Diversity of Useful Mexican Legumes: Analyses of Herbarium Specimen Records
Abstract
1. Introduction
2. Material and Methods
Data Scoring and Statistical Analysis
3. Results
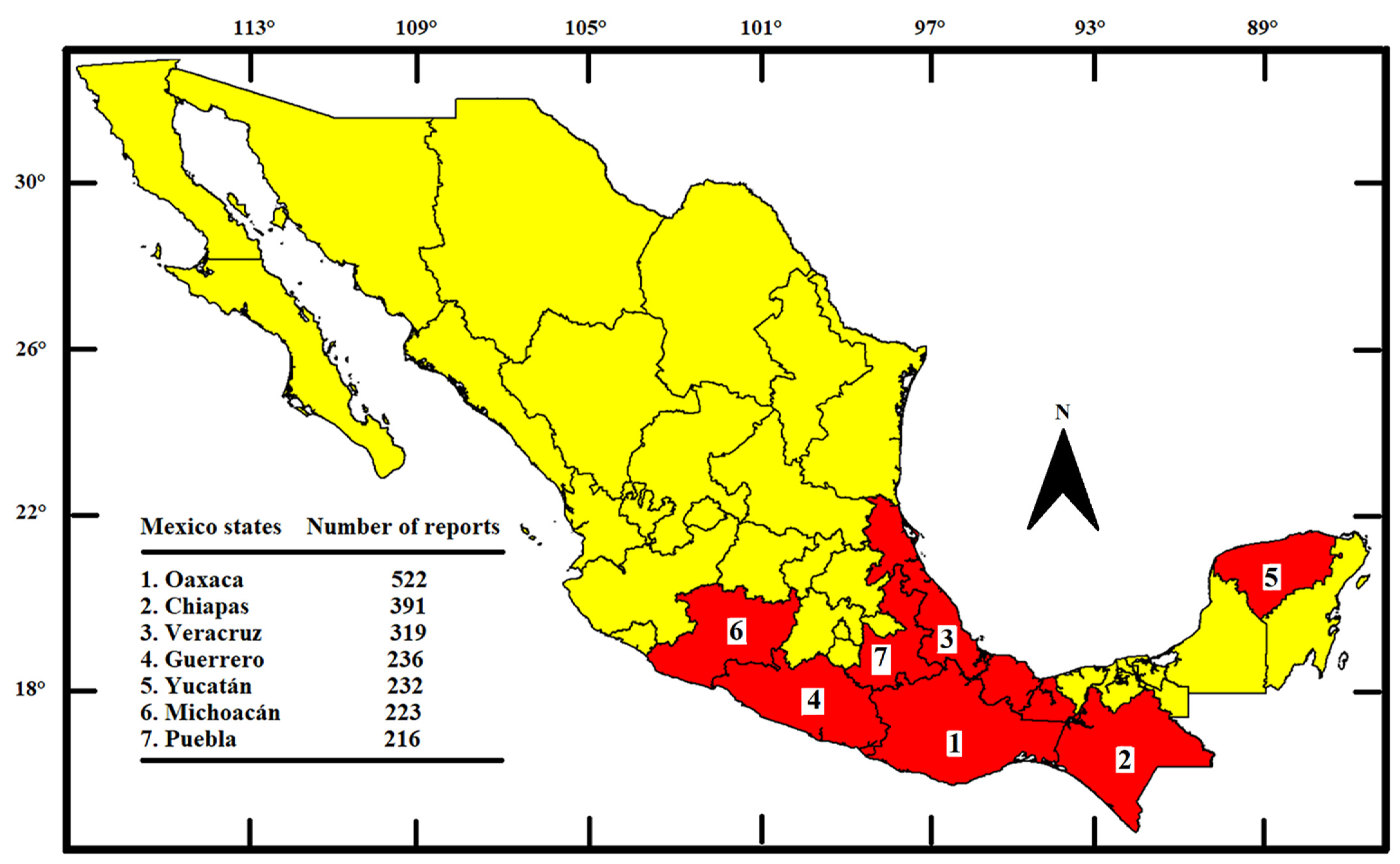
3.1. Mexican Representation and Biocultural Distribution of Useful Legumes at MEXU
3.2. Similarity between Use Categories
3.3. Similarity between Species Based on the Use Categories
3.4. Assessement of Use Values in Sufamilies Cercidoideae, Detarioideae, and Dialioideae
3.5. Analyses of Useful Species of Caesalpinioideae and Papilionoideae
4. Discussion
4.1. Diversity of Species and Their Systematics
4.2. Growth Forms: Importance of Woody Plants
4.3. Relevant Medicine Use
4.4. Food and Domestication
4.5. Animal Food for Livestock
4.6. Other Important Cook’s Use Categories
4.6.1. Gene Sources
4.6.2. Bee Plants
4.6.3. Invertebrate Food
4.7. Threatened or Endangered Species
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Voeks, R.A. Are Women Reservoirs of Traditional Plant Knowledge? Gender, Ethnobotany, and Globalization in Northeast Brazil. Singap. J. Trop. Geogr. 2007, 28, 7–20. [Google Scholar] [CrossRef]
- Brazilian Flora (in construction). Rio de Janeiro Botanical Garden. Available online: http://floradobrasil.jbrj.gov.br/ (accessed on 15 February 2020).
