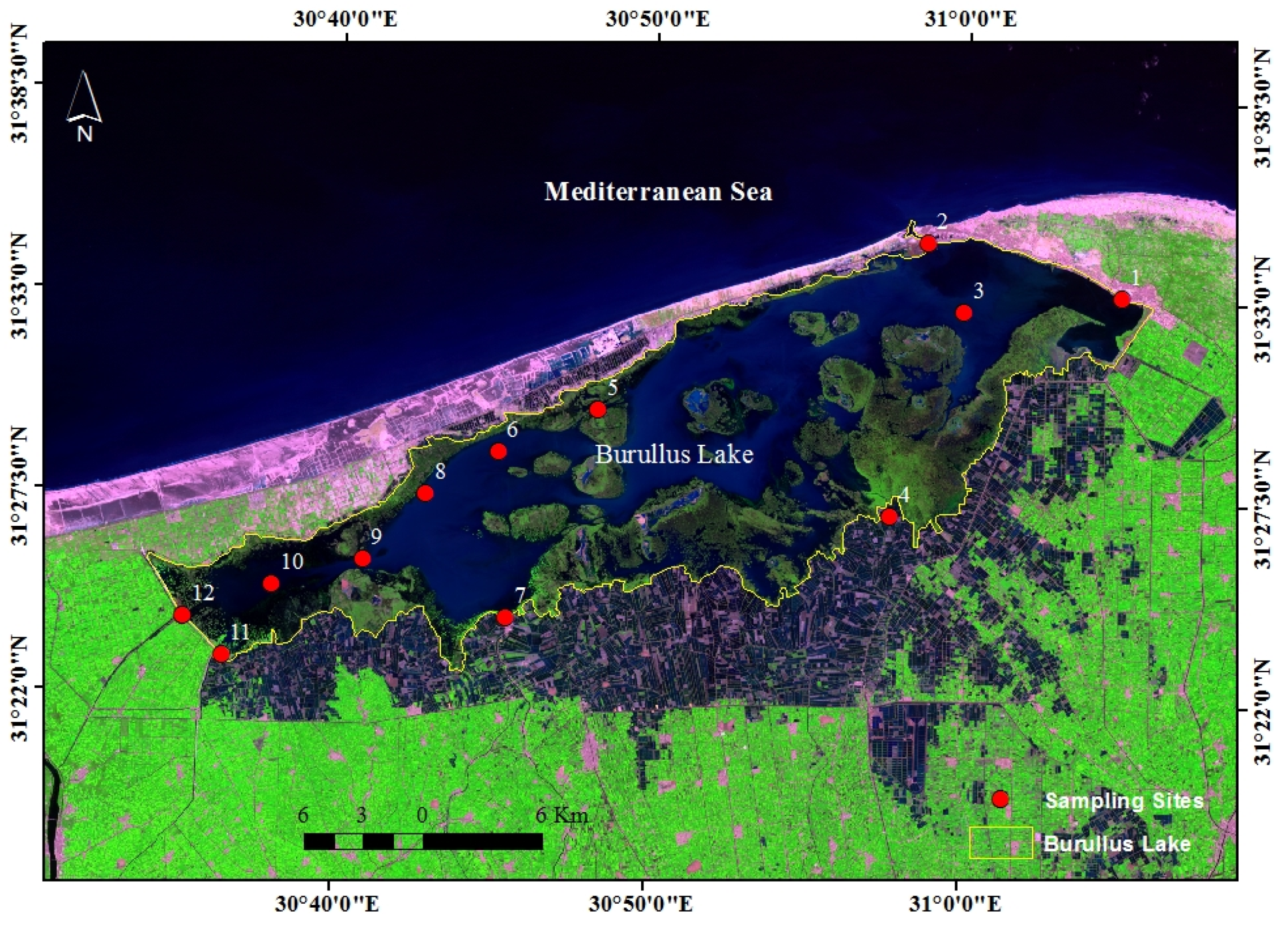
3.1. Water Characteristics and Physicochemical Parameters
The physicochemical parameters recorded at the sampled stations from 2017 to 2019 are summarized in
Table 3. The temperature value is a crucial parameter for all biological processes [
16]. The mean water temperature in the lakes fluctuated between a minimum mean value of 14.47 °C in the winter season of 2018 and a high of 31.23 °C in the summer. In the current study, the maximum value was observed in the year 2017 in the summer season, and the annual average of three years was 21.77 °C. According to EPA [
29], the water temperature ranged from 15.1 to 32.6 °C in lake water, which is suitable for fish growth.
