Influence of Geographical and Climatic Factors on Quercus variabilis Blume Fruit Phenotypic Diversity
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sample Collection
2.3. Geographic Information and Climate Data of the Sample Plot
2.4. Determination of Fruit Morphological Characters
2.5. Statistical Analysis
3. Results
3.1. Fruit Morphological Characters and Variation Characteristics
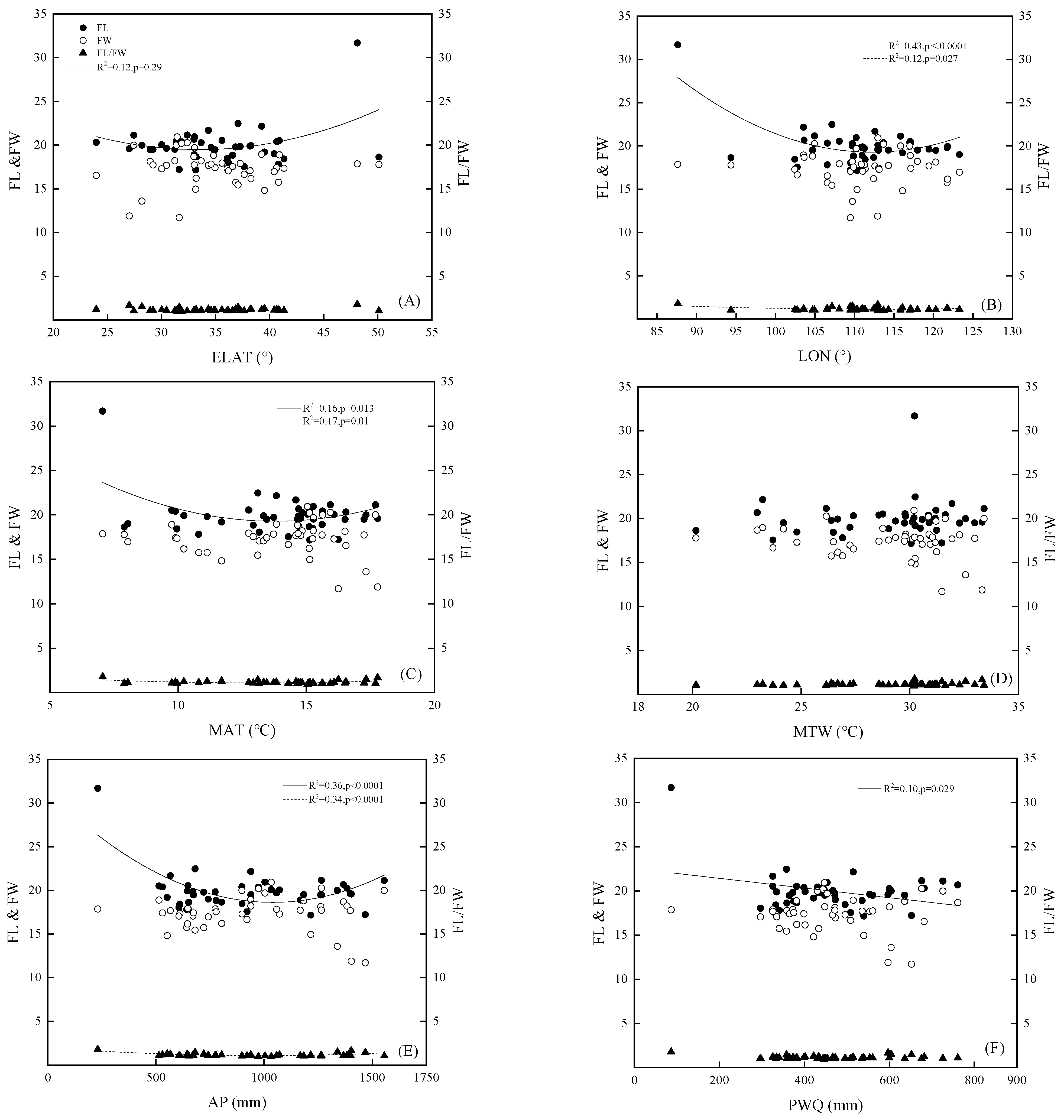
3.2. The Relationship in the Variation Pattern between Fruit Morphology and Geographical and Environmental Factors
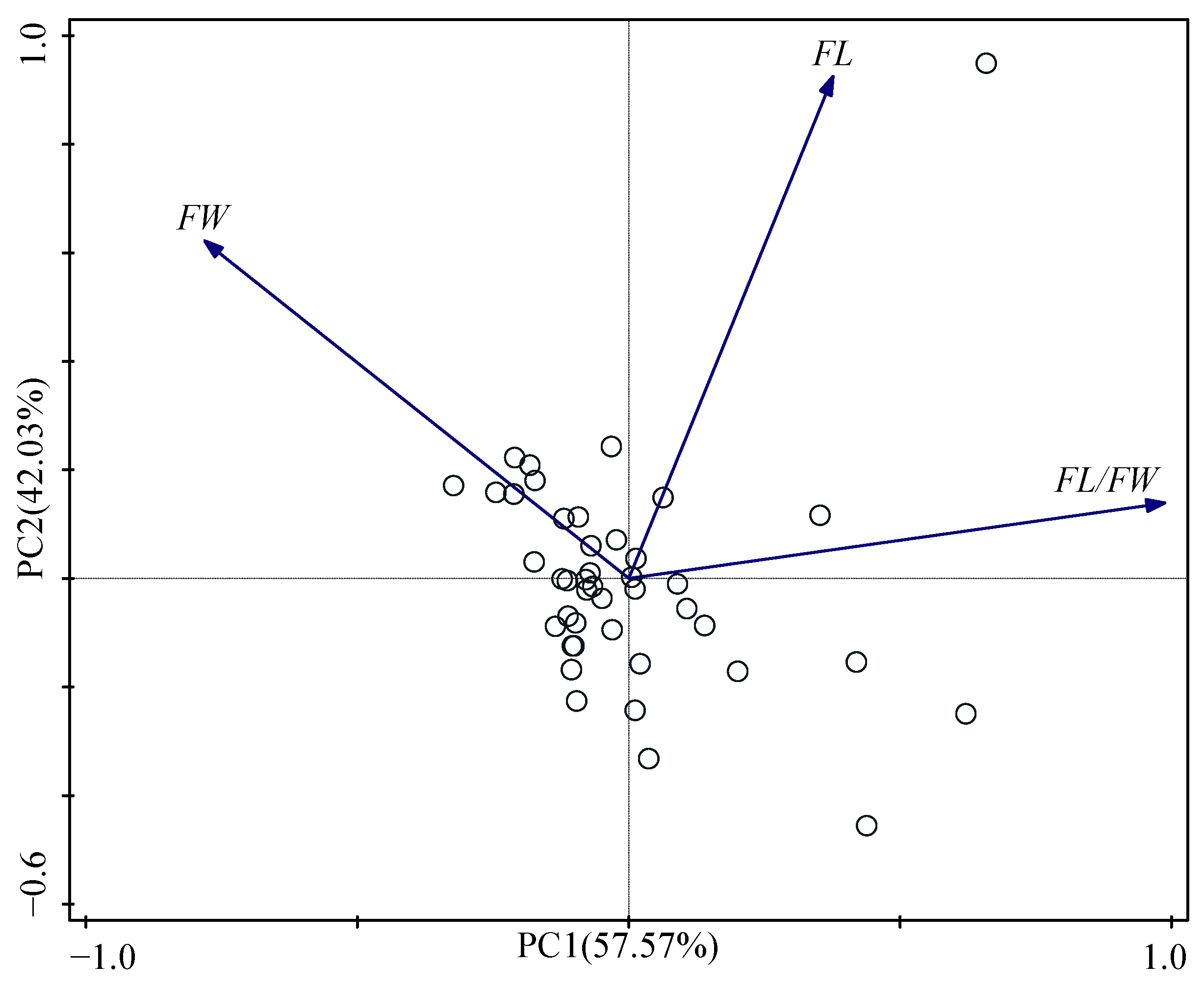
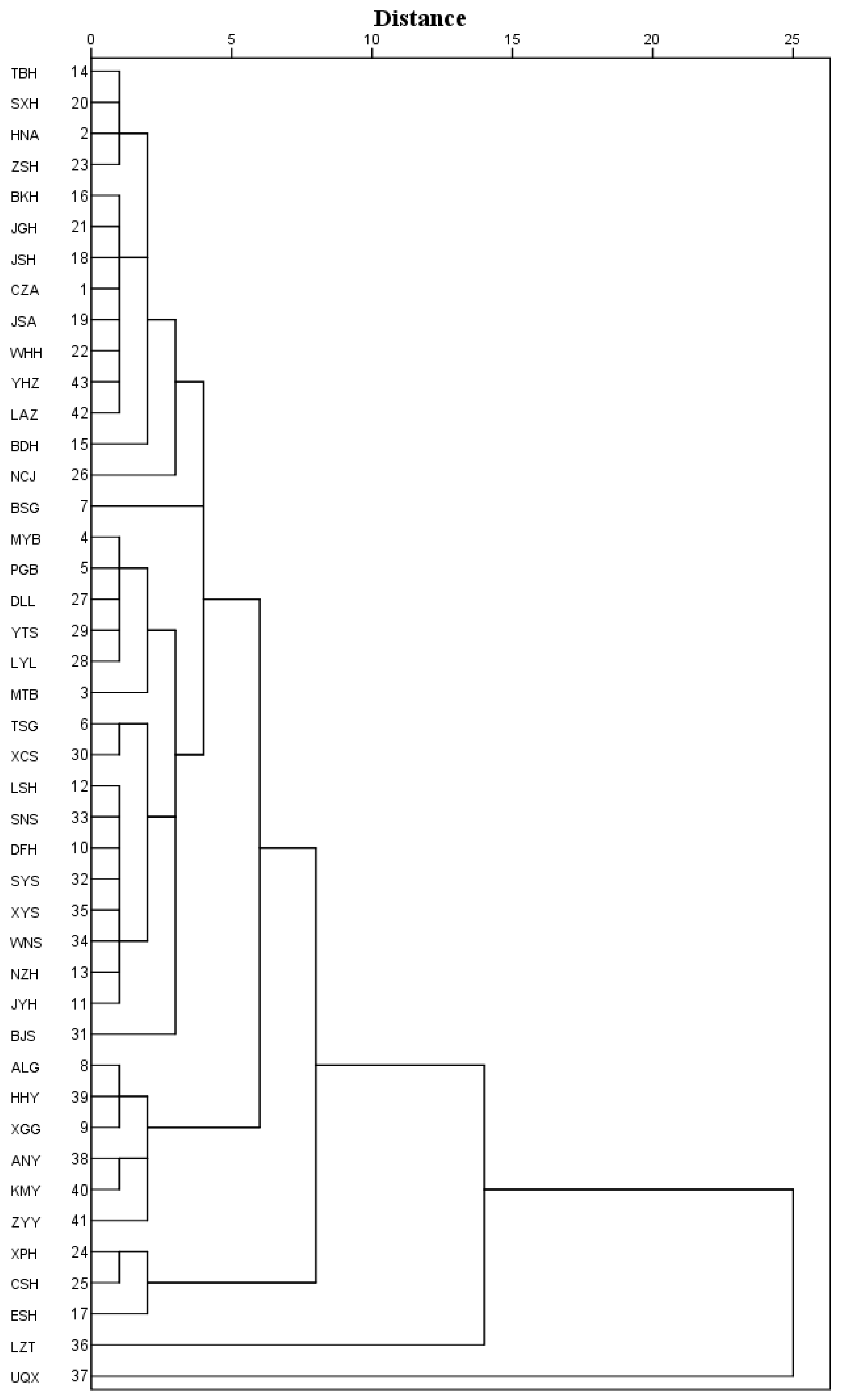
3.3. Principal Component Analysis and Cluster Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Y.; Li, S.; Lu, X.H.; Wang, Q.Q.; Han, H.Y.; Zhang, X.M.; Ma, Y.H.; Gan, X.H. Leaf phenotypic variation of endangered plant Tetracentron sinense Oliv. And influence of geographical and climatic factors. J. For. Res. 2020, 32, 623–636. [Google Scholar] [CrossRef]
- Alcántara-Ayala, O.; Oyama, K.; Ríos-Muñoz, C.; Rivas, G.; Ramirez-Barahona, S.; Luna-Vega, I. Morphological variation of leaf traits in the Ternstroemia lineata species complex (Ericales: Penthaphylacaceae) in response to geographic and climatic variation. PeerJ 2020, 8, e830. [Google Scholar] [CrossRef]
- Thomas, C.L.; Alcock, T.D.; Graham, N.S.; Hayden, R.; Matterson, S.; Wilson, L.; Young, S.D.; Dupuy, L.X.; White, P.J.; Hammond, J.P.; et al. Root morphology and seed and leaf ionomic traits in a Brassica napus L. diversity panel show wide phenotypic variation and are characteristic of crop habit. BMC Plant Biol. 2016, 16, 214. [Google Scholar] [CrossRef]
- Jasińska, A.K.; Rucińska, B.; Kozlowski, G.; Bétrisey, S.; Safarov, H.; Boratyńska, K.; Boratyński, A. Morphological differentiation of leaves in the relict tree Zelkova carpinifolia (Ulmaceae). Dendrobiology 2015, 74, 109–122. [Google Scholar] [CrossRef][Green Version]
- Miljković, D.; Čortan, D. Morphometric and morphological analysis of Populus nigra L. leaves in flooded regions. Sumar. List 2020, 144, 139–147. [Google Scholar] [CrossRef]
- Poljak, I.; Kajba, D.; Ljubić, I.; Idžojtić, M. Morphological variability of leaves of Sorbus domestica L. in Croatia. Acta Soc. Bot. Pol. 2015, 84, 249–259. [Google Scholar] [CrossRef]
- Gómez, J.M.; Perfectti, F.; Armas, C.; Narbona, E.; González-Megías, A.; Navarro, L.; DeSoto, L.; Torices, R. Within-individual phenotypic plasticity in flowers fosters pollination niche shift. Nat. Commun. 2020, 11, 4019. [Google Scholar] [CrossRef] [PubMed]
- Daničić, V.; Kovačević, B.; Ballian, D. Variability in fruit morphology of European sweet chestnut (Castanea sativa Mill.) in natural populations in Bosnia and Herzegovina. Sumar. List 2018, 142, 517–528. [Google Scholar] [CrossRef]
- Pluess, A.R.; Schütz, W.; Stocklin, J. Seed weight increases with altitude in the Swiss Alps between related species but not populations of individual species. Oecologia 2005, 144, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Yi, F.Y.; Wang, Z.R.; Baskin, C.C.; Baskin, J.M.; Ye, R.H.; Sun, H.L.; Zhang, Y.Y.; Ye, X.H.; Liu, G.F.; Yang, X.J.; et al. Seed germination responses to seasonal temperature and drought stress are species-specific but not related to seed size in a desert steppe: Implications for effect of climate change on community structure. Ecol. Evol. 2019, 9, 2149–2159. [Google Scholar] [CrossRef] [PubMed]