- Sutjaritjai, N.; Wangpakapattanawong, P.; Balslev, H.; Inta, A. Traditional Uses of Leguminosae among the Karen in Thailand. Plants 2019, 8, 600. [Google Scholar] [CrossRef]
- Grijalva, A. Flora útil Etnobotánica de Nicaragua, 1st ed.; Marena: Managua, Nicaragua, 2006; pp. 100–112. [Google Scholar]
- Van Wyk, B.-E. The Diversity and Multiple Uses of Southern African Legumes. Aust. Syst. Bot. 2019, 32, 519–546. [Google Scholar] [CrossRef]
- LPWG. Phylogeny and Classification of the Leguminosae. Taxon 2017, 66, 44–77. [Google Scholar]
- Casas, A.; Valiente-Banuet, A.; Viveros, J.L.; Caballero, J.; Cortes, L.; Dávila, P.; Lira, R.; Rodríguez, I. Plant Resources of the Tehuacán-Cuicatlán Valley, Mexico. Econ. Botany 2000, 55, 129–166. [Google Scholar] [CrossRef]
- Lewis, G.; Schrire, B.; Mackinder, B.; Lock, M. Legumes of the World; Royal Botanic Gardens: Kew, UK, 2005. [Google Scholar]
- Cook, F.E.M. Economic Botany Data Collection Standard; Royal Botanic Gardens: Kew, UK, 1995. [Google Scholar]
- Peck, J.E. Multivariate Analysis for Community Ecologists: Step-by-Step Using PC-ORD; MjM Software Design: Gleneden Beach, OR, USA, 2010. [Google Scholar]
- Sneath, P.H.A.; Sokal, R.R. Numerical Taxonomy, the Principles and Practice of Numerical Classification; Freeman: San Francisco, CA, USA, 1973. [Google Scholar]
- McCune, B.; Mefford, M.J. Multivariate Analysis of Ecological Data; PC-ORD v. 6. MjM Software Design: Gleneden Beach, OR, USA, 2011. [Google Scholar]
- Sokal, R.R.; Rohlf, F.J. The Comparison of Dendrograms by Objective Methods. Taxon 1962, 11, 33–40. [Google Scholar] [CrossRef]
- Rohlf, F.J. NTSYS-pc, Numerical Taxonomy and Multivariate Analysis System, Version 2.1. Exeter Software; Applied Biostatistics Inc.: New York, NY, USA, 2000. [Google Scholar]
- Mantel, N.A. The Detection of Disease Clustering and a Generalized Regression Approach. Cancer Res. 1967, 27, 209–220. [Google Scholar]
- Balzarini, M.G.; González, L.; Tablada, M.; Casanoves, F.; Di Rienzo, J.A.; Robledo, C.W. InfoStat, Manual del Usuario versión 2008; Editorial Brujas: Córdoba, Argentina, 2008. [Google Scholar]
- Instituto Nacional de Estadística y Geografía. INEGI. Available online: https://www.inegi.org.mx/ (accessed on 23 November 2020).
