What Makes a Hot-Spring Habitat “Hot” for the Hot-Spring Snake: Distributional Data and Niche Modelling for the Genus Thermophis (Serpentes, Colubridae)
Abstract
:1. Introduction
2. Materials and Methods
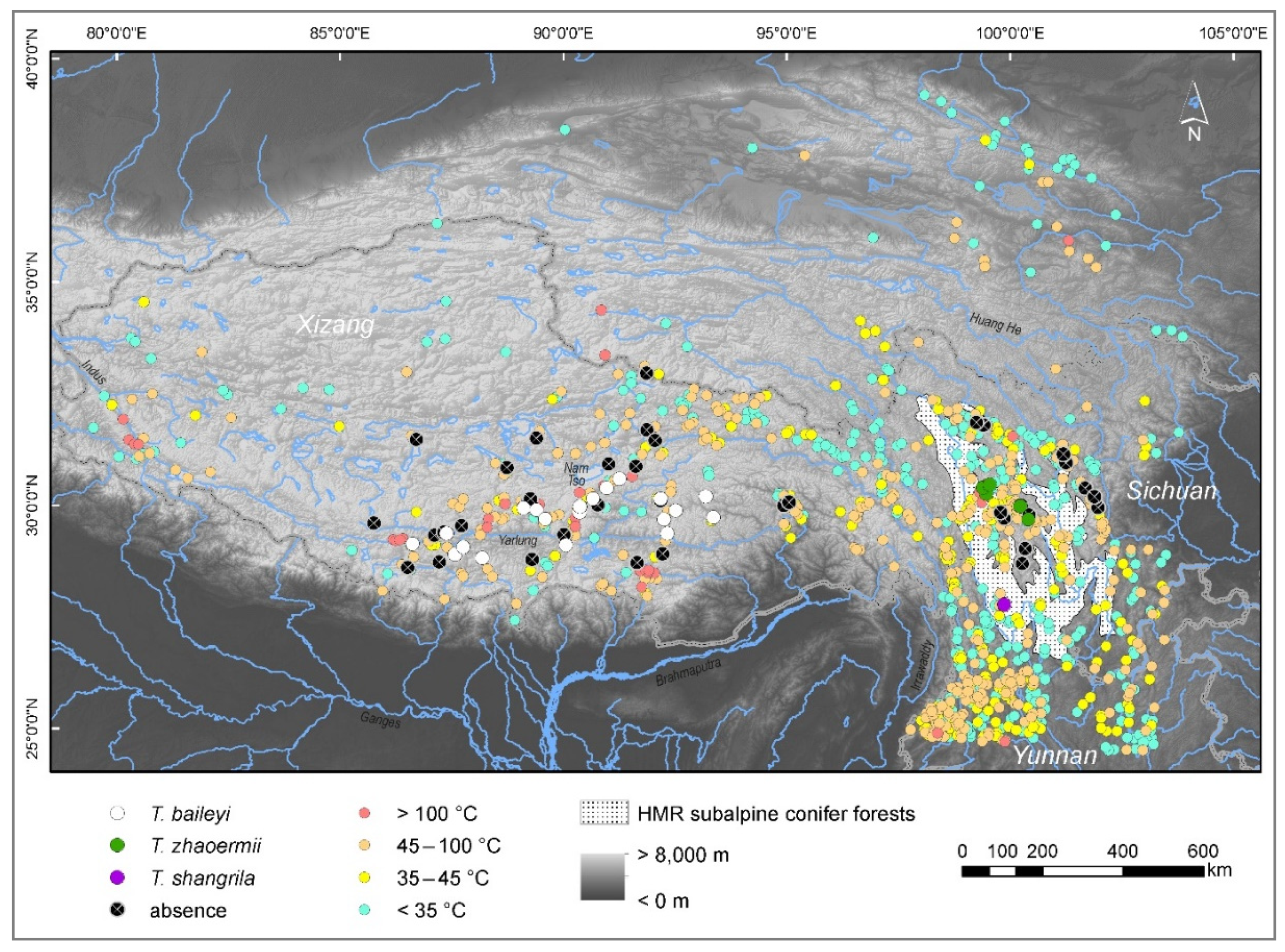
2.1. Occurrence Data and Ecological Niche Analyses
2.2. Hot-Spring Water Characteristics
2.3. Logistic Regression Models
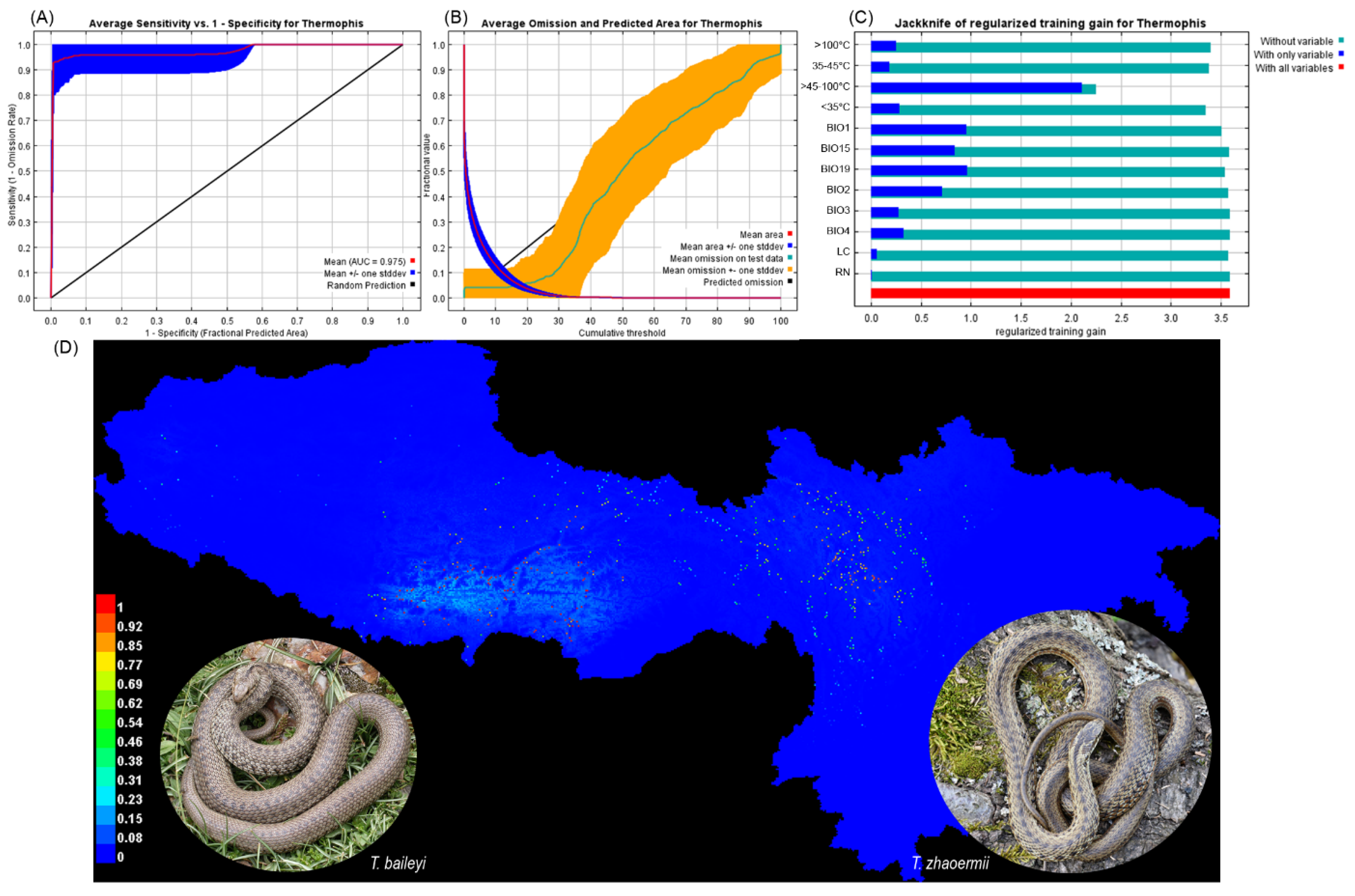
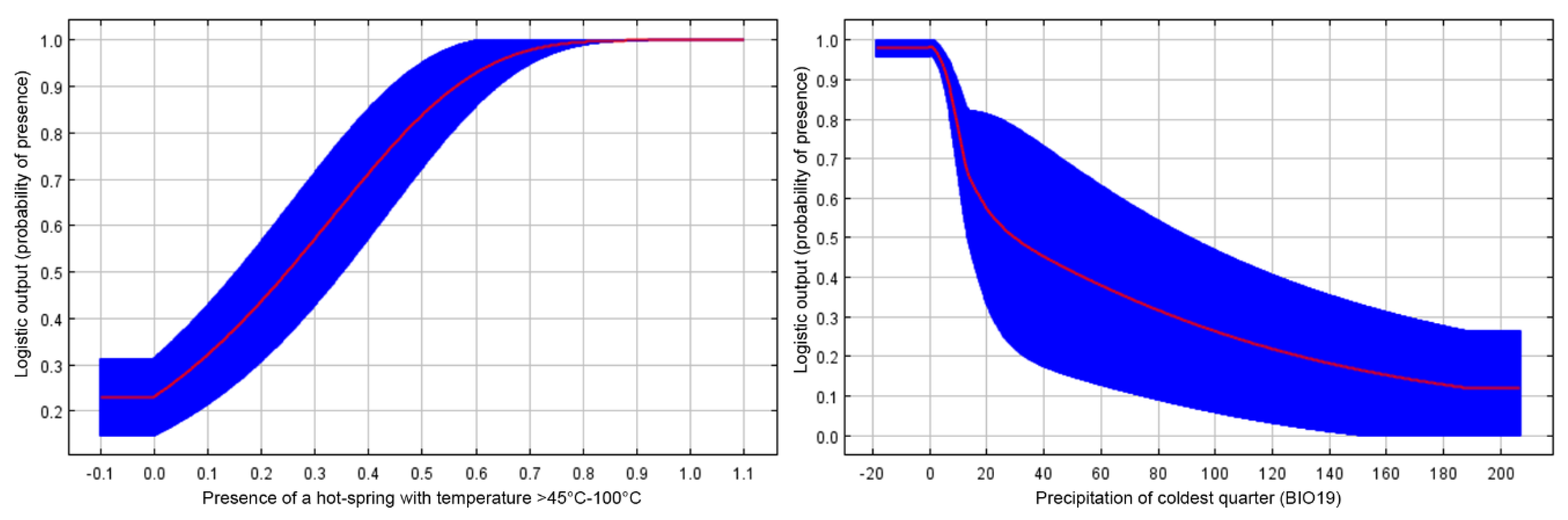
3. Results
3.1. Climate Heterogeneity, Maxent Models
3.2. Habitat Characteristics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Krebs, C.J. Ecology: The Experimental Analysis of Distribution and Abundance, 4th ed.; Harper Collins College Publishers: New York, NY, USA, 1994. [Google Scholar]
- Lomolino, M.V.; Riddle, B.R.; Whittaker, R.J.; Brown, J.H. Biogeography; Sinauer Associates: Sunderland, UK, 2010. [Google Scholar]
- Boufford, D.E.; Van Dyck, P.P. South-Central China in Hotspots: Earth’s Biologically Richest and Most Endangered Terrestrial Ecoregions; Mittermeier, R.A., Myers, N., Mittermeier, C.G., Eds.; CEMEX: Mexico City, Mexico, 1999; pp. 338–351. [Google Scholar]
- Tang, Z.; Wang, Z.; Zheng, C.; Fang, J. Biodiversity in China’s mountains. Front. Ecol. Environ. 2006, 4, 347–352. [Google Scholar] [CrossRef]
- Mittermeier, R.A.; Robles Gil, P.; Hoffman, M.; Pilgrim, J.; Brooks, T.; Mittermeier, C.G.; Lamoreux, J.; da Fonseca, G.A.B. Hotspots Revisited; CEMEX: Mexico City, Mexico, 2004. [Google Scholar]
- Zhang, Y.L.; Li, B.Y.; Zheng, D. A discussion on the boundary and area of the Tibetan Plateau in China. Geogr. Res. 2002, 21, 1–8. [Google Scholar]