- Wirth, L.R.; Graf, R.; Gugerli, F.; Landergott, U.; Holdergger, R. Between-year variation in seed weights across altitudes in the high-alpine plant Eritrichium nanum. Plant Ecol. 2010, 207, 227–231. [Google Scholar] [CrossRef]
- Zhou, X.; He, Z.B.; Kang, H.Z.; Sun, X.; Liu, C.J. Variations of seed morphology related to climate for Quercus variabilis across temperate subtropical China. Chin. J. Plant Ecol. 2013, 37, 481–491. [Google Scholar] [CrossRef]
- Liu, M.L.; Yu, W.B.; Li, D.Z.; Mill, R.; Wang, H. Seed morphological diversity of Pedicularis (Orobanchaceae) and its taxonomic significance. Plant Syst. Evol. 2013, 299, 1645–1657. [Google Scholar] [CrossRef]
- Kaliniewicz, Z.; Markowski, P.; Anders, A.; Jadwisieńczak, K.; Żuk, Z.; Krzysiak, Z. Physical properties of seeds of eleven fir species. Forests 2019, 10, 142. [Google Scholar] [CrossRef]
- Velázquez-Rosas, N.; Ruiz-Guerra, B.; Sánchez-Coronado, M.E.; Buen, A.G.; Orozco-Segovia, A. Morphological variation in fruits and seeds of Ceiba aesculifolia and its relationship with germination and seedling biomass. Bot. Sci. 2017, 95, 81–91. [Google Scholar] [CrossRef]
- Kijowska-Oberc, J.; Staszak, A.M.; Wawrzyniak, M.K.; Ratajczak, E. Changes in proline levels during seed development of orthodox and recalcitrant seeds of genus Ace in a climate change scenario. Forests 2020, 11, 1362. [Google Scholar] [CrossRef]
- Liu, G.F.; Zang, R.G.; Liu, H.; Bai, Z.Q.; Guo, Z.J.; Ding, Y. Geographic variation of seed morphological traits of Picea schrenkiana var. tianschanica in Tianshan Mountains, Xinjiang of Northwest China. Chin. J. Appl. Ecol. 2012, 23, 1455–1461. [Google Scholar]
- Wang, Y.J.; Wang, J.J.; Lai, L.M.; Jiang, L.H.; Zhuang, P.; Zhang, L.H.; Zheng, Y.R.; Jerry, M.; Baskin, J.M.; Baskin, C.C. Geographic variation in seed traits within and among forty-two species of Rhododendron (Ericaceae) on the Tibetan plateau: Relationships with altitude, habitat, plant height, and phylogeny. Ecol. Evol. 2014, 4, 1913–1923. [Google Scholar] [CrossRef]
- Maranz, S.; Wiesman, Z. Evidence for indigenous selection and distribution of the shea tree, Vitellaria paradoxa and its potential significance to prevailing parkland savanna tree patterns in sub-Saharan Africa north of the equator. J. Biogeogr. 2003, 30, 1505–1516. [Google Scholar] [CrossRef]
- Wu, H.; Meng, H.J.; Wang, S.T.; Wei, X.Z.; Jiang, M.X. Geographic patterns and environmental drivers of seed traits of a relict tree species. For. Ecol. Manag. 2018, 422, 59–68. [Google Scholar] [CrossRef]
- Liu, Z.L.; Yu, M.K.; Ma, Y.; Tang, L.Z.; Fang, S.Z. A trend surface analysis of geographic variation in the traits of seeds and seedlings from different Quercus acutissima provenances. Acta Ecol. Sin. 2011, 31, 6796–6804. [Google Scholar]
- Leal-Sáenz, A.; Waring, K.M.; Menon, M.; Cushman, S.A.; Eckert, A.; Flores-Rentería, L.; Hernández-Díaz, J.C.; López-Sánchez, C.A.; Martínez-Guerrero, J.H.; Wehenkel, C. Morphological differences in Pinus strobiformis across latitudinal and elevational gradients. Front. Plant Sci. 2020, 11, 559–697. [Google Scholar] [CrossRef] [PubMed]
- Poljak, I.; Idžojtić, M.; Zebec, M.; Perković, N. The variability of European sweet chestnut (Castanea sativa Mill.) in the region of northwest Croatia according to morphology of fruits. Sumar. List 2012, 136, 479–488. [Google Scholar]
- Liu, M.S.; Hong, B.G. The distribution of Fagaceae in China and its relationship with climatic and geographic characters. Acta Phytoecol. Sin. 1998, 22, 41–50. [Google Scholar]
