Seasonal Changes in the Photosynthetic Activity of Terrestrial Lichens and Mosses in the Lichen Scots Pine Forest Habitat
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fieldwork with Measurement of the Maximum Quantum Efficiency of PSII Photochemistry in Lichens and Mosses
2.2. Statistical Analysis
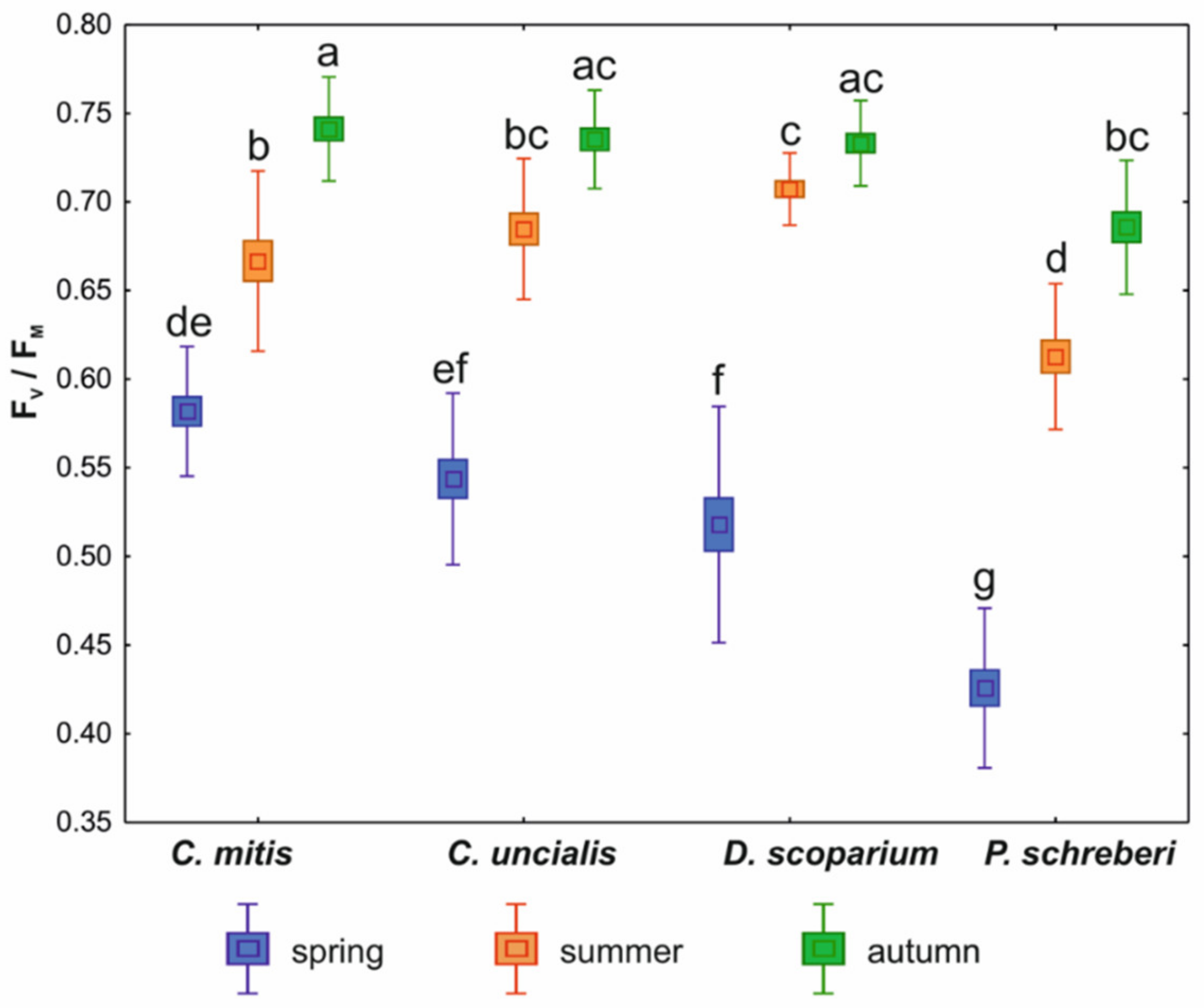
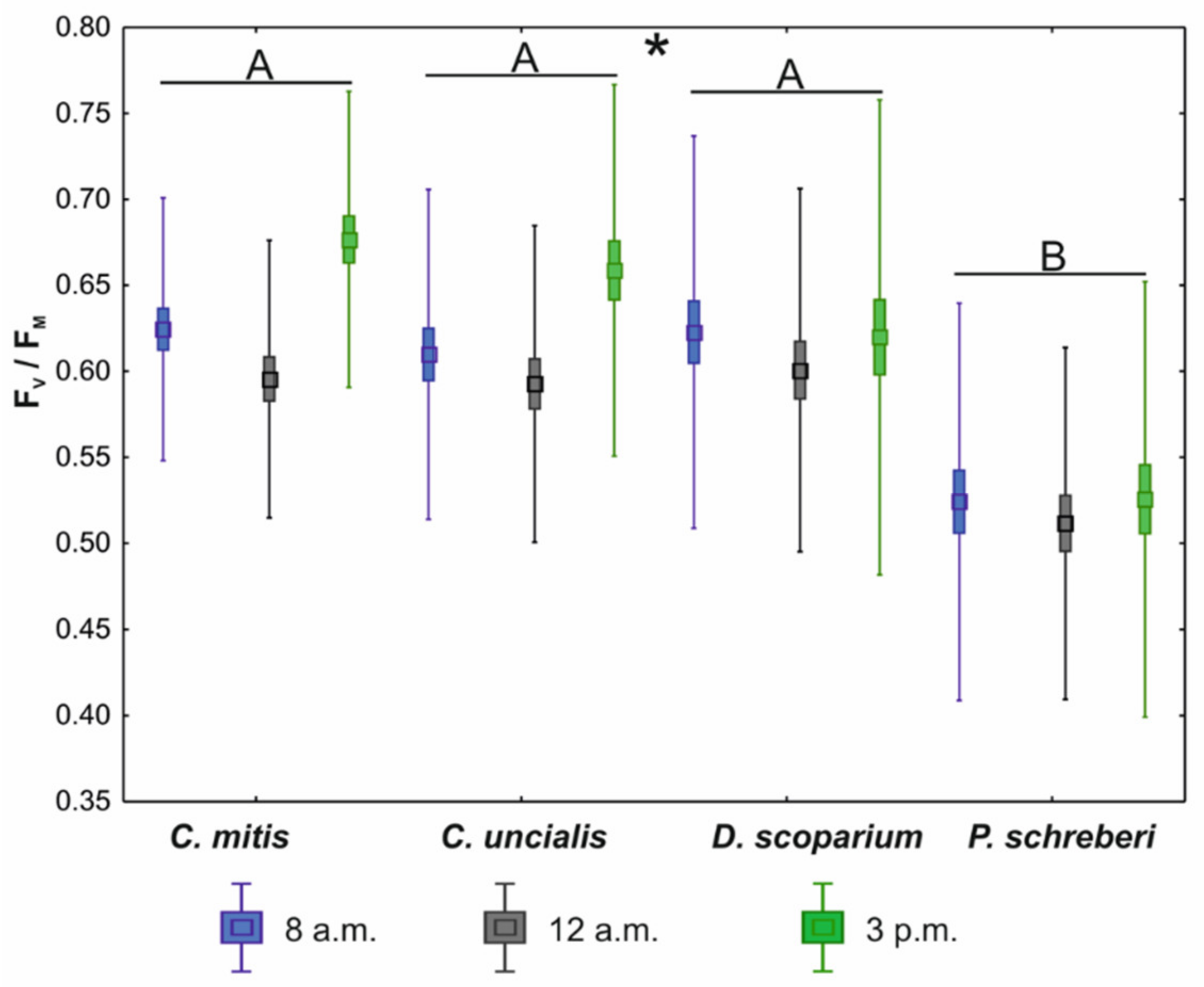
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Noss, R.F. Assessing and monitoring forest biodiversity: A suggested framework and indicators. For. Ecol. Manag. 1999, 115, 135–146. [Google Scholar] [CrossRef]
- McCune, B. Lichen communities as indicators of forest health. Bryologist 2000, 103, 353–356. [Google Scholar] [CrossRef]
- Will-Wolf, S.; Esseen, P.A.; Neitlich, P. Monitoring biodiversity and ecosystem function: Forests. In Monitoring with Lichens–Monitoring Lichens; Springer: Dordrecht, The Netherlands, 2002; pp. 203–222. [Google Scholar] [CrossRef]
- Aber, J.; Neilson, R.P.; McNulty, S.; Lenihan, J.M.; Bachelet, D.; Drapek, R.J. Forest processes and global environmental change: Predicting the effects of individual and multiple stressors: We review the effects of several rapidly changing environmental drivers on ecosystem function, discuss interactions among them, and summarize predicted changes in productivity, carbon storage, and water balance. BioScience 2001, 51, 735–751. [Google Scholar] [CrossRef]
- Lowman, M.D.; Schowalter, T.D. Plant science in forest canopies–the first 30 years of advances and challenges (1980–2010). New Phytol. 2012, 194, 12–27. [Google Scholar] [CrossRef] [PubMed]
- Russell, B.D.; Connell, S.D. Origins and consequences of global and local stressors: Incorporating climatic and non-climatic phenomena that buffer or accelerate ecological change. Mar. Biol. 2012, 159, 2633–2639. [Google Scholar] [CrossRef]
- Valladares, F.; Laanisto, L.; Niinemets, Ü.; Zavala, M.A. Shedding light on shade: Ecological perspectives of understory plant life. Plant Ecol. Divers. 2016, 9, 237–251. [Google Scholar] [CrossRef] [Green Version]
- Alba, C.; Fahey, C.; Flory, S.L. Global change stressors alter resources and shift plant interactions from facilitation to competition over time. Ecology 2019, 100, e02859. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Vegetation stress: An introduction to the stress concept in plants. J. Plant Physiol. 1996, 148, 4–14. [Google Scholar] [CrossRef]