- Miehe, G.; Schleuss, P.M.; Seeber, E.; Babel, W.; Biermann, T.; Braendle, M.; Chen, F.; Coners, H.; Foken, T.; Gerken, T.; et al. The Kobresia pygmaea ecosystem of the Tibetan highlands—Origin, functioning and degradation of the world’s largest pastoral alpine ecosystem: Kobresia pastures of Tibet. Sci. Total Environ. 2019, 648, 754–771. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Li, Z.M.; Sun, H. Seed Plants of the Alpine Subnival Belt from the Hengduan Mountains; SW China; China Science Publishing & Media Ltd. Group: Beijing, China, 2013. [Google Scholar]
- Yang, Y.; Tian, K.; Hao, J.; Pei, S.; Yang, Y. Biodiversity and biodiversity conservation in Yunnan, China. Biodivers. Conserv. 2004, 13, 813–826. [Google Scholar] [CrossRef]
- Li, Z.; He, Y.; An, W.; Song, L.; Zhang, W.; Catto, N.; Wang, Y.; Wang, S.; Liu, H.Q.; Cao, W.; et al. Climate and glacier change in southwestern China during the past several decades. Environ. Res. Lett. 2011, 6, 1–24. [Google Scholar] [CrossRef]
- Qiu, J. Double threat for Tibet—Climate change and human development are jeopardizing the plateau’s fragile environment. Nature 2014, 512, 240–241. [Google Scholar] [CrossRef] [Green Version]
- Sherman, R.; Mullen, R.; Haomin, L.; Zhendong, F.; Yi, W. Alpine ecosystems of northwest Yunnan, China: An initial assessment for conservation. J. Mt. Sci. 2007, 4, 181–192. [Google Scholar] [CrossRef]
- Sherman, R.; Mullen, R.; Haomin, L.; Zhendong, F.; Yi, W. Spatial patterns of plant diversity and communities in Alpine ecosystems of the Hengduan Mountains, northwest Yunnan, China. J. Plant Ecol. 2008, 1, 117–136. [Google Scholar] [CrossRef]
- Watson, J.E.M.; Rao, M.; Ai-Li, K.; Yan, X. Climate Change Adaptation Planning for Biodiversity Conservation: A Review. Adv. Clim. Chang. Res. 2012, 3, 1–11. [Google Scholar] [CrossRef]
- The IUCN Red List of Threatened Species. IUCN. Version 3.1. 2014. Available online: www.iucnredlist.org (accessed on 7 June 2021).
- Huang, S.; Peng, L. Tibetan hot-spring snakes under threat. Science 2019, 366, 193–194. [Google Scholar] [CrossRef]