- Gao, W.Q.; Liu, J.F.; Xue, M.Z.; Zhang, Y.T.; Gao, Z.H.; Ni, Y.Y.; Wang, X.F.; Jiang, Z.P. Geographical patterns and drivers of growth dynamics of Quercus variabilis. For. Ecol. Manag. 2018, 429, 256–266. [Google Scholar] [CrossRef]
- Yuan, J.; Sun, N.X.; Du, H.M.; Yin, S.; Kang, H.Z.; Umair, M.; Liu, C.J. Roles of metabolic regulation in developing Quercus variabilis acorns at contrasting geologically-derived phosphorus sites in subtropical China. BMC Plant Biol. 2020, 20, 389. [Google Scholar] [CrossRef]
- Shi, W.H.; Villar-Salvador, P.; Li, G.L.; Jiang, X.X. Acorn size is more important than nursery fertilization for outplanting performance of Quercus variabilis container seedlings. Ann. For. Sci. 2019, 76, 1–12. [Google Scholar] [CrossRef]
- Sun, X.; Kang, H.; Chen, H.Y.H.; Björn, B.; Samuel, B.F.; Liu, C. Biogeographic patterns of nutrient resorption from Quercus variabilis Blume leaves across China. Plant Biol. 2016, 18, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.W.; Yang, L.N.; Wang, D.M.; Li, D.W. Structure elucidation and properties of different lignins isolated from acorn shell of Quercus variabilis Bl. Int. J. Biol. Macromol. 2018, 107, 1193–1202. [Google Scholar] [CrossRef]
- Sun, Q.Y.; Dumroese, R.K.; Liu, Y. Container volume and subirrigation schedule influence Quercus variabilis seedling growth and nutrient status in the nursery and field. Scand. J. For. Res. 2018, 33, 560–567. [Google Scholar] [CrossRef]
- Liu, J.F.; Deng, Y.P.; Wang, X.F.; Ni, Y.Y.; Wang, Q.; Xiao, W.F.; Lei, J.P.; Jiang, Z.P.; Li, M.H. The concentration of non-structural carbohydrates, N, and P in Quercus variabilis does not decline toward its northernmost distribution range along a 1500 km transect in China. Front. Plant Sci. 2018, 9, 1444. [Google Scholar] [CrossRef] [PubMed]
- Llanderal-Mendoza, J.; Gugger, P.F.; Oyama, K.; Uribe-Salas, D.; González-Rodríguez, A. Climatic determinants of acorn size and germination percentage of Quercus rugosa (Fagaceae) along a latitudinal gradient in Mexico. Bot. Sci. 2017, 95, 37–45. [Google Scholar] [CrossRef]
- Long, T.J.; Jones, R.H. Seedling growth strategies and seed size effects in fourteen oak species native to different soil moisture habitat. Trees Struct. Funct. 1996, 11, 1–8. [Google Scholar] [CrossRef]
- Hijmans, R.J.; Cameron, S.E.; Parra, J.L.; Jones, P.G.; Jarvis, A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 2005, 25, 1965–1978. [Google Scholar] [CrossRef]
- Slave, C. Using arcmap to create a cadastral database. Case study. J. Inf. Syst. Oper. Manag. 2018, 12, 180–190. [Google Scholar]
- Alena, J.; Gösta, E.; Ingegerd, D.; Jan, I. Studies on frost hardiness of Pinus contorta Dougl. seedlings grown in climate chambers. Studia For. Suec. 1981, 157, 4–47. [Google Scholar]
- Wagstaff, K.; Cardie, C.; Rogers, S.; Schroedl, S. Constrained K-means clustering with background knowledge. Int. Conf. Mach. Learn. 2001, 1, 577–584. [Google Scholar]
- Smith, I.M.; Forbes, A.B. Algorithms and software for fitting polynomial functions constrained to pass through the origin. J. Phys. Conf. Ser. 2018, 1065, 212022. [Google Scholar] [CrossRef]
- Lepš, J.; Šmilauer, P. Multivariate analysis of ecological data using canoco 5. Bull. Ecol. Soc. Am. 2014, 85, 5. [Google Scholar]