- Hofmann, G.E.; Todgham, A.E. Living in the now: Physiological mechanisms to tolerate a rapidly changing environment. Annu. Rev. Genet. 2010, 72, 127–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rejeb, I.B.; Pastor, V.; Mauch-Mani, B. Plant responses to simultaneous biotic and abiotic stress: Molecular mechanisms. Plants 2014, 3, 458–475. [Google Scholar] [CrossRef]
- Pereira, A. Plant abiotic stress challenges from the changing environment. Front. Plant Sci. 2016, 7, 1123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raza, A.; Ashraf, F.; Zou, X.; Zhang, X.; Tosif, H. Plant adaptation and tolerance to environmental stresses: Mechanisms and perspectives. In Plant Ecophysiology and Adaptation under Climate Change: Mechanisms and Perspectives I; Springer: Singapore, 2020; pp. 117–145. [Google Scholar] [CrossRef]
- William, W.A., III; Deming-Adams, B. Chlorophyll Fluorescence as a Tool to Monitor Plant Response to the Environment. In Chlorophyll a Fluorescence; Springer: Dordrecht, The Netherlands, 2004; pp. 583–604. [Google Scholar] [CrossRef]
- Bolhàr-Nordenkampf, H.R.; Öquist, G. Chlorophyll fluorescence as a tool in photosynthesis research. In Photosynthesis and Production in a Changing Environment; Springer: Dordrecht, The Netherlands, 1993; pp. 193–206. [Google Scholar] [CrossRef]
- Cosgrove, J.; Borowitzka, M.A. Chlorophyll fluorescence terminology: An introduction. In Chlorophyll a Fluorescence in Aquatic Sciences: Methods and Applications; Springer: Dordrecht, The Netherlands, 2010; pp. 1–17. [Google Scholar] [CrossRef]
- Nikolić, B.R.; Pavlović, D.M.; Đurović, S.; Waisi, H.; Marisavljević, D.; Anđelković, A. Chlorophyll as a measure of plant health: Agroecological aspects. Pestic. Fitomed. 2014, 29, 21–34. [Google Scholar]
- Adak, M.K. Analysis of chlorophyll fluorescence: A reliable technique in determination of stress on plants. In Eco-friendly Agro-biological Techniques for Enhancing Crop Productivity; Springer: Singapore, 2018; pp. 63–88. [Google Scholar] [CrossRef]
- McCree, K.J. The measurement of photosynthetically active radiation. Solar Energy 1973, 15, 83–87. [Google Scholar] [CrossRef]
- McCree, K.J. Photosynthetically active radiation. In Physiological Plant Ecology I; Springer: Berlin/Heidelberg, Germany, 1981; pp. 4–155. [Google Scholar] [CrossRef]