The pH values can affect the biological activities of microbial metabolites that can influence the further utilization of succeeding metabolic products. In the current study, the values of pH of water samples ranged between 7.32 and 9.36 with an annual mean of 8.09. The highest value was observed in front of El-Kashaa drain in the summer season of 2017, while the lowest result was noted at station 11 in the winter season of 2019.
According to the annual average values, the hot season (summer) recorded a higher pH level than the cold season (winter), which is correlated, mainly, to the increase in CO
2 uptake because of the rise in the photosynthetic activity, which leads to high pH values [
3,
30]. High pH values occur according to the nature of effluent in the lake in addition to the fermentation process, decay of organic matter, and release of organic acids by bacterial activity [
28]. These results were in the same ranges stated by Okbah and Hussein [
31]. El-Alfy et al. [
32] at the Burullus Lake showed that the dominant alkaline pH was >7.0. Additionally, Khairy et al. [
33] showed that the average of pH values in Burullus Lake was 8.1.
Salinity has a significant influence on measuring numerous conditions of the aquatic biological processes and the chemistry of natural waters [
34,
35]. In the present study, salinities exhibited wide variations between 0.62 and 38.9‰, with the maximum value being noted in station 2, in front of El-Boughaz in 2017 in the winter season; whereas, the highest values were related to marine water intrusion along the Boughaz area. Conversely, the lowest value was recorded in front of drain El-Hoks (station 11) in 2018 in the summer season, with an average value of 0.62‰. Low salinity values indicate that the water content in the lake was mainly freshwater due to human activities and the quantity and quality of drainages runoff in the lake. Salinity distribution in the lagoon water reflects a decreased gradient from the east to the west. This gradient depends on the amount of drainage water that comes from the south drains, fresh Nile water from Brimbal Canal at the west, and sea water from the sea outlet at the east, and therefore, marine plankton species are restricted only in the eastern basin and in some cases dominate the community. According to previous references, the salinity of the lagoon increased from year to year, which creates high brackish water conditions especially in the eastern basin (>17 PSU) [
36]. El-Shinnawy [
37] reported that the high salinity and presence of marine forms in the eastern basin at Burullus Lake could be due to the closure policy of the pumping drain stations, which diminish the water level to be below sea level and permits seawater to enter the lagoon. As well, Nassar and Gharib [
38] reported that the average salinity of the Burullus Lake in the year 2013 was 3.6‰.
Dissolved oxygen (DO) is considered as one of the maximum important factors controlling the biota in the aquatic habitat [
16]. In the current study, DO values varied from 0.9 to 19.87 mg L
−1. The higher value was obtained at the front of El-Kashaa drain in the year 2017 in the summer season, while the lowest value was documented at station 11 in the winter season of 2018, with an annual mean of the study period of 8.83 mg L
−1. Under low dissolved oxygen, many marine plants and animals may not survive [
39]. The decrease in oxygen supply in the water showed a negative effect on aquatic life. The highest value occurs during the summer season due to the increase in photosynthetic activity during this season, which liberates an important quantity of oxygen to the surrounding water ecosystem, since the photosynthetic method was regarded as the highest source of oxygen in the aquatic environment [
33]. Moreover, the quantity of DO in water could be based on the salinity and temperature of the water, as cold liquid can hold more DO than warmish liquid [
40]. These results agreed with those achieved by Hereher et al. [
41] and Khairy et al. [
33] who showed that the average concentration of DO along the Burullus Lake was 7.6 mg L
−1.