- Li, H.C.; Gan, X.H.; Zhang, Z.P.; Zhang, C.; Song, L. Effects of altitudes and the DBH of seed trees on biological characteristics of Tetracentron sinense (Tetracentraceae) seeds. Plant Divers. Resour. 2015, 37, 177–183. [Google Scholar]
- Wright, S. Isolation by distance. Genetics 1943, 28, 139–156. [Google Scholar] [CrossRef] [PubMed]
- DeWoody, J.; Trewin, H.; Taylor, G. Genetic and morphological differentiation in Populus nigra L. Isolation by colonization or isolation by adaptation? Mol. Ecol. 2015, 24, 2641–2655. [Google Scholar] [CrossRef]
- Poljak, I.; Idžojtić, M.; Šapić, I.; Korijan, P.; Vukelić, J. Diversity and structure of Croatian continental and Alpine-Dinaric populations of grey alder (Alnus incana/L./Moench subsp. incana): Isolation by distance and environment explains phenotypic divergence. Sumar. List 2018, 142, 19–31. [Google Scholar] [CrossRef]
- Chen, Y.T.; Wang, S.F.; Chen, Y.C.; Yuan, J.; Wang, T.; Chen, B.; Qian, Y.N. Variation and stability of seed yield, fall-off process, seed size and form in live oak. For. Res. 2015, 28, 524–530. [Google Scholar]
- Li, Y.Q.; Li, Y.C.; Wu, Z.Z. Variation in phenotype characters and starch content of Quercus mongolica Fisch seed from different provenances. For. Res. 2013, 26, 528–532. [Google Scholar]
- Chang, E.F.; Zhang, Q.; Xiao, G.Y.; Li, P.R.; Li, Y.; Ding, Y.X.; Huang, C.L.; Jing, Y.B. Morphological characteristics and variation analysis of seeds from different provenances and families of Quercus cocciferoides. Seed 2020, 39, 53–58. [Google Scholar]
- Rao, G.R.; Shanker, A.K.; Srinivas, I.; Korwar, G.R.; Venkateswarlu, B. Diversity and variability in seed characters and growth of Pongamia pinnata (L.) Pierre accessions. Trees 2011, 25, 725–734. [Google Scholar] [CrossRef]
- Fu, X.X.; Liu, H.N.; Zhou, X.D.; Hong, X.T. Morphological variation of Cornus Officinalis seeds in relation to environmental factors. Chin. J. Ecol. 2013, 32, 27–32. [Google Scholar]
- Rewicz, A.; Bomanowska, A.; Magda, J.; Rewicz, T. Morphological variability of Consolida regalis seeds of south-eastern and central Europe. Syst. Biodivers. 2016, 15, 25–34. [Google Scholar] [CrossRef]
- Ji, M.F.; Zhang, X.W.; Wang, Z.Q.; Zhang, Q.; Deng, J.M. Intra- versus inter-population variation of cone and seed morphological traits of Pinus tabulaeformis Carr in northern China: Impact of climate-related conditions. Pol. J. Ecol. 2011, 59, 717–727. [Google Scholar]
- Rodríguez-Gómez, F.; Oyama, K.; Ochoa-Orozco, M.; Mendoza-Cuenca, L.; Gaytán-Legaria, R.; González-Rodríguez, A. Phylogeography and climate-associated morphological variation in the endemic white oak Quercus deserticola (Fagaceae) along the trans-Mexican volcanic belt. Botany 2018, 96, 121–133. [Google Scholar] [CrossRef]
- Du, B.M.; Zhu, Y.H.; Kang, H.Z.; Liu, C.J. Spatial variations in stomatal traits and their coordination with leaf traits in Quercus variabilis across eastern Asia. Sci. Total Environ. 2021, 789, 147757. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.B.; Dong, X.; Xin, Z.M.; Liu, M.H.; Zhang, R.H.; Huang, Y.R.; Sun, F. Effects of artificial simulated precipitation on seed characters and germination of Nitraria tangutorum. Southwest China J. Agric. Sci. 2019, 5, 1181–1186. [Google Scholar]
- Shu, X.; Yang, Z.L.; Yang, X.; Duan, H.P.; Yu, H.H.; Huang, J.C.; Li, S.C. Variation in seed characters of Magnolia officinalis from different locations. For. Res. 2010, 23, 457–461. [Google Scholar]