- Wandji Nyamsi, W.; Espinar, B.; Blanc, P.; Wald, L. Estimating the photosynthetically active radiation under clear skies by means of a new approach. Adv. Sci. Res. 2015, 12, 5–10. [Google Scholar] [CrossRef] [Green Version]
- Zhen, S.; Bugbee, B. Far-red photons have equivalent efficiency to traditional photosynthetic photons: Implications for redefining photosynthetically active radiation. Plant Cell Environ. 2020, 43, 1259–1272. [Google Scholar] [CrossRef] [PubMed]
- Grossman, A.R.; Bhaya, D.; Apt, K.E.; Kehoe, D.M. Light-harvesting complexes in oxygenic photosynthesis: Diversity, control, and evolution. Annu. Rev. Genet. 1995, 29, 231–288. [Google Scholar] [CrossRef] [PubMed]
- Schmid, V.H. Light-harvesting complexes of vascular plants. Cell. Mol. Life Sci. 2008, 65, 361–3639. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Schansker, G.; Ladle, R.J.; Goltsev, V.; Bosa, K.; Allakhverdiev, S.; Brestic, M.; Bussotti, F.; Calatayud, A.; Dąbrowski, P.; et al. Frequently asked questions about in vivo chlorophyll fluorescence: Practical issues. Photosynth. Res. 2014, 122, 121–158. [Google Scholar] [CrossRef] [Green Version]
- Kautsky, H.; Hirsch, A. Neue versuche zur kohlensäureassimilation. Naturwissenschaften 1931, 19, 964. [Google Scholar] [CrossRef]
- Chukhutsina, V.U.; Holzwarth, A.R.; Croce, R. Time-resolved fluorescence measurements on leaves: Principles and recent developments. Photosynth. Res. 2019, 140, 355–369. [Google Scholar] [CrossRef] [PubMed]
- Rola, K.; Latkowska, E.; Myśliwa-Kurdziel, B.; Osyczka, P. Heavy-metal tolerance of photobiont in pioneer lichens inhabiting heavily polluted sites. Sci. Total Environ. 2019, 679, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Coe, R.A.; Lin, H.C. Light-Response Curves in Land Plants. Photosynthesis 2018, 1770, 83–94. [Google Scholar] [CrossRef]
- Longton, R.E. The role of bryophyte and lichens in terrestrial ecosystems. In Bryophyte and Lichens in a Changing Environment Oxford; Bates, J.W., Farmer, A.M., Eds.; Clarendon Press: Oxford, UK, 1992; pp. 32–76. [Google Scholar]
- Sveinbjörnsson, B.; Sonesson, M. Photosynthesis and respiration in mosses and lichens. Glob. Change Arctic Terrest. Ecosyst. 1997, 124, 113–128. [Google Scholar] [CrossRef]
- Lange, O.; Green, T.G. Photosynthetic performance of a foliose lichen of biological soil-crust communities: Long-term monitoring of the CO2 exchange of Cladonia convoluta under temperate habitat conditions. Bibl. Lichenol. 2003, 86, 257–280. [Google Scholar]