Biological oxygen demand (BOD) gives data on the biological convertible amount of the content of organic content in marine samples. In the current work, BOD in the lakeshores habitat varied from 1.67 to 176.60 mg L
−1, with an average value of 23.63 mg L
−1. The maximum value was recorded at station 9 (Abou Amer) in the year 2018 in the autumn season, and the lowest result was showed in facing the El-Boughaz outlet in the spring season of 2019 due to the dilution effect of low organic matter loaded seawater. BOD values of 3 mg L
−1 indicate pure water, but values that reach 5 mg L
−1 give an indication of doubtful purity of water [
42]. The high values of DO may be because of the abundance of plankton, which enhanced water quality with oxygen throughout photosynthesis activity [
15,
43,
44]. In addition, augmented oxygen reduction is essential for oxidation of the organic matter in the aquatic body [
45,
46]. Younis and Nafea [
47] recorded that the concentration of biological oxygen demand in the water samples at Burullus Lake ranged from 11.7 to 36.66 mg L
−1.
Chemical oxygen demand (COD) is an important parameter for determining the quality of water and wastewater quality. The COD test is used to monitor water treatment plant efficiency. In the current study, COD showed different values between different habitats. It varied from 30.12 to 423.98 mg L
−1. The maximum value was recorded in the year 2018 in the winter season, and the lowest value was recorded in the year 2018 in the autumn season, with an annual average value of 100.39 mg L
−1. The permissible limit for COD in marine water is 100 mg L
−1 [
48]. The average data of COD parameter in all environments were exceeding 40 mg L
−1, hence the lake is measured as polluted water as reported by Hassan et al. [
45] who stated that water bodies contain COD > 40 mg L
−1 are considered as polluted waters; however, those containing COD > 120 mg L
−1 are extremely polluted.
Nitrogen and phosphorous are the nutrients most likely to remain deficient in the contaminated environment. Nutrients from anthropogenic pollution can degrade water quality and alter the balance of marine food webs [
16,
19]. Ammonia (NH
4) contents in the water of lakeshores habitat varied from 0.013 to 4.45 mg L
−1, which was the maximum value recorded in station 7 in front of downstream 8 and 9 drains in the winter season in the year 2017. The annual average value during the three-year period of the study was 0.613 mg L
−1. The minimum values may be related to increases in plankton biomass. The high phytoplankton population in water bodies utilizes a high content of ammonium ions in preference to other inorganic nitrogen [
49]. Our results agree with Okbah and Hussien [
25], who showed that in the regional variations of NH
4, the minimum mean concentration was 3.70 μmol L
−1. In contrast, the maximum mean value of NH
4 was 12.33 μmol L
−1.
Nitrite (NO
2) is the noxious via-creation of nitrifying bacteria (nitrospiration) in substrate consuming NH
3 or a filter. In the current study, nitrite ranged from 2.98 (summer season) to 419.71 mg L
−1 in station 6, in the spring season in the year 2018, with a mean value throughout the three-year stage of this study of 126.93 mg L
−1. The highest value of water nitrite is related to human activities of diverse origin mainly from domestic drainage. It also occurs due to the nitrification of free ammonia and a reduction in nitrate to nitrite and use of ammonium fertilizers. Minimum values of nitrite are explicating to these places that are far away from any pollution sources. Nassar and Gharib [
38] recorded that the average concentration of NO
2 in Burullus Lake was 0.1 µmol L
−1.
Nitrate (NO
3) is considered as the most stable and predominant inorganic nitrogen form in seawater, in addition to major nutrients for the phytoplankton growth [
50]. Nitrate serves as another electron acceptor under anoxic conditions. The present values of nitrate ranged from 0.13 to 1.64 mg L
−1. The present study found that the highest value of nitrate was observed in the year 2017 in the winter season in station 6 in the north of the lake. The increase in nitrate values may be attributed to the discharge of sewage wastes and the increase in mineralization of organic matter. Additionally, it is noticed that the high results of nitrate are related to drainage 9 and 8 of the agricultural wastewater that are loaded by high amounts of nitrogenous fertilizers, discharged into these sites. The minimum value was recorded in station 5 in the middle of the lake, which related to the increase in aquatic plants that feed on nutrients and cause depletion in their concentration [
51,
52,
53]. Abd El-Hamid [
54] reported that NO
3 concentrations in water samples at Burullus Lake ranged from 0.15 to 0.47 mg L
−1.