- Gao, Z.Y.; Zhang, H.F.; Chen, G.P.; Feng, X.M.; Zhao, T.J.; Gao, X.; Shi, F.C. Fruit stone morphology and geographic variation in Juglans mandshurica populations. Chin. J. Appl. Environ. Biol. 2017, 23, 609–615. [Google Scholar]
- Scrivanti, L.R.; Mestre, L.; Anton, A.M. Phenotypical variation and taxonomic correlates of five closely related andean species of Poa (Poaceae) along geographic and climatic gradients. Phytotaxa 2014, 183, 121–144. [Google Scholar] [CrossRef]
- Malanson, G.P.; Cheney, A.B.; Kinney, M. Climatic and geographic relations of alpine tundra floras in western North America. Alp. Bot. 2015, 125, 21–29. [Google Scholar] [CrossRef]
| Site Number | Site Location | Code | LON (° E) | LAT (° N) | ALT (m) | MAT (°C) | MTW (°C) | AP (mm) | PWQ (mm) | ELAT (°) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Chuzhou City, Anhui Province | CZA | 117.97 | 32.35 | 80 | 15.28 | 30.89 | 939 | 448 | 31.25 |
| 2 | Huainan County, Anhui Province | HNA | 117.00 | 32.63 | 49 | 15.64 | 31.64 | 897 | 431 | 31.37 |
| 3 | Mentougou District, Beijing | MTB | 116.09 | 39.96 | 213 | 11.70 | 30.25 | 552 | 422 | 39.53 |
| 4 | Miyun County, Beijing | MYB | 117.07 | 40.50 | 357 | 9.75 | 28.76 | 514 | 382 | 40.90 |
| 5 | Pinggu District, Beijing | PGB | 117.13 | 40.28 | 353 | 9.90 | 28.59 | 530 | 399 | 40.66 |
| 6 | Tianshui City, Gansu Province | TSG | 106.55 | 34.47 | 1189 | 10.81 | 26.91 | 642 | 341 | 40.82 |
| 7 | Baise City, Guangxi Province | BSG | 106.55 | 24.77 | 142 | 16.55 | 27.41 | 1270 | 682 | 23.98 |
| 8 | Anlong county, Guizhou Province | ALG | 104.70 | 24.85 | 1697 | 14.66 | 24.19 | 1183 | 636 | 34.82 |
| 9 | Xingren County, Guizhou Province | XGG | 104.95 | 25.25 | 1298 | 15.94 | 26.16 | 1265 | 676 | 32.38 |
| 10 | Dengfeng City, Henan Province | DFH | 113.05 | 34.45 | 371 | 13.46 | 29.76 | 673 | 365 | 34.96 |
| 11 | Jiyuan City, Henan Province | JYH | 112.60 | 35.07 | 155 | 14.60 | 31.95 | 567 | 326 | 34.34 |
| 12 | Lushi County, Henan Province | LSH | 111.05 | 34.05 | 880 | 13.33 | 30.58 | 671 | 335 | 38.20 |
| 13 | Nanzhao County, Henan Province | NZH | 112.43 | 33.46 | 251 | 15.11 | 31.24 | 804 | 382 | 33.21 |
| 14 | Tongbai County, Henan Province | TBH | 113.68 | 32.53 | 170 | 15.13 | 30.86 | 974 | 444 | 31.88 |
| 15 | Badong County, Hubei Province | BDH | 110.34 | 31.04 | 598 | 15.14 | 30.07 | 1216 | 540 | 33.17 |
| 16 | Baokang County, Hubei Province | BKH | 111.26 | 31.88 | 680 | 13.72 | 29.35 | 1058 | 472 | 34.59 |
| 17 | Enshi City, Hubei Province | ESH | 109.49 | 30.28 | 491 | 16.26 | 31.48 | 1468 | 652 | 31.65 |
| 18 | Jianshi County, Hubei Province | JSH | 109.73 | 30.60 | 730 | 14.84 | 29.81 | 1383 | 600 | 33.67 |
| 19 | Jingshan City, Hubei Province | JSA | 113.12 | 31.02 | 103 | 16.08 | 31.17 | 1071 | 467 | 30.03 |
| 20 | Suixian County, Hubei Province | SXH | 112.98 | 31.53 | 287 | 15.05 | 30.20 | 1033 | 447 | 31.46 |
| 21 | Jiangui County, Hubei Province | JGH | 110.98 | 30.83 | 610 | 15.62 | 30.49 | 1167 | 536 | 33.04 |
| 22 | Wuhan City, Hubei Province | WHH | 114.31 | 30.59 | 27 | 17.26 | 33.00 | 1265 | 561 | 29.23 |
| 23 | Zhushan County, Hubei Province | ZSH | 110.23 | 32.22 | 418 | 15.28 | 31.21 | 1004 | 454 | 33.07 |
| 24 | Xiangxi Prefecture, Hunan Province | XPH | 109.74 | 28.31 | 277 | 17.33 | 32.57 | 1339 | 604 | 28.20 |
| 25 | Changsha City, Hunan Province | CSH | 112.94 | 28.23 | 61 | 17.78 | 33.33 | 1403 | 597 | 27.03 |
| 26 | Nanchang City, Jiangxi Province | NCJ | 115.83 | 28.76 | 37 | 17.70 | 33.42 | 1556 | 726 | 27.44 |