- Veres, K.; Farkas, E.; Csintalan, Z. The bright and shaded side of duneland life: The photosynthetic response of lichens to seasonal changes is species-specific. Mycol. Prog. 2020, 19, 629–641. [Google Scholar] [CrossRef]
- Tuba, Z.; Csintalan, Z.; Proctor, M.C. Photosynthetic responses of a moss Tortula ruralis ssp. ruralis, and the lichens Cladonia convoluta and C. furcata to water deficit and short periods of desiccation and their ecophysiological significance: A baseline study at present-day concentration. New Phytol. 1996, 133, 353–361. [Google Scholar] [CrossRef]
- Węgrzyn, M.H.; Fałowska, P.; Kołodziejczyk, J.; Alzayany, K.; Wężyk, P.; Zięba-Kulawik, K.; Hawryło, P.; Turowska, A.; Grzesiak, B.; Lipnicki, L.; et al. Tree height as the main factor causing disappearance of the terricolous lichens in the lichen Scots pine forests. Sci. Total Environ. 2021, 771, 144834. [Google Scholar] [CrossRef] [PubMed]
- Mangerud, J.; Jakobsson, M.; Alexanderson, H.; Astakhov, V.; Clarke, G.K.; Henriksen, H.C.; Krinner, G.; Lunkka, J.P.; Möller, P.; Murray, A.; et al. Ice-dammed lakes and rerouting of the drainage of northern Eurasia during the Last Glaciation. Quat. Sci. Rev. 2004, 23, 1313–1332. [Google Scholar] [CrossRef]
- Słowiński, M.; Błaszkiewicz, M.; Brauer, A.; Noryśkiewicz, B.; Ott, F.; Tyszkowski, S. The role of melting dead ice on landscape transformation in the early Holocene in Tuchola Pinewoods, North Poland. Quat. Int. 2015, 388, 64–75. [Google Scholar] [CrossRef]
- Directive, H. Council Directive 92/43/EEC of 21 May 1992 on the conservation of natural habitats and of wild fauna and flora. Off. J. Europ Commun. 1992, 206, 7–50. [Google Scholar]
- Kolbek, J.; Chytrý, M.; Kučera, T.; Kočí, M.; Grulich, V.; Lustyk, P. Suché Bory—dry pine forests. In Katalog Biotopu Cēské Republiky, 2nd ed.; Agentura Ochrany Přírody a Krajiny ČR: Prague, Czech Republic, 2010; pp. 331–334. [Google Scholar]
- Węgrzyn, M.H.; Kołodziejczyk, J.; Fałowska, P.; Wężyk, P.; Zięba-Kulawik, K.; Szostak, M.; Turowska, A.; Grzesiak, B.; Wietrzyk-Pełka, P. Influence of the environmental factors on the species composition of lichen Scots pine forests as a guide to maintain the community (Bory Tucholskie National Park, Poland). Glob. Ecol. Conserv. 2020, 22C, e01017. [Google Scholar] [CrossRef]