- Monroy Gamboa, A.G.; Sánchez-Cordero, V.; Briones-Salas, M.; Lira-Saade, R.; Maass Moreno, J.M. Representatividad de los Tipos de Vegetación en Distintas Iniciativas de Conservación en Oaxaca, México. Bosque 2015, 36, 199–210. [Google Scholar] [CrossRef][Green Version]
- Sousa, S.M.; Medina, L.R.; Andrade, M.G.; Rico, A.M.L. Leguminosas. In Biodiversidad de Oaxaca; García-Mendoza, A.J., Ordoñez, M.J., Briones-Salas, M., Eds.; Instituto de Biología, Universidad Nacional Autónoma de México: Mexico City, México; Fondo Oaxaqueño para la Conservación de la Naturaleza and World Wildlife Fund: Mexico City, México, 2004; pp. 249–269. [Google Scholar]
- Kruskal, J.B. Nonmetric Multidimensional Scaling: A Numerical Method. Psychometrika 1964, 29, 115–129. [Google Scholar] [CrossRef]
- Avendaño Reyes, S.; Acosta Rosado, I. Plantas Utilizadas como Cercas Vivas en el Estado de Veracruz. Madera Bosques 2000, 6, 55–71. [Google Scholar] [CrossRef]
- Hubbard, T. Natural History of the Desert Ironwood Tree (Olneya tesota). A Synopsis of Published Literature; Desert Museum Organization: Tucson, AZ, USA, 2015. [Google Scholar]
- Mendez, M.O.; Maier, R.M. Phytostabilization of Mine Tailings in Arid and Semiarid Environments—An Emerging Remediation Technology. Environ. Health Perspect. 2008, 116, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Lavin, M.; Sousa, S.M. Phylogenetic Systematics and Biogeography of the Tribe Robinieae (Leguminosae). Syst. Bot. Monogr. 1995, 45, 1–165. [Google Scholar] [CrossRef]
- Nashed-Toral, J.; Valdivieso-Pérez, A.; Aguilar-Jiménez, R.; Cámara-Cordova, J.; Grande-Cano, D. Silvopastoral Systems with Traditional Management in Southeastern Mexico: A Prototype of Livestock Agroforestry for Cleaner Production. J. Clean. Prod. 2013, 57, 266–279. [Google Scholar] [CrossRef]
- Peacock, C. Improving Goat Production in the Tropics: A Manual for Development Workers; Oxfam: Oxford, UK, 1996. [Google Scholar]
- Basurto-Peña, F.; Villalobos, G.; Martínez, M.A.; Sotelo, A.; Gil, L.; Delgado Salinas, A. Use and Nutritive Value of Talet Beans, Amphicarpaea bracteata (L. ) Fern. (Fabaceae: Phaseoleae) as Human Food in Puebla, México. Econ. Bot. 1999, 53, 427–434. [Google Scholar]
- Sotuyo, S. El Palo Morado (Peltogyne mexicana), una Leguminosa Maderable con Futuro Incierto y Parientes Lejanos. Rev. Digit. Univ. 2014, 4, 15. [Google Scholar]
- Hastings, R.B. Medicinal Legumes of Mexico: Fabaceae, Papilionoideae, Part One. Econ. Botany 1990, 44, 336–348. [Google Scholar] [CrossRef]
- Grace, O.M.; Prendergast, H.D.V.; Van Staden, J.; Jäger, A.-K. The Status of Bark in South African Health Care. South Afr. J. Bot. 2002, 68, 21–30. [Google Scholar] [CrossRef][Green Version]
- Catarino, S.; Duarte, M.C.; Costa, E.; García-Carrero, P.; Romeiras, M.M. Conservation and Sustainable Use of the Medicinal Leguminosae Plants from Angola. Peer J. 2019, 7, e6736. [Google Scholar] [CrossRef]
- Duke, J.A. Handbook of Legume of Economic Importance; Plenum Press: New York, NY, USA, 1992; p. 345. [Google Scholar]
- Graham, P.H.; Vance, C.P. Legumes: Importance and Constraints to Greater Use. Plant Physiol. 2003, 131, 872–877. [Google Scholar] [CrossRef] [PubMed]
- Bitocchi, E.; Rau, D.; Bellucci, E.; Rodriguez, M.; Murgia, M.L.; Gioia, T.; Santo, D.; Nanni, L.; Attene, G.; Papa, R. Beans (Phaseolus ssp.) as a Model for Understanding Crop Evolution. Front. Plant Sci. 2017, 8, 722. [Google Scholar] [CrossRef]
- Wiersema, J.H.; León, B. World Economic Plants: A Standard Reference, 2nd ed.; CRC Press: Boca Ratón, FL, USA, 2016. [Google Scholar]
- Clement, C.R.; Casas, A.; Parra-Rondinel, F.A.; Levis, C.; Peroni, N.; Hanazaki, N.; Cortés-Zárraga, L.; Rangel-Landa, S.; Alves, R.P.; Ferreira, M.J.; et al. Disentangling Domestication from Food Production Systems in the Neotropics. Quaternary 2021, 4, 4. [Google Scholar] [CrossRef]