| 27 | Dalian City, Liaoning Province | DLL | 121.79 | 39.10 | 137 | 10.22 | 26.69 | 646 | 402 | 38.28 |
| 28 | Liaoyang City, Liaoning Province | LYL | 123.30 | 41.08 | 171 | 8.04 | 27.25 | 742 | 473 | 40.43 |
| 29 | Yantai City, Shandong Province | YTS | 121.74 | 37.26 | 222 | 11.13 | 26.39 | 721 | 434 | 36.87 |
| 30 | Xia County, Shanxi Province | XCS | 111.37 | 35.01 | 1185 | 9.95 | 26.49 | 611 | 333 | 41.33 |
| 31 | Baoji City, Shaanxi Province | BJS | 107.14 | 34.37 | 680 | 13.11 | 30.25 | 681 | 358 | 37.08 |
| 32 | Shanyang County, Shaanxi Province | SYS | 109.88 | 33.53 | 726 | 12.93 | 29.03 | 778 | 375 | 36.58 |
| 33 | Shangnan County, Shaanxi Province | SNS | 110.88 | 33.53 | 826 | 14.69 | 31.05 | 774 | 373 | 37.29 |
| 34 | Weinan County, Shaanxi Province | WNS | 109.50 | 34.50 | 536 | 13.16 | 30.94 | 606 | 297 | 36.19 |
| 35 | Xianyang City, Shaanxi Province | XYS | 108.08 | 34.27 | 486 | 12.76 | 29.78 | 648 | 326 | 35.60 |
| 36 | Linzhi City, Tibet | LZT | 94.36 | 29.65 | 3164 | 7.90 | 20.16 | 651 | 359 | 50.11 |
| 37 | Urumqi, Xinjiang | UQX | 87.62 | 43.83 | 899 | 7.06 | 30.22 | 231 | 87 | 48.10 |
| 38 | Anning City, Yunnan Province | ANY | 102.45 | 24.99 | 1852 | 15.25 | 24.81 | 898 | 496 | 36.07 |
| 39 | Honghe Prefecture, Yunnan Province | HHY | 103.61 | 23.33 | 1655 | 14.71 | 22.98 | 1367 | 761 | 33.01 |
| 40 | Kunming, Yunnan Province | KMY | 102.75 | 25.14 | 2051 | 14.30 | 23.70 | 921 | 509 | 37.65 |
| 41 | Zhanyi County, Yunnan Province | ZYY | 103.55 | 25.59 | 2214 | 13.84 | 23.23 | 938 | 515 | 39.27 |
| 42 | Lin’an District, Zhejiang Province | LAZ | 119.44 | 30.33 | 320 | 14.78 | 30.13 | 1399 | 554 | 30.47 |
| 43 | Yuhang District, Zhejiang Province | YHZ | 120.30 | 30.42 | 9 | 16.51 | 32.29 | 1262 | 472 | 28.96 |
| Site | FL | FW | FL/FW | Sample Size | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Max | Min | SD | CV (%) | Mean | Max | Min | SD | CV (%) | Mean | Max | Min | SD | CV (%) | ||
| 1 | 19.51 defghijk | 23.56 | 15.02 | 1.71 | 8.74 | 18.21 ijk | 26.78 | 13.13 | 1.58 | 8.70 | 1.07 bcde | 1.58 | 0.70 | 0.10 | 9.08 | 100 |
| 2 | 20.42 hijkl | 24.22 | 15.88 | 1.13 | 5.53 | 19.99 lmn | 27.17 | 13.82 | 1.50 | 7.51 | 1.04 abc | 1.32 | 0.76 | 0.06 | 6.21 | 120 |
| 3 | 19.20 cdefghij | 22.94 | 15.49 | 1.49 | 7.79 | 14.82 bc | 18.46 | 10.87 | 1.26 | 8.50 | 1.30 i | 1.60 | 1.05 | 0.07 | 5.56 | 80 |
| 4 | 20.52 ijkl | 22.75 | 18.68 | 0.97 | 4.74 | 18.88 klm | 21.90 | 16.33 | 1.43 | 7.56 | 1.09 bcde | 1.36 | 0.94 | 0.10 | 9.26 | 20 |
| 5 | 20.40 hijkl | 24.27 | 16.69 | 1.89 | 9.26 | 17.42 fghijk | 20.97 | 15.00 | 1.41 | 8.09 | 1.18 efgh | 1.50 | 0.86 | 0.14 | 11.55 | 20 |
| 6 | 17.81 abc | 21.52 | 13.36 | 2.12 | 11.88 | 15.75 cde | 19.52 | 12.44 | 1.77 | 11.26 | 1.14 cdefg | 1.40 | 0.94 | 0.12 | 10.55 | 20 |
| 7 | 20.31 hijkl | 24.49 | 14.14 | 2.13 | 10.48 | 16.53 defghi | 20.69 | 13.75 | 1.57 | 9.52 | 1.23 fghi | 1.52 | 1.00 | 0.13 | 10.46 | 30 |
| 8 | 19.53 defghijk | 22.80 | 13.45 | 1.92 | 9.82 | 18.82 klm | 24.56 | 14.26 | 2.07 | 10.99 | 1.04 abc | 1.24 | 0.85 | 0.10 | 9.37 | 30 |
| 9 | 21.15 klmn | 23.84 | 18.20 | 1.42 | 6.71 | 20.26 mn | 22.89 | 17.97 | 1.02 | 5.03 | 1.04 abc | 1.23 | 0.90 | 0.07 | 6.89 | 30 |
| 10 | 19.49 defghijk | 26.80 | 13.97 | 1.60 | 8.23 | 17.42 fghijk | 22.08 | 12.57 | 1.48 | 8.48 | 1.12 bcdef | 1.61 | 0.82 | 0.10 | 8.72 | 438 |
| 11 | 21.69 lmn | 26.10 | 17.08 | 1.61 | 7.44 | 17.68 fghijk | 21.57 | 13.97 | 1.23 | 6.94 | 1.23 fghi | 1.50 | 0.98 | 0.09 | 6.94 | 60 |