- Hofmann, S.; Kraus, S.; Dorge, T.; Nothnagel, M.; Fritzsche, P.; Miehe, G. Effects of Pleistocene climatic fluctuations on the phylogeography, demography and population structure of a high-elevation snake species, Thermophis baileyi, on the Tibetan Plateau. J. Biogeogr. 2014, 41, 2162–2172. [Google Scholar] [CrossRef]
- Guo, P.; Liu, S.Y.; Feng, J.C.; Miao, H.E. The description of a new species of Thermophis (Serpentes: Colubridae). Sichuan J. Zool. 2008, 27, 321. [Google Scholar]
- Hofmann, S. Population genetic structure and geographic differentiation in the hot spring snake Thermophis baileyi (Serpentes, Colubridae): Indications for glacial refuges in southern-central Tibet. Mol. Phylogenet. Evol. 2012, 63, 396–406. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Lu, C.; Huang, S.; Guo, P.; Zhang, Y. A new species of the genus Thermophis (Serpentes: Colubridae) from Shangri-La, Northern Yunnan, China, with a proposal for an eclectic rule for species delimitation. Asian Herpetol. Res. 2014, 5, 228–239. [Google Scholar]
- Li, J.-T.; Gao, Y.-D.; Xie, L.; Deng, C.; Shi, P.; Guan, M.-L.; Huang, S.; Ren, J.-L.; Wu, D.-D.; Ding, L.; et al. Comparative genomic investigation of high-elevation adaptation in ectothermic snakes. Proc. Natl. Acad. Sci. USA 2018, 115, 8406–8411. [Google Scholar] [CrossRef] [Green Version]
- CAS. Atlas of Tibet Plateau; CAS, Institute of Geography: Bejing, China, 1990. [Google Scholar]
- Olson, D.M.; Dinerstein, E.; Wikramanayake, E.D.; Burgess, N.D.; Powell, G.V.N.; Underwood, E.C.; D’Amico, J.A.; Itoua, I.; Strand, H.E.; Morrison, J.C.; et al. Terrestrial ecoregions of the world: A new map of life on Earth. Bioscience 2001, 51, 933–938. [Google Scholar] [CrossRef]
- Kou, Z. Preliminary reports on the herpetofauna of Shuitang and Zhelong districts of the eastern slope of Mt. Ailao, with description of a new Species. Acta Herpetol. Sin. Chengdu 1984, 3, 39–45. [Google Scholar]