Total nitrogen (TN) concentration in the investigated area showed narrow variation, ranging between a minimum value of 0.52 mg L
−1 at sampling site 2 in front of El-Boughaz in the summer season and a maximum value of 10.33 mg L
−1 at El-Shakhlouba in the winter season as a result of the effect of industrial, agricultural, and demotic wastes through drains 8 and 9, with an average concentration of 3.40 mg L
−1. It is obvious that the water of this lake surrounds a considerable quantity of particulate nitrogen related to organisms and products of their metabolism, besides decay [
31]. These results agree with El-Zeiny and El-Kafrawy [
55]; they showed that the highest concentrations of TN have been noticed in the parts polluted by fertilizer, run-off animal wastes, and domestic sewage at the southeastern and western part of the lake.
Phosphorous is needed for the formation of cellular enzymatic compounds used in the processes of synthesis and degradation. The most common sources of phosphorous in bacterial sources are K
2HPO
4, KH
2PO
4, NaH
2PO
4, Na
2HPO
4, or mixtures of them [
56]. The current results found that total phosphorus content showed its high values in water in the year 2019, with a high value (60.96 mg L
−1) in winter in station 7 and a low value (2105.77 mg L
−1) in summer in station 2 at summer season. While the annual average through the three-year time of the study was 607.23 mg L
−1. Maximum values are related to industrial effluents and domestic sewage disposal to these sites. In contrast, the minimum values are related to an increase in plankton, which feed on nutrients and cause depletion in its concentration [
56]. El-Zeiny and El-Kafrawy [
55] documented that the concentration of total phosphorous in Burullus Lake ranged from 4.42 to 53.6 mg L
−1.
Chlorophyll-a (
Chlo-a) is considered as the main pigment that can be used for the determination of phytoplankton biomass, and it is used as a trophic state indicator and reflected the water quality in the aquacultures ecosystem [
57]. In the current study, the
Chlo-a contents ranged from 6.25 to 383.81 μg L
−1. The average value for three years was 62.93 μg L
−1. In general, the higher result was found in station 4 in front of drain 7 in the autumn season, while the lowest value was exhibited at station 2 in the spring season of 2017. The high values of
Chlo-a in the investigated area are undoubtedly due to the rich supply of reactive silicate and reactive phosphate; these nutrient salts contribute to the growth of phytoplankton expressed in high levels of
Chlo-a, which lead to the eutrophication process in the Lake. Abd El-Hamid et al. [
54] reported that chlorophyll-a concentrations in aquatic samples of Burullus Lake ranged from 3.1 to 108 µg L
−1.
Generally, it is notable that regarding the Chlo-a and NH4 in Burullus Lake, the minimum results were recorded at the northern part of the lake especially in the next El-Boughaz part due to it receiving water from the sea by the Al-Boughaz outlet. On the other hand, the maximum values of Chlo-a and NH4 were noted at the southern part of Burullus Lake; this may be due to a dense population of phytoplankton as a result of the huge amounts of discharged wastewater that is heavily loaded with nutrient. Moreover, NH4, TN, and total P exhibited high contents in station 7 in front of drains 8 and 9 compared with the sampling sites.
3.2. Heavy Metals
The maximum, minimum, and average concentrations of heavy metal ions in the water samples collected from Burullus Lake are shown in
Table 4.
The different heavy metals, including Pb
3+, Cr
3+, Ni
2+, and Cd
2+, are toxic ions to living organisms even at quite low concentrations, whereas Zn
2+ and Cu
2+ are more biologically essential as natural constituents of aquatic ecosystems and, generally, only become toxic at very high concentrations. Heavy metals are normally entering the aquatic environment through erosion of the geological matrix, or due to anthropogenic activities caused by industrial effluents, domestic sewage, and mining wastes [
58]. Fe
2+ compounds in aquatic environments resulting in Fe
2+ precipitate in alkaline and oxidizing conditions [
34]. In the current work, the Fe
2+ values in the water varied from 6.68 to 220.71 μg L
−1. The maximum value was recorded in the year 2018, while the annual average for three years was 49.29 μg L
−1. The high concentration of oxygen leads to Fe
2+ oxidation and subsequent hydrolysis to form insoluble Fe(OH)
3. The maximum concentration of Fe
2+ observed in the current study may be due to the industrial wastewater discharged to the coastal area of the Mediterranean Sea. High levels of Fe
2+ may decrease the microbial degradation of hydrocarbon in seawater because excessive concentrations break down enzyme action [
59]. Eid et al. [
60] found that Fe
2+ concentrations in Burullus Lake were high during the growing season.
