]
- Pate, J.S.; Gunning, B.E.S. Transfer cells. Annu. Rev. Plant. Physiol. 1972, 23, 173–196. [Google Scholar] [CrossRef]
- Offler, C.E.; McCurdy, D.W.; Patrick, J.W.; Talbot, M.J. Transfer cells: Cells Specialized for a Special Purpose. Annu. Rev. Plant Biol. 2003, 54, 431–454. [Google Scholar] [CrossRef]
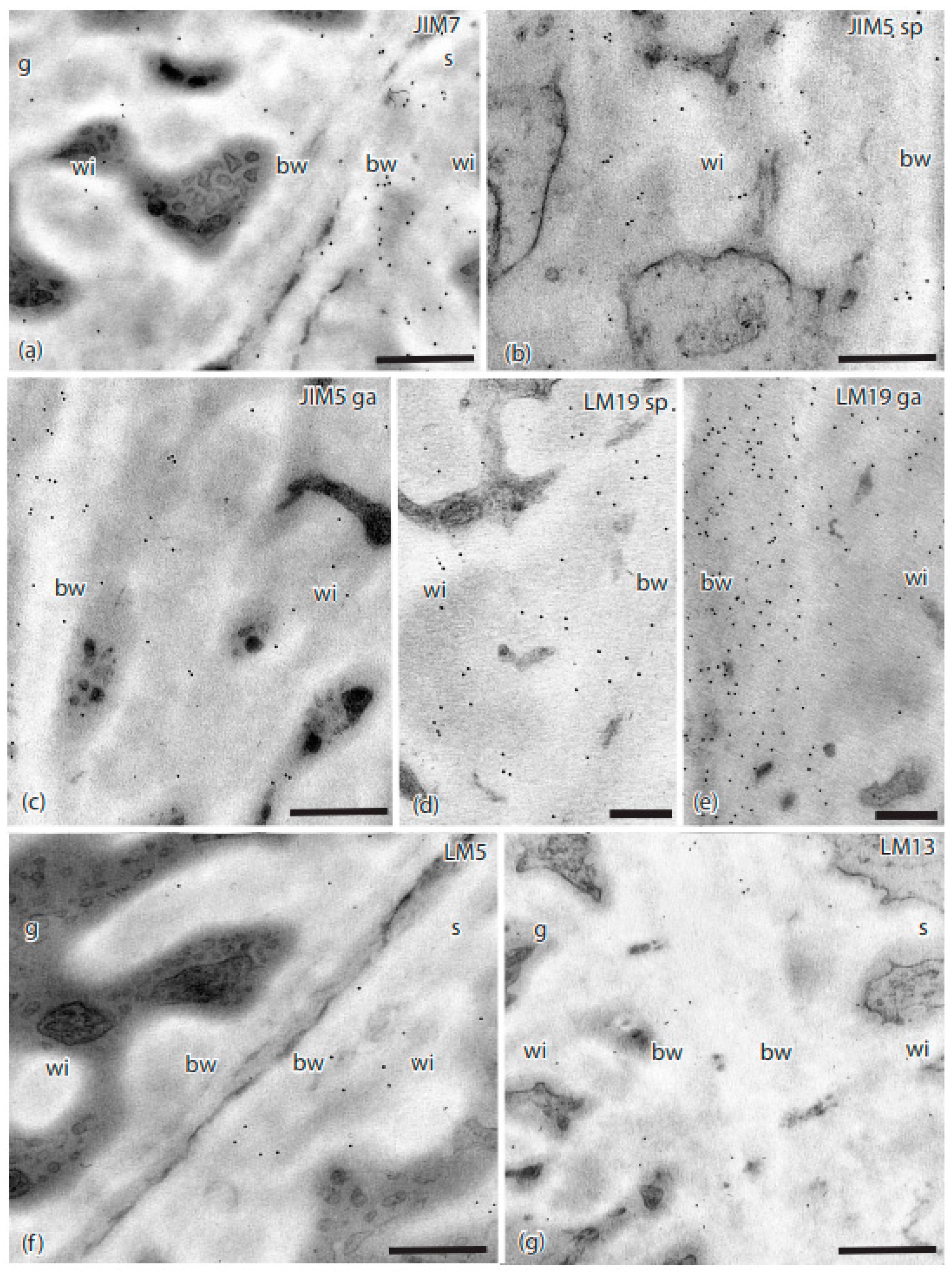
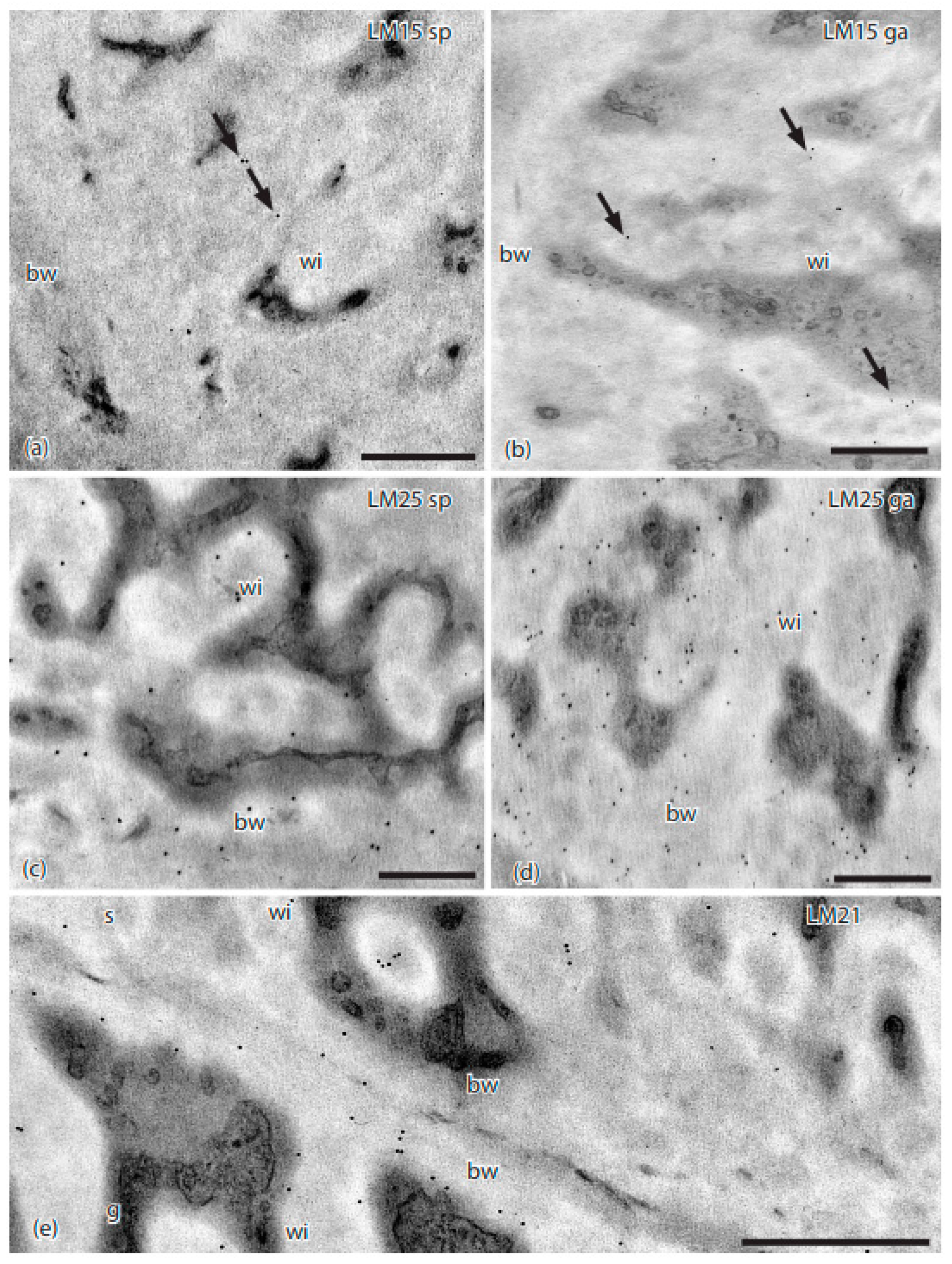

| Antibody | Antigen(s)/Epitope | Reference/Source |
|---|---|---|
| JIM7 | Homogalacturonan/Methyl-esterified | [21] |
| JIM5 | Homogalacturonan/Unesterified | [22] |
| LM19 | Homogalacturonan/Unesterified | [23] |
| LM20 | Homogalacturonan/Methyl-esterified | [23] |
| LM5 | Galactan, rhamnogalacturonan-I/(1-4)-β-d-galactan | [24] |
| LM13 | Arabinan, rhamnogalacturonan-I/(1-5)-α-L-arabinan (linear) | [25] |
| LM15 | XXXG motif of xyloglucan | [26] |
| LM25 | Galactoxylated xyloglucans | [27] |
| LM21 | Mannan/β-(1,4)-manno-oligosaccharide | [28] |
| LM28 | Glucuronoxylan | [29] |
| JIM13 | Arabinogalactan protein (AGP)/β-d-GlcA-(1,3)-α-d-GalpA-(1,2)-l-Rha(glucuronicacid-galacturonicacid-rhamnose) | [30] |