- Chen, X.; Huang, Y. A herpetological survey of Muli county, Sichuan. Acta Herpetol. Sin. Guiyang 1995, 4–5, 117–123. [Google Scholar]
- Chen, L.M.; Gao, Z.F.; Ou, W.F.; Chen, W.L.; Ma, W.-H. Surveys of amphibians and reptiles in Tangjiahe Nature Reserve, Sichuan Province. Sichuan J. Zool. 1999, 18, 132–134. [Google Scholar]
- Pan, X.-F.; Zhou, W.; Zhou, Y.-W.; Wu, F.; Zhang, Q. Amphibian and Reptile in Zhongdian Area of Northwest Yunnan. Sichuan J. Zool. 2002, 21, 88–91. [Google Scholar]
- Wang, D.; Song, Z.; Yue, B.; Ye, H.; Liu, S. A survey of herpetological resources in National Dafengding Nature Reserve, Meigu Co., Sichuan, China. Sichuan J. Zool. 2004, 23, 238–242. [Google Scholar]
- Dorge, T.; Hofmann, S.; Wangdwei, M.; Duoje, L.; Solhøy, T.; Miehe, G. The ecological specialist, Thermophis baileyi (Wall, 1907)—New records, distribution and biogeographic conclusions. Herpetol. Bull. 2007, 101, 8–12. [Google Scholar]
- Kéry, M. Inferring the Absence of a Species: A Case Study of Snakes. J. Wildl. Manag. 2002, 66, 330. [Google Scholar] [CrossRef]
- Hijmans, R.J.; Cameron, S.E.; Parra, J.L.; Jones, P.G.; Jarvis, A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 2005, 25, 1965–1978. [Google Scholar] [CrossRef]
- Brown, J.L. SDMtoolbox: A python-based GIS toolkit for landscape genetic, biogeographic and species distribution model analyses. Methods Ecol. Evol. 2014, 5, 694–700. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef] [Green Version]
- Phillips, S.J.; Dudık, M.; Schapire, R.E. A maximum entropy approach to species distribution modeling. In Proceedings of the Twenty-First International Conference on Machine Learning, New York, NY, USA, 4–8 July 2004; pp. 655–662. [Google Scholar]