Copper complexation in water is affected by dense phytoplankton blooms [
61]. In the current study, the mean values of Cu
2+ content in Burullus Lake ranged from 1.64 to 34.56 μg L
−1. The maximum value was recorded in the year 2017 in station 7, and the annual average of the three years was 8.81 μg L
−1. The maximum concentration of Cu
2+ may lead to a negative impact on microbial degradation of hydrocarbon in seawater, which excessively breaks down the action of the enzyme [
62]. Nafea and Zyada [
63] noticed that the concentration of copper content in the water of Burullus Lake ranged between 19.2 and 35.8 36.9 mg L
−1.
Zinc is an essential ion for the growth of marine organisms, and its concentration is affected by plankton communities [
59]. The current work observed that Zn
2+ showed its high mean value in water (34.56 μg L
−1) in station 2 in the year 2017 and the low one (0.12 μg L
−1), and the average low value was 11.28 μg L
−1. The relatively high Zn
2+ level is suggestive of the influence of refuse dump and domestic sewage sources. It could also be attributed to industrial effluents and urbanization. High temperature and low dissolved oxygen concentration lead to an increase in toxicity of Zn
2+ [
22]. Cr is one of the biochemically active transition metals in the aquatic environment [
64]. In the current study, dissolved chromium values in the water of the lake varied from 2.29 to 34.98 μg L
−1. The maximum value was recorded in 2019, and the annual average of three years was 10.19 μg L
−1. The values in the lake are within the EPA limit (100 μg L
−1) [
64]. Cr oxidizes easily from trivalent to hexavalent. Cr
3+ ion is not toxic, but an essential nutrient, but Cr
6+ ion is very toxic and may damage adrenals, livers, and lungs.
Ni
2+ contents in water bodies are attributed as results from industrial and urban activities and may accumulate in many types of fishes and macrophytes [
65]. In the current study, Ni
2+ ions contents ranged between 1.59 and 12.98 μg L
−1. The maximum value was observed in the year 2018, and the annual average of three years was 5.44 μg L
−1. The higher value of Ni is associated with Fe and Mn, because of the fact that Ni
2+ has been scavenged directly from water by hydrous MnO
2 [
59]. El-Amier et al. [
64] showed that nickel concentration along Burullus Lake ranged from 1.26 to 9.37 µg L
−1 with an average value of 5.10 µg L
−1. Cd
2+ is one of the greatest poisonous metals with widespread carcinogenic effects in humans and is considered to be toxic if its concentration exceeds 0.01 mg L
−1 both in drinking and irrigation water [
9,
12]. Cd
2+ ions are extremely toxic to fish. Cd
2+ contents ranged from 0.04 to 3.35 μg L
−1 in front of Burullus east drain in the year 2018 as a result of agricultural wastes, especially agricultural fertilizers, and the annual average was 1.186 μg L
−1.
Pb
2+ is a very significant ion in the aquatic system [
66]. Lead contents in the current study showed its high mean values in water (18.51 μg L
−1) at station 7 in the year 2019, while it showed a low mean value of 0.29 μg L
−1 with annual averages from three years of 4.87 μg L
−1. The highest values of lead can disrupt the health system of phytoplankton, which is an important source of oxygen production in seas. The great level of lead may be attributed to the agricultural and industrial effluents in addition to the spill of fishing boats, which are distributed along the coast of the study area. However, many ships have been painted by a dye containing a high concentration of Pb
2+ metal. Pb
2+ in high concentrations causes hemorrhages and congestion of the gastrointestinal tract and kidneys of fish [
67]. Masoud et al. [
68] recorded that the concentration of lead content along Burullus Lake ranged from 4.5 to 10.1 µg L
−1.