| 12 | 19.89 efghijk | 20.74 | 18.92 | 0.46 | 2.29 | 17.07 efghij | 18.83 | 14.47 | 1.27 | 7.41 | 1.17 defgh | 1.36 | 1.00 | 0.09 | 7.85 | 20 |
| 13 | 18.65 abcdefg | 23.00 | 13.90 | 1.63 | 8.76 | 16.20 cdef | 21.50 | 10.45 | 1.53 | 9.43 | 1.16 cdefgh | 1.76 | 0.85 | 0.11 | 9.25 | 80 |
| 14 | 20.36 hijkl | 24.45 | 15.70 | 1.29 | 6.35 | 20.20 mn | 23.42 | 16.6 | 1.49 | 7.35 | 1.01 ab | 1.20 | 0.86 | 0.05 | 5.15 | 60 |
| 15 | 17.17 a | 24.35 | 9.96 | 1.43 | 8.33 | 14.95 bc | 20.08 | 9.78 | 1.26 | 8.44 | 1.14 cdefg | 1.44 | 0.80 | 0.12 | 10.82 | 60 |
| 16 | 19.71 fghijkl | 22.82 | 14.62 | 1.32 | 6.70 | 17.82 ghijk | 20.40 | 14.26 | 1.05 | 5.89 | 1.11 cdefg | 1.41 | 0.90 | 0.09 | 8.55 | 40 |
| 17 | 17.22 a | 20.79 | 11.56 | 1.64 | 9.54 | 11.70 a | 13.79 | 9.28 | 0.91 | 7.74 | 1.48 j | 1.99 | 1.03 | 0.16 | 10.62 | 60 |
| 18 | 20.27 ghijkl | 23.89 | 17.58 | 1.91 | 9.41 | 18.19 ijk | 21.99 | 15.31 | 1.66 | 9.14 | 1.12 bcde | 1.41 | 0.99 | 0.10 | 8.87 | 30 |
| 19 | 20.05 fghijkl | 23.58 | 17.02 | 1.05 | 5.25 | 17.29 efghijk | 21.63 | 13.55 | 1.16 | 6.69 | 1.17 defgh | 1.62 | 0.94 | 0.09 | 7.31 | 60 |
| 20 | 20.08 fghijkl | 22.02 | 16.65 | 1.21 | 6.04 | 20.93 n | 23.13 | 18.49 | 1.12 | 5.36 | 0.96 a | 1.12 | 0.82 | 0.07 | 7.25 | 30 |
| 21 | 18.90 bcdefghi | 22.82 | 14.62 | 1.95 | 10.34 | 17.72 fghijk | 19.88 | 15.30 | 1.19 | 6.70 | 1.07 abcde | 1.36 | 0.90 | 0.13 | 12.22 | 20 |
| 22 | 19.51 cdefghijk | 24.49 | 13.79 | 1.28 | 6.57 | 17.73 fghijk | 23.33 | 12.72 | 1.20 | 6.75 | 1.10 bcde | 1.45 | 0.81 | 0.07 | 6.78 | 99 |
| 23 | 20.95 jklm | 22.73 | 19.19 | 0.90 | 4.30 | 19.70 lmn | 21.72 | 17.22 | 1.06 | 5.38 | 1.06 abcde | 1.13 | 0.96 | 0.05 | 4.49 | 20 |
| 24 | 19.99 efghijk | 23.93 | 15.12 | 1.50 | 7.52 | 13.58 b | 18.20 | 9.46 | 1.34 | 9.84 | 1.50 j | 2.01 | 1.08 | 0.13 | 8.47 | 49 |
| 25 | 19.58 defghijk | 26.79 | 12.87 | 1.35 | 6.90 | 11.89 a | 20.17 | 7.96 | 0.85 | 7.11 | 1.68 k | 2.25 | 1.01 | 0.14 | 8.26 | 180 |
| 26 | 21.12 klmn | 23.61 | 17.46 | 1.51 | 7.14 | 19.99 lmn | 22.34 | 16.79 | 1.54 | 7.68 | 1.06 abcde | 1.22 | 0.95 | 0.06 | 5.95 | 20 |
| 27 | 19.93 efghijk | 22.45 | 16.91 | 1.49 | 7.49 | 16.16 cdefg | 18.21 | 12.74 | 1.34 | 8.27 | 1.24 ghi | 1.44 | 0.96 | 0.11 | 8.62 | 20 |
| 28 | 19.00 bcdefghi | 24.73 | 14.30 | 2.06 | 10.85 | 16.96 defghij | 21.32 | 13.19 | 2.08 | 12.27 | 1.13 bcdefg | 1.42 | 0.93 | 0.12 | 10.85 | 30 |
| 29 | 19.79 efghijk | 26.69 | 15.66 | 1.69 | 8.55 | 15.73 cde | 19.03 | 12.22 | 1.27 | 8.08 | 1.26 hi | 1.86 | 1.04 | 0.11 | 8.82 | 80 |
| 30 | 18.42 abcdef | 20.94 | 15.68 | 1.42 | 7.71 | 17.34 efghijk | 21.42 | 14.32 | 1.53 | 8.81 | 1.07 abcde | 1.22 | 0.91 | 0.07 | 6.65 | 20 |
| 31 | 22.46 n | 24.86 | 20.48 | 1.29 | 5.74 | 15.45 cd | 17.23 | 13.68 | 1.06 | 6.86 | 1.46 j | 1.58 | 1.34 | 0.06 | 4.38 | 20 |
| 32 | 18.84 abcde | 21.78 | 14.62 | 1.43 | 7.56 | 17.54 efghijk | 21.42 | 12.07 | 1.44 | 8.19 | 1.08 abcde | 1.35 | 0.84 | 0.08 | 7.69 | 60 |
| 33 | 19.83 efghijk | 21.78 | 17.02 | 1.01 | 5.12 | 17.88 ghijk | 21.42 | 14.16 | 1.58 | 8.81 | 1.11 bcde | 1.30 | 0.91 | 0.08 | 7.55 | 20 |
| 34 | 18.04 abcd | 22.62 | 14.08 | 1.78 | 9.89 | 17.06 efghij | 21.37 | 14.09 | 1.46 | 8.54 | 1.06 abcde | 1.28 | 0.81 | 0.11 | 10.09 | 60 |
| 35 | 20.55 ijkl | 24.04 | 16.24 | 1.91 | 9.30 | 17.93 hijk | 20.88 | 13.72 | 1.53 | 8.54 | 1.16 cdefg | 1.44 | 0.86 | 0.15 | 13.25 | 30 |