- Saslis-Lagoudakis, C.H.; Savolainen, V.; Williamson, E.M.; Forest, F.; Wagstaff, S.J.; Baral, S.R.; Watson, M.F.; Pendry, C.A.; Hawkins, J.A. Phylogenies Reveal Predictive Power of Traditional Medicine in Bioprospecting. Proc. Natl. Acad. Sci. USA 2012, 109, 15835–15840. [Google Scholar] [CrossRef]
- Souza, E.N.F.; Williamson, E.M.; Hawkins, J.A. Which Plants Used in Ethnomedicine are Characterized? Phylogenetic Patterns in Traditional Use Related to Research Effort. Front. Plant Sci. 2018, 9, 834. [Google Scholar] [CrossRef] [PubMed]
- Dowes, E.; Crouch, N.R.; Edwards, T.J.; Mulholland, D.A. Regression Analyses of Southern African Ethnomedicinal Plants: Informing the Targeted Selection of Bioprospecting and Pharmacological Screening Subjects. J. Ethnopharamacology 2008, 119, 356–364. [Google Scholar] [CrossRef]
- Selegato, D.M.; Monteiro, A.F.; Vieira, N.C.; Cardoso, P.; Pavani, V.D.; Bolzani, V.S.; Castro-Gamboa, I. Update: Biological and Chemical Aspects of Senna spectabilis. J. Braz. Chem. Soc. 2017, 28, 415–426. [Google Scholar] [CrossRef]
- Marazzi, B.; Endress, P.K.; de Queiroz, L.P.; Conti, E. Phylogenetic Relationships within Senna (Leguminosae, Cassiinae) Based on Three Chloroplast DNA Regions: Patterns in the Evolution of Floral Symmetry and Extrafloral Nectaries. Am. J. Bot. 2006, 93, 288–303. [Google Scholar] [CrossRef]
- Southon, I.W.; Bisby, F.A.; Buckingham, J.; Harborne, J.B. (Eds.) Phytochemical Dictionary of the Leguminosae; Chapman & Hall: London, UK, 1994. [Google Scholar]
- Moerman, D.E. Native North American Food and Medicinal Plants: Epistemological Considerations. In Plants for Food and Medicine; Prendergast, H.D.V., Etkin, N.L., Harris, D.R., Houghton, P.J., Eds.; Royal Botanic Gardens: Kew, UK, 1998; pp. 69–74. [Google Scholar]
- Souza, E.N.F.; Hawkins, J.A. Comparison of Herbarium Label Data and Published Medicinal Use: Herbaria as an Underutilized Source of Ethnobotanical Information. Econ. Bot. 2017, 71, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wink, M. Evolution of Secondary Metabolites in Legumes (Fabaceae). South Afr. J. Bot. 2013, 89, 164–175. [Google Scholar] [CrossRef]
- Acosta-Gallegos, J.A.; Kelly, J.D.; Gepts, P. Prebreeding In Common Bean And Use Of Genetic Diversity From Wild Germplasm. Crop Sci. 2017, 47, S44–S59. [Google Scholar] [CrossRef]
- Zamora-Natera, J.F.; Rodríguez-Macías, R.; Salcedo-Pérez, E.; García-López, P.; Barrientos-Ramírez, L.; Vargas-Radillo, J.; Soto-Velasco, C.; Ruiz-López, M.A. Forage Potential of Three Wild Species of Genus Lupinus (Leguminosae) from Mexico. Legume Res. Int. J. 2020, 43, 93–98. [Google Scholar]
- Suso, M.J.; Bebeli, P.J.; Christmann, S.; Mateus, C.; Negri, V.; Pinheiro de Carvalho, M.A.A.; Torricelli, R.; Veloso, M.M. Enhancing Legume Ecosystem Services through an Understanding of Plant-pollinator Interplay. Front. Plant Sci. 2016, 7, 333. [Google Scholar] [CrossRef] [PubMed]
- Villanueva-Gutiérrez, R.; Roubik, D.W.; Colli-Ucán, W.; Tuz-Novelo, M. The Value of Plants for the Mayan Stingless honeybee Melipona beecheii (Apidae: Meliponini): A Pollen-based Study in the Yucatán Peninsula, Mexico. In Pot-Pollen in Stingless Bee Melittology; Vit, P., Pedro, S.R.M., Roubik, D.W., Eds.; Springer: Cham, Switzerland, 2018. [Google Scholar]
- Ramos-Elorduy, J. Threatened Edible Insects in Hidalgo, Mexico and Some Measures to Preserve Them. J. Ethnobiol. Ethnomedicine 2006, 2, 51. [Google Scholar] [CrossRef] [PubMed]
- Norma Oficial Mexicana NOM-059-Secretaría de Medio Ambiente y Recursos Naturales (SEMARNAT) México. Available online: https://dof.gob.mx/nota_detalle_popup.php?codigo=5173091 (accessed on 20 November 2010).