- Tobias, M.; Niinemets, Ü. Acclimation of photosynthetic characteristics of the moss Pleurozium schreberi to among-habitat and within-canopy light gradients. Plant Biol. 2010, 12, 743–754. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Rastogi, A.; Živčák, M.; Brestic, M.; Daszkowska-Golec, A.; Sitko, K.; Alsharafa, K.Y.; Lotfi, R.; Stypiński, P.; Samborska, I.A.; et al. Prompt chlorophyll fluorescence as a tool for crop phenotyping: An example of barley landraces exposed to various abiotic stress factors. Photosynthetica 2018, 56, 953–961. [Google Scholar] [CrossRef] [Green Version]
- Schansker, G.; Tóth, S.Z.; Strasser, R.J. Dark-recovery of the Chl a fluorescence transient (OJIP) after light adaptation: The qT- component of non-photochemical quenching is related to an activated photosystem I acceptor side. Biochim. Biophys. Acta 2006, 1757, 787–797. [Google Scholar] [CrossRef] [Green Version]
- Angelini, G.; Ragni, P.; Esposito, D.; Giardi, P.; Pompili, M.L.; Moscardelli, R.; Giardi, M.T. A device to study the effect of space radiation on photosynthetic organisms. Physica Med. 2001, 17, 267–268. [Google Scholar]
- Garty, J. Biomonitoring atmospheric heavy metals with lichens: Theory and application. Crit. Rev. Plant Sci. 2001, 20, 309–371. [Google Scholar] [CrossRef]
- Dzubaj, A.; Bačkor, M.; Tomko, J.; Peli, E.; Tuba, Z. Tolerance of the lichen Xanthoria parietina (L.) Th. Fr. to metal stress. Ecotox. Environ. Saf. 2008, 70, 319–326. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Gauslaa, Y.; Solhaug, K.A. High-light damage in air-dry thalli of the old forest lichen Lobaria pulmonaria—interactions of irradiance, exposure duration and high temperature. J. Exper. Bot. 1999, 50, 697–705. [Google Scholar] [CrossRef]
- Palmqvist, K.; Sundberg, B. Light use efficiency of dry matter gain in five macro-lichens: Relative impact of microclimate conditions and species-specific traits. Plant Cell Environ. 2000, 23, 1–14. [Google Scholar] [CrossRef]
- Singh, R.; Ranjan, S.; Nayaka, S.; Pathre, U.V.; Shirke, P.A. Functional characteristics of a fruticose type of lichen, Stereocaulon foliolosum Nyl. in response to light and water stress. Act. Physiol. Plant. 2013, 35, 1605–1615. [Google Scholar] [CrossRef]
- Wietrzyk-Pełka, P.; Rola, K.; Patchett, A.; Szymański, W.; Węgrzyn, M.H.; Björk, R.G. Patterns and drivers of cryptogam and vascular plant diversity in glacier forelands. Sci. Total Environ. 2021, 770, 144793. [Google Scholar] [CrossRef] [PubMed]