| 36 | 18.63 abcdefgh | 22.50 | 13.84 | 1.92 | 10.32 | 17.78 fghijk | 21.34 | 13.45 | 1.87 | 10.51 | 1.05 abcd | 1.25 | 0.86 | 0.09 | 8.76 | 30 |
| 37 | 31.69 o | 35.04 | 24.65 | 2.43 | 7.67 | 17.86 ghijk | 20.97 | 14.53 | 1.76 | 9.86 | 1.78 l | 2.31 | 1.56 | 0.16 | 8.97 | 30 |
| 38 | 18.46 a | 20.64 | 15.27 | 1.36 | 7.38 | 17.29 defgh | 19.48 | 15.52 | 1.04 | 6.02 | 1.07 abc | 1.25 | 0.89 | 0.06 | 6.02 | 50 |
| 39 | 20.67 ijkl | 23.33 | 18.00 | 0.93 | 4.52 | 18.69 jkl | 21.09 | 15.77 | 1.40 | 7.50 | 1.11 bcde | 1.34 | 0.96 | 0.10 | 9.16 | 30 |
| 40 | 17.54 ab | 20.42 | 11.78 | 1.80 | 10.29 | 16.65 defghi | 20.91 | 12.28 | 1.51 | 9.09 | 1.06 abcde | 1.24 | 0.81 | 0.09 | 8.93 | 47 |
| 41 | 22.16 mn | 25.48 | 17.78 | 1.50 | 6.77 | 18.95 klm | 20.83 | 15.67 | 1.16 | 6.14 | 1.17 defgh | 1.42 | 1.03 | 0.09 | 7.85 | 30 |
| 42 | 19.62 defghijk | 24.70 | 14.76 | 2.02 | 10.29 | 17.69 fghijk | 21.61 | 15.53 | 1.44 | 8.15 | 1.11 bcde | 1.26 | 0.91 | 0.08 | 7.54 | 30 |
| 43 | 19.48 defghijk | 21.79 | 16.01 | 1.30 | 6.68 | 18.14 hijk | 21.46 | 13.25 | 1.74 | 9.60 | 1.08 bcde | 1.42 | 0.91 | 0.11 | 10.16 | 30 |
| Total | 19.97 | 23.65 | 15.77 | 1.53 | 7.72 | 17.35 | 21.09 | 13.77 | 1.40 | 8.11 | 1.17 | 1.46 | 0.94 | 0.10 | 8.41 | - |
| CV betweenPopulations(%) | 10.80 | 11.21 | 14.48 | |||||||||||||
| Variance Source | df | SS | MS | F | p | |
|---|---|---|---|---|---|---|
| Fruit Length | Inter-group | 7183 | 42 | 171.015 | 39.28 | <0.001 |
| Intra-group | 10,232 | 2350 | 4.354 | |||
| Total | 17,415 | 2392 | ||||
| Fruit Width | Inter-group | 11,452 | 42 | 272.667 | 67.70 | <0.001 |
| Intra-group | 9464 | 2350 | 4.027 | |||
| Total | 20,916 | 2392 | ||||
| Fruit Length-to-Width Ratio | Inter-group | 84 | 42 | 2.009 | 105.42 | <0.001 |
| Intra-group | 45 | 2350 | 0.019 | |||
| Total | 129 | 2392 | ||||
| LON (°) | ELAT (°) | ALT (m) | MAT (°C) | MTW (°C) | AP (mm) | PWQ (mm) | |
|---|---|---|---|---|---|---|---|
| FL | −0.402 ** | 0.243 | −0.040 | −0.261 | 0.103 | −0.282 | −0.333 * |
| FW | −0.038 | 0.042 | 0.126 | −0.019 | −0.155 | −0.055 | −0.042 |
| FL/FW | −0.244 | 0.071 | −0.174 | −0.103 | 0.263 | −0.088 | −0.150 |
| Comparison | r | p-Value |
|---|---|---|
| Morphological, Geographic | 0.633 | <0.001 |
| Morphological, Climate | 0.988 | <0.001 |
| Principal Component | PC1 | PC2 |
|---|---|---|
| FL | 0.38 | 0.93 |
| FW | −0.78 | 0.62 |
| FL/FW | 0.99 | 0.14 |
| Eigenvalue | 1.73 | 1.26 |
| Variance (%) | 57.57 | 42.03 |
| % Total Variance | 57.57 | 99.60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, S.; Ren, Y.; Masabni, J.; Zou, F.; Xiong, H.; Zhu, J. Influence of Geographical and Climatic Factors on Quercus variabilis Blume Fruit Phenotypic Diversity. Diversity 2021, 13, 329. https://doi.org/10.3390/d13070329
Gao S, Ren Y, Masabni J, Zou F, Xiong H, Zhu J. Influence of Geographical and Climatic Factors on Quercus variabilis Blume Fruit Phenotypic Diversity. Diversity. 2021; 13(7):329. https://doi.org/10.3390/d13070329
Chicago/Turabian StyleGao, Shuang, Yue Ren, Joseph Masabni, Feng Zou, Huan Xiong, and Jingle Zhu. 2021. "Influence of Geographical and Climatic Factors on Quercus variabilis Blume Fruit Phenotypic Diversity" Diversity 13, no. 7: 329. https://doi.org/10.3390/d13070329
APA StyleGao, S., Ren, Y., Masabni, J., Zou, F., Xiong, H., & Zhu, J. (2021). Influence of Geographical and Climatic Factors on Quercus variabilis Blume Fruit Phenotypic Diversity. Diversity, 13(7), 329. https://doi.org/10.3390/d13070329