- Red List of Threatened Species, IUCN. Version 2009. IUCN. International Union for Conservation of Nature. Available online: https://www.iucnredlist.org (accessed on 23 March 2021).
- Barstow, M. Olneya tesota. The IUCN Red List of Threatened Species. 2018. Available online: https://dx.doi.org/10.2305/IUCN.UK.2018-2.RLTS.T62026716A62026718.en (accessed on 15 March 2021).
- Cervantes, A.; Linares, J.; Quintero, E. An Updated Checklist of the Mexican Species of Dalbergia (Leguminosae) to Aid in Its Conservation Efforts. Rev. Mex. Biodivers. 2019, 90, e902528. [Google Scholar] [CrossRef]
- Delgado-Salinas, A.; Alejandre-Iturbide, G.; Azurdia, C. Wild Species of Phaseolus. The IUCN Red List of Threatened Species. Available online: https://dx.doi.org/10.2305/IUCN.UK.2019-3.RLTS.T71777142A71777145.en (accessed on 20 December 2019).




| Subfamily | Tribe | useGen. | MxGen. | %taxa | useSpp. | MxSpp. | %taxa |
|---|---|---|---|---|---|---|---|
| Cercidoideae | Cercidieae | 2 | 3 | 66 | 7 | 35 | 20 |
| Detarioideae | Amherstieae | 1 | 1 | 100 | 1 | 3 | 33 |
| Detarioideae | Detarieae | 2 | 2 | 100 | 2 | 2 | 100 |
| Dialioideae | Dialiineae | 1 | 2 | 50 | 1 | 2 | 50 |
| Caesalpinioideae | Acacieae | 4 | 4 | 100 | 40 | 86 | 46 |
| Caesalpinioideae | Caesalpinieae | 12 | 15 | 80 | 22 | 74 | 29 |
| Caesalpinioideae | Cassieae | 3 | 3 | 100 | 38 | 85 | 44 |
| Caesalpinioideae | Ingeae | 16 | 17 | 94 | 72 | 155 | 46 |
| Caesalpinioideae | Mimoseae | 9 | 12 | 75 | 70 | 165 | 42 |
| Papilionoideae | Amorpheae | 4 | 7 | 57 | 32 | 197 | 16 |
| Papilionoideae | Brongniartieae | 2 | 2 | 100 | 7 | 66 | 10 |
| Papilionoideae | Crotalarieae | 1 | 1 | 100 | 13 | 23 | 56 |
| Papilionoideae | Dalbergieae | 13 | 14 | 92 | 38 | 117 | 32 |
| Papilionoideae | Desmodieae | 1 | 3 | 33 | 23 | 98 | 23 |
| Papilionoideae | Galegeae | 1 | 3 | 33 | 2 | 99 | 2 |
| Papilionoideae | Genisteae | 1 | 2 | 50 | 6 | 83 | 7 |
| Papilionoideae | Indigofereae | 1 | 1 | 100 | 9 | 34 | 26 |
| Papilionoideae | Millettieae | 3 | 5 | 60 | 35 | 139 | 25 |
| Papilionoideae | Phaseoleae | 20 | 29 | 69 | 74 | 250 | 29 |
| Papilionoideae | Psoraleeae | 2 | 4 | 50 | 2 | 10 | 20 |
| Papilionoideae | Robinieae | 6 | 9 | 66 | 18 | 51 | 35 |
| Papilionoideae | Sesbanieae | 1 | 1 | 100 | 1 | 5 | 20 |
| Papilionoideae | Sophoreae | 7 | 9 | 77 | 10 | 43 | 23 |
| Papilionoideae | Swartzieae | 1 | 2 | 50 | 1 | 6 | 16 |
| Papilionoideae | Trifolieae | 1 | 1 | 100 | 1 | 18 | 5 |