- Elith, J.; Graham, C.H.; Anderson, R.P.; Dudík, M.; Ferrier, S.; Antoine, G.; Hijmans, R.J.; Huettmann, F.; Leathwick, J.R.; Lehmann, A.; et al. Novel methods improve prediction of species’ distributions from occurrence data. Ecography 2006, 29, 129–151. [Google Scholar] [CrossRef] [Green Version]
- Pearson, R.G.; Raxworthy, C.J.; Nakamura, M.; Peterson, A.T. Original article: Predicting species distributions from small numbers of occurrence records: A test case using cryptic geckos in Madagascar. J. Biogeogr. 2006, 34, 102–117. [Google Scholar] [CrossRef]
- Hanley, J.A.; McNeil, B.J. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 1982, 143, 29–36. [Google Scholar] [CrossRef] [Green Version]
- Robin, X.; Turck, N.; Hainard, A.; Tiberti, N.; Lisacek, F.; Sanchez, J.-C.; Muller, M.J. pROC: An open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinform. 2011, 12, 77. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudik, M. Modeling of species distributions with Maxent: New extensions and a comprehensive evaluation. Ecography 2008, 31, 161–175. [Google Scholar] [CrossRef]
- Préau, C.; Trochet, A.; Bertrand, R.; Isselin-Nondedeu, F. Modeling potential distributions of three European amphibian species comparing ENFA and MaxEnt. Herpetol. Conserv. Biol. 2018, 13, 91–104. [Google Scholar]
- Lobo, J.; Jiménez-Valverde, A.; Real, R. AUC: A misleading measure of the performance of predictive distribution models. Glob. Ecol. Biogeogr. 2008, 17, 145–151. [Google Scholar] [CrossRef]
- Hanczar, B.; Hua, J.; Sima, C.; Weinstein, J.; Bittner, M.; Dougherty, E.R. Small-sample precision of ROC-related estimates. Bioinformatics 2010, 26, 822–830. [Google Scholar] [CrossRef] [Green Version]
- Akaike, H. Autoregressive model fitting for control. Ann. Inst. Stat. Math. 1971, 23, 163–180. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 20 June 2021).