- Wietrzyk-Pełka, P.; Cykowska-Marzencka, B.; Maruo, F.; Szymański, W.; Węgrzyn, M.H. Mosses and liverworts in the glacier forelands and mature tundra of Svalbard (High Arctic): Diversity, ecology, and community composition. Pol. Polar Res. 2020, 41, 37–64. [Google Scholar] [CrossRef]
- Wietrzyk-Pełka, P.; Rola, K.; Szymański, W.; Węgrzyn, M.H. Organic carbon accumulation in the glacier forelands with regard to variability of environmental conditions in different ecogenesis stages of High Arctic ecosystems. Sci. Total Environ. 2020, 717, 135151. [Google Scholar] [CrossRef] [PubMed]
- Phinney, N.H.; Solhaug, K.A.; Gauslaa, Y. Photobiont-dependent humidity threshold for chlorolichen photosystem II activation. Planta 2019, 250, 2023–2031. [Google Scholar] [CrossRef] [PubMed]
- Dilks, T.J.; Proctor, M.C. Seasonal variation in desiccation tolerance in some British bryophyte. J. Bryol. 1976, 9, 239–247. [Google Scholar] [CrossRef]
- Proctor, M.C. Experiments on the effect of different intensities of desiccation on bryophyte survival, using chlorophyll fluo- rescence as an index of recovery. J. Bryol. 2003, 25, 201–210. [Google Scholar] [CrossRef]
- Green, T.A.; Sancho, L.G.; Pintado, A. Ecophysiology of desiccation/rehydration cycles in mosses and lichens. Plant Desiccation Toler. 2011, 215, 89–120. [Google Scholar] [CrossRef]
- Csintalan, Z.; Péli, E.R. Seasonality and Small Spatial-Scale Variation of Chlorophyll a Fluorescence in Bryophyte Syntrichia ruralis [Hedw.] in Semi-Arid Sandy Grassland, Hungary. Plants 2020, 9, 92. [Google Scholar] [CrossRef] [Green Version]
- Baruffo, L.; Tretiach, M. Seasonal variations of Fo, Fm and Fv/Fm in an epiphytic population of the lichen Punctelia subrudecta (Nyl.) Krog. Lichenologist 2007, 39, 555–565. [Google Scholar] [CrossRef]
- Čabrajic, A.V.; Liden, M.; Lundmark, T.; Palmqvist, K. Modelling hydration and photosystem II activation in relation to in situ rain and humidity patterns: A tool to compare performance of rare and generalist epi- phytic lichens. Plant Cell Environ. 2010, 33, 840–850. [Google Scholar] [CrossRef]
- Moser, T.J.; Nash, T.H., III; Link, S.O. Diurnal gross photosynthetic patterns and potential seasonal CO2 assimilation in Cladonia stellaris and Cladonia rangiferina. Canad. J. Bot. 1983, 61, 642–655. [Google Scholar] [CrossRef]