| LM6 | Arabinan, rhamnogalacturonan-I/(1-5)-α-L-arabinan(also labels AGP) | [31] |
| JIM8 | Arabinogalactan protein (AGP)/unknown | [32] |
| LM2 | Arabinogalactan protein (AGP) β-d-GlcA (glucuronic acid) | [33] |
| Anticallose | Callose/(1,3)-β-linked penta-to-hexa-glucan | [34] |
| JIM12 | Extensin | [35] |
| Primary Antibody | Sporophyte | Gametophyte |
|---|---|---|
| JIM7 | ++ | + |
| JIM5 | + | + |
| LM19 | + | ++ |
| LM20 | - | - |
| LM5 | + | ± |
| LM13 | ± | ± |
| LM15 | ± | ± |
| LM25 | ± | + |
| LM21 | + | + |
| LM28 | - | - |
| JIM13 | ± | ± |
| LM6 * | ± | + |
| JIM8 | - | - |
| LM2 | - | - |
| Callose | + | + |
| JIM12 | - | - |
| Cell Wall Polymer | MAbs | Wall Properties | References | |
|---|---|---|---|---|
| Esterified | JIM7, LM20 |
| [23,41,42] | |
| HG Pectin | De-esterified | LM19, JIM5 | Ca2+ binding
| [23,42,43] |
| RG-I Pectin | Arabinan |
|
| [42,43,44,45] |
| Galactan |
|
| [42,44] | |
| Hemicellulose | Xyloglucan |
|
| [41,46,47,48,49,50] |
| Mannan |
|
| [28,51,52,53] | |
| AGP |
|
| [44,50,54,55,56,57,58] | |
| Extensin |
|
| [59,60,61,62] | |
| Callose |
| Stress response
| [25,63,64,65,66,67,68,69,70,71,72,73,74] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Henry, J.S.; Renzaglia, K.S. The Placenta of Physcomitrium patens: Transfer Cell Wall Polymers Compared across the Three Bryophyte Groups. Diversity 2021, 13, 378. https://doi.org/10.3390/d13080378
Henry JS, Renzaglia KS. The Placenta of Physcomitrium patens: Transfer Cell Wall Polymers Compared across the Three Bryophyte Groups. Diversity. 2021; 13(8):378. https://doi.org/10.3390/d13080378
Chicago/Turabian StyleHenry, Jason S., and Karen S. Renzaglia. 2021. "The Placenta of Physcomitrium patens: Transfer Cell Wall Polymers Compared across the Three Bryophyte Groups" Diversity 13, no. 8: 378. https://doi.org/10.3390/d13080378
APA StyleHenry, J. S., & Renzaglia, K. S. (2021). The Placenta of Physcomitrium patens: Transfer Cell Wall Polymers Compared across the Three Bryophyte Groups. Diversity, 13(8), 378. https://doi.org/10.3390/d13080378