3.4. Zooplankton Community
Zooplankton serve in the evaluation and productivity of the ecosystem, since they are considered as an essential food for other organisms. The studies of the distributions of zooplankton are beneficial for the monitoring of definite characteristics of the environment such as hydrographic, pollution, eutrophication, and long-term changes, which are signs of environmental disturbances. Therefore, zooplankton studies becoming very important in both marine and freshwater ecosystems [
27,
28]. In the current study, the comparison among the three years (2017 to 2019 seasons) in the distribution, density, and occurrence of zooplankton was conducted. The zooplankton assemblage was dominated by Rotifera, Copepoda, Protozoa, and Cladocera, respectively, while other groups have rare occurrence so appear and disappear in different years and stations. Over the study period, mean zooplankton density was higher in the western basin (333,579 ind. M
−3) in the same trend with Khalil [
71], Dumont and El-Shabrawy [
72] and Saad et al. [
73]. The middle and eastern basins (290,707 and 142,978 ind. M
−3, respectively) may be increased for phytoplankton in this area [
28], while mean zooplankton density was higher in the 2017 year (301,664 ind. M
−3) than the 2019 and 2018 years (238,703 and 226,897 ind. M
−3, respectively) as shown in
Table 8.
In 2017, a total of 93 taxa from 52 genera and 10 groups were noted for the study, namely, Protozoa (16 taxa); Rotifera (34 taxa), the same number in Saad et al. [
73], the dominant species being
Brachionus angularis,
Brachionus calyciflorus, and
Keratella valga; Copepoda (28 taxa), the dominant species being
Acanthocyclpos vernalis,
Mesocyclops leuckarti, and
Thermocyclops crassus; Cladocera (nine taxa), the dominant species being
Daphnia hyaline and
Daphnia longispina, and taxon for each of Ostacoda, Cirripeda, Nematoda, Annelid, Decapoda, and Velliger of lamellibranchs.
In 2018, a total of 98 taxa from 59 genera and 10 groups with larvae were documented for the study, namely, Protozoa (20 taxa from 16 genera); Rotifera (35 taxa), the dominant species being Brachionus angularis, Brachionus calyciflorus, Keratella valga, and Keratella quadrata; Copepoda (28 taxa), the dominant species being Acanthocyclpos vernalis, Mesocyclops leuckarti, and Thermocyclops crassus; Cladocera (nine taxa), the dominant species being Moina micrura, and taxon for each of Ostacoda, Cirripeda, Nematoda, Annelid, Decapoda, and Velliger of lamellibranchs.
In 2019, a total of 98 taxa from 59 genera and 10 groups with larvae were detailed for the study, namely, Protozoa (20 taxa from 16 genera); Rotifera (35 taxa), the dominant species being
Brachionus angularis, Brachionus calyciflorus, Keratella valga, and
Keratella quadrata; Copepoda (28 taxa), the dominant species being
Acanthocyclpos vernalis, Mesocyclops leuckarti, and
Thermocyclops crassus; Cladocera (nine taxa), the dominant species being
Moina micrura, and taxon for each of Ostacoda, Cirripeda, Nematoda, Annelid, Decapoda, and Velliger of lamellibranchs. While, Shaltout [
74] recorded 90 taxa in Burullus Lake.
The lowest population densities of zooplankton at the sites of the eastern sector are perhaps due to their vertical migration far of sunlight. Many authors concluded that the zooplankton has a high population density in the summer season, but their lowest one happens in winter [
14,
16,
75]. Additionally, it is difficult to distinguish between the effects of most environmental factors on the abundance at most aquatic ecosystems due to inter-relation between them; also, the biological and ecological factors have the same effects of environmental factors on zooplankton abundance inside any aquatic system [
12,
76]. So, it is not surprising that the standing crop of zooplankton decline in autumn, although environmental conditions are good; this is perhaps due to the great amounts of fish fries that are received at these zones. Heneash et al. [
77] reported that Copepoda were related (twice) mainly to domestic activities, while Rotifera were related to aquaculture activities [
12,
16].
3.6. Similarity between Sites
During the year 2017, the data indicated the presence of three clusters (
Figure 2A). The first cluster included station 3 in the seaside of El-Burullus Boughaz with relatively low similarity to the other stations being 75% on average. The second cluster included three stations 9,4, and 10 with 92% similarity between them, but a sub-cluster was found between stations 4 and 10 with 97.5% being the highest similarity in this period. However, the third cluster included other stations being 86% (stations 7, 8, 1, 5, 12, 2, 6, and 11). However, this cluster contains three sub-clusters (between stations 7and 8, sub-cluster station 1, sub-cluster between 5, 12, 2, 6, and 11).