- Gloyd, H.K.; Conant, R. Snakes of the Agkistrodon Complex. A Monographic Review. Contributions to Herpetology, No. 6; Society for the Study of Amphibians and Reptile: Oxford, OH, USA, 1990; Volume 614, p. 52. [Google Scholar]
- Pyron, R.A.; Burbrink, F.T.; Wiens, J.J. A phylogeny and revised classification of Squamata, including 4161 species of lizards and snakes. BMC Evol. Biol. 2013, 13, 1–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, M.; Feng, J.C.; Liu, S.Y.; Guo, P.; Zhao, E.M. The phylogenetic position of Thermophis (Serpentes: Colubridae), an endemic snake from the Qinghai-Xizang Plateau, China. J. Nat. Hist. 2009, 43, 479–488. [Google Scholar] [CrossRef]
- Sinervo, B.; Méndez-de-la-Cruz, F.; Miles, D.B.; Heulin, B.; Bastiaans, E.; Villagrán-Santa Cruz, M.; Lara-Resendiz, R.; Martínez-Méndez, N.; Calderón-Espinosa, M.L.; Meza-Lázaro, R.N.; et al. Erosion of lizard diversity by climate change and altered thermal niche. Science 2010, 328, 894–899. [Google Scholar] [CrossRef] [Green Version]
- Rao, D.Q. Complimentary survey of the herpetofauna of Xizang Autonomous Region (Tibet) with discussion of their distribution and current status. Sichuan J. Zool. 2000, 19, 107–112. [Google Scholar]
- Hofmann, S.; Fritzsche, P.; Solhøy, T.; Dorge, T.; Miehe, G. Evidence of sex-biased dispersal in Thermophis baileyi inferred from microsatellite markers. Herpetologica 2012, 68, 514–522. [Google Scholar] [CrossRef]
- Rajabizadeh, M.; Nagy, Z.T.; Adriaens, D.; Avcı, A.; Masroor, R.; Schmidtler, J.; Nazarov, R.; Esmaeili, H.R.; Christiaens, J. Alpine-Himalayan orogeny drove correlated morphological, molecular, and ecological diversification in the Persian dwarf snake (Squamata: Serpentes: Eirenis persicus). Zool. J. Linn. Soc. Lond. 2015, 176, 878–913. [Google Scholar] [CrossRef] [Green Version]
- O’Connor, J.H.; Rittenhouse, T.A.G. Snow cover and late fall movement influence wood frog survival during an unusually cold winter. Oecologia 2015, 181, 635–644. [Google Scholar] [CrossRef] [PubMed]
- Simmons, A.M.; Goulet, J.M.; Bellehumeur, K.F. The effect of snow depth on overwinter survival in Lobelia inflata. Oikos 2010, 119, 1685–1689. [Google Scholar] [CrossRef]
- Zeisset, I.; Bebee, T.J.C. Determination of biogeographical range: An application of molecular phylogeography to the European pool frog Rana lessonae. Proc. R. Soc. B Biol. Sci. 2001, 268, 933–938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gassert, F.; Schulte, U.; Husemann, M.; Ulrich, W.; Rödder, D.; Hochkirch, A.; Engel, E.; Meyer, J.; Habel, J.C. From southern refugia to the northern range margin: Genetic population structure of the common wall lizard, Podarcis muralis. J. Biogeogr. 2013, 40, 1475–1489. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hofmann, S.; Fritzsche, P.; Dorge, T.; Miehe, G.; Nothnagel, M. What Makes a Hot-Spring Habitat “Hot” for the Hot-Spring Snake: Distributional Data and Niche Modelling for the Genus Thermophis (Serpentes, Colubridae). Diversity 2021, 13, 325. https://doi.org/10.3390/d13070325
Hofmann S, Fritzsche P, Dorge T, Miehe G, Nothnagel M. What Makes a Hot-Spring Habitat “Hot” for the Hot-Spring Snake: Distributional Data and Niche Modelling for the Genus Thermophis (Serpentes, Colubridae). Diversity. 2021; 13(7):325. https://doi.org/10.3390/d13070325
Chicago/Turabian StyleHofmann, Sylvia, Peter Fritzsche, Tsering Dorge, Georg Miehe, and Michael Nothnagel. 2021. "What Makes a Hot-Spring Habitat “Hot” for the Hot-Spring Snake: Distributional Data and Niche Modelling for the Genus Thermophis (Serpentes, Colubridae)" Diversity 13, no. 7: 325. https://doi.org/10.3390/d13070325
APA StyleHofmann, S., Fritzsche, P., Dorge, T., Miehe, G., & Nothnagel, M. (2021). What Makes a Hot-Spring Habitat “Hot” for the Hot-Spring Snake: Distributional Data and Niche Modelling for the Genus Thermophis (Serpentes, Colubridae). Diversity, 13(7), 325. https://doi.org/10.3390/d13070325