- Glime, J.M. (Ed.) Adaptive Strategies: Growth and Life Forms. In Bryophyte Ecology; Michigan Technological University: Houghton, MI, USA, 2017; Volume 1: Physiological Ecology, Chapters 4–5. [Google Scholar]
- Lange, O.L.; Kilian, E.; Ziegler, H. Water vapour uptake and photosynthesis of lichens: Performance differences in species with green and blue-green algae as phycobionts. Oecologia 1986, 71, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Lange, O.L.; Bilger, W.; Rimke, S.; Schreiber, U. Chlorophyll fluorescence of lichens containing green and blue-green algae during hydration by water vapor uptake and by addition of liquid water. Bot. Acta 1989, 102, 306–313. [Google Scholar] [CrossRef]
- Smith, C.W. Lichens of Great Britain and Ireland; British Lichen Society: London, UK, 2009. [Google Scholar]


| Variables | F | p | |
|---|---|---|---|
| (a) | Species | 16.238 | <0.001 |
| Sample type | 37.091 | <0.001 | |
| Species × sample type | 2.729 | 0.043 | |
| (b) | Species | 55.393 | <0.001 |
| Season | 543.092 | <0.001 | |
| Species × season | 5.626 | <0.001 | |
| (c) | Species | 28.891 | <0.001 |
| Daytime | 7.416 | <0.001 | |
| Species × daytime | 1.247 | 0.281 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Węgrzyn, M.H.; Fałowska, P.; Alzayany, K.; Waszkiewicz, K.; Dziurowicz, P.; Wietrzyk-Pełka, P. Seasonal Changes in the Photosynthetic Activity of Terrestrial Lichens and Mosses in the Lichen Scots Pine Forest Habitat. Diversity 2021, 13, 642. https://doi.org/10.3390/d13120642
Węgrzyn MH, Fałowska P, Alzayany K, Waszkiewicz K, Dziurowicz P, Wietrzyk-Pełka P. Seasonal Changes in the Photosynthetic Activity of Terrestrial Lichens and Mosses in the Lichen Scots Pine Forest Habitat. Diversity. 2021; 13(12):642. https://doi.org/10.3390/d13120642
Chicago/Turabian StyleWęgrzyn, Michał H., Patrycja Fałowska, Karima Alzayany, Karolina Waszkiewicz, Patrycja Dziurowicz, and Paulina Wietrzyk-Pełka. 2021. "Seasonal Changes in the Photosynthetic Activity of Terrestrial Lichens and Mosses in the Lichen Scots Pine Forest Habitat" Diversity 13, no. 12: 642. https://doi.org/10.3390/d13120642
APA StyleWęgrzyn, M. H., Fałowska, P., Alzayany, K., Waszkiewicz, K., Dziurowicz, P., & Wietrzyk-Pełka, P. (2021). Seasonal Changes in the Photosynthetic Activity of Terrestrial Lichens and Mosses in the Lichen Scots Pine Forest Habitat. Diversity, 13(12), 642. https://doi.org/10.3390/d13120642