| Papilionoideae | Loteae | 0 | 2 | 0 | 0 | 32 | 0 |
| Papilionoideae | Thermopsideae | 0 | 2 | 0 | 0 | 3 | 0 |
| Papilionoideae | Vicieae | 0 | 2 | 0 | 0 | 12 | 0 |
| Use Categories | Cercidioideae * | Detarioideae * | Dialoideae * | Total (%) |
|---|---|---|---|---|
| FOOD | 2 | 1 | 1 | 4 (12%) |
| ENVIRONMENTAL | 5 | 0 | 0 | 5 (15.5%) |
| MATERIALS | 3 | 2 | 1 | 6 (18%) |
| MEDICINES | 7 | 2 | 1 | 10 (30%) |
| ANIMAL FOOD | 6 | 0 | 0 | 5 (15.5%) |
| FUELS | 2 | 0 | 0 | 2 (6%) |
| FOOD ADDITIVES | 0 | 0 | 0 | 0 |
| SOCIAL | 0 | 0 | 0 | 0 |
| Caesalpinioideae * | |||
|---|---|---|---|
| 97 spp. Single—Use (40%) | 147 spp. Multipurpose (60%) | ||
| Use Categories | Total Species, (%) | Total Species, (%) | Total |
| FOOD | 17 (7) | 51 (21) | 68 |
| ENVIRONMENTAL | 12 (5) | 65 (26.6) | 77 |
| MATERIALS | 15 (6.1) | 91 (37.4) | 106 |
| MEDICINES | 20 (8.1) | 91 (37.2) | 111 |
| ANIMAL FOOD | 28 (11.4) | 67 (27.4) | 95 |
| FUELS | 5 (2) | 84 (34.4) | 89 |
| FOOD ADDITIVES | 0 | 3 (1.2) | 3 |
| SOCIAL | 0 | 6 (2.4) | 6 |
| Papilionoideae * | |||
|---|---|---|---|
| 154 spp. Single—Use (57%) | 117 spp. Multipurpose (43%) | ||
| Use Categories | Total Species, (%) | Total Species, (%) | Total |
| FOOD | 7 (2.5) | 35 (13) | 42 |
| ENVIRONMENTAL | 11 (4) | 46 (17) | 57 |
| MATERIALS | 19 (7) | 66 (24.3) | 85 |
| MEDICINES | 58 (21.4) | 72 (26.5) | 130 |
| ANIMAL FOOD | 56 (20.6) | 49 (18) | 105 |
| FUELS | 2 (0.7) | 22 (8.11) | 24 |
| FOOD ADDITIVES | 0 | 6 (2.2) | 6 |
| SOCIAL | 1 (0.3) | 8 (3) | 9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delgado-Salinas, A.; Torres-Colín, L.; Luna-Cavazos, M.; Bye, R. Diversity of Useful Mexican Legumes: Analyses of Herbarium Specimen Records. Diversity 2021, 13, 267. https://doi.org/10.3390/d13060267
Delgado-Salinas A, Torres-Colín L, Luna-Cavazos M, Bye R. Diversity of Useful Mexican Legumes: Analyses of Herbarium Specimen Records. Diversity. 2021; 13(6):267. https://doi.org/10.3390/d13060267
Chicago/Turabian StyleDelgado-Salinas, Alfonso, Leticia Torres-Colín, Mario Luna-Cavazos, and Robert Bye. 2021. "Diversity of Useful Mexican Legumes: Analyses of Herbarium Specimen Records" Diversity 13, no. 6: 267. https://doi.org/10.3390/d13060267
APA StyleDelgado-Salinas, A., Torres-Colín, L., Luna-Cavazos, M., & Bye, R. (2021). Diversity of Useful Mexican Legumes: Analyses of Herbarium Specimen Records. Diversity, 13(6), 267. https://doi.org/10.3390/d13060267