During the year 2018, the data indicated the presence of four clusters (
Figure 2B). The first cluster included station 3 in the seaside of El-Boughaz with relatively low similarity to the other stations being 76% on average. The second cluster included station 1 with 82% on average near to El-Boughaz. The third cluster included six stations with 88% (stations 12, 9, 10, 11, 2, and 4), and the highest similarity was between station 9 and 10 with 98%. The fourth cluster was between stations 7, 8, 5, and 6, but the highest similar one was between 5 and 6 with 94%.
During the year 2019, the data were found to be similar to that obtained in 2018 with slight differences in the arrangement of stations. Four major clusters were documented during this year. The first cluster was El-Boughaz station 3 with 76%. The second cluster included three stations with 81% on average nearby El-Boughaz. The third cluster included some the rest of the stations with four sub-clusters. The first included station 12, which had 93.5%. The second sub-cluster was between stations 9 and 10, with 98% highest similarity. The third sub-cluster included stations 11 with 90% (
Figure 2C). The fourth sub-cluster was between stations 2 and 4 with 93% on average. The fourth cluster was between stations 7, 8, 5, and 6, but the highest similarity was between 5 and 6 with 94%.
The changes in the character of the water are also reflected in the zooplankton population. Changes in the zooplankton population may be due to the changes in water conditions, introducing freshwater species and eliminating marine ones. Dumont and El-Shabrawy [
72] conclude that, from the early onset of dam construction in the Nile valley, slight changes happened in the zooplankton of Lake Burullus and three other delta lakes.
3.8. The Correlation between Physicochemical Parameters and Zooplankton Groups
Correlation analysis was achieved by Pearson’s correlation coefficient. The correlation matrix was calculated for the water quality parameters. A result of the correlation coefficient was evaluated as follows: 0.0 (no); 0.3–0.5 (low); 0.5–0.7 (medium); 0.7–0.9 (high); 0.9–1 (very high). In the present study, there were high significant correlations between many parameters such as Protozoa with Rotifera, Cirriped larvae, Velliger of lamellibranchs, salinity, and Zn ions (r = 0.925, 0.991, 0.946, 0.998, 0.897, respectively). Rotifera also showed a significant correlation with Copepoda, Cirriped larvae, pH, salinity, Chlo-a, and Pb ions (r = 0.954, 0.984, 0.925, 0.968, 0.961, 0.814, respectively). There is a significant positive correlation between Copepoda and Cirriped larvae, Velliger of lamellibranchs, salinity, and Zn ions (r = 0.992, 0.944, 0.999, 0.894, respectively). Cirriped larvae correlated negatively and some parameters such as salinity, NO3, and Chlor-a (r = −0.995, −0.970, −0.999, respectively). Ostracoda showed a negatively significant correlation with BOD, NH4, NO3, and Fe (r = −0.995, −0.981, −0.970, −0.959, respectively).
Cladocera correlated positively with parameters such as Ostracoda and Annelida with r = 0.97 and 0.94, respectively; while, it correlated negatively with parameters such as BOD, NH4, NO3, and Fe ions (r = −0.041, −0.91, −1.00, −0.999, respectively).
Velliger of lamellibranch showed correlations with DO, NO
2, PO
4, Cu, and Zn (r = 1, 0.96, −0.88, 0.89, and −0.975, respectively). Salinity has negative correlations with
Chlor-a and Cr (r = −1 and −0.925, respectively). The variations in correlation coefficients between salinity and zooplankton groups are due to the difference in species. The variations of mean salinity of the lake water are a result of the amount of drain water entering the lake from the southern part (this part receives freshwater supply from six drains) and seawater inflow to the lake from the northern part through El-Boughaz as well as the amount of evaporation from the external area of the lake. Salinity can be considered as a limiting factor for the abundance and diversity of zooplankton populations in the lake and the reverse relation between salinity and zooplankton. The pH concentration lies on the alkaline side, the minimum value recorded in the western sector showing an inverse relation to zooplankton. El-Enany [
79] stated that Protozoa, Cladocera, and Copepoda were abundant in the stations characterized by high TS, EC, PO
4, and Ca. The high numbers of drains from the River Nile, which carries high amounts of nutrients and salts, lead to the eutrophication of water.